Abstract
The ketogenic diet (KD) is a non-pharmacological treatment for specific types of epilepsy. In addition, it has been shown to be effective in mitigating other neurologic disorders. The KD is also effective in reducing body mass, leading to an increase in use by the general population for weight loss. As the popularity of the clinical and general use of the KD has increased, it is important to develop adequate mouse models to better understand the effects of the KD in both normal and diseased states. Many times, the best outcome for disorders treatable with the KD would be achieved by commencing treatment in early life. Few studies have evaluated the cognitive effect of starting the KD in early life. To better understand these effects, male C57BL6/J mice were placed on a KD from postnatal day (P) 21 through young adulthood (~P90). KD-fed mice had increased blood ketone levels, reduced blood glucose, and reduced weight gain versus mice fed a control diet (CD). The weight loss in the KD-fed mice was not accompanied by a change in body fat percentage, suggesting that there was a loss of lean mass. Behavioral testing began on P60 while the mice were still on the diet. KD-fed mice were hypoactive with CD-fed mice. In the Morris water maze, KD-fed mice showed decreased path efficiency, suggesting a spatial learning deficits. No differences were observed in spatial memory or in novel object recognition memory. In a contextual and conditioned fear paradigm, the KD-fed mice had an increase in freezing behavior. These data suggest that early-life exposure to a KD leads to impaired body composition and long-term cognitive changes.
Keywords: Ketogenic, Spatial learning, Memory, Perinatal, Fear memory
1. Introduction
The ketogenic diet (KD) is a high-fat, low-carbohydrate diet that stimulates the production of ketone bodies (KB) to fuel energy production. It has been long used as a treatment for certain types of intractable epilepsy (Wilder, 1921). The metabolism of KBs as a primary fuel source reduces oxidative stress and inhibits the apoptosis-mediating mitochondrial membrane permeability transition pore (Gano et al., 2014; Pinto et al., 2018). Due to its purported anti-oxidant and mitochondrially-protective properties, the effects of a KD have been evaluated in several neurologic diseases related to metabolic dysfunction, including Parkinson’s disease (Gasior et al., 2006), Alzheimer’s disease (Broom et al., 2019), and brain cancer (Schwartz et al., 2018). While the KD is clinically effective, the long-term effects of the KD as well as the mechanisms in which the KD confers therapeutic benefit require further investigation. This would support a need for additional animal models of the KD particularly across the lifespan. A comprehensive model will allow for screening the effectiveness of the KD in neurologic disorders and refining the target populations. In particular, there is a need for a more thorough understanding of the effects of a KD on behavior, with a focus on cognition.
The behavioral effects of feeding rats and mice a KD vary based on the species and age of the subjects tested. When started in mice at 2–3 months of age, KD feeding for 3 months did not affect locomotor activity, forced swim test/tail suspension, or spatial learning (Huang et al., 2019). In aged (one-year old) mice, 14 months of KD feeding improved novel object recognition (NOR) performance, increased longevity; coordination, strength, and endurance were improved in hanging wire and grip strength tests; and increased muscle mass when compared with mice on a control or a high-fat diet (Roberts et al., 2017). Similarly, one-year old mice that were placed on a cyclic KD (alternating between control and KD weekly) had reduced mortality and less memory decline (Newman et al., 2017). Three weeks of the KD in older rats (22 months old) improved NOR memory when compared with rats fed the CD; however, the KD in older rats did not restore NOR performance to that of younger (3 months old) rats (Xu et al., 2010). These findings suggest that the KD partially mitigates age related cognitive decline. While the KD diet is well tolerated adult animals and may improve performance in age-related cognitive function, the effects of the KD on developing animals, which have unique metabolic demands, have not been fully elucidated. These effects are important as many disorders that could benefit from the KD are diagnosed and treated during childhood.
The KD is an effective treatment for certain types of intractable epilepsy in children. In children with epilepsy, patients often self-report improvement in cognitive function following commencement of the KD, though objective clinical testing showed no change in cognitive ability (van Berkel et al., 2018). Neuropsychological testing showed there was an improvement in scores related to alertness, which could be attributed to a reduction in seizures, the KD itself, or a combination of both. A retroactive study conducted by Patel et al assesses the long-term risks and benefits of patients prescribed a KD during early life. Survey responses suggest that there are no long-term adverse effects associated with early-life use of the KD for seizure control purposes. However, it is important to note that this study did not include cognitive assessments (Patel et al., 2010). There are little to no studies that investigate the effects of KD on otherwise healthy children.
In addition to its therapeutic use in neurologic disorders, the KD has gained popularity as a diet for weight loss (Bueno et al., 2013). With the increase in childhood obesity in the US, there is the potential for early-life exposure to the KD. Obese children placed on a KD had greater weight loss and reductions in insulin resistance than children fed a hypocaloric diet that was balanced in carbohydrates, fats, and proteins (Partsalak et al., 2012). Further, the children placed on a KD had increased levels of high-molecular weight adiponectin, a hormone involved in regulating glucose and fatty acid oxidation, than children placed on the hypocaloric diet (Partsalaki et al., 2012). Similarly, implementing a KD in (adult) obese patients can curtail hunger, while allowing for satiety, and improve oxidative metabolism of fat which leads to decreased body weight (Paoli, 2014).
While the KD is well-tolerated in infants and younger children (Kim et al., 2016; Nordli et al., 2001), the cognitive effects of prolonged early-life exposure to the KD are still somewhat unknown. The developing brain is able to utilize KBs better than the adult brain, likely due to developmentally-regulated expression of monocarboxylate transporter genes (Barry et al., 2018). The perinatal brain appears to prefer using KB for energy as KB oxidation provides the majority of the energy consumption by the developing rat brain (Hawkins et al., 1971). The utilization of KB reduces quickly after birth, with peak KB levels seen in humans between 1 and 6 months of age and peaks in rats at ~P7 (Girard et al., 1973). The significant differences in KB dependence between the adult and developing brain would suggest that behavioral findings resulting from adult exposure to the KD may not be relevant to early-life exposure. Therefore, it is important to determine the behavioral effects of the KD when started during a developmental stage.
The purpose of this study was to better understand the behavioral effect of KD exposure from early life through adulthood, leading to an effective model for early-life commencement of a KD at P21. The results of this study suggest that starting the KD at weaning reduces stature, decreases locomotor activity and impairs spatial learning while enhancing contextual and cued memory.
2. Results
2.1. BHB and glucose
To ensure that the young mice would undergo ketosis on the KD, we ran an initial experiment to measure KB and glucose over time in mice that were not used for behavioral testing. KD-fed mice had increased blood ß-hydroxybuterate (BHB) levels (main effect of diet: F (1,18) = 41.25; p < 0.0001) that increased over time (diet × time (F (2,36) = 20.79; p < 0.0001); Fig. 1A). KD-fed mice had lower blood glucose levels compared with CD-fed mice (F(1,18) = 10.07; p = 0.0053; Fig. 1B). There was no difference in blood glucose prior to the start of the diet; on P31 and P60 blood glucose decreased in KD fed mice when compared with CD-fed mice (diet × time interaction (F (2,36) = 5.496; p = 0.0023)).
Fig. 1.
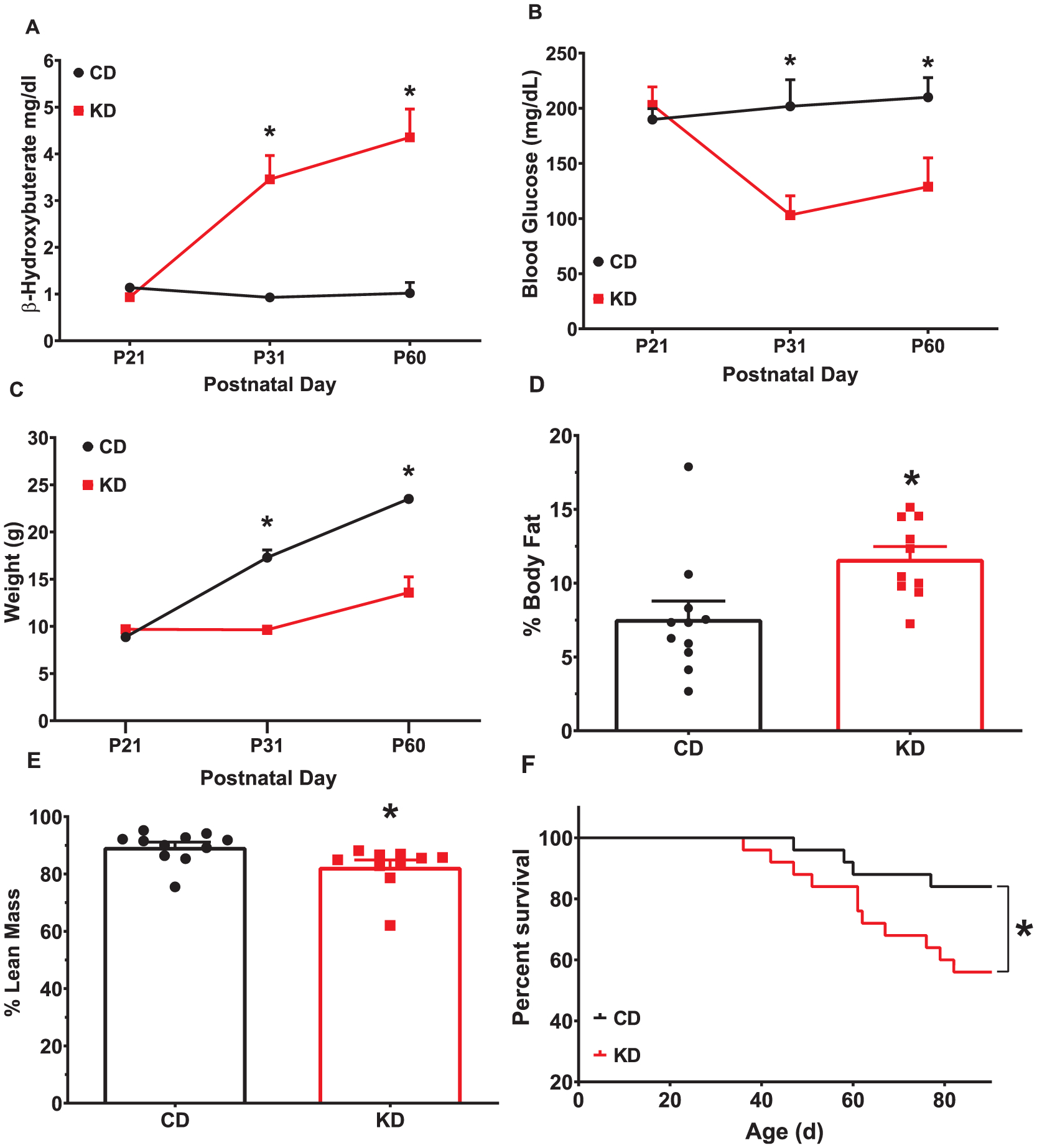
Effects of KD on weight and body composition in naïve mice. (A) KD fed mice have increased blood BHB (B) and decreased blood glucose levels after 10-day exposure to the KD. (C) KD decreased total body weight, (D) increased body fat, and (E) reduced lean mass. N = 10–11/group (KD) (F) KD fed mice show decreased survival compared with CD fed mice. Data are MEANS ± SEM N = 25/group, *p < 0.05.
2.2. Body composition
On P31 and P60, KD-fed mice weighed less than CD-fed mice (time × diet, (F(3,51) = 40.19, p < 0.0001; Fig. 1C). Body composition was measured on P60 with KD-fed mice having an increased body fat percentage (p = 0.0101; Fig. 1D) and reduced lean mass percentage (p = 0.0062; Fig. 1E) compared with CD-fed mice. Total fat mass was unchanged between groups (Mean ± SEM: CD = 1.78 ± 0.28 g, KD = 1.71 ± 0.25 g; t(17) = 0.18, p = 0.86) while the total lean mass (Mean ± SEM: CD = 20.59 ± 1.294 g, KD = 10.94 ± 1.294 g; t(19) = 7.463, p < 0.0001) was reduced in the KD-fed mice. Water content was increased in KD-fed mice (p = 0.0127; data not shown), however, there was no difference in hydration ratio, a measure of water content in organs (p = 0.3144; data not shown).
2.3. Survival curve
Survival rates were lower in KD fed mice (p = 0.0024, log rank test; Fig. 1F).
2.4. Open field locomotor
Open-field activity was evaluated for 1 h in order to assess not only the initial reaction to the arena but to measure the mouse’s ability to habituate to a novel environment. KD fed mice had fewer beam breaks during the open field task than CD fed mice (F(1,51) = 9.74, p = 0.003; Fig. 2A). There was a main effect of time as both groups had fewer beam breaks as time progressed, a sign of habituation (F(5.531,282.1) = 52.59; p < 0.0001). In addition, there was a time × diet interaction (F(11,561) = 5.910; p < 0.0001) with the KD-fed mice having fewer beam breaks than CD-fed mice during the first 55 min of testing.
Fig. 2.
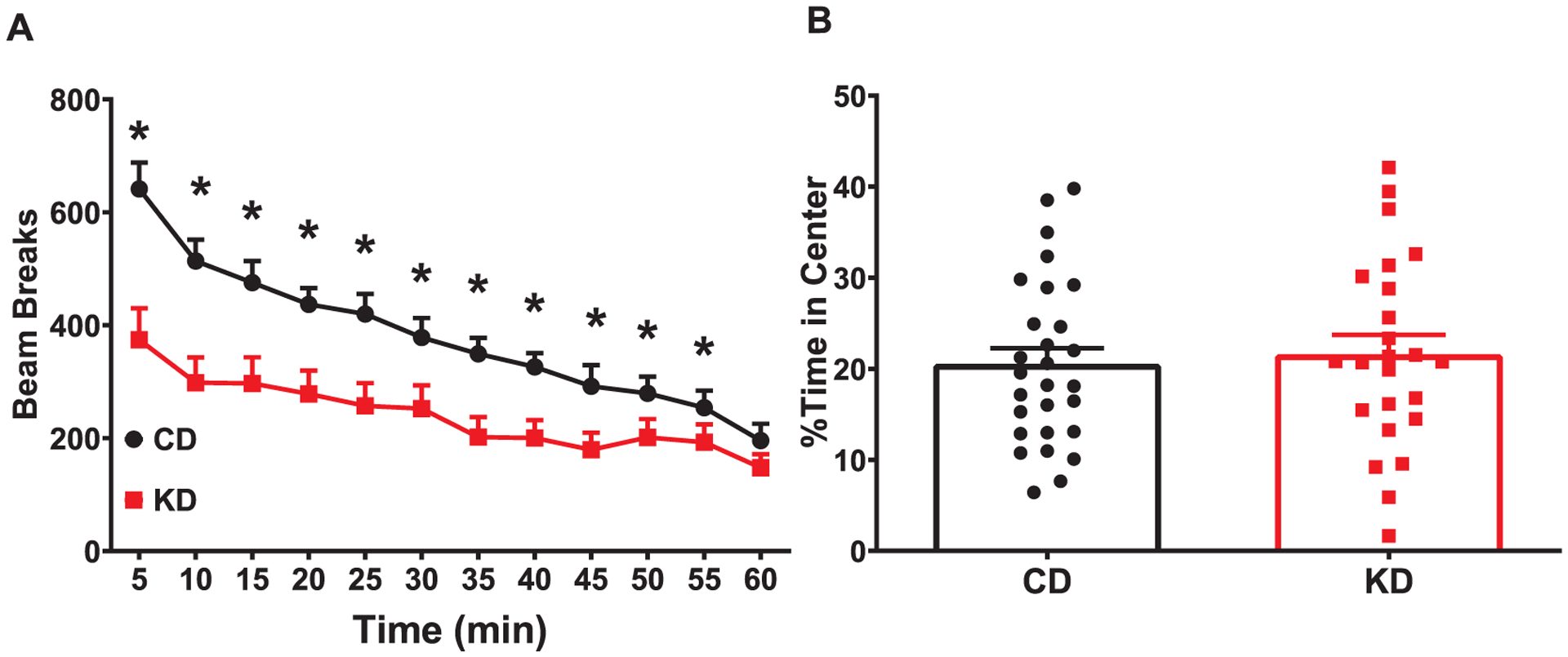
Reduced activity in open field with KD. Locomotor activity was assessed in mice for 1 h during the light phase. (A) KD fed mice show hypoactivity when compared with CD fed mice. (B) There was no difference in time spent in the center. Data are MEANS ± SEM N = 25–28/group *p < 0.05.
To determine if the reduction in beam breaks were a sign of reduced exploration of the chamber, time in the center of the arena was evaluated. There was no effect of diet on ambulation in the center of the arena (main effect of diet (F(1,51) = 0.08, p = 0.777; Fig. 2B), suggesting the KD-fed mice did not simply stay against the wall and not explore the chamber.
2.5. Morris water maze
During the acquisition phase of hidden platform testing, no differences were observed for latency (F(1,41) = 2.175; p = 0.1479; not shown) or path length (F(1,41) = 0.09338; p = 0.7615; Fig. 3A) to the platform. Path efficiency, which measures the deviation from a direct path to the platform and measures spatial accuracy, was decreased in KD fed mice (F(1,41) = 7.925; p = 0.0075; Fig. 3B). During the acquisition probe trial there was no difference in average distance from the platform between groups (p = 0.4755; Fig. 3C). During the reversal phase of testing, KD fed mice had longer latencies (F (1,41) = 13.26; p = 0.0008; data not shown) and path lengths (F(1,41) = 8.795; p < 0.0005; Fig. 3D) to the platform compared with CD fed mice. KD fed mice also had a lower path efficiencies during reversal (F (1,41) = 19.91; p < 0.0001; Fig. 3E). No differences were observed during the reversal probe trial (average distance p = 0.6130; Fig. 3F). KD fed mice had an increased latency to the platform during visible platform training, or cued learning (main effects of diet (F (1,51) = 5.87; p < 0.0001; Fig. 3G). Reduced swim speed was observed in the KD-fed mice during both the acquisition (F (1,41) = 10.86; p = 0.0020; Fig. 3H) and reversal phases of testing (F (1,41) = 4.522; p = 0.0395; Fig. 3I).
Fig. 3.
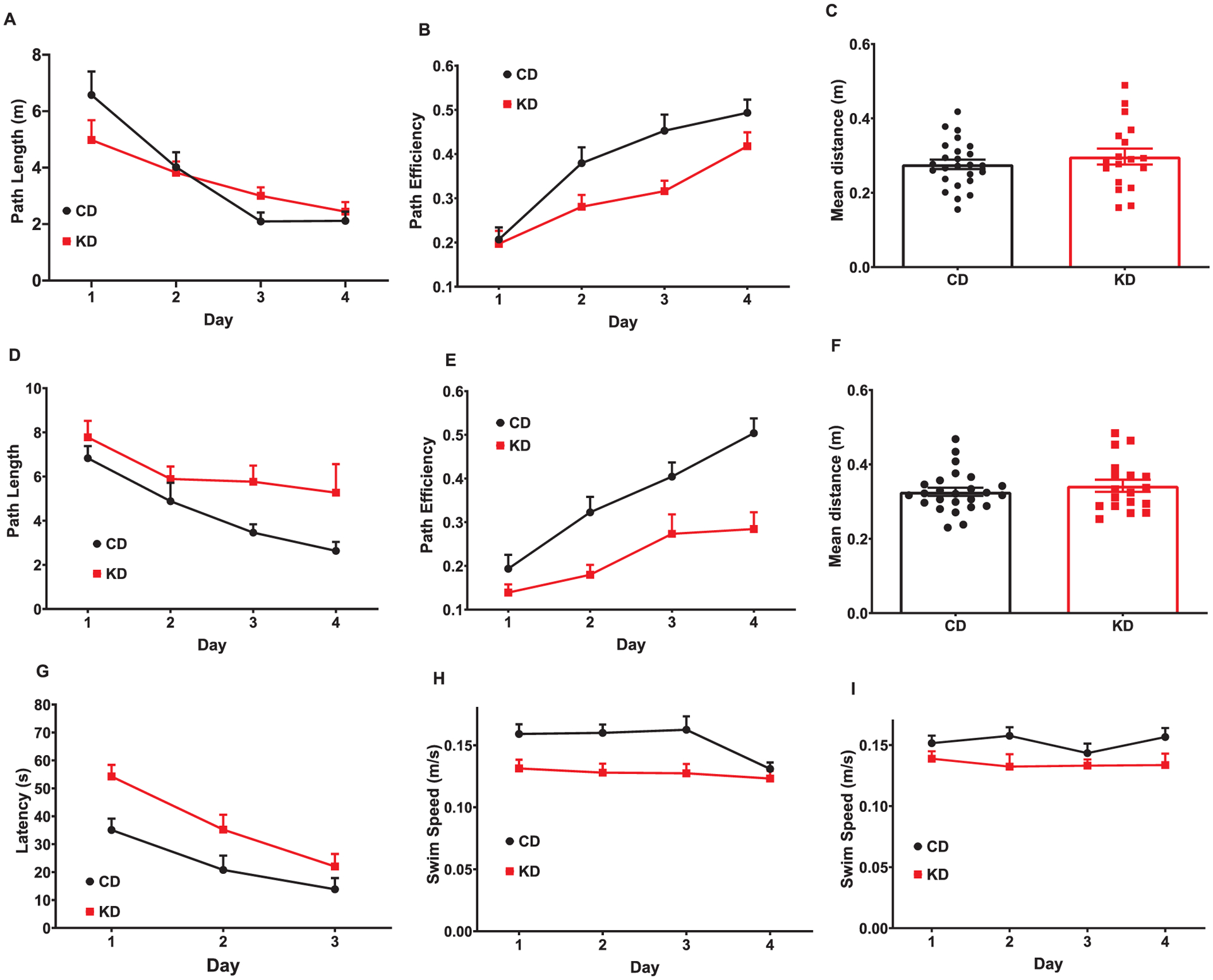
KD led to decreased spatial navigation but no changes in reference memory during MWM. (A) During hidden platform testing, there were no significant differences in path length during acquisition; (B) KD-fed mice have overall lower path efficiencies than CD-fed mice during acquisition. (C) There was no effect of diet for average distance from the platform during the acquisition probe trial. (D) KD-fed mice had overall longer lengths and (E) reduced path efficiency compared with CD-fed mice during the reversal phase of hidden platform testing. (F) No effect of diet was observed for average distance from the platform during the reversal probe trial. (G) The KD increased overall latency to the platform during visible platform training (cued learning). KD-fed mice had a slower swim speed during acquisitionff(H) and reversal (I). Data are MEANS ± SEM N = 18–25/group, p values for main effects are in text.
2.6. Novel object recognition testing
There were no differences in the discrimination index between groups (t(36) = 0.6637; p = 0.51; mean ± SEM CD = 0.34 ± 0.07 KD = 0.28 ± 0.04)
2.7. Conditioned fear
Mice were conditioned to the tone-footshook pairings on day 1. As with open field, KD-fed mice had fewer beam breaks than CD-fed mice during habituation (initial 8 min on day 1) (F (1,33) = 4.645; p = 0.0385; Fig. 4A). On day 2, mice were returned to the arena for 6 min to measure contextual fear. The KD-fed mice had fewer beam breaks than CD-fed mice in response to the original arena (F (1,34) = 4.384; p = 0.0438; data not shown). To control for the hypoactivity seen during day 1 and during the locomotor testing, the percent time freezing was evaluated. KD fed mice had an increase in the percent time freezing in response to the contextual cues (t = 2.371, df = 33; p = 0.0237; Fig. 4B). While there was a significant correlation (r2 = 0.13, p < 0.05) between percent time freezing and beam breaks, the low coefficient of determination would suggest that there is a great deal of variability between these measures and both should be reported (data not shown). On day 3, mice were acclimated to the modified arena for 3 min prior to the tone presentation. KD mice had fewer beam breaks than CD mice during this baseline measurement (F (1,34) = 7.203, p < 0.001; data not shown). During the tone presentation, there was a main effect of diet (F (1,34) = 11.33; p = 0.0019; data not shown) with KD-fed mice having fewer beam breaks than CD-fed mice. To control for the initial hypoactivity, the beam break activity during the tone presentation was analyzed as a percent of the pre-tone activity. The KD mice had a 60% reduction in activity relative to baseline compared with the 43% reduction seen in CD mice (F (1,34) = 20.60, p < 0.0001; data not shown). Additionally, KD-fed mice showed increased freezing when the tone was presented following the acclimation period (t = 2.520, df = 34; p = 0.0166; Fig. 4C). On day 3, reduced beam breaks correlated to increased freezing time (r2 = 0.5615; p < 0.0001; data not shown).
Fig. 4.
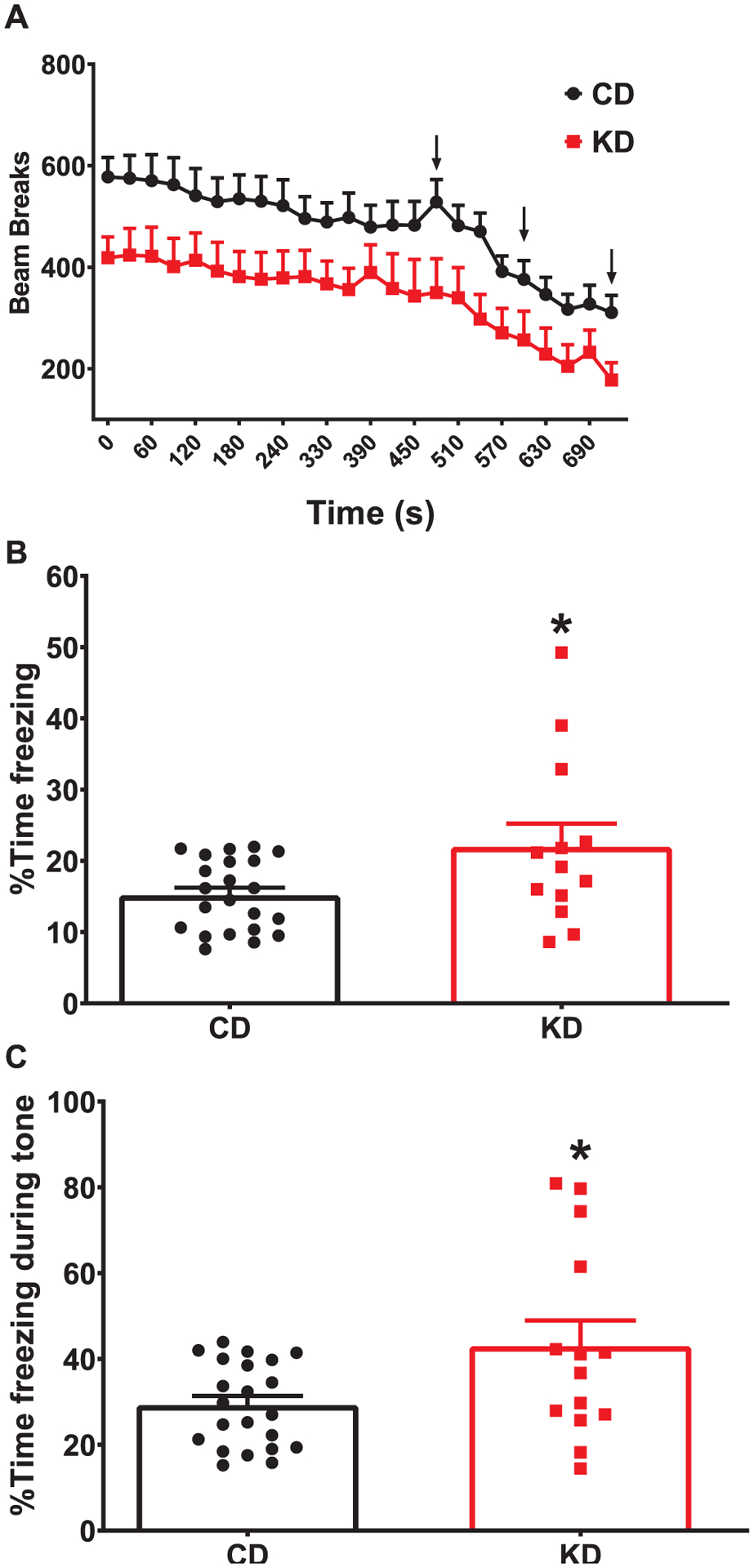
Increased fear memory following continuous exposure to KD. (A). KD fed mice show fewer beam breaks on test day. Arrows indicate the time the footshock was administered. (B) KD fed mice show increased freezing behavior during contextual and (C) cued fear testing. Data are MEANS ± SEM N = 14–22/group *p < 0.0001.
3. Discussion
The purpose of this study was to determine the behavioral and cognitive effects of starting a KD in early life. KD-fed mice were hypoactive during open field, showed deficits in the MWM, and had increased freezing during a test of fear memory. This suggests that continuous exposure to the KD, from P21 to P90, leads to spatial learning deficits but an improved fear memory. However, weight reductions and reduced activity could indicate that the KD-fed mice have deficits in physical performance which could confound some of the interpretations of the observed results. It is important to note that the deficits persisted across multiple tests and were altered in several measures in these tasks. Together, these results suggest that early-life exposure to a KD could impair learning and memory. A limitation of this study is that female mice were not tested. Sexually dimorphic effects in the KD have been observed in adult mice, though many studies have excluded females. In rats, starting the KD at 12 weeks of age led to a reduction of bone volume in males but not in females (Zengin et al., 2016). A better understanding of the KD in male and female mice is necessary, though it may be better served in a more therapeutically relevant model.
The KD has been linked to weight loss in humans (Greene et al., 2018; LaFountain et al., 2019) and adult mice (Roberts et al., 2017). A KD led to reduced bone mass (Simm et al., 2017) and growth rate in children with intractable epilepsy (Groleau et al., 2014). Similar to humans on a KD, mice fed a KD in this study had reduced weight, and overall growth, from P21 to P60. The weight deficits appear to be only due to a loss of lean body mass, as body fat mass did not differ between groups. The similar hydration ratio between groups suggests that the KD did not cause dehydration.
One of the main limitations of this study is that there was reduced survival in the KD-fed mice. The protein content in the diets may have contributed to the increased mortality in KD-fed mice. The diets used for this study were not protein matched; the KD was 8.6% protein while the CD was 18.3% protein. Low protein diets have been used to model malnourishment. It has been shown that protein restriction (4–6% of diet) in post-weaning mice and rats leads to a reduced body weight but we have found no mention of an increase in mortality with these studies (Batista et al., 2012, 2013; Dalvi et al., 2018). We found the KD fed mice had reduced lean mass which could also be due to the limited amount of protein in the diet. However, a KD with limited protein(9.5% protein) was shown to decrease weight in mice and this weight loss was due to an increase in metabolism, as the mice consumed a similar amount of calories across groups (Kennedy et al., 2007). It is possible that the lower body weights were due to a change in the metabolism of the mice, however, without indirect calorimetry measurements this is somewhat speculative. Another caveat to be considered is that we did not measure food consumption in the KD-fed vs CD-fed mice. It is possible that the diet was less palatable or that the mice were not calorically matched. Another potential difference between human KD studies and this study is related to the fluid intake of the mice. The mice in this study were given free access to water. Traditionally, fluid intake was restricted in epilepsy patients placed on the KD, though there was a lack of scientific rationale for fluid restriction and most guidelines do not recommend fluid restriction (Kossoff et al., 2009; Wirrell, 2008). While this may confound the comparison of some of the results in humans and rodent models, moving forward the best model one that matches current clinical guidelines.
KD-fed mice were hypoactive compared with CD-fed mice. When combined with the reduced size, it is possible that the KD-fed mice did not explore the arena, a potential sign of illness. Time in the center was used to determine if the reduction in beam breaks were due to the mice simply staying next to the wall and not moving. Time in the center did not differ, suggesting the mice did explore the arena, albeit at a much slower pace than their CD-fed counterparts. Young adult rats showed less exploratory behavior and spent more time immobile during open-field after 24 h on a KD (Murphy and Burnham, 2006). Rats placed on a KD for 10 weeks starting at P30 were hyperactive in a 5 min open-field test (Ziegler et al., 2005) the KD did not change locomotor activity (Brownlow et al., 2013; Huang et al., 2019). It is unlikely that the difference in protein content between the diets played a role in the hypoactivity as mice placed on a low-protein diet after weaning did not show differences in activity levels compared with mice on a balanced diet (Borck et al., 2017). Taken together, these studies suggest that KD exerts variable changes in locomotor activity that could be due to the species, age, and duration of treatment.
In this study, the KD-fed mice had reduced path efficiency during the acquisition phase and had increased latency and path length, with lower path efficiency during the reversal phase of the MWM-a well-established test for assessing spatial learning and memory. Path efficiency is calculated by determining the distance between the start point and the center of the platform for each mouse and dividing it by the path length. A mouse that swims directly to the platform would have a path efficiency of 1 (Vorhees and Williams, 2006). This can be used as a measurement of spatial accuracy. The lower path efficiency in the KD-fed mice indicate that they employed a broader search strategy, suggesting a spatial learning deficit. KD-fed mice had reduced swim speed in the MWM consistent with the size differences and hypoactivity observed. Reduced swim speed can confound the interpretation of latency related MWM data. To mitigate this confound, path length and path efficiency were also measured as they are independent of swim speed. The convergence of multiple measures allows for a higher degree of confidence that the KD-fed mice do have a spatial learning deficit. No differences between groups were seen during the probe trial, suggesting that spatial memory is intact. The appearance of spatial learning and memory deficits does appear to be age related. Rats started on a KD at P20 had spatial learning and memory deficits when tested at ~P53 (Zhao et al., 2004), while adult KD-fed mice did not have spatial learning deficits (Huang et al., 2019). The protein levels of the diet must be considered in this behavior as well. Mice placed on a reduced protein diets have MWM deficits (Fukuda et al., 2002; Reyes-Castro et al., 2018). This could suggest that there is a critical period in which the hippocampus requires glucose, though the lack of deficits in spatial memory and NOR may suggest that other brain regions could be affected by the KD.
There was no difference in NOR performance between KD- or CD- fed mice. The NOR test is considered a test of short-term memory and is thought to be hippocampally-dependent (Clark et al., 2000), though other regions have been shown to be necessary. Aged (26 months old) KD-fed mice show improvements in NOR compared with age-matched controls, suggesting KBs or a reduction in blood glucose may be neuroprotective during aging (Roberts et al., 2017). In addition, KD fed young adult rats had increased novel object recognition time using a 24 h interval between familiarization and retention (Brownlow et al., 2017). This suggests that the benefits of KD are limited to mitigating the deterioration of short-term recall that is associated with aging.
KD-fed mice spent more time freezing in response to both the environment (contextual freezing) and to the shock-associated tone (cued freezing) compared with CD-fed mice. Changes in freezing behavior are often interpreted as fear-based memory and these behaviors require an intact amygdala and hippocampus (Rusta et al., 2008). A potential confound to the interpretation of increased contextual and cued freezing is the noted hypoactivity in KD-fed mice. In addition to having fewer beam breaks during the open-field test, the KD-fed mice had fewer beam breaks in the arena prior to the conditioning paradigm. To mitigate this confound, we calculated the percent change in activity from habituation to tone presentation. KD mice had a greater reduction from pre-tone activity, suggesting that the increase in freezing behavior is due to associating the arena and the tone with the foot shock. There were no changes in contextual or cued freezing in rats fed the KD from P21–42. Interestingly, the calorie restricted controls did show deficits compared with KD or standard diet fed rats (Thio et al., 2010). In this study, mice were on the KD for approximately 10 weeks prior to fear memory testing, suggesting that a longer exposure to the diet could be required to affect fear memory.
Our findings suggest that exposure to a KD from P21 throughout young adulthood causes spatial learning deficits while increasing cue-conditioned memory. However, the KD led to a reduction in the rate of growth along with an increase in mortality. In addition, further work is needed to delineate if the lower protein concentration in the KD used in this study contributed to the findings. These findings do not appear to be in concert with data from studies conducted in (epileptic) humans, where the diet appears to be well tolerated in infants and during early childhood. Since the KD has been proposed as a potential therapy for several neurologic disorders, developing a rodent model in which to test its efficacy is important. Further refinement is likely needed to generate an early-life, long-term mouse model of the KD and to delineate the contribution of neurological versus somatic contribution to behavioral findings.
4. Methods
4.1. Subjects
Male and female C57BL/6J mice were bred in-house and the male offspring were the subjects of these studies. Date of birth was considered P0 and mice were weaned ~4/cage (1 male/litter * diet) on P21. Prior to weaning, dams were kept on NIH-07 diet (LabDiet 5001- calories provided by: 13.8% fat, 28.7% protein, 57.94% carbohydrates) At weaning, male mice were provided with either the KD (Bioserv S366;75.1% fat, 8.6% protein, 3.2% carbohydrates) or a CD (Test Diet AIN-93 growth pellets; 7.1% fat, 18.3% protein, 63.2% carbohydrates) ad libitum. While fluid restriction has been traditionally prescribed along with a ketogenic diet, the use of fluid restriction has diminished (Paul et al., 2010; Wirrell, 2008). The modified Atkins diet, with no fluid restriction, has also been shown to be effective in treating epilepsy (Kossoff et al., 2013). Based on these studies, mice were given ad libitum access to water. Mice were maintained on a 14 h light:10 h dark cycle (lights on 0700) with the room temperature at 21 + 1 °C. Mice remained on the diet throughout their lifetime. The vivarium is fully accredited by the AAALAC and all protocols were approved by the Institutional Animal Care and Use Committee. All behavioral testing was performed between 1200 h and 1500 h.
4.2. Blood glucose and ketone body measurements
Blood glucose and ketone bodies (KB) were measured using a Precision Xtra glucometer (Abbott, Alameda, CA) on P21, P31, and P60 which allows for the simultaneous measurement of glucose and KB. Blood from non-fasted mice was taken by tail nick 4 h into the light period of the day.
4.3. EchoMRI
On P60, body composition was determined using EchoMRI (Houston, TX) which analyzes total body fat, lean mass, and water content. Body fat percentage and lean mass were calculated by dividing the total body fat, or lean mass, by total mass and multiplying by 100. The hydration ratio was calculated by subtracting free water from total water and dividing by lean mass.
4.4. Open field locomotor
Spontaneous locomotor activity was tested for 1 h in the Photobeam Activity System (PAS) – Open-Field (San Diego Instruments, San Diego, CA). The 1 h time was selected to evaluate both the initial response to a novel arena and the mouse’s ability to habituate to its environment. Chambers were 40 cm (W) × 40 cm (D) × 38 cm (H) with 16 LED-photodetector beams spaced 2.5 cm apart in X and Y planes. The dependent variable is the total number of photobeam interruptions per 5 min interval. PAS distinguishes between fine and ambulatory movements; ambulation is defined as any movement when the subject leaves the square defined by box size (line crossings), while fine motor movements are classified as stereotypic, or grooming behaviors. Within the software a 20 × 20 cm center area was defined. Time spent in the center of the maze was calculated to ensure that mice ambulated throughout the arena.
4.5. Morris water maze
The MWM is a test of spatial learning and reference memory (Vorhees and Williams, 2006); mice were tested as described (Skelton et al., 2011). The tank was constructed of white plastic, 122 cm in diameter, and filled with room temperature water (21 ± 1 °C). Visible platform training (cued learning) was conducted for 3 days. During this phase, curtains were closed around the maze to obscure prominent distal cues. The escape platform was 10 cm in diameter and was submerged ~1.5 cm under the water. An orange ball was mounted to the platform (7 cm above the water) using a steel rod to serve as the visible cue. On the first day, mice were given 6 trials (90 s) with a fixed start and platform position. On day-2 and day-3, 2 trials per day were given with the start and platform positions randomized. Latency to the platform was recorded with a 90 s maximum per trial.
Hidden platform testing was conducted in two phases with each phase consisting of four, 90 s, trials per day for four days followed by a single probe trial (no platform) on day five. The reversal phase began the day after the probe trial for the acquisition phase for a total of 10 d of hidden platform testing. Distal cues for each phase were large black shapes painted on the wall alongside distinctive commercially available posters. Mice were tested in rotation, e.g. all mice completed trial 1 prior to the first mouse starting trial 2. The inter-trial interval depended on the number of mice but was approximately 8–10 min. For the acquisition phase, the platform was 10 cm in diameter and placed in a fixed position in the SW quadrant of the maze (with S representing the point closet to the door-side wall). The center of the platform was placed 30.5 cm from the wall using custom-made sleeves that were mounted to the bottom of the tank. The start positions were N, E, SE, and NW. The start position patter was kept consistent for that day of the phase and was randomized by day to ensure that the pattern was not repeated over the 4 days. During the probe trial on day 5 of testing, the start position was NE corner. Mice were placed into the tank facing the wall of the maze for each trial. For the reversal phase, which began one day following the probe trial, the 10 cm platform was replaced by 7 cm platform. The platform was placed in the NE quadrant and the start positions were S, W, NW, and SE. For the probe trial the mouse was placed in the SW quadrant. Latency to the platform, path length, and swim speed was measured using AnyMaze software (Stoelting Company, Wood Dale, IL). Path efficiency was calculated by dividing the straight-line distance from the start to the platform by the path length.
4.6. Novel object recognition
NOR is a test of incidental learning and short-term memory (Clark et al., 2000). The testing arena was a 40 × 40 cm constructed of black plastic. Mice were habituated to the empty arena for 2 d (10 min/d), followed by 2 d in which they were placed in the arena with two identical objects to reduce neophobia. On test day, mice were presented with 2 new identical objects for the familiarization stage for 10 min. One hour later, memory was tested by presenting mice with an identical copy of one of the familiar objects and a novel object. The test concluded when 30 s of total observation time between both objects was accrued. All testing was video recorded; videos were manually scored using ODLog software. Observation time was defined as being within ~2 cm of and oriented toward the object. A discrimination index was calculated by subtracting the time observing the familiar object from time spent observing the novel object and dividing by the total observation time.
4.7. Conditioned fear
Conditioned fear (CF) was assessed as described by Peters et al. (2010) with modification. On day 1, mice were placed in the chamber for 12 min. Following an 8 min acclimation period, mice were exposed to three tone-footshock pairings with each separated by 120 s (designated by arrows in Fig. 4A). The pairing consisted of the 30 s tone (82 dB, 2 kHz) accompanied during the last second by a scrambled footshock (0.5 mA for 1 s) delivered through the floor and a 90 s rest period. On the second day, mice were returned to the chamber with no tone or shock as a test of contextual fear. On the third day, mice were placed in the chamber with a novel floor as a test of cued fear. Following 3 min of acclimation, the tone was presented for 3 min. Beam breaks and percent time freezing (defined as > 2 s of no beam breaks) were analyzed.
4.8. Statistics
All data were tested for either a normal distribution or sphericity prior to analysis. Data with a single variable were analyzed as using two-tailed t tests. Data with multiple measures taken from the same mouse were analyzed using a mixed model, repeated-measures ANOVA (GraphPad Prism, La Jolla, CA) with diet, as the between subject factor and trial or day as the repeated measure (weight, blood glucose and KB, MWM, CF data were analyzed using a repeated measures ANOVA). For repeated measures data the Geisser-Greenhouse correction was applied, which may result in fractional degrees of freedom. Significant interactions were examined using false discovery rate (FDR) corrections. Data are presented as mean ± SEM.
HIGHLIGHTS.
Starting the KD on postnatal day (P) 21 in male mice reduced their body weight and lean mass.
KD-fed mice were hypoactive in the open field.
KD fed mice show deficits in spatial learning.
KD increases contextual and cued-based freezing behavior.
Acknowledgements
This work was supported by NIH grants HD080910 and NS111217 (MRS) and HD092224 (KNM), a CARE grant from the Association for Creatine Deficiencies (MRS), and internal support from the Division of Neurology (MRS). The authors thank Zuhair Abdulla and Dr. Marla Perna for their editorial support.
Footnotes
Disclosures
The authors declare no competing interests. KNM and MRS designed the research, analyzed and interpreted the data, and wrote the manuscript. KNM performed the research.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brainres.2020.146697.
References
- Barry D, Ellul S, Watters L, Lee D, Haluska R, White R, 2018. The ketogenic diet in disease and development. Int. J. Dev. Neurosci 10.1016/j.ijdevneu.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Batista TM, Ribeiro RA, Amaral AG, de Oliveira CAM, Boschero AC, Carneiro EM, 2012. Taurine supplementation restores glucose and carbachol-induced insulin secretion in islets from low-protein diet rats: involvement of Ach-M3R, Synt 1 and SNAP-25 proteins. J. Nutr. Biochem 10.1016/j.jnutbio.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Batista TM, Ribeiro RA, da Silva PMR, Camargo RL, Lollo PCB, Boschero AC, Carneiro EM, 2013. Taurine supplementation improves liver glucose control in normal protein and malnourished mice fed a high-fat diet. Mol. Nutr. Food Res 10.1002/mnfr.201200345. [DOI] [PubMed] [Google Scholar]
- Borck PC, Batista TM, Vettorazzi JF, Camargo RL, Boschero AC, Vieira E, Carneiro EM, 2017. Protein malnutrition after weaning disrupts peripheral clock and daily insulin secretion in mice. J. Nutr. Biochem 10.1016/j.jnutbio.2017.08.013. [DOI] [PubMed] [Google Scholar]
- Broom GM, Shaw IC, Rucklidge JJ, 2019. The ketogenic diet as a potential treatment and prevention strategy for Alzheimer’s disease. Nutrition. 10.1016/j.nut.2018.10.003. [DOI] [PubMed] [Google Scholar]
- Brownlow ML, Benner L, D’Agostino D, Gordon MN, Morgan D, 2013. Ketogenic diet improves motor performance but not cognition in two mouse models of Alzheimer’s pathology. PLoS One. 10.1371/journal.pone.0075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlow ML, Jung SH, Moore RJ, Bechmann N, Jankord R, 2017. Nutritional ketosis affects metabolism and behavior in Sprague-Dawley rats in both control and chronic stress environments. Front. Mol. Neurosci 10.3389/fnmol.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno NB, De Melo ISV, De Oliveira SL, Da Rocha Ataide T, 2013. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: a meta-analysis of randomised controlled trials. Br. J. Nutr 10.1017/S0007114513000548. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR, 2000. Impaired recognition memory in rats after damage to the hippocampus. J. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvi PS, Yang S, Swain N, Kim J, Saha S, Bourdon C, Bandsma RHJ, 2018. Long-term metabolic effects of malnutrition: liver steatosis and insulin resistance following early-life protein restriction. PLoS One. 10.1371/journal.pone.0199916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda MTH, Françolin-Silva AL, Almeida SS, 2002. Early postnatal protein malnutrition affects learning and memory in the distal but not in the proximal cue version of the Morris water maze. Behav. Brain Res. 10.1016/S0166-4328(02)00010-4. [DOI] [PubMed] [Google Scholar]
- Gano LB, Patel M, Rho JM, 2014. Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res 10.1194/jlr.r048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Rogawski MA, Hartman AL, 2006. Neuroprotective and disease-modifying effects of the ketogenic diet. Behav. Pharmacol 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard JR, Cuendet GS, Marliss EB, Kervran A, Rieutort M, Assan R, 1973. Fuels, hormones, and liver metabolism at term and during the early postnatal period in the rat. J. Clin. Invest 10.1172/JCI107519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene DA, Varley BJ, Hartwig TB, Chapman P, Rigney M, 2018. A low-carbohydrate ketogenic diet reduces body mass without compromising performance in powerlifting and olympic weightlifting athletes. J. Strength Condition. Res 10.1519/JSC.0000000000002904. [DOI] [PubMed] [Google Scholar]
- Groleau V, Schall JI, Stallings VA, Bergqvist CA, 2014. Long-term impact of the ketogenic diet on growth and resting energy expenditure in children with intractable epilepsy. Dev. Med. Child Neurol 10.1111/dmcn.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RA, Williamson DH, Krebs HA, 1971. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem. J 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Li Y, Wu C, Zhang Y, Zhao S, Chen Y, Sun X, 2019. The effect of ketogenic diet on behaviors and synaptic functions of naive mice. Brain Behav. 9 (4), e01246. 10.1002/brb3.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AR, Pissios P, Otu H, Xue B, Asakura K, Furukawa N, et al. , 2007. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am. J. Physiol. Endocrinol. Metab 10.1152/ajpendo.00717.2006. [DOI] [PubMed] [Google Scholar]
- Kim JA, Yoon JR, Lee EJ, Lee JS, Kim JT, Kim HD, Kang HC, 2016. Efficacy of the classic ketogenic and the modified Atkins diets in refractory childhood epilepsy. Epilepsia. 10.1111/epi.13256. [DOI] [PubMed] [Google Scholar]
- Kossoff EH, Cervenka M, Henry B, Haney C, Turner Z, 2013. A decade of the modified Atkins diet (2003–2013): results, insights, and future directions. Epilepsy Behav. 29 (3), 437–442. 10.1016/j.yebeh.2013.09.032. [DOI] [PubMed] [Google Scholar]
- Kossoff EH, Zupec-Kania BA, Amark PE, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. , 2009. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. 10.1111/j.1528-1167.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- Reyes-Castro LA, Padilla-Gomez E, Parga-Martiínez NJ, Castro-Rodriguez D, Quirarte G, Diaz-Cintra S, Zambrano E, 2018. Hippocampal mechanisms in impaired spatial learning and memory in male offspring of rats fed a low-protein iso-caloric diet in pregnancy and/or lactation. Hippocampus 28 (1), 18–30. 10.1002/hipo.22798. [DOI] [PubMed] [Google Scholar]
- LaFountain RA, Miller VJ, Barnhart EC, Hyde PN, Crabtree CD, McSwiney FT, Volek JS, 2019. Extended ketogenic diet and physical training intervention in military personnel. Mil. Med 10.1093/milmed/usz046. [DOI] [PubMed] [Google Scholar]
- Murphy P, Burnham WM, 2006. The ketogenic diet causes a reversible decrease in activity level in Long-Evans rats. Exp. Neurol 201 (1), 84–89. 10.1016/j.expneurol.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Newman J, Covarrubias A, Zhao M, Huang Y, Haldar S, Verdin E, 2017. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Met. 26 (3), 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordli DR Jr, Kuroda MM, Carroll J, Koenigsberger DY, Hirsch LJ, Bruner HJ, et al. , 2001. Experience with the ketogenic diet in infants. Pediatrics. 10.1542/peds.108.1.129. [DOI] [PubMed] [Google Scholar]
- Paoli A, 2014. Ketogenic diet for obesity: friend or foe? Int. J. Environ. Res. PublicHealth 10.3390/ijerph110202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partsalaki I, Karvela A, Spiliotis BE, 2012. Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents. J. Pediatr. Endocrinol. Metab 10.1515/jpem-2012-0131. [DOI] [PubMed] [Google Scholar]
- Patel A, Pyzik P, Turner Z, Rubenstein J, Kossoff E, 2010. Long‐term outcomes of children treated with the ketogenic diet in the past. Epilepsia 51 (7), 1277–1282. [DOI] [PubMed] [Google Scholar]
- Paul E, Conant KD, Dunne IE, Pfeifer HH, Lyczkowski DA, Linshaw MA, Thiele EA, 2010. Urolithiasis on the ketogenic diet with concurrent topiramate or zonisamide therapy. Epilepsy Res. 10.1016/j.eplepsyres.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ, 2010. Induction of fear extinction with hippocampal-lnfralimbic BDNF. Science. 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Bonucci A, Maggi E, Corsi M, Businaro R, 2018. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer’s disease. Antioxidants. 10.3390/antiox7050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, et al. , 2017. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 10.1016/j.cmet.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustay N, Browman K, Curzon P, 2008. In: Cued and Contextual Fear Conditioning forRodents, 10.1201/noe1420052343.ch2. [DOI] [PubMed]
- Schwartz KA, Noel M, Nikolai M, Chang HT, 2018. Investigating the ketogenic diet as treatment for primary aggressive brain cancer: challenges and lessons learned. Front. Nutr 10.3389/fnut.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simm PJ, Bicknell-Royle J, Lawrie J, Nation J, Draffin K, Stewart KG, et al. , 2017. The effect of the ketogenic diet on the developing skeleton. Epilepsy Res. 10.1016/j.eplepsyres.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Skelton MR, Schaefer TL, Graham DL, deGrauw TJ, Clark JF, Williams MT, Vorhees CV, 2011. Creatine transporter (CrT; Slc6a8) knockout mice as a model of human CrT deficiency. PLoS One. 10.1371/journal.pone.0016187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thio LL, Rensing N, Maloney S, Wozniak DF, Xiong C, Yamada KA, 2010. A ketogenic diet does not impair rat behavior or long-term potentiation: BRIEF COMMUNICATION. Epilepsia. 10.1111/j.1528-1167.2009.02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkel AA, Ijff DM, Verkuyl JM, 2018. Cognitive benefits of the ketogenic diet in patients with epilepsy: a systematic overview. Epilepsy Behav. 87, 69–77. 10.1016/j.yebeh.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT, 2006. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder RM, 1921. High fat diets in epilepsy. Mayo Clin. Bull 308. [Google Scholar]
- Wirrell EC, 2008. Ketogenic ratio, calories, and fluids: do they matter? Epilepsia. 10.1111/j.1528-1167.2008.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Sun X, Eroku BO, Tsipis CP, Puchowicz MA, LaManna JC, 2010. Dietinduced ketosis improves cognitive performance in aged rats. Adv. Exp. Med. Biol 10.1007/978-1-4419-1241-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin A, Kropp B, Chevalier Y, Junnila R, Sustarsic E, Herbach N, Fanelli F, Mezzullo M, Milz S, Bidlingmaier M, Beilohuby M, 2016. Low-carbohydrate, high-fat diets have sex-specific effects on bone health in rats. Eur. J. Nutr 55 (7), 2307–2320. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Stafstrom CE, Fu DD, Hu Y, Holmes GL, 2004. Detrimental effects of the ketogenic diet on cognitive function in rats. Pediatr. Res 10.1203/01.PDR.0000112032.47575.D1. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Gamaro GD, Araújo E, Bassani MG, Perry MLS, Dalmaz C, Gonçalves CA, 2005. Nociception and locomotor activity are increased in ketogenic diet fed rats. Physiol. Behav 10.1016/j.physbeh.2005.01.003. [DOI] [PubMed] [Google Scholar]


