Supplemental Digital Content is available in the text.
Keywords: Air pollution, Noise, Traffic-related, Metabolic dysfunction, Cognitive impairment, Dementia
Background:
Cognitive impairment has been linked to traffic-related air pollution and noise exposure as well as to metabolic syndrome or some of its individual components. Here, we investigate whether the presence of metabolic dysfunction modifies associations between air pollution or noise exposures and incident dementia or cognitive impairment without dementia (CIND).
Methods:
For 1,612 elderly Mexican-American participants of the Sacramento Area Latino Study on Aging (SALSA) followed for up to 10 years, we estimated residential-based local traffic-related exposures relying on the California Line Source Dispersion Model version 4 (CALINE4) for nitrogen oxides (NOx) and the SoundPLAN software package (Version 8.0; NAVCON, Fullerton, CA) that implements the Federal Highway Administration Traffic Noise Model (TNM) for noise, respectively. We used Cox proportional hazard models to estimate the joint effects of NOx or noise exposures and obesity, hyperglycemia, or low high-density lipoprotein (HDL) cholesterol.
Results:
The risk of developing dementia/CIND among participants with hyperglycemia who also were exposed to high levels of NOx (≥3.44 parts per billion [ppb] [75th percentile]) or noise (≥65 dB) was 2.4 (1.4, 4.0) and 2.2 (1.7, 3.9), respectively. For participants with low HDL-cholesterol, the estimated hazard ratios for dementia/CIND were 2.5 (1.4, 4.3) and 1.8 (1.0, 3.0) for those also exposed to high levels of NOx (≥3.44 ppb) or noise (≥65 dB), respectively, compared with those without metabolic dysfunction exposed to low traffic-related air pollution or noise levels.
Conclusions:
Exposure to traffic-related air pollution or noise most strongly increases the risk of dementia/CIND among older Mexican-Americans living in California who also exhibit hyperglycemia or low HDL-cholesterol.
What this study adds
There is substantial evidence that metabolic dysfunction and emerging evidence that traffic-related air pollution and noise exposures are threats to brain health. However, we know little about the joint action of these factors. In a cohort of older Mexican-Americans, we found that traffic-related air pollution or noise exposure strongly increase the risk of dementia or cognitive impairment without dementia among those who were hyperglycemic or had low high-density lipoprotein cholesterol. Thus, multiple risk factors may come together to exacerbate the risk of cognitive decline in elderly minority populations and, as there are currently no treatments for dementia available, preventative measures are needed.
Introduction
Air pollution is a complex mixture of toxic compounds from different sources. Exposure to air pollution has been linked to endothelial dysfunction, microvasculature damage, and atherosclerosis in both clinical and animal experiments.1 There is growing epidemiologic evidence that both short- and long-term exposures to ambient air pollutants, namely nitrogen dioxides (NO2), particular matter (PM2.5), and ozone (O3), may increase the risk of neurodegenerative disease including cognitive impairment.2–6 Recently, concerns have been raised that noise exposure—also originating from traffic—may result in cardiovascular diseases7,8 and cognitive impairment.3,4,9
Metabolic syndrome describes a cluster of reversible pathophysiologic conditions including insulin resistance, obesity, dyslipidemia, and hypertension that are widely recognized in clinical practice and research for their potential to increase risk of chronic diseases including cardiovascular and neurodegenerative diseases10–12 and late-life cognitive impairment.13 Accurately capturing the complex temporal aspects of blood pressure or triglyceride changes in older age participants when assessing their influence on cognitive impairment has been shown to be important in previous studies.14–17
Mexican-Americans, especially those age 60 years or older, have a particularly high prevalence of obesity18 and diabetes19–21 and are also among the most highly environmentally exposed populations in California, including to traffic-related air pollution and noise.22 Thus far, very few studies investigated the influence of noise exposures on cognition function, and to our knowledge, no study has examined whether metabolic dysfunction contributes to the associations between noise exposure and cognition function, that is, increases their vulnerability for cognitive decline. The Sacramento Area Latino Study on Aging (SALSA) cohort offers the opportunity to examine this hypothesis in a longitudinal cohort that enrolled and followed elderly Mexican-Americans.
Methods
Study population
All procedures described here were approved by the Institutional Review Boards of University of California San Francisco, Los Angeles, and Davis, University of North Carolina, and University of Michigan.
We are using data from the Sacramento Area Latino Study on Aging (SALSA), a prospective population-based cohort study of older Mexican-Americans living in the Sacramento Area of California (1998–2007). A total of 1,789 participants were originally recruited. Participants were enrolled if they were 60 years or older, resided in the California Sacramento Valley, and self-identified as Latino; they were followed with interviews and exams at their homes every 12–15 months for up to seven visits, and every 6 months, they were contacted in a 10-minute phone call to update contact information, health status, and medication information between home visits. More information about the sampling process has been detailed elsewhere.23 In this study, those who (1) did not participate in the interview at baseline (n = 3), (2) lived too far away from traffic sources to generate air pollution or noise measures (n = 3), (3) already had dementia/cognitive impairment without dementia (CIND) at baseline (n = 114), and (4) did not have any follow-up visit (n = 57) were excluded, leaving 1,612 participants in total as our baseline sample. For analysis purposes, we further excluded those who at baseline did not have information on high-density lipoprotein (HDL) levels, obesity, or hyperglycemia (Figure).
Figure.
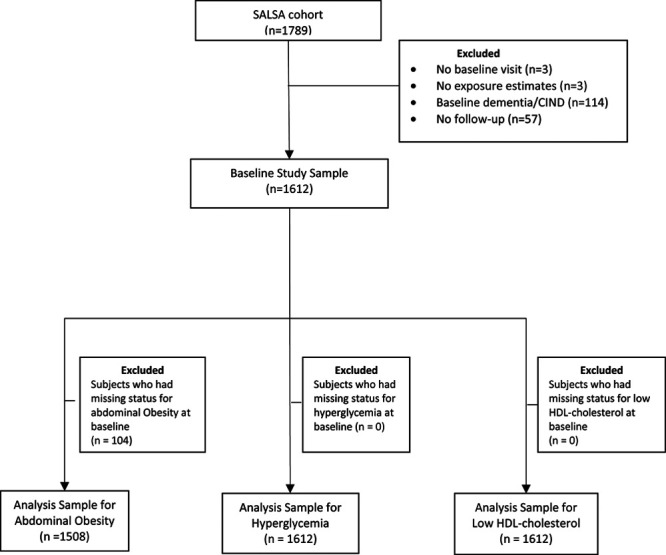
Flow chart of the study population, SALSA, 1998–2007.
Exposure assessment
All air pollution and noise exposure levels were estimated based on participants’ geocoded residential addresses at baseline. Because the spatial pattern of traffic in the Sacramento area did not change much during the study period, and the average duration of participants’ living at the baseline address was 22 years with more than 80% not changing their addresses during the study period, we expect our exposure measures to serve as good indicators for exposures due to long-term traffic patterns around each participants’ residence, that is, the decades before and the decade of follow-up.
Estimation of traffic-related nitrogen oxides
Details about the generation of traffic-related nitrogen oxides (NOx) exposure measures for SALSA have been provided elsewhere.24 In brief, traffic-related NOx was estimated by the California Line Source Dispersion Model version 4 (CALINE4), which captures local traffic emissions within 1,500 meters of a participant’s baseline address using traffic volume data in 2002 from the California Department of Transportation (DOT), while taking into account meteorological influences such as wind speed and direction, mixing height, and temperature. The emission factors were obtained from the California Air Resources Board (CARB)’s EMFAC2011 model.25 Meteorological data were obtained from the CARB Air Quality and Meteorological Information System.26
Estimation of traffic-related noise
The creation of traffic-related noise exposure in SALSA has also been detailed elsewhere.24 Briefly, noise exposure was assessed using the SoundPLAN (Version 8.0; NAVCON, Fullerton, CA) software package that implemented the Federal Highway Administration Traffic Noise Model (TNM) for noise prediction—based on input of Annual Average Daily Traffic (AADT) data from the local Metropolitan Planning Organizations (MPOs). The TNM incorporates vehicle speed, distance between receiver (geocoded residential address of study subjects) and roadway, ground classification (soft vs. hard),27 and counts of different types of vehicles. Continuous roadway traffic was considered the only source for our noise estimates. The 2002 State DOT hourly traffic counts were used to generate average diurnal patterns and to adjust the MPO AADT values to hour-of-day specific traffic counts for each noise receptor location, to account for differences in noise exposure depending on the time of day.28
Metabolic dysfunction
As SALSA participants were, on average, already 70 years old at enrollment, we are only assessing the influence of three metabolic dysfunction indicators here, that is, obesity, hyperglycemia, and low high-density lipoprotein (HDL) cholesterol. These three metabolic dysfunction components were defined according to the Third Adult Treatment Panel of the National Cholesterol Education Program (NCEP ATP III)29 as (1) obesity: waist circumference of ≥40 inches in men or ≥35 inches in women; (2) hyperglycemia: fasting glucose ≥100 mg/dl or use of glucose-lowering medications; and (3) low HDL-cholesterol: HDL-cholesterol <40 mg/dl in men or <50 mg/dl in women or use of statins.
Dementia and CIND
The main outcome of interest is incident dementia/CIND. Cognition function was first evaluated with two cognitive screening tests (Modified Mini–Mental State Examination [3MSE] and Spanish English Verbal Learning Test [SEVLT]) administered to each participant at baseline and again during each follow-up visit. Participants were referred for a neuropsychological test battery and a standard neuropsychological examination (Informant Questionnaire on Cognitive Decline in the Elderly) by a geriatrician if their scores (1) on the 3MSE or SEVLT were below the 20th percentile at baseline or (2) had decreased ≥8 points on 3MSE or ≥3 points on SEVLT compared with baseline. A team of neurologists and a neuropsychologist reviewed and classified them as cognitively normal, dementia, or CIND, according to standard diagnostic criteria. Those diagnosed with CIND or dementia were also referred for a magnetic resonance imaging (MRI) examination (details have been published elsewhere).23 For the following analyses, all-cause dementia and CIND were combined to improve statistical power and CIND captures the onset of cognitive decline before dementia.
Other covariates
Demographic information was collected during enrollment including age, sex, birthplace (Mexico, United States, or other), years of education, occupation held longest during the lifetime (nonmanual, manual or other), household income (<1,000, 1,000–1,499, 1,500–1,999, 2,000–2,499, or 2,500 or more US dollars/month), and residential county (Sacramento county or others). At each interview, participants were asked about lifestyle behaviors such as smoking (never/nonsmoker, former smoker, or current smoker) and alcohol drinking (frequent [daily], moderate [weekly], occasional [monthly], or yearly/rarely/never drinker), medical diagnoses, and medication use. Participants’ standing height and weight were also measured to obtain the body mass index (BMI; kg/m2). Depressive symptoms were evaluated using the Center for Epidemiologic Studies Depression (CESD) scale (range 0–60). We derived a neighborhood socioeconomic status (NSES) indicator calculated as a score ranging from 1 (low NSES) to 5 (high NSES), according to six census block (2000) measures including the percentages of individuals 25+ years old without a high school diploma, population living below the poverty line, individuals 16+ years old who at one time had been in the workforce but are unemployed, households with ownership of their home, vacant housing units, and the median number of rooms in a household.30 Physical activity level was evaluated according to spending time on 18 different activities in which older adults commonly engage in during a regular week.31
Statistical analysis
We employed Cox proportional hazards regression models with calendar time as the time scale and calculated hazard ratios (HRs) and 95% confidence intervals. Participants were censored at their last date of contact if they did not return for a follow-up examination or at their time of death if they died before the end of 2007.
The influence of traffic-related NOx and noise with each metabolic dysfunction (obesity, hyperglycemia, and low HDL-cholesterol) on incident dementia/CIND were first explored separately. We entered traffic-related NOx and noise exposure into separate (single exposure) Cox regression models treating them as continuous variables normalized by their respective interquartile ranges (IQRs). For models with two-way interactions between each metabolic dysfunction (present vs. absent) and environmental exposures, we dichotomized NOx exposure as low and high, comparing the first three to the last (4th) quartile (<3.44 parts per billion [ppb] vs. ≥3.44 ppb) and the noise exposure cut-point (<65 vs. ≥65 dB) was chosen according to the World Health Organization community noise guidelines (2009) and in studies from the United States and is comparable to what previous studies from the United States and European countries used to define high noise exposure.32–34 We explored two-way interactions between NOx or noise exposure and obesity, hyperglycemia, and low HDL-cholesterol, respectively. Age, sex, education, occupation, household income level, smoking and alcohol status, physical activity levels, NSES, and residential location were entered into all models as covariates. We also calculated the relative excess risk due to interaction (RERI) to evaluate interactions on an additive scale.35
As a secondary analysis, the estimated effect for traffic-related NOx or 24-hour noise exposure by presence/absence of each metabolic dysfunction were similar; thus, we redefined metabolic dysfunction in the following manner: two multi-level categorical variables were generated for hyperglycemia and low HDL-cholesterol status, respectively; the four categories were (1) normal, (2) untreated, (3) treated and well-controlled, and (4) treated but not well-controlled. Traffic-related NOx and 24-hour noise exposures were entered into Cox regression models as continuous variables normalized by their interquartile ranges (IQRs), and an interaction term between the respective exposure and the ordinal variable representing multiple categories for hyperglycemia or low HDL-cholesterol was also included; the hazard ratios were calculated for developing incident dementia/CIND per IQR increase in NOx or noise exposure for each category of metabolic dysfunction.
In sensitivity analyses, we ignored medication information to define metabolic dysfunction (Table S1; http://links.lww.com/EE/A112) and also repeated two-way interaction analyses using 75 dB as cutoff points to define high noise exposure and also contrasted the highest versus the two lower tertiles (<2.68 vs. ≥2.68 ppb) for traffic-related NOx exposure.
Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Participants with prevalent obesity, hyperglycemia, or low HDL-cholesterol at baseline were of similar average age but had less education, a lower household income and neighborhood socioeconomic status than those unaffected. There were more current smokers and moderate/frequent alcohol drinkers among those without metabolic dysfunction. At baseline, females had a higher prevalence of obesity, hyperglycemia, and low HDL-cholesterol, while average exposure to traffic-NOx or to noise was similar across all groups (Table 1). The distribution of air pollutants and noise was described in Table S2 (http://links.lww.com/EE/A112); traffic-related NOx and noise exposures were moderately correlated with each other (Pearson r = 0.43).
Table 1.
Characteristics of the study population at baseline by metabolic dysfunction statusa, SALSA, 1998–2007
| Characteristics; mean ± SD/N (%) | Total | Obesity | Hyperglycemia | Low HDL-cholesterol | |||
|---|---|---|---|---|---|---|---|
| (n = 1,612) | No (n = 607) | Yes (n = 901) | No (n = 840) | Yes (n = 772) | No (n = 1,026) | Yes (n = 586) | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Baseline age (year); mean (SD) | 70.2 (±6.8) | 70.2 (±6.9) | 70.0 (±6.6) | 70.8 (±7.0) | 69.7 (±6.5) | 70.3 (±7.0) | 70.1 (±6.5) |
| Male | 680 (42) | 340 (56) | 287 (32) | 338 (40) | 342 (44) | 486 (47) | 194 (33) |
| Education (year); mean (SD) | 7.4 (±5.3) | 8.0 (±5.4) | 7.2 (±5.3) | 7.3 (±5.3) | 7.5 (±5.4) | 7.6 (±5.3) | 7.0 (±5.4) |
| Sacramento county residence | 1,255 (78) | 485 (80) | 694 (77) | 644 (77) | 611 (79) | 787 (77) | 468 (80) |
| Urban residence | 1,400 (87) | 528 (87) | 783 (87) | 728 (87) | 672 (87) | 895 (87) | 505 (86) |
| NSES; mean (SD) | 2.1 (1.0) | 2.1 (1.0) | 2.1 (1.0) | 2.1 (1.0) | 2.1 (1.0) | 2.1 (1.0) | 2.1 (1.0) |
| Birth country | |||||||
| Mexico | 721 (45) | 269 (44) | 399 (44) | 400 (48) | 321 (42) | 462 (45) | 259 (44) |
| United States | 797 (50) | 299 (49) | 459 (51) | 375 (45) | 422 (55) | 502 (49) | 295 (50) |
| Others | 88 (6) | 39 (6) | 43 (5) | 59 (7) | 29 (4) | 56 (6) | 32 (6) |
| Occupation held during most of the lifetime | |||||||
| Nonmanual | 346 (22) | 144 (24) | 185 (21) | 177 (22) | 169 (22) | 216 (22) | 130 (22) |
| Manual | 960 (61) | 374 (63) | 519 (58) | 507 (62) | 453 (59) | 631 (63) | 329 (57) |
| Other | 282 (18) | 80 (13) | 189 (21) | 141 (17) | 141 (19) | 159 (16) | 123 (21) |
| Household income (US dollar/month) | |||||||
| <1,000 | 691 (44) | 227 (38) | 409 (46) | 355 (43) | 336 (44) | 432 (43) | 259 (45) |
| 1,000–1,499 | 321 (20) | 117 (20) | 179 (20) | 177 (22) | 144 (19) | 198 (20) | 123 (21) |
| 1,500–1,999 | 184 (12) | 80 (13) | 95 (11) | 101 (12) | 83 (11) | 125 (12) | 59 (10) |
| 2,000–2,499 | 154 (10) | 68 (11) | 85 (10) | 81 (10) | 73 (10) | 92 (9) | 62 (11) |
| 2,500 or more | 233 (15) | 107 (18) | 119 (13) | 106 (13) | 127 (17) | 158 (16) | 75 (13) |
| Baseline smoking status | |||||||
| Never/nonsmoker | 735 (46) | 256 (42) | 435 (48) | 401 (48) | 334 (43) | 470 (46) | 265 (45) |
| Former smoker | 681 (42) | 254 (42) | 389 (43) | 328 (39) | 353 (46) | 431 (42) | 250 (43) |
| Current smoker | 189 (12) | 96 (16) | 77 (9) | 104 (13) | 85 (11) | 118 (12) | 71 (12) |
| Baseline alcohol status | |||||||
| Frequent drinker | 146 (9) | 76 (13) | 58 (7) | 90 (11) | 56 (7) | 124 (12) | 22 (4) |
| Moderate drinker | 172 (11) | 87 (14) | 81 (9) | 106 (13) | 66 (9) | 130 (13) | 42 (7) |
| Occasional drinker | 158 (10) | 63 (10) | 88 (10) | 78 (9) | 80 (10) | 102 (10) | 56 (10) |
| Yearly/rarely/never drinker | 1,125 (70) | 378 (63) | 672 (75) | 557 (67) | 568 (74) | 663 (65) | 462 (79) |
| Baseline physically active | 341 (21) | 158 (26) | 156 (17) | 203 (24) | 138 (18) | 240 (23) | 101 (17) |
| Baseline CESD; mean (SD) | 9.8 (±10.4) | 8.9 (±9.9) | 10.1 (±10.7) | 9.5 (±10.3) | 10.1 (±10.5) | 9.4 (±10.1) | 10.5 (±10.9) |
| Baseline BMI; mean (SD) | 29.9 (±6.0) | 26.2 (±4.1) | 32.3 (±5.8) | 28.5 (±5.6) | 31.2 (±6.1) | 29.4 (±6.1) | 30.6 (±5.7) |
| 24-hour noise (dB); mean (SD) | 68.5 (±8.9) | 68.6 (±8.9) | 68.4 (±8.9) | 68.2 (±8.8) | 68.7 (±9.0) | 68.5 (±8.8) | 68.4 (±9.0) |
| Traffic-related NOx (ppb); mean (SD) | 2.6 (±2.2) | 2.6 (±2.2) | 2.6 (±2.1) | 2.5 (±2.1) | 2.7 (±2.2) | 2.6 (±2.2) | 2.5 (±2.1) |
aDefinitions for metabolic dysfunction: (1) obesity: waist circumference of ≥40 inches in men and ≥35 inches in women; (2) hyperglycemia: fasting glucose ≥100 mg/dl or use of glucose-lowering medications; and (3) low HDL-cholesterol: men <40 mg/dl and women <50 mg/dl or use of statins.
dB indicates decibels.
Among 1,612 participants without dementia/CIND at baseline, 159 developed dementia/CIND during follow-up. The average length of follow-up time was 6.5 years, and the average annual attrition rate in SALSA was 5%. The risk of incident dementia/CIND increased ~18% (HR = 1.2 [1.0, 1.4]) per 2.29 ppb increase in traffic-related NOx exposure and 23% (HR = 1.2 [1.0, 1.5]) for each 11.6 dB increase in the 24-hour noise level, respectively (Table S3; http://links.lww.com/EE/A112). As expected, obesity, hyperglycemia, or low HDL-cholesterol were positively associated with the risk of incident dementia/CIND, respectively, although some of the 95% CIs included the null (Table S3; http://links.lww.com/EE/A112).
When examining the joint effects for each environmental exposure and each metabolic dysfunction that affected CIND/dementia, hazard ratios (HRs) for incident dementia/CIND among participants exposed to high levels of traffic-related NOx (≥3.44 ppb) who were obese or had hyperglycemia or low HDL-cholesterol compared with those exposed to low level of traffic-related NOx without the respective metabolic dysfunction were 1.7 (0.99, 3.0), 2.4 (1.41, 4.0), and 2.5 (1.4, 4.3), respectively. For participants exposed to high 24-hour noise (≥65 dB) levels and had hyperglycemia or low HDL-cholesterol, the hazard ratios for incident dementia/CIND compared with those exposed to low levels of noise and without the respective metabolic dysfunction were 2.2 (1.3, 3.9) and 1.8 (1.0, 3.0), respectively (Table 2). However, only the interaction of low HDL-cholesterol and high traffic-related NOx was formally statistically significant and suggested superadditivity (RERI = 1.1 [0.03, 2.1]) (Table S4; http://links.lww.com/EE/A112). Results changed minimally when NOx and noise exposures were mutually adjusted for in the models. The joint effects for obesity, hyperglycemia, or low HDL-cholesterol remained similar when we used alternative cutoff thresholds for NOx and 24-hour noise exposure (Table S5; http://links.lww.com/EE/A112). Analyses based on alternative definitions for hyperglycemia and low HDL-cholesterol (Tables S6 and S7; http://links.lww.com/EE/A112) also did not make a difference for our effect estimates.
Table 2.
Joint effectsa for traffic-related NOx (<3.44 vs. ≥3.44 ppb) or 24-hour noise (<65 vs. ≥65 dB) exposure and metabolic dysfunction on incident dementia/CIND
| Risk factor | Traffic-related NOx | 24-hour noise | ||||||
|---|---|---|---|---|---|---|---|---|
| NOx <3.44 ppb | NOx ≥3.44 ppb | 24-hour noise <65 dB | 24-hour noise ≥65 dB | |||||
| Case/total | HR (95% CI) | Case/total | HR 95% CI | Case/total | HR (95% CI) | Case/total | HR (95% CI) | |
| Obesityb | ||||||||
| No | 38/463 | Reference | 13/144 | 1.3 (0.67, 2.6) | 16/226 | Reference | 35/381 | 1.5 (0.76, 2.8) |
| Yes | 69/678 | 1.1 (0.72, 1.8) | 25/223 | 1.7 (0.99, 3.0) | 31/339 | 1.3 (0.67, 2.5) | 63/562 | 1.7 (0.89, 3.1) |
| Hyperglycemiab | ||||||||
| No | 56/656 | Reference | 17/184 | 1.1 (0.57, 2.2) | 23/325 | Reference | 50/515 | 1.4 (0.75, 2.4) |
| Yes | 59/562 | 1.5 (0.96, 2.3) | 27/210 | 2.4 (1.4, 4.0) | 27/273 | 1.7 (0.90, 3.1) | 59/499 | 2.2 (1.7, 3.9) |
| Low HDL-cholesterolb | ||||||||
| No | 72/759 | Reference | 22/267 | 1.0 (0.58, 1.8) | 30/379 | Reference | 64/647 | 1.4 (0.84, 2.3) |
| Yes | 43/459 | 1.1 (0.71, 1.7) | 22/127 | 2.5 (1.4, 4.3) | 20/219 | 1.5 (0.79, 2.7) | 45/367 | 1.8 (1.0, 3.0) |
aAll the models were adjusted with baseline age, sex, education, occupation held during most of the life, NSES, smoking status, alcohol status, residential county, physical activity and household income, and baseline cognition function.
bDefinitions for metabolic dysfunction: (1) obesity: waist circumference of ≥40 inches in men and ≥35 inches in women; (2) hyperglycemia: fasting glucose ≥100 mg/dl or use of glucose-lowering medications; and (3) low HDL-cholesterol: men <40 mg/dl and women <50 mg/dl or use of statins.
95% CI indicates 95% confidence interval, dB, decibels.
Finally, we estimated that participants treated with glucose-lowering medications who still had glucose levels ≥126 mg/dl were at highest risk (HR = 1.4 [1.0, 2.0]) of developing dementia/CIND when exposed to traffic-related NOx, followed by those with treated and well-controlled glucose levels (<126 mg/dl), then those untreated with higher (≥126 mg/dl) or borderline higher glucose levels (100 mg/dl ≤fasting glucose level <126 mg/dl) and finally those with normal glucose levels (Table 3). Also, the risk of developing incident dementia/CIND when exposed to traffic-related NOx exposure was higher among those having low HDL-cholesterol who were treated with medications (Table 4); however, no difference was found in obese versus nonobese (obese: HR = 1.1 [0.9, 1.5]; nonobese: HR = 1.1 [0.8, 1.5] per 2.29 ppb increase in traffic-related NOx exposure). For 24-hour noise exposure, no difference in risk of dementia/CIND was found across levels of hyperglycemia, low HDL-cholesterol, or obesity, respectively.
Table 3.
Effect estimates (and 95% CIs) from Cox modelsa for traffic-related NOx (per 2.29 ppb increase) and dementia/CIND, according to hyperglycemia status
| Hyperglycemia status | N | No. cases | Traffic-related NOx, per 2.29 ppb increase |
|---|---|---|---|
| HR (95% CI) | |||
| Normal glucose level | 840 | 73 | 1.0 (0.71, 1.4) |
| Untreated but hyperglycemiab | 440 | 39 | 1.2 (0.88, 1.7) |
| Treated and well-controlledc | 108 | 16 | 1.3 (0.70, 2.5) |
| Treated but not well-controlledd | 220 | 31 | 1.4 (1.04, 2.0) |
aAll the models were adjusted for baseline age, sex, education, occupation held during most of the life, NSES, smoking status, alcohol status, residential county, physical activity and household income, and baseline cognition function.
bIncludes the untreated participants whose fasting glucose level were either ≥126 mg/dl or 100 mg/dl ≤fasting glucose level <126 mg/dl.
cIncludes the treated participants whose fasting glucose level were either <100 mg/dl or 100 mg/dl ≤fasting glucose level <126 mg/dl.
dIncludes the treated participants whose fasting glucose level were ≥126 mg/dl.
95% CI indicates 95% confidence interval.
Table 4.
Effect estimates (and 95% CIs) from Cox modelsa for traffic-related NOx (per 2.29 ppb increase) and dementia/CIND, according to low HDL-cholesterol status
| HDL-Cholesterol Status | N | No. cases | Traffic-related NOx, per 2.29 ppb increase |
|---|---|---|---|
| HR (95% CI) | |||
| Normal HDL-cholesterol level | 1,026 | 94 | 1.0 (0.8, 1.4) |
| Untreated but low HDL-cholesterol | 453 | 52 | 1.1 (0.8, 1.6) |
| Treated low HDL-cholesterolb | 132 | 13 | 1.7 (1.0, 2.8) |
aAll models were adjusted for baseline age, sex, education, occupation held during most of the life, NSES, smoking status, alcohol status, residential county, physical activity and household income, and baseline cognition function.
bIncludes treated participants whose HDL-cholesterol level were either ≥40 mg/dl or <40 mg/dl in men and either ≥50 mg/dl or <50 mg/dl in women.
95% CI indicates 95% confidence interval.
Discussion
We found that high exposure to traffic-related air pollution or noise among older Mexican-Americans with hyperglycemia, low HDL-cholesterol, and obesity exacerbates the risk of developing incident dementia/CIND. We further observed that cohort participants whose glucose levels were not well-controlled when treated and similarly those who are obese or with low HDL-cholesterol even when treated with statins were more likely to develop incident dementia/CIND when exposed to high traffic-related air pollution.
Air pollution, especially traffic-related air pollution, is a growing global problem along with other detrimental aspects of urbanization such as noise exposure; these exposures have been connected with various chronic health outcomes including adverse effects on cognition.4,36–41 Both experimental and animal studies have shown that air pollutants provoke oxidative stress and systemic inflammatory responses, and can disrupt the blood–brain barrier, precipitate β-amyloid and activate microglia.42–45 Recently, concerns have been raised about the role that traffic-related air pollution and noise exposures play in neurodegenerative diseases.46 Possible mechanisms affected by noise include sleep disturbance and stress, which in turn lead to an activation of the autonomic nervous system and the hypothalamic–pituitary–adrenal axis, that is, stress-responsive regulatory systems including those involved in insulin resistance.47–50 Noise has also been shown to reduce brain volume in the medial prefrontal cortex area and cortical thickness in the hippocampus and amygdala in animal experiments,51,52 along with elevated level of noradrenaline and dopamine from the activation of stress pathways in the hypothalamus and brainstem, followed by prefrontal cortex dysregulation.53–55 Epidemiologic studies have thus far provided some limited evidence for a link between air pollution or noise exposure and cognitive impairment. A cohort study (Ontario Population Health and Environment Cohort [ONPHEC]) in Canada used the health records of 20,666,639 subjects and found that per 1.2 ppb increase in NO2, dementia incidence increased by 10% (1.08, 1.12).37 The longitudinal Betula study in Northern Sweden (1,806 participants) reported a risk for incident dementia of 1.6 (1.0, 2.1) among those with highest traffic-related NOx exposure (>26 μg/m3) compared to those with the lowest exposure (4.8–9 μg/m3), and estimated a hazard ratio of 1.1 (1.0, 1.1) per 10 μg/m3 increase in NOx.6 A longitudinal cohort study in England reported a 2% increase in risk of dementia per 2.68 dB increase in traffic-related nighttime noise exposure.4 A cross-sectional study in Germany of 4,086 participants 50–80 years old found that higher residential noise from traffic (per 10 dB(A) increase) was positively associated with mild cognitive impairment (MCI) (odds ratio [OR] = 1.4 [1.0, 1.9] and amnestic MCI: OR = 1.5 [1.1, 2.2]).9
Metabolic dysfunctions, including obesity, hyperglycemia, and dyslipidemia, have widely been considered to play a role in the development of dementia. Insulin resistance, one of the key pathological features of metabolic syndrome, is closely related to oxidative stress and inflammation and may induce alterations in β-amyloid deposition or clearance, a pathological mechanism considered important for dementia and cognitive impairment.46,56 Evidence linking metabolic syndrome to decline in cognitive performance is growing and includes structural changes such as volume loss in the hippocampus and frontal lobes, white matter alterations, and altered brain metabolism.57,58 Our findings agree with previous studies that reported on metabolic syndrome and cognition. The longitudinal Aging Study Amsterdam (1,183 participants 65–88 years old) reported that hyperglycemia was most strongly associated with decline in cognition function.59 The population-based PROgnostic indicator OF cardiovascular and cerebrovascular events study in France (n = 895) observed a positive association between low HDL-cholesterol (HDL-cholesterol <1.03 mmol/L in men or <1.29 mmol/L in women) and poor executive function (OR = 2.6 [1.7, 4.0]).60 In a substudy of the Longitudinal Older Veteran (LOVE) study in Taiwan with 276 men 75 years old or older, central obesity was positively associated with cognitive decline (OR = 4.2 [1.3, 13.9]).61
We also investigated the roles of obesity, hyperglycemia, and low HDL-cholesterol after accounting for treatment effects and found that the risk of developing incident dementia/CIND when exposed to traffic-related NOx exposure was higher among those treated but with glucose levels that suggested their diabetes was not well-controlled, and among those who were obese, or those treated with statins who still had low HDL-cholesterol levels. However, we did not see the same pattern with 24-hour noise exposure, possible due to different pathophysiologic mechanisms underlying air pollution (inflammation pathway) and noise exposure (stress pathway)62 or alternatively due to random measurement errors and small sample size. Since our study is the first study to investigate the role that different metabolic dysfunctions play for noise exposure and cognitive impairment, further investigations are needed.
The SALSA study is a population-based longitudinal cohort study with up to 10 years of follow-up and one of few studies focusing on brain health in older Mexican-Americans. To our knowledge, no study has thus far investigated the combined effects of high levels of traffic-related air pollution and noise exposure and several metabolic dysfunctions, and our study is one of few investigating the modification of effect measures by metabolic dysfunction on cognitive impairment and to account for treatment effects. For air pollution and noise, we derived exposure estimates based on geocoded residential addresses using Global Positioning System (GPS) readings performed at home during visits. Thus, these measures have high geo-location quality. Additionally, we employed the CALINE4 dispersion model—a well-validated model—to characterize pollutant exposures from traffic sources in close proximity to homes. We used anthropometric, biochemical measurements, and medication information to assess metabolic function and repeated cognitive function testing and imaging (MRI) to diagnose incident dementia/CIND, thus, guaranteeing high accuracy for metabolic dysfunction and dementia/CIND diagnoses.
There are some limitations. First, because SALSA study is a cohort of older Mexican-Americans with an average age of 70 years, we excluded hypertension from the metabolic disorders we studied as the temporal relationship between hypertension and dementia/cognitive impairment is complex and likely not well characterized this late in life. Specifically, while positive association between midlife hypertension and late-life dementia/cognitive impairment are well established, many studies report null or protective associations between late-life hypertension and dementia/cognitive impairment,14,15 possibly due to a loss of cerebral autoregulation to maintain adequate blood flow to the brain.63 As for hypertriglyceridemia, complex temporal relationships were also reported in epidemiologic studies16,17 such that triglyceride levels were seen to be increased before amyloid beta accumulation, which starts 10–20 years before symptom onset. It is also possible that low lipid levels could be part of a prodromal stage of dementia/cognitive impairment due to alterations in the energetic profile and studies with a high baseline age of participants are not able to detect any mid-life related harmful effect of high lipid levels.64 Similar as in previous studies, our study found no associations between baseline hypertension (definition: blood pressure ≥130/85 mmHg or use of antihypertensive medication; HR = 0.88 [0.59, 1.3] or hypertriglyceridemia [definition: triglycerides ≥150 mg/dl or use of statins; HR = 0.78 (0.56, 1.1)]) and incident dementia/CIND. Thus, since we lack health information for mid-life in SALSA, we only evaluated the metabolic dysfunctions obesity, hyperglycemia, and low HDL-cholesterol. We also were unable to estimate participants’ historical exposures to air pollution and noise before enrollment in the study due to the lack of lifetime residential addresses and of adequate air monitoring or traffic density data before 1990. However, SALSA participants’ low residential mobility suggests that by using baseline addresses, we likely generated spatially distinct long-term exposure measures. Additionally, we only used 2002 traffic data to generate our exposure estimates; however, traffic counts, meteorological and emission factors are highly correlated across the years of follow-up in the Sacramento area, and the traffic counts changes likely only have had a minor influence on absolute exposure estimates over the years; thus, we assume that relative exposure levels based mainly on location of the residence remained the same across time. Furthermore, we lack information about use of protective measures including window insulation, bedroom orientation (facing to street or not), and use of ear plugs, which might contribute to exposure measurement error. As for NOx exposure, our CALINE4 dispersion model only captures local traffic emissions within 1,500 m of the residence, without taking into account background air pollution and emissions farther away. Thus, while the estimated concentrations are very low, they serve as a spatially dense proxy for local traffic pollution. Noise exposure estimates only considered major roadway (freeways, highways, and major roads) traffic as a source and did not include railway and airport noises or contributions from other sources such as construction sites. Thus, overall noise exposures are possibly underestimated for some participants. The environmental exposures, metabolic syndrome, and cognitive impairment status were not self-reported, making selection bias less likely. Last, residual confounding cannot be completely ruled out even though we have adjusted for a number of important covariates that are related to both exposures and dementia incidence including demographic and lifestyle factors and NSES.
In conclusion, our study indicates that high levels of exposure to traffic-related air pollution or noise among older Mexican-Americans who are obese or suffer from hyperglycemia or low HDL-cholesterol increases the risk of developing cognitive impairment disproportionately. These findings provide some evidence that metabolic dysfunction may not only act as a risk factors for incident dementia/cognitive impairment but also modifies the negative impacts of environmental exposures. Early identification and treatment of people with metabolic dysfunction and interventions that reduce traffic-related exposures might be needed to mitigate cognitive impairment in older adults.
Conflicts of interest statement
The authors declare that they have no conflicts of interest with regard to the content of this report.
This work was supported by grants R01ES023451 from National Institute of Environmental Health Sciences, AG012975, AG033751 and AG053410 from National Institute on Aging, and DK060753 from National Institute of Diabetes and Digestive and Kidney Diseases.
The data for these cohorts are available on the server of Inter-university Consortium for Political and Social Research (ICPSR) at University of Michigan and the exposure data will be available upon request to the authors. The analytical code used was standard SAS code (e.g., data steps, proc phreg).
Supplementary Material
Footnotes
Published online 3 December 2020
Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
References
- 1.Kilian J, Kitazawa M. The emerging risk of exposure to air pollution on cognitive decline and Alzheimer’s disease - evidence from epidemiological and animal studies. Biomed J. 2018; 41:141–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein J, Schettler T, Rohr B, Valenti M. Environmental Threats to Healthy Aging. 2008. Greater Boston physicians for social responsibility and science and environmental health network [Google Scholar]
- 3.Tzivian L, Winkler A, Dlugaj M, et al. Effect of long-term outdoor air pollution and noise on cognitive and psychological functions in adults. Int J Hyg Environ Health. 2015; 218:1–11 [DOI] [PubMed] [Google Scholar]
- 4.Carey IM, Anderson HR, Atkinson RW, et al. Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open. 2018; 8:e022404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med. 2012; 172:219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oudin A, Forsberg B, Adolfsson AN, et al. Traffic-related air pollution and dementia incidence in Northern Sweden: a longitudinal study. Environ Health Perspect. 2016; 124:306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahad O, Kröller-Schön S, Daiber A, Münzel T. The cardiovascular effects of noise. Dtsch Arztebl Int. 2019; 116:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Münzel T, Schmidt FP, Steven S, Herzog J, Daiber A, Sørensen M. Environmental noise and the cardiovascular system. J Am Coll Cardiol. 2018; 71:688–697 [DOI] [PubMed] [Google Scholar]
- 9.Tzivian L, Dlugaj M, Winkler A, et al. ; Heinz Nixdorf Recall study Investigative Group. Long-term air pollution and traffic noise exposures and mild cognitive impairment in older adults: a cross-sectional analysis of the Heinz Nixdorf Recall Study. Environ Health Perspect. 2016; 124:1361–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014; 2014:943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005; 365:1415–1428 [DOI] [PubMed] [Google Scholar]
- 12.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2005; 48:1684–1699 [DOI] [PubMed] [Google Scholar]
- 13.Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: common links. J Intern Med. 2006; 260:211–223 [DOI] [PubMed] [Google Scholar]
- 14.Iadecola C. Hypertension and dementia. Hypertension. 2014; 64:3–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abell JG, Kivimäki M, Dugravot A, et al. Association between systolic blood pressure and dementia in the Whitehall II cohort study: role of age, duration, and threshold used to define hypertension. Eur Heart J. 2018; 39:3119–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol. 2000; 20:2255–2260 [DOI] [PubMed] [Google Scholar]
- 17.Nägga K, Gustavsson AM, Stomrud E, et al. Increased midlife triglycerides predict brain β-amyloid and tau pathology 20 years later. Neurology. 2018; 90:e73–e81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. 2017Available at: https://www.cdc.gov/nchs/data/databriefs/db288.pdf. Accessed 19 November 2020
- 19.Benjamin EJ, Virani SS, Callaway CW, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018; 137:e67–e492 [DOI] [PubMed] [Google Scholar]
- 20.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA. 2015; 313:1973–1974 [DOI] [PubMed] [Google Scholar]
- 21.Chukwueke I, Cordero-Macintyre Z. Overview of type 2 diabetes in Hispanic Americans. Int J Body Compos Res. 2010; 8supp77–81 [PMC free article] [PubMed] [Google Scholar]
- 22.California EPA Office of Environmental Health Hazard Assessment (OEHHA). Analysis of Race/Ethnicity, Age, and CalEnviroScreen 3.0 Scores. 2018Available at: https://oehha.ca.gov/media/downloads/calenviroscreen/document-calenviroscreen/raceageces3analysis.pdf. Accessed 19 November 2020
- 23.Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003; 51:169–177 [DOI] [PubMed] [Google Scholar]
- 24.Yu Y, Paul K, Arah OA, et al. Air pollution, noise exposure, and metabolic syndrome - a cohort study in elderly Mexican-Americans in Sacramento area. Environ Int. 2020; 134:105269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.California EPA. 2011. California Environmental Protection Agency (EPA)air resources board. Emfac2011 - technical documentation. 2013. California Air Resources Board [Google Scholar]
- 26.CARB. California Air Resources Board (CARB), air quality and meteorological in-formation system. 2015. California Air Resources Board [Google Scholar]
- 27.FHWA. U.S. Department of Transportation Federal Highway Administration (FHWA). NHI Course No. 132034. Ground Modification Methods Reference Manual – Volume II. Publication No. FHWA-NHI-16-028 FHWA GEC 013. 2017Available at: https://www.fhwa.dot.gov/engineering/geotech/pubs/nhi16028.pdf. Accessed 26 July 2019.
- 28.Fecht D, Hansell AL, Morley D, et al. Spatial and temporal associations of road traffic noise and air pollution in London: implications for epidemiological studies. Environ Int. 2016; 88:235–242 [DOI] [PubMed] [Google Scholar]
- 29.Grundy SM, Cleeman JI, Daniels SR, et al. ; American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112:2735–2752 [DOI] [PubMed] [Google Scholar]
- 30.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001; 12:703–711 [DOI] [PubMed] [Google Scholar]
- 31.Shih IF, Paul K, Haan M, Yu Y, Ritz B. Physical activity modifies the influence of apolipoprotein E ε4 allele and type 2 diabetes on dementia and cognitive impairment among older Mexican Americans. Alzheimers Dement. 2018; 14:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee EY, Jerrett M, Ross Z, Coogan PF, Seto EY. Assessment of traffic-related noise in three cities in the United States. Environ Res. 2014; 132:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seong JC, Park TH, Ko JH, et al. Modeling of road traffic noise and estimated human exposure in Fulton County, Georgia, USA. Environ Int. 2011; 37:1336–1341 [DOI] [PubMed] [Google Scholar]
- 34.Paul KC, Haan M, Yu Y, et al. Traffic-related air pollution and incident dementia: direct and indirect pathways through metabolic dysfunction. J Alzheimers Dis. 2020; 76:1477–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007; 17:227–236 [DOI] [PubMed] [Google Scholar]
- 36.Jung CR, Lin YT, Hwang BF. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: a population-based cohort study in Taiwan. J Alzheimers Dis. 2015; 44:573–584 [DOI] [PubMed] [Google Scholar]
- 37.Chen H, Kwong JC, Copes R, et al. Exposure to ambient air pollution and the incidence of dementia: a population-based cohort study. Environ Int. 2017; 108:271–277 [DOI] [PubMed] [Google Scholar]
- 38.Jerrett M, Brook R, White LF, et al. Ambient ozone and incident diabetes: a prospective analysis in a large cohort of African American women. Environ Int. 2017; 102:42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H, Kwong JC, Copes R, et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: a population-based cohort study. Lancet. 2017; 389:718–726 [DOI] [PubMed] [Google Scholar]
- 40.Jerrett M, McConnell R, Wolch J, et al. Traffic-related air pollution and obesity formation in children: a longitudinal, multilevel analysis. Environ Health. 2014; 13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jerrett M, Burnett RT, Pope CA, 3rd, et al. Long-term ozone exposure and mortality. N Engl J Med. 2009; 360:1085–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genc S, Zadeoglulari Z, Fuss SH, Genc K. The adverse effects of air pollution on the nervous system. J Toxicol. 2012; 2012:782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Block ML, Wu X, Pei Z, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004; 18:1618–1620 [DOI] [PubMed] [Google Scholar]
- 44.Calderón-Garcidueñas L, Mora-Tiscareño A, Ontiveros E, et al. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn. 2008; 68:117–127 [DOI] [PubMed] [Google Scholar]
- 45.Levesque S, Taetzsch T, Lull ME, et al. Diesel exhaust activates and primes microglia: air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ Health Perspect. 2011; 119:1149–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul KC, Haan M, Mayeda ER, Ritz BR. Ambient air pollution, noise, and late-life cognitive decline and dementia risk. Annu Rev Public Health. 2019; 40:203–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt FP, Basner M, Kröger G, et al. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur Heart J. 2013; 34:3508–3514a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griefahn B, Robens S. Experimental studies on the effects of nocturnal noise on cortisol awakening response. Noise Health. 2010; 12:129–136 [DOI] [PubMed] [Google Scholar]
- 49.Björntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000; 16:924–936 [DOI] [PubMed] [Google Scholar]
- 50.Cui B, Gai Z, She X, Wang R, Xi Z. Effects of chronic noise on glucose metabolism and gut microbiota-host inflammatory homeostasis in rats. Sci Rep. 2016; 6:36693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czéh B, Müller-Keuker JI, Rygula R, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007; 32:1490–1503 [DOI] [PubMed] [Google Scholar]
- 52.Jafari Z, Kolb BE, Mohajerani MH. Chronic traffic noise stress accelerates brain impairment and cognitive decline in mice. Exp Neurol. 2018; 308:1–12 [DOI] [PubMed] [Google Scholar]
- 53.Arnsten AF, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry. 1998; 55:362–368 [DOI] [PubMed] [Google Scholar]
- 54.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009; 10:410–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jafari Z, Kolb BE, Mohajerani MH. Noise exposure accelerates the risk of cognitive impairment and Alzheimer’s disease: adulthood, gestational, and prenatal mechanistic evidence from animal studies. Neurosci Biobehav Rev. 2019Apr 9. doi: 10.1016/j.neubiorev.2019.04.001. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 56.Yaffe K, Weston AL, Blackwell T, Krueger KA. The metabolic syndrome and development of cognitive impairment among older women. Arch Neurol. 2009; 66:324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bokura H, Nagai A, Oguro H, Kobayashi S, Yamaguchi S. The association of metabolic syndrome with executive dysfunction independent of subclinical ischemic brain lesions in Japanese adults. Dement Geriatr Cogn Disord. 2010; 30:479–485 [DOI] [PubMed] [Google Scholar]
- 58.Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arterioscler Thromb Vasc Biol. 2012; 32:2060–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dik MG, Jonker C, Comijs HC, et al. Contribution of metabolic syndrome components to cognition in older individuals. Diabetes Care. 2007; 30:2655–2660 [DOI] [PubMed] [Google Scholar]
- 60.Rouch I, Trombert B, Kossowsky MP, et al. Metabolic syndrome is associated with poor memory and executive performance in elderly community residents: the PROOF study. Am J Geriatr Psychiatry. 2014; 22:1096–1104 [DOI] [PubMed] [Google Scholar]
- 61.Liu CL, Lin MH, Peng LN, et al. Late-life metabolic syndrome prevents cognitive decline among older men aged 75 years and over: one-year prospective cohort study. J Nutr Health Aging. 2013; 17:523–526 [DOI] [PubMed] [Google Scholar]
- 62.Fuks KB, Weinmayr G, Basagaña X, et al. Long-term exposure to ambient air pollution and traffic noise and incident hypertension in seven cohorts of the European study of cohorts for air pollution effects (ESCAPE). Eur Heart J. 2017; 38:983–990 [DOI] [PubMed] [Google Scholar]
- 63.Gottesman RF. Should hypertension be treated in late life to preserve cognitive function? Con side of the argument. Hypertension. 2018; 71:787–792 [DOI] [PubMed] [Google Scholar]
- 64.Gustafson D. A life course of adiposity and dementia. Eur J Pharmacol. 2008; 585:163–175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


