Abstract
Objective
Regulatory T cells (Tregs) play a central role in immune responses to infectious agents and tumors. Paradoxically, Tregs protect self‐cells from the immune response as a part of peripheral tolerance and prevents autoimmune disorders, whereas during the process of carcinogenesis, they are exploited by tumor cells for protection against antitumor immune responses. Therefore, Tregs are often considered as a major obstacle in anticancer therapy. The objective of this review is to provide a current understanding on Tregs as a potential cellular target for achieving therapeutic gain and discuss various approaches that are implicated at preclinical and clinical scenario.
Recent findings
Several approaches like immunotherapy and adjuvant chemotherapy, which reduce Tregs population, have been found to be useful in improving local tumor control. Our recent observations with the glycolytic inhibitor, 2‐deoxy‐D‐glucose, established as an adjuvant in radiotherapy and chemotherapy of tumors also show that potential of 2‐deoxy‐D‐glucose to improve local tumor control is linked with its ability to reduce the Tregs pool.
Conclusions
Several published studies and emerging evidences indicate that suppression of Treg numbers, infiltration into the tumors, and function can improve the cancer therapy by enhancing the antitumor immunity.
Keywords: 2‐deoxy‐D‐glucose, antitumor immunity, cancer therapy, immune modulation, regulatory T cells
1. INTRODUCTION
Cancer is the second biggest cause of morbidity and mortality after cardiovascular diseases in the world.1 It is well established that cancer primarily arises because of uncontrolled and rampant growth of self‐cells leading to what is loosely defined as “malignancy” besides the other hallmarks of cancer as described by Hanahan and Weinberg.2 At the initial stages of tumor progression, tumor cells are recognized by the cells of the innate immune system, while at later stages, tumor cells use several strategies to evade immune responses.3, 4 The adaptive immune response is initiated and matured as a result of the expression of tumor‐associated antigens recognized by the immune system of the host.5, 6 Nevertheless, tumors can escape immune surveillance. The process of evasion (immune editing) results from various mechanisms indicating that the immune responses to tumor cells create selective pressures that decide their survival and progression including spread.7, 8 Among the several mechanisms known, suppression of cytotoxic T cell response to tumors by regulatory T cells (Tregs) plays an important role. Evidences from mouse models and cancer patients show that the population of Tregs increases in parallel with the tumor growth, while depletion of Tregs in mice increases the antitumor immunity and reduces tumor growth.9, 10
Treg cells (CD4+CD25+FoxP3+) are a specialized subset of CD4+ T cells and are different from other immune cells because of high expression of CD25 (interleukin [IL]‐2 receptor) and transcription factor FoxP311 (forkhead box P3). The role of Tregs has been extensively investigated in autoimmunity, allergy, and microbial infections.12, 13, 14 Tregs are mainly classified into 2 types on the basis of their origin: n‐Tregs (naturally occurring Tregs) and i‐Tregs (inducible Tregs). n‐Tregs are mainly generated in thymus and suppress immune effector cells in a contact‐dependent manner, while i‐Tregs are found to be generated in the periphery and suppress immune responses by several ways, which include production of inhibitory cytokines, such as IL‐10, IL‐35, and transforming growth factor (TGF)‐β, induction of cytolysis by the production of granzymes, disruption of effector cell metabolism, and dendritic cell–mediated suppression.15, 16, 17 Several new subsets of Tregs have been discovered and extensively studied.18
Tregs on the one hand save self‐cells from recognition by immune system and protect body from autoimmunity, and on the other hand, they compromise the antitumor immunity.13, 14 It is well established that in mouse and human malignancies, body's own cells become deregulated and avoid death from chemotherapeutic agents by up‐regulating the pool of Tregs and escaping immune response.19, 20, 21 Therefore, strategies that target Treg cells have been found to be successful in achieving local tumor control. Targeting Tregs‐specific molecules using monoclonal antibodies and several chemotherapeutic drugs have been found to deplete Tregs, besides their established antitumor effects.22, 23, 24, 25, 26, 27 Our recent studies have shown that the glycolytic inhibitor 2‐deoxy‐D‐glucose (2‐DG) that target metabolic reprogramming of tumors also reduce the levels of i‐Tregs.28, 29 All these studies suggest that suppression of Tregs level and/or function can restore antitumor immunity, which can be exploited for the treatment of tumors.
Other interesting approaches such as directing naïve T cells and Tregs into Th17 and other subpopulation of cell fates have been the topic of several recent reviews. This review summarizes the current status of Tregs‐based targeted therapies as well as conventional chemotherapeutic agents that reduce Tregs thereby leading to improved therapeutic gain.
2. TREGS IN SURVIVAL AND PROGNOSIS
Increased presence of Tregs is generally associated with reduced survival and poor prognosis in many types of cancer30, 31 such as renal cell carcinoma32 primarily due to their capacity to inhibit antitumor immunity. However, in colorectal carcinoma33, 34, 35, 36, 37 and B‐cell lymphoma38 patients, high Treg cell infiltration is associated with a favorable prognosis. In case of B‐cell lymphomas or other hematological cancers, this could be due to an additional role of Tregs in regulating malignant immune cell by recognizing tumor antigens on MHCII on the tumor cell leading to tumor cell killing.39 Similarly, in large intestine, the presence of microbiological flora results in increased Th17‐mediated inflammatory antimicrobial response that can promote cancer growth. Attenuation of this tumor‐enhancing response by Tregs may provide an explanation for favorable prognosis in colorectal carcinoma patients with high Treg cell numbers in the tumor.34 Therefore, it appears that Tregs play diverse roles in different types of cancers and may be useful in some settings, which need a thorough understanding of their relationship with other cells, effects of their anatomic distribution, and other factors.
3. SPECIFIC AND NONSPECIFIC APPROACHES FOR THE MODULATION OF TREGS
Tumors use numerous mechanisms to suppress host immunity including altered antigen‐presenting cell function, fostering dysfunctional T cell co‐signaling and generating an immune‐subversive cytokine milieu.3 Moreover, accumulating evidences suggest that the effects of anticancer treatments on the immune system significantly contribute to their overall efficacy, besides their direct effects on the tumor cells/tissues.40 Therefore, agents that have the potential to restore host immunity have been investigated as immunotherapeutic as well as adjuvant to other therapeutic modalities. Among several approaches to modulate immune status, eliminating CD4+CD25+ T cells has been found to enhance antitumor immunity by abrogating immunological unresponsiveness of syngeneic tumor in‐vivo and in‐vitro.41 This can be achieved through specific depletion of Tregs by blocking the specific surface receptors like CTLA‐4, CD25, or various chemokine receptors (eg, CCR5, which induces migration of Tregs to tumor sites)22, 23, 24, 25 (Figure 1). Administration of agonist anti‐GITR or anti‐OX40 antibodies is also capable of evoking antitumor immunity and resulting in the eradication of established tumor25, 26 (Figure 1). Interestingly, several chemotherapeutic agents like aromatase inhibitor, cyclophosphamide, fludarabine, gemcitabine, and mitoxantrone have also been found to deplete Tregs, besides their established antitumor effects27 (Figure 1). Our recent studies have shown that systemically administered 2‐DG significantly enhances the antitumor effects of radiation and anticancer drugs42, 43, 44 that appears to be related to the immunomodulation induced by the combination.28 Interestingly, the complete response (tumor‐free survival; cure) observed only in nearly 50% of the animals following the combined treatment with tumor irradiation and 2‐DG was found to be strongly linked to the immune stimulation characterized by selective lymphodepletion, shift from Th2 to Th1 and attenuation of the immune‐suppressive network.28 Interestingly, the immune stimulation appeared to be related to the depletion of i‐Tregs brought about by the combined treatment28, 29 in spleen, peripheral blood, lymph node, and in the tumor.44 However, natural Tregs (Tregs in nontumor‐bearing mice) were not influenced, suggesting a differential effect on n‐Tregs and i‐Tregs by the combination. Although inhibition of glycolysis (including by 2‐DG) is known to reduce the FoxP3 level, essential for the functioning of Tregs,45 the differential effects on n‐Tregs and i‐Tregs are not understood. These differential effects, however, could be related to differences in the metabolic profile as metabolic reprogramming of lymphocytes in general has been suggested to be context and environment dependent.46 Thus, 2‐DG not only sensitizes tumor cells by modifying glycolysis‐dependent and glycolysis‐independent damage response pathways47 but also stimulates antitumor immunity mainly by reducing the Tregs (Figure 1). Therefore, 2‐DG appears to be a good candidate as an adjuvant for immunotherapy besides its established role as an adjuvant for chemotherapy and radiotherapy.48, 49
Figure 1.
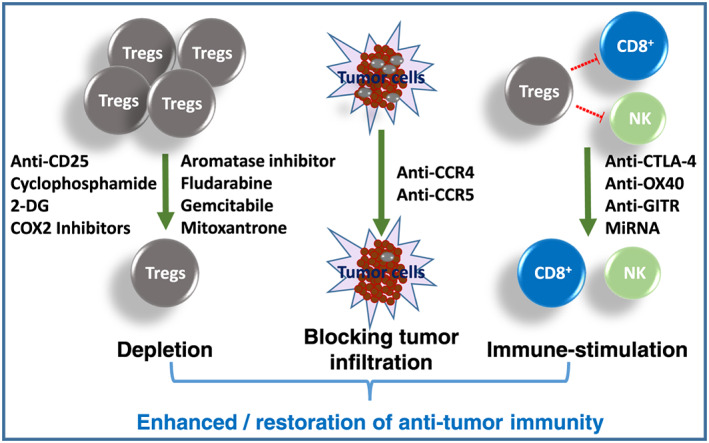
Specific and nonspecific strategies to attenuate the numbers or function of regulatory T cells (Tregs). The depletion of Tregs, blocking of their recruitment to the tumor tissues or inhibition of their immunosuppressive function leads to restoration of antitumor immunity
In addition, several studies have shown that disruption of chemokine signaling leads to reduced migration of Tregs to the tumors50, 51 (Figure 1). Reducing CCL5 production by tumors or CCR5 inhibition by TAK‐779 resulted in reduction in tumor growth in human and murine pancreatic tumor models.50 Targeting of CCL17/22‐CCR4 axis, which is important in several types of cancers including lymphoma, lung, ovarian, gastric, breast, and prostate,52 by CCR4‐targeted antibodies has shown effective Treg depletion and antitumor response53, 54, 55, 56, 57, 58 (Figure 1).
Collectively, these studies suggest that suppressing the Treg function can restore antitumor immunity, which can be used as an approach for therapy.
4. REGULATION OF ANTITUMOR IMMUNITY BY TREGS AND IMPLICATIONS ON THE OUTCOME OF THERAPIES
Studies in mouse models suggest that Tregs are major regulators of antitumor immunity.59, 60, 61 They not only inhibit natural killer cell–mediated cytotoxicity but also suppress the proliferation of and IFN‐γ production by CD4+ and CD8+ T cells thus impairing the antitumor immune response.59, 60 However, in the clinical scenario, increased level of Tregs has also been shown to be associated with reduced survival and poor prognosis in patients of different types of cancers.30, 62, 63, 64, 65 As discussed in this review, one of the obstacles for specific depletion of FoxP3+ Tregs is that both FoxP3+ Tregs and effector T cells exhibit an activated phenotype, especially in expression patterns of cell surface molecules. Both are high in expression of CD25, an activation marker; CTLA‐4, an immune checkpoint; and OX40 and GITR, immune co‐stimulatory molecules.25, 66, 67, 68, 69 Therefore, there may be a limitation in differentially targeting each molecule alone for Treg depletion. With current knowledge, however, the search is under way to find a highly specific cell surface marker for FoxP3+ Tregs. Another way of augmenting tumor immunity is to design combination therapy targeting both Tregs and non‐Treg cells and alter numerical or functional balance between the 2 populations, ie, to deplete Tregs or attenuate Treg suppressive function and to simultaneously expand effector T cells or augment their effector activity.65 Therefore, it appears that a delicate balance between Treg and effector T cells is important, as a very low number of Tregs will result in autoimmunity, while a higher number can suppress the immune system and result in tumor chemoresistance leading to poor prognosis and reduced survival of patients.
5. CROSS TALK BETWEEN TREGS AND INFLAMMATION
Tregs are known to suppress the antitumor immune response during cancer progression. They not only suppress innate but also the adaptive immune responses activated during the cancer progression.70 Although Tregs have always been a fascinating target for cancer therapy, manipulating them as a therapeutic approach has received relatively less attention.71 Targeting Tregs without addressing the cause of their up‐regulation might even lead to more inflammation and aggressive cancer progression. Indeed, we recently reported this in tumor‐bearing mice following the administration of 2‐DG alone.72 Up‐regulation of Tregs during heightened inflammation as in the case of cancer is a defense mechanism to protect the collateral damage, but suppression induced by the Tregs becomes nonspecific and also suppresses the antitumor immune response. Therefore, approaches that target the source of inflammation (source of induction of Tregs) besides decreasing (depleting) Tregs may be more efficient in enhancing the therapeutic gain, as observed following the combination treatment with 2‐DG and radiation.28 These studies also indicate that the extent of decrease in Tregs coupled with reduction in the inflammation are essential in achieving local tumor control as modifying any one of them did not elicit complete response.28, 72 Further, induction of Tregs associated with different malignancies has been shown to polarize macrophages toward M2 type,73, 74 suggesting that depletion of Tregs may lead to macrophagic polarization toward M1 and correlate with local tumor control, as has been reported by us recently. 72
6. NEW APPROACHES FOR TARGETING TREGS
Although many approaches are currently available for the depletion of Tregs, relentless search for novel targets or depletors of Tregs are increasing the hope for an effective cancer immunotherapy. In line with this, RANKL expression on Tregs has been shown to activate RANK (NF‐κB) receptor on cancer cells75. RANKL activity is greatly increased in the cancers that metastasize to bones such as breast and prostate cancers.76, 77 Furthermore, RANKL‐RANK signaling appears to have a role in the functioning of the immune system as RANKL is expressed by T helper cells as well as Tregs and are also involved in dendritic cell maturation.75, 78 Therefore, inhibitors of RANK signaling, like anti‐RANKL antibody denosumab, holds promising potential in preventing Tregs‐assisted metastases of established cancers.75 Another novel strategy for Tregs immunotherapy is targeting FoxP3, a master transcription factor of Tregs, through RNA interference (Figure 1). This window of hope opened up when it was shown that administration of miR‐31, a negative regulator of FoxP3, through a lentiviral vector abrogated Treg function.79 The issue to be addressed while targeting FoxP3 is that it is also expressed in T effector population80 as well as in some cancer cells,81 so the outcome can also be therapeutically detrimental. Furthermore, up‐regulated COX‐2 expression is a characteristic feature of tumor environment82 and several COX‐2 inhibitors have been shown to deplete Tregs (Figure 1).83
Tumor‐derived exosomes have been found to influence the levels and functioning of Tregs in the effusions from malignant cells, with the cell surface TGF‐β1 mediating the FoxP3 expression.84 Therefore, containing malignant effusion‐derived exosomes expressing TGF‐β1 appears to be an attractive immunotherapeutic strategy for overcoming Treg‐induced immune suppression.84 Furthermore, recent evidences point toward the contribution of vascular endothelial growth factor receptor 2 in the induction of Tregs,85 suggesting thereby that vascular endothelial growth factor receptor 2 may be a useful target in overcoming immune suppression caused by tumors and improve therapy. The safety of combining peptide vaccination using VEGFR1/2 with or without chemotherapy has been established in phase I clinical trials in patients with pancreatic cancer and renal cell carcinoma,86, 87 while the clinical efficacy is yet to be established.
7. CONCLUSIONS
Emerging evidences are convincing enough to support the notion that attenuating the impact of Tregs (by depletion, blocking their infiltration into tumors and reducing differentiation and signaling) has the potential to enhance tumor immunity88, 89, 90, 91 thereby improving the efficacy of cancers therapy and, in particular, the immunotherapy92 (Figure 1). Unfortunately, it has the risk of enhancing normal tissue toxicity and induction of autoimmunity due to the sharing of the same receptor by other effector T cells leading to autoimmune disorders and diseases because of uncontrolled pathological immune responses.93, 94, 95, 96, 97, 98, 99, 100, 101, 102 Therefore, a careful consideration is required on the extent of depletion and the context (ie, location, homing, and cytokine milieu) and an approach that selectively depletes tumor‐associated i‐Tregs, while maintaining the naturally occurring n‐Tregs. Several interesting approaches including the conversion of inducible Tregs into other antitumor T cell subpopulations have been found to be useful, besides certain chemotherapeutic agents, which reduce Tregs in addition to their primary target (tumor).25, 27 Our recent observations with the glycolytic inhibitor, 2‐DG42, 44, 48, 49, 103, 104, 105, 106 suggest that metabolic modifiers like this glucose analog can also effectively deplete Tregs and may be related to their antitumor efficacy. Since 2‐DG/metabolic modifiers have little or no undesirable effects on the normal cells, they can be used more successfully to boost antitumor immune response and enhance the efficacy of radiotherapy, chemotherapy, and immunotherapy.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
FUNDING SOURCES
Earlier work in the laboratory of B.S.D. was supported by grants from DRDO, Government of India (INM‐301 and INM‐311).
AUTHORS' CONTRIBUTION
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, B.S.D., A.F., S.G.; Methodology, B.S.D., S.G.; Investigation, B.S.D., S.G.; Formal Analysis, B.S.D., S.G.; Resources, B.S.D., A.F., S.G.; Writing ‐ Original Draft, B.S.D., S.G.; Writing ‐ Review & Editing, B.S.D., S.G.; Visualization, B.S.D., A.F., S.G.; Supervision, B.S.D., S.G.; Funding Acquisition, B.S.D.
ACKNOWLEDGEMENTS
B.S.D. thanks Dr Jiade Lu, Executive Vice President and Dr Xiaodong Wu, Shanghai Proton and Heavy Ion Center, Shanghai, China for their encouragement and support.
Dwarakanath BS, Farooque A, Gupta S. Targeting regulatory T cells for improving cancer therapy: Challenges and prospects. Cancer Reports. 2018;1:e21105. 10.1002/cnr2.1105
Authorship of Dr Abdullah is posthumously included, who contributed in the initial conception of the review and unfortunately met with an untimely death.
Contributor Information
Bilikere S. Dwarakanath, Email: dwarakanath@sphic.org.cn.
Seema Gupta, Email: sg1335@georgetown.edu.
REFERENCES
- 1. Eyre H, Kahn R, Robertson RM, American Cancer Society tADA , the American Heart Association , Collaborative Writing C . Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Diabetes Care. 2004;27(7):1812‐1824. [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. 10.1016/j.cell.2011.02.013, S0092–8674(11)00127–9[pii] [DOI] [PubMed] [Google Scholar]
- 3. Cavallo F, De Giovanni C, Nanni P, Forni G, Lollini PL. 2011: the immune hallmarks of cancer. Cancer Immunol Immunother. 2011;60(3):319‐326. 10.1007/s00262-010-0968-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22(1):329‐360. 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 5. de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24‐37. 10.1038/nrc1782 [DOI] [PubMed] [Google Scholar]
- 6. Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced toll‐like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6(6):579‐586. 10.1038/ni1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer. 2018;18(3):139‐147. 10.1038/nrc.2017.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35(Suppl):S185‐S198. 10.1016/j.semcancer.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 9. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015;16(13):e498–e509. 10.1016/S1470-2045(15)00007-8, S1470‐2045(15)00007‐8 [pii]. [DOI] [PubMed] [Google Scholar]
- 10. Viehl CT, Moore TT, Liyanage UK, et al. Depletion of CD4+CD25+ regulatory T cells promotes a tumor‐specific immune response in pancreas cancer‐bearing mice. Ann Surg Oncol. 2006;13(9):1252‐1258. 10.1245/s10434-006-9015-y [DOI] [PubMed] [Google Scholar]
- 11. Zhao H, Liao X, Kang Y. Tregs: where we are and what comes next? Front Immunol. 2017;8:1578. 10.3389/fimmu.2017.01578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28(8):401‐409. 10.1093/intimm/dxw025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol. 2005;116(5):961‐968. quiz 9. 10.1016/j.jaci.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 14. Demengeot J, Zelenay S, Moraes‐Fontes MF, Caramalho I, Coutinho A. Regulatory T cells in microbial infection. Springer Semin Immunopathol. 2006;28(1):41‐50. 10.1007/s00281-006-0024-5 [DOI] [PubMed] [Google Scholar]
- 15. Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10(7):689‐695. 10.1038/ni.1760 [DOI] [PubMed] [Google Scholar]
- 16. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330‐336. 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- 17. Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006;16(2):115‐123. 10.1016/j.semcancer.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 18. Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003;171(12):6323‐6327. [DOI] [PubMed] [Google Scholar]
- 19. Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108(3):804‐811. 10.1182/blood-2006-02-002774 [DOI] [PubMed] [Google Scholar]
- 20. Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92(5):913‐920. 10.1038/sj.bjc.6602407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T‐cell–mediated suppression of infiltrating CD4+ T cells in B‐cell non‐Hodgkin lymphoma. Blood. 2006;107(9):3639‐3646. 10.1182/blood-2005-08-3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Callahan MK, Wolchok JD, Allison JP. Anti–CTLA‐4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. 2010;37(5):473‐484. 10.1053/j.seminoncol.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shevach EM. Mechanisms of foxp3+ T regulatory cell‐mediated suppression. Immunity. 2009;30(5):636‐645. 10.1016/j.immuni.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 24. McHugh RS, Whitters MJ, Piccirillo CA, et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid‐induced TNF receptor. Immunity. 2002;16(2):311‐323. [DOI] [PubMed] [Google Scholar]
- 25. Liu C, Workman CJ, Vignali DA. Targeting regulatory T cells in tumors. FEBS J. 2016;283(14):2731‐2748. 10.1111/febs.13656 [DOI] [PubMed] [Google Scholar]
- 26. Ko K, Yamazaki S, Nakamura K, et al. Treatment of advanced tumors with agonistic anti‐GITR mAb and its effects on tumor‐infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202(7):885‐891. 10.1084/jem.20050940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune‐based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale‐based combined treatments against cancer. Cell Death Differ. 2014;21(1):15‐25. 10.1038/cdd.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Farooque A, Singh N, Adhikari JS, Afrin F, Dwarakanath BS. Enhanced antitumor immunity contributes to the radio‐sensitization of Ehrlich ascites tumor by the glycolytic inhibitor 2‐deoxy‐D‐glucose in mice. PLoS one. 2014;9(9):e108131. 10.1371/journal.pone.0108131, PONE‐D‐14‐16581 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farooque, A. S. N. , Verma, A. , Agrahari, S. , et al. eds (2012). Immuno‐modulation linked to the depletion of T regulatory cells contributes to the radio‐sensitization of tumors by the glycolytic inhibitor 2‐Deoxy‐D‐Glucose. International Conference on Radiation Biology (ICRB‐2012) on Cosmic Radiation to Cancer Therapeutics and 11th Biennial Meeting of the Indian Society of Radiation Biology; 2012; Mumbai, India: J. Cancer Res. Therap.
- 30. Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high‐risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373‐5380. 10.1200/JCO.2006.05.9584 [DOI] [PubMed] [Google Scholar]
- 31. Miyara M, Sakaguchi S. Human FoxP3+CD4+ regulatory T cells: their knowns and unknowns. Immunol Cell Biol. 2011;89(3):346‐351. 10.1038/icb.2010.137 [DOI] [PubMed] [Google Scholar]
- 32. Siddiqui SA, Frigola X, Bonne‐Annee S, et al. Tumor‐infiltrating Foxp3−CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res. 2007;13(7):2075‐2081. 10.1158/1078-0432.CCR-06-2139 [DOI] [PubMed] [Google Scholar]
- 33. Frey DM, Droeser RA, Viehl CT, et al. High frequency of tumor‐infiltrating FOXP3+ regulatory T cells predicts improved survival in mismatch repair‐proficient colorectal cancer patients. Int J Cancer. 2010;126(11):2635‐2643. 10.1002/ijc.24989 [DOI] [PubMed] [Google Scholar]
- 34. Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother. 2011;60(7):909‐918. 10.1007/s00262-011-1046-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salama P, Phillips M, Grieu F, et al. Tumor‐infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27(2):186‐192. JCO.2008.18.7229 [pii], 10.1200/JCO.2008.18.7229 [DOI] [PubMed] [Google Scholar]
- 36. Correale P, Rotundo MS, Del Vecchio MT, et al. Regulatory (FoxP3+) T‐cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. 2010;33(4):435‐441. 10.1097/CJI.0b013e3181d32f01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suzuki H, Chikazawa N, Tasaka T, et al. Intratumoral CD8+ T/FOXP3+ cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother. 2010;59(5):653‐661. 10.1007/s00262-009-0781-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center‐like diffuse large B‐cell lymphoma, follicular lymphoma and classical Hodgkin's lymphoma. Haematologica. 2008;93(2):193‐200. 10.3324/haematol.11702 [DOI] [PubMed] [Google Scholar]
- 39. Lindqvist CA, Loskog AS. T regulatory cells in B‐cell malignancy—tumour support or kiss of death? Immunology. 2012;135(4):255‐260. 10.1111/j.1365-2567.2011.03539.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, Tesniere A, Kroemer G. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118(6):1991‐2001. 10.1172/JCI35180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tanaka H, Tanaka J, Kjaergaard J, Shu S. Depletion of CD4+ CD25+ regulatory cells augments the generation of specific immune T cells in tumor‐draining lymph nodes. J Immunother. 2002;25(3):207‐217. [DOI] [PubMed] [Google Scholar]
- 42. Dwarakanath BS, Singh S, Jain V. Optimization of tumour radiotherapy: part V—radiosensitization by 2‐deoxy‐D‐glucose and DNA ligand Hoechst‐33342 in a murine tumour. Indian J Exp Biol. 1999;37(9):865‐870. [PubMed] [Google Scholar]
- 43. Gupta S, Mathur R, Dwarakanath BS. The glycolytic inhibitor 2‐deoxy‐D‐glucose enhances the efficacy of etoposide in Ehrlich ascites tumor‐bearing mice. Cancer Biol Ther. 2005;4(1):87‐94. [DOI] [PubMed] [Google Scholar]
- 44. Gupta S, Farooque A, Adhikari JS, Singh S, Dwarakanath BS. Enhancement of radiation and chemotherapeutic drug responses by 2‐deoxy‐D‐glucose in animal tumors. J Cancer Res Ther. 2009;5(Suppl 1):S16‐S20. 10.4103/0973-1482.55135, JCanResTher_2009_5_9_16_55135 [pii] [DOI] [PubMed] [Google Scholar]
- 45. De Rosa V, Galgani M, Porcellini A, et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat Immunol. 2015;16(11):1174‐1184. 10.1038/ni.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Donnelly RP, Finlay DK. Glucose, glycolysis and lymphocyte responses. Mol Immunol. 2015;68(2 Pt C):513‐519. 10.1016/j.molimm.2015.07.034 [DOI] [PubMed] [Google Scholar]
- 47. Dwarakanath BS. Cytotoxicity, radiosensitization, and chemosensitization of tumor cells by 2‐deoxy‐D‐glucose in vitro. J Cancer Res Ther. 2009;5(Suppl 1):S27‐S31. 10.4103/0973-1482.55137, JCanResTher_2009_5_9_27_55137 [pii] [DOI] [PubMed] [Google Scholar]
- 48. Dwarakanath B, Jain V. Targeting glucose metabolism with 2‐deoxy‐D‐glucose for improving cancer therapy. Future Oncol. 2009;5(5):581‐585. 10.2217/fon.09.44 [DOI] [PubMed] [Google Scholar]
- 49. Dwarakanath BS, Singh D, Banerji AK, et al. Clinical studies for improving radiotherapy with 2‐deoxy‐D‐glucose: present status and future prospects. J Cancer Res Ther. 2009;5(Suppl 1):S21‐S26. 10.4103/0973-1482.55136, JCanResTher_2009_5_9_21_55136 [pii] [DOI] [PubMed] [Google Scholar]
- 50. Tan MC, Goedegebuure PS, Belt BA, et al. Disruption of CCR5‐dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol. 2009;182(3):1746‐1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chaudhary B, Elkord E. Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines (Basel). 2016;4(3). 10.3390/vaccines4030028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mailloux AW, Young MR. Regulatory T‐cell trafficking: from thymic development to tumor‐induced immune suppression. Crit Rev Immunol. 2010;30(5):435‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sugiyama D, Nishikawa H, Maeda Y, et al. Anti‐CCR4 mAb selectively depletes effector‐type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S a. 2013;110(44):17945‐17950. 10.1073/pnas.1316796110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kurose K, Ohue Y, Sato E, et al. Increase in activated Treg in TIL in lung cancer and in vitro depletion of Treg by ADCC using an antihuman CCR4 mAb (KM2760). J Thorac Oncol. 2015;10(1):74‐83. 10.1097/JTO.0000000000000364 [DOI] [PubMed] [Google Scholar]
- 55. Kurose K, Ohue Y, Wada H, et al. Phase Ia study of FoxP3+ CD4 Treg depletion by infusion of a humanized anti‐CCR4 antibody, KW‐0761, in cancer patients. Clin Cancer Res. 2015;21(19):4327‐4336. 10.1158/1078-0432.CCR-15-0357 [DOI] [PubMed] [Google Scholar]
- 56. Ishida T, Joh T, Uike N, et al. Defucosylated anti‐CCR4 monoclonal antibody (KW‐0761) for relapsed adult T‐cell leukemia‐lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30(8):837‐842. 10.1200/JCO.2011.37.3472 [DOI] [PubMed] [Google Scholar]
- 57. Ogura M, Ishida T, Hatake K, et al. Multicenter phase II study of mogamulizumab (KW‐0761), a defucosylated anti‐cc chemokine receptor 4 antibody, in patients with relapsed peripheral T‐cell lymphoma and cutaneous T‐cell lymphoma. J Clin Oncol. 2014;32(11):1157‐1163. 10.1200/JCO.2013.52.0924 [DOI] [PubMed] [Google Scholar]
- 58. Ni X, Jorgensen JL, Goswami M, et al. Reduction of regulatory T cells by Mogamulizumab, a defucosylated anti‐CC chemokine receptor 4 antibody, in patients with aggressive/refractory mycosis fungoides and Sezary syndrome. Clin Cancer Res. 2015;21(2):274‐285. 10.1158/1078-0432.CCR-14-0830 [DOI] [PubMed] [Google Scholar]
- 59. Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32(11):3267‐3275. [DOI] [PubMed] [Google Scholar]
- 60. Lin YC, Chang LY, Huang CT, et al. Effector/memory but not naive regulatory T cells are responsible for the loss of concomitant tumor immunity. J Immunol. 2009;182(10):6095‐6104. 10.4049/jimmunol.0803829 [DOI] [PubMed] [Google Scholar]
- 61. Turk MJ, Guevara‐Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200(6):771‐782. 10.1084/jem.20041130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Okita R, Saeki T, Takashima S, Yamaguchi Y, Toge T. CD4+CD25+ regulatory T cells in the peripheral blood of patients with breast cancer and non‐small cell lung cancer. Oncol Rep. 2005;14(5):1269‐1273. [PubMed] [Google Scholar]
- 63. Bergmann C, Strauss L, Wang Y, et al. T regulatory type 1 cells in squamous cell carcinoma of the head and neck: mechanisms of suppression and expansion in advanced disease. Clin Cancer Res. 2008;14(12):3706‐3715. 10.1158/1078-0432.CCR-07-5126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12(18):5423‐5434. 10.1158/1078-0432.CCR-06-0369 [DOI] [PubMed] [Google Scholar]
- 65. Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127(4):759‐767. 10.1002/ijc.25429 [DOI] [PubMed] [Google Scholar]
- 66. Rodriguez‐Perea AL, Arcia ED, Rueda CM, Velilla PA. Phenotypical characterization of regulatory T cells in humans and rodents. Clin Exp Immunol. 2016;185(3):281‐291. 10.1111/cei.12804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kmieciak M, Gowda M, Graham L, et al. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory/suppressor function. J Transl Med. 2009;7(1):89. 10.1186/1479-5876-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McCoy KD, Le Gros G. The role of CTLA‐4 in the regulation of T cell immune responses. Immunol Cell Biol. 1999;77(1):1‐10. 10.1046/j.1440-1711.1999.00795.x [DOI] [PubMed] [Google Scholar]
- 69. Sanmamed MF, Pastor F, Rodriguez A, et al. Agonists of co‐stimulation in Cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015;42(4):640‐655. 10.1053/j.seminoncol.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 70. Kanterman J, Sade‐Feldman M, Baniyash M. New insights into chronic inflammation‐induced immunosuppression. Semin Cancer Biol. 2012;22(4):307‐318. 10.1016/j.semcancer.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 71. Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20(2):241‐246. 10.1016/j.coi.2008.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Farooque A, Afrin F, Adhikari JS, Dwarakanath BS. Polarization of macrophages towards M1 phenotype by a combination of 2‐deoxy‐d‐glucose and radiation: implications for tumor therapy. Immunobiology. 2016;221(2):269‐281. 10.1016/j.imbio.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 73. Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S a. 2007;104(49):19446‐19451. 10.1073/pnas.0706832104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu G, Ma H, Qiu L, et al. Phenotypic and functional switch of macrophages induced by regulatory CD4+CD25+ T cells in mice. Immunol Cell Biol. 2011;89(1):130‐142. 10.1038/icb.2010.70 [DOI] [PubMed] [Google Scholar]
- 75. Tan W, Zhang W, Strasner A, et al. Tumour‐infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL‐RANK signalling. Nature. 2011;470(7335):548‐553. 10.1038/nature09707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Brown JM, Corey E, Lee ZD, et al. Osteoprotegerin and rank ligand expression in prostate cancer. Urology. 2001;57(4):611‐616. [DOI] [PubMed] [Google Scholar]
- 77. Thomas RJ, Guise TA, Yin JJ, et al. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999;140(10):4451‐4458. 10.1210/endo.140.10.7037 [DOI] [PubMed] [Google Scholar]
- 78. Leibbrandt A, Penninger JM. Novel functions of RANK (L) signaling in the immune system. Adv Exp Med Biol. 2010;658:77‐94. 10.1007/978-1-4419-1050-9_9 [DOI] [PubMed] [Google Scholar]
- 79. Amendola M, Passerini L, Pucci F, Gentner B, Bacchetta R, Naldini L. Regulated and multiple miRNA and siRNA delivery into primary cells by a lentiviral platform. Mol Ther. 2009;17(6):1039‐1052. 10.1038/mt.2009.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Passerini L, Allan SE, Battaglia M, et al. STAT5‐signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25− effector T cells. Int Immunol. 2008;20(3):421‐431. 10.1093/intimm/dxn002 [DOI] [PubMed] [Google Scholar]
- 81. Karanikas V, Speletas M, Zamanakou M, et al. Foxp3 expression in human cancer cells. J Transl Med. 2008;6(1):19. 10.1186/1479-5876-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18(55):7908‐7916. 10.1038/sj.onc.1203286 [DOI] [PubMed] [Google Scholar]
- 83. Sharma S, Yang SC, Zhu L, et al. Tumor cyclooxygenase‐2/prostaglandin E2‐dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Res. 2005;65(12):5211‐5220. 10.1158/0008-5472.CAN-05-0141 [DOI] [PubMed] [Google Scholar]
- 84. Wada J, Onishi H, Suzuki H, et al. Surface‐bound TGF‐beta1 on effusion‐derived exosomes participates in maintenance of number and suppressive function of regulatory T‐cells in malignant effusions. Anticancer Res. 2010;30(9):3747‐3757. [PubMed] [Google Scholar]
- 85. Wada J, Yamasaki A, Nagai S, et al. Regulatory T‐cells are possible effect prediction markers of immunotherapy for cancer patients. Anticancer Res. 2008;28(4C):2401‐2408. [PubMed] [Google Scholar]
- 86. Miyazawa M, Ohsawa R, Tsunoda T, et al. Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci. 2010;101(2):433‐439. 10.1111/j.1349-7006.2009.01416.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yoshimura K, Minami T, Nozawa M, Uemura H. Phase I clinical trial of human vascular endothelial growth factor receptor 1 peptide vaccines for patients with metastatic renal cell carcinoma. Br J Cancer. 2013;108(6):1260‐1266. 10.1038/bjc.2013.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942‐949. 10.1038/nm1093 [DOI] [PubMed] [Google Scholar]
- 89. Gallimore A, Sakaguchi S. Regulation of tumour immunity by CD25+ T cells. Immunology. 2002;107(1):5‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti‐CD25 (interleukin‐2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59(13):3128‐3133. [PubMed] [Google Scholar]
- 91. Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163(10):5211‐5218. [PubMed] [Google Scholar]
- 92. Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109‐118. 10.1038/cr.2016.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tree TI, Roep BO, Peakman M. A mini meta‐analysis of studies on CD4+CD25+ T cells in human type 1 diabetes: report of the immunology of diabetes society T cell workshop. Ann N Y Acad Sci. 2006;1079(1):9‐18. 10.1196/annals.1375.002. [DOI] [PubMed] [Google Scholar]
- 94. Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155(2):173‐181. 10.1111/j.1365-2249.2008.03860.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Izcue A, Powrie F. Special regulatory T‐cell review: regulatory T cells and the intestinal tract—patrolling the frontier. Immunology. 2008;123(1):6‐10. 10.1111/j.1365-2567.2007.02778.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057‐1061. 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- 97. Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen‐specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169(9):4712‐4716. [DOI] [PubMed] [Google Scholar]
- 98. Morgan ME, Sutmuller RP, Witteveen HJ, et al. CD25+ cell depletion hastens the onset of severe disease in collagen‐induced arthritis. Arthritis Rheum. 2003;48(5):1452‐1460. 10.1002/art.11063 [DOI] [PubMed] [Google Scholar]
- 99. Buckner JH. Mechanisms of impaired regulation by CD4+CD25+FOXP3+ regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10(12):849‐859. 10.1038/nri2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bovenschen HJ, van Vlijmen‐Willems IM, van de Kerkhof PC, van Erp PE. Identification of lesional CD4+ CD25+ Foxp3+ regulatory T cells in Psoriasis. Dermatology. 2006;213(2):111‐117. 10.1159/000093849 [DOI] [PubMed] [Google Scholar]
- 101. Yan KX, Fang X, Han L, et al. Foxp3+ regulatory T cells and related cytokines differentially expressed in plaque vs. guttate psoriasis vulgaris. Br J Dermatol. 2010;163(1):48‐56. 10.1111/j.1365-2133.2010.09742.x [DOI] [PubMed] [Google Scholar]
- 102. Zenclussen AC. CD4+CD25+ T regulatory cells in murine pregnancy. J Reprod Immunol. 2005;65(2):101‐110. 10.1016/j.jri.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 103. Mohanti BK, Rath GK, Anantha N, et al. Improving cancer radiotherapy with 2‐deoxy‐D‐glucose: phase I/II clinical trials on human cerebral gliomas. Int J Radiat Oncol Biol Phys. 1996;35(1):103‐111. S0360–3016(96)85017–6 [pii] [DOI] [PubMed] [Google Scholar]
- 104. Singh D, Banerji AK, Dwarakanath BS, et al. Optimizing cancer radiotherapy with 2‐deoxy‐d‐glucose dose escalation studies in patients with glioblastoma multiforme. Strahlenther Onkol. 2005;181(8):507‐514. 10.1007/s00066-005-1320-z [DOI] [PubMed] [Google Scholar]
- 105. Venkataramanaa NK, Venkatesh PK, Dwarakanath BS, Vani S. Protective effect on normal brain tissue during a combinational therapy of 2‐deoxy‐d‐glucose and hypofractionated irradiation in malignant gliomas. Asian J Neurosurg. 2013;8(1):9‐14. 10.4103/1793-5482.110274, AJNS‐8‐9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Farooque A, Afrin F, Adhikari JS, Dwarakanath BS. Protection of normal cells and tissues during radio‐ and chemosensitization of tumors by 2‐deoxy‐D‐glucose. J Cancer Res Ther. 2009;5(Suppl 1):S32‐S35. 10.4103/0973-1482.55138, JCanResTher_2009_5_9_32_55138 [pii] [DOI] [PubMed] [Google Scholar]


