Abstract
Background
Coeliac plexus block (CPB) is an interventional pain management option for patients with pancreatic or other upper abdominal malignancy.
Aims
To assess the safety, utilization, and outcomes of CPBs in the local context.
Methods and Results
We conducted a retrospective case series of all patients with cancer who underwent CPB at 4 Sydney teaching hospitals from March 2010 to February 2016. We recorded baseline demographic data, details of the injectate, procedural approach and survival, as well as pain scores and analgesic use at 4 time points of interest. Thirty‐nine procedures were performed during the study period. Twenty‐four were performed endoscopically, 14 were performed via a bilateral percutaneous posterior approach by Pain Specialists or Radiologists and 1 was performed intraoperatively by a Surgeon. Patients had experienced pain for a mean of 17 weeks prior to CPB. Prior to CPB, the mean pain score was 8.8 out of 10. The mean pain score was reduced at 48 hours, 2 weeks, and 4 weeks following CPB (P < .01). The mean oral morphine equivalent daily dose prior to CPB was 362 mg which was reduced at 48 hours and 2 weeks but increased at the 4 weeks following CPB. One patient developed a bacteremia but otherwise no complications were observed.
Conclusion
CPB is performed by a number of approaches and is well tolerated. The approach selected appears to depend on patient anatomy, preference, and availability of local expertise. Local clinicians could consider CPB earlier in the management of malignant epigastric pain.
Keywords: celiac plexus, coeliac plexus, pain management, palliative care, pancreatic neoplasms
1. INTRODUCTION
Epigastric and back pain due to pancreatic malignancy can prove challenging to manage with systemic analgesia. As the number and doses of systemic analgesic agents rise, so too does the incidence of drug side effects such as drowsiness, dizziness, constipation, pruritus, nausea, and vomiting. 1 , 2 These adverse effects can further impair quality of life, which is important in this patient cohort who have a 5 year survival of just 8%. 3
The coeliac plexus is an established target for injection to provide analgesia for patients with pain arising from unresectable malignancy of the pancreas or other organs in close proximity. 4
Several different coeliac plexus block (CPB) approaches have been described, which include:
Anterior percutaneous transabdominal approach. 5
Bilateral posterior percutaneous approach. 6
Intraoperative approach. 7
Endoscopic approach through the posterior wall of the stomach using endoscopic ultrasound (EUS) guidance. 8
A Cochrane Review published in 2011 included six randomized control trials (RCTs) which compared either percutaneous or intraoperative CPB to medical management in patients with pain due to pancreatic cancer. 9 This concluded that at 4 weeks, the mean difference in visual analog scale scores was −0.42 in favor of the CPB group which was statistically significant, but of unclear clinical significance. 10 Mean oral morphine equivalent daily dose (oMEDD) was significantly reduced in the CPB group both at 4 weeks post CPB and just before death. There was also a significant reduction in constipation in the patients treated with CPB.
Results of a systematic review by Zhong et al were consistent with the above findings. 11
CPB is generally safe and well tolerated. Common adverse events are transient diarrhea and hypotension (9% and 8%, respectively) 12 likely due to unopposed parasympathetic activity following predominant sympathetic blockade. 13 The incidence of bleeding requiring a transfusion has been reported as 1.2% and the incidence of paraplegia as 0.15% in large retrospective case series. 14 , 15
To the authors' knowledge, there has not been a RCT comparing safety and efficacy of percutaneous vs endoscopic CPB in the setting of malignancy.
A systematic review by Nagels et al reported that it is unclear what impact CPB has on quality of life. 16 There have been conflicting results regarding the impact of CPB on survival. 17 , 18
2. AIM
To assess the safety, utilization and outcomes of CPBs in the local context for patients with malignancy, and, secondarily, to assess whether outcomes are influenced by the route via which CPB is performed.
3. METHODS AND MATERIALS
All patients who underwent CPB for treatment of malignant pain at Royal Prince Alfred, St Vincent's, Prince of Wales and Bankstown Hospitals in Sydney between March 2010 and February 2016 were identified.
Baseline demographic data, duration of pain prior to CPB, time between referral and performance of CPB, details of the injectate, procedural approach and imaging used for CPB, specialty of the proceduralist, performance status at the time of CPB, complications and survival after CPB, as well as pain scores, presence of nausea, vomiting, constipation, diarrhea and analgesic use were collected at 4 time points.
T0 was defined as immediately prior to CPB.
T1 was defined as 48 hours after CPB.
T2 was defined as 2 weeks after CPB.
T3 was defined as 4 weeks after CPB.
If a pain score was documented in the notes, this score was recorded. If only a verbal descriptor of pain intensity was documented (41% of procedures), this was transformed into a numerical score to facilitate analysis. The descriptor “none” was allocated a 0 out of 10, the descriptor “mild” was allocated a 2 out of 10, the descriptor “moderate” was allocated a 5 out of 10 and the descriptor “severe” was allocated an 8.5 out of 10. 10
Baseline and follow‐up numerical variables were summarized using the summary statistics, mean, minimum, and maximum. In comparing means, the independent t test was used. Baseline and follow‐up categorical variables were summarized using number and percentages and the Pearson chi‐square test. All percentages have as their denominator the number of procedures for which data on the variable in question was available, rather than the total number of procedures. Repeated measures mixed models were used to analyze the change in the pain scores and oMEDD at the 4 time points.
4. RESULTS
Between January 2010 and March 2016, 36 patients underwent CPB at Royal Prince Alfred, St Vincent's, Prince of Wales and Bankstown Hospitals for malignant epigastric or back pain. There were three patients who underwent neurolytic CPB twice, totaling 39 procedures. All patients had malignant masses in the upper abdomen, 71% of which were pancreatic in origin.
Complete follow‐up data was available for 17 of 39 procedures.
Figure 1 depicts the number of CPBs performed during the study period and the approaches taken. As one of our aims was to compare different approaches, the single patient who underwent intraoperative CPB was excluded from further analysis.
FIGURE 1.
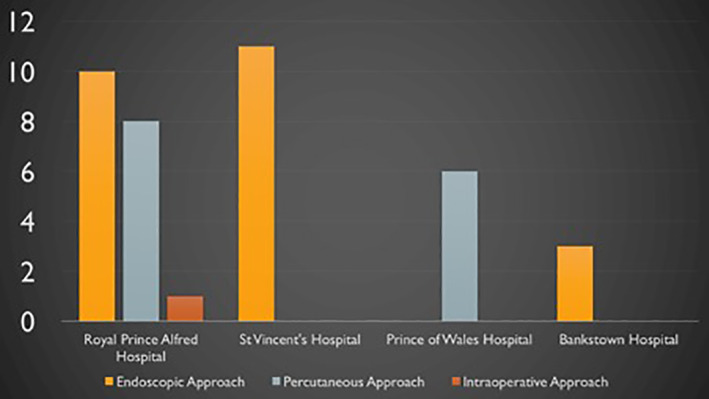
Number of coeliac plexus blocks performed at each hospital during the study period and approaches by which these were performed
Baseline demographics are presented in Table 1.
TABLE 1.
Baseline demographics
| Endoscopic CPB (approach 1) n (%) | Percutaneous posterior CPB (approaches 2 + 3) n (%) | Overall n (%) | ||
|---|---|---|---|---|
| Variable | Statistic/level | N = 24 | N = 14 | N = 38 |
| Age in years | Mean (range) | 63 (41‐87) | 55 (36‐74) | 60 (36‐87) |
| Female | 9 (41) | 3 (23) | 12 (32) | |
| Type of cancer | Pancreatic cancer | 17 (71) | 10 (71) | 27 (71) |
| Poorly differentiated carcinoma of likely biliary origin | 2 (8) | 0 (0) | 2 (5) | |
| Metastatic nonsmall cell lung cancer | 1 (4) | 1 (8) | 2 (5) | |
| Gastric cancer | 1 (4) | 2 (15) | 3 (8) | |
| Ovarian cancer | 1 (4) | 0 (0) | 1 (3) | |
| Unknown | 2 (8) | 1 (0) | 3 (8) | |
| Duration of pain (weeks) | Mean (range) | 12 (4‐66) | 26 (5‐78) | 17 (4‐78) |
Abbreviation: CPB, coeliac plexus block.
The injectate used was alcohol and local anesthetic in 25 procedures (65%). The total volume of alcohol injected varied between 4 and 40 mL and the concentration of alcohol varied between 30% and 100%. The other injectate used was 10% phenol and local anesthetic in 11 procedures (29%). The total volume of phenol injected varied between 10 and 20 mL. A mixture of local anesthetic, steroid and clonidine was injected in 2 (4%) procedures and local anesthetic and steroid was injected in 1 (2%) procedure. The local anesthetic used was bupivacaine in 19 procedures and ropivacaine in 10 procedures and was not specified in the remaining procedures.
Patients waited a mean of 9 (range 1‐37) days after referral for the CPB before this took place.
At T0, 89% of patients had epigastric pain and 62% had back pain. The mean pain scores at T0, T1, T2, and T3 are displayed in Figure 2.
FIGURE 2.
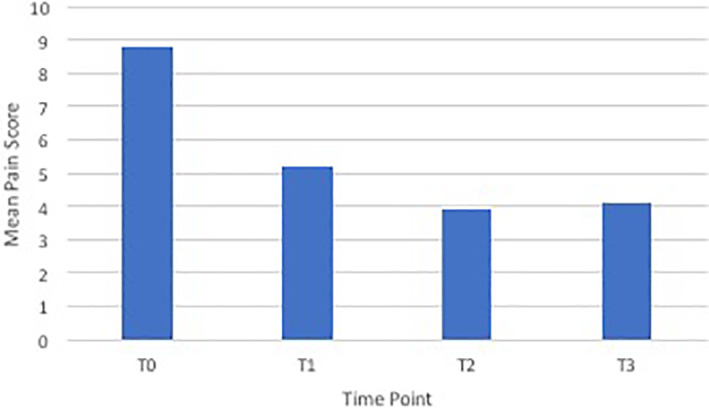
Mean pain scores at different time points
The mean oMEDD at T0, T1, T2, and T3 are displayed in Figure 3. The mean number of prescribed adjuvant analgesic agents was 1.7 at T0, 1.9 at T1, 1.3 at T2 and 1.4 at T3.
FIGURE 3.
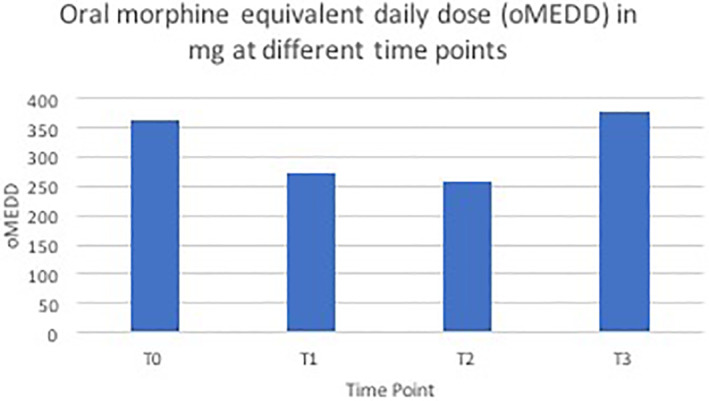
Oral morphine equivalent daily dose (oMEDD) in mg at different time points
When comparing percutaneous vs endoscopic CPB procedures, there was no significant difference between the groups in pain scores at T0, T1 or T2 or in oMEDD at any time point. Patients who underwent percutaneous block were slightly younger (55 vs 63 years old) and had lower pain scores at T3 (2.2 vs 5.1 out of 10) despite having experienced a longer duration of pain at the time of CPB (26 weeks vs 12 weeks).
Nausea or vomiting was documented in 44% of patients at T0, 14% at T1, 38% at T2 and 41% at T3. Constipation was documented in 42% of patients at T0, 21% at both T1 and T2 and 33% at T3. At T0, there were four patients who had diarrhea. At T1, two patients had diarrhea, at T2, a different two patients had diarrhea, and one patient had diarrhea at T3.
CPB was well tolerated. Importantly, there were no neurological complications recorded and no patients required blood transfusion. One patient who underwent bilateral percutaneous CPB via the posterior approach in theater developed an Escherichia coli bacteremia necessitating treatment with intravenous antibiotics. The patient who underwent CPB intraoperatively developed hypotension requiring a short period of vasopressors postoperatively. This patient had a history of left ventricular failure and had also received 200 μg of intrathecal morphine preoperatively.
Patients lived an average of 107 days following CPB (range 12‐365 days) as depicted in a Kaplan‐Meier curve in Figure 4.
FIGURE 4.
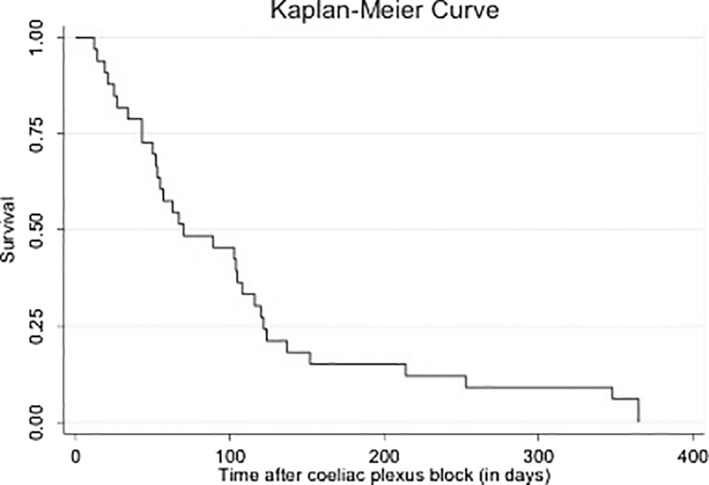
Survival after coeliac plexus block
5. DISCUSSION
The challenge for clinicians caring for patients with pain arising from unresectable pancreatic or other malignancy in close proximity to the pancreas, is to not only decide which patients might benefit from CPB and when those patients are most likely to benefit, but also, if a patient chooses to pursue CPB, to which proceduralist they should be referred—a Gastroenterologist, a Pain Specialist, or an Interventional Radiologist.
This retrospective case series adds to our current knowledge about CPB referral patterns, waiting times, outcomes and complications in four major teaching hospitals in Australia's largest city. The patients who underwent CPB were symptomatic at baseline with high pain scores and high doses of opioid analgesia. The observed baseline pain scores are consistent with pain scores of patients included in other CPB case series, 19 , 20 although the observed baseline oMEDD of 362 mg is higher than described elsewhere (0‐200 mg). 21 , 22
Optimum timing of CPB in the context of malignancy is uncertain. There are two RCTs which demonstrate that early CPB (prior to failure of strong opioids) is better than late CPB in terms of pain outcomes. 23 , 24 Patients in our series had experienced a mean of 17 weeks of pain prior to CPB and had a higher oMEDD than reported elsewhere. This suggests that in the local context, CPB may be considered more of a salvage management strategy rather than an early treatment option.
Following CPB, there was a reduction in the mean pain score from baseline to T1, T2, and T3 of greater than 3.5 points out of 10 which is clinically significant. This is consistent with published systematic review evidence that CPB can provide analgesic benefit for at least 4 weeks and possibly until death. Our series noted a reduction in oMEDD from baseline to T1 and T2 but not at T3. This is different from the results of the Cochrane review which demonstrated a reduction in opioid dose at 4 weeks and the day before death. 21 Our study is retrospective and has a small sample size which may account for these differences. The reduction in oMEDD observed at T1 and T2 is a plausible explanation for the observed trend toward less nausea, vomiting, and constipation at these time points.
What is original about this case series is that it demonstrates the variation in the content and volume of the injectate used for CPB in the local context. This variation reflects the lack of consensus in the literature as to the optimal substance and concentration for CPB injectate. 25 It is interesting to note that 65% of procedures were performed with alcohol and only 28% were performed with phenol, as alcohol is painful to inject whereas phenol is not. 25 This may relate to challenges with local availability of phenol. It also demonstrates that the availability of local proceduralist expertise and/or referring clinician preference were likely relevant in determining the CPB approach any particular patient underwent. Patients at Prince of Wales Hospital only underwent CPB via a percutaneous approach whereas patients at St Vincent's and Bankstown Hospitals only underwent endoscopic CPB.
The limitations of this study are that it is an uncontrolled retrospective case series and has a small sample size. Information on QOL was not available. Complete data was only available in 17 of 39 episodes of care. The main reason for this was that many patients referred for CPB at these four teaching hospitals were not followed by the proceduralist.
6. CONCLUSION
This retrospective case series demonstrates outcomes following CPB consistent with those reported in the literature. The procedure is well tolerated. The approach and injectate varies. The optimal approach is yet to be determined, and currently seems to depend on patient anatomy, availability of local expertise and the preference of the patient and referring clinician. In terms of timing, local clinicians could consider CPB earlier in the management of malignant abdominal pain. We propose to undertake a prospective national case series to allow for larger scale collection of data on the utilization, efficacy, and complications of CPBs at multiple sites across Australia. Ultimately, a RCT comparing percutaneous and endoscopic CPB in the context of malignancy will be considered.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
AUTHORS' CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, A.D., R.C. and A.A.; Methodology, A.D., R.C. and A.A.; Investigation, A.D., R.C. and A.A.; Resources, A.D., R.C. and A.A.; Writing – Original Draft, A.D.; Writing – Review & Editing, A.D., R.C. and A.A.; Visualization A.D., R.C. and A.A.; Supervision, R.C. and A.A.
ETHICS STATEMENT
Approval was obtained from Sydney Local Health District Human Research Ethics Committee (X16‐0054; LNR/16/RPAH/64). The need for patient consent was waived given the deidentified nature of the data to be retrospectively collected about deceased patients' routine clinical care.
ACKNOWLEDGMENT
Dr Mario D'Souza, Statistician, RPA Hospital.
Dumitrescu A, Aggarwal A, Chye R. A retrospective case series of patients who have undergone coeliac plexus blocks for the purpose of alleviating pain due to intra‐abdominal malignancy. Cancer Reports. 2020;3:e1265. 10.1002/cnr2.1265
Arun Aggarwal and Richard Chye should be considered joint senior authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Marret E, Kurdi O, Zufferey P. Effects of nonsteroidal anti‐inflammatory drugs on patient‐controlled analgesia morphine side effects: meta‐analysis of randomized controlled trials. Anesthesiology. 2005;102(6):1249‐1260. [DOI] [PubMed] [Google Scholar]
- 2. Kawamata M, Ishitani K, Ishikawa K, et al. Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer pain. Pain. 1996;64(3):597‐602. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2017;67(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 4. Dobosz L, Kaczor M, Stefaniak TJ. Pain in pancreatic cancer: review of medical and surgical remedies. ANZ J Surg. 2016;86(10):756‐761. [DOI] [PubMed] [Google Scholar]
- 5. Gimenez A, Martinez‐Noguera A, Donoso L, Catala E, Serra R. Percutaneous neurolysis of the celiac plexus via the anterior approach with sonographic guidance. Am J Roentgenol. 1993;161(5):1061‐1063. [DOI] [PubMed] [Google Scholar]
- 6. Ischia S, Ischia A, Polati E, Finco G. Three posterior percutaneous celiac plexus block techniques. A prospective, randomized study in 61 patients with pancreatic cancer pain. Anesthesiology. 1992;76(4):534‐540. [DOI] [PubMed] [Google Scholar]
- 7. Lillemoe KD, Cameron JL, Kaufman HS, Yeo CJ, Pitt HA, Sauter PK. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann Surg. 1993;217(5):447‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiersema M, Wong G, Croghan G. Endoscopic technique with ultrasound imaging for neurolytic celiac plexus block. Reg Anesth Pain Med. 2001;26:159‐163. [DOI] [PubMed] [Google Scholar]
- 9.. Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA. Celiac plexus block for pancreatic cancer pain in adults (Review). Cochrane Database of Systematic Reviews [Internet] 2011;(3). CD007519. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD007519.pub2/full. Accessed April 24, 2018. [DOI] [PMC free article] [PubMed]
- 10. Breivik H, Borchgrevink P, Allen S, et al. Assessment of pain. BJA. 2008;101(1):17‐24. [DOI] [PubMed] [Google Scholar]
- 11. Zhong W, Yu Z, Zeng J, et al. Celiac plexus block for treatment of pain associated with pancreatic cancer: a meta‐analysis. Pain Pract. 2014;14(1):43‐51. [DOI] [PubMed] [Google Scholar]
- 12. Yan B, Myers R. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol. 2007;102:430‐438. [DOI] [PubMed] [Google Scholar]
- 13. Wyse J, Chen Y, Sahai A. Celiac plexus neurolysis in the management of unresectable pancreatic cancer: when and how? World J Gastroenterol. 2014;20(9):2186‐2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Warner N, Moeschler S, Warner M, et al. Bleeding complications in patients undergoing celiac plexus block. Reg Anesth Pain Med. 2016;41(4):488‐493. [DOI] [PubMed] [Google Scholar]
- 15. Davies D. Incidence of major complications of neurolytic coeliac plexus block. J R Soc Med. 1993;86(5):264‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagels W, Pease N, Bekkering G, Cools F, Dobbels P. Celiac plexus neurolysis for abdominal cancer pain: a systematic review. Pain Med. 2013;14:1140‐1163. [DOI] [PubMed] [Google Scholar]
- 17. Oh T, Lee W, Woo S, Kim N, Yim J, Kim D. Impact of celiac plexus neurolysis on survival in patients with unresectable pancreatic cancer: a retrospective, propensity score matching analysis. Pain Physician. 2017;20:E357‐E365. [PubMed] [Google Scholar]
- 18. Fujii‐Lau L, Bamlet W, Eldrige J, et al. Impact of celiac neurolysis on survival in patients with pancreatic cancer. Gastrointest Endosc. 2015;82:46‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao L, Yang Y, Xu H, et al. A randomized clinical trial of nerve block to manage end‐stage pancreatic cancerous pain. Tumor Biol. 2014;35:2297‐2301. [DOI] [PubMed] [Google Scholar]
- 20. Gunaratnam N, Sarma A, Norton I, Wiersema M. A prospective study of EUS‐guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54(3):316‐324. [DOI] [PubMed] [Google Scholar]
- 21. Wong Y, Schroeder D, Carns P. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer. A randomized controlled trial. Jama. 2004;291(9):1092‐1099. [DOI] [PubMed] [Google Scholar]
- 22. Ahmed A, Arora D. Fluoroscopy‐guided neurolytic splanchnic nerve block for intractable pain from upper abdominal malignancies in patients with distorted celiac axis anatomy: an effective alternative to celiac plexus neurolysis—a retrospective study. Indian J Palliat Care. 2017;23(3):274‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amr Y, Makharita M. Neurolytic sympathectomy in the management of cancer pain‐time effect: a prospective, randomized multicenter study. J Pain Symptom Manage. 2014;48(5):944e‐956e. [DOI] [PubMed] [Google Scholar]
- 24. Wyse J, Carone M, Paquin S, Usatii M, Sahai A. Randomized, double‐blind, controlled trial of early endoscopic ultrasound‐guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. JCO. 2011;29(26):3541‐3546. [DOI] [PubMed] [Google Scholar]
- 25. Koyyalagunta D, Engle M, Yu J, Feng L, Novy D. The effectiveness of alcohol versus phenol based splanchnic nerve neurolysis for the treatment of intra‐abdominal cancer pain. Pain Physician. 2016;19:281‐292. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


