Abstract
Background
Patients with lung cancer (LC) report lower quality of life (QoL) and higher levels of psychological distress compared with other cancer populations. Lung cancer stigma (LCS) may in part explain these findings.
Aim
We investigated the prevalence of patient‐perceived lung cancer stigma (LCS) and its relationships to symptom burden/severity, depression, and deficits in health‐related quality of life (HR‐QoL).
Methods
In this descriptive, observational, and cross‐sectional study, 201 participants were sent questionnaires. These included the Cataldo Lung Cancer Stigma Scale (CLCSS), the Lung Cancer Symptom Scale, the Centre for Epidemiologic Studies‐Depression Scale, and the Quality of Life Inventory.
Results
Participants were on average 69 years old, 52% women, 95% ever smokers, and 18.5% current smokers. The mean total CLCSS score was 53.1 (SD = 14.1; range = 31‐94). LCS was significantly correlated with younger age (P < .001), greater social deprivation (P < .05), being unemployed (P < .001), depression (P < .001), symptom burden (P < .001), and HR‐QoL deficits (P < .001). Symptom burden explained 18% of variance in LCS (P < .001). LCS explained 8.5% and 14.3% of the variance in depression (P < .001) and HR‐QoL (P < .001), respectively.
Conclusion
Patients with lung cancer are vulnerable to LCS. Symptom burden can directly contribute to greater perceived LCS. Greater perceived LCS can be directly related to greater levels of depression and lower HR‐QoL. A tailored approach is required to screen for LCS and implement interventions to enhance the psychosocial well‐being of patients with perceived LCS.
Keywords: cancer support, clinical care, health‐related stigma, oncology, psychological well‐being
1. INTRODUCTION
Compared with patients with a history of other cancers, patients with lung cancer (LC) are more likely to report only fair or poor health, and exhibit significant psychological problems.1 Psychological morbidity has been associated with increased symptom burden and lack of social support,2 being the precursor of poor health‐related quality of life (HR‐QoL) and increased risk for mortality.3 Specific to LC, up to 55% of patients may meet the criteria for clinical depression.4 In addition, the association between depression and mortality is stronger amongst patients exhibiting more symptoms and those who report less social support.2
Psychological morbidity in patients with LC may have its origins in perceived lung cancer stigma (LCS). In his classic work about stigma, Goffman5 defined stigma as “an attribute that is deeply discrediting.” This can result in patients feeling unjustly blamed for their illness and particularly stigmatised because the disease is so strongly associated with smoking.6, 7 Blame has often been cited as a major stressor of having LC.8, 9 Indeed, patients with LC are more likely to report internal causal attributions for their cancer than patients with breast or prostate cancer.10 Previous US‐based research has demonstrated a link between LCS and poor patient outcomes. Specifically, LCS was associated with greater depressive symptomatology,11, 12, 13 poorer HR‐QoL,12 and higher symptom severity.4 Furthermore, LCS was found to account for unique and significant variability of 5%, 1.2%, and 1.3% in depression,13 HR‐QoL,11 and symptom severity,4 respectively.
Health‐related stigma is a culturally derived phenomenon.14 Therefore, it is important to understand the effects of perceived LCS in a UK sample to inform health care providers of the needs of their patients with the intention to develop support pathways for these patients. To date, studies that quantify LCS are limited to studies from the USA and Australia.11, 15, 16, 17, 18 No research has been conducted in the United Kingdom to explore LCS and its relationship with symptom burden/severity, depression, and HR‐QoL. Investigating and understanding these relationships is a necessary first step to facilitate the development of possible targeted LCS interventions. This study aimed to address this existing gap in knowledge in the UK as follows.
1.1. Aims and hypothesis
Our aim was to investigate the prevalence of patient‐perceived LCS and the role of LCS as a covariate of symptom severity and/or burden, depression, and HR‐QoL.
We aimed to address the following research questions:
What are the prevalence and characteristics of perceived LCS in LC patients in Scotland?
What are the effects of LC symptom severity and/or burden on perceived LCS, controlling for patient sociodemographic characteristics and clinical variables?
What are the effects of perceived LCS on depression, controlling for patient sociodemographic characteristics and clinical variables?
What are the effects of perceived LCS on HR‐QoL, controlling for patient sociodemographic characteristics and clinical variables?
We specifically hypothesised that greater symptom burden and/or severity would be adversely related to greater perceived LCS. In turn, greater perceived LCS would be associated with greater levels of depression and lower HR‐QoL.
2. METHODS
2.1. Study design and setting
We conducted a descriptive, observational, cross‐sectional study. We recruited a convenience sample of patients with LC from two NHS Health Boards in Scotland, and within these Boards, recruitment took place at six regional hospitals. Reporting of the study was guided by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement (Von Elm et al, 2014).
2.2. Eligibility criteria
Potential participants had to (a) be aged ≥18 years, (b) have a clinical diagnosis of LC, (c) be able to understand study information, and (d) be physically and psychologically fit to participate as deemed by a member of the health care team. We excluded patients unable to meet any of the above criteria.
2.3. Procedures
The study received a favourable ethical opinion from the NRES Committee East of England ‐ Norfolk (Ref 14/EE/1099). Health professionals identified potential participants during routinely scheduled clinic visits. They briefly outlined the purposes of the study and provided a study pack which contained the patient information sheet, a consent form to be contacted for research interviews (details reported elsewhere), self‐reported questionnaires, and a stamped envelope to return the completed pack anonymously to the researcher. Completion of the questionnaires acted as study consent. If the patient did not return the study packs within 14 days, a reminder letter was sent.
2.4. Outcome measures
2.4.1. Cataldo Lung Cancer Stigma Scale (CLCSS)
The CLCSS is a valid and reliable measure to assess the experiences of stigma.12 It comprises 31 items and four subscales (stigma and shame, social isolation, discrimination, and smoking) that are rated on a four‐point Likert scale (“strongly disagree,” “disagree,” “agree,” and “strongly agree”). A higher score indicates greater experience of stigma (possible score range 31‐124). Cronbach's alpha was 0.93. Construct validity was demonstrated with expected relationships with depression in our study as well as other studies.12, 13
2.4.2. Lung Cancer Symptom Scale (henceforth “LC symptom scale”)
The LC symptom scale measures the severity of LC specific symptoms and has demonstrated good reliability and content validity.19 The scale has nine items (rated from 0 to 100), which measure symptoms, symptom burden, and symptom severity. Higher scores indicate greater symptom severity. Cronbach's alpha was 0.84.
2.4.3. Centre for Epidemiologic Studies‐Depression Scale (CES‐D)
The CES‐D is a widely used tool to assess depression, with established validity and reliability in a wide range of populations including cancer.20 Twenty items are rated on a four‐point scale. Higher scores indicate greater depression (score range 0‐60). Cronbach's alpha was 0.89.
2.5. Quality of Life Inventory (QLI)
The QLI has four subscales (physical, psychological, social, and spiritual) and consists of 33 items.21 This measure was previously validated with a population with LC.22 Higher scores indicate better HR‐QoL (score range 0‐10). Cronbach's alpha was 0.91.
We collected self‐reported sociodemographic information (eg, smoking history) and clinical data from case note review, including LC type and stage, presenting symptoms, mode of presentation, date of diagnosis, time since being diagnosed, previous treatment for LC, current management/treatment of LC, and any comorbidities.
2.6. Data analysis
We first investigated the factor structure of the CLCSS in our sample to account for any cultural diversity compared with the original factor structure based on US‐based data.12 Compared with Cataldo et al12 who reported a four‐factor structure (Stigma and Shame, Social Isolation, Discrimination, and Smoking), our analyses resulted in five factors (Social Isolation, Smoking, Stigma and Shame, Health care provider Stigma, and Discrimination) (Table 1). The number of items in the scale per factor is reported in Table 1 (see Supplementary file S1 and Figure S2 for details).
Table 1.
Descriptive statistics for the CLCSS total and component scores
| CLCSS Component (N = 201) | N Items | Mean (SD); Median | Actual Range | Possible Range |
|---|---|---|---|---|
| Component 1—Social isolation | 14 | 22.1 (7.6); 20.0 | 14‐48 | 14‐56 |
| Component 2—Smoking | 3 | 7.6 (2.4); 8.0 | 3‐12 | 3‐12 |
| Component 3—Stigma and shame | 8 | 12.6 (4.2); 12.0 | 8‐26 | 8‐32 |
| Component 4—Health care provider stigma | 2 | 3.6 (1.5); 3.0 | 2‐8 | 2‐8 |
| Component 5—Discrimination | 4 | 7.2 (2.3); 7.0 | 4‐16 | 4‐16 |
| Scale | 31 | 53.1 (14.1); 53.0 | 31‐94 | 31‐124 |
Abbreviation: CLCSS, Cataldo Lung Cancer Stigma Scale.
Descriptive statistics and graphs were used to analyse CLCSS data. Frequency counts and percentages of responses were generated to describe response patterns and quantify missing data. Total and subscale CLCSS scores were treated as interval‐ratio variables and are presented as means, standard deviation, and range.
Bivariate associations were explored between sociodemographic characteristics and clinical factors (interval‐ratio or categorical), and all patient outcome variables (interval‐ratio). Relationships between variables were determined using two‐sample t‐test, one‐way analysis of variance, or correlation coefficient calculation. Relationships amongst patient outcome variables were tested through correlation analysis. The functional form of these relationships and any necessary transformations were explored and assumptions of normality checked.
Sociodemographic characteristics and clinical factors statistically significantly associated with patient outcome variables were retained and their effects controlled for in subsequent hierarchical regression modelling to investigate: (a) the effects of LC symptom burden on perceived LCS and (b) the effects of perceived LCS on depression and HR‐QoL after controlling for significant sociodemographic and clinical covariates identified in the previous step. Assumptions relating to the normal distribution of errors and multicollinearity were investigated; no modifications to the analysis were required. Cumulative R2 and R2 change (ΔR2) was reported for each model to indicate the unique contribution of each predictor variable in explaining the variance of each dependent variable. The level of significance was set at 0.05. IBM SPSS (IBM Inc. Chicago, IL) was used for the statistical analysis.
3. RESULTS
From 368 potential participants approached, 201 (54.6%) returned questionnaire packs. Case notes were accessible and reviewed for 195 participants. The total sample had a mean age of 69.2 ± 9.1 years (range 41‐89 years), 52% were women, 63.5% were married/partnered, and 71.2% were in a cohabitating situation(Table 2). Ninety‐five percent were ever‐smokers, 18.5% current smokers, and 76.9% were quitters. The majority of participants had localised LC (51.9%), non‐small cell LC (91.2%), and received surgery alone or in combination with systemic treatment (49.7%).
Table 2.
Characteristics of all study participants (n = 201)
| Variable | Mean (SD); Median | Min‐Max |
|---|---|---|
| Age in years (N = 190) | 69.2 (9.1); 70.5 | 41‐89 |
| Years since diagnosis (N = 187) | 2.6 (1.8); 1.9 | 0.2‐11.0 |
| Variable | N | % |
| Gender (N = 196) | ||
| Male | 94 | 48.0 |
| Female | 102 | 52.0 |
| Age categories (N = 190) | ||
| 41‐50 years | 8 | 4.2 |
| 51‐60 years | 25 | 13.2 |
| 61‐70 years | 62 | 32.6 |
| 71‐80 years | 75 | 39.5 |
| >80 years | 20 | 10.5 |
| Ethnicity (N = 198) | ||
| White | 196 | 99.0 |
| Other | 2 | 1.0 |
| Marital status (N = 200) | ||
| Married/partnered | 127 | 63.5 |
| Divorced/separated | 24 | 12.0 |
| Widowed | 36 | 18.0 |
| Single | 13 | 6.5 |
| Living status (N = 198) | ||
| Alone | 57 | 28.8 |
| With partner/family/friend | 141 | 71.2 |
| Highest educational attainment (N = 197) | ||
| Primary school | 28 | 14.2 |
| High school | 122 | 61.9 |
| Higher education college | 33 | 16.8 |
| University | 14 | 7.1 |
| Employment status (N = 199) | ||
| Employed (full‐time/part‐time) | 24 (18/6) | 12.0 |
| Unemployed | 17 | 8.5 |
| Retired | 149 | 75.0 |
| Other (eg, home maker) | 9 | 4.5 |
| Annual income in £ (N = 164) | ||
| <10 000 | 64 | 39.0 |
| 10 001‐20 000 | 65 | 39.6 |
| 20 001‐50 000 | 30 | 18.3 |
| >50 000 | 5 | 3.1 |
| Deprivation index (SIMD)a (N = 185) | ||
| Fifth 1 (most deprived) | 71 | 38.4 |
| Fifth 2 | 35 | 18.8 |
| Fifth 3 | 29 | 15.7 |
| Fifth 4 | 24 | 13.0 |
| Fifth 5 (least deprived) | 26 | 14.1 |
| Smoking status (N = 195) | ||
| Ever smoker | 185 | 94.9 |
| Smoking quitter | 150 | 76.9 |
| Current smoker | 36 | 18.5 |
| Never‐smoker | 10 | 5.1 |
| Symptomatic prior to diagnosis (N = 199) | ||
| Yes | 153 | 76.9 |
| Presentation (N = 195) | ||
| GP | 151 | 77.4 |
| Emergency | 8 | 4.1 |
| Incidental | 36 | 18.5 |
| Suspected asbestos exposure (N = 197) | 36 | 18.3 |
| Disease status (N = 183) | ||
| Local | 95 | 51.9 |
| Locally advanced | 52 | 28.4 |
| Metastatic | 31 | 16.9 |
| Not staged | 5 | 2.8 |
| Treatment (N = 195) | ||
| Surgery only | 62 | 31.8 |
| Surgery in combination with other treatment | 35 | 17.9 |
| Chemotherapy | 87 | 44.6 |
| Radiotherapy | 74 | 37.9 |
| No. of comorbid illnesses (N = 188) | ||
| None | 40 | 21.3 |
| One | 83 | 44.1 |
| Two | 44 | 23.4 |
| Three or more | 21 | 11.2 |
“Local disease” is defined as any tumour that has not spread to the nodes; “Locally advanced” is defined as the spread to lymph nodes; “Metastatic” is defined as disease that is not localised and has spread to other parts of the body.
Abbreviation: SD, Standard deviation.
3.1. Prevalence of LCS, symptom severity/burden, depression, and HR‐QoL deficits
CLCSS score was 53.1 (SD = 14.1; Table 1). Perceptions of LCS more frequently reflected eight CLCSS items/statements, endorsed by at least 20% of the sample (Figure 1). Most frequently, respondents agreed/strongly agreed that others assumed that LC was caused by smoking even if the patient had stopped smoking years ago (61.7%) or never smoked at all (55.2%). Just over half the respondents “agreed”/“strongly agreed” that LC is viewed as a self‐inflicted disease (51.2%). Eighteen per cent revealed that others had told them that being diagnosed with LC was what they deserved for smoking. One in five respondents agreed that they felt guilty for having LC (20%) and felt the need to hide their LC (20%). Nearly a quarter of participants (23.9%) believed that older people are less likely to be blamed for LC compared with younger ones, that health professionals do not take smoker's cough seriously (26.9%), and that they put off seeking medical help because they were afraid (23.9%).
Figure 1.
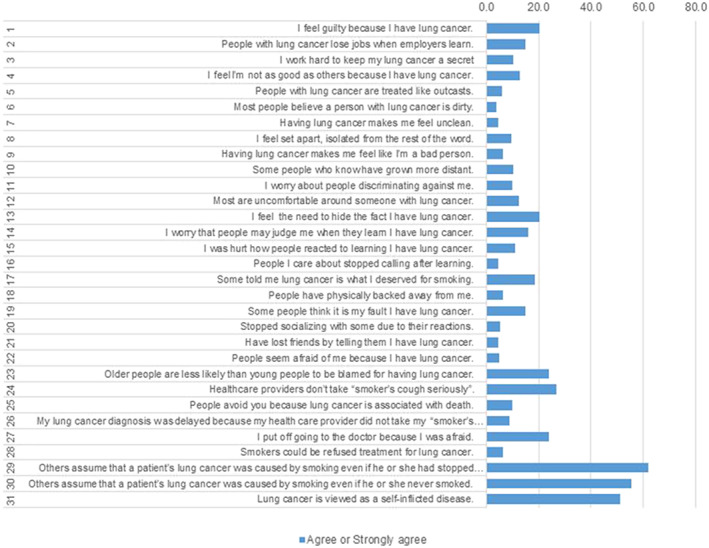
Item‐by‐item prevalence of “agree” or “strongly agree” responses (indicating perceived LCS) on the LCSS
Symptom burden and symptom severity were low (mean: 31.1, 21.9) (Table 3). Shortness of breath and fatigue were on average reported as the most severe symptoms, followed by appetite loss and cough. Over half (55.2%) of respondents met the criteria for clinical depression (score > 16). HR‐QoL was moderate to good on average.
Table 3.
Descriptive statistics for depression, symptom burden, and HR‐QoL
| Variable (N = 201) | Mean (SD); Median | Actual Range | Possible Range |
|---|---|---|---|
| Depression | 16.2 (10.9); 14.0 | 0‐48 | 0‐60 |
| Symptom overall score | 32.1 (18.0); 31.6 | 0‐84 | 0‐100 |
| Symptom burden index | 31.1 (17.2); 29.7 | 0‐85 | 0‐100 |
| Symptom severity | 21.9 (5.4); 22.0 | 16‐89 | 0‐100 |
| Individual symptoms | |||
| Appetite loss | 31.9 (26.9); 27.0 | 0‐96 | 0‐100 |
| Fatigue | 49.1 (28.3); 49.0 | 0‐100 | 0‐100 |
| Cough | 29.5 (28.5); 21.0 | 0‐100 | 0‐100 |
| Shortness of breath | 49.2 (32.1); 51.0 | 0‐100 | 0‐100 |
| Haemoptysis | 4.8 (12.5); 1.0 | 0‐83 | 0‐100 |
| Pain | 23.0 (29.2); 8.0 | 0‐100 | 0‐100 |
| Quality of life | |||
| Overall score | 5.8 (1.4); 5.8 | 0‐9 | 0‐10 |
| Physical | 6.5 (1.8); 6.4 | 1‐10 | 0‐10 |
| Psychological | 5.5 (1.7); 5.6 | 0‐10 | 0‐10 |
| Social | 6.7 (1.9); 7.0 | 0‐10 | 0‐10 |
| Spiritual | 4.7 (2.1); 4.4 | 0‐10 | 0‐10 |
Abbreviations: HR‐QoL, health‐related quality of life; LCS, LC Stigma.
3.2. Relationships amongst study variables
Positive correlations were demonstrated with depression, symptom burden, and all LC symptoms except for appetite loss (Table 4). A moderate‐to‐strong negative correlation of perceived LCS with overall HR‐QoL and all HR‐QoL sub‐domains was found.
Table 4.
Relationships amongst the study variables
| Variable | LCS | Depression | HR‐QoL |
|---|---|---|---|
| Age (N = 190) | r = −0.28** | r = −0.19* | r = 0.35** |
| Time since diagnosis (N = 187) | r = 0.09 | r = 0.17 | r = −0.02 |
| Gender (N = 196) | t = 0.08 | t = −2.22* | t = 0.93 |
| Marital status (N = 200) | F = 1.15 | F = 1.93 | F = 0.75 |
| Living status (N = 198) | t = 0.97 | t = 2.95** | t = −1.23 |
| Highest educational attainment (N = 197) | F = 0.57 | F = 0.61 | F = 0.02 |
| Employment status (N = 199) | F = 8.81*** | F = 6.88** | F = 13.51*** |
| Annual income (N = 164) | F = 0.52 | F = 4.18* | F = 2.28 |
| Deprivation index (SIMD) (N = 185) | t = 2.23* | t = 1.50 | t = −0.79 |
| Ever smoker (N = 195) | t = 0.91 | t = 1.18 | t = −0.97 |
| Smoking quitter (N = 195) | t = −1.64 | t = −3.43** | t = 3.00** |
| Current smoker (N = 194) | t = 1.81 | t = 3.56*** | t = −3.12** |
| Symptomatic prior to diagnosis (N = 199) | t = 0.20 | t = 0.66 | t = −1.98* |
| Presentation (N = 195) | F = 0.43 | F = 0.26 | F = 1.02 |
| Asbestos exposure (N = 197) | t = 0.43 | t = −1.09 | t = 1.08 |
| Localised disease (N = 183) | t = −1.55 | t = −0.65 | t = 1.83 |
| Locally advanced (N = 183) | t = 1.20 | t = 0.25 | t = −1.22 |
| Metastatic disease (N = 183) | t = 0.59 | t = 0.56 | t = −0.93 |
| Surgery only (N = 195) | t = −0.32 | t = −0.98 | t = 1.84 |
| Surgery in combination with other treatment (N = 195) | t = 0.75 | t = 0.42 | t = −0.35 |
| Chemotherapy (N = 195) | t = 0.68 | t = 0.68 | t = −1.18 |
| Radiotherapy (N = 195) | t = 0.64 | t = 0.90 | t = −2.04* |
| No. of comorbid illnesses (N = 188) | F = 0.94 | F = 0.17 | F = 0.25 |
| Quality of life (N = 201) | |||
| Overall score | r = −0.52*** | r = −0.69** | ‐ |
| Physical | r = −0.32*** | r = −0.61** | ‐ |
| Psychological | r = −0.49*** | r = −0.65** | ‐ |
| Social | r = −0.52*** | r = −0.58** | ‐ |
| Spiritual | r = −0.18** | r = −0.16** | ‐ |
| Depression (N = 201) | r = 0.40*** | ‐ | r = −0.69** |
| Symptom overall score (N = 201) | r = 0.29*** | r = 0.67** | r = −0.67** |
| Symptom burden index (N = 201) | r = 0.26*** | r = 0.64** | r = −0.61** |
| Symptom severity (N = 201) | r = 0.07 | r = 0.10 | r = −0.07 |
| Individual symptoms | |||
| Appetite loss (N = 201) | r = 0.14 | r = 0.47** | r = −0.42** |
| Fatigue (N = 201) | r = 0.21*** | r = 0.56** | r = −0.53** |
| Cough (N = 201) | r = 0.19*** | r = 0.34** | r = −0.31** |
| Shortness of breath (N = 201) | r = 0.15* | r = 0.38** | r = −0.40** |
| Haemoptysis (N = 201) | r = 0.19*** | r = 0.26** | r = −0.21** |
| Pain (N = 201) | r = 0.16* | r = 0.46** | r = −0.46** |
“Local disease” is defined as any tumour that has not spread to the nodes; “Locally advanced” is defined as the spread to lymph nodes; “Metastatic” is defined as disease that is not localised and has spread to other parts of the body.
Abbreviations: G, general practitioner; LCS, LC Stigma; QoL, quality of life; SIMD, Scottish Index of Multiple Deprivation.
P < .05.
P < .01.
P < .001.
Negative correlations between LCS scores and sociodemographic variables were found with age, (Table 4). Positive correlations with LCS scores were observed for unemployed/homemaker status and deprivation index. Although not statistically significant, current smokers reported on average greater perceived LCS than people who were not currently smokers.
We identified significant relationships of higher levels of depression with younger age, female gender, living alone, being unemployed or homemaker, having a low annual income, being a smoking non‐quitter or a current smoker, lower HR‐QoL, and higher symptom burden (Table 4).
Significant relationships of poorer HR‐QoL with younger age, being unemployed or a homemaker, being a smoking quitter or a current smoker, being symptomatic prior to LC diagnosis, having received radiotherapy, higher depression levels, and higher symptom burden were found.
3.3. Symptom burden as a predictor of greater perceived LCS
Accounting for the effects of age, employment status and social deprivation, and hierarchical regression showed that the overall regression model of the second step was statistically significant with 18% of the variance in symptom burden being explained, F(4, 174) = 9.24, P < .001. More details are shown in Table 5. Incremental R2 for symptom burden was significant (ΔR2 = 0.05).
Table 5.
Summary of hierarchical regression analysis for symptom burden predicting LCS
| Step | Predictor | β | ΔR2 |
|---|---|---|---|
| 1 | Sociodemographic characteristics | 0.13*** | |
| Age | −0.23** | ||
| Unemployed or home maker | 0.11* | ||
| Most deprived socioeconomic area | −0.12 | ||
| 2 | Patient outcome variables | 0.05*** | |
| Symptom burden index | 0.22*** | ||
| Cumulative R2 | 0.18*** |
Dependent variable: LCS.
Notes: Betas shown are for the last step; Employment status was coded as a binary variable (unemployed/home maker vs employed/retired); Social deprivation was coded as a binary variable (1st fifth vs all other).
P < .05.
P < .01.
P < .001.
3.4. Perceived LCS as a predictor of greater patient depression
Accounting for the effects of covariates, hierarchical regression showed that the overall regression model of the third step was statistically significant with 22% of the variance in depression being explained, F(8, 138) = 4.96, P < .001 (Table 6). Sociodemographic characteristics explained 12.1% (P = .006) of the total variance in depression, clinical factors explained 1.8% (P > .05), and perceived LCS contributed uniquely and significantly explaining 8.5% (P < .001) of the total variance in depression.
Table 6.
Summary of hierarchical regression analysis for LCS predicting depression and QoL
| Regression 1—dependent variable: Depression | |||
|---|---|---|---|
| Step | Predictor | β | ΔR2 |
| 1 | Sociodemographic characteristics | 0.12** | |
| Age | −0.07 | ||
| Female | 0.16* | ||
| Living alone | −0.11 | ||
| Unemployed or home maker | −0.01 | ||
| Earning <£10 000 annually | 0.20 | ||
| Earning £10 001‐20 000 annually | 0.12 | ||
| 2 | Clinical factors | 0.02 | |
| Current smoker | −0.15 | ||
| 3 | Patient outcome variables | 0.09*** | |
| Perceived LCS | 0.31*** | ||
| Cumulative R2 | 0.23*** | ||
| Regression 2—dependent variable: QoL | |||
|---|---|---|---|
| Step | Predictor | β | ΔR2 |
| 1 | Sociodemographic characteristics | 0.14*** | |
| Age | 0.15* | ||
| Unemployed or home maker | −0.10 | ||
| 2 | Clinical factors | 0.06** | |
| Current smoker | 0.12 | ||
| Symptomatic prior to LC diagnosis | 0.13* | ||
| Previous radiotherapy | 0.12 | ||
| 3 | Patient outcome variables | 0.15*** | |
| Perceived LCS | −0.40*** | ||
| Cumulative R2 | 0.35*** | ||
Notes: Betas shown are for the last step; Employment status was coded as a binary variable (unemployed/home maker vs employed/retired).
P < .05.
P < .01.
P < .001.
3.5. Perceived LCS as a predictor of lower patient HR‐QoL
Accounting for the effects of covariates, hierarchical regression showed that the overall regression model of the third step was statistically significant with 35% of the variance in HR‐QoL being explained, F(6, 174) = 15.36, P < .001 (Table 6). Sociodemographic characteristics explained 14.4% (P < .001), clinical factors explained 5.9% (P = .005), and perceived LCS contributed uniquely and significantly explaining 14.3% (P < .001) of the total variance in HR‐QoL.
4. DISCUSSION
These findings support our hypotheses that greater perceived LCS can be directly associated with greater levels of depression and lower HR‐QoL amongst patients with a LC diagnosis. Moreover, we were able to confirm that symptom burden (but not symptom severity) can directly contribute to greater perceived LCS. These observations have clear implications for clinical practice and research.
As in previous research,4, 11, 23 analysis of CLCSS scores indicated that patients with LC are vulnerable to stigmatisation after diagnosis, although in this study, mean LCS scores were below the midrange of the scale. Other studies have shown CLCSS scores of 75 to 102 (vs 53.1 in this study).4, 11, 16 This observation may indicate that the perceived LCS was generally low in this study's sample. Low scores were also reported in two Australian samples of LC patients with scores ranging from 52 to 5517, 18 and one American sample with a mean stigma score of 68.24 In these samples, patients had a confirmed diagnosis of LC and were either recruited via oncology clinics18, 24 or local support groups.17 The participants in studies with high stigma scores recruited a convenience sample via online cancer support websites, which could have influenced the LCS score because those participants may not be a typical LC population.
Rose et al18 suggested that the LCS scores were low due to their sample being newly diagnosed with LC. However, our sample was diagnosed on average 2.6 years prior to study participation. It could be suggested that gender may contribute to the findings of different scores of stigma as those studies with higher scores had a higher proportion of women.4, 11, 16 However, an Australian study that reported lower stigma scores had a higher proportion of men18 and another study with an Australian sample had a higher proportion of women.17 Both genders were evenly presented in this study making gender a less likely explanation. One factor that studies with lower stigma score had in common is the age of participants. In these studies, including the present study, participants' mean age was 65 to 69 years.17, 18
However, this observation should be interpreted with caution, as there is no consensus on what is low or high stigma using the CLCSS. It may also indicate that participants with high levels of perceived LCS did not return their questionnaires. A recent study validated a new LCS tool to establish cut‐off values to identify patients with clinically meaningful LCS.25 They found a value of 37.5 (possible range of 25‐125) and above to be clinically meaningful. The evidence is clear that perceived stigma is associated with poorer psychological well‐being, and therefore it is important that these patients receive intervention. Patients who only score high on a few items of the CLCSS resulting in an overall low score are still expressing perceptions of stigma. Thus, a lower score should also be recognised, and those patients should be offered intervention. It is therefore important to establish a meaningful cut‐point to identify patients in need for support. Future research could explore the cut‐off points for the CLCSS which are clinically relevant for patients with LC.
Findings for the role of LCS as predictor for depression and poorer HR‐QoL in patients with LC are consistent with the literature.11, 13 Greater scores of stigma were predictive of higher levels of depression and poorer HR‐QoL. This population has been found to exhibit poorer psychological well‐being than other cancer populations.26 The present findings assist in the identification of contributing factors to this high depressive symptomatology. Although only 8.5% of the variance in depression was explained by perceived LCS, this significant finding gives credence to the notion that the social implications and adverse social experiences of living with LC can well build on patients' feelings of low mood. It is possible that the longer patients live with perceived LCS, the greater the chances for them developing chronic depression.
In addition to depression, we found that perceived LCS uniquely contributed to poorer HR‐QoL. Further research is warranted to investigate a mediation path that leads from perceived LCS to depression and subsequently to HR‐QoL, and investigate possible additional mediators such as social anxiety. Indeed, anxiety has previously been found to be related to higher levels of stigma.4, 11 Anxiety as an additional stressor could further negatively affect health and increase the risk for stress‐related illnesses.27
Attempting to identify the actual onset of perceived LCS, we identified a significant relationship between symptom burden and perceived LCS. Overt symptoms, such as cough, dyspnoea, or haemoptysis may interfere with social interactions and lead to overt or covert reactions from others that may trigger experiences of LCS in patients with LC. These relationships warrant future investigation. Interestingly, symptom severity did not make a significant contribution to perceived LCS over and above symptom burden. This may indicate that even mild symptoms, when manifested during social interactions and in everyday life (eg, cough, dyspnoea), can be seen as a source of LCS for patients with LC.28 These relationships have not been explored previously in people with LC, but these findings may partly explain the higher perceived stigma in this group of patients compared with other cancers.29
Personalised care for patients with LC who manifest perceived LCS is required. Our findings indicate that patients with poorer socioeconomic backgrounds have higher levels of LCS. Whilst data on smoking rates across socioeconomic groups exist30 the association with LCS has not been demonstrated. Stuber et al31 investigated the associations between smoking stigma and educational status. Their findings indicate that better socioeconomic background is associated with higher levels of smoking‐related stigma. It is thought that people with social deprivation have more exposure to smoke‐free laws32 thus leading to higher perceived stigma. Their participants were smokers and nonsmokers from New York City neighbourhoods answering a survey about relationships between neighbourhood characteristics and drug use. The design of their study (different measure, sample without LC) may explain the difference to our data. Further investigation of this relationship with LC patients is needed.
We also found that younger patients are more likely to experience LCS. Older participants may feel less stigma due to the social acceptability of smoking when they first started smoking.6 We did not find a significant difference in perceived LCS between current and past smokers or non‐smokers. Previous studies also failed to demonstrate a relationship between smoking status and LCS,16 which is in support of findings from qualitative research where participants reported LCS regardless of smoking status.6 Smoking cigarettes is recognised as the main contributing risk factor for LC, and national and international health policies have targeted the tobacco industry to attempt to reduce the burden of this disease. In doing so, this may have come with a cost: that these health policies lead to experiences of stigma in people diagnosed with LC.6, 31, 33 Thus, it is likely that smoking history is irrelevant for experiences of perceived stigma if people with LC are being queried about their smoking history by strangers, acquaintances, or health care professionals after a diagnosis.34 However, there is qualitative evidence that smoking history is relevant in the experience of internalised stigma.34 It is likely that the small number of never‐smokers in our study contributed to the lack of relationship. Associations between smoking status and depression, however, were significant, and this demonstrates that these patients have a need for care to address psychological morbidities, whether this is related to the experience of stigma or not. Further research should be considered to investigate the role of smoking and the experience of stigma.
In this study, we, as well as other research groups, have demonstrated that perceived LCS can originate from various sources and take various forms.6, 15, 34, 35, 36 Perceived stigma from health care professionals can be detrimental, as patients can feel discomfort communicating their symptoms to health professionals, which in turn can lead to delay in presentation, in diagnosis, and in treatment, or even low uptake of treatment.35, 37, 38 Supportive and empathetic communication is important to promote adherence to treatment.39 Furthermore, shame and blame can also lead to high levels of distress and depression amongst family members and partners.40, 41 Therefore, it is important not to exclude family members from such discussions, and to assess for the families'/carers' own perceptions of LCS and how this may impact on their own feelings. It is thus important for health professionals to enter into sensitive discussions with patients and their families to explore the prevalence, nature, and chronicity of LCS perceptions.
Currently, interventions to reduce stigma amongst these patients are lacking, but individual counselling or group interventions have been suggested to help the stigmatized to protect themselves from the impact of stigma and reduce their vulnerability to encounters of stigmatisation.42 In this group of patients, a combined intervention addressing depressive symptomatology and stigma should be considered because of the relationship between the two. A recent wellness intervention with patients with LC, which was based on cognitive behaviour therapy (CBT), focused on psycho‐education, skills in stress reduction, problem‐solving, cognitive challenging, and enhancing relationship support17 and resulted in improvements in health‐related stigma, as well as depression and cancer‐specific distress.17
Such interventions may also be delivered via the internet. Online CBT interventions have been studied in the context of mental health to treat, for example, depression and anxiety.43 Results demonstrate that such interventions can achieve similar outcomes as conventional face‐to‐face delivered therapies.44 Potential benefits of digital interventions could reduce health costs as less hours of therapists are required, but this largely depends on the design of the intervention.45 Furthermore, considering the stigma surrounding seeking help for mental health, the digital setting could overcome the barrier of low adherence to mental health treatments and increase its reach to people in need of psychological treatments.46
4.1. Strengths and limitations
In this study, we used a relatively large dataset from a patient sample from six hospitals and with diverse demographic and clinical characteristics that allowed for statistical examination of relationships amongst the multiple covariates. However, a large proportion of patients in this study received surgical treatment which is atypical for this population. Although we achieved a fairly high response rate, we are unable to make comparisons between respondents and nonrespondents to identify potential bias in our sample and data. It is possible that people who did feel stigmatised in relation to their LC diagnosis found the study of greater interest than those who had no LCS perceptions. Alternatively, those with experiences of LCS may have chosen not to participate.
In addition, we did not collect information on additional covariates of potential interest, such as self‐esteem, self‐efficacy, anxiety, or treatment adherence. Therefore, future research should explore the above factors and their relationships with perceived LCS. Finally, this was a cross‐sectional investigation of perceived LCS and covariates, which prevents us from establishing the “causal” pathways that involve precipitating and perpetuating triggers of LCS and its longitudinal impact on patient outcomes. Further longitudinal research is thus warranted.
5. CONCLUSIONS
Perceived LCS is a significant predictor of poorer outcomes (symptom burden, depression, and HR‐QoL) in patients with LC, when controlling for demographic and clinical variables. Perceived LCS is a potentially important psychosocial factor when considering the psychosocial well‐being of patients with LC. Incorporating systematic assessments of perceived LCS in the plan of care for patients with LC is required to ensure that adverse effects are tackled in a timely fashion at the time of diagnosis, during treatment and post‐treatment.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Research data are not shared.
AUTHOR CONTRIBUTION
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualisation, R.M., R.M., J.C., J.P.; Methodology, R.M., J.C.; Investigation, L.L., J.M., R.M.; Formal Analysis, G.K., L.L..; Resources, J.M., R.M.; Writing—Original Draft, L. L, G.K.; Writing—Review & Editing, R.M., R.M., J.M., J.C..; Visualisation, G.K., L.L.; Supervision, R.M..; Funding Acquisition, R.M.
FUNDING
We would also like to thank the funders, the Royal College of Physicians and Surgeons of Glasgow, the West of Scotland Lung Cancer Research Group, and NHS Lanarkshire, for their support with this study.
Supporting information
Data S1 Supporting Information
ACKNOWLEDGEMENTS
We acknowledge the support of the clinical staff who supported the recruitment to this study at the participating NHS hospitals. We also thank the participants of this study to commit their time to complete to this study.
Maguire R, Lewis L, Kotronoulas G, McPhelim J, Milroy R, Cataldo J. Lung cancer stigma: A concept with consequences for patients. Cancer Reports. 2019;2:e1201. 10.1002/cnr2.1201
REFERENCES
- 1. Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58(1):M82‐M91. [DOI] [PubMed] [Google Scholar]
- 2. Sullivan D, Forsberg C, Ganzini L, et al. Depression symptom trends and health domains among lung cancer patients in the CanCORS study. Lung Cancer. 2016;100:102‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients. Cancer. 2009;115(22):5349‐5361. [DOI] [PubMed] [Google Scholar]
- 4. Cataldo JK, Brodsky JL. Lung cancer stigma, anxiety, depression and symptom severity. Oncology. 2013;85(1):33‐40. [DOI] [PubMed] [Google Scholar]
- 5. Goffman E. Stigma: Notes on the management of spoiled identity: Simon and Schuster; 2009.
- 6. Chapple A, Ziebland S, McPherson A. Stigma, shame, and blame experienced by patients with lung cancer: qualitative study. BMJ. 2004;328(7454):1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sasco A, Secretan M, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45:S3‐S9. [DOI] [PubMed] [Google Scholar]
- 8. Marlow LA, Waller J, Wardle J. Variation in blame attributions across different cancer types. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1799‐1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor CLC, Badr H, Lee JH, et al. Lung cancer patients and their spouses: psychological and relationship functioning within 1 month of treatment initiation. Ann Behav Med. 2008;36(2):129‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Else‐Quest NM, LoConte NK, Schiller JH, Hyde JS. Perceived stigma, self‐blame, and adjustment among lung, breast and prostate cancer patients. Psychol Health. 2009;24(8):949‐964. [DOI] [PubMed] [Google Scholar]
- 11. Brown Johnson CG, Brodsky JL, Cataldo JK. Lung cancer stigma, anxiety, depression, and quality of life. J Psychosoc Oncol. 2014;32(1):59‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cataldo JK, Slaughter R, Jahan TM, Pongquan VL, Hwang WJ (Eds). Measuring stigma in people with lung cancer: psychometric testing of the cataldo lung cancer stigma scale. Oncol Nurs Forum. 2011;38(1):E46‐E54. NIH Public Access [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gonzalez BD, Jacobsen PB. Depression in lung cancer patients: the role of perceived stigma. Psychooncology. 2012;21(3):239‐246. [DOI] [PubMed] [Google Scholar]
- 14. Van Brakel WH. Measuring health‐related stigma—a literature review. Psychol Health Med. 2006;11(3):307‐334. [DOI] [PubMed] [Google Scholar]
- 15. Brown C, Cataldo J. Explorations of lung cancer stigma for female long‐term survivors. Nurs Inq. 2013;20(4):352‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cataldo JK, Jahan TM, Pongquan VL. Lung cancer stigma, depression, and quality of life among ever and never smokers. Eur J Oncol Nurs. 2012;16(3):264‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chambers SK, Baade P, Youl P, et al. Psychological distress and quality of life in lung cancer: the role of health‐related stigma, illness appraisals and social constraints. Psychooncology. 2015;24(11):1569‐1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rose S, Kelly B, Boyes A, Cox M, Palazzi K, Paul C (Eds). Impact of perceived stigma in people newly diagnosed with lung cancer: a cross‐sectional analysis. Oncol Nurs Forum. 2018;45(6):737‐747. Oncology Nursing Society [DOI] [PubMed] [Google Scholar]
- 19. Hollen P, Gralla R, Kris M, McCoy S, Donaldson G, Moinpour C. A comparison of visual analogue and numerical rating scale formats for the Lung Cancer Symptom Scale (LCSS): does format affect patient ratings of symptoms and quality of life? Qual Life Res. 2005;14(3):837‐847. [DOI] [PubMed] [Google Scholar]
- 20. Sheehan TJ, Fifield J, Reisine S, Tennen H. The measurement structure of the Center for Epidemiologic Studies Depression scale. J Pers Assess. 1995;64(3):507‐521. [DOI] [PubMed] [Google Scholar]
- 21. Ferrell BR, Wisdom C, Wenzl C. Quality of life as an outcome variable in the management of cancer pain. Cancer. 1989;63(11):2321‐2327. [DOI] [PubMed] [Google Scholar]
- 22. Sarna L, Brown JK, Cooley ME, et al. (Eds). Quality of life and meaning of illness of women with lung cancer. Oncol Nurs Forum. 2005;32(1):E9‐E19. [DOI] [PubMed] [Google Scholar]
- 23. Lebel S, Castonguay M, Mackness G, Irish J, Bezjak A, Devins GM. The psychosocial impact of stigma in people with head and neck or lung cancer. Psychooncology. 2013;22(1):140‐152. [DOI] [PubMed] [Google Scholar]
- 24. Carter‐Harris L, Hermann CP, Schreiber J, Weaver MT, Rawl SM (Eds). Lung cancer stigma predicts timing of medical help‐seeking behavior. Oncol Nurs Forum. 2014;41(3):E203‐E210. NIH Public Access [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ostroff J, Riley K, Shen M, Atkinson T, Williamson T, Hamann H. Lung cancer stigma and depression: validation of the Lung Cancer Stigma Inventory. Psychooncology. 2019;28(5):1011‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19‐28. [DOI] [PubMed] [Google Scholar]
- 27. Link BG, Phelan JC. Stigma and its public health implications. Lancet. 2006;367(9509):528‐529. [DOI] [PubMed] [Google Scholar]
- 28. Maguire R, Stoddart K, Flowers P, McPhelim J, Kearney N. An interpretative phenomenological analysis of the lived experience of multiple concurrent symptoms in patients with lung cancer: a contribution to the study of symptom clusters. Eur J Oncol Nurs. 2014;18(3):310‐315. [DOI] [PubMed] [Google Scholar]
- 29. Marlow LA, Waller J, Wardle J. Does lung cancer attract greater stigma than other cancer types? Lung Cancer. 2015;88(1):104‐107. [DOI] [PubMed] [Google Scholar]
- 30. Centers for Disease Control . Cigarette smoking among adults—United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(45):1221. [PubMed] [Google Scholar]
- 31. Stuber J, Galea S, Link BG. Smoking and the emergence of a stigmatized social status. Soc Sci Med. 2008;67(3):420‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barbeau EM, Krieger N, Soobader M‐J. Working class matters: socioeconomic disadvantage, race/ethnicity, gender, and smoking in NHIS 2000. Am J Public Health. 2004;94(2):269‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bell K, Salmon A, Bowers M, Bell J, McCullough L. Smoking, stigma and tobacco ‘denormalization’: further reflections on the use of stigma as a public health tool. A commentary on Social Science & Medicine's Stigma, Prejudice, Discrimination and Health Special Issue (67: 3). Soc Sci Med. 2010;70(6):795‐799. [DOI] [PubMed] [Google Scholar]
- 34. Hamann HA, Ostroff JS, Marks EG, Gerber DE, Schiller JH, Lee SJC. Stigma among patients with lung cancer: a patient‐reported measurement model. Psychooncology. 2014;23(1):81‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lehto RH. Patient views on smoking, lung cancer, and stigma: a focus group perspective. Eur J Oncol Nurs. 2014;18(3):316‐322. [DOI] [PubMed] [Google Scholar]
- 36. Weiss J, Yang H, Weiss S, et al. Stigma, self‐blame, and satisfaction with care among patients with lung cancer. J Psychosoc Oncol. 2017;35(2):166‐179. [DOI] [PubMed] [Google Scholar]
- 37. Corner J, Hopkinson J, Roffe L. Experience of health changes and reasons for delay in seeking care: a UK study of the months prior to the diagnosis of lung cancer. Soc Sci Med. 2006;62(6):1381‐1391. [DOI] [PubMed] [Google Scholar]
- 38. Tod AM, Craven J, Allmark P. Diagnostic delay in lung cancer: a qualitative study. J Adv Nurs. 2008;61(3):336‐343. [DOI] [PubMed] [Google Scholar]
- 39. Zolnierek KBH, DiMatteo MR. Physician communication and patient adherence to treatment: a meta‐analysis. Med Care. 2009;47(8):826‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Milbury K, Badr H, Carmack CL. The role of blame in the psychosocial adjustment of couples coping with lung cancer. Ann Behav Med. 2012;44(3):331‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siminoff LA, Wilson‐Genderson M, Baker S. Depressive symptoms in lung cancer patients and their family caregivers and the influence of family environment. Psychooncology. 2010;19(12):1285‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weiss MG, Ramakrishna J, Somma D. Health‐related stigma: rethinking concepts and interventions. Psychol Health Med. 2006;11(3):277‐287. [DOI] [PubMed] [Google Scholar]
- 43. Andersson G. Using the internet to provide cognitive behaviour therapy. Behav Res Ther. 2009;47(3):175‐180. [DOI] [PubMed] [Google Scholar]
- 44. Andersson G. The Internet and CBT: A Clinical Guide. Boca Raton, FL: CRC Press; 2014. [Google Scholar]
- 45. Fairburn CG, Patel V. The impact of digital technology on psychological treatments and their dissemination. Behav Res Ther. 2017;88(Supplement C):19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Muñoz RF, Bunge EL, Chen K, et al. Massive open online interventions: a novel model for delivering behavioral‐health services worldwide. Clin Psychol Sci. 2016;4(2):194‐205. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting Information


