Abstract
Numerous studies have documented tactile and proprioceptive deficits in children with cerebral palsy (CP) and linked these with weaker somatosensory cortical activity. However, whether such aberrations in somatosensory processing extend and/or progress into adulthood remains poorly understood. In the current study, we used magnetoencephalography (MEG) to investigate the primary somatosensory responses in a sample of individuals with CP (N=42; Age = 9 – 28 years) and a cohort of healthy controls (N=23; Age range = 11 – 23 years). Briefly, transient electrical stimulation was applied to the right tibial nerve, and standardized low-resolution brain electromagnetic tomography (sLORETA) was used to image the dynamic somatosensory cortical response. We found that the strength of somatosensory cortical activity within the 112 – 252 ms time window was significantly reduced in the individuals with CP compared with the healthy controls (HC = 286.53 ± 30.51, 95% CI [226.74, 346.32]; CP = 208.30 ± 19.66,CI [169.77, 246.83], P = 0.0126). These results corroborate previous findings of aberrant somatosensory cortical activity in individuals with CP. Our results also suggest that the somatosensory cortical activity tends to become weaker with age, with a similar rate of neurophysiological change in individuals with CP and healthy controls (P = 0.8790). Visualization of regression models fit to the data imply that youth with CP may have somatosensory cortical activity similar to adult controls. These findings suggest that some individuals with CP exhibit an aberrant developmental trajectory of their somatosensory system.
Keywords: Sensorimotor, sLORETA, magnetoencephalography, lower extremity
Introduction
Cerebral palsy (CP) refers to a general class of movement disorders resulting from an early insult to the developing brain (Rosenbaum et al., 2007). Approximately 3 out of every 1,000 children have CP, making it one of the most prevalent and costly pediatric neurological disorders diagnosed in the United States (Kirby et al., 2011; Christensen et al., 2014). In addition to the motor deficits, impairments in proprioception, stereognosis, and tactile discrimination have been noted in the clinic (Cooper et al., 1995; Clayton et al., 2003; Sanger & Kukke, 2007; Wingert et al., 2008; Auld et al., 2012; Maitre et al., 2012; Robert et al., 2013). Therefore, the classification of CP has expanded to not only include motor deficits but also sensory and perceptual deficits. Although CP is a non-progressive neurological disorder, the musculoskeletal system appears to show aberrant developmental effects that lead to increased mobility impairments across the lifespan (Jahnsen et al., 2004). In particular, the transition from adolescence to adulthood appears to be a critical window where there is often a marked decline in gross motor function and mobility (Hanna et al., 2009). It is possible that somatosensory processing also declines during this critical window since many adults with CP report sedentary lifestyles, fatigue, and balance problems (Jahnsen et al., 2004; Morgan & McGinley, 2014; Lundh et al., 2018; Verschuren et al., 2018). Such lifestyle and physiological changes may limit the richness of the daily sensory experiences. Despite this conjecture, we still have a substantial knowledge gap in our understanding of the cortical somatosensory processing of adults with CP, let alone what happens during this critical transition period.
Numerous magnetoencephalographic (MEG) and electroencephalographic (EEG) studies have shown that somatosensory cortical activity is diminished in youth with CP relative to their typically developing peers. These studies have illustrated that somatosensory cortical oscillations are weaker in the theta-alpha (4–14 Hz) and beta (18–34 Hz) frequency bands following both tactile and electrical stimulation of the feet and hands (Guo et al., 2012; Kurz et al., 2014; Pihko et al., 2014; Kurz et al., 2015a; Kurz et al., 2015b). These attenuated frequency-specific neuronal oscillations appear to be related to deficits in the ankle plantarflexor strength and mobility of youth with CP (Kurz et al., 2014; Kurz et al., 2015b). This observation implies that individuals with higher Gross Motor Function Classification System (GMFCS) levels (i.e., greater mobility impairments) would likely have weaker somatosensory cortical oscillations. In addition to measuring oscillatory activity, several studies have also evaluated the phase-locked somatosensory-evoked cortical responses in individuals with CP. These investigations have consistently found similar outcomes for youth with CP, in that the somatosensory-evoked cortical activity for both upper and lower extremities are attenuated and, in some cases, have longer response latencies (Kurz & Wilson, 2011; Teflioudi et al., 2011; Guo et al., 2012; Maitre et al., 2012; Papadelis et al., 2014; Papadelis et al., 2018). However, these studies have broadly focused on children and adolescent populations, which has significantly limited the field’s understanding of how the transition to adulthood affects the strength of such somatosensory cortical responses in individuals with CP.
Previous studies by Riquelme and colleagues aimed to address this knowledge gap by assessing whether the somatosensory processing of tactile sensations applied to the hand differed between youth (5–14 years) and adults (22–55 years) with CP (Riquelme & Montoya, 2010; Riquelme et al., 2011). Their clinical assessments suggested that upper extremity proprioception and finger tactile sensitivity deficiencies were not appreciably different between youth and adults with CP. Their EEG results corroborated this notion by showing that the amplitude of the P50 and P100 somatosensory-evoked cortical responses following stimulation of the finger were similar between the two groups (Riquelme & Montoya, 2010). This implies that the somatosensory processing of the afferent feedback from the hands may not further decline as individuals with CP maturate into adults. However, of note, the progressive motor declines reported in the clinical literature are predominantly centered on mobility and not hand function (Jahnsen et al., 2004; Hanna et al., 2009; Opheim et al., 2013; Morgan & McGinley, 2014; Lundh et al., 2018). Thus, there may be a stronger connection between somatosensory processing declines and age for lower extremity areas such as the feet in individuals with CP.
In the current study, we used MEG to image and quantify the primary somatosensory response following electrical stimulation of the tibial nerve in a cohort of individuals with CP and healthy controls. Based on the prior MEG literature, we hypothesized that these somatosensory responses would be weaker in those with CP compared to the healthy controls. Additionally, we postulated that there would be a link between the magnitude of the somatosensory response and age, and that older participants with CP would tend to have weaker cortical responses. Lastly, we hypothesized that the magnitude of the somatosensory response would be tightly coupled with the GMFCS levels, such that individuals with greater mobility impairments would have more diminished somatosensory cortical activity.
Methods
Ethical Approval
The study protocol conformed with the standards set by the Declaration of Helsinki, except the study was not registered in a database. The Institutional Review Board at the University of Nebraska Medical Center reviewed and approved this investigation. Informed consent was acquired from the adult participants and parents of the youth participants, and the youth assented to participate in the experiment. This study was not registered in a database.
Participants
Forty-two individuals with a diagnosis of spastic diplegic CP and GMFCS levels between I-IV completed this study (Age = 9 – 28 years, mean = 15.23 ± 4.55 yrs.). Individuals with GMFCS levels of I and II typically ambulate independently, although with slowed gait speed and abnormal gait patterns. Individuals with GMFCS level of III often require assistive devices to ambulate, such as crutches, ankle-foot orthoses, or wheelchairs. Individuals with GMFCS levels IV and V often require powered mobility devices. An additional twenty-three healthy youth and young adults (Age range = 11 – 23 years, mean = 15.03 ± 3.10 years) served as a control group (HC). The two groups did not significantly differ by age (P = 0.4313). Exclusionary criteria included any medical illness affecting CNS function, any neurological disorder, history of head trauma, any non-removable metal implant that would adversely affect data acquisition, and current substance abuse. Furthermore, the included participants with CP did not have noticeable volume loss on the MRI that would have impacted the integrity of the cortices.
MEG Acquisition and Experimental Paradigm
Throughout the somatosensory experiment, the participants were seated in a custom-made nonmagnetic chair with their head positioned within the MEG helmet-shaped sensor array while focusing on a fixation cross. A single pulse, unilateral electrical stimulation was applied using electrodes that were affixed to the skin overlying the right tibial nerve. The intensity of stimulation was set to the individual’s motor threshold to control for impedance differences among participants. To find the motor threshold, the intensity of stimulation was gradually increased until an overt muscle twitch from the toes was elicited. During the experiment, a single-pulse of stimulation was applied every two seconds for four minutes, yielding a total of 120 trials.
All recordings were conducted in a one-layer magnetically-shielded room with active shielding engaged for advanced environmental noise compensation. During data acquisition, participants were monitored via real-time audio-video feeds from inside the shielded room. With an acquisition bandwidth of 0.1 – 330 Hz, neuromagnetic responses were sampled continuously at 1 kHz using an Elekta MEG system (Helsinki, Finland) with 306 sensors, including 204 planar gradiometers and 102 magnetometers. Each MEG data set was individually corrected for head motion during task performance and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu & Simola, 2006).
MEG Coregistration and Structural MRI Processing
Four coils were affixed to the head of the participant and were used for continuous head localization during the experiment. Prior to the experiment, the location of these coils, three fiducial points and the scalp surface were digitized to determine their three-dimensional position (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the participant was positioned for the MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the four coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the recording session. Since the coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system (including the scalp surface points), each participant’s MEG data was coregistered with structural T1-weighted MRI data prior to source reconstruction. Structural MRI data were aligned parallel to the anterior and posterior commissures and transformed into a standardized space. Structural MRI data were acquired using a Siemens Skyra 3T scanner. High-resolution T1-weighted sagittal images were obtained with a 32-channel head coil using a 3D fast field echo sequence with the following parameters: TR: 2400 ms; TE: 1.94 ms; flip angle = 8 deg; FOV: 256 mm; slice thickness: 1 mm slice with no gap; in-plane resolution: 1.0 mm3.
MEG Preprocessing
The raw MEG recordings were initially filtered with a 200 Hz zero order low pass digital filter, and a 0.5 zero order high pass digital filter. Additionally, a notch filter was applied to remove the 60 Hz line noise. Cardiac artifacts were subsequently removed from the data using signal-space projection, which was accounted for during source reconstruction (Uusitalo & Ilmoniemi, 1997). The continuous magnetic time series was divided into epochs of 1100 ms duration, from −500 to 600 ms with the baseline being defined as −400 to −100 ms and 0.0 ms being stimulation onset. Epochs containing artifacts (e.g., eye blinks, muscle artifacts, etc.) were rejected based on a fixed-threshold method using individual amplitude and gradient thresholds, supplemented with visual inspection. An independent samples t-test revealed that the number of trials accepted between groups was not significantly different (CP = 102.31 ± 2.60, HC = 104.68 ± 2.65, P = 0.6374).
Sensor-level Analysis
The artifact-free epochs were next averaged across trials to generate a mean time series per sensor and participant, and the specific time windows used for subsequent source imaging were determined by statistical analysis of the sensor-level time series across all participants using the entire array of gradiometers. Each data point in the time series was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false positive results while maintaining reasonable sensitivity, a two-stage procedure was followed to control for Type 1 error. In the first stage, paired-sample t-tests were conducted to test for differences from baseline at each data point and the output time series of t-values was threshold at P < 0.05 to define time bins containing potentially significant phase-locked activity across all participants. In stage two, the time points that survived the threshold were clustered with temporally and/or spatially neighboring time points that were also above the threshold (P < 0.05), and a cluster value was derived by summing all of the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster-values and the significance level of the observed clusters (from stage one) were tested directly using this distribution (Maris & Oostenveld, 2007). For each comparison 1,000 permutations were computed to build a distribution of cluster values. Based on these analyses, the time windows that contained significant phase-locked events across all participants were used to guide subsequent time-domain source level analysis.
Source Imaging (sLORETA)
Time domain source images were computed using standardized low resolution brain electromagnetic tomography (sLORETA). (Pascual-Marqui, 2002) The resulting whole-brain maps were 4-dimensional estimates of current density per voxel, per time sample across the experimental epoch. These data were normalized to the sum of the noise covariance and theoretical signal covariance, and thus the units are arbitrary. These maps were then averaged temporally over the time windows identified in the sensor-level analysis. The resulting maps were then grand-averaged across all participants to determine the location of the peak voxel. From this peak voxel, the sLORETA units were extracted to derive estimates of the time-domain response amplitude for each participant. All imaging procedures were done with the Brain Electrical Source Analysis (BESA) software (BESA v7.0; Grafelfing, Germany). For additional methodological detail, please see our recent paper (Wiesman & Wilson, 2020).
Statistical Analysis
The Shapiro-Wilk test of normality was used to determine whether the data were normally distributed. The data that failed the test were subsequently logarithmically transformed for statistical testing. An ANCOVA model with group as the independent variable, age as a covariate, and the extracted peaks from the sLORETA analysis as the dependent variable was calculated. Spearman correlation coefficients were also calculated between the overall severity of gross motor function (i.e., GMFCS) and the magnitude of the somatosensory cortical response, as well as GMFCS and age. The statistical analyses were conducted with SPSS (Version 22.0; IBM Corporation, Armonk, NY). Data within the text and the figures are presented as the mean plus/minus the standard error of the mean.
Results
MEG Findings
Robust somatosensory cortical responses appeared in a wide array of sensors within the frontal and parietal cortices, with the strongest activity present within the medial sensors near what was likely the leg region of the contralateral (left) somatosensory cortex. Permutation testing of the sensor-level data revealed that the somatosensory cortical response was significantly different from baseline during the 112 – 252 ms time window; thus, source activity estimates were averaged across this window and then across all participants. Not surprisingly, the resulting grand-averaged sLORETA data revealed that the peak neural response emanated from the contralateral somatosensory cortices. To highlight the similarity, we show the images separately for each group in Figure 1. These images clearly show that the somatosensory cortical response was weaker in the individuals with CP, although the location of the peak response was similar. We subsequently extracted the peak voxel (Talairach coordinates: −6, −32, 49) from the grand-averaged image to confirm this observation. Our ANCOVA model confirmed that there was a significant main effect of group (HC = 286.53 ± 30.51, 95% CI [226.74, 346.32]; CP = 208.30 ± 19.66, CI [169.77, 246.83], P = 0.0126), indicating that somatosensory cortical activity was weaker in individuals with CP (Figure 2). To visualize the difference in the dynamics, we subsequently extracted the neural time course from the peak voxel; note that this time course was extracted per participant, once the coordinates of interest were known from the grand-averaged image, and subsequently averaged within group. As shown in Figure 2, these time courses indicate that the somatosensory cortical activity was clearly weaker in the individuals with CP.
Figure 1:
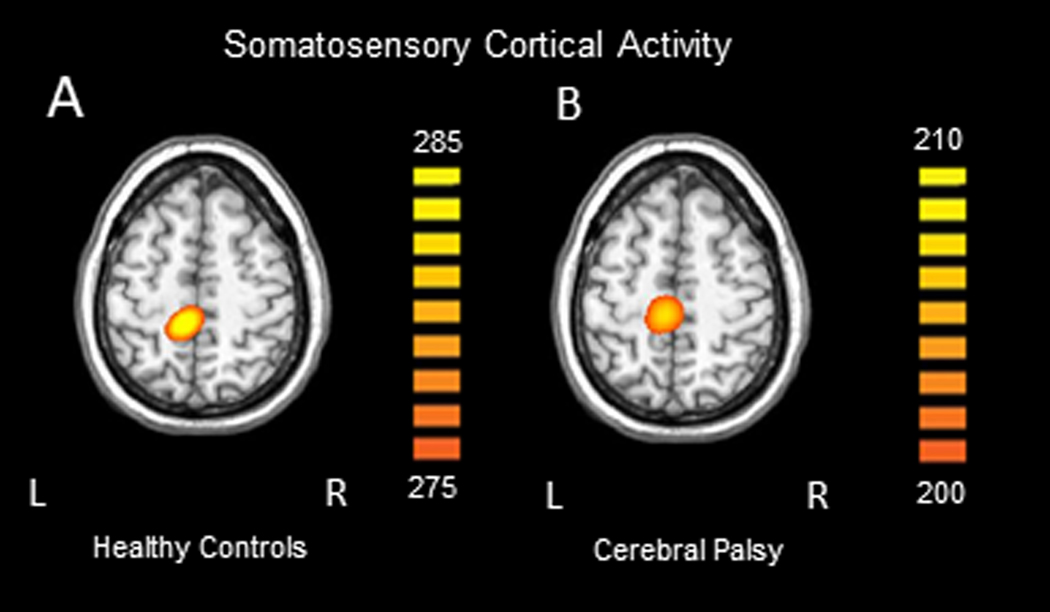
Group averaged sLORETA images across the time window identified through cluster based permutation testing of the sensor level results (112 – 252ms) for the healthy controls (A) and individuals with CP (B). All responses were located in the leg region of the somatosensory cortex contralateral (left) to stimulation. Note that the units are arbitrary, and the threshold is lower for the group with CP in order to visualize the response.
Figure 2:
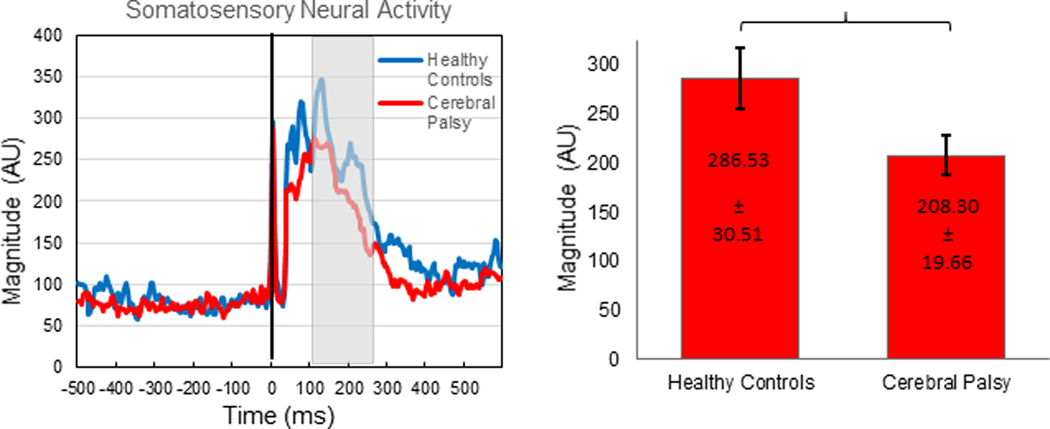
Left: Peak voxel time series. The stimulus was administered at time 0.0 ms (black line). The main somatosensory response, depicted in arbitrary units (AU), begins around 40 ms and diverges group-wise shortly thereafter. The time bin containing significantly different activity relative to baseline, which was subsequently used for further imaging analysis, is demarcated by the gray box (112 – 252 ms). Blue depicts the healthy controls and red depicts the individuals with cerebral palsy. The somatosensory response was notably weaker in the individuals with cerebral palsy. Right: Bar graph representing the difference in magnitude of the response between the healthy controls and the individuals with CP. The healthy controls had a significantly stronger response than the individuals with CP (P = 0.0126).
The ANCOVA model additionally confirmed that the somatosensory cortical activity covaried with age (P = 0.0168). Our post-hoc Pearson correlation indicated that the somatosensory cortical activity tended to decrease with age in all participants (r = −0.30, P = 0.0164). The interaction effect between group and age was not significant (P = 0.8790), indicating that the change in somatosensory cortical activity with age displayed parallel changes in the respective groups. To further conceptualize these results, we fit linear regression models to the data from the respective groups (Figure 3). Inspection of these models conveyed that the slopes were relatively similar between groups, but the intercept was notably shifted downward for the individuals with CP. This indicates that the individuals with CP had weaker somatosensory cortical activity overall, and it implies that youth with CP may have somatosensory cortical activity that is similar to an older adult controls. For example, the regression lines depict that an individual with cerebral palsy 9.8 years old has predicted somatosensory cortical activity similar to that of a neurotypical individual that is 16.8 years old. This suggests that at least some individuals with CP exhibit aberrant maturation of their somatosensory system.
Figure 3:
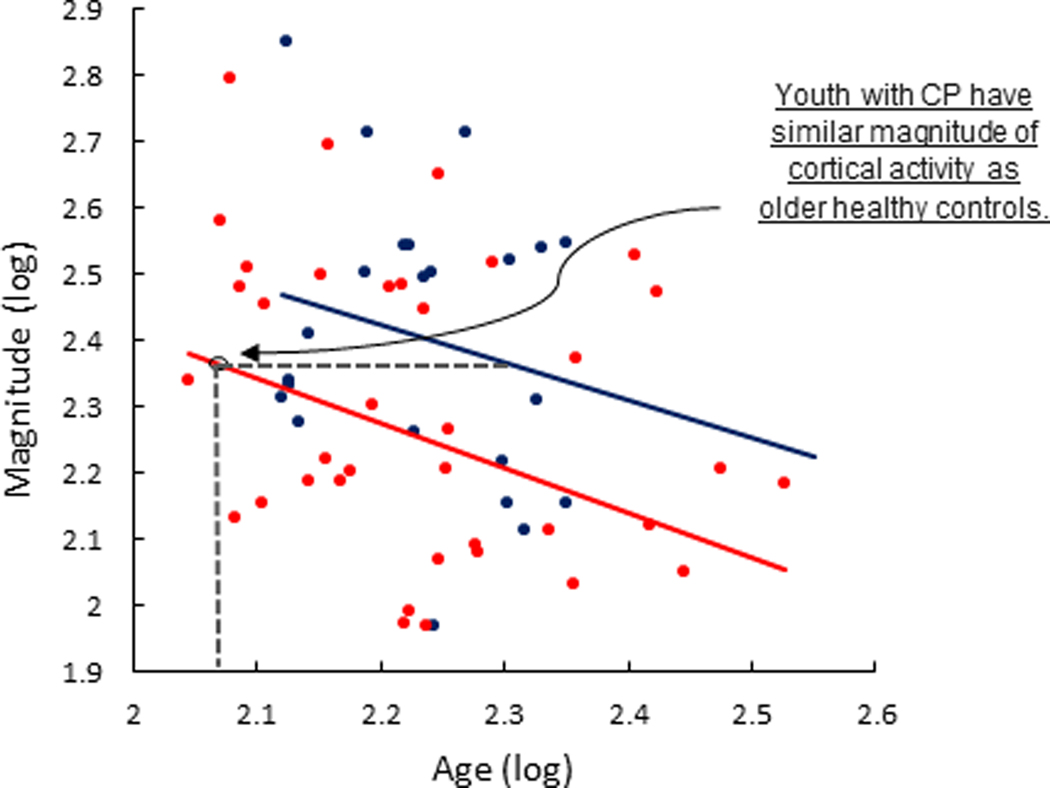
There were parallel age related declines in somatosensory cortical activity between controls (blue) and individuals with CP (red). Inspection of linear regression models fit to the log transformed data of the respective groups conveyed that the slopes were relatively similar between the respective groups, but the intercept was shifted downward for the individuals with CP. The shifted intercept reflects the weaker somatosensory cortical activity seen in the individuals with CP across the examined age range. Inspection of the respective models indicates that the youth with CP exhibited somatosensory cortical activity that was similar to much older controls. This suggests that some of those with CP exhibit accelerated aging in the somatosensory system.
Correlation Analysis
GMFCS levels and the magnitude of the somatosensory cortical responses were not significantly associated with each other (r = −0.18, P = 0.2603), suggesting that the strength of the somatosensory response was likely not connected with the degree of the participant’s mobility impairment. Age was also not significantly associated with GMFCS level (r = 0.16, P = 0.3087), suggesting that GMFCS level did not change as a function of age.
Discussion
We used MEG brain imaging to evaluate the magnitude of the somatosensory response that was evoked after a peripheral stimulation was applied to the tibial nerve in a cohort of individuals with CP and healthy controls. Overall, our results align with prior research that has shown that somatosensory cortical activity is diminished in individuals with CP (Kurz & Wilson, 2011; Teflioudi et al., 2011; Guo et al., 2012; Maitre et al., 2012; Papadelis et al., 2014). Our results also suggest that somatosensory cortical activity tends to become weaker with age; yet, the rate of this neurophysiological change in individuals with CP appears to parallel that seen in healthy controls, at least within the age range that we examined. Nonetheless, the overall age-related trajectories suggest that youth with CP have an altered maturation trajectory within the somatosensory system that emerges during youth and is maintained into early adulthood. Further discussion of this premise and the implications of these experimental findings appear in the following sections.
Compared with healthy controls, our results show that the somatosensory cortical responses seen in the leg region of the somatosensory cortices are reduced in amplitude in individuals with CP. This is in line with several neuroimaging studies that have previously identified alterations in somatosensory processing in individuals with CP, in response to both tactile and electrical stimulation paradigms (Cooper et al., 1995; Hirayama et al., 1999; Park et al., 2002; Kurz & Wilson, 2011; Teflioudi et al., 2011; Auld et al., 2012; Guo et al., 2012; Maitre et al., 2012; Kurz et al., 2014; Papadelis et al., 2014; Kurz et al., 2015a; Kurz et al., 2018; Papadelis et al., 2018). Thus, it is likely that this altered activity contributes to the somatosensory deficits that are reported clinically for this patient population. The previous MEG studies that have evaluated the somatosensory cortical responses have primarily utilized sensor space or dipole analyses, or have focused only on youth with CP. While these prior experimental outcomes have provided valuable insights on the differences in amplitude and latency of somatosensory processing in youth, the sample demographics and imaging methods used in the current investigation significantly enhance these prior outcomes by verifying the precise neural tissue underlying the response across youth and early adulthood.
Overall, our results identified that the somatosensory cortical activity tended to become weaker as adolescents transitioned into adulthood. These results align with a prior study that reported the strength of the somatosensory cortical responses are reduced in healthy adults compared with newborns (Pihko et al., 2009). Hence, corroborating the notion that the cortical activity decreases as the somatosensory system matures. There are several anatomical changes that occur within the brain throughout development which may contribute to this maturation-related reduction. Cortical gray matter thickness tends to increase during the pre-adolescent period but then decreases in frontal and parietal lobes around 12 years of age (Giedd et al., 1999; Sowell et al., 2002; Sowell et al., 2003; Lenroot & Giedd, 2006; Wilke et al., 2007). Furthermore, synaptic density within gray matter tends to peak between four and eight years of age and then declines into adulthood (Huttenlocher, 1979; Goldman-Rakic, 1987; Wilke et al., 2007; Pang, 2011). Altogether these structural changes might partially account for the reduction in somatosensory activity across development.
Our results illustrated that individuals with CP also display a reduction in the somatosensory cortical activity with age. Despite having a parallel trajectories with the healthy controls, the younger individuals with CP tended to have somatosensory cortical activity that was more aligned with what was seen in the older healthy controls. For example, our results showed that an individual with CP that is 9.8 years old has predicted somatosensory cortical activity similar to that of a healthy control that is 16.8 years old. This implies that at least some youth with CP have an aberrant developmental trajectory in regard to somatosensory processing that might set them up for having an aberrant profile later in life. That being said, a diffusion weighted imaging (DWI) and transcranial magnetic stimulation (TMS) study showed the corticospinal tracts in both hemispheres appeared to arrest in maturation in children with hemiplegic CP when compared with typically developing children (Papadelis et al., 2019). This would imply that there would be a point in development where there would not be further changes in the cortical activity with age. Potentially, this discrepancy might be due to the different time windows for the maturation of the sensory and motor fibers.
We suggest that the aberrant maturation of the somatosensory cortices may partially be a contributing factor to the degraded motor actions often reported as youth with CP transition into young adulthood (Hanna et al., 2009). Furthermore, we imply that the aberrant trajectory of the somatosensory cortical activity could be partially attributable to the more sedentary lifestyles seen in individuals with CP, which would make the system prematurely degenerate (Jahnsen et al., 2004; Morgan & McGinley, 2014; Lundh et al., 2018; Verschuren et al., 2018). However, this inference is at odds with previous studies reporting that somatosensory tactile sensitivity and evoked potentials do not significantly differ between children and adults with CP (Riquelme & Montoya, 2010; Riquelme et al., 2011). Of note, these prior outcomes were based on studies that focused on somatosensory processing of the hand and not the foot, and it is well recognized that individuals with CP are more likely to have lower extremity versus upper extremity impairments (Aicardi & Bax, 1992). Hence, the connection between the reduced somatosensory cortical activity and age found in this investigation may be more representative of the average case with CP.
Previous studies utilizing MEG brain imaging have reported that the strength of somatosensory cortical oscillations in the theta-alpha range are related to diminished ankle strength and mobility (Kurz et al., 2014; Kurz et al., 2015b). Based on these previous outcomes, we presumed that the magnitude of the somatosensory responses would be related to the GMFCS levels that were used to clinically classify the degree of mobility impairments seen in individuals with CP (e.g., weaker responses for higher GMFCS levels). However, surprisingly, our results suggested that GMFCS levels were not directly related to the magnitude of somatosensory cortical responses following tibial nerve stimulation. It is plausible that the GMFCS levels provide a clinical gestalt on the overall presentation, but may lack the specificity for separating the patient’s presentation on a continuum. In other words, although two patients may have similar GMFCS levels, their individualized sensorimotor presentations can be quite different.
Despite our novel findings, several limitations should be kept in mind. First, the heterogeneity seen in CP inherently leads to variability in the cortical function within this population, which may then affect the somatosensory responses examined in this study. Nevertheless, as previous studies have consistently confirmed, somatosensory cortical activity is reduced in those with CP across a range of presentations, substantiating the robustness of this finding. Additionally, the participants included in this investigation were between 9–28 years of age. It is possible that assessing a larger cohort of individuals across a broader age range would allow for stronger conclusions to be made regarding somatosensory cortical activity, its trajectory of change, and the notion of accelerated development across the lifespan. That said, this is one of the few studies to date that has evaluated cortical activity in adults with CP, or considered the dimension of age as an important neurophysiological variable.
Conclusions
Our experimental results illustrate that the magnitude of somatosensory cortical responses in the feet are reduced in individuals with CP and tend to become weaker with maturation. We suggest that the weaker somatosensory cortical activity seen in youth with CP may reflect an altered developmental course than typically seen within the somatosensory system. Currently, specialized treatments for adults with CP are extremely limited and have gathered less attention compared with the pediatric population with CP (Liptak, 2008). The results of this investigation imply that attention to the aberrant somatosensory processing seen in youth as they transition toward adulthood is of critical importance. Potentially, somatosensory training (i.e., limb awareness, heightened tactile sensations during gait) might be a key ingredient that could alter the trajectory of the aberrant maturation of the somatosensory system noted in this investigation.
Supplementary Material
Key Points.
-
-
Individuals with cerebral palsy (CP) have a reduced somatosensory cortical response
-
-
Somatosensory cortical response strength decreases from adolescence to early adulthood
-
-
Somatosensory cortical responses in youth with CP are similar to adult controls
-
-
Individuals with CP may have aberrant maturation of the somatosensory system
Acknowledgments
Funding
This work was partially supported by the National Institutes of Health (1R01-HD086245, 1R01-HD101833, R21-HD096390).
Footnotes
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing Interests
There were no competing interests for this study.
References
- Aicardi J & Bax M. (1992). Diseases of the Nervous System in Childhood.s Cambridge, UK: Mac Keith Press. [Google Scholar]
- Auld ML, Boyd RN, Moseley GL, Ware RS & Johnston LM. (2012). Impact of tactile dysfunction on upper-limb motor performance in children with unilateral cerebral palsy. Arch Phys Med Rehabil 93, 696–702. [DOI] [PubMed] [Google Scholar]
- Christensen D, Van Naarden Braun K, Doernberg NS, Maenner MJ, Arneson CL, Durkin MS, Benedict RE, Kirby RS, Wingate MS, Fitzgerald R & Yeargin-Allsopp M. (2014). Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning - Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol 56, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton K, Fleming JM & Copley J. (2003). Behavioral responses to tactile stimuli in children with cerebral palsy. Phys Occup Ther Pediatr 23, 43–62. [PubMed] [Google Scholar]
- Cooper J, Majnemer A, Rosenblatt B & Birnbaum R. (1995). The determination of sensory deficits in children with hemiplegic cerebral palsy. J Child Neurol 10, 300–309. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC & Rapoport JL. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2, 861–863. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. (1987). Development of cortical circuitry and cognitive function. Child Dev 58, 601–622. [PubMed] [Google Scholar]
- Guo X, Xiang J, Mun-Bryce S, Bryce M, Huang S, Huo X, Wang Y, Rose D, Degrauw T, Gartner K, Song T, Schmit J & Vargus-Adams J. (2012). Aberrant high-gamma oscillations in the somatosensory cortex of children with cerebral palsy: a meg study. Brain Dev 34, 576–583. [DOI] [PubMed] [Google Scholar]
- Hanna SE, Rosenbaum PL, Bartlett DJ, Palisano RJ, Walter SD, Avery L & Russell DJ. (2009). Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Dev Med Child Neurol 51, 295–302. [DOI] [PubMed] [Google Scholar]
- Hirayama Y, Takahashi H, Yasuhara A & Ochi A. (1999). [Somatosensory evoked potential (SEP) to posterior tibial nerve stimulation in children with cerebral palsy]. Rinsho Byori 47, 76–82. [PubMed] [Google Scholar]
- Huttenlocher PR. (1979). Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res 163, 195–205. [DOI] [PubMed] [Google Scholar]
- Jahnsen R, Villien L, Egeland T, Stanghelle JK & Holm I. (2004). Locomotion skills in adults with cerebral palsy. Clin Rehabil 18, 309–316. [DOI] [PubMed] [Google Scholar]
- Kirby RS, Wingate MS, Van Naarden Braun K, Doernberg NS, Arneson CL, Benedict RE, Mulvihill B, Durkin MS, Fitzgerald RT, Maenner MJ, Patz JA & Yeargin-Allsopp M. (2011). Prevalence and functioning of children with cerebral palsy in four areas of the United States in 2006: a report from the Autism and Developmental Disabilities Monitoring Network. Res Dev Disabil 32, 462–469. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Becker KM, Heinrichs-Graham E & Wilson TW. (2015a). Children with cerebral palsy have uncharacteristic somatosensory cortical oscillations after stimulation of the hand mechanoreceptors. Neuroscience 305, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Heinrichs-Graham E, Arpin DJ, Becker KM & Wilson TW. (2014). Aberrant synchrony in the somatosensory cortices predicts motor performance errors in children with cerebral palsy. J Neurophysiol 111, 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Heinrichs-Graham E, Becker KM & Wilson TW. (2015b). The magnitude of the somatosensory cortical activity is related to the mobility and strength impairments seen in children with cerebral palsy. J Neurophysiol 113, 3143–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ, Wiesman AI, Coolidge NM & Wilson TW. (2018). Children with Cerebral Palsy Hyper-Gate Somatosensory Stimulations of the Foot. Cereb Cortex 28, 2431–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz MJ & Wilson TW. (2011). Neuromagnetic activity in the somatosensory cortices of children with cerebral palsy. Neurosci Lett 490, 1–5. [DOI] [PubMed] [Google Scholar]
- Lenroot RK & Giedd JN. (2006). Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev 30, 718–729. [DOI] [PubMed] [Google Scholar]
- Liptak GS. (2008). Health and well being of adults with cerebral palsy. Curr Opin Neurol 21, 136–142. [DOI] [PubMed] [Google Scholar]
- Lundh S, Nasic S & Riad J. (2018). Fatigue, quality of life and walking ability in adults with cerebral palsy. Gait Posture 61, 1–6. [DOI] [PubMed] [Google Scholar]
- Maitre NL, Barnett ZP & Key AP. (2012). Novel assessment of cortical response to somatosensory stimuli in children with hemiparetic cerebral palsy. J Child Neurol 27, 1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E & Oostenveld R. (2007). Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164, 177–190. [DOI] [PubMed] [Google Scholar]
- Morgan P & McGinley J. (2014). Gait function and decline in adults with cerebral palsy: a systematic review. Disabil Rehabil 36, 1–9. [DOI] [PubMed] [Google Scholar]
- Opheim A, McGinley JL, Olsson E, Stanghelle JK & Jahnsen R. (2013). Walking deterioration and gait analysis in adults with spastic bilateral cerebral palsy. Gait Posture 37, 165–171. [DOI] [PubMed] [Google Scholar]
- Pang EW. (2011). Practical aspects of running developmental studies in the MEG. Brain Topogr 24, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadelis C, Ahtam B, Nazarova M, Nimec D, Snyder B, Grant PE & Okada Y. (2014). Cortical somatosensory reorganization in children with spastic cerebral palsy: a multimodal neuroimaging study. Front Hum Neurosci 8, 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadelis C, Butler EE, Rubenstein M, Sun L, Zollei L, Nimec D, Snyder B & Grant PE. (2018). Reorganization of the somatosensory cortex in hemiplegic cerebral palsy associated with impaired sensory tracts. Neuroimage Clin 17, 198–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadelis C, Kaye H, Shore B, Snyder B, Grant PE & Rotenberg A. (2019). Maturation of Corticospinal Tracts in Children With Hemiplegic Cerebral Palsy Assessed by Diffusion Tensor Imaging and Transcranial Magnetic Stimulation. Front Hum Neurosci 13, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ES, Park CI, Kim DY & Kim YR. (2002). The effect of spasticity on cortical somatosensory-evoked potentials: changes of cortical somatosensory-evoked potentials after botulinum toxin type A injection. Arch Phys Med Rehabil 83, 1592–1596. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. (2002). Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. In Methods Find Exp Clin Pharmacol, pp. 5–12. Spain. [PubMed] [Google Scholar]
- Pihko E, Nevalainen P, Stephen J, Okada Y & Lauronen L. (2009). Maturation of somatosensory cortical processing from birth to adulthood revealed by magnetoencephalography. Clin Neurophysiol 120, 1552–1561. [DOI] [PubMed] [Google Scholar]
- Pihko E, Nevalainen P, Vaalto S, Laaksonen K, Maenpaa H, Valanne L & Lauronen L. (2014). Reactivity of sensorimotor oscillations is altered in children with hemiplegic cerebral palsy: A magnetoencephalographic study. Hum Brain Mapp 35, 4105–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme I, Cifre I & Montoya P. (2011). Age-related changes of pain experience in cerebral palsy and healthy individuals. Pain Med 12, 535–545. [DOI] [PubMed] [Google Scholar]
- Riquelme I & Montoya P. (2010). Developmental changes in somatosensory processing in cerebral palsy and healthy individuals. Clin Neurophysiol 121, 1314–1320. [DOI] [PubMed] [Google Scholar]
- Robert MT, Guberek R, Sveistrup H & Levin MF. (2013). Motor learning in children with hemiplegic cerebral palsy and the role of sensation in short-term motor training of goal-directed reaching. Dev Med Child Neurol 55, 1121–1128. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B & Jacobsson B. (2007). A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 109, 8–14. [PubMed] [Google Scholar]
- Sanger TD & Kukke SN. (2007). Abnormalities of tactile sensory function in children with dystonic and diplegic cerebral palsy. J Child Neurol 22, 289–293. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL & Toga AW. (2003). Mapping cortical change across the human life span. Nat Neurosci 6, 309–315. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Rex D, Kornsand D, Tessner KD, Jernigan TL & Toga AW. (2002). Mapping sulcal pattern asymmetry and local cortical surface gray matter distribution in vivo: maturation in perisylvian cortices. Cereb Cortex 12, 17–26. [DOI] [PubMed] [Google Scholar]
- Taulu S & Simola J. (2006). Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51, 1759–1768. [DOI] [PubMed] [Google Scholar]
- Teflioudi EP, Zafeiriou DI, Vargiami E, Kontopoulos E & Tsikoulas I. (2011). Somatosensory evoked potentials in children with bilateral spastic cerebral palsy. Pediatr Neurol 44, 177–182. [DOI] [PubMed] [Google Scholar]
- Uusitalo MA & Ilmoniemi RJ. (1997). Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput 35, 135–140. [DOI] [PubMed] [Google Scholar]
- Verschuren O, Smorenburg ARP, Luiking Y, Bell K, Barber L & Peterson MD. (2018). Determinants of muscle preservation in individuals with cerebral palsy across the lifespan: a narrative review of the literature. J Cachexia Sarcopenia Muscle 9, 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesman AI & Wilson TW. (2020). Attention modulates the gating of primary somatosensory oscillations. Neuroimage 211, 116610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Krägeloh-Mann I & Holland SK. (2007). Global and local development of gray and white matter volume in normal children and adolescents. Exp Brain Res 178, 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert JR, Burton H, Sinclair RJ, Brunstrom JE & Damiano DL. (2008). Tactile sensory abilities in cerebral palsy: deficits in roughness and object discrimination. Dev Med Child Neurol 50, 832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


