ABSTRACT
Anemia is a leading cause of morbidity in sub-Saharan Africa. The etiologies of anemia are multifactorial, and it is unclear what proportion of anemia is attributable to malaria in children of different ages in Malawi. We evaluated the population attributable fraction (PAF) of anemia due to malaria using multiple cross-sectional surveys in southern Malawi. We found a high prevalence of anemia, with the greatest proportion attributable to malaria among school-age children (5–15 years) in the rainy season (PAF = 18.8% [95% CI: 16.3, 21.0], compared with PAF = 5.2% [95% CI: 4.0, 6.2] among young children pooled across season [< 5 years] and PAF = 9.7% [95% CI: 6.5, 12.4] among school-age children in the dry season). Malaria control interventions will likely lead to decreases in anemia, especially among school-age children.
Anemia affects more than one-third of the population in sub-Saharan Africa and is associated with clinical morbidity as well as impaired childhood development, with potential long-term impacts on productivity in adulthood.1,2 Children younger than 5 years, women of childbearing age, and pregnant women have the highest prevalence of anemia; however, the burden extends across all ages.1 The etiology of anemia is multifactorial and, in sub-Saharan Africa, includes nutritional deficiencies, helminth infections, and malaria.
The prevalence and relative contribution of different etiologies to anemia varies by age.1 Because hemoglobin is measured in young children (6–59 months old) and women of childbearing age in routine Demographic and Health Surveys, most data come from these high-risk groups.3 Among young children, in whom the prevalence of anemia is the highest, nutritional deficiencies are the primary cause of anemia.1 The proportion of anemia attributable to malaria in young children, known as the attributable fraction, varies widely from 2.5% in Rwanda to an estimated 15% in West Africa.4,5 A meta-analysis of early studies showed that malaria control interventions, namely insecticide-treated bed nets and chemoprophylaxis, reduced the risk of anemia in young children by 27%.6
Available data on anemia in older, school-age children predominantly come from assessments of helminth infections undertaken as a part of school-based deworming programs, which substantially reduce the contribution of these infections to anemia in school-aged children.7 There is increasing evidence that, in many malaria-endemic settings, the prevalence of Plasmodium falciparum infection peaks in school-age children.8,9 Malaria control interventions targeting this age-group should also decrease anemia. The potential reduction in anemia by malaria-specific interventions depends on the attributable fraction of anemia due to P. falciparum infection. We used data from malaria surveillance in southern Malawi to estimate the contribution of P. falciparum to anemia in children. Quantifying the attributable fraction of anemia due to P. falciparum infection in school-age children is essential for estimating the impacts of malaria control interventions targeting this age-group.
We evaluated the attributable fraction of anemia due to P. falciparum infection in children aged 6 months to 15 years with hemoglobin measured as a part of repeated household-based cross-sectional surveys in three neighboring districts in Malawi from April 2012 to May 2016. The studies were approved by the University of Maryland Baltimore, Michigan State University, and the University of Malawi College of Medicine. Details of these surveys have been published previously.9 In brief, biannual surveys were conducted at the end of the rainy (April–May) and dry (October–November) seasons in Blantyre (urban to peri-urban, highland, and low malaria transmission), Chikwawa (rural lowland, moderate–high transmission), and Thyolo (rural highland, moderate transmission) districts. Using two-stage sampling, 10 clusters of 30 households in each district were visited from 2012 to 2015. Surveys conducted in 2015–2016 included only four of the original clusters plus 50 households near the local primary school in each cluster. Blood samples were collected by finger prick for hemoglobin measurement (Hemocue®, Ängelholm, Sweden), preparation of a thick blood smear, and PCR detection of P. falciparum lactate dehydrogenase gene from approximately 50 mL of blood spotted on a filter paper. Hemoglobin was measured in young children (< 5 years old) in all surveys, but only measured systematically in school-age children (5–15 years old) in 2014–2016. Analyses of all surveys are presented here. Results of analyses including only the 2014–2016 surveys in which hemoglobin was measured in both age-groups were similar (Supplemental Information). Anemia was defined using WHO age-specific definitions after hemoglobin was adjusted for altitude: mild (age < 5 years: 10.0–10.9 g/dL; age 5–11 years: 11.0–11.4 g/dL; females age 12–15 years and males age 12–14 years: 11.0–11.9 g/dL; males age 15 years: 11.0–12.9 g/dL), moderate (age < 5 years: 7.0–9.9 g/dL; age 5–15 years: 8.0–10.9 g/dL), and severe (age < 5 years: < 7.0 g/dL; age 5–15 years: < 8.0 g/dL).10 Thick smear microscopy slides were stained with Giemsa stain and read by trained, blinded microscopists. Quality control was conducted using published procedures.11 We used univariate analyses to assess characteristics of study participants stratified by age-group (young versus school-age children). We used log-binomial models to estimate the prevalence ratio (PR) of anemia (outcome) comparing children with P. falciparum infection (exposure) with those without infection. Linear regression was used to estimate the association between hemoglobin and parasite density (log transformed). Significance was determined by two-sided P-value with an alpha ≤ 0.05.
Interactions terms were tested to determine if the association between P. falciparum infection and anemia differed by age-group, net use, season, or gender. There was a significant three-way interaction among P. falciparum infection, age-group, and season (P = 0.019), suggesting that the association between P. falciparum infection and anemia differed by age and season. Therefore, results were stratified by age-group and season. Further investigation found a significant interaction between P. falciparum infection and season among school-age children (P = 0.0003), but not among young children (P = 0.43). Consequently, a pooled estimate across season among young children was also calculated. Interaction terms between P. falciparum infection and net use or gender were not significant. Bivariate analyses found significant associations between each of the following variables with both P. falciparum infection and anemia: district, education level, wealth, survey, and net use. Therefore, these variables, as well as survey clusters, were adjusted for in multivariable models.
To estimate the excess anemia cases in the population that can be attributed to P. falciparum infection, the population attributable fraction (PAF) of anemia was computed as
where Pd is the proportion of anemia cases exposed to P. falciparum infection, and aPR is the adjusted PR of anemia comparing children with P. falciparum infection with those without P. falciparum infection.12 To compute the 95% CIs for PAF, the aforementioned formula was applied to the 95% CI of the corresponding adjusted PR.
Among the 7,842 observations from children with hemoglobin results, 28 were missing PCR results and 318 were in school-age children before systematic measurement began in this age-group and were, thus, excluded. The remaining 7,496 observations were included in the analysis (Table 1). The prevalence of anemia was higher in younger children (40.6% [95% CI: 39.1, 42.2]) than that in school-age children (34.8% [95% CI: 33.3, 36.3]). Conversely, the prevalence of P. falciparum infection was higher in school-age children (23.8% [95% CI: 22.5, 25.2]) than that in younger children (11.7% [95% CI: 10.7, 12.8]).
Table 1.
Characteristics of study participants by age-group
| Children younger than 5 years (N = 3,737) | School-age children (N = 3,759) | |
|---|---|---|
| Child factors | ||
| Median age (IQR) (years) | 2.3 (2.0) | 9.4 (5.0) |
| Female, n (col %) | 1,861 (49.8) | 1,937 (51.5) |
| Mean hemoglobin level (SD) | 11.2 (1.4) | 12.1 (1.4) |
| P. falciparum infection, n (col %) | 437 (11.7) | 895 (23.8) |
| Smear positive, n (col %) | 311 (8.4) | 578 (15.4) |
| Geometric mean (SD) | 7.3 (2.0) | 6.8 (1.7) |
| Fever, n (% col) | 1,260 (33.8) | 653 (17.4) |
| Antimalarial drugs, n (% col) | 377 (10.1) | 239 (6.4) |
| Anemia,* n (col %) any | 1,519 (40.6) | 1,309 (34.8) |
| Mild | 902 (24.1) | 625 (16.6) |
| Moderate | 596 (15.9) | 666 (17.7) |
| Severe | 21 (0.6) | 18 (0.5) |
| Using bed nets, n (col %)† | 2,486 (66.5) | 1,852 (49.3) |
| Household factors | ||
| Median number of people in the household (IQR) | 4.8 (2.0) | 5.3 (2.0) |
| Mean wealth index (SD)‡ | −0.07 (2.5) | 0.10 (2.6) |
| Highest education level of household head or spouse, n (col %) | ||
| No schooling | 398 (10.6) | 582 (15.5) |
| Primary education | 2,148 (57.5) | 2,192 (58.3) |
| Secondary and college | 1,191 (31.9) | 985 (26.2) |
| Study factors | ||
| Season, n (col %) | ||
| Rainy | 1,946 (52.1) | 2,046 (54.4) |
| Dry | 1,791 (47.9) | 1,713 (45.6) |
| District, n (col %) | ||
| Blantyre (urban, peri-urban highland) | 990 (26.5) | 786 (20.9) |
| Chikwawa (rural, lowland) | 1,642 (43.9) | 1,938 (51.6) |
| Thyolo (rural, highland) | 1,105 (29.6) | 1,035 (27.5) |
School-age defined as children aged 5–15 years.
Mild anemia (age < 5 years: 10.0–10.9 g/dL; age 5–11 years: 11.0–11.4 g/dL; females age 12–15 years and males age 12–14 years: 11.0–11.9 g/dL; males age 15 years: 11.0–12.9 g/dL), moderate anemia (age < 5 years: 7.0–9.9 g/dL; age 5–15 years: 8.0–10.9 g/dL), and severe anemia (age < 5 years: < 7.0 g/dL; age 5–15 years: < 8.0 g/dL).
Individual bed net use was assessed by asking whether the individual slept under a bed net the previous night.
Wealth index based on household assets was created using principal component analysis and the Filmer and Pritchett method.26 Asset indicators were assessed using questions on ownership of house, phone radio, television, bike, and/or car, availability of electricity in the house, food security, source of income, and highest level of education of head of household or spouse.
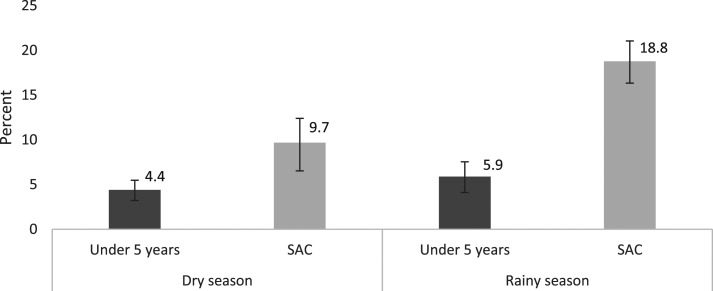
P. falciparum infection was associated with an increased prevalence of anemia in both seasons in young children (aPR rainy season 1.42 [95% CI: 1.26, 1.61]; aPR dry season 1.55 [95% CI: 1.35, 1.79]) and school-age children (aPR rainy season 1.88 [95% CI: 1.68, 2.09]; aPR dry season 1.48 [95% CI: 1.28, 1.71]) (Table 2). The magnitude of the association and the PAF of anemia due to P. falciparum infection were greatest in school-age children in the rainy season (Figure 1). The adjusted attributable fraction was higher in school-age children in the rainy season (PAF: 18.8% [95% CI: 16.3, 21.0]) than in school-age children in the dry season (PAF: 9.7% [95% CI: 6.5, 12.4]) and young children (PAF: 5.2% [95% CI: 4.0, 6.2] pooled across season). Among participants with P. falciparum infection detected by microscopy, a log increase in parasite density was associated with a 0.396 g/dL and 0.433 g/dL decrease in hemoglobin among younger children and school-age children, respectively (both P < 0.0001; Supplemental Information).
Table 2.
Prevalence of anemia among children with P. falciparum infection compared with children without P. falciparum infection
| Age-groups | Exposure groups | Total, n (col %) | Anemia, n (row %) | Unadjusted PR of anemia (95% CI) | Adjusted* PR of anemia (95% CI) | |
|---|---|---|---|---|---|---|
| Dry season | Younger than 5 years | P. falciparum infection | 151 (8.4) | 90 (59.6) | 1.54 (1.33, 1.78)† | 1.55 (1.35, 1.79)† |
| No infection | 1,640 (91.6) | 635 (38.7) | ||||
| School-age | P. falciparum infection | 371 (21.7) | 166 (44.7) | 1.54 (1.34, 1.77)† | 1.48 (1.28, 1.71)† | |
| No infection | 1,342 (78.3) | 390 (29.1) | ||||
| Rainy season | Younger than 5 years | P. falciparum infection | 286 (14.7) | 158 (55.2) | 1.44 (1.28, 1.63)† | 1.42 (1.26, 1.61)† |
| No infection | 1,660 (85.3) | 636 (38.3) | ||||
| School-age | P. falciparum infection | 524 (25.6) | 304 (58.0) | 1.97 (1.77, 2.19)† | 1.88 (1.68, 2.09)† | |
| No infection | 1,522 (74.4) | 449 (29.5) |
Log-binomial models controlling for district, education, net use, wealth, and survey cluster. PR = prevalence ratio; school-age defined as children aged 5–15 years
P-value < 0.001
Figure 1.
Adjusted population attributable fraction of anemia associated with P. falciparum infection by age-group and season.
These results show that anemia is prevalent throughout childhood, and the contribution of P. falciparum infection to anemia is greater in school-age children than that in younger children in southern Malawi, particularly during the rainy season. The larger PAF of anemia due to P. falciparum infection in older children has several potential explanations: 1) other causes of childhood anemia, such as nutritional deficiencies, may be relatively more common in younger children, and school-based deworming programs may make coinfections less common; 2) younger children have a lower prevalence of P. falciparum infection in this population, potentially due to higher levels of use of existing malaria control interventions, that is, sleep under bed nets more frequently and receive effective malaria diagnosis and treatment more often than school-age children13–15; and 3) naturally acquired immunity to malaria disease results in school-age children more frequently having subclinical infections with prolonged and untreated parasitemia, which is associated with anemia.16 In younger children, the PAF of anemia in our study was lower than that in the 2014 nationally representative Malaria Indicator Survey, where the PAF was 11.6% in the setting of an overall prevalence of P. falciparum infection by microscopy of 26% (Supplemental Information).17 Neither malaria prevalence nor anemia was assessed in older children, limiting comparisons to our results.
One limitation to our analysis is that we did not measure other causes of anemia. Concomitant nutritional deficiencies and/or coinfection with helminths can increase the contribution of P. falciparum infection to anemia.18,19 The prevalence of Schistosomiasis haematobium in southern Malawi is heterogeneous, but ranges from 1.8 to 28.6% and peaks in prevalence and intensity in school-age children.20,21 The prevalence of soil-transmitted helminths, predominantly hookworm, is also heterogeneous, but increases with age through adulthood.22–24 Furthermore, there may be confounding as other causes of anemia may also be associated with P. falciparum infection. Although having measured other etiologies of anemia would enhance our understanding of these interrelationships and might find elevated PAF in groups with coinfections, our results nonetheless support the importance of decreasing the burden of P. falciparum infection in school-age children. A second limitation is that the surveys were repeated in many of the same households, but individuals were not linked over time. Thus, we were not able to account for repeated measures. Repeated measures among individuals imply some dependent observations were treated as independent, suggesting CIs may truly be wider than we estimated; however, this should not bias our estimates of effect.
In this setting, highly effective malaria control interventions targeting school-age children have the potential to reduce anemia in this age-group by as much as 20%. This is consistent with meta-analyses suggesting that school-based preventive treatment of P. falciparum reduced the overall prevalence of anemia by 15%.25
Our findings highlight the importance of addressing the burden of P. falciparum infection to improve the health of school-age children by decreasing anemia. Combining malaria control interventions with existing interventions, for example, school-based deworming and school feeding programs, will increase this benefit. It is not clear which malaria control interventions are most effective for this purpose; however, understanding the attributable fraction of P. falciparum infection to anemia in this age-group will aid in the evaluation of future interventions.
Supplemental information
ACKNOWLEDGMENTS
We are grateful for the hard work and dedication of the field team, study nurses, teachers, school administrators who made the study possible. We thank the participants for their commitment and patience. We thank Stephen Kumwenda for assistance obtaining data from the Malaria Indicator Survey.
Note: Supplemental information appears at www.ajtmh.org.
REFERENCES
- 1.Kassebaum NJ, et al. 2016. The global burden of anemia. Hematol Oncol Clin North Am 30: 247–308. [DOI] [PubMed] [Google Scholar]
- 2.Balarajan Y, Ramakrishnan U, Özaltin E, Shankar AH, Subramanian SV, 2011. Anaemia in low-income and middle-income countries. Lancet 378: 2123–2135. [DOI] [PubMed] [Google Scholar]
- 3.The DHS Program , 2020. Research Topics - Anemia. Available at: https://dhsprogram.com/topics/Anemia.cfm. Accessed July 6, 2020. [Google Scholar]
- 4.Nkulikiyinka R, Binagwaho A, Palmer K, 2015. The changing importance of key factors associated with anaemia in 6- to 59-month-old children in a sub-Saharan African setting where malaria is on the decline: analysis of the Rwanda Demographic and Health Survey 2010. Trop Med Int Heal 20: 1722–1732. [DOI] [PubMed] [Google Scholar]
- 5.Soares Magalhães RJ, Clements ACA, 2011. Mapping the risk of anaemia in preschool-age children: the contribution of malnutrition, malaria, and helminth infections in West Africa. PLoS Med 8: e1000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korenromp EL, Armstrong-Schellenberg JRM, Williams BG, Nahlen BL, Snow RW, 2004. Impact of malaria control on childhood anaemia in Africa -- a quantitative review. Trop Med Int Health 9: 1050–1065. [DOI] [PubMed] [Google Scholar]
- 7.Guyatt HL, Brooker S, Kihamia CM, Hall A, Bundy DAP, 2001. Evaluation of efficacy of school-based anthelmintic treatments against anaemia in children in the United Republic of Tanzania. Bull World Health Organ 79: 695–703. [PMC free article] [PubMed] [Google Scholar]
- 8.Bundy DAP, de Silva N, Horton S, Jamison DT, Patton GC, eds., 2017. Child and Adolescent Health and Development. Disease Control Priorities (third edition), Volume 8. Washington, DC: World Bank. 10.1596/978-1-4648-0423-6. [Google Scholar]
- 9.Walldorf JA, et al. 2015. School-age children are a reservoir of malaria infection in Malawi. PLoS One 10: e0134061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO , 2011. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Geneva, Switzerland: World Health Organization. (WHO/NMH/NHD/MNM/11.1). Available at: www.who.int/vmnis/indicators/haemoglobin.pdf. Accessed July 12, 2020. [Google Scholar]
- 11.The RTS,S Clinical Trials Partnership , 2012. A phase 3 trial of RTS,S/AS01 malaria vaccine in african infants. N Engl J Med 367: 2284–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockhill B, Newman B, Weinberg C, 1998. Use and Misuse of Population Attributable Fractions. Am J Public Health 88: 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olapeju B, et al. 2018. Age and gender trends in insecticide-treated net use in sub-Saharan Africa: a multi-country analysis. Malar J 17: 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchwald AG, et al. 2016. Bed net use among school-aged children after a universal bed net campaign in Malawi. Malar J 15: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coalson JE, Cohee LM, Walldorf JA, Bauleni A, Mathanga DP, Taylor TE, Wilson ML, Laufer MK, 2019. Challenges in treatment for fever among school-age children and adults in Malawi. Am J Trop Med Hyg 100: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen I, Clarke SE, Gosling R, Hamainza B, Killeen G, Magill A, O’Meara W, Price RN, Riley EM, 2016. “Asymptomatic” malaria: a chronic and debilitating infection that should be treated. PLoS Med 13: e1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malawi National Malaria Control Program and ICF International , 2015. Malawi: Malaria Indicator Survey (MIS) 2014. Available at: http://dhsprogram.com/pubs/pdf/MIS18/MIS18.pdf. Accessed November 15, 2020. [Google Scholar]
- 18.Ehrhardt S, Burchard GD, Mantel C, Cramer JP, Kaiser S, Kubo M, Otchwemah RN, Bienzle U, Mockenhaupt FP, 2006. Malaria, anemia, and malnutrition in african children—defining intervention priorities. J Infect Dis 194: 108–114. [DOI] [PubMed] [Google Scholar]
- 19.Valice EM, et al. 2018. Relative contribution of schistosomiasis and malaria to anemia in western Kenya. Am J Trop Med Hyg 99: 713–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makaula P, Sadalaki JR, Muula AS, Kayuni S, Jemu S, Bloch P, 2014. Schistosomiasis in Malawi: a systematic review. Parasit Vectors 7: 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chipeta MG, Ngwira B, Kazembe LN, 2013. Analysis of Schistosomiasis haematobium infection prevalence and intensity in Chikhwawa, Malawi: an application of a two part model. PLoS Negl Trop Dis 7: e2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowie C, Purcell B, Shaba B, Makaula P, Perez M, 2004. A national survey of the prevalence of schistosomiasis and soil transmitted helminths in Malaŵi. BMC Infect Dis 4: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Msyamboza K, Ngwira B, Banda R, Mkwanda S, Brabin B, 2010. Sentinel surveillance of lymphatic filariasis, schistosomiasis, soil transmitted helminths and malaria in rural southern Malawi. Malawi Med J 22: 12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phiri BBW, Ngwira B, Kazembe LN, 2016. Analysing risk factors of co-occurrence of Schistosomiasis haematobium and hookworm using bivariate regression models: case study of Chikwawa, Malawi. Parasite Epidemiol Control 1: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohee LM, et al. 2020. Preventive malaria treatment among school-age children in sub-Saharan Africa: a systematic review and meta-analyses. Lancet Glob Heal 8: e1499–e1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filmer D, Pritchett LH, 2001. Estimating wealth effects without expenditure data - or tears: an application to educational enrollments in states of India. Demography 38: 115–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.