Abstract
Objective
The current standard D2 lymphadenectomy for gastric cancer (GC) includes dissection of lymph nodes (LNs) along the proper hepatic artery (No. 12a), however, the survival benefit remains controversial. The purpose of this study was to evaluate the pattern of No. 12a LN metastasis (LNM) in GC and explore the indications for No. 12a LN dissection.
Methods
Medical records of 413 consecutive GC patients who underwent curative surgery in Zhongshan Hospital, Fudan University between January 2015 and December 2018 were enrolled and reviewed retrospectively. The correlation between No. 12a LNM and clinicopathologic characteristics of patients was analyzed.
Results
The overall incidence of No. 12a LNM was 2.67% (11/413). Tumor location (P=0.012), depth of tumor infiltration (P<0.01) and N stage (P=0.018) were significant factors associated with No. 12a LNM. All the tumors with No. 12a LNM involved the lower third of the stomach and were in T3−4 stages. Patients with No. 12a LNM had extensive LNM than those without (20.91±4.25vs. 5.0±0.54, P<0.001). For advanced GC patients (stage III/IV) with tumors involving the lower third of the stomach, the incidence of No. 12a LNM increased to 10.7% (11/103). Patients with No. 12a LNM had a significantly poorer recurrence-free survival (RFS) (P=0.005) and overall survival (OS) (P=0.017). According to the result of multivariable Cox regression, No. 12a LNM was not an independent impact factor on RFS and OS.
Conclusions
The overall incidence of No. 12a LNM was low but it was much higher in GC patients who had very advanced tumors involving the lower third of the stomach. No. 12a LN dissection should be considered for these patients to improve the survival outcomes.
Keywords: Gastric cancer, No. 12a lymph node, lymph node metastasis, lymphadenectomy
Introduction
Lymph node metastasis (LNM) is the most common metastasis type in gastric cancer (GC). Lymphadenectomy has been proven beneficial to the survival of GC patients (1). However, the extent of lymph node (LN) dissection remains controversial. Recently, the Dutch Gastric Cancer Group Trial reported their finding after a median follow-up of 15 years; they reported that D2 lymphadenectomy was associated with lower local recurrence and cancer-related mortality compared with D1 lymphadenectomy (12% vs. 22%; 37% vs. 48%), but not with significantly improved overall survival (OS) (2). Compared with D1 lymphadenectomy, D2 lymphadenectomy is associated with more surgical complications and a higher perioperative mortality rate, which may compromise its survival benefit (3,4). Although some studies have shown that the morbidity and mortality of D2 lymphadenectomy were significantly reduced in high-volume centers (5-7), this procedure remains a formidable challenge even for very skilled surgeons.
Different GC patients may have different LN metastatic patterns due to their specific characteristics (8). To avoid unnecessary LN dissection and to reduce surgical complications, the extent of D2 lymphadenectomy is constantly modified with the accumulation of research evidence. For example, after the JCOG0110 Trial reported that splenectomy for No. 10 LN dissection could increase the operative morbidity without improving the survival of proximal GC patients without greater curvature invasion, the Japanese Gastric Cancer Treatment Guidelines (5th version) recommended that radical total gastrectomy should not include the dissection of LNs at the splenic hilum (No. 10) in standard D2 lymphadenectomy for those cases (9).
The current standard D2 lymphadenectomy includes LN dissection along the proper hepatic artery (No. 12a) for both distal and total gastrectomies. However, the survival benefit of No. 12a LN dissection remains controversial (10,11). In this study, we aimed to evaluate the pattern of No. 12a LNM and to explore the indications for No. 12a LN dissection.
Materials and methods
Patient selection
Consecutive GC patients who underwent curative surgery in Zhongshan Hospital, Fudan University (Shanghai, China) from January 2015 to December 2018 were retrospectively screened. All enrolled patients had histologically confirmed gastric adenocarcinoma and they received radical gastrectomy with standard D2 lymphadenectomy according to the Japanese Gastric Cancer Treatment Guidelines. Remnant GC patients and patients with advanced tumors who only received exploration or palliative surgery were excluded. Once No. 12a LNs were dissected, they were isolated from the specimen immediately during the operation and sent separately for pathological evaluation to avoid confusion with the other groups of LNs. Patients without No. 12a LN dissection (D1/D1+ lymphadenectomy) or without No. 12a LN isolation were not included in this study. The stepwise process of data extraction is depicted in Figure 1.
1.
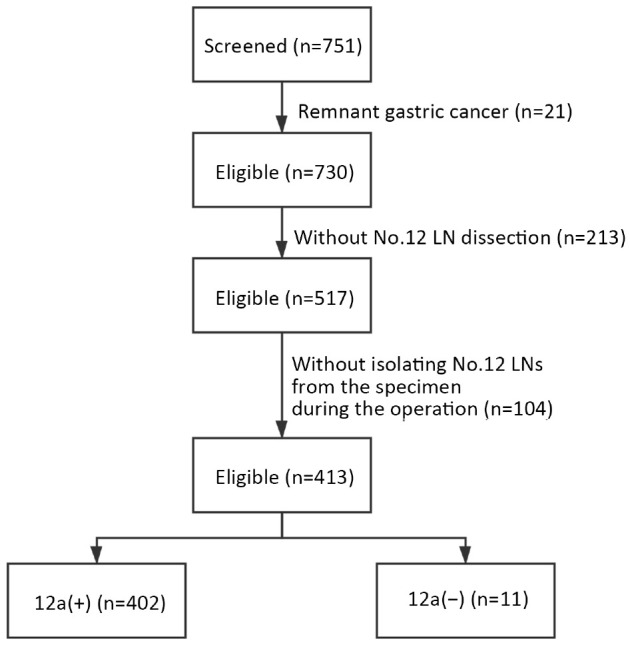
Diagram of cohort selection in this study. LN, lymph node.
Data collection
Variables of patients including age, sex, tumor site, pathological types and surgical procedure were collected from the medical records. We subdivided patients into the upper third, the middle third and the lower third of the stomach according to the tumor locations. The numbers of harvested LNs and metastatic LNs were investigated based on the postoperative pathologies. Pathological staging was performed according to the 8th edition of the American Joint Committee on Cancer (AJCC) stage system for GC.
Follow-up
Recurrence and survival status was last updated in June 2020. Follow-up observations were carried out through outpatient basis or telephone interviews. Abdominal and pelvic CT/MRI images were usually obtained every 3 months. The observation endpoint was recurrence-free survival (RFS) and OS. RFS was calculated from the date of primary tumor resection to the date of recurrence (including local relapse and metastasis) or the last follow-up date. OS was defined as the time from primary treatment to patient death or the last follow-up date. The use of patients data was approved by the ethics committee of Zhongshan Hospital, Fudan University, and the study was performed in accordance with the ethics statements presented in the 1964 Declaration of Helsinki and its later amendments. Informed written consent was obtained from all participants.
Statistical analysis
All statistical analyses were carried out using SPSS software (Version 17.0, SPSS Inc., Chicago, IL, USA). Quantitative variables were described as
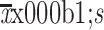 or median with range and categorical variables were described as frequency and percentages. Quantitative data were compared using t-test or Mann-Whitney U test. Chi-square test, Continuity correction, or Fisher’s exact test was performed for categorical data, as appropriate. Survival was compared using the log-rank test, and survival curves were generated using the Kaplan-Meier method. Univariate and multivariate analyses were conducted using the Cox proportional hazards regression model. Hazard ratio (HR) with 95% confidence intervals (95% CI) was assessed for determining the relationship between dichotomous variables. All tests were two-tailed, and P<0.05 was considered statistically significant.
or median with range and categorical variables were described as frequency and percentages. Quantitative data were compared using t-test or Mann-Whitney U test. Chi-square test, Continuity correction, or Fisher’s exact test was performed for categorical data, as appropriate. Survival was compared using the log-rank test, and survival curves were generated using the Kaplan-Meier method. Univariate and multivariate analyses were conducted using the Cox proportional hazards regression model. Hazard ratio (HR) with 95% confidence intervals (95% CI) was assessed for determining the relationship between dichotomous variables. All tests were two-tailed, and P<0.05 was considered statistically significant.
Results
Patient clinicopathological characteristics
A total of 751 patients were screened and 413 patients were finally enrolled in the study. The characteristics of the included patients are shown in Table 1. The median age of the patients was 63 (range, 25−87) years at the time of surgery. Among them, 305 (73.8%) patients were males and 108 (26.2%) were females. A total of 26.2% (108/413) of tumors were located in the upper third of the stomach, 8.0% (33/413) in the middle third of the stomach, 59.8% (247/413) in the lower third of the stomach, and 6.0% (25/413) in the whole stomach. Distal radical gastrectomy was performed in 249 (60.3%) patients, and total gastrectomy was performed in the other 164 (39.7%) patients. Two patients had distant metastatic lesions, including one with ovarian metastasis and another with liver metastasis. Despite the existing controversy on surgery in such cases, we still performed radical gastrectomy with resection of the metastatic lesions. The percentage of LNM in T1a stage patients was 11.6%. The metastasis proportion of the T1b stage was 33.9%. The metastasis proportion of T2, T3 and T4 stage were 40.0%, 75.7%, and 81.6%, respectively. The patients with a higher T stage tended to have a higher possibility of LNM.
1. Clinicopathologic characteristics of included patients (N=413).
| Variables | n (%) |
| NA, not available; P, pathology;TNM was based on the T, N and M elements defined by the American Joint Committee on Cancer (AJCC), the 8th edition. | |
| Age (median, range) (year) | 63 (25−87) |
| Sex | |
| Male | 305 (73.8) |
| Female | 108 (26.2) |
| Location of lesion | |
| Upper | 108 (26.2) |
| Middle | 33 (8.0) |
| Lower | 247 (59.8) |
| Entire | 25 (6.0) |
| Surgical procedure | |
| Distal gastrectomy | 249 (60.3) |
| Total gastrectomy | 164 (39.7) |
| Differentiation | |
| Well and moderate | 84 (20.3) |
| Poor | 320 (77.5) |
| NA | 9 (2.2) |
| Lauren’s classification | |
| Intestinal | 154 (37.3) |
| Diffuse | 82 (19.9) |
| Mixed | 152 (36.8) |
| NA | 25 (6.1) |
| pT stage | |
| T1a | 69 (16.7) |
| T1b | 56 (13.6) |
| T2 | 60 (14.5) |
| T3 | 103 (24.9) |
| T4a | 118 (28.6) |
| T4b | 7 (1.7) |
| pN stage | |
| N0 | 183 (44.3) |
| N1 | 60 (14.5) |
| N2 | 53 (12.8) |
| N3a | 64 (15.5) |
| N3b | 53 (12.8) |
| pTNM stage | |
| I (Ia+Ib) | 145 (35.1) |
| II (IIa+IIb) | 103 (24.9) |
| III (IIIa+IIIb+IIIc) | 163 (39.5) |
| IV | 2 (0.5) |
Correlations between No. 12a LNM metastasis and clinicopathologic characteristics
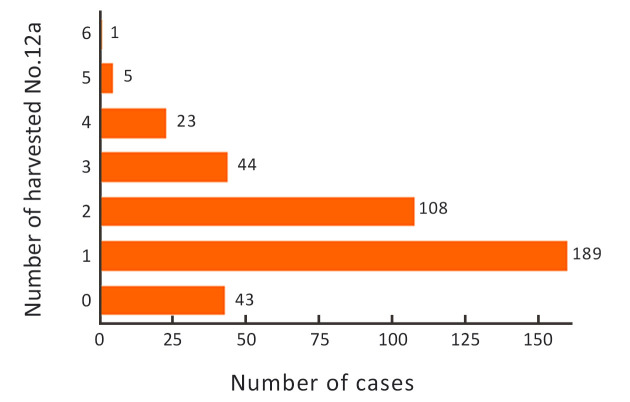
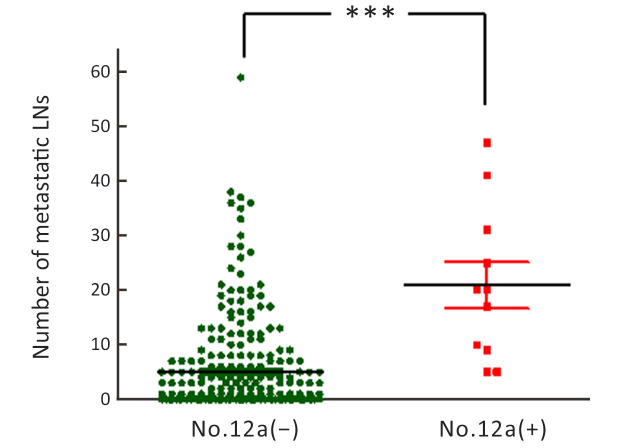
The number of harvested No. 12a LNs in each case is shown in Figure 2. The majority of patients (93%) had ≤3 No. 12a harvested LNs. The mean number of harvested LNs was 1.6±1.1. Forty three patients did have a No. 12a LN resection, but no LN was detected from the samples submitted by the pathology department. We considered that these patients may have anatomic variations and No. 12a LNs are indeed absent. Exclusion of these patients may result in inaccurate prognostic analysis of GC patients with No. 12a LN dissection. Therefore, these patients were included in the study according to the inclusion criteria and exclusion criteria. Furthermore, the main purpose of our study was to analyze the pattern of No. 12a LNM in GC patients who underwent No. 12a LN dissection, so we included the 43 patients in the No. 12a (−) group. The overall incidence of No. 12a LNM was only 2.67% [11/413, 95% confidence interval (95% CI): 0.0140−0.0485] in this series. The correlation between No. 12a LNM and clinicopathologic characteristics of patients is shown inTable 2. Tumor location (P=0.012), the depth of invasion (P<0.01), and N stage (P=0.018) were significantly associated factors, indicating that No. 12a LNM was prone to occur in tumors located at the lower third of the stomach (L/UML) and penetrating to the deeper layer of the stomach wall (T3−4). Most of the patients (9/11, 81.8%) with No. 12a LNM had extensive LNM (N3 stage). The number of metastatic LNs in No. 12a LN (+) patients was significantly higher than that in No. 12a LN (−) patients (20.91±4.25vs. 5.00±0.54, P<0.001) (Figure 3). The patients with positive No. 12a LNs were all stratified to very advanced stages (stage III/IV) by the AJCC 8th staging system. For those advanced cases (stage III/IV) with tumors involving the lower third of the stomach, the incidence of No. 12a LNM increased to 10.7% (11/103). In addition, diffused or mixed type of GC by Lauren’ s classification tended to have No.12a LNM than the intestinal type, although the difference was not statistically significant (P=0.096).
2.
Frequency of patients distinguished by number of harvested No. 12a lymph nodes.
2. Correlations between No. 12a LNs metastasis and clinicopathologic characteristics.
| Variables | n (%) | P | |
| No. 12 (+) | No. 12 (−) | ||
| P, pathology; TNM was based on the T, N and M elements defined by the American Joint Committee on Cancer (AJCC), the 8th edition. | |||
| Age (year) | 0.538 | ||
| ≤60 | 6 (54.5) | 168 (41.8) | |
| >60 | 5 (45.5) | 234 (58.2) | |
| Gender | 0.735 | ||
| Male | 9 (81.8) | 296 (73.6) | |
| Female | 2 (18.2) | 106 (26.4) | |
| Location | 0.012 | ||
| Upper | 0 (0) | 108 (26.8) | |
| Middle | 0 (0) | 33 (8.2) | |
| Lower | 8 (72.7) | 239 (59.5) | |
| Entire | 3 (27.3) | 22 (5.5) | |
| Differentiation | 0.472 | ||
| Well and moderate | 1 (9.1) | 83 (21.1) | |
| Poor | 10 (90.9) | 310 (78.9) | |
| Lauren’s classification | 0.096 | ||
| Intestinal | 1 (9.1) | 153 (40.6) | |
| Diffuse/Mixed | 10 (90.9) | 224 (59.4) | |
| pT stage | <0.010 | ||
| T1−T2 | 0 (0) | 185 (46.0) | |
| T3−T4 | 11 (100) | 217 (54.0) | |
| pN stage | 0.018 | ||
| N0 | 0 (0) | 183 (45.5) | |
| N1 | 0 (0) | 60 (14.9) | |
| N2 | 2 (18.2) | 51 (12.7) | |
| N3a | 2 (18.2) | 62 (15.4) | |
| N3b | 7 (63.6) | 46 (11.5) | |
| pTNM stage | <0.010 | ||
| I−II | 0 (0) | 248 (61.7) | |
| III−IV | 11 (100) | 154 (38.3) | |
3.
Number of metastatic LNs in No. 12a LN (−) group and (+) group. LN, lymph node. ***, P<0.001.
Oncological outcomes
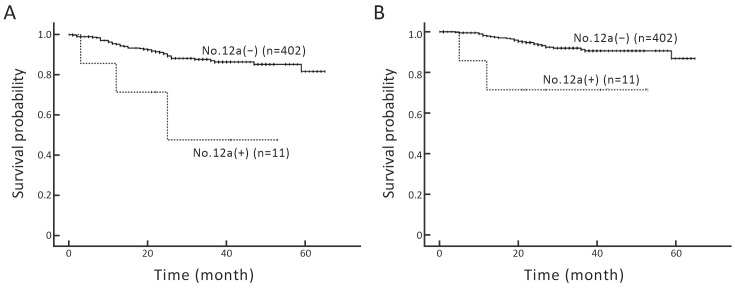
The median follow-up period was 29 (range, 21−40) months. At the time of the last follow-up, recurrence and metastasis occurred in 48 patients. The number of tumor-related deaths was 31. The 3-year RFS and OS rates in all patients of this series were 86.4% and 90.9%, respectively, and the median RFS and OS were not reached. A comparison of survival between patients with or without No. 12a LNM revealed that those with No. 12a LNM had a significantly poorer RFS (Log-rank, P=0.005) and OS (Log-rank, P=0.017) (Figure 4). The Cox regression model was used to determine the factors affecting RFS and OS. Univariate Cox analysis showed that RFS was correlated with pTNM stage, surgical procedure, and No. 12a LN metastasis (Table 3). OS was correlated with pTNM stage and No. 12a LNM (Table 4). To minimize the impact of selection bias on the survival outcome, multivariate Cox regression analysis was used. The result showed that pTNM stage was an independent factor leading to poor RFS (HR: 6.147, 95% CI: 3.098−12.198, P<0.001) and OS (HR: 6.193, 95% CI: 2.625−14.610, P<0.001). No. 12a LNM did not have a significant impact on RFS and OS outcome (Tables 3,4).
4.
RFS (A) and OS (B) curves of patients with gastric cancer according to No. 12a group lymph nodes. RFS, recurrence-free survival; OS, overall survival.
3. Univariate and multivariate Cox regression analyses on variables affecting RFS.
| Variable | Univariate analysis | Multivariate analysis | |||
| HR (95% CI) | P | HR (95% CI) | P | ||
| RFS, recurrence-free survival; P, pathology; LN, lymph node; HR, hazard ratio; 95% CI, 95% confidence interval; TNM was based on the T, N and M elements defined by the American Joint Committee on Cancer (AJCC), the 8th edition. | |||||
| Age (year) | 0.485 | 0.352 | |||
| ≤60 | 1 | 1 | |||
| >60 | 0.817 (0.463−1.441) | 0.755 (0.417−1.366) | |||
| Gender | 0.720 | 0.380 | |||
| Female | 1 | 1 | |||
| Male | 0.890 (0.470−1.684) | 0.743 (0.382−1.444) | |||
| pTNM stage | <0.001 | <0.001 | |||
| I−II | 1 | 1 | |||
| III−IV | 6.794 (3.462−13.332) | 6.147 (3.098−12.198) | |||
| Surgical procedure | 0.006 | 0.018 | |||
| Distal | 1 | 1 | |||
| Total | 2.218 (1.253−3.925) | 2.030 (1.130−3.644) | |||
| No. 12a LNs | 0.011 | 0.162 | |||
| Negative | 1 | 1 | |||
| Positive | 4.550 (1.411−14.673) | 2.342 (0.711−7.715) | |||
4. Univariate and multivariate Cox regression analyses on variables affecting OS.
| Variable | Univariate analysis | Multivariate analysis | |||
| HR (95% CI) | P | HR (95% CI) | P | ||
| OS, overall survival; P, pathology; LN, lymph node; HR, hazard ratio; 95% CI, 95% confidence interval; TNM was based on the T, N, and M elements defined by the American Joint Committee on Cancer (AJCC), the 8th edition. | |||||
| Age (year) | 0.896 | 0.713 | |||
| ≤60 | 1 | 1 | |||
| >60 | 0.954 (0.467−1.947) | 0.870 (0.414−1.829) | |||
| Gender | 0.825 | 0.801 | |||
| Female | 1 | 1 | |||
| Male | 1.100 (0.473−2.556) | 0.894 (0.373−2.143) | |||
| pTNM stage | <0.001 | <0.001 | |||
| I−II | 1 | 1 | |||
| III−IV | 6.810 (2.932−15.817) | 6.193 (2.625−14.610) | |||
| Surgical procedure | 0.093 | 0.213 | |||
| Distal | 1 | 1 | |||
| Total | 1.831 (0.904−3.708) | 1.587 (0.768−3.281) | |||
| No. 12a LNs | |||||
| Negative | 1 | 0.031 | 1 | 0.244 | |
| Positive | 4.839 (1.152−20.329) | 2.391 (0.552−10.358) | |||
Discussion
According to the Japanese guidelines for GC treatment, standard D2 lymphadenectomy includes the dissection of No. 12a LNs, whether for total or distal gastrectomy. However, the benefit of routine dissection of No. 12a LNs remains controversial.
Currently, there is a large discrepancy in the incidence of No. 12a LNM reported in different studies and it ranges from 1.7% to 18.2% (12-15), which is partly due to the heterogeneity of tumors. Additionally, it is also difficult to distinguish No. 12a LNs from LNs along the right gastric artery (No. 5) and the common hepatic artery (No. 8) after all these LNs are dissected. No. 5 and No. 8 LNs are more prone to metastasis in GC patients. If these LNs are mistaken for No. 12a LNs for pathological evaluation, it would result in a higher positive ratio of No. 12a LNM (16). In this study, we isolated No. 12a LNs during the operation once they were dissected to avoid confusion with the other LNs. Our result showed that the number of harvested No. 12a LNs was low (1.6±1.1), and the incidence of No.12a LNM was only 2.67%.
In this study, we identified that several tumor characteristics were associated with No. 12a LNM. The most significant factor was the degree of tumor progression. We found that all patients with No. 12a LNM were in very advanced (T3−4, stage III−IV) stages, suggesting that No. 12a LNM is a sign of disease progression, which explained why No. 12a LN positive patients usually have a poor prognosis (13,17). In addition, No. 12a LN status is also associated with tumor location. All tumors with No. 12a LNM involved the lower third of the stomach, but they were not confined to the upper or middle third in our study. The reason might be partially attributed to the fact that tumors located in the upper part of the stomach were distant to No. 12 LNs; thus the main lymphatic flux was not preferentially converged into the upper pyloric LNs and then to No. 12 LNs. The result was similar to the finding reported by Yamashita et al. (11), who stated that No. 12a LNM was not found in 102 patients with Siewert type II esophagogastric junction (EGJ) carcinoma after routine dissection. Another study including 35 GC patients with tumors in the upper or middle third of the stomach also detected a very low incidence of No.12a LNM (4). However, in tumors involving the lower third, our results indicated that the incidence of No.12a LNM increased to 4.0% (11/272), which was similar to the study by Kong et al. reporting a rate of 3.6% (18). In patients with more advanced stages (stage III−IV), the incidence was as high as 6.7% (11/165), Additionally, the diffused or mixed type of tumors tended to metastasize to No. 12a LNs more easily than patients with the intestinal type, although the difference is not statistically significant (P=0.096). In our series, No. 12a LNM did not seem to be an independent prognostic factor, but the patients with No. 12a LNM had a poorer prognosis than those with No. 12a LNs negative. As mentioned above, No. 12a LN had not been identified in the dissected specimens in 43 cases who did undergo a dissection. Considering these patients as another group, we analyzed the remaining 370 patients. As expected, the results were coincident with those mentioned above. Therefore, No. 12a LN dissection should be beneficial to survival for such selected patients with the characteristics associated with No. 12a LNM mentioned above. But high-level evidence should be obtained to confirm this conclusion.
Due to the nature of the retrospective design, our study had several limitations. Firstly, all analyses were subject to selection biases and imbalances in unquantified variables. Secondly, the number of patients included in this study was not large enough. Thus, the significance of this study lies in further expansion of the sample size in the follow-up experiments for further observation.
Conclusions
According to our clinical experiences and practice,the present study has analyzed the pattern of No. 12a LNM in gastric cancer patients who underwent No. 12a LN dissection. The present data suggest that the No. 12a LNM did not seem to be an independent prognostic factor, but patients with No. 12a LNM had poorer prognosis than those without. The overall incidence of No. 12a LNM was low but it was much higher in patients who had very advanced tumors involving the lower third of the stomach. No. 12a LN dissection should be considered for these patients to improve the survival outcomes.
Acknowledgements
This study was supported by National Natural Science Fund (No. 31842033).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Fenglin Liu, Email: liu.fenglin@zs-hospital.sh.cn.
Yong Fang, Email: fang.yong@zs-hospital.sh.cn.
References
- 1.Yang L, Ying X, Liu S, et al Gastric cancer: Epidemiology, risk factors and prevention strategies. Chin J Cancer Res. 2020;32:695–704. doi: 10.21147/j.issn.1000-9604.2020.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Songun I, Putter H, Kranenbarg EM, et al Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49. doi: 10.1016/S1470-2045(10)70070-X. [DOI] [PubMed] [Google Scholar]
- 3.Cuschieri A, Weeden S, Fielding J, et al Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–30. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galizia G, Lieto E, De Vita F, et al Modified versus standard D2 lymphadenectomy in total gastrectomy for nonjunctional gastric carcinoma with lymph node metastasis. Surgery. 2015;157:285–96. doi: 10.1016/j.surg.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Degiuli M, Sasako M, Ponti A, et al Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg. 2014;101:23–31. doi: 10.1002/bjs.9345. [DOI] [PubMed] [Google Scholar]
- 6.Degiuli M, Sasako M, Ponti A, on behalf of the Italian Gastric Cancer Study Group Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg. 2010;97:643–9. doi: 10.1002/bjs.6936. [DOI] [PubMed] [Google Scholar]
- 7.National Health Commission of the People’s Republic of China Chinese guidelines for diagnosis and treatment of gastric cancer 2018 (English version) Chin J Cancer Res. 2019;31:707–37. doi: 10.21147/j.issn.1000-9604.2019.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claassen Y, van Sandick JW, Hartgrink HH, et al Association between hospital volume and quality of gastric cancer surgery in the CRITICS trial. Br J Surg. 2018;105:728–35. doi: 10.1002/bjs.10773. [DOI] [PubMed] [Google Scholar]
- 9.Sano T, Sasako M, Mizusawa J, et al Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma. Ann Surg. 2017;265:277–83. doi: 10.1097/SLA.0000000000001814. [DOI] [PubMed] [Google Scholar]
- 10.Goto H, Tokunaga M, Miki Y, et al The optimal extent of lymph node dissection for adenocarcinoma of the esophagogastric junction differs between Siewert type II and Siewert type III patients. Gastric Cancer. 2014;18:375–81. doi: 10.1007/s10120-014-0364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita H, Katai H, Morita S, et al Optimal extent of lymph node dissection for Siewert type II esophagogastric junction carcinoma. Ann Surg. 2011;254:274–80. doi: 10.1097/SLA.0b013e3182263911. [DOI] [PubMed] [Google Scholar]
- 12.Yang K, Chen HN, Liu K, et al The survival benefit and safety of No. 12a lymphadenectomy for gastric cancer patients with distal or total gastrectomy. Oncotarget. 2016;7:18750–62. doi: 10.18632/oncotarget.7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai S, Chen J, Chen C, et al Survival of proper hepatic artery lymph node metastasis in patients with gastric cancer: implications for D2 lymphadenectomy. PLoS One. 2015;10:e118953. doi: 10.1371/journal.pone.0118953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumagai K, Hiki N, Nunobe S, et al Metastasis to the lymph nodes along the proper hepatic artery from adenocarcinoma of the stomach. Langenbecks Arch Surg. 2016;401:677–85. doi: 10.1007/s00423-016-1429-9. [DOI] [PubMed] [Google Scholar]
- 15.Lee SL, Lee HH, Ko YH, et al Relevance of hepatoduodenal ligament lymph nodes in resectional surgery for gastric cancer. Br J Surg. 2014;101:518–22. doi: 10.1002/bjs.9438. [DOI] [PubMed] [Google Scholar]
- 16.Lin GT, Chen QY, Zheng CH, et al Lymph node noncompliance affects the long-term prognosis of patients with gastric cancer after laparoscopic total gastrectomy. J Gastrointest Surg. 2020;24:540–50. doi: 10.1007/s11605-019-04199-9. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Zhu G, Zheng W, et al Scope definition and resection significance of No. 12a group lymph nodes in gastric cancer. Mol Clin Oncol. 2016;5:257–62. doi: 10.3892/mco.2016.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong SH, Yoo MW, Kim JW, et al Validation of limited lymphadenectomy for lower-third gastric cancer based on depth of tumour invasion. Br J Surg. 2011;98:65–72. doi: 10.1002/bjs.7266. [DOI] [PubMed] [Google Scholar]