Supplemental Digital Content is available in the text.
Keywords: Birth weight, Perfluorooctane sulfonic acid, Random effects, Meta-regression, Metaanalysis
Background:
Perfluorooctane sulfonic acid (PFOS) is a ubiquitous environmental contaminant. Most people in developed countries have detectable serum concentrations. Lower birth weight has been associated with serum PFOS in studies world-wide, many of which have been published only recently.
Methods:
To facilitate a causal assessment of the birth weight and PFOS association, we updated previous meta-analyses of the association and employed a method that facilitated inclusion of all available data in one analysis. Our analysis was based on observations from 29 studies.
Results:
The random effects summary was −3.22 g/ng/ml (95% confidence interval [CI] = −5.11, −1.33). In a subgroup analysis stratified by when in pregnancy the PFOS concentration was measured, the summary for the early group was −1.35 (95% CI = −2.33, −0.37) and for the later group was −7.17 (95% CI = −10.93, −3.41). In a meta-regression model including a term for timing of blood draw, the intercept was slightly positive but essentially zero (0.59 g/ng/ml, 95% CI = −1.94, 3.11). In other words, the model indicated that when blood was drawn at the very beginning of pregnancy, there was essentially no relation of birth weight to PFOS. The results from the subgroup analyses differed from those from the model because the average gestational age at blood draw in the early group was 14 weeks, when bias would still be expected. A stronger inverse association in Asian studies was not completely explained by their blood draws being from later in pregnancy.
Conclusions:
The evidence was weakly or not supportive of a causal association.
What this study adds.
We performed a meta-analysis of the association between birth weight and perfluorooctane sulfonic acid (PFOS). Using sub group analysis, we found that the association was present, but attenuated, when PFOS was measured early in pregnancy. Meta-regression showed that the time of blood draw was a key factor in the association and that there was no significant association present when PFOS is measured at the beginning of pregnancy, which supports the possibility of confounding related to timing of specimen sampling.
Introduction
Perfluorooctane sulfonic acid (PFOS) and the similar compound perfluorooctanoic acid (PFOA) are environmental contaminants of the general chemical class perfluorinated alkyl substances (PFAS).1 These compounds were used or have ongoing use in manufacturing of a variety of products, including consumer-use products.2 Almost everyone in developed countries has a detectable amount of PFOS and PFOA in their serum, due to exposure via contaminated food and other sources.3 The health advisory levels of PFOA and PFOS in drinking water have recently been reduced because of concern that exposure is linked to a variety of potential health effects.4
The association of maternal or cord blood serum (or plasma) PFOS concentration in relation to offspring birth weight was examined in two previous meta-analyses and was found to be inverse.5,6 Whether the evidence supported a causal association was questioned by both groups, but for different reasons. Based on pharmacokinetic simulations, Verner et al. predicted that the association would be more inverse when the specimen assayed for PFOS or PFOA was obtained later in pregnancy, demonstrating a confounding effect related to the timing of specimen sampling. Verner et al’s meta-regression analysis showed that timing of specimen draw was a statistically significant predictor of the association of PFOS with birthweight, as hypothesized. In their subsequent meta-analysis of birth weight and PFOS, Negri et al. found that among the studies with the specimen drawn in the first or second trimester of pregnancy, no statistically significant association was present (see their Table 11 in Negri et al6), whereas when a specimen was obtained from the mother later in pregnancy, at delivery, or from cord blood, the association was inverse and statistically significant.6 However, Negri et al6 concluded in their abstract that for PFOA and PFOS together, “No consistent pattern emerged for … timing of blood sampling,” apparently based on weaker evidence for the importance of timing among a subset of studies on PFOS, and no evidence of the importance of timing for PFOA. Their questioning of causality in humans was largely based on animal experiments showing that much larger doses of PFAS were needed to lower birth weight. Birth weight reduction in mice occurred at a slightly lower dose with PFOA than for PFOS; the comparison of slopes in epidemiologic studies using measured serum concentrations, however, did not clearly indicate a stronger association for PFOA as compared with PFOS.6 Another recent meta-analysis, of birth weight and PFOA, included a larger number of studies and provided evidence that timing of specimen draw was important for PFOA.7 Steenland et al7 observed that studies with specimens obtained earlier in pregnancy showed little support for an association, consistent with the earlier suggestion of pharmacokinetic bias by Verner et al.5 Steenland et al7 also introduced a methodologic advance whereby results from studies using a logarithmic transformation of exposure could be combined with those using no transformation, allowing a more statistically powerful analysis. The meta-analysis on birth weight and PFOS by Negri et al. was based on data for about 8,000 subjects; since then relevant data for more than 10,000 additional subjects have been published. Given the large number of recent publications not included in the previous meta-analyses on birth weight and PFOS, the possibility of using more advanced methods, and the pharmacokinetic evidence supporting the importance of timing in the association, we revisited the question of whether birth weight is related to PFOS, and whether this association varies by timing of blood draw.
Methods
Our meta-analysis protocol was registered with PROSPERO (International Prospective Register of Systematic Reviews) and is described below following a recommended format.8 The PROSPERO registration number is CRD42019140382. Italicized text below indicates amendments to the protocol, which are justified in the Supplemental Digital Content (SDC); http://links.lww.com/EE/A84.
Eligibility criteria
Participants: mother-child pairs. Intervention: observed concentration of PFOS in serum or plasma. Comparators: timing of blood draw used for measurement of PFOS. Outcome: birth weight. Study design: longitudinal with a blood measure before birth, or cross-sectional with blood collected at birth.
The measure of association must be from an analysis with birth weight as a continuous measure, and its relation to PFOS was with the exposure on a continuous scale, or a scale that could be re- expressed that way (e.g., if beta coefficients were given for categories of exposure, and mean or median exposure within category was given). The measure of exposure must have been obtained from a blood specimen that was drawn either before pregnancy, during pregnancy, or at the time of delivery, including cord blood. A measured serum or plasma concentration of PFOS in the mother or cord blood must be examined in relation to child birth weight. A central tendency indicator of when the blood draw for PFOS measurement was done, in relation to pregnancy onset, must be reported or estimable based on the reported data. The study must be conducted in humans, the date of publication must be 1 July, 2019 or before, and the report must be in English.
Information sources
All studies must be in PubMed and published online or in print as full reports.
Search strategy
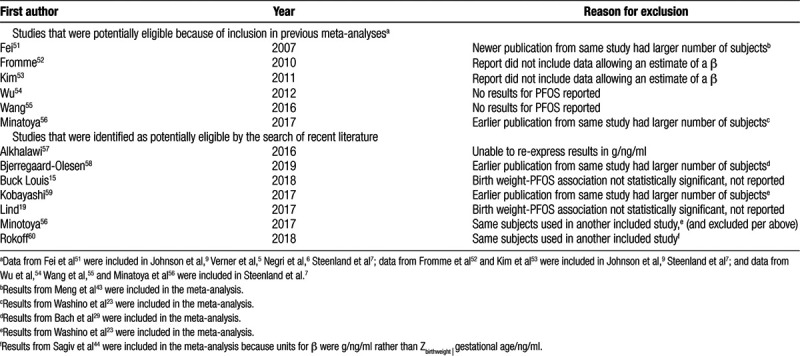
As noted above, two meta-analyses on birth weight and PFOS have already been published, and all studies included in those meta-analyses were potentially eligible. In addition, we reviewed the recent meta-analysis of Steenland et al. on birth weight and PFOA, and an older meta-analysis on birth weight and PFOA, to see if any of the studies identified by their formal search and included in their meta-analysis also had data on birth weight and PFOS.7,9
We also conducted a PubMed search based on a search strategy modified from the approach of Steenland et al, to discover any additional primary research on birth weight and PFOS published since 11-20-15 (the final date of the search conducted by Negri et al).6 The search algorithm is listed in the SDC.
The search was conducted independently by two authors (L.C. and M.P.L.) and discrepancies were resolved by discussion.
Study records
Data were abstracted into DistillerSR (Version 2.29.0, Evidence Partners, Inc., Ottawa, Canada). Study records were annotated with the reason for exclusion, if applicable.
Study selection process
All studies included in the four previous meta-analyses were reviewed to verify eligibility. With respect to studies potentially eligible for inclusion identified by the PubMed search, we reviewed the titles and abstracts to determine relevancy. If the study appeared to have the type of data required, we reviewed the entire report.
When the same participants were included in more than one eligible report, the report with the largest number of participants was included. The study selection was conducted independently by the same two authors and discrepancies were resolved by discussion.
Data collection process
Data for each study were summarized in a spreadsheet. The data for each study were abstracted independently by the same two authors and discrepancies were resolved by discussion. Piloting of the data collection process was done by one author (M.P.L.).
Data items
The study attributes and results that were recorded in the spreadsheet were: name of first author and year of publication, number of participants, location, design, sex of offspring, mean or median birth weight in study population, type of specimen analyzed for PFOS, timing of specimen draw, data on spread of timing of specimen draw, median or geometric mean PFOS, data on spread of concentration of PFOS, beta coefficient, and 95% confidence interval (CI) relating change in birth weight to PFOS and units thereof (using the most-adjusted coefficient presented), adjustment for gestational age, adjustment for parity, other adjustment factors, and other information as seemed appropriate (e.g., limitation of study to term births).
Outcomes and prioritization
The only outcome for which data were sought was birth weight.
Risk of bias in individual studies
We did not attempt to evaluate risk of bias in individual studies.
Given the inclusion criteria for studies, we suspected little potential bias among results other than that attributable to timing of blood draw or perhaps lack of adjustment for parity. Negri et al6 evaluated risk of bias in individual studies but little of use came from it. Instead, we characterized studies according to specific items that we thought might influence results and examined these in meta-regression analyses, described below. The approach we used was recommended by Greenland and O’Rourke.10
Data synthesis
The summary measure of association a was a beta coefficient relating change in birth weight in grams to ng/ml increase in serum or plasma PFOS. We used a random effects model based on the method of moments estimate of the between-study variance.11 Heterogeneity was quantified by the Q, I2, and T statistics.12,13
In some studies, the authors log-transformed the serum or plasma PFOS concentration before they fit the regression of birth weight on PFOS. In such instances, and related instances where the scale of birth weight or PFOS or log(PFOS) was altered before fitting the regression, we re-expressed the results so that they had the desired units. The method was like the one used by Steenland et al. for PFOA, with the main differences being that our method fitted a β in g/ng/ml to the reported β in g/log(ng/ml) using an algorithmic optimization over 6 points from the 25th to the 75th percentiles of the estimated PFOS distribution.7 Our methods of re-expressing results are described in detail in the SDC.
We also note that if the original authors measured PFOS in whole blood rather than serum or plasma, we rescaled their β coefficient to account for the difference in matrix.14
Additional analyses
The simple contrast that was used to evaluate the effect of timing of blood draw on the association was between pre- or early pregnancy (prepregnancy, first trimester, or first and second trimester combined) and later pregnancy (second trimester, third trimester, second and third trimester combined, or cord blood), as was done in Steenland et al.7 This subgroup analysis and one by continent was augmented by a random effects meta-regression analysis with effect modification of the birth weight-PFOS association by mean or median time of blood draw.12 We examined variation in the coefficient relating birth weight to PFOS concentration, after accounting for the effect of timing, according to: adjustment for gestational age, parity, median PFOS concentration, spread in timing of blood draw used for PFOS measurement, continent, mean birth weight in study population, inclusion of only term births, and re-expression needed for coefficient relating birth weight to log PFOS concentration. We conducted sensitivity analyses to evaluate the change in results after excluding certain groups of studies: (1) studies that used cord blood for measurement of PFOS; (2) studies from Asia; and (3) those for which timing of blood draw did not fit entirely within the timing group definitions give above. We also conducted sensitivity analyses where we added 1.26 g/ng/ml to β coefficients from studies using cord serum or plasma, because Verner et al. calculated that use of cord serum or plasma would bias βs by −1.26 g/ng/ml compared with maternal serum measurements at 40 weeks of gestation. We conducted sensitivity analyses using combinations of the bias adjustment and exclusions, as described above (e.g., after excluding studies that used cord blood, we fit a regression model that adjusted for gestational week of blood draw). We conducted a sensitivity analysis after including imputed null results for a large study that was not eligible for inclusion because the results were reported as not statistically significant.15 The criteria for statistical significance was a two-sided P value < 0.05.
Meta-bias(es)
We used a funnel plot to assess the possibility of publication bias.
Confidence in cumulative evidence
The strength of the evidence for an association between birth weight and serum PFOS concentration among studies with the blood specimen obtained before or early in pregnancy was assessed and characterized using a GRADE-type approach.16
Data analyses were conducted using Comprehensive Meta-analysis, version 3.17
Results
Nineteen previously-identified studies met our eligibility criteria and were included in the meta-analysis (eTable1; http://links.lww.com/EE/A84; Table 1). Six of the reports included in previous meta-analyses did not meet our criteria for inclusion; the reasons for exclusion are described in Table 2. As shown in eFigure 1; http://links.lww.com/EE/A84, our search of recent literature identified 191 records that needed screening, of which 164 were excluded. The reasons for exclusion of the 164 are shown in detail in eTable 2; http://links.lww.com/EE/A84. Of the 27 potentially eligible studies identified from our literature search, 10 had previously been designated for inclusion (included in previous meta-analysis), and seven were excluded for various reasons (Table 2), which left 10 new reports for inclusion. A total of 29 reports presented data that were included in the meta-analysis. Of these, 19 had been included in one or more previous meta-analyses, and 10 new reports were identified by our literature search. eTable 1; http://links.lww.com/EE/A84 shows the source(s) of the 29 included studies. Robledo et al18 and Lind et al19 presented results stratified by sex; Lauritzen et al20 presented results from a multicenter study, stratified by country (Norway or Sweden). For that reason, our analysis was based on 32 observations from 29 studies (Table 1).
Table 1.
Studies included in the meta-analysis of birth weight and PFOS and their characteristics.
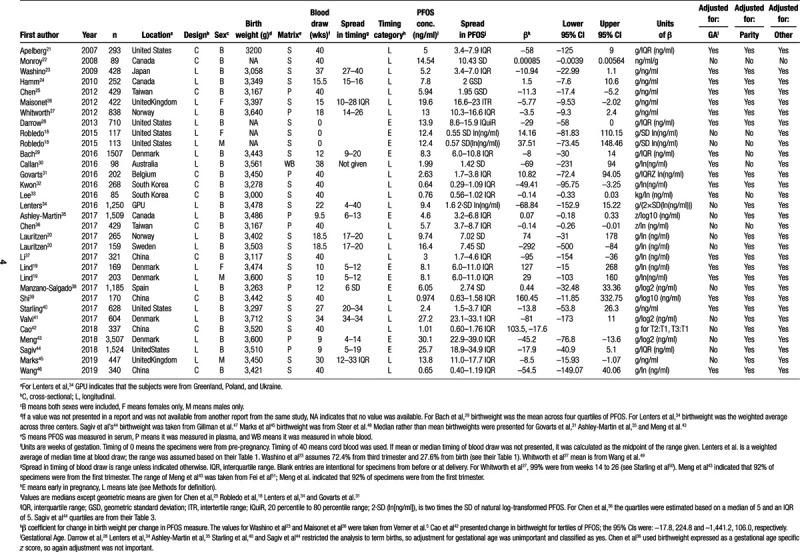
Table 2.
Studies identified as potentially eligible that were excluded from the meta-analysis, and reason for exclusion.
The studies that were included in the meta-analysis were published from 2007 to 2019 and ranged in size from 85 to 3,507 participants; the distribution of studies by continent was: Europe, 11; Asia, 9; North America, 8; and Australia, 1. Eighteen studies were longitudinal and 11 studies were cross-sectional. Most studies presented results for both sexes combined. Mean or median birth weight tended to be lowest in Japan and South Korea and the highest in Scandinavia. Two studies had measures of PFOS before pregnancy, six studies had a measure of PFOS from early in pregnancy, 10 studies were from later in pregnancy, and 11 had measures at delivery. Of the 11 with measures at delivery, all used cord blood except Monroy et al,22 who used maternal blood (not shown in table). Of the nine studies from Asia, only one had blood from before delivery. Median PFOS concentration tended to be the lowest among studies in Asia (the Australian study was also low) and the highest in studies from Europe and North America, especially when those pregnancies were in 1990–2002.
The units of the β coefficients from regression analyses of birth weight on PFOS varied across studies, as did the variables used to adjust the β coefficients. For 7 studies, the units of the β were g/ng/ml (g birth weight per ng/ml PFOS), the metric we chose for summarizing the results. For 4 studies that expressed β in g/interquartile range (ng/ml), the β was divided by the interquartile range to get results in g/ng/ml. For the study that presented a coefficient from a regression of PFOS on birth weight, we re-expressed the results as a β in g/ng/ml using the method described by Negri et al.6 For the study that presented the β coefficient for categories of PFOS in tertiles, we estimated the mean PFOS concentration in each tertile, calculated the distance between the means of the first and second and first and third tertiles, rescaled the two βs as per g/ng/ml, and then took the weighted average of the rescaled β for the two tertiles to estimate an overall β (see SDC for details). For the remaining 16 studies, the denominator of the original β included a logarithmic transformation of PFOS. Before re-expressing the log-based β to a g/ng/ml scale, initial re-expressions were required in some cases. The numerators of the β coefficient were re-expressed as g in 3 cases (see SDC for details). In some cases, a variant of log(PFOS) was used in the original denominator, such as SD of log(PFOS). These variant denominators were re- expressed as log(ng/ml), and then the β coefficients were re-expressed as g/ng/ml, using the method described in the SDC.
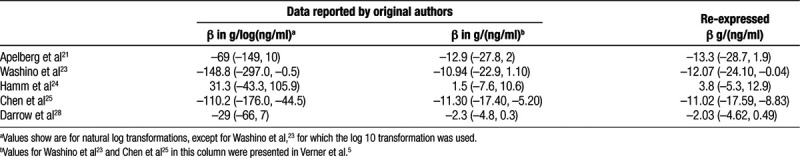
We identified 5 studies with a β coefficient in g/ng/ml and in g/log(ng/ml) available from the original authors and used these to calibrate and evaluate our re-expression procedure (Table 3). The average difference between the original β in g/ng/ml and the re-expressed β in g/ng/ml was 0.26. Eight of the 29 studies had the blood drawn before or early in pregnancy; among these, half the study results were re-expressed with the algorithm and half were not. Twenty-one of the 29 had the blood drawn later in pregnancy; among these, 11 had the results re-expressed. The Pearson’s correlation coefficient of the z score (β/standard error of β) between the original and re-expressed results was 0.99. Given the advantage of increasing our statistical power by combining β regardless of original units, we considered the degree of error introduced by the re-expression to be acceptable.
Table 3.
Observed β coefficients (and 95% CIs) from regressions of birthweight on PFOS and corresponding estimates calculated using the re-expression algorithm
For the 29 studies (32 observations) as a group the βs were heterogenous; the Q was 74.4 with 31 d.f., P < 0.00002; the I2 = 58.3; and the T = 3.1 (Figure 1; Table 3). To remind the reader about the statistics used in meta-analysis and their interpretation, we have included a brief didactic overview in the SDC. The random effects summary was −3.22 g/ng/ml (95% CI = −5.11, −1.33). When we stratified the observations by when in pregnancy the PFOS concentration was measured (subgroup analysis), the summary for the early group was −1.35 (95% CI = −2.33, −0.37) and for the later group was −7.17 (95% CI = −10.93, −3.41; Figure 2). The difference between groups (5.82) was strongly supported by the heterogeneity Q, with a corresponding P value of 0.003. The summary β for the subgroup analysis of studies from Asia was much more negative than for the subgroup analyses of studies from Europe or North America, and this difference was supported by the heterogeneity Q, with a corresponding P value of 0.02 (Table 4).
Figure 1.
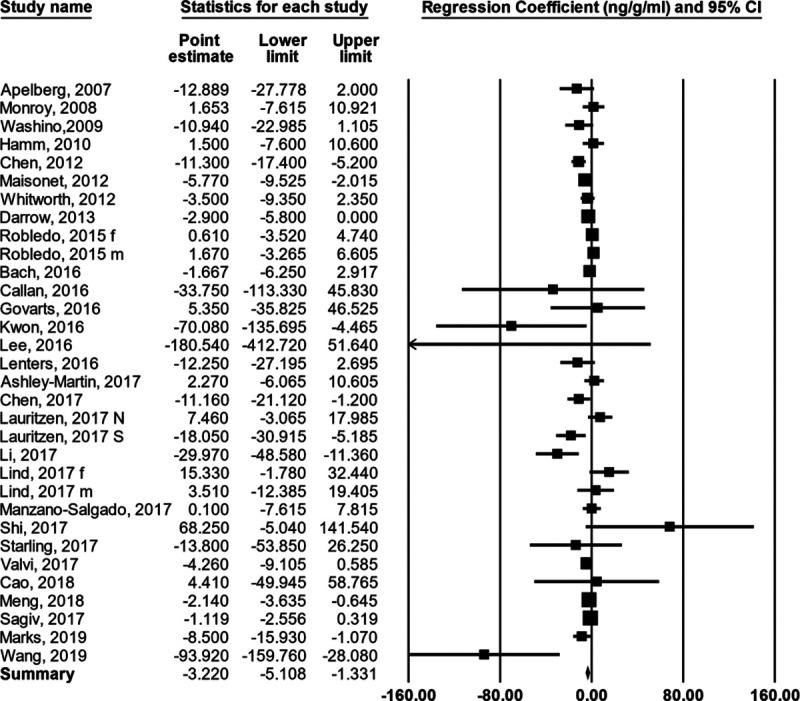
Forest plot showing the regression coefficient relating birth weight to PFOS concentration in each study (and 95% CI) and an estimate of the mean value after a random effects analysis.
Figure 2.
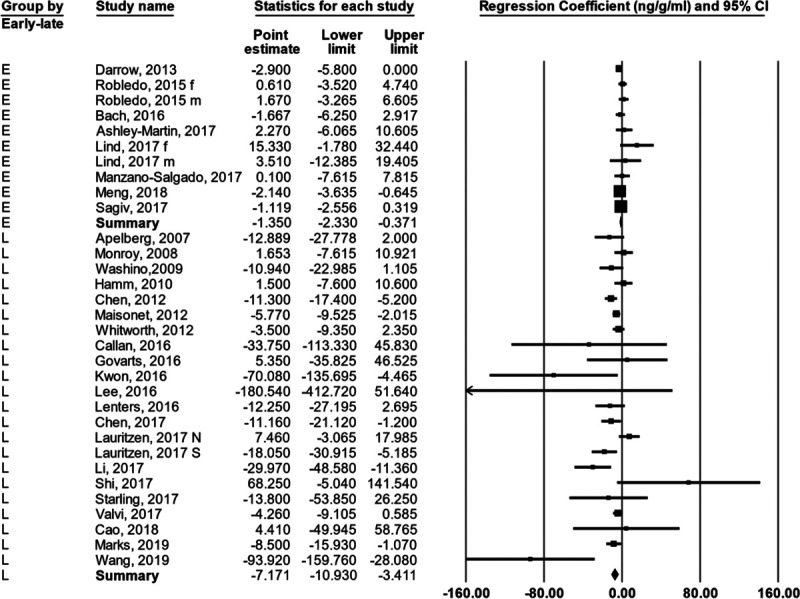
Forest plot showing the regression coefficient relating birth weight to PFOS concentration in each study (and 95% CI) and an estimate of the mean value using a random effects model with stratification by the timing of blood draw for PFOS analysis. See Methods section for definition of early and later.
Table 4.
Results of overall meta-analysis of birth weight and serum PFOS concentration, and for key subgroup analyses.a
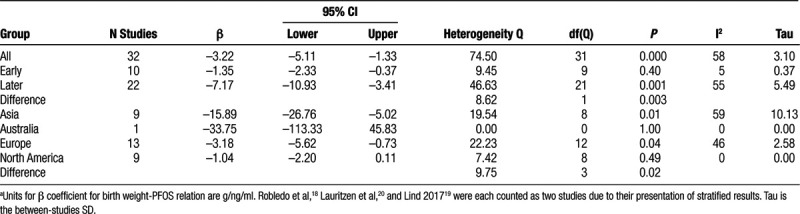
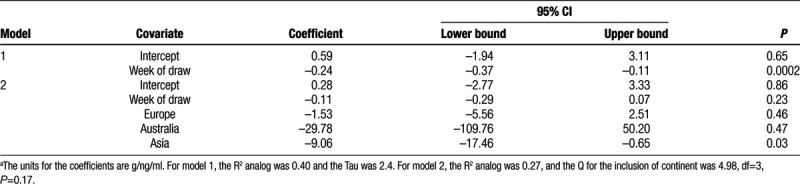
Based on the strong relation between timing of blood draw and the β for the birth weight-PFOS association, and the evidence of heterogeneity among the “later draw” group, we fit a random effects meta-regression model with timing of draw as a continuous variable. The intercept was slightly positive but essentially zero, and the coefficient for timing of draw was −0.24 (95% CI = −0.37, −0.11; Table 5). The coefficient indicates that for each week later in pregnancy that the blood was collected, the measured association of birth weight with PFOS would decrease by 0.24 g/ng/ml. In other words, the model indicated that when blood was drawn at the very beginning of pregnancy, there was essentially no relation of birth weight to PFOS concentration. Although the subgroup analysis (Table 4) suggested that β was less than zero for studies with blood drawn early in pregnancy, the average gestational week at blood draw (random effects weights) among those studies was 14. Addition of a quadratic term to the model for timing of blood draw showed no support for non-linearity (not shown). The discrepancy between the “early” subgroup and the intercept from the timing-adjusted regression model was due to the model’s ability to address the question: what would we expect to find at the very beginning of pregnancy? Addition of continent to the model attenuated the coefficient for timing of blood draw (Table 5). The average gestational age at blood draw (random effects weights) among studies from Asia was 40 weeks; eight of the 10 studies using cord blood were from Asia. Addition of other potentially modifying variables to the model with timing showed that none were important (eTable 3; http://links.lww.com/EE/A84).
Table 5.
Results of fitting key random-effects meta-regression models of birthweight in relation to serum PFOS concentration.a
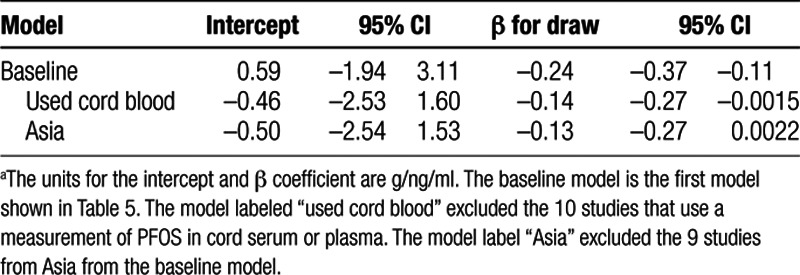
In the sensitivity analyses, after exclusion of the 10 studies using cord blood, the intercept was indistinguishable from 0 and week of blood draw had a coefficient of −0.14 (95% CI = −0.27, −0.002; Table 6). When continent was added as a covariate to this model, as before, the blood draw timing covariate was attenuated and not statistically significant, and the continent variable as a group did not improve the model fit (not shown). When we excluded studies from Asia (Table 6), the intercept was indistinguishable from 0 and the coefficient for week of blood draw was −0.13 (95% CI = −0.26, 0.002). Exclusion of the three studies that did not fit perfectly into the “early-late” blood draw categories did not change the results (not shown). When we repeated the analysis after adjusting the cord blood results for the estimated bias from using cord blood, the results were like those shown in Table 5 (not shown).
Table 6.
Results of selected meta-regression models of birth weight in relation to serum PFOS concentration examined in the sensitivity analysis.a
A funnel plot of the results (eFigure 2; http://links.lww.com/EE/A84) suggested that a few small studies with positive β coefficients may have remained unpublished. When we conducted the sensitivity analysis with a null result imputed for the large study by Buck Louis et al,15 which had blood drawn early in pregnancy, the results were essentially the same (not shown).
Discussion
Birth weight was associated with measured concentrations of PFOS in serum. The overall association was −3.22 g/ng/ml, 95% CI = −5.11, −1.33. The results confirmed our hypothesis that timing of blood draw for PFOS measurement would modify the association. Among those with blood measurements before or early in pregnancy, however, PFOS was still inversely associated with birth weight (−1.35, 95% CI = −2.33, −0.37). When we used meta-regression to estimate the association at the beginning of pregnancy, then it was indistinguishable from zero. This analysis, however, was confounded by the inclusion of studies from Asia, which had both more inverse associations and later blood draws. After exclusion of studies from Asia, the meta-regression still showed an association at the beginning of pregnancy that was indistinguishable from zero. In our protocol, we said we would focus on assessment of the birth weight-PFOS association on data from studies with the blood specimen obtained before or earlier in pregnancy, and that this would be supplemented by meta-regression results. Here, however, we have emphasized the intercept from the meta-regression as the primary finding, which could be construed as slightly at odds with the protocol. Our post-hoc preference for meta-regression results reflects a deficiency in the original protocol; overly strict adherence would diminish the importance of learning.
Classifying studies as having the blood drawn early or later in pregnancy was suboptimal. The dichotomy, as defined by Steenland et al7 and followed here, put some studies with blood draws in the second trimester in the early group. Second trimester blood draws, according to Verner et al,5 were expected to be negatively biased. A related problem affected the regression analysis, which depended on a biased timing variable. For example, in Bach et al,29 classified in the early group, most draws were in the 12th week of pregnancy, but subjects with a draw up to 20 weeks of pregnancy were included. This would result a negative bias in timing of draw. Analysis of individual level data would allow a better assessment of the bias due to timing.
Although Verner et al5 found that timing of blood draw was related to the size of the birth weight-PFOS association in a meta-regression analysis, their meta-analysis included only seven studies and did not consider the predicted association at the beginning of pregnancy. Negri et al6 included 14 studies in their meta-analysis; they presented subgroup analyses that supported both the timing and Asia effects reported here. In their analysis, they considered these both independently and in subgroups, according to whether log transformation of PFOS had been employed in the analysis in the original studies.
The proposed mechanism of bias due to the timing of the blood draw is that PFAS concentrations drop over the course of pregnancy, and the extent of that drop is likely proportional to the size of the newborn. The amount of confounding is a combination of timing and size of the mother, where larger mothers tend to have larger newborns. The reason for the drop in PFAS concentration over pregnancy has been attributed to plasma volume expansion-related dilution and to increased excretion.5,61 These two phenomena are inextricably linked.62 Although some authors have adjusted for glomerular filtration rate (excretion) in models of birth weight as a function of PFAS, this adjustment would not be expected to have an effect unless the blood draw was later in pregnancy, especially given the measurement error in estimated glomerular filtration rate.38,44
Relevant data on the toxicity of PFOS in rodents has recently been reviewed in detail by Negri et al.6 Administration of PFOS to pregnant rodents generally reduces offspring birth weight, but this occurs at serum concentrations that are 2–3 orders of magnitude higher than found in humans. In animals, the toxic effect may be mediated by binding with the peroxisome proliferator-activated receptor alpha. Such binding in rodents and humans, however, has different effects.63 If PFOS reduces birth weight in humans, it may be due to binding with other receptors or its affinity for membranes.64
In keeping with PRISMA guidelines, we characterized the quality of evidence as low for the birth weight-PFOS association when the timing of blood draw was before or early in pregnancy.16 Results were inconsistent, the association was small (or null, based on the meta-regression), and may be confounded. The associations in studies from Asia are more inverse than would be expected based on the timing of the blood draw, possibly due to the different mixture of PFAS in that region. Additional data from studies with blood drawn before or early in pregnancy, especially from Asia, might have an important impact on the assessment of the birth weight-PFOS association.
Conflict of interest statement
3M was not involved in the preparation of the manuscript. The authors retained sole control of the manuscript content and the findings, and statements in this paper are those of the authors and not those of the author’s employer or the sponsors. No authors were directly compensated by 3M. This project was funded through a contract between 3M and Ramboll, an international science and engineering company that provided salary compensation to the authors. None of the authors are currently engaged to testify as experts on behalf of the sponsors in litigation related to the compound discussed in this manuscript.
Supplementary Material
Footnotes
Published online 23 April 2020
This work was funded by 3M.
The data files used to prepare the analysis are available on request.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.Buck RC, Franklin J, Berger U, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011; 7:513–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Interstate Technology Regulatory Council (ITRC) History and Use of Per- and Polyfluoroalkyl Substances (PFAS). 2017, Washington, DC: ITRC [Google Scholar]
- 3.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Env Epid. 2019; 29:131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Environmental Protection Agency Drinking Water Health Advisories for PFOA and PFOS. 2016, Washington, DC: US EPA [Google Scholar]
- 5.Verner MA, Loccisano AE, Morken NH, et al. Associations of Perfluoroalkyl Substances (PFAS) with lower birth weight: an evaluation of potential confounding by glomerular filtration rate Using a Physiologically Based Pharmacokinetic Model (PBPK). Environ Health Perspect. 2015; 123:1317–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Negri E, Metruccio F, Guercio V, et al. Exposure to PFOA and PFOS and fetal growth: a critical merging of toxicological and epidemiological data. Crit Rev Toxicol. 2017; 47:482–508 [DOI] [PubMed] [Google Scholar]
- 7.Steenland K, Barry V, Savitz D. Serum perfluorooctanoic acid and birthweight: an updated meta-analysis with bias analysis. Epidemiology. 2018; 29:765–776 [DOI] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009; 151:W65–W94 [DOI] [PubMed] [Google Scholar]
- 9.Johnson PI, Sutton P, Atchley DS, et al. The navigation guide - evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect. 2014; 122:1028–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenland S, O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics. 2001; 2:463–471 [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188 [DOI] [PubMed] [Google Scholar]
- 12.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Meta-regression. Introduction to Meta-Analysis. 2009, Chichester, UK: John Wiley and Sons, 187–203 [Google Scholar]
- 13.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 14.Ehresman DJ, Froehlich JW, Olsen GW, Chang SC, Butenhoff JL. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ Res. 2007; 103:176–184 [DOI] [PubMed] [Google Scholar]
- 15.Buck Louis GM, Zhai S, Smarr MM, et al. Endocrine disruptors and neonatal anthropometry, NICHD Fetal Growth Studies - Singletons. Environ Int. 2018; 119:515–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. GRADE Working Group What is “quality of evidence” and why is it important to clinicians? BMJ. 2008; 336:995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-Analysis Version 3. 2013, Englewood, NJ: Biostat [Google Scholar]
- 18.Robledo CA, Yeung E, Mendola P, et al. Preconception maternal and paternal exposure to persistent organic pollutants and birth size: the LIFE study. Environ Health Perspect. 2015; 123:88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lind DV, Priskorn L, Lassen TH, et al. Prenatal exposure to perfluoroalkyl substances and anogenital distance at 3 months of age in a Danish mother-child cohort. Reprod Toxicol. 2017; 68:200–206 [DOI] [PubMed] [Google Scholar]
- 20.Lauritzen HB, Larose TL, Øien T, et al. Maternal serum levels of perfluoroalkyl substances and organochlorines and indices of fetal growth: a Scandinavian case-cohort study. Pediatr Res. 2017; 81:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apelberg BJ, Witter FR, Herbstman JB, et al. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect. 2007; 115:1670–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monroy R, Morrison K, Teo K, et al. Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples. Environ Res. 2008; 108:56–62 [DOI] [PubMed] [Google Scholar]
- 23.Washino N, Saijo Y, Sasaki S, et al. Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ Health Perspect. 2009; 117:660–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamm MP, Cherry NM, Chan E, Martin JW, Burstyn I. Maternal exposure to perfluorinated acids and fetal growth. J Expo Sci Environ Epidemiol. 2010; 20:589–597 [DOI] [PubMed] [Google Scholar]
- 25.Chen MH, Ha EH, Wen TW, et al. Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PloS One. 2012; 7:e42474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maisonet M, Terrell ML, McGeehin MA, et al. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ Health Perspect. 2012; 120:1432–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitworth KW, Haug LS, Baird DD, et al. Perfluorinated compounds in relation to birth weight in the Norwegian Mother and Child Cohort Study. Am J Epidemiol. 2012; 175:1209–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darrow LA, Stein CR, Steenland K. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005-2010. Environ Health Perspect. 2013; 121:1207–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bach CC, Bech BH, Nohr EA, et al. Perfluoroalkyl acids in maternal serum and indices of fetal growth: the Aarhus Birth Cohort. Environ Health Perspect. 2016; 124:848–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callan AC, Rotander A, Thompson K, et al. Maternal exposure to perfluoroalkyl acids measured in whole blood and birth outcomes in offspring. Sci Total Environ. 2016; 569-570:1107–1113 [DOI] [PubMed] [Google Scholar]
- 31.Govarts E, Remy S, Bruckers L, et al. Combined effects of prenatal exposures to environmental chemicals on birth weight. Int J Environ Res Public Health. 2016; 13:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon EJ, Shin JS, Kim BM, et al. Prenatal exposure to perfluorinated compounds affects birth weight through GSTM1 polymorphism. J Occup Environ Med. 2016; 58:e198–e205 [DOI] [PubMed] [Google Scholar]
- 33.Lee ES, Han S, Oh JE. Association between perfluorinated compound concentrations in cord serum and birth weight using multiple regression models. Reprod Toxicol. 2016; 59:53–59 [DOI] [PubMed] [Google Scholar]
- 34.Lenters V, Portengen L, Rignell-Hydbom A, et al. Prenatal phthalate, perfluoroalkyl acid, and organochlorine exposures and term birth weight in three birth cohorts: multi-pollutant models based on elastic net regression. Environ Health Perspect. 2016; 124:365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashley-Martin J, Dodds L, Arbuckle TE, et al. Maternal concentrations of perfluoroalkyl substances and fetal markers of metabolic function and birth weight. Am J Epidemiol. 2017; 185:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen MH, Ng S, Hsieh CJ, Lin CC, Hsieh WS, Chen PC. The impact of prenatal perfluoroalkyl substances exposure on neonatal and child growth. Sci Total Environ. 2017; 607-608:669–675 [DOI] [PubMed] [Google Scholar]
- 37.Li M, Zeng XW, Qian ZM, et al. Isomers of perfluorooctanesulfonate (PFOS) in cord serum and birth outcomes in China: Guangzhou Birth Cohort Study. Environ Int. 2017; 102:1–8 [DOI] [PubMed] [Google Scholar]
- 38.Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, et al. Prenatal exposure to perfluoroalkyl substances and birth outcomes in a Spanish birth cohort. Environ Int. 2017; 108:278–284 [DOI] [PubMed] [Google Scholar]
- 39.Shi Y, Yang L, Li J, et al. Occurrence of perfluoroalkyl substances in cord serum and association with growth indicators in newborns from Beijing. Chemosphere. 2017; 169:396–402 [DOI] [PubMed] [Google Scholar]
- 40.Starling AP, Adgate JL, Hamman RF, et al. Perfluoroalkyl substances during pregnancy and offspring weight and adiposity at birth: examining Mediation by Maternal Fasting Glucose in the Healthy Start Study. Environ Health Perspect. 2017; 125:067016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valvi D, Oulhote Y, Weihe P, et al. Gestational diabetes and offspring birth size at elevated environmental pollutant exposures. Environ Int. 2017; 107:205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao W, Liu X, Liu X, et al. Perfluoroalkyl substances in umbilical cord serum and gestational and postnatal growth in a Chinese birth cohort. Environ Int. 2018; 116:197–205 [DOI] [PubMed] [Google Scholar]
- 43.Meng Q, Inoue K, Ritz B, Olsen J, Liew Z. Prenatal exposure to perfluoroalkyl substances and birth outcomes; an updated analysis from the Danish National Birth Cohort. Int J Environ Res Public Health. 2018; 15:1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sagiv SK, Rifas-Shiman SL, Fleisch AF, et al. Early-pregnancy plasma concentrations of perfluoroalkyl substances and birth outcomes in project viva: confounded by pregnancy hemodynamics? Am J Epidemiol. 2018; 187:793–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marks KJ, Cutler AJ, Jeddy Z, Northstone K, Kato K, Hartman TJ. Maternal serum concentrations of perfluoroalkyl substances and birth size in British boys. Int J Hyg Environ Health. 2019; 222:889–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Du H, Yang J, et al. PFOS, PFOA, estrogen homeostasis, and birth size in Chinese infants. Chemosphere. 2019; 221:349–355 [DOI] [PubMed] [Google Scholar]
- 47.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004; 144:240–245 [DOI] [PubMed] [Google Scholar]
- 48.Steer CD, Sayers A, Kemp J, Fraser WD, Tobias JH. Birth weight is positively related to bone size in adolescents but inversely related to cortical bone mineral density: findings from a large prospective cohort study. Bone. 2014; 65:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Starling AP, Haug LS, et al. Association between perfluoroalkyl substances and thyroid stimulating hormone among pregnant women: a cross-sectional study. Environ Health. 2013; 12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Starling AP, Engel SM, Whitworth KM, et al. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study. Environ Int. 2014; 62:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007; 115:1677–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fromme H, Mosch C, Morovitz M, et al. Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ Sci Technol. 2010; 44:7123–7129 [DOI] [PubMed] [Google Scholar]
- 53.Kim S, Choi K, Ji K, et al. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ Sci Technol. 2011; 45:7465–7472 [DOI] [PubMed] [Google Scholar]
- 54.Wu K, Xu X, Peng L, Liu J, Guo Y, Huo X. Association between maternal exposure to perfluorooctanoic acid (PFOA) from electronic waste recycling and neonatal health outcomes. Environ Int. 2012; 48:1–8 [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Adgent M, Su PH, et al. Prenatal exposure to perfluorocarboxylic acids (PFCAs) and Fetal and Postnatal Growth in the Taiwan Maternal and Infant Cohort Study. Environ Health Perspect. 2016; 124:1794–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minatoya M, Itoh S, Miyashita C, et al. Association of prenatal exposure to perfluoroalkyl substances with cord blood adipokines and birth size: the Hokkaido Study on environment and children’s health. Environ Res. 2017; 156:175–182 [DOI] [PubMed] [Google Scholar]
- 57.Alkhalawi E, Kasper-Sonnenberg M, Wilhelm M, Völkel W, Wittsiepe J. Perfluoroalkyl acids (PFAAs) and anthropometric measures in the first year of life: results from the Duisburg Birth Cohort. J Toxicol Environ Health A. 2016; 79:1041–1049 [DOI] [PubMed] [Google Scholar]
- 58.Bjerregaard-Olesen C, Bach CC, Long M, et al. Associations of fetal growth outcomes with measures of the combined xenoestrogenic activity of maternal serum perfluorinated alkyl acids in Danish pregnant women. Environ Health Perspect. 2019; 127:17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kobayashi S, Azumi K, Goudarzi H, et al. Effects of prenatal perfluoroalkyl acid exposure on cord blood IGF2/H19 methylation and ponderal index: the Hokkaido Study. J Expo Sci Environ Epidemiol. 2017; 27:251–259 [DOI] [PubMed] [Google Scholar]
- 60.Rokoff LB, Rifas-Shiman SL, Coull BA, et al. Cumulative exposure to environmental pollutants during early pregnancy and reduced fetal growth: the Project Viva cohort. Environ Health. 2018; 17:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glynn A, Berger U, Bignert A, et al. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996-2010. Environ Sci Technol. 2012; 46:9071–9079 [DOI] [PubMed] [Google Scholar]
- 62.Baylis C, Davison JM. The normal renal physiological changes which occur during pregnancy. The Oxford Textbook of Clinical Nephrology. 2005, New York: Oxford, 2216 [Google Scholar]
- 63.Gonzalez FJ, Shah YM. PPARalpha: mechanism of species differences and hepatocarcinogenesis of peroxisome proliferators. Toxicology. 2008; 246:2–8 [DOI] [PubMed] [Google Scholar]
- 64.Bjork JA, Butenhoff JL, Wallace KB. Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology. 2011; 288:8–17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


