ABSTRACT
The WHO recommends single low-dose (SLD) primaquine as a gametocytocide to reduce Plasmodium falciparum transmission in areas of low transmission. Despite this recommendation, uptake of SLD primaquine has been low because of concerns of glucose-6-phosphate dehydrogenase (G6PD) deficiency. Individuals with G6PD deficiency can experience hemolysis when exposed to primaquine. In Southern Province, Zambia, malaria transmission has declined significantly over the past decade. Single low-dose primaquine may be an effective tool, but there is limited information on G6PD deficiency. We screened 137 residents in Macha, Southern Province, Zambia, and the prevalence of G6PD (A−) was 15%. We also revisited data collected from 2008 to 2013 in the same area and found the highest gametocyte burden among those aged 5–15 years. The findings from this study suggest that SLD primaquine targeted to school-aged children may be an effective tool to help achieve malaria elimination in southern Zambia.
Malaria continues to pose a major public health threat in sub-Saharan Africa. Increased funding of malaria control initiatives made a significant impact by decreasing transmission in some areas, but the decline has been stagnant in recent years.1 To further decrease transmission and achieve elimination, the WHO recommends administration of single low-dose (SLD) primaquine to kill Plasmodium falciparum gametocytes and reduce parasite transmission from humans to mosquitoes.2 Single low-dose primaquine is given together with artemisinin combination therapy in pre-elimination or elimination settings. Unlike standard radical cure for Plasmodium vivax and Plasmodium ovale infections that requires administration of primaquine daily for 14 days (0.25 mg/kg weight per day; total dose of 3.5 mg base/kg body weight),3 SLD primaquine as a gametocytocide is a single dose of 0.25 mg base/kg body weight.2
When used for radical cure, guidelines recommend testing for glucose-6-phosphate dehydrogenase (G6PD) deficiency, as primaquine can cause severe hemolysis in those affected.4,5 Glucose-6-phosphate dehydrogenase is an enzyme responsible for homeostasis of glutathione, a major antioxidant.5 Glucose-6-phosphate dehydrogenase enzymopathy is X-chromosome linked, and female heterozygous G6PD-deficient individuals may have some protection against uncomplicated malaria.6 Glucose-6-phosphate dehydrogenase deficiency is a widely prevalent enzymopathy and is often asymptomatic unless hemolysis is triggered by certain foods or drugs, including 8-aminoquinolines such as primaquine.7 Unlike radical cure, the WHO recommendations do not mandate testing for G6PD deficiency before administrating SLD primaquine.8
Approximately 140 variants of G6PD deficiency have been reported worldwide.5 The A-variant is the most prevalent variant in sub-Saharan Africa, accounting for almost 90% of G6PD deficiency.9 Currently, the Zambian National Malaria Elimination Programme has not adopted a policy of SLD primaquine in pre-elimination or low-transmission settings.1 In this study, we estimated the prevalence of G6PD (A−) genotype in the catchment area of Macha Hospital in Choma district, Southern Province, Zambia. The study was approved as part of a larger study under the International Centers of Excellence in Malaria Research (ICEMR) project approved by the Tropical Disease Research Centre Ethics Review Committee in Ndola, Zambia, and the Institutional Review Board of the Johns Hopkins Bloomberg School of Public Health.
In the study area, the peak malaria transmission season is from November through March, followed by a cool dry season (April–July) and a hot dry season (August–October). The area is populated by subsistence farmers, living in small scattered homesteads. Anopheles arabiensis is the primary vector responsible for malaria transmission,10 but recently, P. falciparum was detected in Anopheles squamosus.11 The area was historically meso-endemic, but parasite prevalence and malaria incidence decreased substantially over the past 15 years, and the region is considered pre-elimination.
Participants were enrolled through two different study designs: random sampling based on satellite imagery in 2014 and reactive case detection in 2015. For random sampling, details of the household selection were described previously.12 In brief, the sampling frame was constructed using a Quickbird™ satellite image obtained from DigitalGlobe Services, Inc. (Denver, CO). The image was imported into ArcGIS 9.2 (Redlands, CA), and locations of households were identified and enumerated manually. For the sample collection through reactive case detection and test-and-treat, the ICEMR field study team and community health workers visited rapid diagnostic test (RDT)-positive cases identified at health centers (index cases) at their household (index case households). The team screened residents in the index case household as well as residents in all households within 250 m of the index case household.13
For those who agreed to participate, written informed consent was obtained from all adults or caregivers of children. Tympanic temperature was taken, and participants were tested for malaria by a PfHRP2-based RDT (SD Bioline Malaria Ag P.f [Standard Diagnostics Inc., Gyeonggi-do, Republic of Korea]). All RDT-positive participants were offered treatment according to the guidelines of the Zambian Ministry of Health. Blood was collected by finger prick using a capillary tube (Microvette CB300, Sarstedt, Nümbrecht, Germany). Fifty-six and 81 whole blood samples were collected in June 2014 and July 2015, respectively. Blood in capillary tubes was kept in a cooler box with ice packs, transported to the laboratory, and centrifuged to separate the cell fraction and plasma. Samples were stored at −20°C until processing.
DNA was extracted from the cell fractions using a QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The G6PD A-allele (202G→A) was characterized using previously published primers and touchdown program with modifications.14 The amplification reaction included 3 μL of the extracted DNA, 5 μL of Phusion HF Buffer (New England Biolabs, Ipswich, MA), 2 μL of 5 mM deoxynucleoside triphosphate, 1.25 μL of 10 μM forward and reverse primers, 0.75 μL of DMSO, and 0.25 μL of Phusion HF DNA-Polymerase (Thermo Scientific), in a total of 25 μL. The PCR product was digested by NlaIII restriction enzyme (New England Biolabs, Ipswich, MA) at 37°C for 1 hour followed by an enzyme deactivation step at 65°C. Digested products were analyzed using 2% agarose gel electrophoresis for allele-specific fragment size (G6PD [A−]: 173 bp and 123 bp; G6PD [B]: 296 bp and 165 bp).14 To compare differences in the prevalence of G6PD (A−) genotype between the two different sampling methods, Fisher’s exact test was used. Statistical analyses were performed using Stata version 14 (StataCorp LP, College Station, TX).
Of the 137 samples screened, 11 were G6PD (A−) hemizygous. None of the female participants were G6PD (A−) heterozygous or homozygous. There was no statistically significant difference in G6PD (A−) genotype prevalence between the two sampling methods, and, therefore, the results were combined (Table 1). Glucose-6-phosphate dehydrogenase deficiency in the studied population was estimated by calculating the prevalence of G6PD (A−) hemizygote among male participants. Eleven of 73 male participants screened were G6PD (A−) hemizygous, for a prevalence of G6PD deficiency of 15% (95% CI: 7.8, 25). The prevalence of G6PD deficiency in Zambia was previously estimated to be 20–25%, which is in agreement with what we observed.9 With a national index of relative hemolytic risk of level 3, Zambia is a low-risk country but with high uncertainty.9 Our data can enhance the precision of the relative hemolytic risk index that was previously modeled.
Table 1.
Characteristics of participants and prevalence of G6PD deficiency
| Screened participants (N = 137) | |
|---|---|
| Males, N (%) | 73 (53) |
| RDT positive, N (%) | 4 (2.9) |
| Median age (IQR) | 17 (8, 36) |
| G6PD (A−) hemizygote | |
| N | 11 |
| Prevalence in male (95% CI) | 15 (7.8, 25) |
IQR = interquartile range; G6PD = glucose-6-phosphate dehydrogenase.
Single low-dose primaquine is used to eliminate gametocytes, and the age distribution of gametocyte prevalence in the study area may be useful information to guide elimination strategies. For this purpose, gametocyte-specific mRNA data from previous work was reanalyzed.15 In brief, samples were collected in the same study area between 2008 and 2013. Finger-prick blood samples were collected for preparation of dried blood spot (DBS) and to measure hemoglobin concentrations using HemoCue® Hb 201 + Hemoglobin Analyzer (HemoCue AB, Ängelholm, Sweden). Dried blood spot were used for molecular detection of P. falciparum parasite DNA and gametocyte-specific transcript (pfs25) following a previously published protocol.16 All samples collected were subjected to molecular detection of gametocyte up to 2009. Starting from 2010, only samples positive by nested PCR detecting pfcytb were tested for gametocyte specific transcript because of reduced transmission.
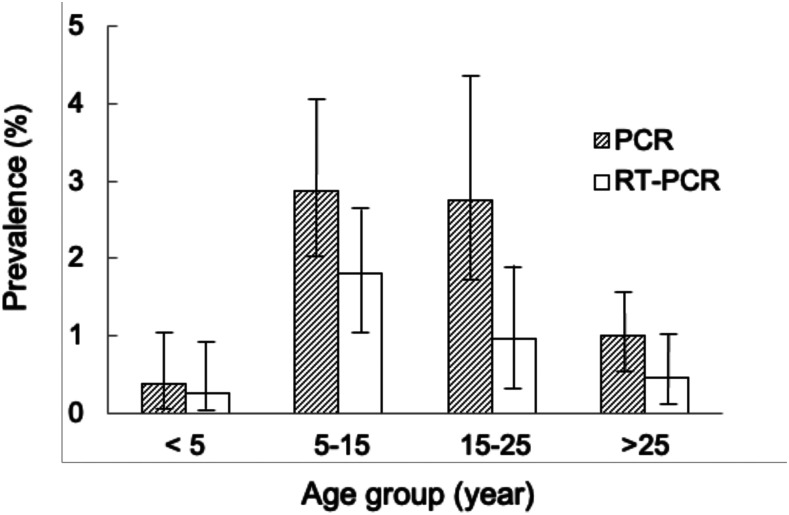
Of 3,934 samples collected between 2008 and 2013 and tested, 72 (1.8%) were positive for Plasmodium DNA and 36 (0.9%) were positive for gametocyte-specific pfs25 mRNA. The prevalence of parasites by PCR and the prevalence of gametocytemia by reverse transcriptase (RT)-PCR were stratified by age-groups, with those aged 5–15 years having the highest gametocyte prevalence (Figure 1).
Figure 1.
Prevalence of all-stage malaria parasites or late gametocyte stage by age-group. Columns represent the prevalence of all-stage malaria parasites by pan-Plasmodium nested PCR (light gray), and the prevalence of gametocytes-specific pf25s mRNA by RT-PCR (dark gray). Bars on columns are 95% CI.
Previous reports suggested that gametocyte prevalence was higher among children younger than 10 years as they typically had higher levels of parasitemia.17 However, in this low-transmission setting, school-age children and adolescents had the highest gametocyte prevalence. This age-group is also the least likely to use insecticide-treated bed nets.18
Of the samples tested, 743 were from children younger than 5 years and for whom hemoglobin concentrations were available. The prevalence of severe anemia (hemoglobin < 8 gm/dL) in this age-group was 0.3% (95% CI: 0.03, 0.97), significantly lower than the 5% (P < 0.001) prevalence of severe anemia among children younger than 5 years reported in the 2018 Malaria Indicator Survey.19
The use of SLD primaquine in Southern Province, Zambia, was previously modeled, in which the addition of SLD primaquine or/and ivermectin to currently used antimalarial regimens was simulated.20 The main finding was the importance of population coverage instead of specific drug combinations. Nevertheless, these findings do not preclude the potential use of SLD primaquine in the study area.
This study has some limitations, including the small sample size for estimation of the prevalence of G6PD deficiency and lack of enzymological confirmation of deficiency. Despite the small sample size, the representative sampling strategy allows the results to be generalizable for the local population. The association between G6PD residual activity and A− (376G, 202A) genotype has been described, although in this study we did not assess the prevalence of other less severe genotypes or female heterozygous for their deficiency levels.
Given the reported high tolerability of SLD primaquine, we hope our data foster renewed discussion on the use of SLD primaquine to help achieve malaria elimination in southern Zambia, with a particular focus on school-age children who are the major gametocyte reservoirs.
ACKNOWLEDGMENTS
We thank the field teams and laboratory staff at Macha Research Trust. Most importantly, we thank the residents of the Macha community who participated in this study.
REFERENCES
- 1.WHO , 2019. World Malaria Report 2019 . Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/publications/i/item/world-malaria-report-2019. Accessed November 11, 2020. [Google Scholar]
- 2.WHO , 2012. Updated WHO Policy Recommendation: Single Dose Primaquine as a Gametocytocide in Plasmodium Falciparum Malaria. Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/malaria/publications/atoz/who_pq_policy_recommendation/en/. Accessed November 11, 2020. [Google Scholar]
- 3.WHO , 2015. Guidelines for the Treatment of Malaria, 3rd edition. Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/malaria/publications/atoz/9789241549127/en/. Accessed November 11, 2020. [Google Scholar]
- 4.WHO , 2016. Testing for G6PD deficiency for safe use of primaquine in radical cure of P. vivax and P. ovale malaria. Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/malaria/publications/atoz/g6pd-testing-pq-radical-cure-vivax/en/. Accessed November 11, 2020. [Google Scholar]
- 5.Howes RE, Battle KE, Satyagraha AW, Baird JK, Hay SI, 2013. G6PD deficiency. Global distribution, genetic variants and primaquine therapy. Adv Parasitol 81: 133–201. [DOI] [PubMed] [Google Scholar]
- 6.Mbanefo EC, et al. 2017. Association of glucose-6-phosphate dehydrogenase deficiency and malaria: a systematic review and meta-analysis. Sci Rep 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cappellini M, Fiorelli G, 2008. Glucose-6-phosphate dehydrogenase deficiency. Lancet 371: 64–74. [DOI] [PubMed] [Google Scholar]
- 8.WHO , 2015. Policy Brief on Single-Dose Primaquine as a Gametocytocide in Plasmodium Falciparum Malaria. Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/malaria/publications/atoz/policy-brief-single-dose-primaquine-pf/en/. Accessed November 11, 2020. [Google Scholar]
- 9.Howes RE, et al. 2012. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med 9: e1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kent RJ, Thuma PE, Mharakurwa S, Norris DE, 2007. Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. Am J Trop Med Hyg 76: 267–274. [PMC free article] [PubMed] [Google Scholar]
- 11.Stevenson JC, Norris DE, 2017. Implicating cryptic and novel anophelines as malaria vectors in Africa. Insects 8: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moss WJ, Hamapumbu H, Kobayashi T, Shields T, Kamanga A, Clennon J, Mharakurwa S, Thuma PE, Glass G, 2011. Use of remote sensing to identify spatial risk factors for malaria in a region of declining transmission: a cross-sectional and longitudinal community survey. Malar J 10: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deutsch-feldman M, et al. 2018. Efficiency of a malaria reactive test-and-treat program in southern Zambia: a prospective, observational study. Am J Trop Med Hyg 98: 1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanello CI, Karema C, Avellino P, Bancone G, Uwimana A, Lee SJ, d’Alessandro U, Modiano D, 2008. High risk of severe anaemia after chlorproguanil-dapsone+artesunate antimalarial treatment in patients with G6PD (A-) deficiency. PLoS One 3: e4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi T, Kanyangarara M, Laban NM, Phiri M, Hamapumbu H, Searle KM, Stevenson JC, Thuma PE, Moss WJ, 2019. Characteristics of subpatent malaria in a pre-elimination setting in southern Zambia. Am J Trop Med Hyg 100: 280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mlambo G, Vasquez Y, Leblanc R, Sullivan D, Kumar N, 2008. Short report: a filter paper method for the detection of Plasmodium falciparum gametocytes by reverse transcription – polymerase chain reaction. Am J Trop Med Hyg 78: 114–116. [PubMed] [Google Scholar]
- 17.Bousema T, Drakeley C, 2011. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 24: 377–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinchoff J, Hamapumbu H, Kobayashi T, Simubali L, Stevenson JC, Norris DE, Colantuoni E, Thuma PE, Moss WJ, 2015. Factors associated with sustained use of long-lasting insecticide-treated nets following a reduction in malaria transmission in southern Zambia. Am J Trop Med Hyg. 93: 954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.PMI , 2020. US President’s Malaria Initiative Zambia: Malaria Operational Plan FY 2020. 2020: 1-136. Available at: https://www.pmi.gov/where-we-work/zambia. Accessed November 11, 2020. [Google Scholar]
- 20.Stuckey EM, Miller JM, Littrell M, Chitnis N, Steketee R, 2016. Operational strategies of anti-malarial drug campaigns for malaria elimination in Zambia’s southern province: a simulation study. Malar 15: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]