Abstract
OBJECTIVES:
Little is known about frailty that develops following critical illness. We sought to describe the prevalence of newly-acquired frailty, its clinical course, and the co-occurrence of frailty with disability and cognitive impairment in survivors of critical illness.
DESIGN:
Longitudinal prospective cohort study
SETTING:
Medical and surgical ICUs at 5 US Centers
PATIENTS:
Adult patients treated for respiratory failure and/or shock.
MEASUREMENTS AND MAIN RESULTS:
We measured frailty with the Clinical Frailty Scale (CFS) at baseline (i.e., study enrollment) and 3- and 12-months post-discharge. We constructed alluvial diagrams to describe the course of frailty and Venn diagrams to describe the overlap of frailty with disability in activities of daily living and cognitive impairment.
We included 567 participants a median [interquartile range] of 61 [51–70] years old with a high severity of illness (Acute Physiology and Chronic Health Evaluation II of 23). Frailty (CFS scores ≥5) was present in 135/567 (24%) at baseline, 239/530 (45%) at 3 months, and 163/445 (37%) at 12 months. Of those with frailty at 3- or 12-month follow-up, 61% were not frail at baseline. Transition to a worse frailty state occurred in 242/530 (46%) of patients between baseline and 3 months and in 179/445 (40%) of patients between baseline and 12 months. There were 376 patients with frailty, disability or cognitive impairment at 3-month follow-up. Of these, 53 (14%) had frailty alone. At 12 months, 276 patients had frailty, disability or cognitive impairment, 37 (13%) of whom had frailty alone.
CONCLUSIONS:
Frailty is common among survivors of critical illness. In the majority, frailty is newly-acquired. Roughly 1 in 7 had frailty without co-occurring disability or cognitive impairment. Studies to understand outcomes of frailty that develops as the result of a critical illness and to identify modifiable risk factors for this potentially reversible syndrome are needed.
Keywords: Frailty, Critical Illness, Survivors, Post-Intensive Care Syndrome
INTRODUCTION
Frailty is a syndrome characterized by the loss of physiological reserve across multiple organ systems that reduces one’s ability to maintain or to restore homeostasis in the setting of an acute stressor (1, 2). Though an important proportion of patients with critical illness have frailty before their admission to the intensive care unit (ICU), 70% or more become critically ill without evidence of preexisting frailty (3–8). Nevertheless, because many survivors of critical illness often develop new or worsened chronic organ dysfunction (e.g., cardiovascular (9, 10), respiratory (11, 12), renal (13), immunologic(14)), disabilities in activities of daily living (15, 16), impairments in physical (17, 18) and cognitive function (16, 19) following their critical illness, they are likely at high risk for developing new-onset frailty, a syndrome related to, but distinct from, previously defined adverse long-term outcomes of critical illness.
In those without critical illness, frailty is associated with a greater risk for falls, new onset disability, admission to long-term care, hospitalization and death (20, 21). Moreover, frailty may be reversible (22–24), therefore, identification and treatment of frailty in survivors of critical illness could serve as a target by which to reduce the burdens of critical illness survivorship. The extent to which newly-acquired frailty is present in adult survivors of critical illness, the clinical course of frailty, and the degree to which frailty overlaps with disability or cognitive impairment in this population, however, is unclear (25).
To address these knowledge gaps, we conducted a prospective, multicenter, cohort study of survivors of critical illness, assessing frailty at enrollment and at 3 and 12 months after hospital discharge. We hypothesized that adult survivors of critical illness, age 18 years and older, would transition to more severe states of frailty after their illness, that frailty would be present in those without disability or cognitive impairment, and that factors present before and during critical illness would be associated with frailty severity at follow-up.
METHODS
We tested these hypotheses among participants enrolled in the identical (but with different enrolling sites) Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors (BRAIN-ICU, NCT00392795)(6, 19) and Delirium and Dementia in Veterans Surviving ICU Care (MIND-ICU, NCT00400062)(6, 26) studies.
Settings and Participants
As described in the online supplement and in prior publications (15, 19), each day, trained study personnel screened the censuses from the medical or surgical ICUs at five US centers for patients age 18 years or older, treated for respiratory failure and/or shock for less than 72 hours. We excluded those who were moribund, had organ dysfunction for >72 hours, severe cognitive impairment, an inability to communicate in English, substance abuse disorder, psychotic disorder, homelessness, or who resided >200 miles from an enrolling site. Participants or their proxies provided consent. The institutional review boards at each center approved the study.
Study Procedures
At study enrollment, we obtained sociodemographics and data on coexisting illness, disabilities in basic and instrumental ADLs, and cognitive function. During the index hospitalization, we prospectively collected physiologic and laboratory data (for up to 30 days). At 3 and 12 months after hospital discharge, study personnel who were blinded to the baseline Clinical Frailty Scale score, performed in-person follow-up assessments at the enrolling centers or in patients’ homes.
Measuring Frailty
We used the well-validated, highly reliable, Clinical Frailty Scale (CFS) to rate patients along the fitness to frailty continuum (27, 28). CFS scores range from 1 (very fit) to 7 (severe frailty, Table S1 in the online supplement), where scores of 5 or greater represent frailty (27, 28). Study personnel were trained by a geriatrician with expertise in frailty assessment (A.M.). These personnel used all available clinical and study-related data (e.g., patient/proxy interviews, medical records, and clinical and study-related measurements of comorbidity, disability, and cognitive function) to complete the CFS at baseline (i.e., at study enrollment, based on the participant’s status during the 2 months prior to ICU admission) and again 3 and 12 months after hospital discharge. The Vanderbilt Coordinating Center Follow-up Core maintained standardization of follow-up assessments.
Determining the Co-occurrence of Frailty with Disability and Cognitive Impairment
At 3 and 12 months we assessed disability in activities of daily living (ADLs) using the Katz Index of Independence (Katz ADL)(29) and cognition using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)(30). We considered disability to be present if the Katz ADL score was equal to 1 or greater (i.e., requiring assistance in at least 1 ADL) (31). We considered cognitive impairment to be present if the RBANS score was 78 or less (i.e., 1.5 standard deviations below the age-adjusted norm) (19, 31). Detailed descriptions of these instruments are presented in the online supplement.
Factors Associated with Frailty Severity after Critical Illness
A priori, we selected seven baseline and six critical illness-related factors to evaluate their potential associations with CFS scores at follow-up: baseline CFS score, age, years of education, sex, Charlson comorbidity index score (32), baseline Katz ADL score (29), baseline Functional Activities Questionnaire (FAQ) score,(33) duration of delirium (i.e., the number of days the Confusion Assessment Method for the ICU [CAM-ICU] (34) was positive), duration of coma (i.e., the number of days the Richmond Agitation-Sedation Scale [RASS] (35) was −4 or −5), duration of sepsis (i.e., the number of days Sepsis-2 criteria were met) (36), duration of mechanical ventilation, daily modified Sequential Organ Failure Assessment (SOFA) score (37, 38), and discharge location. Detailed descriptions of these factors are presented in the online supplement.
Statistical Analysis
To determine the proportion of patients who developed newly-acquired frailty after their critical illness, we compared CFS scores at 3- and 12-months to CFS scores at baseline. We considered those patients who had CFS scores of ≥5 at either 3- or 12-month follow-up, but who had CFS scores of <5 at baseline, to have newly-acquired frailty.
To determine transitions between frailty states, we used previously published CFS score-based frailty categories (27). We considered patients to be fit if the CFS score was 1–3, vulnerable if the CFS score was 4, and frail if the CFS score was 5 to 7. To illustrate transitions between these frailty states from baseline to 3- and 12-month follow-up, we constructed alluvial flow diagrams. We considered transitions from fit to vulnerable, fit to frail, or from vulnerable to frail to be transitions to a worse frailty state. We considered transitions from frail to vulnerable, frail to fit, and from vulnerable to fit to be transitions to a better frailty state. Those remaining in the same frailty state were considered to have had no transition.
To describe the extent to which frailty overlaps with disability in basic activities of daily living and/or cognitive impairment at 3- or 12-month follow-up, we constructed Venn diagrams, restricted to patients with one of these syndromes using the cutoffs defined above (31). We categorized patients in to seven groups: 1) frailty alone, 2) frailty with disability, 3) frailty with cognitive impairment, 4) frailty with disability and cognitive impairment, 5) disability alone, 6) disability with cognitive impairment, and 7) disability and cognitive impairment.
To evaluate potential associations between baseline and critical illness-related factors and CFS scores at follow-up, we used multivariable regression. We adjusted for the likelihood a patient would be alive, remain in the study, and participate in follow-up using inverse probability of attrition weighting (IPAW) (39, 40). We used multiple imputation to account for missing covariate and incomplete outcome data (41). Continuous risk factors were allowed to be non-linear using restricted cubic splines. Model assumptions were verified using graphical techniques. All model assumptions were met. We used R version 3.4.1 (R Project, Vienna, Austria) for all analyses. Descriptive data are reported as median and interquartile ranges (IQR). P values less than 0.05 were considered significant.
RESULTS
Between March 2007 and December 2010, we enrolled 1,047 patients. After accounting for death, study withdraw, and loss to follow-up, we assessed CFS scores in 530/711 (75%) of survivors at 3 months and in 445/631 (70%) of survivors at 12 months (Figure 1).
Figure 1.
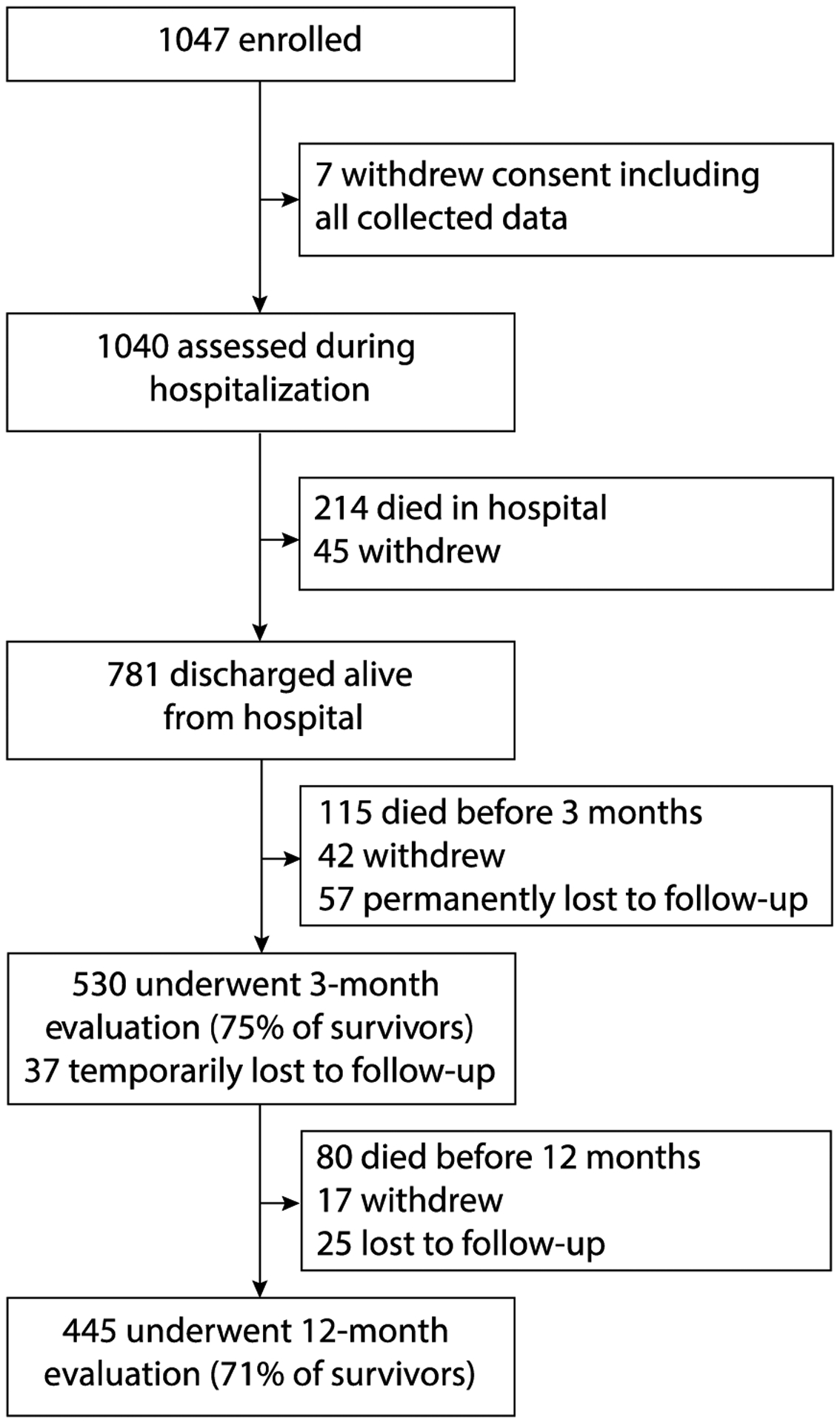
Enrollment and Follow-up
Overall, 567 unique patients contributed data to these analyses. As shown in Table 1, the median age was 61 years (IQR 51 to 70), 41% were female, and severity of illness was high (median APACHE II score of 23 [IQR 17 to 29]).
Table 1.
Demographic and Clinical Characteristics
| Characteristic | N=567 |
|---|---|
| Age, years | 61 (51 to 70) |
| Female sex, n (%) | 232 (41%) |
| CFS Score at baseline, n (%) | |
| 1-Very Fit | 21 (4%) |
| 2-Well | 93 (16%) |
| 3-Well with treated comorbid disease | 193 (34%) |
| 4-Apparently vulnerable | 125 (22%) |
| 5-Mildly frail | 70 (12%) |
| 6-Moderately frail | 53 (9%) |
| 7-Severely frail | 12 (2%) |
| Charleson Comorbidity Index score | 2 (1 to 4) |
| Katz ADL score at baseline | 0 (0 to 1) |
| FAQ score at baseline | 0 (0 to 2) |
| IQCODE score | 3.0 (3.0 to 3.1) |
| APACHE II score at ICU admission | 23 (17 to 29) |
| Mean daily SOFA score in the ICU | 7 (5 to 9) |
| Deliriousc, n (%) | 397 (70%) |
| Duration of deliriuma, days | 3 (2 to 7) |
| Comatosed, n (%) | 294 (52%) |
| Duration of comaa, days | 2 (1 to 5) |
| Septicb, n (%) | 365 (65%) |
| Duration of sepsisa, days | 4 (2 to 8) |
| Mechanically ventilated, n (%) | 499 (88%) |
| Duration of mechanical ventilationa, days | 2 (1 to 6) |
| ICU length of stay, days | 5 (3 to 10) |
| Hospital length of stay, days | 10 (6 to 18) |
Data are median (interquartile range) unless otherwise indicated.
CFS= clinical frailty scale; ADL= activities of daily living; FAQ= functional activities questionnaire of instrumental ADLs; IQCODE= informant questionnaire on cognitive decline in the elderly; APACHE II= acute physiologic and chronic health evaluation, version II; SOFA= sequential organ failure assessment
Among those with the clinical condition
Defined according to Sepsis-2 definition for severe sepsis
Defined as the number of days the Confusion Assessment Method for the ICU was positive
Defined at the number of days the Richmond Agitation-Sedation Scale was −4 or −5.
Frailty Status after Critical Illness
The prevalence of frailty increased following critical illness, and in most, was newly acquired. At enrollment, there were 135/567 (24%) patients with frailty (i.e., CFS score ≥5). At 3 months, however, 239/530 (45%) were frail and at 12 months 162/444 (36%) were frail. Of the 239 patients with frailty at 3-month follow-up 146 (61%) were not frail (i.e., CFS score <5) at enrollment. Likewise, of the 162 patients with frailty at 12-month follow-up, 98 (61%) were not frail at enrollment. Overall, the median increase in CFS scores was 1 (0 to 2) between enrollment and 3 months and 1 (0 to 1) between enrollment at 12 months.
As shown in Figure 2A, transitions between frailty states were common. In general, patients tended to transition to worse states of frailty in the period immediately following their critical illness (i.e., between baseline and 3-month follow-up) and maintained these worse states of frailty over the longer-term (i.e., between 3- and 12-month follow-up). Transitions to worse frailty states occurred in 242/530 (46%) patients between enrollment and 3 months (Figure 2B). There were 80/444 (18%) patients who transitioned to a worse frailty state between 3 and 12 months (Figure 2B). Overall, 178/444 (40%) patients had transitioned to a worse frailty state between enrollment and 12 months. Few patients transitioned to better frailty states (67/530 [13%] between enrollment and 3 months and 98/444 [22%] between 3 and 12 months, [Figure 2C]). No change in frailty state occurred in 221/530 (42%) patients between enrollment and 3 months and in 230/444 (52%) patients between 3 months and 12 months (Figure 2D). There were 203 out of 444 (46%) who had the same frailty state at enrollment and 12 months. Descriptive characteristics of patients according to frailty state at 3 months and at 12 months are presented in Tables S2 and S3 of the online supplement.
Figure 2. Transitions Between Frailty States During the First Year After Hospitalization for Critical Illness.
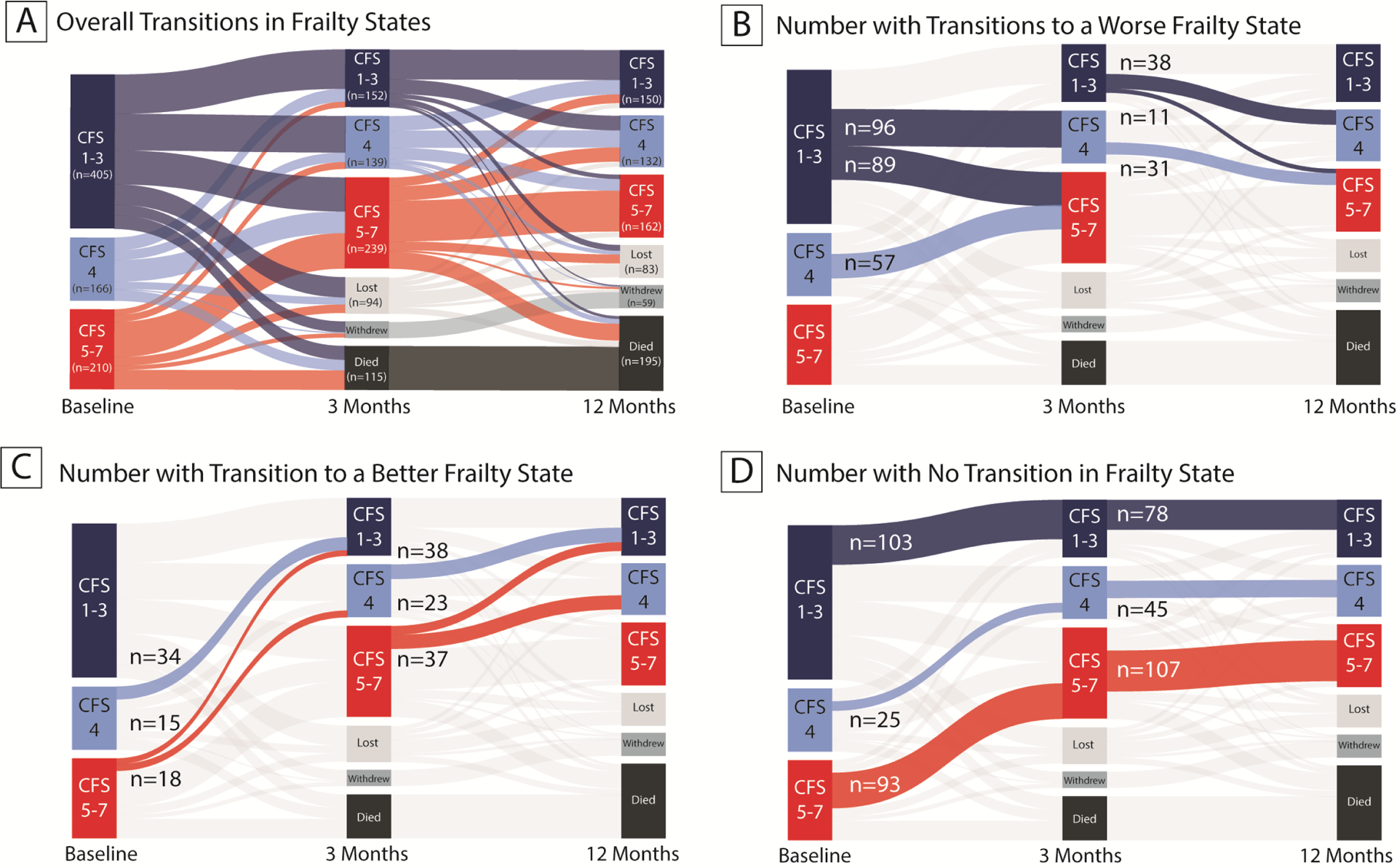
Figure 2A illustrates the changes in frailty states from baseline (i.e., in the 2 months prior to critical illness), to 3-month and 12-month follow-up among survivors of critical illness. Figure 2B shows the number of patients who transitioned to worse frailty states, Figure 2C shows the number of patients who transitioned to better frailty states, and Figure 2D shows the number of patients who had no transition in frailty state.
Overlap of Frailty with Disability and Cognitive Impairment
Of the 530 patients assessed at 3 months, there were 376 (71%) with either frailty, disability in ADLs, or cognitive impairment (Figure 3A). Of these, 53 (14%) had frailty alone, 85 (23%) had both frailty and disability, 46 (12%) had frailty and cognitive impairment, and 55 (15%) patients had all three syndromes. At 12-months, 276/445 (62%) of patients had frailty, disability, or cognitive impairment (Figure 3B). As at 3- months, 37 (13%) patients had frailty alone, 60 (22%) had frailty and disability, 31 (11%) had frailty and cognitive impairment, and 35 (13%) had all three syndromes.
Figure 3. Overlap of Frailty, Disability, and Cognitive Impairment at 3- and 12-Month Follow-Up.
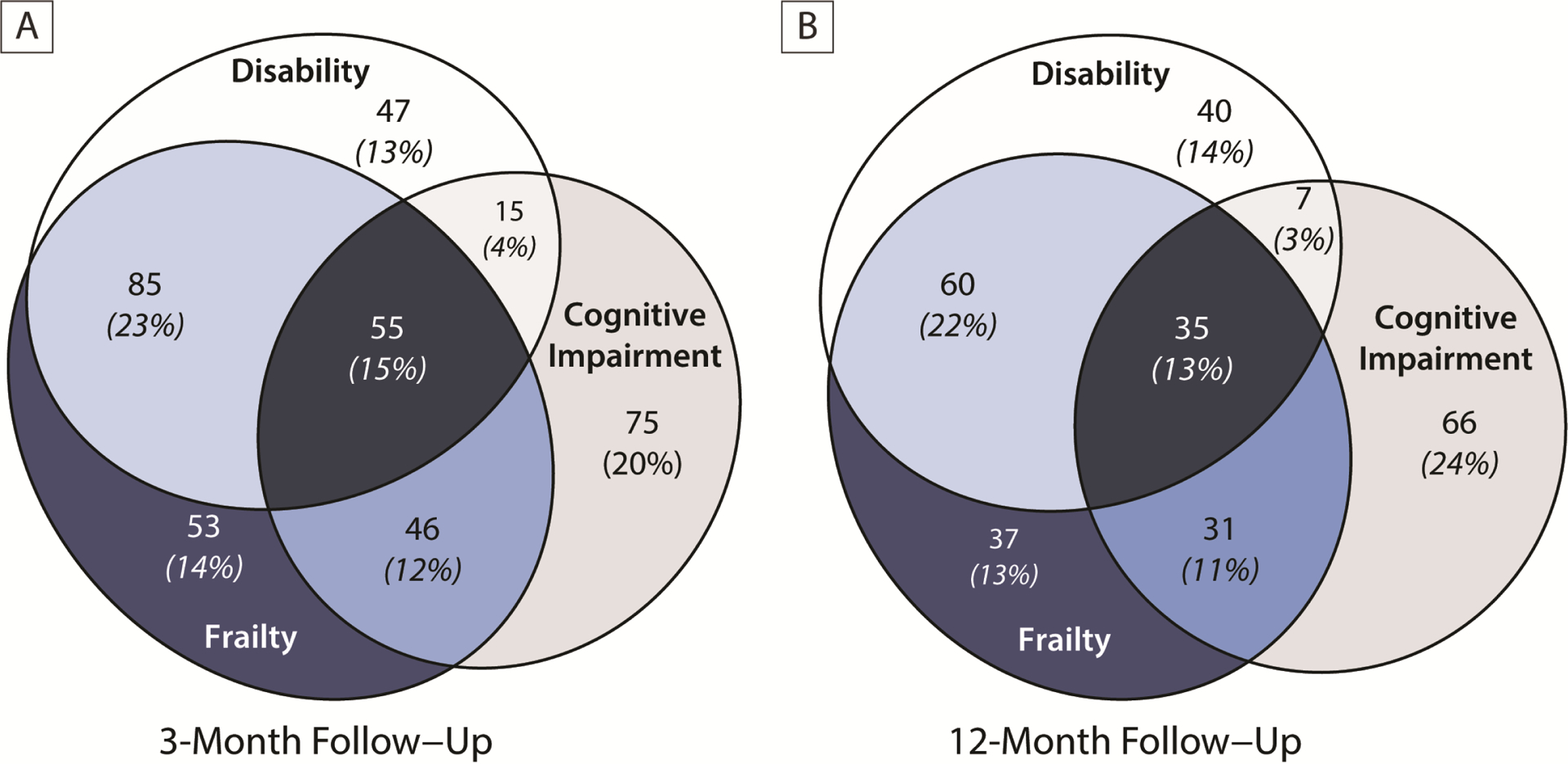
Figure 3 shows the overlap of frailty with disability in activities of daily living and cognitive impairment among those who had one of these three syndromes at 3-months (Panel A) or 12-months (Panel B). We defined disability as a Katz ADL score of ≥1 and cognitive impairment as a Repeatable Battery for the Assessment of Neuropsychological Status score of <78. At both time points, 1 out of 7 patients had frailty without also having disability and/or cognitive impairment.
Factors Associated with Severity of Frailty after Critical Illness
Three factors, baseline CFS score, baseline ADL score, and modified SOFA score during the ICU stay, were associated with frailty severity at both 3- and 12-month follow-up, though the magnitude of these associations were small and were not of clinical significance (Table 2 and Figure S1). After adjusting for all covariates, for example, patients with a baseline CFS score of 4 (the 75th percentile) had 3-month and 12-month CFS scores that were 0.2 points (95% CI, 0.1 to 0.3) higher than that of patients with a baseline CFS score of 3 (the 25th percentile). Compared with a baseline Katz ADL score of 0, a score of 1 was associated with a 0.2 point (95% CI, 0.1 to 0.2) higher CFS score at both 3 and 12 months. In contrast, greater modified SOFA scores during the ICU stay were associated lower CFS scores at follow-up (nonlinear association; p <0.001 at 3 months and p <0.001 at 12 months; Table 2 and Figure S1).
Table 2.
Factors Associated with Clinical Frailty Scale scores at 3 and 12 months.
| 3 Months | 12 Months | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Reference | Comparison | Point Estimate | 95% CI | P | Point Estimate | 95% CI | P | ||||
| Baseline CFS score | 3 | 4 | 0.2 | 0.1 to 0.3 | 0.007 | 0.2 | 0.1 to 0.3 | 0.002 | ||||
| Age | 51 | 70 | 0.1 | −0.1 to 0.3 | 0.14 | 0.2 | 0.1 to 0.4 | 0.003 | ||||
| Education | 12 | 14 | −0.1 | −0.2 to 0.0 | 0.09 | −0.1 | −0.2 to 0.0 | 0.23 | ||||
| Sex | Male | Female | −0.1 | −0.3 to 0.1 | 0.28 | 0.0 | −0.2 to 0.2 | 0.99 | ||||
| Charlson Index score | 1 | 4 | 0.3 | 0.1 to 0.5 | 0.004 | 0.2 | 0.0 to 0.4 | 0.21 | ||||
| Katz ADL score | 0 | 1 | 0.2 | 0.1 to 0.2 | <0.001 | 0.2 | 0.1 to 0.2 | <0.001 | ||||
| FAQ score | 0 | 2 | 0.0 | 0.0 to 0.1 | 0.15 | 0.1 | 0.0 to 0.1 | 0.04 | ||||
| Duration of mechanical ventilation | 1 | 5 | 0.3 | −0.1 to 0.6 | 0.25 | −0.3 | −0.6 to 0.1 | 0.12 | ||||
| Duration of sepsis | 0 | 5 | −0.1 | −0.4 to 0.3 | 0.94 | 0.2 | −0.1 to 0.5 | 0.33 | ||||
| Duration of delirium | 0 | 5 | 0.0 | −0.3 to 0.3 | 0.89 | 0.1 | −0.3 to 0.4 | 0.16 | ||||
| Duration of coma | 0 | 2 | −0.2 | −0.5 to 0.1 | 0.22 | 0.1 | −0.2 to 0.4 | 0.02 | ||||
| Discharge location | Home | Facility | 0.3 | 0.1 to 0.6 | 0.01 | 0.2 | −0.1 to 0.5 | 0.12 | ||||
| Modified SOFA score | 4 | 7 | −0.1 | −0.3 to 0.1 | <0.001 | −0.2 | −0.4 to 0.1 | <0.001 | ||||
CFS= Clinical Frailty Scale; ADL= activities of daily living; FAQ= functional activities questionnaire for instrumental ADLs.
Reference values are those at the 25th percentile and comparison values are those at the 75th percentile (except for sex and discharge location). Positive point estimates represent an increase in CFS score at follow-up, indicating more severe frailty. For example, in a comparison of two patients, alike in all other ways (i.e., other factors adjusted to their median or mode value), a patient with a baseline CFS score of 4 would have 0.2-point higher CFS score at 12 months compared with a patient with a baseline CFS score of 3. Though a number of factors demonstrate statistical significance, the magnitude of these changes in CFS scores are small and below the clinically significant change in CFS score of 1. In cases where the confidence interval crosses 0, but the p-value is significant, the p-value is correct.
None of the remaining factors (i.e., age, years of education, sex, Charlson Comorbidity Index, baseline FAQ, duration of delirium, coma, sepsis, or mechanical ventilation, or discharge location) were consistently associated with CFS scores at both 3 and 12 months (Table 2).
DISCUSSION
In this large, multicenter prospective cohort of survivors of critical illness, we found that 4 out of 10 survivors were frail at 3 and 12 months after hospital discharge. Among those with frailty at follow-up, 60% were not frail prior to their critical illness, supporting our hypothesis that critical illness promotes newly-acquired frailty. Even among those who were not frail per traditional cutoffs, many transitioned to a worse frailty state after critical illness. Finally, frailty was not limited only to survivors with disability or cognitive impairment, as 1 in 7 survivors without these syndromes had frailty.
The present study advances the understanding of frailty across the continuum of critical illness and survivorship by building on a previous Dutch study, to our knowledge, the only other study to perform longitudinal measures of frailty in survivors of critical illness (42). In contrast to our finding that 45% of survivors of critical illness were frail at 3 months and 36% were frail at 12 months, the prior study reported frailty to be present 18% and 10%, respectively. We assessed frailty using personnel who were trained by a geriatrician with expertise in the assessment of frailty and performed in-person assessments that included patient/proxy interviews, a review of medical records, and the assessment of disabilities and cognitive and physical function to complete the CFS, whereas the prior study relied on patient and proxies to assess frailty. Because patients/proxies and clinicians frequently disagree in their assessments of frailty during critical illness (with proxies typically rating patients less frail than clinicians) (43, 44) our more comprehensive approach to frailty measurement may account for the higher prevalence seen in our study. Alternatively, our cohort characterized by diverse reasons for acute medical and surgical critical illness and had greater exposure to critical illness characterized by median ICU length of stay of 5 days and high proportions receiving mechanical ventilation, and experiencing sepsis, delirium, and coma. In contrast, the majority of patients in the previous study were admitted following elective surgery, three-quarters were in the ICU for two days or fewer, and a lower proportion were mechanically ventilated. Thus, the overall ‘dose’ of critical illness could account for the higher prevalence of frailty in the current study. Nevertheless, despite these differences, both studies report that the majority of survivors with frailty did not have the syndrome prior to their critical illness. Thus, future studies are needed to understand clinical outcomes associated with newly acquired frailty.
Our findings also build on previous work by Gill and colleagues who, in a 738-patient cohort of community dwelling, non-critically ill, adults age 70 years or older, found that hospitalization increased the odds that patients would transition to a worse state of frailty and decreased the odds they would recover (45). In the present study, we found that a large number of survivors of critical illness, half of whom were younger than 61 years old, transitioned to worse frailty states. That survivors of critical illness of younger chronologic age develop frailty, a syndrome considered to be age-related, suggests that critical illness may accelerate biological processes of aging (46, 47). Future studies are needed to evaluate the underlying biological mechanisms by which hospitalization for critical illness contributes to the development of frailty in both older and younger adults.
We also found that, while frailty overlapped with disability and cognitive impairment in many survivors, 14% of patients in this cohort had frailty without either of these syndromes. Although frailty is a distinct clinical syndrome from disability and multimorbidity (21), it is not currently considered as part of the Post-Intensive Care Syndrome (PICS). Coupled with our finding that frailty is newly-acquired in the majority survivors with frailty, that survivors of critical illness have frailty without other PICS syndromes suggests that screening for frailty should occur in survivors of critical illness. Moreover, because frailty is associated with poor long-term outcomes and is potentially reversible when detected early, identifying this syndrome in the post-ICU setting could serve as a means by which to improve outcomes for vulnerable population.
Fourth, we hypothesized that factors present before and during critical illness would be associated with the severity of frailty at follow-up. Of the 13 a priori selected risk factors, only 3 were associated with CFS scores at follow-up, none of which were associated with clinically significant changes in CFS scores. Future studies to identify potentially modifiable factors present during critical illness (e.g., immobility, nutritional status, inflammation, perceived stress) which may be associated with worse frailty are needed.
Several limitations of our study are worthy of mention. First, we used the CFS to identify frailty, one of a number of different approaches to measuring frailty (1, 48). The single systematic review on frailty assessment in those who become critically ill did not identify a preferred frailty measure, it did, however, identify the CFS as the most frequently used frailty assessment tool (7). Nevertheless, because different frailty instruments may identify different patient populations, future work is needed to understand the subtypes of frailty and their causes in survivors of critical illness (49). Second, although the CFS provides a brief set of descriptors for each category which rely on coexisting illnesses, disabilities in activities of daily living, and cognition, the instrument is intended to have an element of judgement, such that different aspects of health can be considered to determine the presence of frailty (27). Thus, factors present during the acute illness such as physical appearance and acute decrease in function could bias CFS scoring. Such bias, however, would most likely affect baseline CFS scores. Follow-up assessments, in contrast, were performed by study personnel blinded to events of the critical illness and occurred months after hospital discharge. Thus, our findings of an increase in frailty after critical illness may, if anything, be conservative. Third, CFS (and its parent tool, the Frailty Index) rely on measurement of a heterogenous group of domains and are thus best suited for overall risk assessment (50). Therefore, future studies building upon our data, are needed to increase understanding of the mechanisms by which frailty develops in survivors of critical illness.
In addition to the strengths of our study detailed above, we enrolled a large multicenter cohort of adult patients from academic, community, and Veterans Affairs hospitals across the U.S., thereby enhancing the generalizability to our findings. Finally, we achieved high rates of in-person follow-up over 12 months after hospital discharge and employed modern statistical techniques to reduce bias related to death and study drop-out.
CONCLUSIONS
In conclusion, we found that frailty is common among adult survivors of critical illness, in the majority of these survivors, frailty is worsened or newly acquired. Studies to understand outcomes of critical illness-associated frailty and to identify factors which can be targeted for intervention in this potentially reversible syndrome are needed.
Supplementary Material
Funding/Support:
The National Institutes of Health under awards (K76AG054864, R01AG027472, R01AG035117, R01HL111111, K07AG043587 and P30AG21342), Vanderbilt University Medical Center Department of Medicine Academic Support Funds, Department of Veterans Affairs Tennessee Valley Health Care System Geriatric Research, Education and Clinical Center (GRECC).
Footnotes
Institutions Where Work Was Performed:
Vanderbilt University Medical Center (Nashville, TN, USA), Saint Thomas Hospital (Nashville, TN, USA), Department of Veterans Affairs (VA) Tennessee Valley Healthcare System (Nashville, TN, USA), the George E. Wahlen Department of Veterans Affairs Medical Center (Salt Lake City, UT, USA), and the VA Puget Sound Health Care System (Seattle, WA, USA).
Statement regarding whether reprints will be ordered:
We do not plan to order reprints.
Declaration of interests:
NEB has performed advisory board activities for Arjo and Merck. PPP has received a research grant from Hospira. EWE has received honoraria from Orion and Hospira for continuing medical education activities. CGH and EWE have received a research grant from Chemie GMBH. The remaining authors report no financial conflicts of interest.
The contents of this paper are solely the responsibility of the authors and do not necessarily represent those of the Department of Veterans Affairs, the National Institutes of Health, The Ohio State University, the University of Pittsburgh, Vanderbilt University Medical Center, Vanderbilt University, or Yale University.
Dr. Brummel had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES:
- 1.Morley JE, Vellas B, van Kan GA, Anker SD, et al. : Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14(6):392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walston J, Hadley EC, Ferrucci L, Guralnik JM, et al. : Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006; 54(6):991–1001 [DOI] [PubMed] [Google Scholar]
- 3.Bagshaw SM, Stelfox HT, McDermid RC, Rolfson DB, et al. : Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ 2014; 186(2):E95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heyland DK, Garland A, Bagshaw SM, Cook D, et al. : Recovery after critical illness in patients aged 80 years or older: a multi-center prospective observational cohort study. Intensive Care Med 2015; 41(11):1911–1920 [DOI] [PubMed] [Google Scholar]
- 5.Hope AA, Gong MN, Guerra C, Wunsch H: Frailty Before Critical Illness and Mortality for Elderly Medicare Beneficiaries. J Am Geriatr Soc 2015; 63(6):1121–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brummel NE, Bell SP, Girard TD, Pandharipande PP, et al. : Frailty and Subsequent Disability and Mortality among Patients with Critical Illness. Am J Respir Crit Care Med 2017; 196(1):64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muscedere J, Waters B, Varambally A, Bagshaw SM, et al. : The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med 2017; 43(8):1105–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, et al. : The Association of Frailty With Post-ICU Disability, Nursing Home Admission, and Mortality: A Longitudinal Study. Chest 2018; 153(6):1378–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergh C, Fall K, Udumyan R, Sjoqvist H, et al. : Severe infections and subsequent delayed cardiovascular disease. Eur J Prev Cardiol 2017; 24(18):1958–1966 [DOI] [PubMed] [Google Scholar]
- 10.Yende S, Linde-Zwirble W, Mayr F, Weissfeld LA, et al. : Risk of cardiovascular events in survivors of severe sepsis. Am J Respir Crit Care Med 2014; 189(9):1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neff TA, Stocker R, Frey HR, Stein S, et al. : Long-term assessment of lung function in survivors of severe ARDS. Chest 2003; 123(3):845–853 [DOI] [PubMed] [Google Scholar]
- 12.Heyland DK, Groll D, Caeser M: Survivors of acute respiratory distress syndrome: relationship between pulmonary dysfunction and long-term health-related quality of life. Crit Care Med 2005; 33(7):1549–1556 [DOI] [PubMed] [Google Scholar]
- 13.Kellum JA, Sileanu FE, Bihorac A, Hoste EA, et al. : Recovery after Acute Kidney Injury. Am J Respir Crit Care Med 2017; 195(6):784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotchkiss RS, Monneret G, Payen D: Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013; 13(12):862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson JC, Pandharipande PP, Girard TD, Brummel NE, et al. : Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: a longitudinal cohort study. Lancet Respir Med 2014; 2(5):369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwashyna TJ, Ely EW, Smith DM, Langa KM: Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA 2010; 304(16):1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herridge MS, Tansey CM, Matte A, Tomlinson G, et al. : Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med 2011; 364(14):1293–1304 [DOI] [PubMed] [Google Scholar]
- 18.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, et al. : Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med 2014; 42(4):849–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandharipande PP, Girard TD, Jackson JC, Morandi A, et al. : Long-term cognitive impairment after critical illness. N Engl J Med 2013; 369(14):1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermeiren S, Vella-Azzopardi R, Beckwee D, Habbig AK, et al. : Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J Am Med Dir Assoc 2016; 17(12):1163 e1161–1163 e1117 [DOI] [PubMed] [Google Scholar]
- 21.Fried LP, Tangen CM, Walston J, Newman AB, et al. : Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56(3):M146–156 [DOI] [PubMed] [Google Scholar]
- 22.Cesari M, Vellas B, Hsu FC, Newman AB, et al. : A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J Gerontol A Biol Sci Med Sci 2015; 70(2):216–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill TM, Baker DI, Gottschalk M, Gahbauer EA, et al. : A prehabilitation program for physically frail community-living older persons. Arch Phys Med Rehabil 2003; 84(3):394–404 [DOI] [PubMed] [Google Scholar]
- 24.Ng TP, Feng L, Nyunt MS, Feng L, et al. : Nutritional, Physical, Cognitive, and Combination Interventions and Frailty Reversal Among Older Adults: A Randomized Controlled Trial. Am J Med 2015; 128(11):1225–1236 e1221 [DOI] [PubMed] [Google Scholar]
- 25.Hogan DB, Maxwell CJ, Afilalo J, Arora RC, et al. : A Scoping Review of Frailty and Acute Care in Middle-Aged and Older Individuals with Recommendations for Future Research. Can Geriatr J 2017; 20(1):22–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ely EW: MIND-ICU Study: Delirium and Dementia in Veterans Surviving ICU Care. Accessed [Google Scholar]
- 27.Rockwood K, Song X, MacKnight C, Bergman H, et al. : A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173(5):489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rockwood K, Fox RA, Stolee P, Robertson D, et al. : Frailty in elderly people: an evolving concept. CMAJ 1994; 150(4):489–495 [PMC free article] [PubMed] [Google Scholar]
- 29.Katz S, Ford AB, Moskowitz RW, Jackson BA, et al. : Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. JAMA 1963; 185:914–919 [DOI] [PubMed] [Google Scholar]
- 30.Randolph C, Tierney MC, Mohr E, Chase TN: The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 1998; 20(3):310–319 [DOI] [PubMed] [Google Scholar]
- 31.Marra A, Pandharipande PP, Girard TD, Patel MB, et al. : Co-Occurrence of Post-Intensive Care Syndrome Problems Among 406 Survivors of Critical Illness. Crit Care Med 2018; 46(9):1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40(5):373–383 [DOI] [PubMed] [Google Scholar]
- 33.Pfeffer RI, Kurosaki TT, Harrah CH Jr., Chance JM, et al. : Measurement of functional activities in older adults in the community. J Gerontol 1982; 37(3):323–329 [DOI] [PubMed] [Google Scholar]
- 34.Ely EW, Inouye SK, Bernard GR, Gordon S, et al. : Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001; 286(21):2703–2710 [DOI] [PubMed] [Google Scholar]
- 35.Ely EW, Truman B, Shintani A, Thomason JW, et al. : Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA 2003; 289(22):2983–2991 [DOI] [PubMed] [Google Scholar]
- 36.Levy MM, Fink MP, Marshall JC, Abraham E, et al. : 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003; 31(4):1250–1256 [DOI] [PubMed] [Google Scholar]
- 37.Vincent JL, Moreno R, Takala J, Willatts S, et al. : The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22(7):707–710 [DOI] [PubMed] [Google Scholar]
- 38.Vasilevskis EE, Pandharipande PP, Graves AJ, Shintani A, et al. : Validity of a Modified Sequential Organ Failure Assessment Score Using the Richmond Agitation-Sedation Scale. Crit Care Med 2016; 44(1):138–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mansournia MA, Altman DG: Inverse probability weighting. BMJ 2016; 352:i189. [DOI] [PubMed] [Google Scholar]
- 40.Seaman SR, White IR: Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res 2013; 22(3):278–295 [DOI] [PubMed] [Google Scholar]
- 41.Little RJ, D’Agostino R, Cohen ML, Dickersin K, et al. : The prevention and treatment of missing data in clinical trials. N Engl J Med 2012; 367(14):1355–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geense W, Zegers M, Dieperink P, Vermeulen H, et al. : Changes in frailty among ICU survivors and associated factors: Results of a one-year prospective cohort study using the Dutch Clinical Frailty Scale. J Crit Care 2020; 55:184–193 [DOI] [PubMed] [Google Scholar]
- 43.Hope AA, Ng Gong M: Discordance about Frailty Diagnosis between Surrogates and Physicians and its relationship to Hospital Mortality in Critically Ill Older Adults. J Frailty Aging 2019; 8(4):176–179 [DOI] [PubMed] [Google Scholar]
- 44.Hope AA, Munoz M, Hsieh SJ, Gong MN: Surrogates’ and Researchers’ Assessments of Prehospital Frailty in Critically Ill Older Adults. Am J Crit Care 2019; 28(2):117–123 [DOI] [PubMed] [Google Scholar]
- 45.Gill TM, Gahbauer EA, Han L, Allore HG: The relationship between intervening hospitalizations and transitions between frailty states. J Gerontol A Biol Sci Med Sci 2011; 66(11):1238–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belsky DW, Caspi A, Houts R, Cohen HJ, et al. : Quantification of biological aging in young adults. Proc Natl Acad Sci U S A 2015; 112(30):E4104–4110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levine ME: Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci 2013; 68(6):667–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malmstrom TK, Miller DK, Morley JE: A comparison of four frailty models. J Am Geriatr Soc 2014; 62(4):721–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue QL, Tian J, Walston JD, Chaves PHM, et al. : Discrepancy in Frailty Identification: Move Beyond Predictive Validity. J Gerontol A Biol Sci Med Sci 2020; 75(2):387–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walston JD, Bandeen-Roche K: Frailty: a tale of two concepts. BMC Med 2015; 13:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


