Abstract
As highlighted by the ongoing COVID-19 pandemic, vaccination is critical for infectious disease prevention and control. Obesity is associated with increased morbidity and mortality from respiratory virus infections. While obese individuals respond to influenza vaccination, what is considered a seroprotective response may not fully protect the global obese population. In a cohort vaccinated with the 2010–2011 trivalent inactivated influenza vaccine, baseline immune history and vaccination responses were found to significantly differ in obese individuals compared to healthy controls, especially towards the 2009 pandemic strain of A/H1N1 influenza virus. Young, obese individuals displayed responses skewed towards linear peptides versus conformational antigens, suggesting aberrant obese immune response. Overall, these data have vital implications for the next generation of influenza vaccines, and towards the current SARS-CoV-2 vaccination campaign.
One Sentence Summary:
Obese individuals have altered baseline and post-vaccination influenza antibody repertoires.
Obesity is an independent risk factor for increased morbidity and mortality after influenza infection, demonstrating the convergence of infectious disease (influenza) with a noncommunicable disease (obesity) (1). Similarly, obesity has been shown to be an independent-risk factor for severe outcomes from SARS-CoV-2 infection in the ongoing global COVID-19 pandemic (2, 3). The expansive prevalence of obesity worldwide (>500 million obese adults) coupled with significant influenza mortality even in non-pandemic years (3,000–56,000 annual deaths in the US alone) make identifying factors that affect influenza outcomes a critical need. Although vaccination is the primary method of influenza prevention, we have demonstrated that influenza vaccinated adults with obesity have an increased risk of influenza or influenza-like illness compared to vaccinated non-obese adults despite generating what is generally considered a seroprotective response (4). To further understand how obesity may impact antibody responses to influenza vaccination, we conducted an in-depth analysis of influenza-specific IgG and IgA antibody repertoires of 205 subjects, 100 with healthy weight (HW: Body mass index (BMI) = 18.5 to 24.9 kg/m2), and 105 with obesity (OB; BMI≥30kg/m2) prior to, and 30 days post-vaccination with split virus trivalent influenza vaccine preparations (TIV; vaccine disrupted by detergent; Table 1). The vaccine was enriched for hemagglutinin (HA) surface protein that contains major conformational neutralizing antigenic epitopes of influenza viruses. The three influenza strains in the 2010–2011 TIV were A/California/7/2009-like virus (A/H1N1; Cal/09), A/Perth/16/2009-like virus (A/H3N2; Perth09) and B/Brisbane/60/2008-like virus (Victoria lineage; BrisB). Of note, this vaccine was the first usage of the Cal09 pandemic strain in the seasonal vaccine, presenting the opportunity to study antibody response to a novel strain (Cal09) and two longer circulating strains (Perth09 and BrisB).
Table 1.
Demographics of study participants
| group | N | BMI | Age | Gender M/F | Race |
|---|---|---|---|---|---|
| Obese | 105 | 30–46.9 (34.6 median) | 20–82.6 (54.2 median) | 42/63 | Caucasian (55 %) African American (42 %) Hispanic (2 %) Asian (1 %) |
| Healthy-Weight | 100 | 19–24.9 (22.7 median) | 19–87 (57.8 median) | 41/59 | Caucasian (75 %) African American (16 %) Hispanic (2 %) Asian (6 %) |
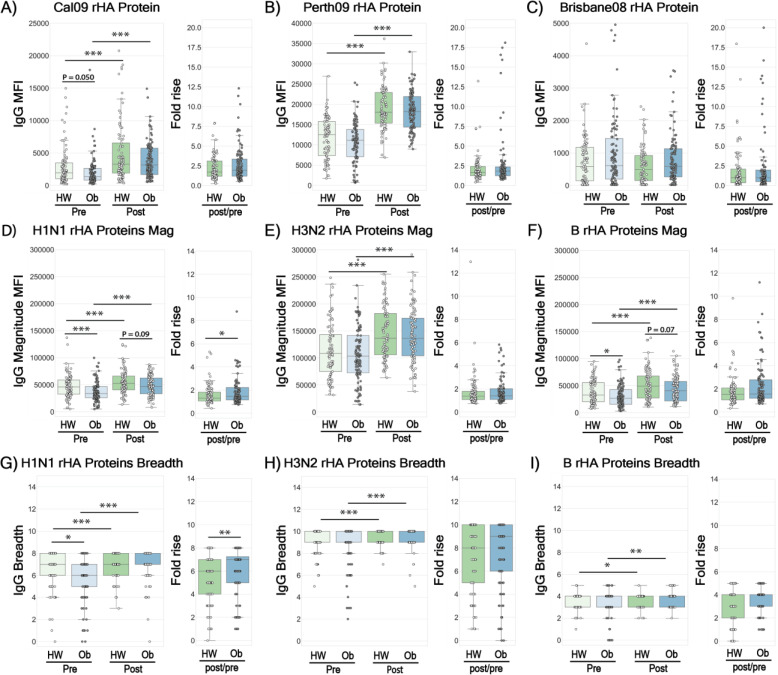
Baseline immune history (BIH), the repertoire of antibodies raised against influenza viruses following previous vaccinations and/or natural exposures, varies between individuals. Therefore, we first measured the BIH for IgG and IgA responses to recombinant hemagglutinin proteins (rHA) from Cal09, Perth09, BrisB and 20 additional rHAs obtained from seasonal vaccine and historical strains from 1933–2016 (Table S1) spotted on microarrays. While baseline IgG levels against Cal09 rHA were marginally lower in the OB group (p=0.050; Fig. 1A), baseline IgG levels against rHA antigens of Perth09 and BrisB were similar in OB and HW individuals (Fig. 1B,–C). There was a significantly lower magnitude (sum of antibody levels to all strains from a given subtype) and breadth (number of strains to which a subject has antibodies) for IgG against rHA proteins of A/H1N1 (Fig. 1D, G pre) and lower IgG magnitude to B rHA(Fig. 1F), but not A/H3N2 strains (Fig 2E, H) in OB adults at baseline. There were no differences in BIH IgA responses between HW and OB adults to rHAs of the 3 TIV strains (Fig. S1A–C) or in the BIH IgA magnitude and breadth for A/H3N2 or B proteins in the panel (Fig. S1E–F, H–I). However, the magnitude and breadth of the baseline IgA repertoire against rHA proteins of A/H1N1 strains were significantly decreased in OB individuals, similarly to baseline IgG levels (Fig. S1D, G pre).
Fig. 1. Baseline Immune History (BIH) and 30-day post vaccination IgG responses in healthy weight (HW) and obese (OB) individuals to a panel of historical influenza recombinant proteins (rHA).
Baseline and post-vaccination serum samples from 89 healthy-weight (HW) and 100 obese (OB) subjects were hybridized with an antigen microarray spotted with 34 BPL-inactivated influenza viruses and 23 recombinant HA (rHA) proteins that included the three vaccine strains used in the study, for profiling of IgG binding (see Table S1 for IgA binding). (A-C) IgG binding to the recombinant HA of H1N1 A/California/7/2009, H3N2 A/Perth/16/2009 and B/Brisbane/60/2008 vaccine strains. (D-F) The magnitude of IgG antibodies bound to a panel of 8 recombinant H1 (rH1) proteins (D); a panel of 10 recombinant H3 (rH3) proteins; (E) and a panel of recombinant HA antigens of 5 B strains (F). (G-I) The breadth of IgG antibodies bound to a panel of recombinant HA proteins, including: 8 rH1 proteins (G); 10 rH3 proteins (H); and 5 recombinant HA proteins of 5 B strains (l) proteins. The four box plots in the left portion of each panel summarize the baseline (L->R: HW: light green, OB: light blue) and the 30-day post-vaccination (L->R: HW: dark green, OB: dark blue) binding responses. The two boxplots on the right side of each panel represent the fold increase (L->R: HW: green, OB: blue). Lines represent the median fluorescence intensity (MFI), the boxes denote the 25th and 75th percentiles, and the error bars represent 1.5 times the interquartile range. Statistical significance was assessed using the Wilcoxon signed rank test (pre vs. post) and the Wilcoxon rank-sum test (HW vs. OB). * p<0.05, ** p<0.005, *** p<0.0005.
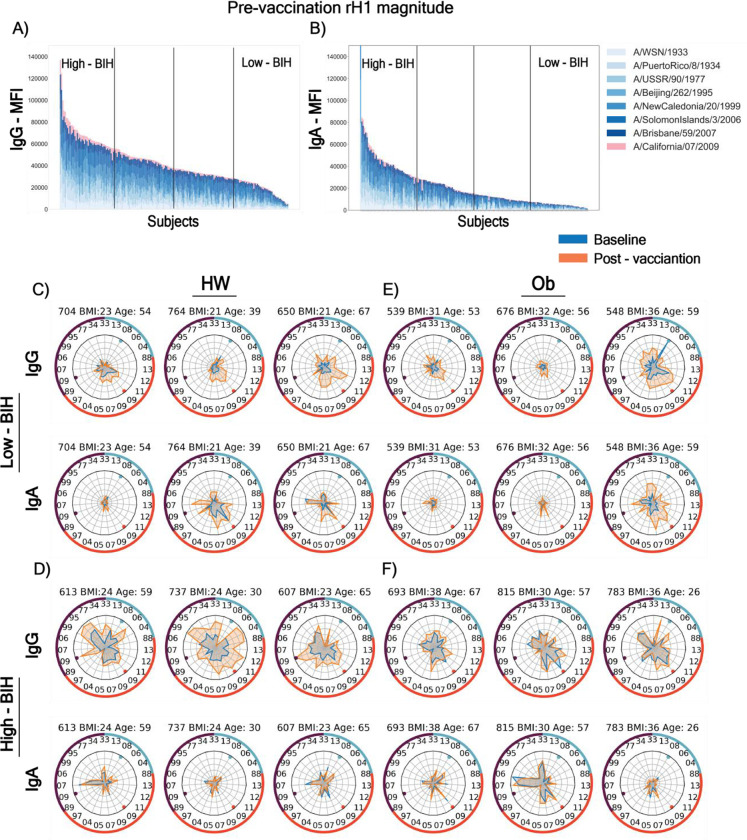
Fig. 2. Heterogeneity of baseline (BIH) and 30-day post-vaccination influenza immune history profiles to recombinant HA (rHA) proteins.
Panels (A-B): The entire cohort of 205 subjects, 89 healthy-weight (HW) and 100 obese (OB) subjects were assigned to quartiles (low-BIH: n=47, mid-BIH: n=95 and high-BIH: n=47) based on the magnitudes of (A) IgG responses and (B) IgA responses (low-BIH: n=44, mid-BIH: n=86 and high-BIH: n=44) to recombinant HA proteins of the H1N1 subtype strains. (C-F) Spider plots of the BIH and post-vaccination binding responses of individual subjects where each vertex represents the normalized binding of IgG or IgA to a single influenza strain rHA. Baseline and post-vaccination antibody repertoires are denoted by blue and orange spider plots, respectively (light and dark grey in Black and white). The ID, BMI and age of each subject is presented above each spider graph. The numbers on the inside of the outer circles denote the year each strain was isolated, and the three vaccine strains are denoted by the colored dots near the inner circles. Counter-clockwise rHA from: H1N1 (purple segment of circle) strains 33–09-33; H3N2 (red segment of circle) strains 89–13; and B (light blue segment of circle) strains 13–88. Isolates appear in the same order as listed in Table S1. Representative spider graphs are presented for HW subjects (C and D) and OB subjects (E and F) for subjects whose responses were in the low IgG BIH group (C and E) or the high IgG BIH group (D and F).
To further define the specific responses, we measured BIH antibody response against whole, inactivated viruses spotted on microarrays including the 3 TIV strains and a panel of additional 31 whole inactivated influenza viruses (Table S1) representing 83 years of antigenic diversity in A/H1N1, A/H3N2, and B strains between 1933 and 2017. Interestingly, OB individuals had significantly lower total levels of IgG to all 3 TIV strains (Fig. S2A–C), and had significantly decreased IgG magnitude (Fig. S2D–F) and IgG breadth (Fig S2G–I) to all 3 subtypes in the panel. In contrast, there were no significant differences in baseline IgA levels between OB and HW individuals for the current strains (Fig. S3A–C), and no differences in magnitude and breadth of responses to whole viruses (Fig. S3D–I).
Taken together, clear differences in baseline responses exist between OB and HW individuals: the OB group have lower levels of IgG against TIV strains; decreased breadth and magnitude of IgG to 34 strains of whole influenza viruses; and, decreased magnitude and breadth of IgA against rHA proteins from 8 historical A/H1N1 strains of influenza. Thus, prior to vaccination, OB subjects displayed a diminished antibody response against influenza, despite multiple exposures and vaccinations, in particular for subtype A/H1N1.
While OB individuals displayed decreased BIH, previous studies show they do respond to influenza vaccination (4, 5). Therefore, to answer whether standard vaccination might overcome obesity-associated BIH deficit in the short term, we measured serum antibody responses at 28–35 days post-vaccination. IgG and IgA levels significantly increased in both HW and OB individuals against rHA of Cal09 and Perth09, but not BrisB, vaccine strains following vaccination (Fig. 1 A–C, S1A–C). Both groups also significantly increased the magnitude and breadth of both IgG and IgA to all three subtypes (Fig. 1D–I; S1D–I). However, vaccine-induced increase in magnitude and breadth of both IgG and IgA against A/H1N1 rHA proteins and IgA against B subtype rHA proteins were significantly stronger in the OB individuals, as reflected by the higher fold-change (Fig. 1 D, G; Fig. S1D, F, G and I). Nevertheless, while the post-vaccination magnitude and breadth to H1N1 rHAs were similar for IgG in both groups (Fig. 1 D, G), they were lower in the OB for IgA (Fig. S1D, G), due to the lower IgA magnitude and breadth at baseline. Post-vaccination increases in breadth or magnitude of IgA or IgG against A/H3N2 or B strains (Fig. S1E–F, H–I, 1E–F, H–I) were not significant between HW and OB individuals.
In terms of whole virus antigens, a significant rise in IgG and IgA levels against all isolates post-vaccination was observed in both OB and HW individuals. However, comparing between groups, total IgG levels and magnitude of response were significantly lower against the three vaccine strains in OB individuals (Fig S2A–F,). In contrast, total IgA levels magnitude and breadth were significantly higher in the OB individuals (Fig. S3D–I). Differences in post-vaccination IgG breadth were noted only for A/H3N2 strains (Fig. S2G–I). In OB individuals, the lower anti-influenza IgG levels post-vaccination were possibly driven by the decreased BIH IgG levels, as the vaccine-induced fold-change in IgG levels, breadth and magnitude were similar between OB and HW (Fig S2). Interestingly, IgA response to the vaccine was stronger in OB individuals reflected by the significantly higher fold-change in IgA to whole influenza viruses, leading to increased IgA levels against whole virus TIV antigens, and higher IgA magnitude and breadth against the three subtypes (Fig S3).
Since antibodies can target linear epitopes in addition to conformational epitopes, we next characterized the BIH and post-vaccination IgG and IgA repertoire using a peptide microarray spotted with a succession of 20-mer peptides with partial overlap of 15 aa spanning the HA and NA protein sequences (H1, N1) of the A/H1N1 Cal09 vaccine strain. This array facilitates characterization of the peptides magnitude, defined as the sum of antibody levels to all peptides from the same protein; peptides breadth, defined as the number of peptides from each protein to which the subject had antibodies; as well as the individual epitopes that were recognized. There was an inverse correlation between recognition of linear epitopes of peptides and recognition of conformational epitopes on whole viruses and rHAs. Specifically, pre- and post-vaccination sera from OB individuals had a higher magnitude and broader repertoire of IgG antibodies to Cal09 H1 and N1 peptides (Fig. S4A–D) in comparison to their lower recognition of Cal09 whole virus and rHA (Fig. 1A, S2A). The pattern for IgAs was more complicated. Pre- and post-vaccination IgA breadth, but not magnitude, to Cal09 H1 and N1 peptides was significantly lower in OB individuals (Fig. S4E–H), while there were no BIH differences for Cal09 whole virus and rHA, but the post-vaccination IgA magnitude and breadth response and fold-responses for H1N1 viruses were greater than for HW individuals (Fig. S3D, G). These findings suggest that an increase in the level of IgG antibodies to conformational antigens is associated with a decrease in the diversity and quantity of IgG antibodies to linear peptides. The differences between the OB and HW antibody response described here were specific to influenza antigens, since the total IgG and IgA levels in the sera of the two groups were similar, as detected by ELISA (Fig. S3J–K).
BIH differs for each individual depending on previous exposure and vaccine history. Indeed, extensive BIH heterogeneity emerged when IgG or IgA baseline magnitude or breadth against specific types of influenza antigens (rHA versus whole viruses versus peptides) was measured in all 205 individuals (e.g. baseline magnitudes of IgG and IgA antibodies to H1N1 rHA (rH1) proteins, Fig. 1D and S1D). To study whether age or obesity affect the BIH to A/H1N1 antigens, the entire cohort was blindly ranked by decreasing BIH IgG and IgA magnitudes of response to whole virus or rHA A/H1N1 antigens, as well as breadth or magnitude of antibodies against Cal09 A/H1 and N1 peptides. For each such ranking, the cohort was divided into quartiles: individuals in the lowest quartile were denoted as low-BIH group, individuals in the highest quartile were denoted as high-BIH group, and the remaining two middle quartiles were denoted as the mid-BIH group (e.g. Fig. 2A–B). To visualize the BIH of each subject, we generated spider plots for a representative subset of low IgG-BIH and high IgG-BIH subjects (Fig. 2C–F), further highlighting that each individual has a unique BIH profile that varies both in the overall magnitude and breadth, but also in the specificity to each subtype and to individual strains within the panel. Interestingly, some subjects with low IgG-BIH to rH1 have low IgG-BIH to all three subtypes (e.g. subjects 704, 539 and 676; Fig. 2C, E), while others have stronger levels of IgG to other subtypes (e.g. subject 548). We found that the antibody response to the vaccine was heavily biased by the individual BIH. While some low-BIH subjects failed to respond to the vaccine (e.g. subject 676), others generated robust vaccine-induced immune responses (e.g. subjects 650 and 548). A comparison between the IgG and IgA spider plots for each subject demonstrated that most subjects with low IgG-BIH to rH1 had also low IgA-BIH to rH1. However, in some cases there were striking differences between IgG and IgA profiles (e.g. subjects 764, 737 and 783, Fig. 2C–F).
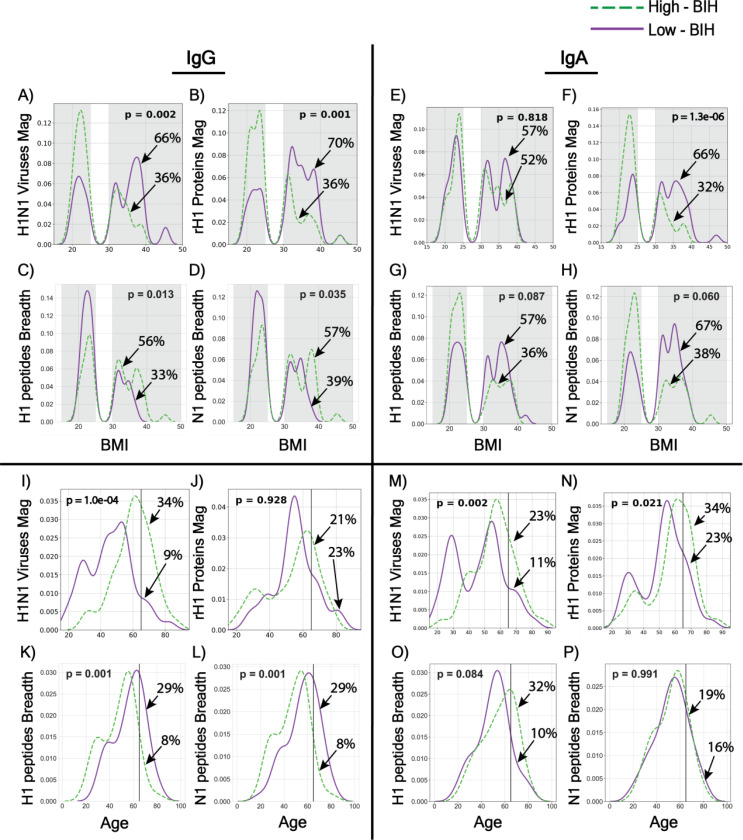
The distribution of BMI or age of individuals in the low-BIH and high-BIH groups were compared for each ranking (Fig. 3). This analysis demonstrated again the inverse correlation between magnitude of IgG antibodies to conformational (viruses and proteins) and linear (peptides) antigens when comparing obese and HW groups and when comparing individuals by age. A significantly higher frequency of OB individuals was present in the low-BIH group for magnitude of IgG antibodies to both A/H1N1 viruses (RR 1.86; 95%CI 1.19 to 2.91) and rH1 proteins (RR 2.07; 95%CI 1.29 to 3.34; Fig. 3A–B and S7A). A significantly higher frequency of OB individuals was also present for magnitude of IgA antibodies to rH1 proteins (RR 2.02; 95%CI 1.27 to 3.21), but not to whole H1N1 viruses (RR 1.10; 95%CI 0.72 to 1.69; Fig. 3E–F and S7A). In contrast, when the individuals were ranked according to H1 and N1 peptide response, obese individuals were less frequent in both low-IgG-BIH groups (H1: RR 0.43; 95%CI 0.25 to 0.73; N1: RR 0.43; 95%CI 0.25 to 0.73; Fig. 3E–H, S5A–D and S7A). OB individuals were also more frequently present in the low-BIH group for magnitude of IgG antibodies to A/H3N2 viruses (RR 1.78; 95%CI 1.14 to 2.78), B viruses (RR 1.88; 95%CI 1.18 to 2.98) and B rHI proteins (RR 1.68; 95%CI 1.10 to 2.58) as shown in Fig. S6A, C, D and S7A). Similarly, the age distribution comparison revealed that a significantly higher frequency of individuals younger than 65 was present in the low-BIH group for magnitude of IgG antibodies to A/H1N1 viruses (RR 2.91; 95%CI 1.18 to 7.13), A/H3N2 (RR 2.42; 95%CI 1.10 to 5.32) and B viruses (RR 1.96; 95%CI 1.02to 3.77) as shown in Figure 3I, S6I, S6M and S7B, respectively. This contrasted with lower frequencies of individuals younger than 65 years in the low IgG-BIH breadth groups for H1 (RR 0.60; 95%CI 0.42 to 0.87) and N1 (RR 0.55; 95%CI 0.39 to 0.77) peptides, in Figures 3M, N and S7B).
Fig. 3. Obesity- and age-associated baseline immune history (BIH) profiles to influenza H1N1 antigens.
(A- H) The distributions of BMI within the low-BIH group (n=47 for IgG and n=44 for IgA, solid purple line) and the high-BIH group (n=47 for IgG and n=44 for IgA, dashed green line) were plotted for IgG and IgA responses to H1N1 antigens as follows: (A) IgG Magnitude against H1N1 viruses; (B) IgG Magnitude to rH1 proteins; (C) IgG breadth to H1 peptides; (D) IgG breadth to N1 peptides; (E) IgA magnitude to H1N1 viruses; (F) IgA magnitude to rH1 proteins; (G) IgA breadth to H1 peptides; (H) IgA breadth to N1 peptides. Overweight subjects (25 < BMI < 30) were excluded from our analysis. The percentage of obese subjects in the low-BIH responders and high-BIH responders quartiles are listed. (I- P): The distributions by subjects age group (<65 y and > 65 y) within the low-BIH group (n=47 for IgG and n=44 for IgA, purple) and the high-BIH group (n=47 for IgG and n=44 for IgA, green) were plotted for their IgG and IgA responses to H1N1 antigens as follows: (I) IgG Magnitude against H1N1 viruses; (J) IgG Magnitude to rH1 proteins; (K) IgG breadth to H1 peptides; (L) IgG breadth to N1 peptides; (M) IgA magnitude to H1N1 viruses; (N) IgA magnitude to rH1 proteins; (O) IgA breadth to H1 peptides; (P) IgA breadth to N1 peptides. p values for differences between the age distributions of the low-BIH and high-BIH groups were determined using the Wilcoxon ranksum test. Obesity was associated with low IgG-BIH to H1N1 viruses and rH1 proteins, high IgA-BIH to rH1 proteins, and high IgG-BIH to peptides of the Cal09 H1 and N1 proteins. Age >65 y was associated with high IgA-BIH to H1N1 viruses and H1 proteins, high IgG-BIH to H1N1 viruses, low IgG- and IgA-BIH to Cal09 H1 peptides, and low IgG-BIH (but not IgA-BIH) to Cal09 N1 peptides. The number of individuals in the low-BIH and high-BIH groups in panels (C-D, G-H, K-L, and O-P) was 47–51 (see Materials and Methods).
The distribution of BMI or age of individuals in the low-BIH and high-BIH groups were then compared for each ranking for IgA responses (Fig. 3 and S6). There were significantly more OB individuals in the low quartile of IgA-BIH to rH1 proteins (Fig. 3F; RR 2.02; 95%CI 1.27 to 3.21, Fig. S7A). Of note, there was a significantly higher number of individuals <65 in the lower IgA-BIH quartiles for IgA responses to magnitude of H1 and N1 peptides (RR 2.18; 95% CI 1.07 to 4.46 for both; Fig. S5G–H and S7B), and there were more individuals <65 in the low quartile of IgA-BIH for H1N1 viruses (Fig 3M), H1N1 proteins (Fig 3N), H3N2 viruses (Fig. S6M), and B viruses (Fig. S6O), although the respective RRs were not significant (1.60, 95% CI 0.76 to 3.38; 1.35, 95% CI 0.79–2.30; 1.60, 95% CI 0.76 to 3.38; and 1.41, 95% CI 0.72 to 2.75 respectively; Fig. S7B). Thus, unlike IgG responses, there was no significant inverse correlation between magnitude of IgA antibodies to conformational (viruses and proteins) and linear (peptides) antigens when taking weight or age into account.
We then checked whether younger and older obese individuals differ by their BIH to H1N1 antigens, since both obese and individuals <65 were enriched in the low-IgG-BIH groups for whole H1N1 viruses, and in the high-IgG-BIH groups for H1 and N1 peptides, as well as in the low-IgA-BIH groups for rH1 proteins (Fig. 3). While no correlation was found between age and BMI (p=0.85), the age distributions of obese individuals in the low-BIH and high-BIH groups were compared. Obese individuals in the low IgG- and IgA-BIH groups to whole viruses were significantly younger than obese subjects in the high-BIH groups to viruses (IgG: p=0.000001 and IgA: p=0.002, respectively). Furthermore, obese individuals in the low-IgA-BIH to rH1 proteins were younger than the obese individuals in the high-IgA-BIH to proteins (p=0.02), while the age of obese individuals in IgG-BIH groups to rH1 proteins was similar (p=0.9). No similar age differences were found when comparing HW individuals from the low-BIH and high-BIH groups.
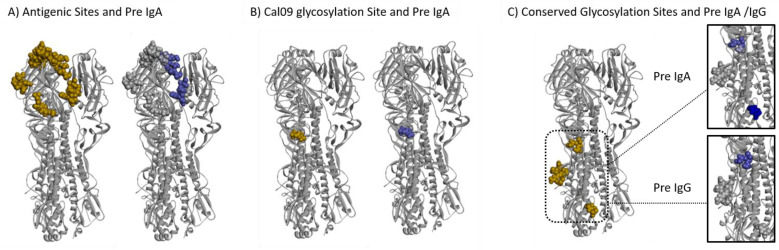
The significant differences between OB and HW was found in the magnitude and breadth of both IgG and IgA antibodies to Cal09 H1 peptides, prompted to ask whether different peptides were targeted in the OB and HW individuals. We mapped the H1 peptides targeted by BIH IgG and IgA antibodies to the structure of the Cal09 HA protein, and epitopes targeted preferentially by OB and HW individuals were compared using a logistic regression model. Relative weights were assigned to responses to individual peptides, and the score for an individual position was based on the maximal absolute weights assigned to the peptides that overlapped that position. We found that HW IgG antibodies preferentially targeted positions in HA1 subunit and a conserved glycosylation site, while only IgG from OB targeted the more conserved HA2 subunit (Table 2 and Fig. S8D–E). Of the eight functional domains analyzed, five domains (Receptor Binding Site, esterase domain, conserved glycosylation sites, Cal09 glycosylation site and the antigenic sites) were preferentially targeted by IgA of HW individuals, while only the Fusion domain was preferentially targeted by IgA antibodies from OB individuals (Table 2, Fig. 4A–C and S8A–C). These findings further demonstrate that the differences between the HW and OB individuals can be observed even at the resolution of specific functional domains, which may have functional implications for providing protection against infection and development of disease.
Table 2.
Targeting of HA functional sites by Obese and healthy weight (HW).
| pre IgA | pre IgG | |||||||
|---|---|---|---|---|---|---|---|---|
| Obese | HW | Residues(n) | P Value | Obese | HW | Residues(n) | P Value | |
| HA protein | 85 | 114 | 498 | 59 | 40 | 498 | ||
| HA1 | 50 | 65 | 322 | 0.7438 | 34 | 40 | 322 | <1 × 10e−4 ** |
| HA2 | 35 | 48 | 174 | 0.3591 | 23 | 0 | 174 | 0.0003 ** |
| Fusion | 76 | 53 | 261 | <1 × 10e−4 ** | 33 | 20 | 261 | 0.3008 |
| RBS | 0 | 20 | 153 | 0.0509 * | 20 | 20 | 153 | 0.9176 |
| Esterase | 7 | 26 | 64 | 0.0006 ** | 0 | 0 | 64 | 0.8066 |
| Conserved glycosylation sites | 0 | 6 | 13 | 0.0152 ** | 0 | 3 | 13 | 0.0255 ** |
| Cal09 glycosylation site | 0 | 3 | 3 | 0.0131 ** | 0 | 0 | 3 | 0.8293 |
| Antigenic sites | 0 | 11 | 50 | 0.0371 ** | 5 | 0 | 50 | 0.1896 |
p value below 0.05
p value below 0.055
Fig. 4. Different domains in the Cal09 HA protein are differentially targeted by BIH antibodies of obese and healthy weight subjects.
A logistic regression model was trained to discriminate between HW and OB individuals using the IgG and IgA antibody profiles to the HA peptides of the Cal509 vaccine strain. The weights assigned by the model were used to score individual amino acids on the HA protein based on the maximal weight of a given position across all of the peptides in which it was included (see Materials and Methods for details). Figures were created using Discovery Studio Visualizer software (XXref) and the crystal structure of the Cal09 HA trimeric protein PDB ID: 3LZG (10.1126/science.1186430). Differential binding to additional regions of interest on the HA protein are presented in Figure S9. Left side in each panel: Dark gold spheres represent amino acid residues belonging to three regions of interest mapped onto one of the three trimeric proteins: (A) Antigenic sites, (B) The Cal09 glycosylation site and (C) Conserved Glycosylation sites. Right side in each Panel: Dark Blue spheres represent amino acid residues within the regions of interest associated with antibodies from HW but not OB individuals according to their scores.
Our study population was not controlled for several potential confounders. While age did not appear to differ significantly between HW and OB subjects (Table 1), there were potential asymmetries for gender and race. In addition, other health factors potentially associated with obesity, such as diabetes, smoking history, or hypertension were not accounted for and could potentially have skewed results. For example, 45% of obese subjects and 7% of healthy weight subjects in our cohort suffered from type 2 diabetes. These factors could impact the specificity of our findings for obesity, but would not diminish their potential relevance or vaccine strategies in an immunologically diverse population.
Despite these caveats, this study demonstrates the power of antigen microarrays spotted with different types of antigens presenting both three-dimensional epitopes (whole viruses and rHA proteins) and linear epitopes (overlapping peptides spanning the entire length of proteins being analyzed) for comparing immune responses of different populations to the same vaccine. Consistent with previous studies(4), we demonstrate that OB individuals do respond to influenza vaccination; however, this in-depth analysis of the influenza-specific antibody repertoire found striking differences in the antibody repertoires of HW and OB subjects, despite the extensive heterogeneity of the baseline immune-history profiles of subjects in both groups. Adults who were repetitively exposed to influenza viruses by vaccines and infections have a greater opportunity to develop a diverse antibody repertoire; however, antibody responses in OB subjects were suboptimal compared with HW. The comparison of the antibody repertoire against linear and conformational H1N1 antigens in HW and OB subjects demonstrated reduced ability in individuals <65, obese individuals to develop an IgG response to whole virus or rHA protein of the pandemic Cal09 strain, which may be associated with a biased IgG repertoire towards linear epitopes.
Since individuals with obesity were reported as more sensitive to Cal09 infection (6), our results suggest that an effective IgG response is associated with IgG antibodies to conformational epitopes. A recent study found that influenza vaccination in humans can recruit memory B cells that were developed following previous exposures to influenza, into the germinal centers, for a further affinity maturation (7). We hypothesize that a successful antibody response following exposure of vaccination may convert immature antibodies to linear epitopes to more effective and possibly better neutralizing antibodies against conformational epitopes. Further research of the memory B cells recruited to germinal centers following influenza vaccination is required to test this hypothesis. On the other hand, a broader IgG repertoire against Cal09 H1 and N1 peptides may confer increased cross-reactivity to historical and future pandemic and endemic strains. Additional functional studies are necessary to understand how to harness differential BIH and vaccine-induced immune responses of the obese population in order to induce more effective antibody responses, especially once it is biased toward linear epitopes for IgG. Our data suggest that different influenza vaccines may be required for achieving optimal protection in an obese population.
The OB IgG antibody repertoire appears to be biased towards linear peptides and not whole viruses and rHA proteins, whereas the baseline OB IgA repertoire to H1N1 antigens is less biased. The IgA repertoire is narrower for both rH1 proteins and Cal09 H1 peptides. Taken together, these findings suggest that the IgG and IgA repertoires develop independently, or may be a function of infection during a novel pandemic. It is unclear why obesity affects the IgG repertoire more than the IgA repertoire, and additional mechanistic studies are required to address this question.
While the exact reason for the difference between obese and HW antibody responses to influenza remains to be studied, childhood obesity and even maternal obesity are associated with increased risk of obesity in adulthood (8–11). These early obesity factors could lead to aberrant immune response to vaccination and/or infection, leading to differential “original antigenic sin” compared to HW children or adults. Indeed, younger OB individuals in this cohort were more frequent in the lowest quartiles of BIH to H1N1 whole viruses and rHA proteins, which could bias responses later in life, especially if these individuals remain obese. Indeed, the fact that the majority of aberrant responses were observed with the relatively “novel” antigen, the 2009 pandemic strain, indicates that primary or early responses to specific antigens can result in significantly skewed responses. Further longitudinal studies are needed on birth cohorts to determine if childhood obesity or other factors may help to predict vaccination failure later in adulthood, even in HW individuals.
In addition accumulated differences in yearly protection against seasonal human influenza strains, and possibly to vaccines against future emergence of avian influenza strains, this work also has direct and timely relevance to the response to the current global COVID-19 pandemic. Influenza vaccines remain the best way to reliably prevent influenza infections. Due to the speculated cocirculation of SARS-CoV-2 and influenza viruses in the Northern Hemisphere during winter months and the similarity of symptoms between disease manifestations, it is imperative for broad coverage of influenza vaccination to reduce clinical and diagnostic burden as well as morbidity and mortality from influenza virus in an already straining healthcare system. In addition, the understanding of humoral immunity and vaccination effectiveness and duration against SARS-CoV-2 is nascent. As obesity is already considered a high risk factor in disease severity and mortality, if there is a differential response to specific vaccines, even with equivalent overall serological response, OB individuals may still be at risk for contracting and spreading SARS-CoV-2 within the population. Finally, the existence of significant numbers of asymptomatic infections is a confounding factor for predicting potential response of vaccines as these individuals are no longer naïve and obesity may skew initial antibody responses, therefore making future vaccination efforts ineffective, or, more concerningly, driving pathogen evolution and antibody escape. Overall, the design and development of different vaccines for OB individuals may need to be seriously taken into consideration, and clinical trials should take potential differences in BIH and vaccine-induced responses into account when choosing a representative “healthy” study population for any phase.
Supplementary Material
Acknowledgements:
The authors thank the Israel Science Foundation grant 882/17 (TH), The Israel America Foundation and the Ben-Gurion University Center for Multidisciplinary Research in Aging (TH), National Institute of Allergy and Infectious Diseases CEIRS contract under HHS contract HHSN27220140006C (EAK and SSC), ALSAC (EAK and SSC), as well as R01 NIH/NIAID/AI078090 (MAB, TLN and SSW) for supporting the study.
Funding: Provide complete funding information, including grant numbers, complete funding agency names, and recipient’s initials. Each funding source should be listed in a separate paragraph.
The National Institute for Biotechnology in the Negev (TH);
Israel Science Foundation Individual Research Grant NO. 882/17 (TH);
The Israel-America Foundation and the Ben-Gurion University Center for Multidisciplinary Research in Aging (TH);
National Institute of Allergy and Infectious Diseases under HHS contract HHSN27220140006C (EAK and SSC);
National Institute of Allergy and Infectious Diseases grant R01 NIH/NIAID/AI078090 (MAB, TLN and SSW);
ALSAC (EAK and SSC).
Footnotes
Competing interests: The authors declare no non-financial interest but declare a competing financial interest. A patent application related to the microarrays used in this research has been filed by TH and LMF.
Data and materials availability:
All row data and code used in the analysis are available in the Hertz Lab website: https://www.hertz-lab.org/
References and Notes
- 1.Green W. D., Beck M. A., Obesity Impairs the Adaptive Immune Response to Influenza Virus. Annals of the American Thoracic Society 14, S406–S409 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seidu S. et al. , The impact of obesity on severe disease and mortality in people with SARS-CoV-2: A systematic review and meta-analysis. Endocrinology, diabetes & metabolism, e00176 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson E. J. et al. , Factors associated with COVID-19-related death using OpenSAFELY. Nature 584, 430–436 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neidich S. D. et al. , Increased risk of influenza among vaccinated adults who are obese. International journal of obesity 41, 1324–1330 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheridan P. A. et al. , Obesity is associated with impaired immune response to influenza vaccination in humans. International journal of obesity 36, 1072–1077 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louie J. K. et al. , A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 52, 301–312 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Turner J. S. et al. , Human germinal centres engage memory and naive B cells after influenza vaccination. Nature 586, 127–132 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo S. S., Roche A. F., Chumlea W. C., Gardner J. D., Siervogel R. M., The predictive value of childhood body mass index values for overweight at age 35 y. The American journal of clinical nutrition 59, 810–819 (1994). [DOI] [PubMed] [Google Scholar]
- 9.Whitaker R. C., Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 114, e29–36 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Whitaker R. C., Wright J. A., Pepe M. S., Seidel K. D., Dietz W. H., Predicting obesity in young adulthood from childhood and parental obesity. The New England journal of medicine 337, 869–873 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Yu Z. et al. , Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PloS one 8, e61627 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paich H. A. et al. , Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity 21, 2377–2386 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisfeld A. J., Neumann G., Kawaoka Y., Influenza A virus isolation, culture and identification. Nature protocols 9, 2663–2681 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All row data and code used in the analysis are available in the Hertz Lab website: https://www.hertz-lab.org/