Supplemental Digital Content is available in the text.
Keywords: Fecundability, Fertility, Preconception cohort, Road proximity, Traffic-related air pollution
Background:
Emerging evidence from animal and human studies indicates that exposure to traffic-related air pollution may adversely affect fertility.
Methods:
Among 7,342 female pregnancy planners from the United States and 1,448 from Canada, we examined the association between residential proximity to major roads and fecundability, the per-cycle probability of conception. From 2013 to 2019, women 21–45 years old who were trying to conceive without fertility treatment completed an online baseline questionnaire and follow-up questionnaires every 8 weeks for up to 12 months or until pregnancy. We geocoded residential addresses reported at baseline and during follow-up, and calculated distance to nearest major roads and length of major roads within buffers of 50, 100, 300, and 400 meters around the residence as proxies for traffic-related air pollution. We used proportional probabilities regression models to estimate fecundability ratios (FRs) and 95% confidence intervals (CIs), adjusting for individual- and neighborhood-level characteristics.
Results:
In the United States, the FR comparing women who lived <50 meters with those who lived ≥400 meters from the closest major road was 0.88 (95% CI = 0.80, 0.98). The association among Canadian women was similar in magnitude, but less precise (FR = 0.93; 95% CI = 0.74, 1.16). Likewise, length of major roads within buffers of 50 and 100 meters was associated with lower fecundability in both countries; associations were attenuated within larger buffers.
Conclusions:
These results are consistent with the hypothesis that traffic-related air pollution or other near-road exposures may adversely affect fecundability.
What this study adds
This is the largest preconception cohort study to prospectively measure the association between road proximity metrics and fecundability. Among participants residing across the United States and Canada, living close to major roads was associated with reduced fecundability, even after adjustment for individual- and neighborhood-level confounding. Our analysis overcomes the limitations of previous studies examining this association, including small sample size, limited geographic diversity, and lack of control for neighborhood-level confounding. In addition, we prospectively measured fecundability in women representing the full range of the fertility spectrum, whereas most other studies have relied on retrospective report of time to pregnancy or have been conducted in fertility clinic populations.
Approximately 10–15% of reproductive-age couples in the United States and Canada experience infertility, defined as the inability to conceive after 12 months of unprotected intercourse.1–3 Infertility can cause substantial psychological and economic hardship. Treatments for infertility cost up to $5 billion annually in the United States4 and may be associated with higher risk of adverse pregnancy and child health outcomes.5–8 As couples delay childbearing, rates of infertility and use of infertility treatments are expected to increase.9 However, few modifiable risk factors for infertility have been identified.
Residential proximity to major roadways may serve as a proxy for a complex set of urban exposures, including traffic-related air pollution, noise, and other spatially-related characteristics, and has been associated with increased risk of cardiovascular10,11 and respiratory disease12,13 and type 2 diabetes.14 Living close to major roadways may also affect reproduction: laboratory evidence demonstrates that diesel exhaust, particulate matter, and other combustion-related pollutants have hormonal activity15–17 and can increase oxidative stress and systemic inflammation.18–20 Mice chronically exposed to urban air in São Paulo, Brazil had fewer antral follicles, longer estrus cycles, greater implantation failure, longer time-to-pregnancy, and fewer live births than mice exposed to air filtered for particulate matter.21,22
Several epidemiologic studies support the hypothesis that living close to major roadways is related to diminished reproductive capacity. Two studies on this topic have been conducted among couples undergoing fertility treatment.23,24 In a study of 7,463 couples undergoing their first autologous in vitro fertilization cycle at four fertility clinics (Seattle, WA; San Francisco, CA; Los Angeles, CA; and Shady Grove, MD), women living within 100 meters of a highway or 50 meters from a major road had 0.90 times the probability of a positive pregnancy test (95% confidence interval [CI] = 0.83, 0.97) and live birth (95% CI = 0.82, 0.99), respectively, relative to women living at least 100 meters from a highway and 50 meters from a major road.24 Likewise, among 423 women undergoing fertility treatment at a Massachusetts hospital (Environment and Reproductive Health [EARTH] study), living <50 meters (vs. ≥400 meters) from a major road or highway was associated with lower probability of implantation (49% vs. 62%) and live birth (33% vs. 46%).23 However, traffic density, characterized by length of roads within 100 meters of the residence multiplied by the annual average daily traffic counts for those roads, was not meaningfully associated with probability of implantation or live birth or intermediate treatment outcomes (e.g., estradiol trigger concentrations or endometrial thickness).23
Unlike studies of couples undergoing fertility treatment, preconception cohort studies of couples attempting to conceive spontaneously have the advantage of capturing the full range of the fertility spectrum, rather than restricting to the least fertile couples. In the Longitudinal Investigation of Fertility and the Environment (LIFE), a preconception cohort study of 500 couples from Michigan and Texas, a 200-meter increase in distance between the residence and a major road was associated with a 3% increase in the per-cycle probability of pregnancy.25 In the Nurses’ Health Study II, living within 200 meters of a major road was associated with an 11% increased risk of infertility (95% CI = 2%, 20%) compared with living at least 200 meters from a major road. Results were stronger for secondary infertility (i.e., infertility among parous women), and appeared to be strongest for ovulatory infertility and weakest for male factor infertility.26
In the present report, we examined the association between residential proximity to major roads and fecundability, the per-cycle probability of conception, in a prospective preconception cohort study of pregnancy planners residing across the United States and Canada.
Methods
Study design and population
Pregnancy Study Online (PRESTO) is a web-based preconception cohort study of pregnancy planners. The study methods have been described in detail elsewhere.27 Briefly, eligible women were 21–45 years old, residing in the United States or Canada, and attempting to conceive without use of fertility treatments. Enrollment began in June 2013 and women were recruited primarily through advertisements placed on social media and health-related websites. After completing a screener questionnaire to determine eligibility, women completed an online baseline questionnaire on sociodemographics, lifestyle, medical and reproductive histories, and medication use. Ten days after baseline, participants were invited to complete a validated food frequency questionnaire (the National Cancer Institute’s Diet History Questionnaire [DHQ] II).28 Every 8 weeks, women completed online follow-up questionnaire on pregnancy status and changes in residential address and other time-varying characteristics.
The study protocol was approved by the Institutional Review Board at the Boston University Medical Campus, and all participants provided informed consent.
Exposure assessment
Women reported their full residential address at baseline and, beginning in October 2014 (84% of participants enrolled), during follow-up. We geocoded all addresses using ArcGIS 10.3 (ESRI, Redlands, CA), and preferentially assigned latitude and longitude to precise parcel locations. We estimated geocodes not matched to a parcel using an automated procedure that calculates the location of the house number. If no house number was available, we assigned the geocode to the location of the nearest intersection or middle of the length of the residential street. Since automated geocoding typically places locations on the centerline of roads, we applied a 9-meter offset to estimate residential location for nonparcel locations.29
We defined major roads in the US-based on Census Feature Class Codes A1 (primary highway with limited access, only accessible via. ramps), A2 (primary road without limited access), and A3 (secondary and connecting roads).30 In Canada, we defined major roads using the 2016 Digital Mapping Technologies, Inc. (DMTI) road network classifications of “expressways,” “highways,” and “major roads.”31 We calculated a number of road proximity metrics informed by previous air pollution measurement studies,32–36 including Euclidean distance to closest major road; Euclidean distance to closest highway (A1–A2 road in the United States and expressways or highways in Canada); length of major roads within buffers of 50, 100, 300, and 400 meters; Euclidean distance to closest intersection; and number of intersections within a buffer of 500 meters. Finally, we calculated a binary “near road residence” variable, defined as living within 50 meters of a major road or within 100 meters of a highway, as has been previously described.24
Outcome assessment
On the baseline questionnaire, women reported the number of menstrual cycles in which they had been trying to conceive. At baseline and over follow-up, we collected data on date of last menstrual period (LMP) and cycle regularity (“Has your menstrual period been regular in a way that you could usually predict about when the next period would start?”). For women with regular cycles, we asked them about their typical cycle length; for women with irregular cycles, we estimated typical cycle length using information on the number of periods in a year, the estimated number of days until their next period, and LMP dates at baseline and over follow-up.
On each follow-up questionnaire, administered every 8 weeks, women reported if they were currently pregnant, if they had initiated fertility treatment, and if they had experienced any intervening pregnancy losses since their last questionnaire. Women who reported a pregnancy were asked about how their pregnancy was confirmed (e.g., urine test, blood test, and ultrasound). Over 96% of participants reported using home pregnancy tests to confirm pregnancy. For women who were not pregnant, we asked if they were still trying to conceive. Among women who were lost to follow-up, we sought outcome information by contacting participants directly via. phone or email, searching for baby registries and birth announcements online, and by linking with birth registries in selected states (CA, FL, MA, MI, OH, PA, and TX). For pregnancies identified without direct participant contact, we assumed that this was the woman’s first pregnancy since enrolling in the study. We calculated time to pregnancy in discrete menstrual cycles using the following formula: (cycles trying to conceive at study entry) + ([LMP date from most recent follow-up questionnaire–date of baseline questionnaire]/cycle length) + 1.
Covariate assessment
We collected individual-level covariate data on the baseline questionnaire and the food frequency questionnaire.28 We ascertained information on sociodemographics (age, educational attainment, annual household income, race/ethnicity), lifestyle (cigarette smoking, passive smoke exposure, alcohol intake, sugar-sweetened soda intake, physical activity, daily multivitamin/folic acid use), anthropometrics (height, weight), reproductive history (parity, history of infertility, last method of contraception), and factors related to intensity of trying to conceive (intercourse frequency, doing something to improve chances of conception [i.e., timing intercourse, charting menstrual cycles, using an ovulation predictor kit]). We calculated body mass index (BMI) as weight (kg) over height (m) squared. From the food frequency questionnaire, we calculated the 2010 version of the Healthy Eating Index (HEI), a measure of diet quality.37
We linked geocoded addresses to the 2010 US Census and the 2016 Canadian Census to obtain information on neighborhood-level factors for each census tract, including percent of residents with less than a high school education, percent non-Hispanic White residents, and tract-level median household income. We also measured whether the participant’s address was in an urban or nonurban census tract. In the United States, we defined urban addresses, based on the definition from the 2010 US Census, as those living in an area with a population density of at least 1,000 people per square mile and with population of at least 2,500 people.38 In Canada, we defined urban addresses as those within a census metropolitan area (an area consisting of one or more neighboring municipalities situated around an urban core with total population of >100,000 of which >50,000 live within urban core).39
Exclusions
Between June 2013 and April 2019, 10,466 eligible women completed the baseline questionnaire. We excluded 175 women with implausible baseline LMP data, 1,168 women who had been attempting to conceive for 12 or more cycles at study entry, and 60 women who resided outside the contiguous United States (i.e., Hawaii, Alaska, US territories). Of the remaining 7,587 women from the contiguous United States, we excluded 245 women (3.2%) because their baseline addresses could not be geocoded. Of the remaining 1,476 Canadian women, the corresponding exclusion was 28 women (1.9%). The final analytic sample comprised 7,342 women from the contiguous United States and 1,448 women from Canada.
Statistical analysis
Women contributed at-risk cycles to the analysis from baseline until pregnancy, regardless of the outcome, or one of the following censoring events: stopped trying to conceive, initiated fertility treatment, loss to follow-up/withdrawal, or 12 cycles, whichever came first. We used life-table methods to calculate the proportion of women who conceived over follow-up, after accounting for censoring.40 We fit restricted cubic splines to determine the monotonicity of the association between road proximity metrics and fecundability. We categorized exposures based on prior research on the decay of pollutants around major roads,41,42 categorization schemes used in previous studies,23,24,26 and the distribution of values in our data. We used the same cut points for the United States and Canadian data. We categorized distance to major roads and highways as <50, 50–99, 100–199, 200–399, and ≥400 meters. For distance to major intersections, we used the same categorization, except we combined the closest two categories (<100 meters) due to small numbers. For length of major roads within varying buffer sizes, we used no major roads within the buffer as the reference group, and then categorized the remaining values as <25th, 25 to <75th, and ≥75th percentiles (defined in the two countries combined). For the 300 and 400 meter buffers, we divided the 25 to <75th percentile group into two categories (25 to <50th and 50 to <75th percentiles). We categorized number of major intersections within 500 meters as none, 1–9, and ≥10.
We used the Andersen-Gill data structure to account for left truncation due to delayed entry into the risk set43,44 and to update exposures and covariates over time. We fit proportional probabilities regression models (i.e., log-binomial regression models with one observation per menstrual cycle) to estimate fecundability ratios (FRs) and 95% confidence intervals (CIs) comparing each category of exposure with the referent group. We modeled indicator variables for cycle at risk to account for the decline in baseline fecundability with increasing attempt time. We updated exposures over time when women changed residences over follow-up (4% of analytic cohort). There were five women whose new residences over follow-up could not be geocoded; for these women, we carried forward their previous address information. We used the weighted copy method to facilitate the convergence of regression models.45
We selected potential confounders a priori based on a literature review and construction of a directed acyclic graph (Supplemental Figure 1; http://links.lww.com/EE/A110). Final models adjusted for the following individual-level variables ascertained at baseline: age (<25, 25–29, 30–34, 35–39, ≥40 years), race/ethnicity (non-Hispanic Black, non-Hispanic Asian, non-Hispanic White, non-Hispanic other/mixed race, Hispanic), education (≤12, 13–15, 16, ≥17 years), annual household income (<50,000, 50,000–99,999, 100,000–149,999, ≥150,000 US dollar [USD]), BMI (<25, 25–29, 30–34, ≥35 kg/m2), physical activity (<10, 10–19, 20–39, ≥40 metabolic equivalent of task [MET]-hours/week), cigarette smoking (not current, current occasional, current regular), sugar-sweetened beverage intake (0, 1, 2–6, ≥7 drinks/week), daily multivitamin or folic acid intake, HEI score (<60, 60–69, 70–79, ≥80),46 parity (nulliparous, parous), intercourse frequency (<1, 1, 2–3, ≥4 times/week), and doing something to improve chances of conception (i.e., timing intercourse, charting menstrual cycles, ovulation predictor kit). We also included three census tract-level variables, modeled individually: median household income (<50,000, 50,000–74,999, 75,000–99,999, ≥100,000 USD), percent non-Hispanic White (<50, 50–74, 75–89, ≥90%), and percent with less than a high school education (<5, 5–14, 15–24, ≥25%). We obtained information on dietary and supplemental intake of folate from the DHQ II.
We accounted for missing covariate and outcome data using multiple imputation. For women who did not complete any follow-up questionnaires (15.6%), we assigned them one cycle of follow-up and imputed their pregnancy status at that cycle. Covariate missingness ranged from 0% (age) to 38% (HEI score, which was measured only among women who completed the food frequency questionnaire). We generated five imputed data sets using Markov chain Monte Carlo methods and statistically combined FRs and standard errors across data sets.47
Because the etiology of primary and secondary infertility may be different, and a previous study documented a stronger association between roadway proximity and risk of secondary infertility relative to primary infertility,26 we stratified final models by parity (nulliparous vs. parous). In addition, studies have found that individuals with low folate intake may be more susceptible to the adverse health effects of air pollution,48,49 including poorer pregnancy outcomes.50 Therefore, we stratified multivariable models by total folate intake (including from dietary and supplemental sources) in dietary folate equivalents (DFEs; a unit of measurement that captures bioavailability of folate). In our primary stratified analysis, we categorized total folate as above versus below 1,000 DFE, the median in our cohort. We conducted additional sensitivity analyses categorizing total folate intake and supplemental folate intake at 400 DFE (the recommended daily intake) and 800 DFE (twice the recommended daily intake).
We used slightly different exposure metrics in the United States and Canada, based on the different road classifications used in the two countries. Therefore, we stratified our primary analyses by country. In secondary analyses, we combined the effect estimates across countries using a fixed effects meta-analysis, which is equivalent to pooling the United States and Canada subpopulations.51,52 We conducted sensitivity analyses restricting to women who had been trying to conceive for ≤6 and ≤2 cycles at cohort entry, as women may change their behaviors in response to difficulty conceiving and may report pregnancy attempt times with more error. Last, because major roads in urban areas generally have a higher traffic volume than major roads in rural areas, we conducted a sensitivity analysis restricted to addresses in an urban census tract.
Results
This analysis includes women from all 48 contiguous US states and 10 Canadian provinces. The states/provinces with the highest proportion of participants were Massachusetts (10%), California (6%), Ontario (6%), Texas (5%), and Michigan (4%). The majority of women in our study population are non-Hispanic White (84%), have a college degree or more (70%), and an annual household income of at least 75,000 USD (59%). However, there was some variability in socioeconomic status: 6% of women had a high school education or less and 8% had an annual household income of less than 25,000 USD. The mean BMI was 28 kg/m2 and 18% of women had a BMI ≥35 kg/m2. Most women were physically active, including outdoor physical activity such as walking (mean hours/week = 5.0), cycling (mean hours/week = 0.3), and gardening (mean hours/week = 0.6). Cigarette smoking (8%) or consuming ≥14 alcoholic beverages on average per week (3%) were uncommon. Most women took a daily multivitamin or folic acid supplement (79%); 49% had never been pregnant and 33% had a previous birth. Most women lived in urban census tracts (85%). The mean census tract median household income was 63,000 USD (range = 9,300–290,000 USD); other census tract characteristics included percent of population with <high school education (mean = 12%, range = 0–72%), percent unemployed (mean = 5%, range = 0–44%), and percent non-Hispanic White (mean = 75%, range = 0–100%).
Fourteen percent of participants from the United States lived within 50 meters of an A1, A2, or A3 road. Among women who lived within 50 meters of a major road, the length of major roads within the 50-meter buffer around their home ranged from 11 to 350 meters (median = 91 meters). The median distance to the closest major intersection was 580 meters (interquartile range [IQR] = 318, 1,058 meters). In Canada, 14% of participants lived within 50 meters of an expressway, highway, or major road. Among women who lived within 50 meters of a major road, the length of major roads in the 50-meter buffer ranged from 1 to 375 meters (median = 86 meters). The median distance to the closest major intersection was 548 meters (IQR = 270, 1,333 meters). In both the United States and Canada, distance to the closest major road was inversely associated with annual household income and positively associated with non-Hispanic White race/ethnicity and parity (Table 1). Residence in an urban census tract and proportion of individuals in the census tract with less than high school education were inversely related to roadway proximity in the United States, whereas census tract-level median household income and proportion non-Hispanic White individuals were positively related to distance to major roads. Associations between roadway proximity and census tract variables in Canada were weaker: only lower median household income was associated with living close to a major road.
Table 1.
Baseline characteristics by distance of residence from nearest major roadway among women residing in the contiguous United States and Canada, Pregnancy Study Online (2013–2019)
| Characteristica | United States (n = 7,342) | Canada (n = 1,448) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Distance from closest A1–A3 road (meters) | Distance from closest expressway, highway, or major road (meters) | |||||||||
| <50 | 50–99 | 100–199 | 200–399 | ≥400 | <50 | 50–99 | 100–199 | 200–399 | ≥400 | |
| Number of women | 1,046 | 863 | 1,488 | 1,750 | 2,195 | 204 | 161 | 291 | 375 | 424 |
| Age (years); mean | 30.1 | 30.3 | 29.9 | 30.0 | 29.8 | 29.0 | 29.9 | 29.6 | 29.4 | 29.6 |
| Race/ethnicity, % | ||||||||||
| Non-Hispanic White | 80.9 | 80.4 | 82.4 | 82.2 | 85.0 | 87.1 | 84.1 | 86.0 | 92.3 | 91.8 |
| Non-Hispanic Black | 4.7 | 3.9 | 4.0 | 3.9 | 3.4 | 1.5 | 1.5 | 2.1 | 0.6 | 0.2 |
| Non-Hispanic Asian | 2.7 | 2.9 | 2.0 | 1.6 | 1.0 | 3.5 | 4.0 | 3.7 | 1.3 | 1.4 |
| Hispanic | 8.2 | 8.7 | 7.6 | 7.8 | 6.2 | 3.4 | 3.1 | 3.0 | 1.9 | 1.9 |
| Other race/ethnicity | 3.5 | 4.2 | 4.0 | 4.5 | 4.4 | 3.7 | 7.3 | 5.1 | 3.9 | 4.7 |
| ≤12 years education, % | 6.4 | 6.5 | 6.7 | 4.8 | 6.2 | 5.8 | 5.1 | 5.9 | 3.9 | 4.7 |
| Household income <50,000 USD/year, % | 24.9 | 24.0 | 21.3 | 20.1 | 22.2 | 19.2 | 14.6 | 17.5 | 13.1 | 15.0 |
| BMI (kg/m2); mean | 28.3 | 28.0 | 28.0 | 28.3 | 28.8 | 26.9 | 27.5 | 27.8 | 27.2 | 27.2 |
| Physical activity (MET-hours/week); mean | 39.3 | 39.5 | 36.9 | 37.3 | 37.2 | 34.4 | 35.3 | 37.1 | 35.1 | 33.8 |
| Current regular smoker, % | 7.7 | 8.1 | 7.6 | 7.2 | 7.3 | 8.8 | 8.8 | 8.3 | 6.9 | 8.2 |
| Alcohol (drinks/week); mean | 3.4 | 3.3 | 3.1 | 3.2 | 3.0 | 3.3 | 2.9 | 3.2 | 3.2 | 3.2 |
| Sugar-sweetened soda (drinks/week); mean | 1.7 | 1.8 | 1.6 | 1.6 | 2.0 | 1.3 | 1.0 | 1.2 | 1.1 | 1.1 |
| Healthy Eating Index 2010 score; mean | 64.6 | 64.3 | 64.1 | 64.4 | 62.6 | 65.9 | 67.5 | 67.2 | 66.9 | 66.5 |
| Daily multivitamins/folic acid, % | 77.0 | 79.0 | 79.2 | 79.4 | 79.4 | 78.7 | 81.1 | 78.9 | 75.9 | 74.4 |
| <7 hours/night of sleep, % | 25.8 | 24.7 | 26.6 | 25.6 | 28.1 | 24.3 | 23.8 | 26.3 | 25.1 | 21.9 |
| Parous, % | 29.9 | 29.4 | 34.5 | 35.7 | 37.3 | 21.6 | 25.7 | 24.5 | 25.2 | 26.5 |
| Intercourse <1 time/week, % | 22.1 | 22.7 | 21.1 | 21.8 | 19.6 | 22.6 | 17.9 | 21.8 | 19.2 | 20.7 |
| Hormonal last contraception, % | 39.8 | 38.7 | 37.8 | 38.2 | 41.5 | 39.3 | 33.3 | 34.9 | 33.0 | 32.1 |
| History of infertility, % | 12.0 | 10.1 | 10.3 | 10.6 | 11.3 | 10.0 | 5.7 | 6.6 | 8.2 | 6.9 |
| Doing something to improve chances, % | 77.0 | 75.3 | 77.6 | 77.5 | 78.7 | 78.1 | 77.2 | 73.9 | 76.4 | 75.5 |
| Urban census tract, % | 89.3 | 92.0 | 93.0 | 92.4 | 72.5 | 73.7 | 87.0 | 80.7 | 81.9 | 74.4 |
| Tract-level median household incomeb | 58,000 | 58,000 | 59,100 | 58,800 | 63,700 | 71,800 | 73,800 | 73,000 | 77,400 | 88,800 |
| Tract-level % <high school education | 12.1 | 12.5 | 11.7 | 11.6 | 10.8 | 16.6 | 15.7 | 16.7 | 16.7 | 16.6 |
| Tract-level % unemployed | 4.9 | 5.0 | 4.9 | 4.9 | 4.5 | 7.7 | 8.1 | 8.1 | 7.8 | 7.4 |
| Tract-level % non-Hispanic White | 72.6 | 70.5 | 71.5 | 71.5 | 77.2 | 80.9 | 77.6 | 79.8 | 81.5 | 83.8 |
aWith the exception of age, all characteristics are standardized to the age of the cohort at baseline.
bUS results reported in USD; Canadian results reported in Canadian dollars.
Among 7,342 US women, 4,099 conceived (66.6% when life-table methods were applied; 55.8% without accounting for censoring) during 27,565 menstrual cycles of follow-up. The remaining women either stopped trying to conceive (2.5%), initiated fertility treatment (7.1%), were lost to follow-up (15.4%), or participated for 12 cycles without conceiving (19.3%). Living within 50 meters of a major road (A1–A3) was associated with 12% lower fecundability compared with living ≥400 meters from a major road (FR = 0.88; 95% CI = 0.80, 0.98), with some evidence of a dose-response trend (Figure 1 and Supplemental Table 1; http://links.lww.com/EE/A110). Results for living close to a highway (A1–A2) were stronger in magnitude, but less precise (FR comparing <50 meters with ≥400 meters was 0.76; 95% CI = 0.59, 0.99). However, the estimated association between distance to highway and fecundability was not monotone, and fecundability was elevated for women living 50–99 and 100–199 meters compared with ≥400 meters from a major highway. Distance to major intersections (i.e., any intersection between two major roads) was also associated with reduced fecundability (FR comparing <100 with ≥400 meters was 0.83; 95% CI = 0.70, 1.00). When examining density of major roads around the home, operationalized as length of major roads within buffers of varying sizes, we found that women with a higher density of major roads around their residence had lower fecundability than women with lower density of major roads (Figure 2 and Supplemental Table 1; http://links.lww.com/EE/A110). Results were generally stronger for buffers of smaller size. For example, women with a length of ≥98 meters (≥75th percentile) of major road within 50 meters of their residence had an FR of 0.82 (95% CI = 0.70, 0.96) compared with women with no major roads within 50 meters of their residence. When using a buffer of 400 meters, the FR comparing the highest and lowest categories was 0.88 (95% CI = 0.80, 0.96).
Figure 1.
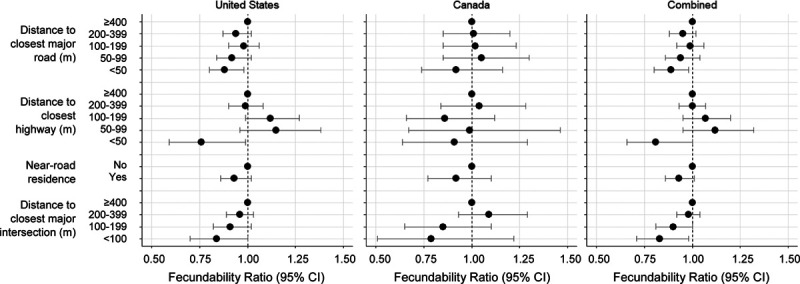
Association between distance to major roads and intersections and fecundability. Near-road residence was defined as living <50 meters from a highway or <100 meters from a major road. The left and center panels show results for women residing in the United States and Canada, respectively. The right panel shows results combined across the two countries using a fixed effects meta-analysis. Fecundability ratios are adjusted for age, race/ethnicity, income, education, BMI, physical activity, smoking, sugar-sweetened soda intake, HEI score, multivitamin/folic acid intake, parity, intercourse frequency, doing something to improve chances of conception, census tract median household income, census tract % with less than a high school education, and census tract % non-Hispanic White.
Figure 2.
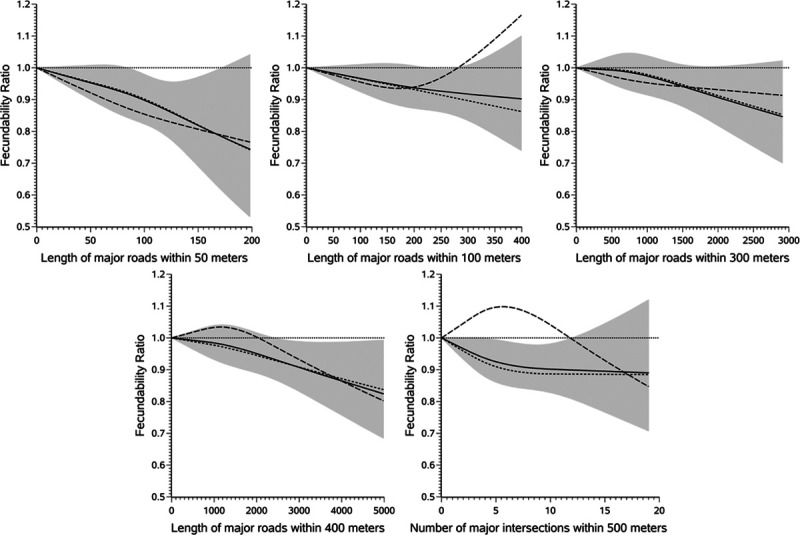
Restricted cubic spline analysis examining the association between density of major roads around the residence and fecundability. The short dashed lines represent the fecundability ratios (FRs) for women residing in the United States. The long dashed lines represent FRs for women from Canada. The solid line represents FRs pooled across the two countries, and the shaded area indicates the 95% confidence band for the pooled data. The reference value for all splines is 0 (in other words, no major roads within the buffer). We included three knots in each spline at the 10th, 50th, and 90th percentile for the pooled data. All splines are adjusted for age, race/ethnicity, income, education, BMI, physical activity, smoking, sugar-sweetened soda intake, HEI score, multivitamin/folic acid intake, parity, intercourse frequency, doing something to improve chances of conception, census tract median household income, census tract % with less than a high school education, and census tract % non-Hispanic White.
Among 1,448 Canadian women, 845 conceived (69.7% when life-table methods were applied; 58.4% without accounting for censoring) over 5,279 menstrual cycles of follow-up. The remaining women either stopped trying to conceive (2.8%), initiated fertility treatment (4.8%), were lost to follow-up (16.2%), or participated for 12 cycles without conceiving (18.0%). Results among Canadian women were generally similar in magnitude to those among US women, but were less precise due to smaller sample size (Figure 1 and Supplemental Table 1; http://links.lww.com/EE/A110). For example, living within 50 meters of a major road (DMTI classifications of expressway, highway, or major road) was associated with 7% lower fecundability compared with living ≥400 meters away (FR = 0.93; 95% CI = 0.74, 1.16), although results for categories of 50–99, 100–199, and 200–399 were different than those from the United States The FR for living <100 meters from a major intersection (compared with ≥400 meters) was 0.79 (95% CI = 0.51, 1.22). Associations with density of major roads (Figure 2 and Supplemental Table 1; http://links.lww.com/EE/A110) were observed in the 50-meter buffer only: comparing ≥98 meters of roads within 50 meters with no roads within 50 meters was associated with 23% lower fecundability (FR = 0.77; 95% CI = 0.48, 1.24). Length of major roads within larger buffers was not appreciably related to fecundability.
The estimates combined across countries using a fixed effects meta-analysis generally supported an association between road proximity and fecundability (Figures 1 and 2 and Supplemental Table 1; http://links.lww.com/EE/A110). The strongest combined results were for distance from highways (FR for <50 vs. ≥400 meters was 0.81; 95% CI = 0.66, 1.00) and length of major roads within a 50 meter buffer (FR for ≥98 vs. 0 meters was 0.82; 95% CI = 0.70, 0.95). Restricted cubic spline models (Figure 2) indicated an inverse association between density of major roads around the residence and fecundability at all examined buffers; results were strongest, but the least precise, when using the 50-meter buffer.
Results were generally stronger among parous women (Figure 3), particularly in the United States (Supplemental Table 2; http://links.lww.com/EE/A110). We did not find strong evidence of effect modification by folate intake, regardless of the cut point used or the assessment of total folate or supplemental folate only (Supplemental Table 3; http://links.lww.com/EE/A110). When we restricted to women who had been trying to conceive for 0–6 cycles and 0–2 cycles, results were similar in magnitude, but less precise (Supplemental Table 4; http://links.lww.com/EE/A110). Last, results were comparable when restricting to urban addresses (Supplemental Table 5; http://links.lww.com/EE/A110).
Figure 3.
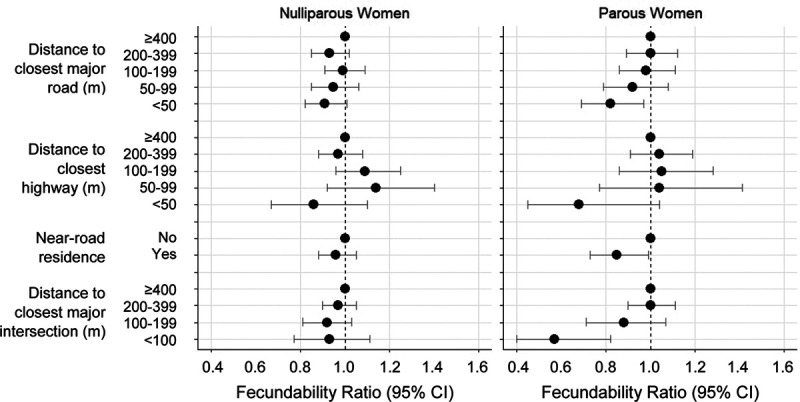
Association between distance to major roads and intersections and fecundability, stratified by parity and pooled across cohorts using a fixed effects meta-analysis. Near-road residence was defined as living <50 meters from a highway or <100 meters from a major road. The left panel shows results for nulliparous women. The right panel shows results for parous women. Fecundability ratios are adjusted for age, race/ethnicity, income, education, BMI, physical activity, smoking, sugar-sweetened soda intake, HEI score, multivitamin/folic acid intake, parity, intercourse frequency, doing something to improve chances of conception, census tract median household income, census tract % with <high school education, and census tract % non-Hispanic White.
Discussion
In this large geographically dispersed cohort of pregnancy planners residing across the United States and Canada, most metrics of residential proximity to major roads, particularly within 100 meters, were associated with lower fecundability. Although the Canadian results were less precise, the effect estimates were generally similar in magnitude across both countries.
These findings are consistent with several cohort studies examining residential proximity to major roads and fertility treatment outcomes,23,24 fecundability,25 and infertility,26 which have found that living close to major roads is associated with subfertility. The primary limitations of previous studies have been small size,23,25 enrollment of participants from restricted geographic areas,23–25 and limited control for confounding by neighborhood-level factors.25 In addition, only one previous study on this topic enrolled couples trying to conceive spontaneously and prospectively measured their time-to-pregnancy25; the other studies either assessed fertility treatment outcomes23,24 or prospectively-measured infertility.26
Not all of the measured exposure metrics showed dose-response associations with fecundability. For example, in the United States, distance to the nearest highway was associated with reduced fecundability in the closest category, but slightly increased fecundability in the middle categories. We do not suspect that the association of increased fecundability in the middle categories is causal, but could stem from unmeasured confounding or chance. Likewise, many of the associations among Canadian women were imprecise and nonmonotonic, and were associated with reduced fecundability only in the extreme categories.
Our finding of a stronger association among parous women compared with nulliparous women, particularly in the United States, agrees with results from the Nurses’ Health Study II, where the association between living within 200 meters of an A1–A3 road and infertility was stronger for secondary infertility (i.e., infertility among parous women) compared with primary infertility. Women at risk for secondary infertility have proven fertility, whereas nulliparous women include a mix of sterile, subfertile, and fertile women. If air pollution is more strongly associated with a specific type of infertility (e.g., ovulatory infertility), and the proportion of this type of infertility is more prevalent in secondary than primary infertility, differences in infertility subtype could explain our finding.
In the EARTH study, a prospective study of couples undergoing fertility treatment at a Massachusetts hospital, the association between residential proximity to major roads and the probability of live birth was stronger among women with low total folate intake,50 which could be due to the effect of both air pollution and folate on deoxyribonucleic acid (DNA) methylation,53,54 oxidative stress,55,56 and/or inflammation.57 We evaluated this hypothesis in PRESTO by stratifying results by total folate intake above or below the median (~1,000 DFE/day). However, we observed similar associations between roadway proximity and fecundability across strata of folate intake. We selected different cut points, including 400 (the daily recommended intake) and 800 (twice the daily recommended intake) DFE/day, but still found no meaningful differences across strata. It is possible that low folate intake only exacerbates the effect of air pollution at higher levels than we were able to observe in this cohort.
While residential proximity to major roads is commonly used as a proxy for exposure to traffic, it is also related to urbanicity, noise, lack of green space, and neighborhood socioeconomic status. Several studies have shown that exposure to individual air pollutants, including fine particulate matter and nitrogen dioxide, is associated with markers of lower fertility.24,26,58–64 Likewise, noise from road traffic has been associated with subfecundity in the Danish National Birth Cohort.65 Noise may influence fertility through shorter sleep duration and poorer sleep quality, greater stress levels, and greater depressive symptoms, all of which have been associated with reduced fecundability in PRESTO.66–69 Green space, while unstudied in relation to fertility, has been associated with improved birth outcomes and child development.70,71 Thus, while our use of a proxy exposure measure effectively captures the complex mixture of exposures found near major roads, we could not identify the contribution of each of these factors to the observed associations.
We based our exposure assessment on each participant’s residential address. We did not collect information on time-activity patterns, housing characteristics influencing pollution infiltration, and other factors that may result in a difference between our exposure metric and an individual’s exposure to traffic-related air pollution. Thus, some misclassification is inevitable. However, we assessed exposure using residential address reported at baseline (i.e., before pregnancy was observed); therefore, misclassification is likely nondifferential, which is expected to bias results towards the null in the extreme categories of exposure.
Our outcome measure was estimated using self-reported attempt time at study entry, LMP dates, usual cycle length, and pregnancy status. To the extent that any of these variables were measured with error, outcome misclassification could have occurred. Attempt time at study entry may be misclassified, particularly for women with longer attempt times at study entry, for whom digit preference is more likely. However, when we restricted analyses to women with 0–2 cycles of attempt at study entry, results were similar, indicating that misclassification is not an important source of bias. Previous validation work in PRESTO has shown that 93% of women reported baseline LMP dates within 1 day of LMP dates prospectively collected in real-time through a menstrual charting app.27 Likewise, among women who conceived, LMP date reported during early pregnancy was within 1 day of LMP date from the menstrual charting app for 98% of women.27 Although we may have missed a small number of conceptions that ended in early losses because we did not have daily measures of urinary human chorionic gonadotropin (hCG), 96% of the women in our cohort report using home pregnancy tests and the average gestational weeks at pregnancy detection was 4.0 (interquartile range = 3.7, 4.4), indicating that women are testing early (i.e., before a missed period). Therefore, we expect outcome misclassification to be minimal.
We controlled for a wide range of potential confounders, including individual- and neighborhood-level sociodemographics, lifestyle, and medical variables. However, we did not observe strong confounding in the analyses. As in any observational study, the possibility of unmeasured confounding exists, but we do not have specific hypotheses regarding which variables could explain the observed associations.
Loss to follow-up was not appreciably associated with living close to major roads (16.0% of women living ≥400 meters from the closest major road were lost to follow-up compared with 15.3% of women living <50 meters from the closest major road). Therefore, differential attrition is likely not an important source of bias in this analysis.
Our study population consisted of volunteer pregnancy planners who enrolled through the internet. Use of internet-based recruitment of participants, methodology that has been used in many cohort studies and randomized trials, should not necessarily bias etiologic associations based on internal comparisons, as we have previously demonstrated.72 However, pregnancy planning is related to higher socioeconomic status and healthier lifestyle. It is possible that residence-based ambient traffic exposure has less of an effect in a higher socioeconomic status cohort, where housing characteristics (e.g., high-quality insulation that prevents filtration into the home) or other built environment features could reduce personal exposure to air pollution and noise. This could therefore modify the association between residential traffic exposure and fecundability, and therefore, our results may not be generalizable to populations with lower socioeconomic status.
Our results lend support to the hypothesis that living close to major roads, as a proxy for traffic-related air pollution, is associated with reduced fecundability. We observed this association in a cohort of women that were largely non-Hispanic White and of high socioeconomic status. The observed association, if causal, could have greater ramifications in more highly polluted areas and in populations with lower socioeconomic status.
Conflicts of interest statement
PRESTO has received in-kind donations from FertilityFriend.com, Kindara.com, Sandstone Diagnostics, and Swiss Precision Diagnostics for primary data collection. L.A.W. serves as a consultant to AbbVie, Inc. for her work on uterine fibroids. The other authors have no conflicts to report.
The results reported herein correspond to specific aim 2 of grant R01-ES028923 to investigators Wise and Hatch from the National Institute of Environmental Health Sciences (NIEHS). This work was also supported by grant P30-ES007033 from the NIEHS, grant R01-HD086742 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), grant R21-HD072326 from the NICHD, grant R01-HD060680 from the NICHD, and grant R21-HD050264 from the NICHD. The authors received funding to support open access publishing.
ACKNOWLEDGMENTS
We are grateful to Michael Bairos for development and maintenance of the web-based infrastructure of PRESTO; Alina Chaiyasarikul for her efforts cleaning residential addresses; Tanran Wang for managing the PRESTO data; and Jessica Levinson for general study support.
Supplementary Material
Footnotes
Published online 11 November 2020
Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
References
- 1.Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982-2010: data from the National Survey of Family Growth. Natl Health Stat Report. 2013;1–18, 1 p following 19. [PubMed] [Google Scholar]
- 2.Thoma ME, McLain AC, Louis JF, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013; 99:1324–1331.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bushnik T, Cook JL, Yuzpe AA, Tough S, Collins J. Estimating the prevalence of infertility in Canada. Hum Reprod. 2012; 27:738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macaluso M, Wright-Schnapp TJ, Chandra A, et al. A public health focus on infertility prevention, detection, and management. Fertil Steril. 2010; 93:16.e1–16.e10. [DOI] [PubMed] [Google Scholar]
- 5.Finnström O, Källén B, Lindam A, Nilsson E, Nygren KG, Olausson PO. Maternal and child outcome after in vitro fertilization–a review of 25 years of population-based data from Sweden. Acta Obstet Gynecol Scand. 2011; 90:494–500. [DOI] [PubMed] [Google Scholar]
- 6.Qin J, Sheng X, Wu D, et al. Adverse obstetric outcomes associated with in vitro fertilization in singleton pregnancies. Reprod Sci. 2017; 24:595–608. [DOI] [PubMed] [Google Scholar]
- 7.Reddy UM, Wapner RJ, Rebar RW, Tasca RJ. Infertility, assisted reproductive technology, and adverse pregnancy outcomes: executive summary of a National Institute of Child Health and Human Development workshop. Obstet Gynecol. 2007; 109:967–977. [DOI] [PubMed] [Google Scholar]
- 8.Hansen M, Kurinczuk JJ, Milne E, de Klerk N, Bower C. Assisted reproductive technology and birth defects: a systematic review and meta-analysis. Hum Reprod Update. 2013; 19:330–353. [DOI] [PubMed] [Google Scholar]
- 9.Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982-2010. Natl Health Stat Report. 2014; 73:1–21. [PubMed] [Google Scholar]
- 10.Kulick ER, Wellenius GA, Boehme AK, Sacco RL, Elkind MS. Residential proximity to major roadways and risk of incident ischemic stroke in NOMAS (The Northern Manhattan Study). Stroke. 2018; 49:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh R, Lurmann F, Perez L, et al. Near-roadway air pollution and coronary heart disease: burden of disease and potential impact of a greenhouse gas reduction strategy in Southern California. Environ Health Perspect. 2016; 124:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauptman M, Gaffin JM, Petty CR, et al. Proximity to major roadways and asthma symptoms in the School Inner-City Asthma Study. J Allergy Clin Immunol. 2020; 145:119–126.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindgren A, Björk J, Stroh E, Jakobsson K. Adult asthma and traffic exposure at residential address, workplace address, and self-reported daily time outdoor in traffic: a two-stage case-control study. BMC Public Health. 2010; 10:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Z, Lin F, Wang B, Cao Y, Hou X, Wang Y. Residential proximity to major roadways and risk of type 2 diabetes mellitus: a meta-analysis. Int J Environ Res Public Health. 2016; 14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh SM, Ryu BT, Chung KH. Identification of estrogenic and antiestrogenic activities of respirable diesel exhaust particles by bioassay-directed fractionation. Arch Pharm Res. 2008; 31:75–82. [DOI] [PubMed] [Google Scholar]
- 16.Sídlová T, Novák J, Janosek J, Andel P, Giesy JP, Hilscherová K. Dioxin-like and endocrine disruptive activity of traffic-contaminated soil samples. Arch Environ Contam Toxicol. 2009; 57:639–650. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Xie P, Kettrup A, Schramm KW. Inhibition of progesterone receptor activity in recombinant yeast by soot from fossil fuel combustion emissions and air particulate materials. Sci Total Environ. 2005; 349:120–128. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012; 10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal A, Rana M, Qiu E, AlBunni H, Bui AD, Henkel R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia. 2018; 50:e13126. [DOI] [PubMed] [Google Scholar]
- 20.Maybin JA, Critchley HO, Jabbour HN. Inflammatory pathways in endometrial disorders. Mol Cell Endocrinol. 2011; 335:42–51. [DOI] [PubMed] [Google Scholar]
- 21.Mohallem SV, de Araújo Lobo DJ, Pesquero CR, et al. Decreased fertility in mice exposed to environmental air pollution in the city of Sao Paulo. Environ Res. 2005; 98:196–202. [DOI] [PubMed] [Google Scholar]
- 22.Veras MM, Damaceno-Rodrigues NR, Guimarães Silva RM, et al. Chronic exposure to fine particulate matter emitted by traffic affects reproductive and fetal outcomes in mice. Environ Res. 2009; 109:536–543. [DOI] [PubMed] [Google Scholar]
- 23.Gaskins AJ, Hart JE, Mínguez-Alarcón L, et al. Residential proximity to major roadways and traffic in relation to outcomes of in vitro fertilization. Environ Int. 2018; 115:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quraishi SM, Lin PC, Richter KS, et al. Ambient air pollution exposure and fecundability in women undergoing in vitro fertilization. Environ Epidemiol. 2019; 3:e036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendola P, Sundaram R, Louis GMB, et al. Proximity to major roadways and prospectively-measured time-to-pregnancy and infertility. Sci Total Environ. 2017; 576:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahalingaiah S, Hart JE, Laden F, et al. Adult air pollution exposure and risk of infertility in the Nurses’ Health Study II. Hum Reprod. 2016; 31:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wise LA, Rothman KJ, Mikkelsen EM, et al. Design and conduct of an internet-based preconception cohort study in North America: pregnancy study online. Paediatr Perinat Epidemiol. 2015; 29:360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol. 2001; 154:1089–1099. [DOI] [PubMed] [Google Scholar]
- 29.Zandbergen PA. Influence of geocoding quality on environmental exposure assessment of children living near high traffic roads. BMC Public Health. 2007; 7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.United States Census Bureau. TIGER/Line(TM) Files, Appendix E. 2011. Available at: https://www2.census.gov/geo/tiger/TIGER1992/Documentation/APPENDXE.txt. Accessed 8 May 2020.
- 31.DMTI Spatial. DMTI Road Network. 2016. Available at: https://www.dmtispatial.com/canmap/. Accessed 8 May 2020.
- 32.HEI Panel on the Health Effects of Traffic-Related Air Pollution. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects. HEI Special Report 17. Health Effects Institute; 2010. Available at: https://www.healtheffects.org/publication/traffic-related-air-pollution-critical-review-literature-emissions-exposure-and-health. Accessed 5 February 2020. [Google Scholar]
- 33.Beckerman B, Jerrett M, Brook JR, Verma DK, Arain MA, Finkelstein MM. Correlation between nitrogen dioxide with other traffic pollutants near a major expressway. Atmos Environ. 2008; 42:275–290. [Google Scholar]
- 34.Gilbert NL, Woodhouse S, Stieb DM, Brook JR. Ambient nitrogen dioxide and distance from a major highway. Sci Total Environ. 2003; 312:43–46. [DOI] [PubMed] [Google Scholar]
- 35.Roorda-Knape MC, Janssen NA, de Hartog J, Van Vliet PH, Harssema H, Brunekreef B. Traffic related air pollution in city districts near motorways. Sci Total Environ. 1999; 235:339–341. [DOI] [PubMed] [Google Scholar]
- 36.Venkatram A, Isakov V, Seila R, Baldauf R. Modeling the impacts of traffic emissions on air toxics concentrations near roadways. Atmos Environ. 2009; 43:3191–3199. [Google Scholar]
- 37.Guenther PM, Kirkpatrick SI, Reedy J, et al. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. J Nutr. 2014; 144:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. 2012. Available at: https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html. Accessed 12 February 2020.
- 39.Statistics Canada. Standard Geographical Classification (SGC) 2016 - Introduction. 2016. Available at: https://www.statcan.gc.ca/eng/subjects/standard/sgc/2016/introduction. Accessed 12 February 2020.
- 40.Cox DR. Regression models and life-tables. R Stat Soc J (B). 1972; 34:187–220. [Google Scholar]
- 41.Karner AA, Eisinger DS, Niemeier DA. Near-roadway air quality: synthesizing the findings from real-world data. Environ Sci Technol. 2010; 44:5334–5344. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002; 52:1032–1042. [DOI] [PubMed] [Google Scholar]
- 43.Schisterman EF, Cole SR, Ye A, Platt RW. Accuracy loss due to selection bias in cohort studies with left truncation. Paediatr Perinat Epidemiol. 2013; 27:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Howards PP, Hertz-Picciotto I, Poole C. Conditions for bias from differential left truncation. Am J Epidemiol. 2007; 165:444–452. [DOI] [PubMed] [Google Scholar]
- 45.Deddens JA, Petersen MR. Approaches for estimating prevalence ratios. Occup Environ Med. 2008; 65:481, 501–481, 506. [DOI] [PubMed] [Google Scholar]
- 46.Guenther PM, Casavale KO, Reedy J, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013; 113:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; 2004. [Google Scholar]
- 48.Stingone JA, Luben TJ, Carmichael SL, et al. ; National Birth Defects Prevention Study. Maternal exposure to nitrogen dioxide, intake of methyl nutrients, and congenital heart defects in offspring. Am J Epidemiol. 2017; 186:719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodrich AJ, Volk HE, Tancredi DJ, et al. Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder. Autism Res. 2018; 11:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaskins AJ, Minguez-Alarcon L, Fong KC, et al. Exposure to traffic-related air pollution, supplemental folate intake, and live birth among women undergoing assisted reproduction. Am J Epidemiol. 2019; 189:1595–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin DY, Zeng D. On the relative efficiency of using summary statistics versus individual-level data in meta-analysis. Biometrika. 2010; 97:321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rice K, Higgins JPT, Lumley T. A re-evaluation of fixed effect(s) meta-analysis. J R Statist Soc A. 2018; 181:205–227. [Google Scholar]
- 53.Alfano R, Herceg Z, Nawrot TS, Chadeau-Hyam M, Ghantous A, Plusquin M. The impact of air pollution on our epigenome: how far is the evidence? (A systematic review). Curr Environ Health Rep. 2018; 5:544–578. [DOI] [PubMed] [Google Scholar]
- 54.Baccarelli A, Wright RO, Bollati V, et al. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009; 179:572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011; 2011:487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joshi R, Adhikari S, Patro BS, Chattopadhyay S, Mukherjee T. Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radic Biol Med. 2001; 30:1390–1399. [DOI] [PubMed] [Google Scholar]
- 57.Midouhas E, Kokosi T, Flouri E. Neighbourhood-level air pollution and greenspace and inflammation in adults. Health Place. 2019; 58:102167. [DOI] [PubMed] [Google Scholar]
- 58.Boulet SL, Zhou Y, Shriber J, Kissin DM, Strosnider H, Shin M. Ambient air pollution and in vitro fertilization treatment outcomes. Hum Reprod. 2019; 34:2036–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaskins AJ, Fong KC, Abu Awad Y, et al. Time-varying exposure to air pollution and outcomes of in vitro fertilization among couples from a fertility clinic. Environ Health Perspect. 2019; 127:77002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Legro RS, Sauer MV, Mottla GL, et al. Effect of air quality on assisted human reproduction. Hum Reprod. 2010; 25:1317–1324. [DOI] [PubMed] [Google Scholar]
- 61.Nieuwenhuijsen MJ, Basagaña X, Dadvand P, et al. Air pollution and human fertility rates. Environ Int. 2014; 70:9–14. [DOI] [PubMed] [Google Scholar]
- 62.Nobles CJ, Schisterman EF, Ha S, Buck Louis GM, Sherman S, Mendola P. Time-varying cycle average and daily variation in ambient air pollution and fecundability. Hum Reprod. 2018; 33:166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qiu J, Dong M, Zhou F, Li P, Kong L, Tan J. Associations between ambient air pollution and pregnancy rate in women who underwent in vitro fertilization in Shenyang, China. Reprod Toxicol. 2019; 89:130–135. [DOI] [PubMed] [Google Scholar]
- 64.Slama R, Bottagisi S, Solansky I, Lepeule J, Giorgis-Allemand L, Sram R. Short-term impact of atmospheric pollution on fecundability. Epidemiology. 2013; 24:871–879. [DOI] [PubMed] [Google Scholar]
- 65.Christensen JS, Raaschou-Nielsen O, Ketzel M, et al. Exposure to residential road traffic noise prior to conception and time to pregnancy. Environ Int. 2017; 106:48–52. [DOI] [PubMed] [Google Scholar]
- 66.Willis SK, Hatch EE, Wesselink AK, Rothman KJ, Mikkelsen EM, Wise LA. Female sleep patterns, shift work, and fecundability in a North American preconception cohort study. Fertil Steril. 2019; 111:1201–1210.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wise LA, Rothman KJ, Wesselink AK, et al. Male sleep duration and fecundability in a North American preconception cohort study. Fertil Steril. 2018; 109:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wesselink AK, Hatch EE, Rothman KJ, et al. Perceived stress and fecundability: a preconception cohort study of North American couples. Am J Epidemiol. 2018; 187:2662–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nillni YI, Wesselink AK, Gradus JL, et al. Depression, anxiety, and psychotropic medication use and fecundability. Am J Obstet Gynecol. 2016; 215:453.e1–453.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Agay-Shay K, Peled A, Crespo AV, et al. Green spaces and adverse pregnancy outcomes. Occup Environ Med. 2014; 71:562–569. [DOI] [PubMed] [Google Scholar]
- 71.Dadvand P, Nieuwenhuijsen MJ, Esnaola M, et al. Green spaces and cognitive development in primary schoolchildren. Proc Natl Acad Sci U S A. 2015; 112:7937–7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hatch EE, Hahn KA, Wise LA, et al. Evaluation of selection bias in an internet-based study of pregnancy planners. Epidemiology. 2016; 27:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


