Abstract
Background
Metastatic uveal melanoma is a highly aggressive disease with no standard of care treatment option. A large proportion of patients have liver‐only metastatic disease which raises the question if liver‐directed therapy can be efficacious in this subpopulation.
Aims
The study aims to evaluate the safety and efficacy of radiosensitizing chemotherapy in combination with yttrium‐90 microspheres in patients with uveal melanoma with liver‐only metastases.
Methods and results
This single arm, open labeled, non‐randomized study enrolled 10 patients with liver‐only metastatic uveal melanoma between November 2012 and January 2018. Eligible patients received intrahepatic yttrium‐90 microspheres followed by intravenous cisplatin (20 mg/m2) for 5 days. Ten patients were enrolled, but nine patients received treatment who were included in the final analysis with a median follow‐up of 30 months (range 7 to 44). Five (50%) were female, five (50%) had an elevated lactate dehydrogenase (LDH), and one (10%) had prior anti‐PD‐1 therapy. The combination was well tolerated with no greater than or equal to grade 3 toxicity observed. The liver objective response rate (ORR) was 33% (3/9), the median progression‐free survival (PFS) in the liver was 3 months (95% CI, 3‐NA), and the extrahepatic PFS was 3 months (95% CI, 3‐NA). Seventy‐eight percent (7/9) received an immune checkpoint inhibitor on disease progression, with no responses seen. The median overall survival (OS) was 10 months (95% CI, 7‐NA).
Conclusion
The combination of cisplatin with yttrium‐90 microspheres was well tolerated; however, it was associated with intrahepatic disease control of relatively short duration. No responses were seen in patients treated with immune checkpoint inhibitors post radioembolization.
Keywords: anti‐PD‐1 therapy, chemotherapy, uveal melanoma, yttrium‐90 microspheres
1. INTRODUCTION
Uveal melanoma is the most common primary intraocular malignancy in adults and is both clinically and genomically distinct from cutaneous melanoma.1 Despite successful local therapy, almost half of all patients develop metastases, with the majority relapsing in the liver.2 Unlike cutaneous melanoma, there are no effective systemic treatments for metastatic uveal melanoma, and indeed, there is no defined standard of care. There have been a number of agents used including chemotherapy, MEK inhibitors, and immune checkpoint inhibitors, but the outcomes remain poor.3, 4 This characteristic of liver‐predominant metastatic disease, coupled with poor response rates to systemic therapy, has led to the use of locoregional therapies directed at the hepatic metastases.5
Liver‐directed therapies such as radioembolization with yttrium‐90 (90Y)‐emitting microspheres (SIR‐Spheres microspheres) has been shown to be an effective therapy for both primary and secondary hepatic malignancies.6 In addition, several studies have shown yttrium‐90 microspheres can be safely combined with platinum‐based chemotherapy.7 There have been a number of other liver‐directed approaches in the treatment of uveal melanoma including intra‐arterial fotemustine, percutaneous isolated hepatic perfusion, and immunoembolization with granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), but none of these studies demonstrated an overall survival (OS) advantage.8, 9, 10
Our study aims to assess the feasibility of combining cisplatin with yttrium‐90 microspheres in uveal melanoma patients with liver‐only metastases. We postulate that the addition of cisplatin chemotherapy not only has a radiosensitizing effect but it also provides systemic control and prevention of extrahepatic disease.
2. METHODS
This open label, non‐randomized pilot study was conducted at the Austin Hospital, Melbourne, Australia, and approved by the institution's ethics committee (H2012/04888). Written informed consent was obtained from all patients, and all methods were performed in accordance with the protocol specified guidelines, the Declaration of Helsinki and guidelines for Good Clinical Practice.
Patients with histologically confirmed liver‐only metastatic uveal melanoma were enrolled between November 2012 and January 2018. Patients had to be 18 years of age or older, have an Eastern Cooperative Oncology Group (ECOG) performance‐status score of 0 or 1, and have adequate hematologic, hepatic, and renal function. Patients underwent angiographic and technetium‐99 macroaggregated albumin assessment of suitability prior to treatment. Exclusion criteria were significant shunting to the lung (greater than 20%), occlusion of the main portal vein, presence of extrahepatic disease, systemic immunosuppression, and previous liver‐directed therapy. Eligible patients received intrahepatic yttrium‐90 microspheres (SIR‐Spheres, Sirtex Medical Ltd., Australia) to an activity and dose determined using the body surface area model of Lau et al.11 Patients then received intravenous cisplatin (20 mg/m2) for 5 days starting immediately after microsphere implantation.
The primary end point of the study was safety, which was evaluated using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. Secondary end points evaluated were liver‐only and overall objective response rate (ORR), progression‐free survival (PFS), and OS. PFS was defined as time between date of commencement of therapy to date of progression or death. Disease assessments were performed utilizing computed tomography (CT) and magnetic resonance imaging (MRI) of the abdomen. Objective response determination was made as per the Response Evaluation Criteria in Solid Tumours (RECIST) v1.1.12 Data on post progression treatment and ORRs were collected to explore any possible continued effects from the trial on survival outcomes.
All patients who received the yttrium‐90 microspheres and cisplatin chemotherapy were evaluated for safety. OS, PFS, and liver‐only PFS were calculated using the Kaplan‐Meier method, and median results and confidence intervals were obtained via the R statistical software. Descriptive statistics were not performed because of the limited sample size and lack of consistent homogenous data.
3. RESULTS
A total of 10 patients were enrolled onto the study. Of those enrolled, nine patients were included in the final analysis; one patient died prior to receiving treatment due to rapid disease progression. The median duration of follow‐up after radioembolization was 30 months (range 7 to 44).
Patient characteristics show a mean age of 68 years; all patients were of ECOG PS 0‐1, and 50% had an elevated baseline lactate dehydrogenase (LDH) (Table 1). The median interval from diagnosis of metastatic disease to radioembolization was 3 months (range 1 to 62). No high grade treatment‐related toxicities were observed. Grades 1 and 2 toxicities were reported in 89% and included abdominal pain (n = 5, 56%), fatigue (n = 4, 44%), anorexia (n = 2, 22%), and nausea (n = 1, 11%) (Table 2). There were no other adverse events noted.
Table 1.
Patient characteristics
| Demographics | Patients (n = 10) |
|---|---|
| Age, y | |
| Mean | 68 |
| Range | 56‐75 |
| Sex, no. (%) | |
| Male | 5 (50%) |
| Female | 5 (50%) |
| ECOG performance status, no. (%) | |
| 0 | 6 (60%) |
| 1 | 4 (40%) |
| LDH, no. (%) | |
| ≤ULN | 5 (50%) |
| >ULN | 5 (50%) |
| Primary ocular therapy, no. (%) | |
| Enucleation | 5 (50%) |
| Radiotherapy/brachytherapy | 5 (50%) |
| Prior treatment for advanced disease, no. (%) | |
| Surgery | 1 (10%) |
| Anti‐PD‐1/PD‐L1 | 1 (10%) |
| Anti‐CTLA‐4 | 1 (10%) |
| None | 7 (70%) |
| Post embolization treatment ‐ no. (%) | |
| Anti‐PD‐1 | 7 (70%) |
| Anti‐CTLA‐4 | 1 (10%) |
| None | 2 (20%) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase, ULN, UpperLimit Normal.
Table 2.
Treatment details and responses
| Patient | Sex | Time from Initial Diagnosis to Liver Metastases, mo | Time from Diagnosis of Liver Metastases to Radioembolization, mo | Microsphere Delivered Activity, GBq | AEs | Best Liver Response | Duration of Response in Liver, mo | Best Overall Response | Subsequent Therapy | Best Response To Subsequent Therapy |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 45 | 4 | 1.8 | G1 fatigue | PD | 1 | PD | Nil | |
| 2 | M | 17 | 1 | 1.7 |
G1 nausea G1 abdominal pain |
SD | 9 | SD |
Pembrolizumab Ipilimumabb |
PD |
| 3 | F | 24 | 3 | 1.42 | G1 abdominal pain | PR | 29 | PR | Pembrolizumab | PD |
| 4 | M | 59 | 67 | 1.8 | G2 abdominal pain | PR | 9 | PR | Pembrolizumab | PD |
| 5 | M | 8 | 3 | 1.65 | G1 abdominal pain | PR | 3 | PDa | Pembrolizumab | PD |
| 6 | M | 14 | 1 | 2.3 |
G2 fatigue G2 anorexia G2 abdominal pain |
PD | 1 | PD | Pembrolizumab | PD |
| 7 | F | 40 | 8 | 2.0 | G1 fatigue | PD | 3 | PD |
Nivolumabb Trametinib |
PD |
| 8 | F | 98 | 2 | 2.5 |
G1 fatigue G1 anorexia |
SD | 3 | PDa | Nil | |
| 9 | F | 7 | 3 | 1.65 | Nil | SD | 6 | SD | Pembrolizumab | NE |
| 10 | M | 109 | 3 | Nil | Nil | 0 | Nil | Nil |
Abbreviations: AE, adverse event; G, grade; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
Extrahepatic disease progression despite ongoing intrahepatic disease control.
Sequential treatment.
The intrahepatic ORR was 33% (n = 3 PR), with no CRs. The intrahepatic disease control rate (DCR) was 68% (n = 3 PR, n = 3 SD) (Figure 1). The median duration of response in the liver was 3 months (range 1 to 29), with one patient achieving a response lasting 29 months. The overall (intra‐ and extrahepatic disease) ORR was 22% (n = 2 PR), and DCR was 44% (n = 2 PR, n = 2 SD) due to extrahepatic progression in two patients despite ongoing intrahepatic disease control (n = 1 intrahepatic PR, n = 1 intrahepatic SD) (Table 2). Of the patients treated with radioembolization, 78% (7/9) received an immune checkpoint inhibitor upon disease progression, with no responses seen (Table 2). The median PFS in the liver post radioembolization was 3 months (95% CI, 3‐NA). The overall median PFS was 3 months (95% CI, 3‐NA), and median OS was 10 months (95% CI, 7‐NA). The 1‐year OS was 44%.
Figure 1.
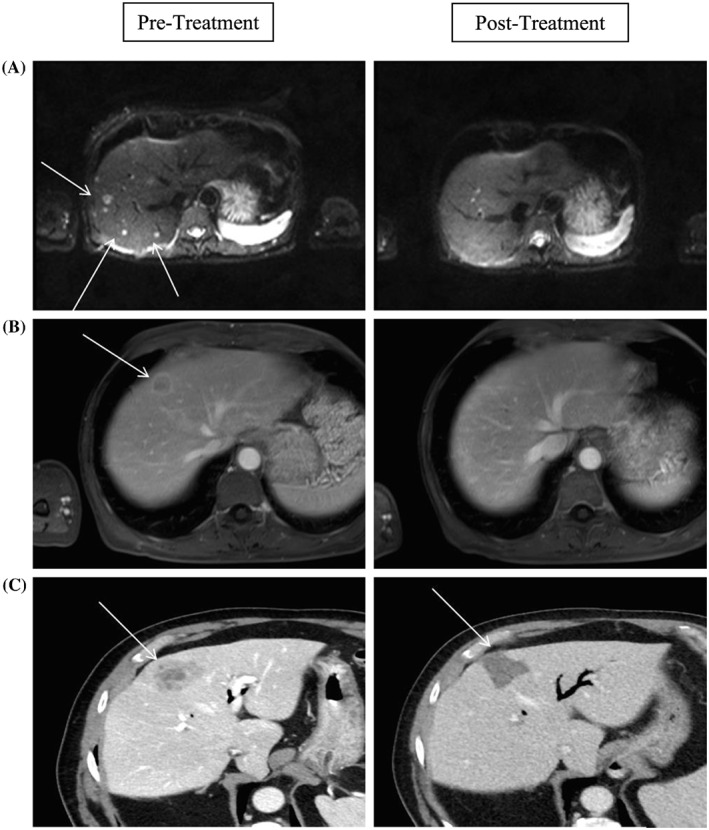
A, MRI diffusion‐weighted images showing partial resolution of pepperpot disease post‐90Y resin microspheres implantation 6 months post treatment. B, Portal venous phase MRI showing partial resolution of the target segment eight lesion post‐90Y resin microspheres implantation 10 months post‐90Y resin microspheres implantation. C, Portal venous phase CT showing complete necrosis of the target lesion 3 months post‐90Y resin microspheres implantation. CT, computed tomography; MRI, magnetic resonance imaging
4. DISCUSSION
This is the first study to evaluate the combination of radiosensitizing chemotherapy with yttrium‐90 microspheres in patients with uveal melanoma and liver‐only metastases. Treatment‐related adverse events reported in this study were mild and are consistent with other reports13, 14; all adverse events were managed with standard supportive care. The addition of low‐dose cisplatin in this study did not result in high grade adverse events. Importantly, there was no radiation‐induced liver disease or treatment‐related deaths in our study.
The PFS and OS reported in our study are similar to those in other studies.13, 14 Higher 1‐year survival and ORR were reported by Kennedy et al15; however, differences in prior treatment as well as imaging modalities used to evaluate responses are likely to account for this.
Preliminary results of a study evaluating various liver‐directed catheter therapies in patients with unresectable liver metastases from chemotherapy‐refractory cutaneous or ocular melanoma reported better PFS (10.1 vs 2.30 months; P = .04) and OS (15.5 vs 5.9 months; P = .04) in those treated with yttrium‐90 microspheres compared with standard transcathether ablation.16 Patients with low‐volume metastatic disease and absence of extrahepatic disease demonstrate greatest survival following yttrium‐90 radioembolization treatment.17 Of note, our study included patients with poor prognostic factors including 50% with an elevated LDH and relatively moderate liver disease burden.
In addition, we were able to evaluate the efficacy of anti‐programmed cell death protein‐1 (PD‐1)‐based therapies post radioembolization. The majority of our cohort received anti‐PD‐1 immune checkpoint blockade therapy following progression after radioembolization/cisplatin. Small prospective and retrospective studies evaluating immune checkpoint inhibitors in metastatic uveal melanoma have been disappointing with poor ORRs and no survival benefit demonstrated.18 In our study, none of the patients that received immune checkpoint inhibitors post radioembolization had disease response. Although not directly assessed, these results suggest that there was no clinically relevant effect of radioembolization on immune priming or generation of immunogenic cell death within treated tumors in what is known to be a typically immunotherapy‐resistant melanoma subtype.
The exact mechanisms by which uveal melanomas evade the immune system yet remain resistant to existing checkpoint blockade immunotherapies remain to be elucidated; however, possible reasons include use of immunosuppressive pathways and checkpoints other than the PD‐1/PD‐L1 axis, and a low somatic mutation burden resulting in lower intrinsic immunogenicity relative to cutaneous melanomas.19 In contrast to cutaneous melanoma, a high proportion of tumor infiltrating lymphocytes (TILs) in uveal melanoma tumors is associated with a poorer outcome which could reflect differences in the functional composition of those TIL, and the presence of incompletely enumerated nonfunctional or regulatory/suppressive cell subsets.20
Intriguingly, a recent meta‐analysis demonstrated that pooled OS was improved with liver‐directed strategies including isolated hepatic perfusion, immunoembolization, and surgery compared with chemotherapy, but OS was inferior in patients who received immune checkpoint inhibitors when compared with chemotherapy.21 Possible reasons for this are the inclusion of studies with small sample sizes, heterogeneous disease assessment tools and differing patient characteristics (ie, patients who were treated with liver‐directed therapy were highly selected, and patients treated with immune checkpoint inhibitors were more likely to have been treated with prior liver‐directed therapy or have extrahepatic disease).
Larger prospective studies are required to confirm the benefit of liver‐directed therapies in this patient population especially given that a third of our study patients rapidly developed extrahepatic progression post‐treatment. Biomarker development is urgently needed to identify these rapidly progressing patients. In addition, translational studies are required to determine if locoregional therapies are capable of enhancing immune responses and thereby improving responses to immune checkpoint therapies.
CONFLICT OF INTEREST
Dr Surein Arulananda has received speaker fees from Merck Sharpe & Dohme, Astra Zeneca and Roche and travel support from Astra Zeneca and Roche. Professor Jonathan Cebon has received honoraria from Amgen, Bristol‐Myers Squibb, GlaxoSmith Kline, Merck; Merck Sharp & Dohme and Novartis. All other authors have declared no conflicts of interest.
AUTHORS' CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, M.C.A., J.C.; Methodology, M.G., M.C.A., J.C.; Investigation, S.A., S.P., M.G., M.C.A., J.C.; Formal Analysis, S.A.; Resources, J.C.; Writing ‐ Original Draft, S.A., S.P.; Writing ‐ Review & Editing, M.C.A., J.C.; Visualization, J.P.; Supervision, J.C.; Funding Acquisition, J.C.
ACKNOWLEDGMENTS
We thank SIRTEX for providing and funding yttrium‐90 microspheres for use in this study. Dr Surein Arulananda received a La Trobe University Postgraduate Research Scholarship for his PhD studies.
Arulananda S, Parakh S, Palmer J, Goodwin M, Andrews MC, Cebon J. A pilot study of intrahepatic yttrium‐90 microsphere radioembolization in combination with intravenous cisplatin for uveal melanoma liver‐only metastases. Cancer Reports. 2019;2:e1183. 10.1002/cnr2.1183
Data Availability Statement: All data generated or analyzed during this study are included in this published article.
S. Arulananda and S. Parakh share first authorship.
M.C. Andrews and J. Cebon share senior authorship.
REFERENCES
- 1. Robertson AG, Shih J, Yau C, et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell. 2017;32(2):204‐220. e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vajdic CM, Kricker A, Giblin M, et al. Incidence of ocular melanoma in Australia from 1990 to 1998. Int J Cancer. 2003;105(1):117‐122. [DOI] [PubMed] [Google Scholar]
- 3. Carvajal RD, Piperno‐Neumann S, Kapiteijn E, et al. Selumetinib in combination with dacarbazine in patients with metastatic uveal melanoma: a phase III, multicenter, randomized trial (SUMIT). J Clin Oncol. 2018;36(12):1232‐1239. [DOI] [PubMed] [Google Scholar]
- 4. Kottschade LA, McWilliams RR, Markovic SN, et al. The use of pembrolizumab for the treatment of metastatic uveal melanoma. Melanoma Res. 2016;26(3):300‐303. [DOI] [PubMed] [Google Scholar]
- 5. Schuster R, Lindner M, Wacker F, et al. Transarterial chemoembolization of liver metastases from uveal melanoma after failure of systemic therapy: toxicity and outcome. Melanoma Res. 2010;20(3):191‐196. [DOI] [PubMed] [Google Scholar]
- 6. Sato KT, Lewandowski RJ, Mulcahy MF, et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres—safety, efficacy, and survival. Radiology. 2008;247(2):507‐515. [DOI] [PubMed] [Google Scholar]
- 7. Sharma RA, Van Hazel GA, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using yttrium‐90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 2007;25(9):1099‐1106. [DOI] [PubMed] [Google Scholar]
- 8. Leyvraz S, Piperno‐Neumann S, Suciu S, et al. Hepatic intra‐arterial versus intravenous fotemustine in patients with liver metastases from uveal melanoma (EORTC 18021): a multicentric randomized trial. Ann Oncol. 2014;25(3):742‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valsecchi ME, Terai M, Eschelman DJ, et al. Double‐blinded, randomized phase II study using embolization with or without granulocyte‐macrophage colony‐stimulating factor in uveal melanoma with hepatic metastases. J Vasc Interv Radiol. 2015;26(4):523‐32 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varghese S, Xu H, Bartlett D, et al. Isolated hepatic perfusion with high‐dose melphalan results in immediate alterations in tumor gene expression in patients with metastatic ocular melanoma. Ann Surg Oncol. 2010;17(7):1870‐1877. [DOI] [PubMed] [Google Scholar]
- 11. Lau W‐Y, Kennedy AS, Kim YH, et al. Patient selection and activity planning guide for selective internal radiotherapy with yttrium‐90 resin microspheres. Int J Radiat Oncol Biol Phys. 2012;82(1):401‐407. [DOI] [PubMed] [Google Scholar]
- 12. Eisenhauer E, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228‐247. [DOI] [PubMed] [Google Scholar]
- 13. Eldredge‐Hindy H, Ohri N, Anne PR, et al. Yttrium‐90 microsphere brachytherapy for liver metastases from uveal melanoma: clinical outcomes and the predictive value of fluorodeoxyglucose positron emission tomography. Am J Clin Oncol. 2016;39(2):189‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klingenstein A, Haug A, Zech C, Schaller U. Radioembolization as locoregional therapy of hepatic metastases in uveal melanoma patients. Cardiovasc Intervent Radiol. 2013;36(1):158‐165. [DOI] [PubMed] [Google Scholar]
- 15. Kennedy AS, Nutting C, Jakobs T, et al. A first report of radioembolization for hepatic metastases from ocular melanoma. Cancer Invest. 2009;27(6):682‐690. [DOI] [PubMed] [Google Scholar]
- 16. Dhanasekaran R, Khanna V, Lawson D, Delman K, Kim H. Abstract no. 167: survival benefits of yttrium‐90 radioembolization (SIR‐spheres) for hepatic metastasis from melanoma: preliminary study. J Vasc Interv Radiol. 2009;20(2):S65. [Google Scholar]
- 17. Xing M, Prajapati HJ, Dhanasekaran R, et al. Selective internal yttrium‐90 radioembolization therapy (90Y‐SIRT) versus best supportive care in patients with unresectable metastatic melanoma to the liver refractory to systemic therapy: safety and efficacy cohort study. Am J Clin Oncol. 2017;40(1):27‐34. [DOI] [PubMed] [Google Scholar]
- 18. Komatsubara KM, Carvajal RD. Immunotherapy for the treatment of uveal melanoma: current status and emerging therapies. Curr Oncol Rep. 2017;19(7):45. [DOI] [PubMed] [Google Scholar]
- 19. Furney SJ, Pedersen M, Gentien D, et al. SF3B1 mutations are associated with alternative splicing in uveal melanoma. Cancer Discov. 2013;3(10):1122‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Javed A, Arguello D, Johnston C, et al. PD‐L1 expression in tumor metastasis is different between uveal melanoma and cutaneous melanoma. Immunotherapy. 2017;9(16):1323‐1330. [DOI] [PubMed] [Google Scholar]
- 21. Rantala ES, Hernberg M, Kivela TT. Overall survival after treatment for metastatic uveal melanoma: a systematic review and meta‐analysis. Melanoma Res. 2019. 10.1097/CMR.0000000000000575 [DOI] [PMC free article] [PubMed] [Google Scholar]


