Abstract
Background
Palmar‐plantar erythrodysesthesia (PPE) is a common adverse event seen with many chemotherapeutic agents as well as targeted therapies which is very debilitating for the patient. To the best of our knowledge there are only a few cases of PPE reported with methotrexate infusion to date.
Case and Conclusion
We report a case of Burkitt Lymphoma, who developed severe PPE with methotrexate infusion and responded very well with conservative management and dose reduction in subsequent cycles.
Keywords: methotrexate, palmar‐plantar erythrodysesthesia
1. INTRODUCTION
The outcome of adult Burkitt lymphoma has dramatically improved in last decade with the advent of intensive multi‐agent chemotherapy protocols that incorporate high‐dose methotrexate (≥1 g/m2/dose) and cytarabine (≥500 mg/m2/dose) as chemotherapeutic agents for extra‐compartmental therapy and CNS prophylaxis. However the administration of these agents is associated with various side effects. The common dermatological side effects include mucositis, alopecia, xerosis, and pruritus. Palmar‐plantar erythrodysesthesia (PPE) also called hand‐foot syndrome (HFS) is a very rare side effect of high‐dose methotrexate therapy. 1 It is a dermatologic toxicity typically affecting the palmar aspects of the hands and the plantar aspects of the feet characterized by erythema, oedema, pain, sometimes ulceration. 2
2. CASE
We report a case of 41 year old male diagnosed as Burkitt Lymphoma Stage 3 who developed severe PPE following high‐dose methotrexate (3 g/m2/dose) infusion. Informed patient consent and institutional approval was taken prior to reporting this case. A 41 year old gentleman came to our clinic with complains of abdominal distension, generalized weakness, and low grade fever since 1 month. On evaluation his laboratory parameters revealed Hb‐11.5 g/dL, WBC 7.2 × 103/μl, Platelet count 3.4 × 103/mm3 with no atypical cells on peripheral smear. Serum creatinine was 1.3 mg/dL with BUN (blood urea nitrogen)‐18, serum potassium‐5.46 meq/L, serum uric acid‐18 mg/dL, LDH‐2570 u/l. The whole body PET CT was suggestive of bulky Stage 3 disease. Histopathological examination of lymph node biopsy specimen was suggestive of Burkitt lymphoma with Ki‐67 index 95% and FISH analysis on biopsy block was positive for c‐MYC gene rearrangement. Patient was immediately started on cyclophosphamide; vincristine and prednisone (COP) based pre‐phase chemotherapy for tumor debulking along with antitumor lysis treatment that included two sessions of hemodialysis and rasburicase. On improvement of his acute renal failure, patient was started on R‐CODOX‐M/R‐IVAC (cyclosphosphamide, doxorubicin, vincristine, methotrexate, ifosfamide, cytarabine, and etoposide) chemotherapy protocol.
On day 10 of chemotherapy protocol, the patient was started on high‐dose methotrexate (HD MTX) infusion (3 g/m2) over 24 hours with adequate hydration and close monitoring of urinary pH. The serum methotrexate levels at 24 and 48 hours were in the therapeutic range and standard leucovorin rescue (12 doses @15 mg/m2 every six hourly) was administered. However, 24 hours after completion of high‐dose methotrexate infusion, the patient developed mild tingling of both palms which was followed by diffuse bilateral palmar erythema and oedema along with severe pain and inability to hold objects. There were no other skin manifestations and soles of feet were not involved. A diagnosis of MTX induced Grade 3 palmar erythrodysesthesia was made (CTCAE 4.0) (Figure 1A). Patient was started on topical steroid ointment with cold compresses, topical lignocaine application and oral pain relief medications. Patient responded to topical steroids dramatically, lesions begin to decrease within 24 hours of initiation of therapy with marked improvement in swelling and pain. Erythematous lesions gradually healed with residual patchy hyperpigmentation over the next 10 days (Figure 1B). Subsequently during the second cycle R‐CODOX‐M, which was administered 8 weeks after the first cycle, HDMTX was given at 50% reduced dose (@1.5 g/m2), the infusion was given over a period of 24 hours as in previous cycle. Other chemotherapeutic agents were given in full dose in view of extensive disease, the patient again developed similar symptoms though of lesser severity (Grade 2, CTCAE 4.0) within 18 hours of initiation of Methotrexate infusion thereby proving the causal association of the drug (high‐dose methotrexate) with the symptoms (Figure 1C,D). The patient tolerated the high‐dose cytarabine infusions during the IVAC chemotherapy administration uneventfully. Presently, the patient has completed his chemotherapy and is in complete remission for his disease at 14 months of follow up.
FIGURE 1.
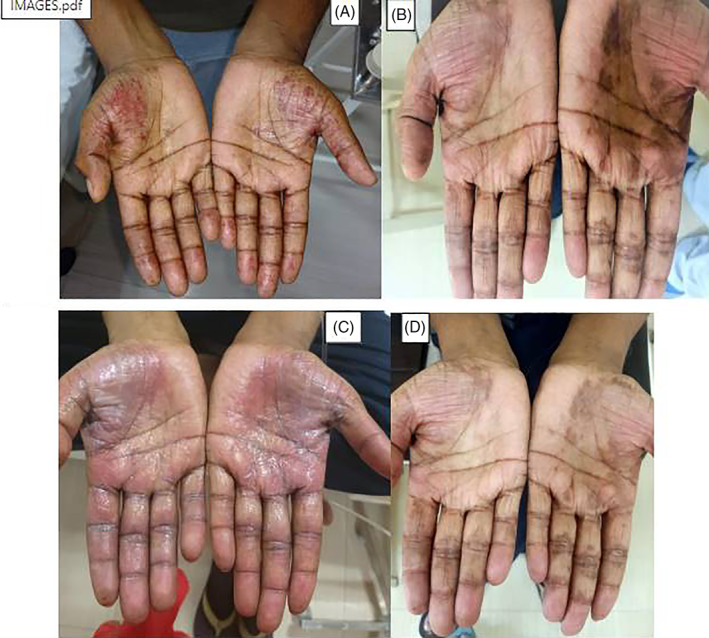
A, Grade 3 PPE post high‐dose methotrexate post first R‐CODOX‐M cycle. B, Resolved PPE after conservative therapy. C, Grade 2 PPE post second cycle of high‐dose MTX in second R‐CODOX‐M cycle. D, Resolved PPE post second R‐CODOX‐M cycle. PPE, palmar‐plantar erythrodysesthesia
3. DISCUSSION
PPE/HFS is a common dermatologic adverse event encountered with certain chemotherapeutic drugs that includes 5‐Flourouracil, liposomal doxorubicin, capecitabine, docetaxel as well as targeted multikinase inhibitors like sorafenib, sunitinib, vemurafenib 2 , 3 with reported incidence of 3% to 64% in various studies. 3 , 4 , 5 , 6 , 7 If not managed timely it can be severely painful, debilitating causing impairment of function and decreased quality of life.
It is very rarely documented side effect of high‐dose methotrexate and cytarabine chemotherapy. Karol et al 1 in their study comprising of 1720 pediatric patients receiving high‐dose methotrexate/cytarabine chemotherapy reported an incidence of 1.3%.
The pathogenesis of this syndrome though poorly understood, has been described as direct toxic effect of chemotherapeutic agent on acral skin. 8 Due to highest concentration of eccrine sweat glands in palms and soles, the drug accumulates in these glands and is secreted causing direct tissue damage. The selective involvement of hands and feet in PPE could be due to thick stratum corneum, vascular anatomy, rapidly dividing epidermis, absence of sebaceous glands and hairs follicles, high concentration of eccrine glands, and wide dermal papillae at this location. 8 , 9 , 10
Karol et al 1 in their cohort of 1720 pediatric cancer cases evaluated the risk factors for the occurrence of PPE during high‐dose methotrexate/cytarabine chemotherapy. They found that patient characteristics (eg, older age), genetic predispositions (eg, Increased skin expression of single nucleotide polymorphism variants that alter skin susceptibility to therapy), and therapy schedule (eg, rapid methotrexate infusion rates ≥1 g/m2/h) are associated with increased propensity for PPE. In some studies, the occurrence of PPE appears to be dose dependent and is determined by both peak drug concentration and total cumulative dose in some patients. 11 However; this association is not seen in all patients. It has also been reported that severity and duration of HFS increases with repetitive use of the causative agent.
Taking into consideration the rarity of this complication with high‐dose methotrexate chemotherapy, the data on its management is scarce. Limited data that is available shows dose intensity modifications along with slow methotrexate infusion rates the most important measures to prevent recurrent toxicity. 1 Treatment options include systemic and topical steroids, local cooling, emollients, topical keratolytics, oral pyridoxine and opioids for pain relief. 2 , 3 , 10 , 11
4. CONCLUSION
PPE is one of the rare toxicities of high‐dose methotrexate administration. High index of suspicion based on typical symptoms is necessary for early diagnosis and management of this adverse event. Reduced methotrexate dosing along with slow infusion rates in subsequent cycles might help in decreasing duration and the severity of this condition.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
PRIYANKA CHAUHAN: Conceptualization; investigation; writing‐original draft. Anshul Gupta: Conceptualization; supervision; writing‐review and editing. Sujeet Kumar: Data curation; investigation. Arijit Bishnu: Data curation. Soniya Nityanand: Conceptualization; supervision; writing‐review and editing.
ETHICS STATEMENT
Informed and written patient consent and institutional approval was taken prior to reporting this case.
Chauhan P, Gupta A, Kumar S, Bishnu A, Nityanand S. Palmar‐plantar erythrodysesthesia associated with high‐dose methotrexate: Case report. Cancer Reports. 2020;3:e1270. 10.1002/cnr2.1270
Contributor Information
Priyanka Chauhan, Email: priyanka.chauhan1785@gmail.com.
Anshul Gupta, Email: anshulhaemat@gmail.com.
Sujeet Kumar, Email: kumar.sujeet781@gmail.com.
Arijit Bishnu, Email: bishnuarijit@gmail.com.
Soniya Nityanand, Email: hodhematology@gmail.com.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Karol SE, Yang W, Smith C, Cheng C, Stewart CF, et al. Palmar‐plantar erythrodysesthesia syndrome following treatment with high‐dose methotrexate or high‐dose cytarabine. Cancer. 2017;123(18):3602‐3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller KK, Gorcey L, McLellan BN. Chemotherapy‐induced hand‐foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol. 2014;71:787‐794. [DOI] [PubMed] [Google Scholar]
- 3. Webster‐Gandy JD, How C, Harrold K. Palmar‐plantar erythrodysesthesia (PPE): a literature review with commentary on experience in a cancer centre. Eur J Oncol Nurs. 2007;11:238‐246. [DOI] [PubMed] [Google Scholar]
- 4. Ozmen S, Dogru M, Bozkurt C, Kocaoglu AC. Probable cytarabine‐induced acral erythema: report of 2 pediatric cases. J Pediatr Hematol Oncol. 2013;35(1):e11‐e13. [DOI] [PubMed] [Google Scholar]
- 5. Giacchero D, Monpoux F, Chiavérini C, Lacour JP. 6‐mercaptopurine‐related hand‐foot syndrome in a four‐year‐old child. Ann Dermatol Venereol. 2008;135(8–9):580‐583. [DOI] [PubMed] [Google Scholar]
- 6. Chidharla A, Kanderi T, Kasi A. Chemotherapy Acral Erythema (Palmar‐Plantar Erythrodysesthesia, Palmoplantar Erythrodysesthesia, Hand‐Foot Syndrome) [Updated 2020 Jul 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459375/ [Google Scholar]
- 7. Hueso L, Sanmartín O, Nagore E, et al. Chemotherapy‐induced acral erythema: a clinical and histopathologic study of 44 cases. Actas Dermosifiliogr. 2008;99(4):281‐290. [PubMed] [Google Scholar]
- 8. Scheithauer W, Blum J. Coming to grips with hand‐foot syndrome. Insights from clinical trials evaluating capecitabine. Oncology (Williston Park). 2004;18(9):1161‐1184. [PubMed] [Google Scholar]
- 9. Milano G, Etienne‐Grimaldi M, Mari M, et al. Candidate mechanisms for capecitabine‐related hand‐foot syndrome. Br J Clin Pharmacol. 2008;66(1):88‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wood LS, Lemont H, Jatoi A, et al. Practical considerations in the management of hand‐foot skin reaction caused by multikinase inhibitors. Commun Oncol. 2010;7(1):23‐29. [Google Scholar]
- 11. Nagore E, Insa A, Sanmartin O. Antineoplastic therapy induced palmar‐plantar erythrodysesthesia (‘hand‐foot’) syndrome. Incidence, recognition and management. Am J Clin Dermatol. 2001;1(4)225‐234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


