Abstract
Purpose of Review:
To introduce recent advances in the understanding of diabetic retinopathy and to summarize current and emerging strategies to treat this common and complex cause of vision loss.
Recent Findings:
Advances in retinal imaging and functional analysis indicate that retinal vascular and neural pathologies exist long before the development of clinically-visible retinopathy. Such diagnostics could facilitate risk stratification and selective early intervention in high-risk patients. Antagonists of the vascular endothelial growth factor pathway effectively reduce vision loss in diabetes and promote regression of disease severity. Promising new strategies to treat diabetic retinopathy involve novel systemic diabetes therapy and ocular therapies that antagonize angiogenic growth factor signaling, improve blood-retina-barrier function and neurovascular coupling, modulate neuroretinal metabolism, or provide neuroprotection.
Summary:
Long considered a pure microvasculopathy, diabetic retinopathy in fact affects the neural and vascular retina as well as neurovascular communication. Emerging therapies include those that target neuroretinal dysfunction in addition to those modulating vascular biology.
Keywords: diabetic retinopathy, diabetic macular edema, neuroretinopathy, neurovascular coupling, vascular endothelial growth factor
Introduction
By the year 2030, nearly 55 million Americans will suffer from diabetes mellitus and 11 million of these individuals will have some form of diabetic retinopathy (DR) [1]. These numbers reflect an increasing prevalence of both diseases, which together represent a significant threat to public health. In the past two decades, tremendous progress in understanding the pathophysiology of DR has led to transformative advances in its treatment. By some measures these new interventions have reduced the incidence of severe vision loss in diabetes [2]. Much of this progress can be directly attributed to the advent and widespread use of agents antagonizing the vascular endothelial growth factor (VEGF) signaling pathway, a key molecular mechanism responsible for late DR, as well as other macular diseases affecting the retinal and choroidal blood vessel network. These agents, used as monotherapy or in combination with other late-acting interventions, are highly safe and effective. However, because their utility is limited to relatively late stages of disease, they are not a panacea for preventing vision loss in diabetes. Much remains to be understood about the early mechanisms leading to DR, especially in the stages that precede outright microvascular lesions. The next generation of paradigm-shifting therapies for DR will be those that act in these early stages. In this review, we will discuss current themes in the understanding of DR pathogenesis, focusing on recent preclinical data elucidating disease processes occurring prior to visible microvascular findings. Then, we will introduce emerging therapies for DR, including those targeting stages prior to the onset of ophthalmoscopic vasculopathy.
Clinical and Experimental Observations
The goal of all therapies for DR is prevention of vision loss. In order of frequency, vision loss in DR occurs from diabetic macular edema (DME), vitreous hemorrhage, traction retinal detachment, and macular ischemia. DME and macular ischemia can develop at any point in the disease once vascular lesions are visible. Treatment for the DME has evolved greatly over the past two decades. No therapies to reverse macular ischemia currently exist. Vision loss from vitreous hemorrhage or tractional retinal detachment occurs in very late stages of disease and largely remains best-treated by surgical intervention. Unfortunately, the outcomes of surgical intervention for tractional retinal detachments are often poor, with roughly one-half of patients having final visual acuity of 20/200 or worse [3, 4]. Although medical and surgical therapy for DR has evolved greatly over the past two decades, resulting in improved outcomes in many series, preventing progression into these late stages is far superior to any currently available therapy. Since the stages preceding outright vision loss in DR are largely asymptomatic, accurate clinical staging based on ophthalmoscopic screening is critical to engaging appropriate preventative intervention.
Retinal microvascular lesions are the hallmarks of DR and are the major standard by which disease progression and response to therapy are gauged. Conventional features of nonproliferative diabetic retinopathy (NPDR) include microaneurysms, intraretinal hemorrhages, venous beading, and intraretinal microvascular anomalies. Also associated with NPDR are hard exudates, cotton wool spots (soft exudates), vascular tortuosity, and breakdown of retinal microvascular barrier properties. Proliferative diabetic retinopathy (PDR) is characterized by neovascularization (NV) emanating from retinal vessels, optic nerve, and/or the iris. PDR can become complicated by vitreous hemorrhage or tractional retinal detachment. The spectrum of DR severity from mild NPDR to end-stage PDR is highly correlated with intraocular VEGF concentration [5], and pharmacologic VEGF antagonism fosters regression on this spectrum [6–8].
Histopathologic analysis demonstrates that with progressive disease, the entire span of the retina is eventually affected by diabetes (Figure 1). Even prior to visible microvascular lesions, vascular pathology is evident by histologic analysis. In donor and autopsy eyes from patients with diabetes but no DR, classic findings include capillary atrophy, pericyte loss, and thickening of the vascular basement membrane [9–11]. More recently, advances in optical coherence tomography angiography (OCT-A) – a modality capable of high-resolution imaging of each of the 3 major plexuses of the retinal capillary network – permit clinical detection of diabetes-associated macular vascular pathology in eyes without DR. Characteristic changes include increased area and irregularity of the foveal avascular zone (FAZ) in the superficial and deep capillary plexuses (SCP and DCP, respectively) among people with diabetes who have no evident retinopathy [12–14]. Upon development of frank DR, and as DR advances, progressive FAZ capillary loss in the SCP and DCP follows [15]. Early studies using OCT-A were limited by flow artifacts, but projection-resolved OCT-A confirms many of the initial findings in eye of patients with diabetes and have been used to predict development of visible DR [16]. Another notable development in clinical care of DR is the ability to detect capillary nonperfusion in the retinal periphery using widefield scanning laser ophthalmoscopy-assisted angiography, findings which may be used to predict which patients are likely to develop nascent neovascularization [17].
Figure 1: Histopathologic features of diabetic retinopathy.
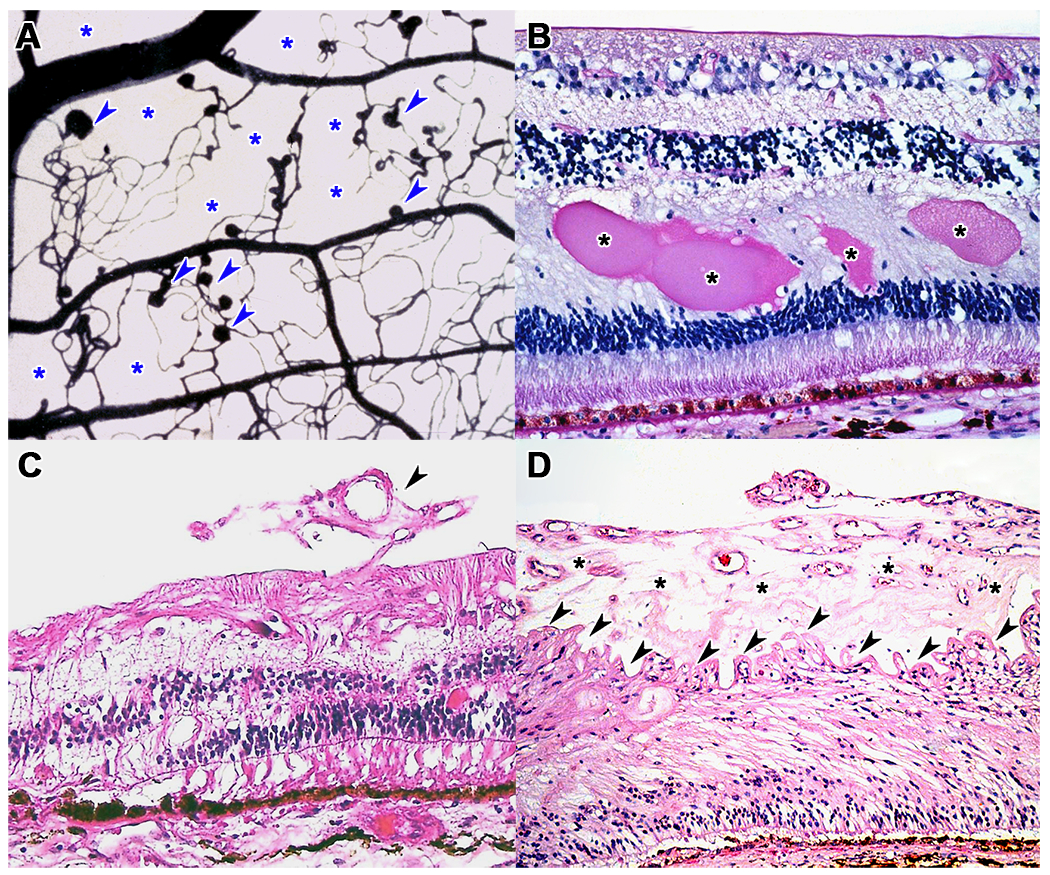
(A) Trypsin digest from a human donor, stained with India ink, showing numerous microaneurysms (arrowheads) and large areas of capillary dropout (asterisks). (B) Hematoxylin and eosin stain showing lipoproteinaceous exudates in the outer plexiform layer (asterisks) in a patient with diabetic macular edema. (C) Early neovascular tuft (arrowheads) in an eye with early proliferative diabetic retinopathy. (D) Thick preretinal neovascular sheet (asterisks) with wrinkling of the ILM (arrowheads) in a patient with advanced proliferative diabetic retinopathy.
Damage to the retinal microvasculature in diabetes is a central focus of pathophysiological studies of DR. Early histopathologic findings such as basement membrane thickening are directly linked to hyperglycemia. Increased glucose flux into polyol-hexosamine pathways, formation of advanced glycated endproducts (AGE), and glucose-induced diacylglycerol (DAG)-protein kinase C (PKC) activation are potential mechanisms whereby retinal damage in diabetes occurs. Sugar alcohols, such as sorbitol, are produced in the polyol-hexosamine pathway regulated by the rate-limiting enzyme aldose reductase. Sorbitol is a cell-impermeable sugar that accumulates in retinal cells and damages tissue via osmotic stress [18]. Sorbitol dehydrogenase exacerbates the effects of sorbitol by converting it to fructose, a sugar with tenfold more glycation activity than glucose [19], and by causing oxidative stress through depletion of nicotinic acid-containing co-enzymes. AGEs commonly detected in human diabetes include carboxy-ethyl-lysine, carboxy-methyl-lysine, and pentosidine, which all have the potential to directly damage vascular basement membranes [20]. Increased plasma levels of carboxy-ethyl-lysine and pentosidine conferred a ~7 fold greater risk of developing diabetes complications in the Joslin Medalist Study, which examined non-canonical risk factors among patients surviving 50 years or more with a diagnosis of T1DM [21]. By binding receptors for AGE (RAGE), these compounds promote inflammation, increase vascular permeability, and stimulate angiogenesis [20]. Increases in glycolytic flux from chronic hyperglycemia result in upregulation of DAG, which in turn activates multiple isoforms of PKC. These serine/threonine kinases exert multiple activities on endothelial cells to promote angiogenesis and have been long-studied targets of drug discovery in diabetes because of their strong association with macrovascular and microvascular endpoints (reviewed in [22]).
Passage of macromolecules into the retina from the bloodstream is tightly controlled by a blood-retina-barrier (BRB), comprised of inner (retinal vasculature) and outer (retinal pigment epithelium) components. DR causes loss of BRB integrity, resulting in angiographic leakage and exudation of plasma macromolecules into the retina – especially plasma lipid. Intercellular tight junctions are a major contributor to the highly selective nature of the BRB, allowing only selective transport along regulated transcellular and paracellular routes [23]. Dysfunction of key components of these junctions, including connexin-43, occludin, claudins and zonula occludens-1 (ZO-1) proteins, contributes to BRB loss (reviewed in [24]). In T1DM animal models, hyperglycemia alone is sufficient to activate Notch1 signaling to induce dissociation of other components of the BRB, namely vascular endothelial cadherin and beta-catenin [25]. Pericytes – mural cells found in a 1:1 ratio with retinal endothelial cells – are another integral component of the BRB [26]. Pericytes are required for development of the blood-brain-barrier [27], a structure with similar properties as the BRB, and selective genetic ablation of pericytes from endothelial tissues reproduces the retinal microvascular abnormalities typical of DR [28]. A transporter of docosahexaenoic acid (DHA) into the retina, called Mfsd2a, was recently identified as the major regulator of transcellular flux through the BRB. Though Mfsd2a function in DR is unknown, dysregulation of very long chain polyunsaturated fatty acid (VLC-PUFA) metabolism, including that of DHA, is a common finding in human and experimental DR [29]. Furthermore, interventions that increase retinal VLC-PUFA reduce severity of experimental DR [30], and some of these benefits are due to direct effects on BRB integrity [31]. Molecular changes leading to BRB dysfunction in diabetes could be secondary to local tissue ischemia itself since a mouse model of ischemia-reperfusion injury, involving transient induction of high pressure intraocular tamponade, recapitulates many aspects of experimental DR such as capillary and pericyte dysfunction as well as loss of occludin and ZO-1 complexes [32–34].
Muller cells are multifunctional glia spanning across the entire width of the retina and are an early target of damage in diabetes [35]. They simultaneously contact and communicate with retinal neurons and blood vessels, making them principle cells in the neurovascular unit [36]. These cells are also very likely involved in maintenance of the BRB, as selective ablation in mice leads to severe retinal vascular leakage [37]. Muller cells actively assist in neurotransmission by taking up and detoxifying synaptic glutamate [38] and by redistributing potassium ions from the synapses (via the inward rectifier channel Kir2.1) to the vasculature and into the vitreous (via Kir4.1) [39]. Diabetes has an impact on both processes to promote neurodegeneration and interstitial fluid imbalance, since potassium flux is a major contributor to osmotic balance in the retina. Accordingly, electron microscopy of eyes of patients with DME shows widespread cytoplasmic swelling within Muller cells [40]. Immunohistochemistry from experimental and clinical samples indicates that Muller cells become reactive early in the course of disease, strongly expressing the marker glial fibrillary acidic protein (GFAP) [41]. The significance of GFAP upregulation in DR is unknown but reactive gliosis could be an important requisite for Muller cell-derived VEGF release, an important source of pro-angiogenic signals in proliferative retinopathy [42]. In addition, Muller cells reacting to diabetes promote a pro-inflammatory milieu by secreting key cytokines such as interleukin-1b, tumor necrosis factor-a, and interleukin-6 and have the potential to contribute to late stage fibrosis [43].
Muller cells serve as an integral bridge between retinal neurons and the blood vessels that feed them. This process, referred to as neurovascular coupling or functional hyperemia, describes how metabolic demand of neural tissues governs blood flow through their dependent vessels [36]. Neurovascular coupling forms the basis of functional neuroimaging, which itself can be performed in the retina. In healthy retina, light stimulation causes vasodilation –detectable by real-time retinal vessel analysis, or by OCT-A. Muller cells respond to neural stimuli by releasing vasoactive arachidonic acid metabolites, nitric oxide, adenosine, and lactate [44]. Patients with diabetes display less vascular reactivity to light [45, 46], a finding recapitulated in an animal model of type 1 DM [47]. Increased Muller cell expression of inducible nitric oxide synthase (iNOS), with resulting elevations in local nitric oxide concentration, is one mechanism accounting for these observed decreases in light-evoked vascular reactivity in the diabetic retina. Accordingly, aminoguanidine – an inhibitor of iNOS – effectively restores retinal neurovascular coupling in diabetic animals when given both acutely and chronically [48].
Diabetes-induced damage to the retina is also detectable by measures of visual function, long before the onset of typical DR. Over half a century ago, Yonemura and colleagues detected delays in the scotopic oscillatory potentials of patients with diabetes but no retinopathy using full-field electroretinography (ERG), when compared to healthy controls. Multiple groups subsequently reported similar effects on inner retinal function in patients with diabetes with normal funduscopic appearance [49–54]. Similarly, prolongation of the implicit time of the oscillatory potentials (another indicator of inner retinal function), is consistently observed in multiple mouse models of diabetes, and precedes detectable retinal vasculopathy by several months [55, 56]. Bresnick and Palta, using patients enrolled in the Early Treatment Diabetic Retinopathy Study (ETDRS), found that the sum of oscillatory potential amplitudes, in addition to more traditional factors such as baseline DR severity, was a significant predictor of progression to severe PDR [57]. In one intriguing study, multifocal ERG implicit time delay was used to predict specific macular locations of later DR development with 86% sensitivity and 84% specificity at 1 year [58].
Aside from ERG, other measures of visual function are also abnormal early in diabetes. Visual field testing by frequency doubling technology detected significant deficits in patients with diabetes but no retinopathy [59]. Other measures of visual function, including color vision [60], contrast sensitivity [61], rate of dark adaptation [62], and pupillary light responses [63] are impaired in diabetes before the development of frank DR. From a structural standpoint, selective inner retinal thinning is observed by OCT in patients with diabetes, even without nascent DR [64]. Disorganization of the retinal inner layers (DRIL) is correlated with vision loss in DR independently of macular edema. Diabetic neuroretinopathy – retinal dysfunction preceding vasculopathy in diabetes – therefore represents a potential target for early interventions that prevent or reduce the need for more exhaustive treatments at later stages [65–67].
The neural retina is commonly considered an innocent bystander in diabetes, impacted slowly by progressive insults to the blood vessels feeding them until they are ultimately pruned of their streams of nutrients and oxygen when outright vision loss ensues [68]. This model is attractive because our best weapons against DR, the anti-VEGF agents, improve vascular health and prevent vision loss [69–72]. However, this model does not explain why particular capillary beds – namely those of the retina, kidneys and peripheral nerves – are more likely to be damaged by diabetes than others. Kern and Engerman, in a classic description of retinopathy in a canine model of experimental diabetes, observed damage in capillaries from the retina but not in those of the cerebral cortex in the same animals [73]. They surmised that this phenomenon could be due to a permissive “local factor” contained within the retina – a hypothesis also raised by Cogan, Toussaint and Kuwabara over thirty years earlier [74].
In the years since such seminal studies, we have learned much about the unique metabolic demands of the neural retina [75–77]. Photoreceptors are among the most metabolically demanding cells in the body and such a metabolic signature may be the “local factor” accounting for the exquisite vulnerability of the retina in diabetes. It is therefore possible that these first cells in the visual pathway are a primary target of diabetes. The neural retina itself is an insulin-responsive tissue [78, 79], and therefore could be directly susceptible to pathology in a disease impacting multiple metabolic pathways controlled by insulin signaling.
Consistent with this notion, evidence in mice and in humans suggests that the very first cell in the visual circuit – the photoreceptor – is impacted by diabetes [80–82]. In rodent models, reactive oxygen species accumulate in the outer nuclear layer after induction of experimental diabetes by streptozotocin [83]. These changes are eliminated in mice lacking rhodopsin or in mice carrying a pathogenic P23H knock-in mutation, mirroring a common form of human rod dystrophy [5, 81, 84]. Since both mutants cause degeneration, the presence of rod photoreceptors may be required for DR. In support of this view, the late visual electrophysiologist Geoffrey Arden noted an absence of retinopathy in patients with coexisting diabetes and retinitis pigmentosa (RP) [80]. Furthermore, by virtue of their need to maintain membrane depolarization in the absence of stimulus, photoreceptors are thought to consume more adenosine triphosphate (ATP) in the dark compared to the light [77]. Arden therefore hypothesized that dark conditions (e.g., during night-time sleep in humans) would exacerbate diseases that induce metabolic stress, such as diabetes. By contrast, light-adapted conditions would improve DR [85]. In line with his hypothesis, light-based DR therapies are currently under clinical investigation (discussed in further detail later).
Clinical and Experimental Interventions
Both systemic and ocular interventions are used to prevent vision loss or to salvage vision lost due to diabetes. Preventative measures tend to be systemic, though recent clinical evidence suggests an important role for intravitreous VEGF antagonism in reversing DR severity [6, 8, 86, 87]. Missing from the current repertoire of DR therapeutics are those specifically targeting early DR and subclinical DR – a phase which is not easily measured using standard clinical tools. Advances in retinal imaging and clinical visual function diagnostics are facilitating the detection of subclinical DR, setting the stage for novel points of intervention in this disease. A summary of therapies currently within clinical testing phases for diabetic eye disease is provided in Table 1.
Table 1.
Therapies currently under clinical investigation for the treatment of various forms of diabetic retinopathy
| Drug | Target/Mechanism | Administration | Disease | Phase | Result | Clinical trial |
|---|---|---|---|---|---|---|
| Propranolol | Decreased NV | Oral | PDR | 1 | No improvement [1] | NCT01535495 |
| Doxycycline | Anti-inflammatory | Oral | Severe NPDR/low risk PDR | 2 | Increased foveal sensitivity [2] | NCT00511875 |
| Octreotide | Multiple (regulation of hormone secretion, inhibition of vascular remodeling and angiogenesis) | Subcutaneous | severe NPDR/low risk PDR | 3 | Decreased progression in euthyroid patients from severe NPDR to PDR requiring laser [3] | NCT00130845 |
| AKB-9778 | Tie2 activation | Subcutaneous | NPDR | 2 | Did not improve DRSS by two or more steps vs placebo [4] | NCT03197870 |
| Emixustat | RPE65 inhibitor | Oral | PDR | 2 | Decreased DME [5] | NCT02753400 |
| Canakinumab | IL-1β inhibitor | Subcutaneous | PDR | 1 | No progression of PDR, stable edema [6] | NCT01589029 |
| Riboxistaurin | PKCβ inhibitor | Oral | CSME | 3 | Reduced vision loss, DME, and need for laser [7] | NCT00133952 |
| Brimonidine + somatostatin | Brimonidine: α2 agonist Somatostatin: decreased NMDA-dependent Ca2+ influx |
Topical | DR | 2+3 | No improvement in neurodegeneration. Somatostatin improves microvascular disease. [8] | NCT01726075 |
| PAN-90806 | VEGFR2 inhibitor | Topical | PDR | 1 | No results | NCT02475109 |
| ALG-1001 | Integrin inhibitor | Intravitreous | DME | 2 | Noninferiority to bevacizumab [9] | NCT02348918 |
| Conbercept | Binds VEGF-A and VEGF-B | Intravitreous | DME (and an ongoing study for PDR) | 3 | Similar clinical efficacy to ranibizumab [10, 11] | NCT02194634 |
| Melatonin | Anti-oxidant, anti-inflammatory | Oral | DR | 3 (currently recruiting) | Currently recruiting for humans, anti-inflammatory in rats [12] | NCT03478306 |
| Aminoguanidine | iNOS inhibitor; improvement of neurovascular coupling | Oral | DR | Animal-only | Decreased vascular damage [13] | |
| Sinemet | Dopamine deficiency leading to inner retinal rod-driven dysfunction in mice | Oral | DR | 1 (currently recruiting) | No results | |
| Sirolimus | mTOR inhibition | Subconjunctival | DME | 1/2 | Safe; no consistent treatment effect [14] | NCT00711490 |
| Squalamine | VEGF and platelet-derived GF inhibitor | Topical | PDR | 2 | No results yet. Improved NV in rat model [15] | NCT01769183 |
| Noctura | Decrease nighttime hypermetabolism | Light mask | DME/PDR | multiple, including 3 | No significant long-term benefit [16] | |
| Candesartan | ARB | Oral | DM | Decreased retinopathy progression without decreased incidence of PDR or DME [17] | NCT00252720 | |
| FOV2304 | Kinin B1 inhbiitor | Topical | DME | 2 | No results. Improved DR in rats [18] | NCT01319487 |
| THR-687 | Integrin inhibitor | Intravitreous | DR/DME | 1 (currently recruiting) | No results | NCT03666923 |
| THR-149 | Plasma kallikrein inhibitor | Intravitreous | DR/DME | 1 | To be released Q3 2019 | NCT03511898 |
| THR-317 | PIGF inhibitor | Intravitreous | DR/DME | 2 | To be released Q3 2019 | NCT03071068 |
| PF-04523655 | siRNA targeting RTP801 | Intravitreous | DR/DME | 2 | Dose-dependent trend toward improvement vs focal/grid laser [19] | NCT00701181 |
| Darapladib | Phosophlipase A2 inhibitor | Oral | DME | 2 | Improvement in DME and BCVA from baseline; not compared to placebo [20] | NCT01506895 |
| Ziv-aflibercept | Higher-osmolarity aflibercept | Intravitreal | DME | 2/3 | Improved DME [21] | NCT02772497 |
| Brolucizumab | Single-chain Ab fragment against VEGF-A | Intravitreal | DME | 3 | Recruiting | NCT03481660 |
| CD34+ | Engraft damaged retina | Intravitreal | DR (among others) | 1 | No results from patients with DR | NCT01736059 |
Plasma Glucose-Directed Therapy
Diabetes therapy, guided by measures of plasma glucose, prevents both incidence and progression of DR in type 1 and type 2 diabetes (T1DM and T2DM, respectively) [88, 89]. DR severity is directly related to mean glycated hemoglobin index (HbA1c) and tight glucose control has durable benefits for slowing or preventing DR, even with subsequent lax control [90]. Molecular mechanisms driving this phenomenon – known as “metabolic memory” – are incompletely understood. The converse effect also occurs, since periods of poor glycemic control are associated with worse long term DR outcomes even when glucose is corrected in the interim. Regarding short term effects, rapid reduction of plasma glucose in the DCCT trial was associated with paradoxical early worsening of DR severity – a transient phenomenon with unresolved mechanisms of action [91, 92].
In recent years, treatment options for all forms of diabetes have rapidly expanded. In T1DM, effective metabolic control can be achieved by the combination of automated insulin pumps (and soon to be released dual-hormone systems incorporating glucagon, achieving far superior outcomes in terms of plasma glucose variability) coupled to continuous glucose monitoring systems in a closed loop circuit. In T2DM, multiple new classes of antihyperglycemics such as dipeptidyl peptidase-4 (DPP4) inhibitors, glucagon-like peptide-1 receptor agonists (GLP-1RA), and sodium/glucose cotransporter 2 (SGLT2) inhibitors are proving highly effective at reducing hyperglycemia. GLP-1RAs may have direct benefits in the retina, since GLP-1 receptors are expressed in photoreceptors and since topical administration of these agents have direct retinal neuroprotective functions in experimental diabetes models [93]. In SUSTAIN6 and LEADER, HbA1c lowering was evaluated among T2DM patients treated with semaglutide and liraglutide, respectively [94, 95]. Both trials demonstrated robust primary endpoint efficacy and have beneficial effects for macrovascular outcomes. However, both studies also showed concerning increases in DR-associated adverse events among patients receiving GLP-1RAs. DCCT also reported similar paradoxical early worsening of DR severity, but the majority of patients experiencing this phenotype did not have sustained effects, had higher baseline HbA1c and had worse baseline DR severity scores [91]. Neither SUSTAIN6 nor LEADER followed patients long enough (each was a 2 year study) to determine if worse DR scores among patients in the GLP-1RA groups were sustained or transient, or if they eventually translated to loss of vision and neither study separately examined subgroups of patients based on initial DR severity score.
Compelling evidence for glucose-centric mechanisms in early DR pathology led to the development of several drugs targeting these pathways. In animal models, aldose reductase inhibitors effectively reduce experimental DR [96]. However, in phase 3 studies, the aldose reductase inhibitor sorbinil failed to halt progression of DR [97]. A more potent and selective aldose reductase inhibitor, ARI-809, is effective in diabetic rats [98], but remains untested in humans. The PKC inhibitor, riboxistaurin, showed efficacy in phase 2 trials, but failed to inhibit progression to proliferative diabetic retinopathy in a subsequent phase 3 trial and was therefore largely abandoned as a DR therapeutic [99]. This is unfortunate, since the drug reduced moderate visual loss by 40%, with benefits first detected after 18 months of therapy [100].
Control of Hypertension
In the UKPDS study, reduction of severe hypertension reduced DR [89], though intensive hypertensive control in the ACCORD trial did not have similar benefits [101]. Multiple mechanisms could contribute to effects of blood pressure on DR, including direct biophysical effects. Hypertension could exacerbate diabetes-associated hemodynamic endothelial damage [102]. Activation of the renin-angiotensin-aldosterone axis in systemic hypertension perturbs local retinal signaling [103], and blockade of this pathway with angiotensin converting enzyme inhibitors or angiotensin receptor blockers is particularly beneficial for DR when compared to other antihypertensive therapies [104].
Lipid-Based Therapy
Hard exudates are commonly found in DR and are likely due to leakage of plasma lipoproteins into the retina. An ETDRS sub-analysis identified an association between hard exudates and worse visual acuity, and found that lowering cholesterol and triglyceride levels decreased hard exudate formation [105]. Subsequently, poor control of all serum lipid types was found to be highly associated with incident macular edema and progression to PDR [106]. Two recent studies strongly suggest that low density lipoprotein lowering with statin therapy effectively reduces DR severity or need for treatment in DR [107, 108].
Two randomized clinical studies independently support the use of oral fenofibrate for the treatment of DR. In the Australia-based FIELD study, patients using fenofibrate plus simvastatin were nearly 30% less likely to require laser therapy for DME than controls using simvastatin alone [109]. The National Eye Institute’s ACCORD EYE study showed similar benefits with fenofibrate, with the most pronounced effects seen among patients with moderate NPDR [101]. Side effects of fenofibrate were minimal in both studies. Based on these data, Australia approved the use of fenofibrate for DR therapy as a sole indication, independent of serum triglyceride level.
Although the rationale for using fenofibrate in DR was that circulating lipid-lowering would benefit, the effects observed in the FIELD and ACCORD studies were independent of serum triglyceride levels. The mechanisms of action accounting for the beneficial effect of fenofibrate remain unresolved. The FIELD investigators themselves postulated that local retinal lipid trafficking could have been impacted by fenofibrate. In fact, the drug is a potent activator of peroxisome proliferator-activated receptor-α (PPARα), a central coordinator of cellular lipid handling and trafficking and PPARα is required for fenofibrate-associated improvement of DR endpoints. In addition, PPARα activity in these models promotes anti-inflammatory and vascular permeability-lowering activities [110]. PPARα also promotes hepatic fibroblast growth factor 21 (FGF21) secretion, which itself is an insulin-sensitizer capable of improving various outcomes in T2DM. Systemic FGF21 administration is sufficient to promote photoreceptor survival and could be interacting with VEGF to promote endothelial health in DR (data presented at the Association for Research in Vision and Ophthalmology Annual Meeting 2019). Therefore, FGF21 could be a key mechanistic link between fenofibrate and retinal health in diabetes and further elucidation of its molecular effects could offer novel therapeutic insights for DR.
In addition to correction of systemic lipoprotein dysmetabolism in diabetes, restoration of dysregulated fatty acid homeostasis in diabetes is also a feasible strategy. Notable recent examples are therapies based on modulation of retinal PUFA metabolism. Soluble epoxide hydrolase, a major catalyst of DHA degradation, negatively impacts BRB health by causing pericyte loss and endothelial damage. Inhibition of this enzyme prevented leakage in a mouse model of DR and is being investigated as clinical therapeutic [111]. In the PREDIMED prospective trial investigating beneficial effects of Mediterranean diet on retinopathy outcomes, dietary supplementation with long-chain PUFA was associated with a nearly 50% decrease in risk of developing sight-threatening DR [112], a finding with substantial biological plausibility based on animal studies [30, 31, 113]. Therefore, modulation of PUFA metabolism by dietary or pharmacologic interventions could become an important component of DR management.
Photocoagulation
Citing recent data, some retinal specialists have suggested that vasogenic antagonism could entirely supplant laser photocoagulation. However, this therapy remains an important part of the DR treatment armament because of their lasting benefits. Macular photocoagulation is postulated to close microaneurysms and stimulate the RPE, but evidence supporting these mechanisms is lacking. It more is likely that macular photocoagulation works in an identical fashion as peripheral pan-retinal photocoagulation (PRP), namely by ablating ischemic retinal tissue to reduce secretion of VEGF from hypoxic sources. Since the pioneering studies of ETDRS and DRS [114, 115], photocoagulation has remained a mainstay of DR treatment. These treatments are associated with nyctalopia, presbyopia, and laser scotoma, including reduction in peripheral field.
Corticosteroids
Inflammation is a strong contributor to DR and corticosteroids are therefore highly effective agents for treatment of DR, especially DME. A landmark study from the Diabetic Retinopathy Clinical Research Network (DRCR.net) proved efficacy of intravitreous triamcinolone in center-involving DME when combined with focal laser photocoagulation [69]. Anatomic outcomes with triamcinolone were similar to those in the group receiving ranibizumab therapy. Although long term visual acuity results were worse compared to ranibizumab, they were similar among the subset of pseudophakic patients, suggesting cataract was a limiting factor [116]. Despite the drawbacks of intraocular pressure elevation and cataract, corticosteroid therapy targeting the retina is an important option for DME refractory to initial anti-VEGF therapy, or among those who cannot tolerate anti-VEGF therapy. Most corticosteroid agents for retinal use are also attractive due to their long durations of activity. Two elegant formulations of ocular corticosteroids, a dexamethasone implant (Ozurdex) [117] and a fluocinolone implant (Iluvien) [118], have shown efficacy in the long-term treatment of DME while requiring fewer injections than the VEGF antagonists [119]. DRCR.net protocol U showed improved central retinal thickness in patients administered Ozurdex implantation with persistent DME despite regular ranibizumab injections, though it failed to improve visual outcomes at 24 weeks [116]. Sustained delivery of corticosteroid via injectable implants is also effective in treating chronic DME, a disease state that tends to be refractory to therapy. Among patients with chronic DME despite prior macular photocoagulation, therapy with fluocinolone implant delivering 0.2 microgram/day more than doubled the number of patients gaining 15 or more letters after 3 years of treatment compared to sham-treated controls [120]. Similarly, patients with chronic DME (mean ~23–25 months) receiving dexamethasone injectable implants were >50% more likely to gain 15 letters of visual acuity than sham-treated controls after 3 years of therapy [117]. When combined with the reduced efficacy of anti-VEGF therapy seen following crossover in the RISE/RIDE and VISTA/VIVID studies, evidence supports a critical role of inflammatory mediators in chronic DME [121, 71]. Phakic patients receiving these long-term steroid treatments invariably develop cataract and some go on to require glaucoma surgery. Fortunately, both side-effects are highly manageable with modern ophthalmic techniques.
Newer, more targeted anti-inflammatories are in development for DR therapy. For instance, ALG-1001 (risuteganib) is an anti-integrin peptide that theoretically would prevent leukocyte adhesion and several subsequent downstream pathways. Initial results have shown non-inferiority to bevacizumab, though when used in combination there does not seem to be an additional benefit [122]. Additionally, despite a DRCR.net study failing to show improvement of central macular edema [123], topical NSAIDs continue to be investigated as a potential therapeutic agent.
Anti-angiogenic agents
A comprehensive map of the molecular mechanisms responsible for the pathogenesis of DR is long from being completely drawn. But all mechanisms – known and unknown – will assuredly converge upon common “executioner” pathways centered on proangiogenic growth factor secretion, principally VEGF. VEGF molecules are encoded by at least six known genes, but it is the vegf-a gene product (referred to as VEGF in this review) that is primarily implicated in regulation of normal and pathogenic ocular angiogenesis and is the main target of therapies to combat retinal neovascularization. Early vascular pathology, neuroglial dysfunction, aberrant neurovascular coupling, retinal inflammation, or a combination of any and all of these factors in the diabetic retina likely cause progressive disruption of retinal capillary flow to induce local hypoxia. Subsequently, low tissue oxygen tension prevents hydroxylation-dependent ubiquitin-mediated degradation of HIF-1α, allowing for its stabilization and activity on the genome to induce a hypoxia-driven gene expression profile – the ultimate molecular switch leading to VEGF induction [124]. VEGF, acting through canonical pro-angiogenic signals, is sufficient to promote retinal vascular permeability, and its blockade is highly effective at reducing permeability. Disintegration of the inner and outer blood-retinal barriers [125] exacerbates interstitial fluid accumulation resulting in cystoid edema and neurosensory retinal detachment. Elucidation of the processes by which hypoxia and VEGF promote neovascular growth in the retina and elsewhere was a substantial achievement of modern visual science. Details of these critical processes have been meticulously studied over the past two decades and have been summarized in recent reviews [126, 127].
VEGF antagonists are now widely used as first-line therapy for center-involving DME, and some have argued that they could be used as the initial therapy for PDR. The three most-commonly employed agents, bevacizumab, ranibizumab, and aflibercept, show similar efficacy for the treatment of DME [128 48]. For the treatment of PDR, ranibizumab [123] or aflibercept [87] are non-inferior to PRP in terms of regression of NV or change in visual acuity and both VEGF antagonists fare better than PRP with regard to peripheral field loss. However, anti-VEGF agents have important limitations. Because of their ocular bioavailability these agents require multiple injections, delivered monthly in some cases. Each injection carries a low risk of endophthalmitis and may be associated with mild and transient ocular discomfort [129]. Costs to the patient and the system are also a factor: aflibercept and ranibizumab combine to account for 12% of all Medicare Part B spending annually [130]. Some of these limitations are being addressed by the development of VEGF antagonists with longer intravitreous half-lives or by implantation of intravitreous drug reservoirs capable of long-term continuous drug release. Brolucizumab, a single-chain antibody variable fragment capable of neutralizing all forms of VEGF-A, is effective in the treatment of exudative age-related macular degeneration [131, 132] and is currently in phase 3 testing for DME [NCT03481660]. Delivery of ranibizumab through a surgically-implanted port delivery system allowed for effective control of exudative macular degeneration in phase 2 studies, comparable to monthly ranibizumab injection, with 80% of patients going >6 months before replenishment of drug into the reservoir [NCT02510794].
In addition to VEGF, other hypoxia-responsive pathways responsible for promoting angiogenesis have recently been targeted for pharmaceutical inhibition in the treatment of DR, most notably those that are related to the tunica interna endothelial cell kinase (Tie2). Tie2 is an endothelial receptor tyrosine kinase important in preserving integrity of vasculature. Activated in a tonic manner by its ligand angiopoietin 1 (Ang1), Tie2 promotes tight junction integrity to maintain the BRB. In a state of hypoxia, Tie2 is inactivated by angiopoietin 2 (Ang2)-mediated competitive inhibition or by vascular endothelial-protein tyrosine phosphatase (VE-PTP)-mediated dephosphorylation. Both of these hypoxia-dependent inhibitory events promote vascular leakage and neovascularization [133]. Two newly-developed pharmaceuticals act on this pathway: faricimab, a humanized monoclonal antibody with specificity to both Ang2 and VEGF, and the small molecule inhibitor AKB-9778, which prevents VE-PTP catalytic activity even in the presence of abundant Ang2 [134]. In the phase 2 BOULEVARD clinical trial for treatment of center-involving DME, monthly faricimab treatment was associated with better visual acuity improvements compared to monthly ranibizumab monotherapy at 20 weeks [135]. Phase 3 trials are currently underway to compare faricimab to aflibercept (YOSEMITE and RHINE trials). AKB-9778, a subcutaneously-administered agent, demonstrated better central subfield thickness and visual outcomes when administered in combination with intravitreal ranibizumab injections compared to ranibizumab alone in the TIME-2 trial [NCT02050828], but failed to meet primary endpoint criteria of two or more step improvement in DR severity score compared to placebo in the phase 2b follow-up study [NCT03197870]. Likely as a function of its systemic delivery in the TIME-2 study, AKB-9778 improved renal function (as measured by urine albumin-to-creatinine ratio) by 21%, with controls showing no such improvement.
A curious finding in DR and other causes of retinal ischemia is the surprising inability of the reflexive retinal angiogenic response to appropriately re-perfuse the neural retina itself (rather than form preretinal neovascularization). Some semaphorins, molecules that serve as developmental guidance cues in many tissues, are induced in ischemic retina and can provide strong intraretinal repulsive cues to newly-formed blood vessels. Under their guidance, new vessels are directed into the vitreous cavity where they cause late-stage manifestations such as pre-retinal fibrosis and contracture (Figure 1). Sema3F and sema6A have been identified as specific molecules capable of guiding vascular repulsion in ischemic retina [136, 137]. In mouse models, sema3A not only serves as a repulsive angiogenic cue but also promotes BRB breakdown, whereas its antagonism improves vascular leakage in a mouse DR model [138]. These data provided biological plausibility for an upcoming line of therapy for DR and other ischemic retinopathy based on sema3A antagonism, and are currently only in preclinical phases.
A common theme among the therapies described above is that they all involve strategies intrinsic to the retina or to the retinal vessels themselves. However, these strategies ignore an important role for bone marrow-derived progenitors in repair of diseased retinal vessels. Elegant work from multiple laboratories indicates that bone marrow-derived hematopoietic stem cells (HSCs) are critical contributors to vascular repair in the healthy retina and that these processes are markedly diminished in diabetes. In individuals with T1DM and T2DM, CD34+ reparative cells exhibit decreased mobility from bone marrow niches compared to healthy controls [139]. Moreover, HSCs from diabetic mice injected into the vitreous of non-diabetic mice have reduced regenerative capability compared to injected HSCs derived from healthy controls [140], suggesting intrinsic differences between these two sources of reparative HSCs. Dyslipidemia in diabetes also increases bone marrow abundance of inflammatory macrophages at the expense of valuable reparative stem cells. Based on these collective findings, there is great interest in bone marrow rejuvenating therapies for DR. Some of these include modulation of acid sphingomyelinase [140] or liver X receptor, to correct DM-associated HSC lipid abnormalities, and supplementation of somatostatin, to correct diabetes-induced dysautonomia to restore appropriate hematopoiesis. Cell-based therapy is also an exciting experimental treatment option for DR (reviewed in [141]), and intravitreous injection of CD34+ HSCs is currently in phase 1 testing for DR, non-exudative macular degeneration and retinal vein occlusion [NCT01736059].
Therapy for Diabetic Neuroretinopathy
One prevailing notion explaining the exquisite vulnerability of photoreceptors and other retinal neurons to pathology in diabetes is that their high metabolic demands that predispose them to oxidative stress induced by hypoperfusion from capillary dysfunction, loss of neurovascular coupling, or direct neural injuries. Rod photoreceptors, which comprise the vast majority of neurons in human and murine retinas, have greater energy requirements in the dark compared to the light. Though this finding is counterintuitive when considering energy requirements of other neurons (which are higher when stimulated, not at rest), photoreceptors are unique because they maintain membrane depolarization in the dark and become hyperpolarized upon engagement of photons. Therefore, photoreceptors require high levels of ATP to drive ion flux and maintain membrane potential in the dark. Limited oxygen supply stemming from diabetes-induced vascular compromise could therefore exacerbate the hypoxic stress incurred by photoreceptors in the dark. Therefore, providing constant levels of light stimulation to patients with diabetes could reduce DR. To this end, clinical trials in patients with DR re currently testing the efficacy of light masks emitting low level photonic emission worn during sleep to reduce eye disease. These masks showed improvements in DR measures in early phase clinical trials [142, 143], however the phase 3 CLEOPATRA trial using a light mask for the therapy of DME failed to meet its primary endpoint [144].
Rather than using light stimulation, pharmacologic strategies to shift photoreceptors into metabolic states requiring less energy in diabetes have been attempted. One such approach posits that visual cycle blockade could mimic the effects of providing low-luminance constant background light stimulation to human eyes by limiting chromophore supply and promoting constitutive apo-opsin signaling. Emixustat, a visual cycle inhibitor targeting the isomerohydrolase RPE65, is currently in phase 3 testing for PDR, though initial results do not support its use for this stage of disease. The choice of the sponsor to target such a late stage of DR for this intervention is puzzling and unfortunate, but does leave an open question as to whether the drug could be more effective when delivered earlier in the course of disease (perhaps before the onset of microvascular lesions).
Another byproduct of the high metabolic demands of photoreceptors is a tendency to generate oxidative stress. Many lines of evidence in mouse models of DR suggest that reactive oxygen species (ROS) accumulate in multiple retina layers, especially the outer retina. ROS promiscuously damage multiple cellular pathways and contribute to destruction of retinal tissue. ROS may be generated in diabetes via an inability of cells to accommodate increased glucose metabolism, leading to decreased glyceraldehyde 3-phosphate dehydrogenase, increased poly ADP ribose polymerase, increased superoxide radicals, and accumulation of glucose metabolic byproducts [145]. Protein Kinase C, polyol/AGE, hexosamine, and matrix metalloproteinases are all activated by accumulation of ROS and could be important effectors of its deleterious actions. Topical therapy with elamipretide (MTP-131, ocuvia), which targets mitochondrial cardiolipin to improve electron transfer efficiency and reduce oxidative stress, reversed visuomotor dysfunction in mouse models of DR without altering diabetes severity [146]. In the ReVIEW study, a phase 1/2 unmasked, single arm trial, twice daily topical elamipretide for one month was associated with ~30% improvement in central macular thickness compared to baseline measurements [NCT02314299]. The drug recently completed phase 2 testing for DME, with results pending [NCT02348918].
Aside from metabolic strategies, therapies that directly promote neural survival during stress could benefit the diabetic retina. Neuroprotection has been a long-sought goal for a multitude of neurological diseases including those due to trauma, ischemia, and chronic degeneration. Preclinical and clinical evidence suggests that neuroretinal injury due to diabetes could be both ischemic and degenerative, making this disease potentially amenable to a variety of neuroprotective strategies. Both somatostatin and brimonidine have shown some efficacy in arresting diabetic neuroretinopathy as measured by multifocal ERG P1 implicit times (EUROCONDOR trial [147]). Topical therapy with a GLP-1RA also showed improvement of retinal function in a mouse model of DR, but is not yet in clinical trials as a local retinal therapy.
Replacement of another key retinal neurotransmitter – dopamine – has exciting potential for neuroprotection in DR. Diabetes causes deficiency of retinal dopamine and dopaminergic retinal neurons, specifically amacrine cells [148, 149]. Though mechanisms causing these specific deficiencies are still being investigated, dopamine replacement corrects aspects of visual dysfunction in diabetes [150]. A phase 1 clinical trial using carbidopa/levodopa, a drug approved for symptomatic therapy in Parkinson’s disease, is currently underway [NCT02706977]. A unique feature of this study is its use of two visual function endpoints as its primary outcome: implicit time of photopic response measurements using a novel handheld ERG device (RETeval), and contrast sensitivity (measured using a tablet application). Therefore, this study could be the first DR interventional clinical trial to use diabetic neuroretinopathy outcome measures as opposed to traditional endpoints, such as central visual acuity, OCT-assessed macular thickness, or DR severity scores based on vascular features.
Conclusions
Recent improvements in treating diabetic retinopathy have paralleled advancements in understanding the pathophysiologic mechanisms responsible for disease onset and progression. Current pharmacotherapies, primarily VEGF pathway inhibitors, used as monotherapy or in combination with glucocorticoids and laser treatment are highly effective in treating late stages of DR and have collectively decreased incidence of diabetes-related vision loss, despite the expanding epidemic of diabetes. Future therapies for DR are being directed at earlier pathophysiology involving preclinical neuroretinal disease of the retina, in addition to those targeting later stages of retinal microvascular pathology. These include novel treatment for diabetes, such as the GLP-1RAs and fenofibrate, which may have salutary effects directly on the retina in addition to those affecting systemic metabolism. In addition to glucose-centric strategies to diabetes management, those that target dyslipidemia are also likely to play a larger role in preventing complications such as DR. For patients who remain unfortunate enough to develop DR despite these new preventative approaches, future ocular therapy will very likely evolve to address more aspects of the multitude of physiologic insults imparted by diabetes on the neural and vascular retina, such as impaired vascular reparative capability, aberrant angiogenic and pro-permeability drive, fatty acid and sterol dysregulation, exacerbation of hypoxia and ROS generation due to photoreceptor energy demand, neurodegeneration, and misdirected signals within the neurovascular unit.
Acknowledgments
Eric Nudleman reports grants from the National Eye Institute (5K08EY028999-02).
Rithwick Rajagopal reports grants from the National Eye Institute (K08EY025269), and grants from Research to Prevent Blindness (Career Development Award).
Footnotes
Conflict of Interest
Avinash Honasoge, Eric Nudleman, Morton Smith, and Rithwick Rajagopal declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers published within the last three years which are of particular interest have been annotated as important (•) or very important (••).
- 1.Centers for Disease Control and Prevention: National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States, 2017. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed June 3, 2019.
- 2.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116(3):497–503. doi: 10.1016/j.ophtha.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson JT, de Bustros S, Michels RG, Rice TA. Results and prognostic factors in vitrectomy for diabetic traction retinal detachment of the macula. Archives of ophthalmology. 1987;105(4):497–502. [DOI] [PubMed] [Google Scholar]
- 4.Abunajma MA, Al-Dhibi H, Abboud EB, Al Zahrani Y, Alharthi E, Alkharashi A et al. The outcomes and prognostic factors of vitrectomy in chronic diabetic traction macular detachment. Clinical ophthalmology. 2016;10:1653–61. doi: 10.2147/OPTH.S98555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. The New England journal of medicine. 1994;331(22):1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 6.Ip MS, Domalpally A, Sun JK, Ehrlich JS. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology. 2015;122(2):367–74. doi: 10.1016/j.ophtha.2014.08.048. [DOI] [PubMed] [Google Scholar]
- 7.•.Mitchell P, McAllister I, Larsen M, Staurenghi G, Korobelnik JF, Boyer DS et al. Evaluating the Impact of Intravitreal Aflibercept on Diabetic Retinopathy Progression in the VIVID-DME and VISTA-DME Studies. Ophthalmology Retina. 2018;2(10):988–96. doi: 10.1016/j.oret.2018.02.011. [DOI] [PubMed] [Google Scholar]; In a sub-analysis of the phase 3 VIVID and VISTA studies in which patients with center-involving DME were treated with two different regimens of aflibercept, more patients receiving aflibercept experienced 2-step improvement in DR severity score compared to those in the laser control arm.
- 8.•.Wykoff CC, Eichenbaum DA, Roth DB, Hill L, Fung AE, Haskova Z. Ranibizumab Induces Regression of Diabetic Retinopathy in Most Patients at High Risk of Progression to Proliferative Diabetic Retinopathy. Ophthalmology Retina. 2018;2(10):997–1009. doi: 10.1016/j.oret.2018.06.005. [DOI] [PubMed] [Google Scholar]; In a post hoc analysis of the phase 3 RISE and RIDE trials in which patients with center-involving DME were treated with two different doses of ranibizumab, more patients receiving ranibizumab experienced 2-step improvement in DR severity score compared to those in the laser control arm. Effects were greatest among patients with baseline severity score of 47/53.
- 9.Ashton N Vascular changes in diabetes with particular reference to the retinal vessels; preliminary report. The British journal of ophthalmology. 1949;33(7):407–20. doi: 10.1136/bjo.33.7.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cogan DG. Diabetic Retinopathy. The New England journal of medicine. 1964;270:787–8. doi: 10.1056/NEJM196404092701508. [DOI] [PubMed] [Google Scholar]
- 11.Toussaint D, Cogan DG, Kuwabara T. Extravascular lesions of diabetic retinopathy. Archives of ophthalmology. 1962;67:42–7. [DOI] [PubMed] [Google Scholar]
- 12.de Carlo TE, Chin AT, Bonini Filho MA, Adhi M, Branchini L, Salz DA et al. Detection of Microvascular Changes in Eyes of Patients with Diabetes but Not Clinical Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Retina. 2015;35(11):2364–70. doi: 10.1097/IAE.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 13.•.Dimitrova G, Chihara E, Takahashi H, Amano H, Okazaki K. Quantitative Retinal Optical Coherence Tomography Angiography in Patients With Diabetes Without Diabetic Retinopathy. Investigative ophthalmology & visual science. 2017;58(1):190–6. doi: 10.1167/iovs.16-20531. [DOI] [PubMed] [Google Scholar]; In this OCT-A analysis of parafoveal vascular parameters, superficial and deep retinal vessel density was decreased in patients with diabetes and no retinopathy compared to healthy subjects.
- 14.Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y. Enlargement of Foveal Avascular Zone in Diabetic Eyes Evaluated by En Face Optical Coherence Tomography Angiography. Retina. 2015;35(11):2377–83. doi: 10.1097/IAE.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 15.Scarinci F, Nesper PL, Fawzi AA. Deep Retinal Capillary Nonperfusion Is Associated With Photoreceptor Disruption in Diabetic Macular Ischemia. American journal of ophthalmology. 2016;168:129–38. doi: 10.1016/j.ajo.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang TS, Zhang M, Bhavsar K, Zhang X, Campbell JP, Lin P et al. Visualization of 3 Distinct Retinal Plexuses by Projection-Resolved Optical Coherence Tomography Angiography in Diabetic Retinopathy. JAMA ophthalmology. 2016;134(12):1411–9. doi: 10.1001/jamaophthalmol.2016.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baxter SL, Ashir A, Nguyen BJ, Nudleman E. Quantification of Retinal Nonperfusion Associated With Posterior Segment Neovascularization in Diabetic Retinopathy Using Ultra-Widefield Fluorescein Angiography. Ophthalmic surgery, lasers & imaging retina. 2019;50(2):86–92. doi: 10.3928/23258160-20190129-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenzi M The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res. 2007;2007:61038. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gugliucci A Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv Nutr. 2017;8(1):54–62. doi: 10.3945/an.116.013912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zong H, Ward M, Stitt AW. AGEs, RAGE, and diabetic retinopathy. Current diabetes reports. 2011;11(4):244–52. doi: 10.1007/s11892-011-0198-7. [DOI] [PubMed] [Google Scholar]
- 21.Sun JK, Keenan HA, Cavallerano JD, Asztalos BF, Schaefer EJ, Sell DR et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50-year medalist study. Diabetes care. 2011;34(4):968–74. doi: 10.2337/dc10-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circulation research. 2010;106(8):1319–31. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayloo S, Gu C. Transcytosis at the blood-brain barrier. Current opinion in neurobiology. 2019;57:32–8. doi: 10.1016/j.conb.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz-Coranguez M, Ramos C, Antonetti DA. The inner blood-retinal barrier: Cellular basis and development. Vision research. 2017;139:123–37. doi: 10.1016/j.visres.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miloudi K, Oubaha M, Menard C, Dejda A, Guber V, Cagnone G et al. NOTCH1 signaling induces pathological vascular permeability in diabetic retinopathy. Proceedings of the National Academy of Sciences of the United States of America. 2019. doi: 10.1073/pnas.1814711116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart PA, Tuor UI. Blood-eye barriers in the rat: correlation of ultrastructure with function. The Journal of comparative neurology. 1994;340(4):566–76. doi: 10.1002/cne.903400409. [DOI] [PubMed] [Google Scholar]
- 27.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–6. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enge M, Bjarnegard M, Gerhardt H, Gustafsson E, Kalen M, Asker N et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. The EMBO journal. 2002;21(16):4307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tikhonenko M, Lydic TA, Wang Y, Chen W, Opreanu M, Sochacki A et al. Remodeling of retinal Fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes. 2010;59(1):219–27. doi: 10.2337/db09-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sapieha P, Chen J, Stahl A, Seaward MR, Favazza TL, Juan AM et al. Omega-3 polyunsaturated fatty acids preserve retinal function in type 2 diabetic mice. Nutrition & diabetes. 2012;2:e36. doi: 10.1038/nutd.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.•.Kady NM, Liu X, Lydic TA, Syed MH, Navitskaya S, Wang Q et al. ELOVL4-Mediated Production of Very Long-Chain Ceramides Stabilizes Tight Junctions and Prevents Diabetes-Induced Retinal Vascular Permeability. Diabetes. 2018;67(4):769–81. doi: 10.2337/db17-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]; In a mouse model of DR, correction of diabetes-induced reduction of VLC-PUFA by AAV-mediated ELOVL4 overexpression restores ceramide-dependent BRB integrity.
- 32.Abcouwer SF, Lin CM, Wolpert EB, Shanmugam S, Schaefer EW, Freeman WM et al. Effects of ischemic preconditioning and bevacizumab on apoptosis and vascular permeability following retinal ischemia-reperfusion injury. Investigative ophthalmology & visual science. 2010;51(11):5920–33. doi: 10.1167/iovs.10-5264. [DOI] [PubMed] [Google Scholar]
- 33.Muthusamy A, Lin CM, Shanmugam S, Lindner HM, Abcouwer SF, Antonetti DA. Ischemia-reperfusion injury induces occludin phosphorylation/ubiquitination and retinal vascular permeability in a VEGFR-2-dependent manner. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34(3):522–31. doi: 10.1038/jcbfm.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakahara T, Hoshino M, Hoshino S, Mori A, Sakamoto K, Ishii K. Structural and functional changes in retinal vasculature induced by retinal ischemia-reperfusion in rats. Experimental eye research. 2015;135:134–45. doi: 10.1016/j.exer.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 35.Puro DG. Diabetes-induced dysfunction of retinal Muller cells. Transactions of the American Ophthalmological Society. 2002;100:339–52. [PMC free article] [PubMed] [Google Scholar]
- 36.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26(11):2862–70. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen W, Fruttiger M, Zhu L, Chung SH, Barnett NL, Kirk JK et al. Conditional Muller cell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J Neurosci. 2012;32(45):15715–27. doi: 10.1523/JNEUROSCI.2841-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harada T, Harada C, Watanabe M, Inoue Y, Sakagawa T, Nakayama N et al. Functions of the two glutamate transporters GLAST and GLT-1 in the retina. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(8):4663–6. doi: 10.1073/pnas.95.8.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karwoski CJ, Lu HK, Newman EA. Spatial buffering of light-evoked potassium increases by retinal Muller (glial) cells. Science. 1989;244(4904):578–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fine BS, Brucker AJ. Macular edema and cystoid macular edema. American journal of ophthalmology. 1981;92(4):466–81. [DOI] [PubMed] [Google Scholar]
- 41.Mizutani M, Gerhardinger C, Lorenzi M. Muller cell changes in human diabetic retinopathy. Diabetes. 1998;47(3):445–9. [DOI] [PubMed] [Google Scholar]
- 42.Bai Y, Ma JX, Guo J, Wang J, Zhu M, Chen Y et al. Muller cell-derived VEGF is a significant contributor to retinal neovascularization. The Journal of pathology. 2009;219(4):446–54. doi: 10.1002/path.2611. [DOI] [PubMed] [Google Scholar]
- 43.Feenstra DJ, Yego EC, Mohr S. Modes of Retinal Cell Death in Diabetic Retinopathy. Journal of clinical & experimental ophthalmology. 2013;4(5):298. doi: 10.4172/2155-9570.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newman EA. Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33(11):1685–95. doi: 10.1038/jcbfm.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lasta M, Pemp B, Schmidl D, Boltz A, Kaya S, Palkovits S et al. Neurovascular dysfunction precedes neural dysfunction in the retina of patients with type 1 diabetes. Investigative ophthalmology & visual science. 2013;54(1):842–7. doi: 10.1167/iovs.12-10873. [DOI] [PubMed] [Google Scholar]
- 46.Lott ME, Slocomb JE, Shivkumar V, Smith B, Quillen D, Gabbay RA et al. Impaired retinal vasodilator responses in prediabetes and type 2 diabetes. Acta ophthalmologica. 2013;91(6):e462–9. doi: 10.1111/aos.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mishra A, Newman EA. Inhibition of inducible nitric oxide synthase reverses the loss of functional hyperemia in diabetic retinopathy. Glia. 2010;58(16):1996–2004. doi: 10.1002/glia.21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mishra A, Newman EA. Aminoguanidine reverses the loss of functional hyperemia in a rat model of diabetic retinopathy. Frontiers in neuroenergetics. 2011;3:10. doi: 10.3389/fnene.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bresnick GH, Korth K, Groo A, Palta M. Electroretinographic oscillatory potentials predict progression of diabetic retinopathy. Preliminary report. Archives of ophthalmology. 1984;102(9):1307–11. [DOI] [PubMed] [Google Scholar]
- 50.Bresnick GH, Palta M. Oscillatory potential amplitudes. Relation to severity of diabetic retinopathy. Archives of ophthalmology. 1987;105(7):929–33. [DOI] [PubMed] [Google Scholar]
- 51.Bresnick GH, Palta M. Temporal aspects of the electroretinogram in diabetic retinopathy. Archives of ophthalmology. 1987;105(5):660–4. [DOI] [PubMed] [Google Scholar]
- 52.Harrison WW, Bearse MA Jr., Ng JS, Jewell NP, Barez S, Burger D et al. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Investigative ophthalmology & visual science. 2011;52(2):772–7. doi: 10.1167/iovs.10-5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holopigian K, Seiple W, Lorenzo M, Carr R. A comparison of photopic and scotopic electroretinographic changes in early diabetic retinopathy. Investigative ophthalmology & visual science. 1992;33(10):2773–80. [PubMed] [Google Scholar]
- 54.Palmowski AM, Sutter EE, Bearse MA Jr., Fung W. Mapping of retinal function in diabetic retinopathy using the multifocal electroretinogram. Investigative ophthalmology & visual science. 1997;38(12):2586–96. [PubMed] [Google Scholar]
- 55.Aung MH, Kim MK, Olson DE, Thule PM, Pardue MT. Early visual deficits in streptozotocin-induced diabetic long evans rats. Investigative ophthalmology & visual science. 2013;54(2):1370–7. doi: 10.1167/iovs.12-10927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajagopal R, Bligard GW, Zhang S, Yin L, Lukasiewicz P, Semenkovich CF. Functional Deficits Precede Structural Lesions in Mice With High-Fat Diet-Induced Diabetic Retinopathy. Diabetes. 2016;65(4):1072–84. doi: 10.2337/db15-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bresnick GH, Palta M. Predicting progression to severe proliferative diabetic retinopathy. Archives of ophthalmology. 1987;105(6):810–4. [DOI] [PubMed] [Google Scholar]
- 58.Bearse MA Jr., Adams AJ, Han Y, Schneck ME, Ng J, Bronson-Castain K et al. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Progress in retinal and eye research. 2006;25(5):425–48. doi: 10.1016/j.preteyeres.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jackson GR, Scott IU, Quillen DA, Walter LE, Gardner TW. Inner retinal visual dysfunction is a sensitive marker of non-proliferative diabetic retinopathy. The British journal of ophthalmology. 2012;96(5):699–703. doi: 10.1136/bjophthalmol-2011-300467. [DOI] [PubMed] [Google Scholar]
- 60.Muntoni S, Serra A, Mascia C, Songini M. Dyschromatopsia in diabetes mellitus and its relation to metabolic control. Diabetes care. 1982;5(4):375–8. doi: 10.2337/diacare.5.4.375. [DOI] [PubMed] [Google Scholar]
- 61.Sokol S, Moskowitz A, Skarf B, Evans R, Molitch M, Senior B. Contrast sensitivity in diabetics with and without background retinopathy. Archives of ophthalmology. 1985;103(1):51–4. [DOI] [PubMed] [Google Scholar]
- 62.Henson DB, North RV. Dark adaptation in diabetes mellitus. The British journal of ophthalmology. 1979;63(8):539–41. doi: 10.1136/bjo.63.8.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jain M, Devan S, Jaisankar D, Swaminathan G, Pardhan S, Raman R. Pupillary Abnormalities with Varying Severity of Diabetic Retinopathy. Scientific reports. 2018;8(1):5636. doi: 10.1038/s41598-018-24015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.••.Sohn EH, van Dijk HW, Jiao C, Kok PH, Jeong W, Demirkaya N et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(19):E2655–64. doi: 10.1073/pnas.1522014113. [DOI] [PMC free article] [PubMed] [Google Scholar]; In patients without visible DR, inner retinal thinning is detectable by high-resolution OCT analysis. These changes were progressive and independent of glycemic control.
- 65.Abramoff MD, Fort PE, Han IC, Jayasundera KT, Sohn EH, Gardner TW. Approach for a Clinically Useful Comprehensive Classification of Vascular and Neural Aspects of Diabetic Retinal Disease. Investigative ophthalmology & visual science. 2018;59(1):519–27. doi: 10.1167/iovs.17-21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lynch SK, Abramoff MD. Diabetic retinopathy is a neurodegenerative disorder. Vision research. 2017;139:101–7. doi: 10.1016/j.visres.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Dijk HW, Verbraak FD, Kok PH, Stehouwer M, Garvin MK, Sonka M et al. Early neurodegeneration in the retina of type 2 diabetic patients. Investigative ophthalmology & visual science. 2012;53(6):2715–9. doi: 10.1167/iovs.11-8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. The New England journal of medicine. 2012;366(13):1227–39. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 69.Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–77.e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elman MJ, Qin H, Aiello LP, Beck RW, Bressler NM, Ferris FL 3rd et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119(11):2312–8. 10.1016/j.ophtha.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 72.The Diabetic Retinopathy Clinical Research N. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. The New England journal of medicine. 2015. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kern TS, Engerman RL. Capillary lesions develop in retina rather than cerebral cortex in diabetes and experimental galactosemia. Archives of ophthalmology. 1996;114(3):306–10. [DOI] [PubMed] [Google Scholar]
- 74.Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Archives of ophthalmology. 1961;66:366–78. [DOI] [PubMed] [Google Scholar]
- 75.••.Joyal JS, Sun Y, Gantner ML, Shao Z, Evans LP, Saba N et al. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nature medicine. 2016;22(4):439–45. doi: 10.1038/nm.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides evidence that photoreceptors oxidize fatty acids to generate ATP, in addition to using the more traditional substrate, glucose. Moreover, disruption of a key lipid sensor necessary for fatty acid uptake into photoreceptors causes abnormal retinal vascularization, suggesting this metabolic pathway could be relevant to neovascular diseases of the retina.
- 76.Kooragayala K, Gotoh N, Cogliati T, Nellissery J, Kaden TR, French S et al. Quantification of Oxygen Consumption in Retina Ex Vivo Demonstrates Limited Reserve Capacity of Photoreceptor Mitochondria. Investigative ophthalmology & visual science. 2015;56(13):8428–36. doi: 10.1167/iovs.15-17901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong-Riley MT. Energy metabolism of the visual system. Eye and brain. 2010;2:99–116. doi: 10.2147/EB.S9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reiter CE, Gardner TW. Functions of insulin and insulin receptor signaling in retina: possible implications for diabetic retinopathy. Progress in retinal and eye research. 2003;22(4):545–62. [DOI] [PubMed] [Google Scholar]
- 79.Reiter CE, Wu X, Sandirasegarane L, Nakamura M, Gilbert KA, Singh RS et al. Diabetes reduces basal retinal insulin receptor signaling: reversal with systemic and local insulin. Diabetes. 2006;55(4):1148–56. [DOI] [PubMed] [Google Scholar]
- 80.Arden GB. The absence of diabetic retinopathy in patients with retinitis pigmentosa: implications for pathophysiology and possible treatment. The British journal of ophthalmology. 2001;85(3):366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Gooyer TE, Stevenson KA, Humphries P, Simpson DA, Gardiner TA, Stitt AW. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Investigative ophthalmology & visual science. 2006;47(12):5561–8. doi: 10.1167/iovs.06-0647. [DOI] [PubMed] [Google Scholar]
- 82.Sternberg P Jr., Landers MB 3rd, Wolbarsht M. The negative coincidence of retinitis pigmentosa and proliferative diabetic retinopathy. American journal of ophthalmology. 1984;97(6):788–9. [DOI] [PubMed] [Google Scholar]
- 83.Berkowitz BA, Kern TS, Bissig D, Patel P, Bhatia A, Kefalov VJ et al. Systemic Retinaldehyde Treatment Corrects Retinal Oxidative Stress, Rod Dysfunction, and Impaired Visual Performance in Diabetic Mice. Investigative ophthalmology & visual science. 2015;56(11):6294–303. doi: 10.1167/iovs.15-16990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.••.Bavinger JC, Dunbar GE, Stem MS, Blachley TS, Kwark L, Farsiu S et al. The Effects of Diabetic Retinopathy and Pan-Retinal Photocoagulation on Photoreceptor Cell Function as Assessed by Dark Adaptometry. Investigative ophthalmology & visual science. 2016;57(1):208–17. doi: 10.1167/iovs.15-17281. [DOI] [PMC free article] [PubMed] [Google Scholar]; Clinical dark adaptometry demonstrated significant phtoreceptor and/or retinal pigment epithelial dysfunction in patients with DR, with abnormalities in dark adaption correlating to severity of disease.
- 85.Arden GB, Sidman RL, Arap W, Schlingemann RO. Spare the rod and spoil the eye. The British journal of ophthalmology. 2005;89(6):764–9. doi: 10.1136/bjo.2004.062547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.••.Gross JG, Glassman AR, Liu D, Sun JK, Antoszyk AN, Baker CW et al. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA ophthalmology. 2018;136(10):1138–48. doi: 10.1001/jamaophthalmol.2018.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this report of five year outcomes in a pivotal trial conducted by the Diabetic Retinopathy Clinical Research Network, therapy with intravitreous ranibizumab was comparable to panretinal phocoagulation in the management of PDR.
- 87.••.Sivaprasad S, Prevost AT, Vasconcelos JC, Riddell A, Murphy C, Kelly J et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389(10085):2193–203. doi: 10.1016/S0140-6736(17)31193-5. [DOI] [PubMed] [Google Scholar]; This landmark study demonstrated that intraviteous aflibercept was comparable to panretinal photocoagulation in the management of PDR.
- 88.The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. New England Journal of Medicine. 1993;329(14):977–86. doi: 10.1056/nejm199309303291401. [DOI] [PubMed] [Google Scholar]
- 89.Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–63. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- 90.Retinopathy and Nephropathy in Patients with Type 1 Diabetes Four Years after a Trial of Intensive Therapy. New England Journal of Medicine. 2000;342(6):381–9. doi: 10.1056/nejm200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Archives of ophthalmology. 1998;116(7):874–86. [DOI] [PubMed] [Google Scholar]
- 92.Feldman-Billard S, Larger E, Massin P, Standards for screeningand surveillance of ocular complications in people with diabetes SFDsg. Early worsening of diabetic retinopathy after rapid improvement of blood glucose control in patients with diabetes. Diabetes & metabolism. 2018;44(1):4–14. doi: 10.1016/j.diabet.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 93.•.Hernandez C, Bogdanov P, Corraliza L, Garcia-Ramirez M, Sola-Adell C, Arranz JA et al. Topical Administration of GLP-1 Receptor Agonists Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes. 2016;65(1):172–87. doi: 10.2337/db15-0443. [DOI] [PubMed] [Google Scholar]; Topical or systemic administration of GLP-1RAs prevented diabetes-associated neurodegeneration and gliosis in this preclinical study performed in a mouse model of T2DM.
- 94.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. The New England journal of medicine. 2016;375(19):1834–44. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 95.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. The New England journal of medicine. 2016;375(4):311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Obrosova IG, Minchenko AG, Vasupuram R, White L, Abatan OI, Kumagai AK et al. Aldose reductase inhibitor fidarestat prevents retinal oxidative stress and vascular endothelial growth factor overexpression in streptozotocin-diabetic rats. Diabetes. 2003;52(3):864–71. [DOI] [PubMed] [Google Scholar]
- 97.A randomized trial of sorbinil, an aldose reductase inhibitor, in diabetic retinopathy. Sorbinil Retinopathy Trial Research Group. Archives of ophthalmology. 1990;108(9):1234–44. [DOI] [PubMed] [Google Scholar]
- 98.Sun W, Oates PJ, Coutcher JB, Gerhardinger C, Lorenzi M. A selective aldose reductase inhibitor of a new structural class prevents or reverses early retinal abnormalities in experimental diabetic retinopathy. Diabetes. 2006;55(10):2757–62. doi: 10.2337/db06-0138. [DOI] [PubMed] [Google Scholar]
- 99.Group P- D, Aiello LP, Davis MD, Girach A, Kles KA, Milton RC et al. Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology. 2006;113(12):2221–30. doi: 10.1016/j.ophtha.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 100.Gardner TW, Antonetti DA. Ruboxistaurin for diabetic retinopathy. Ophthalmology. 2006;113(12):2135–6. doi: 10.1016/j.ophtha.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 101.Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. The New England journal of medicine. 2010;363(3):233–44. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patel V, Rassam S, Newsom R, Wiek J, Kohner E. Retinal blood flow in diabetic retinopathy. Bmj. 1992;305(6855):678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Behl T, Kotwani A. Potential of angiotensin II receptor blockers in the treatment of diabetic retinopathy. Life Sci. 2017;176:1–9. doi: 10.1016/j.lfs.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 104.Wang B, Wang F, Zhang Y, Zhao SH, Zhao WJ, Yan SL et al. Effects of RAS inhibitors on diabetic retinopathy: a systematic review and meta-analysis. The lancet Diabetes & endocrinology. 2015;3(4):263–74. doi: 10.1016/s2213-8587(14)70256-6. [DOI] [PubMed] [Google Scholar]
- 105.Chew EY, Klein ML, Ferris FL 3rd, Remaley NA, Murphy RP, Chantry K et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Archives of ophthalmology. 1996;114(9):1079–84. [DOI] [PubMed] [Google Scholar]
- 106.•.Srinivasan S, Raman R, Kulothungan V, Swaminathan G, Sharma T. Influence of serum lipids on the incidence and progression of diabetic retinopathy and macular oedema: Sankara Nethralaya Diabetic Retinopathy Epidemiology And Molecular genetics Study-II. Clinical & experimental ophthalmology. 2017;45(9):894–900. doi: 10.1111/ceo.12990. [DOI] [PubMed] [Google Scholar]; In this observational study performed in South Asian volunteers with T2DM, serum cholesterol and triglyceride abnormalities were strongly correlated to DR progression and DME.
- 107.Itoh H, Komuro I, Takeuchi M, Akasaka T, Daida H, Egashira Y et al. Intensive Treat-to-Target Statin Therapy in High-Risk Japanese Patients With Hypercholesterolemia and Diabetic Retinopathy: Report of a Randomized Study. Diabetes care. 2018;41(6):1275–84. doi: 10.2337/dc17-2224. [DOI] [PubMed] [Google Scholar]; In this large, multicenter, randomized clinical trial, high dose statin therapy to intensively decrease LDL-C levels was associated with a significant reduction in retinopathy endpoints.
- 108.Kang EY, Chen TH, Garg SJ, Sun CC, Kang JH, Wu WC et al. Association of Statin Therapy With Prevention of Vision-Threatening Diabetic Retinopathy. JAMA ophthalmology. 2019. doi: 10.1001/jamaophthalmol.2018.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370(9600):1687–97. doi: 10.1016/s0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 110.Chen Y, Hu Y, Lin M, Jenkins AJ, Keech AC, Mott R et al. Therapeutic Effects of PPARα Agonists on Diabetic Retinopathy in Type 1 Diabetes Models. Diabetes. 2013;62(1):261–72. doi: 10.2337/db11-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.••.Hu J, Dziumbla S, Lin J, Bibli SI, Zukunft S, de Mos J et al. Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature. 2017;552(7684):248–52. doi: 10.1038/nature25013. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here, soluble epoxide hydrolase was identified as a pro-permeability factor in diabetic mouse models, operating by promoting DHA breakdown. Inhibition of this enzyme improved retinal barrier function, whereas its overexpression in Muller cells exacerbated retinal leakage.
- 112.••.Sala-Vila A, Diaz-Lopez A, Valls-Pedret C, Cofan M, Garcia-Layana A, Lamuela-Raventos RM et al. Dietary Marine omega-3 Fatty Acids and Incident Sight-Threatening Retinopathy in Middle-Aged and Older Individuals With Type 2 Diabetes: Prospective Investigation From the PREDIMED Trial. JAMA ophthalmology. 2016;134(10):1142–9. doi: 10.1001/jamaophthalmol.2016.2906. [DOI] [PubMed] [Google Scholar]; In this randomized trial among patients with T2DM, dietary supplementation with 500 mg/day of long chain PUFA was associated with a 48% relative reduction in risk of sight-threatening DR after a median follow-up of 6 years.
- 113.Tikhonenko M, Lydic TA, Opreanu M, Li Calzi S, Bozack S, McSorley KM et al. N-3 polyunsaturated Fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PloS one. 2013;8(1):e55177. doi: 10.1371/journal.pone.0055177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Group ETDRSR. Early photocoagulation for diabetic retinopathy: ETDRS report number 9. Ophthalmology. 1991;98(5):766–85. [PubMed] [Google Scholar]
- 115.Group DRSR. Photocoagulation treatment of proliferative diabetic retinopathy: clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology. 1981;88(7):583–600. [PubMed] [Google Scholar]
- 116.•.Maturi RK, Glassman AR, Liu D, Beck RW, Bhavsar AR, Bressler NM et al. Effect of Adding Dexamethasone to Continued Ranibizumab Treatment in Patients With Persistent Diabetic Macular Edema: A DRCR Network Phase 2 Randomized Clinical Trial. JAMA ophthalmology. 2018;136(1):29–38. doi: 10.1001/jamaophthalmol.2017.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]; Addition of a long-acting dexamethsone implant (0.7mg) to a regimen of ranibizumab injections was associated with superior anatomic outcomes compared to ranibizumab monotherapy in this 24-month analysis among patients with chronic DME. Patients who were pseudophakic at baseline demonstrated superior visual acuity outcomes in the combination therapy group.
- 117.Boyer DS, Yoon YH, Belfort R Jr., Bandello F, Maturi RK, Augustin AJ et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–14. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 118.Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626–35.e2. doi: 10.1016/j.ophtha.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 119.Fraser-Bell S, Lim LL, Campain A, Mehta H, Aroney C, Bryant J et al. Bevacizumab or Dexamethasone Implants for DME: 2-year Results (The BEVORDEX Study). Ophthalmology. 2016;123(6):1399–401. doi: 10.1016/j.ophtha.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 120.Cunha-Vaz J, Ashton P, Iezzi R, Campochiaro P, Dugel PU, Holz FG et al. Sustained delivery fluocinolone acetonide vitreous implants: long-term benefit in patients with chronic diabetic macular edema. Ophthalmology. 2014;121(10):1892–903. doi: 10.1016/j.ophtha.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 121.Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E et al. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology. 2015;122(10):2044–52. doi: 10.1016/j.ophtha.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 122.Wang W, Lo ACY. Diabetic Retinopathy: Pathophysiology and Treatments. Int J Mol Sci. 2018;19(6). doi: 10.3390/ijms19061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN et al. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. Jama. 2015;314(20):2137–46. doi: 10.1001/jama.2015.15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes & development. 2000;14(16):1983–91. [PubMed] [Google Scholar]
- 125.Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K et al. VEGF-initiated blood–retinal barrier breakdown in early diabetes. Investigative ophthalmology & visual science. 2001;42(10):2408–13. [PubMed] [Google Scholar]
- 126.Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176(6):1248–64. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annual review of pathology. 2014;9:47–71. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 128.Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema: Two-Year Results from a Comparative Effectiveness Randomized Clinical Trial. Ophthalmology. 2016;123(6):1351–9. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kiss S, Dugel PU, Khanani AM, Broder MS, Chang E, Sun GH et al. Endophthalmitis rates among patients receiving intravitreal anti-VEGF injections: a USA claims analysis. Clinical ophthalmology. 2018;12:1625–35. doi: 10.2147/opth.S169143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Patel S Medicare Spending on Anti-Vascular Endothelial Growth Factor Medications. Ophthalmology Retina. 2018;2(8):785–91. doi: 10.1016/j.oret.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 131.Dugel PU, Jaffe GJ, Sallstig P, Warburton J, Weichselberger A, Wieland M et al. Brolucizumab Versus Aflibercept in Participants with Neovascular Age-Related Macular Degeneration: A Randomized Trial. Ophthalmology. 2017;124(9):1296–304. doi: 10.1016/j.ophtha.2017.03.057. [DOI] [PubMed] [Google Scholar]
- 132.Dugel PU, Koh A, Ogura Y, Jaffe G, Schmidt-Erfurth U, Brown D et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2019. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 133.Milam KE, Parikh SM. The angiopoietin-Tie2 signaling axis in the vascular leakage of systemic inflammation. Tissue Barriers. 2015;3(1–2):e957508. doi: 10.4161/21688362.2014.957508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shen J, Frye M, Lee BL, Reinardy JL, McClung JM, Ding K et al. Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. The Journal of clinical investigation. 2014;124(10):4564–76. doi: 10.1172/jci74527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sahni J, Patel SS, Dugel PU, Khanani AM, Jhaveri CD, Wykoff CC et al. Simultaneous Inhibition of Angiopoietin-2 and Vascular Endothelial Growth Factor-A with Faricimab in Diabetic Macular Edema: BOULEVARD Phase 2 Randomized Trial. Ophthalmology. doi: 10.1016/j.ophtha.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 136.Buehler A, Sitaras N, Favret S, Bucher F, Berger S, Pielen A et al. Semaphorin 3F forms an anti-angiogenic barrier in outer retina. FEBS letters. 2013;587(11):1650–5. doi: 10.1016/j.febslet.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Segarra M, Ohnuki H, Maric D, Salvucci O, Hou X, Kumar A et al. Semaphorin 6A regulates angiogenesis by modulating VEGF signaling. Blood. 2012;120(19):4104–15. doi: 10.1182/blood-2012-02-410076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cerani A, Tetreault N, Menard C, Lapalme E, Patel C, Sitaras N et al. Neuron-derived semaphorin 3A is an early inducer of vascular permeability in diabetic retinopathy via neuropilin-1. Cell metabolism. 2013;18(4):505–18. doi: 10.1016/j.cmet.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 139.Thomas J, Liu F, Link DC. Mechanisms of mobilization of hematopoietic progenitors with granulocyte colony-stimulating factor. Current opinion in hematology. 2002;9(3):183–9. [DOI] [PubMed] [Google Scholar]
- 140.•.Chakravarthy H, Navitskaya S, O'Reilly S, Gallimore J, Mize H, Beli E et al. Role of Acid Sphingomyelinase in Shifting the Balance Between Proinflammatory and Reparative Bone Marrow Cells in Diabetic Retinopathy. Stem cells. 2016;34(4):972–83. doi: 10.1002/stem.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]; Acid sphingomyelinase was identified in this study as a key factor promoting aberrant vascular repair by bone-marrow derived hematopoietic stem cells in the diabetic milieu seondary to an upregulation of membrane ceramides. Inhibition of this enzyme corrected these defects in circulating stem cells and prevented pathology associated with DR in a mouse model.
- 141.Park SS, Moisseiev E, Bauer G, Anderson JD, Grant MB, Zam A et al. Advances in bone marrow stem cell therapy for retinal dysfunction. Progress in retinal and eye research. 2017;56:148–65. doi: 10.1016/j.preteyeres.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Arden GB, Jyothi S, Hogg CH, Lee YF, Sivaprasad S. Regression of early diabetic macular oedema is associated with prevention of dark adaptation. Eye. 2011;25(12):1546–54. doi: 10.1038/eye.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Arden GB, Gunduz MK, Kurtenbach A, Volker M, Zrenner E, Gunduz SB et al. A preliminary trial to determine whether prevention of dark adaptation affects the course of early diabetic retinopathy. Eye. 2010;24(7):1149–55. doi: 10.1038/eye.2009.328. [DOI] [PubMed] [Google Scholar]
- 144.•.Sivaprasad S, Vasconcelos JC, Prevost AT, Holmes H, Hykin P, George S et al. Clinical efficacy and safety of a light mask for prevention of dark adaptation in treating and preventing progression of early diabetic macular oedema at 24 months (CLEOPATRA): a multicentre, phase 3, randomised controlled trial. The lancet Diabetes & endocrinology. 2018;6(5):382–91. doi: 10.1016/S2213-8587(18)30036-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this innovative phase 3 trial, light masks worn at night time were used to treat DME by reducing metabolic demands of photoreceptors in patients with diabetes. However, this therapy did not confer signficant benefits.
- 145.Brownlee M The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–25. [DOI] [PubMed] [Google Scholar]
- 146.Alam NM, Mills WCt, Wong AA, Douglas RM, Szeto HH, Prusky GT. A mitochondrial therapeutic reverses visual decline in mouse models of diabetes. Dis Model Mech. 2015;8(7):701–10. doi: 10.1242/dmm.020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.•.Simo R, Hernandez C, Porta M, Bandello F, Grauslund J, Harding SP et al. Effects of Topically Administered Neuroprotective Drugs in Early Stages of Diabetic Retinopathy: Results of the EUROCONDOR Clinical Trial. Diabetes. 2019;68(2):457–63. doi: 10.2337/db18-0682. [DOI] [PubMed] [Google Scholar]; Topical therapy with brimonidine and somatostatin, two compounds with potential neuroprotective effects, was associated with decreased progression of neuroretinopathy endpoints in patients with diabetes.
- 148.Aung MH, Park HN, Han MK, Obertone TS, Abey J, Aseem F et al. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci. 2014;34(3):726–36. doi: 10.1523/JNEUROSCI.3483-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gastinger MJ, Singh RS, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Investigative ophthalmology & visual science. 2006;47(7):3143–50. doi: 10.1167/iovs.05-1376. [DOI] [PubMed] [Google Scholar]
- 150.Pardue MT, Allen RS. Neuroprotective strategies for retinal disease. Progress in retinal and eye research. 2018;65:50–76. doi: 10.1016/j.preteyeres.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Scott IU, Jackson GR, Quillen DA, Larsen M, Klein R, Liao J et al. Effect of doxycycline vs placebo on retinal function and diabetic retinopathy progression in patients with severe nonproliferative or non-high-risk proliferative diabetic retinopathy: a randomized clinical trial. JAMA ophthalmology. 2014;132(5):535–43. doi: 10.1001/jamaophthalmol.2014.93. [DOI] [PubMed] [Google Scholar]
- 152.Stahel M, Becker M, Graf N, Michels S. SYSTEMIC INTERLEUKIN 1beta INHIBITION IN PROLIFERATIVE DIABETIC RETINOPATHY: A Prospective Open-Label Study Using Canakinumab. Retina. 2016;36(2):385–91. doi: 10.1097/IAE.0000000000000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li F, Zhang L, Wang Y, Xu W, Jiao W, Ma A et al. One-Year Outcome of Conbercept Therapy for Diabetic Macular Edema. Current eye research. 2018;43(2):218–23. doi: 10.1080/02713683.2017.1379542. [DOI] [PubMed] [Google Scholar]
- 154.Xu Y, Rong A, Xu W, Niu Y, Wang Z. Comparison of 12-month therapeutic effect of conbercept and ranibizumab for diabetic macular edema: a real-life clinical practice study. BMC ophthalmology. 2017;17(1):158. doi: 10.1186/s12886-017-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Krishnadev N, Forooghian F, Cukras C, Wong W, Saligan L, Chew EY et al. Subconjunctival sirolimus in the treatment of diabetic macular edema. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2011;249(11):1627–33. doi: 10.1007/s00417-011-1694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sjolie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008;372(9647):1385–93. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- 157.Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Basile AS, Klamerus KJ et al. Dose-ranging evaluation of intravitreal siRNA PF-04523655 for diabetic macular edema (the DEGAS study). Investigative ophthalmology & visual science. 2012;53(12):7666–74. doi: 10.1167/iovs.12-9961. [DOI] [PubMed] [Google Scholar]
- 158.Staurenghi G, Ye L, Magee MH, Danis RP, Wurzelmann J, Adamson P et al. Darapladib, a lipoprotein-associated phospholipase A2 inhibitor, in diabetic macular edema: a 3-month placebo-controlled study. Ophthalmology. 2015;122(5):990–6. doi: 10.1016/j.ophtha.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 159.de Andrade GC, de Oliveira Dias JR, Maia A, Farah ME, Meyer CH, Rodrigues EB. Intravitreal Ziv-Aflibercept for Diabetic Macular Edema: 48-Week Outcomes. Ophthalmic surgery, lasers & imaging retina. 2018;49(4):245–50. doi: 10.3928/23258160-20180329-06. [DOI] [PubMed] [Google Scholar]


