ABSTRACT
Intranasal instillation of SE36, a malaria vaccine candidate antigen, in lactating BALB/c strain (derived from the Bagg and albino laboratory inbred mice) female mice resulted in the appearance of the antigen in breast milk as demonstrated by sandwich ELISA and Western blot. Pups born of immunologically naive mice and breastfed on lactating foster mothers exposed intranasally to SE36 developed IgG anti-SE36 antibodies. These data demonstrate that maternal immunization in mice by this route in lactating mothers can result in active immunization of offspring via ingestion of breast milk containing antigen. If confirmed in a nonhuman primate model and in human subjects, this strategy might be transformative for vaccination against malaria and other infant killer infectious diseases.
METHODS, RESULTS, AND DISCUSSION
African infants < 6 months have substantial malaria burdens, with prevalence varying between 0% and 36.1% and mortality based on verbal autopsy ranges between 20% and 46.2%.1–3 The mainstay of malaria control is chemotherapy, but all antimalarial drugs are off-label with unknown dosing, safety, or efficacy for this age-group.1 Although these gaps might be addressed by increasing the number of clinical trials of antimalarial drugs in very young infants, the emergence of drug-resistant malaria parasites still threatens to undermine chemotherapy.4 Another successful malaria control measure is the reduction of mosquito vector/human contact by indoor residual spraying with WHO-approved insecticides and sleeping under long-lasting insecticide-treated bed nets. However, malaria vector control is also constrained by insecticide resistance.5
Malaria vaccines offer a beacon of hope for African infants. RTS,S/AS01 (Mosquirix™; GSK, Brentford, United Kingdom) is the only malaria vaccine candidate that has passed Phase III clinical trials and is under pilot implementation in Africa.6,7 The poor performance of RTS,S in infants aged 6–12 weeks has highlighted some critical determinants of the outcome of immunization in young infants.6 These include the immaturity of the infant immune system, polarization of infant immune responses, maternal antibodies and immune complexes in breast milk at the time of vaccination, and lack of infant-optimized adjuvants.8–11 There is therefore a need to explore other infant immunization strategies.
Maternal immunization to confer active immunity to breastfed infants through ingestion of breast milk containing vaccine antigens is an innovative vaccination concept.12 This concept is supported by a recent report of Plasmodium falciparum blood stage antigens in human breast milk.13 In this report, we present preliminary evidence that intranasal administration of recombinant SE36 antigen to lactating mice results in the appearance of the antigen in breast milk and that pups borne of immunologically naïve mice not exposed to SE36 develop IgG anti-SE36 antibodies following breastfeeding by SE36-exposed foster mothers. SE36 is a promising malaria vaccine candidate derived from P. falciparum serine rich antigen 5. It has demonstrated excellent immunogenicity and safety profiles in phase Ib clinical trials in African children in Burkina Faso and Uganda.14,15
Six-week-old nonimmune lactating BALB/c strain (derived from the Bagg and albino laboratory inbred mice) female mice received a 30 µL mixture of SE36 (42 µg) or phosphate buffered saline (PBS) (N = 10 per group) plus mouse monoclonal IgG1 anti-TGF-β (Sigma Aldrich catalogue no. T0438, clone 9,016.2) in each nare every other day over 21 days. The rationale of administering neutralizing anti-TGF-β antibody was to reduce maternal levels of TGF-β to prevent TGF-β–dependent development of antigen-specific oral tolerance in pups.16 Milk samples were obtained 4–6 hours after each intranasal administration. In brief, lactating mice were separated from their pups for an hour before milking, and 5 minutes before milking, the mice were injected intraperitoneally with oxytocin (0.5 international units; Sigma Aldrich catalogue no. O4375) to stimulate milk let down.17 Individual mice were restrained and milk manually expressed and collected as described.17,18 The milk was stored at −70°C until further analysis.
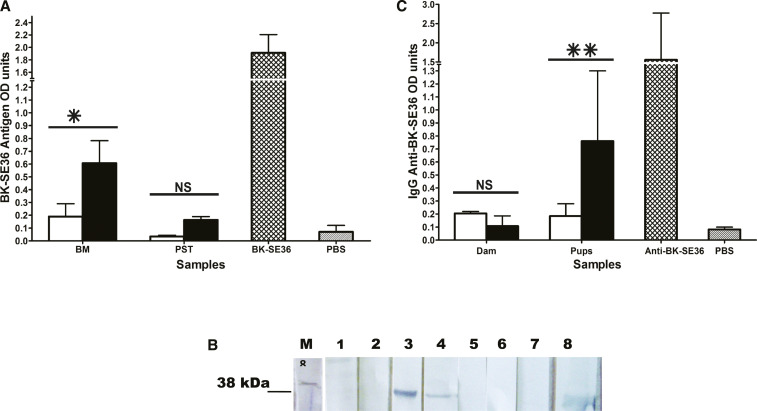
The presence of SE36 in breast milk was demonstrated by sandwich ELISA and Western blot analysis. For ELISA, a mouse polyclonal antiserum against SE36 was used as a source of capture and reporter antibodies. Flat-bottomed 96-well microtiter plates were coated overnight with the polyclonal mouse serum diluted 1: 50 in coating buffer of 0.5 M carbonate bicarbonate buffer pH 9.6. The plates were washed with PBS containing 0.1% Tween 20 (PBS/Tween) and nonspecific binding sites blocked by incubation for 2 hours with 5% bovine serum albumin (BSA) in PBS/Tween. After washing off excess BSA, de-lipidated breast milk samples diluted 2-fold in PBS-Tween, SE36 (as positive control), and PBS (as negative control) were added to designated wells and incubated for further 3 hours. The plates were washed with PBS/Tween, and the polyclonal mouse serum diluted 1:1,000 was added and the plates incubated for 2 hours. The reaction was developed by successive incubations with a goat anti-mouse IgG-alkaline phosphatase conjugate and ρ-nitrophenylphosphate substrate; optical densities (ODs) for SE36 antigen were measured at 405 nm on a spectrophotometric ELISA reader. To demonstrate the presence of SE36 in pup stomachs, an extract of pooled stomach contents of breastfed pups was analyzed alongside breast milk samples in the sandwich ELISA. For Western blots, pooled de-lipidated milk samples from SE36 or PBS-sham–treated lactating dams and SE36 (positive control) were boiled with Laemmli sample buffer and resolved by discontinuous sodium dodecyl sulfate polyacrylamide gel electrophoresis. The resolved proteins were electrophoretically transferred to the nitrocellulose membrane which was blocked with 2% BSA and incubated successively with polyclonal mouse anti-SE36 diluted 1:1,000, a secondary horseradish peroxidase–conjugated goat anti-mouse IgG diluted 1:1,000, and 4-chloro-1-naphthol substrate. SE36 was detectable in breast milk after intranasal antigen administration and in pup stomachs after breastfeeding (Figure 1A). SE36 recovery from pup stomachs was low, which probably reflected the effect of gastric digestion, but the antigen OD in stomach contents of pups breastfed by antigen-exposed mothers was 4-fold higher than background. Western blot analysis demonstrated an antigen of about Mr 38,000 in breast milk which co-migrated with a band of similar Mr in the SE36 antigen–positive control lane. This band was observed only in breast milk of mice which received SE36 but not PBS.
Figure 1.
Detection of Plasmodium falciparum SE36 antigen in breast milk of mice and IgG anti-SE36 in mice pup sera. (A) SE36 optical densities (ODs) in breast milk from mothers (dams) that received PBS (open bar) or SE36 (black bar), stomach extracts (PST) of pups breastfed by foster mothers that received PBS (open bar) or SE36 (black bar), positive control SE36 (cross-hatched bar), and negative control PBS (stripped bar). There was a significant difference in SE36 ODs between milk of PBS and antigen-exposed mothers (P = 0.024) but not between the stomach contents of pups breastfed by PBS and antigen-exposed mothers (P = 0.994). Similar results were obtained in three independent antigen recovery experiments. (B) Western blot demonstration of SE36 in breast milk of mice that received SE36 or PBS. The primary antibody was polyclonal mouse anti-SE36, and the secondary antibody was a goat anti-mouse IgG conjugated to horseradish peroxidase; both were diluted 1:1,000. Lane M, molecular weight standard; lanes 1 and 2, milk from PBS-treated mice; lanes 3 and 4, milk from SE36-treated mice after 4 and 6 hours, respectively, after intranasal instillation showing a band of Mr 38,000; lanes 5 and 6, milk from PBS-treated mice; lane 7, PBS-negative control; lane 8, SE36-positive control showing a band which co-migrates slightly below Mr 38,000 probably because of degradation during processing. (C) Results were reported as means ± standard error of the mean (s.e.m) ODs of serum pools from each of three independent experiments and differences between groups assessed using Student’s t test. A value of P < 0.05 was regarded as statistically significant. One representative experiment is presented. IgG ODs in pooled sera from foster mothers that received PBS (open bar) or SE36 (black bar), in pooled sera from pups breastfed by foster mothers that received PBS (open bar) or SE36 (black bar), positive control polyclonal mouse IgG anti-SE36 (cross-hatched bar) and negative control PBS (stripped bar). There was a significant difference in serum IgG anti-SE36 ODs between pups breastfed by PBS and antigen-exposed mothers (P = 0.002) but not between PBS and antigen-exposed mothers themselves (P = 0.787). Keys for (A and C) *P < 0.05, **P < 0.002. NS = nonsignificant. This figure appears in color at www.ajtmh.org.
Having demonstrated that breast milk of SE36-exposed lactating mice contained the antigen, we next determined whether ingestion of milk containing the antigen induces specific IgG anti-SE36 in breastfed pups. Three-week-old pups born of malaria-naive mothers that did not receive SE36 were foster-nursed daily for 21 days by lactating mice which received SE36 or PBS. Blood samples from breastfed pups and foster mothers were collected after the 3 weeks, and the experiment repeated thrice. Serum pools were made from each independent experiment and examined for the presence of IgG anti-SE36 using indirect ELISA. In brief, microtiter plates were coated overnight with SE36 in coating buffer and blocked with BSA as already described. Pooled sera were diluted 500-fold in PBS/Tween, added to antigen-coated wells, and incubated for 3 hours at room temperature. After washing the plates with PBS/Tween, this was followed by successive incubations with a secondary alkaline phosphatase–conjugated goat anti-mouse IgG and ρ-nitrophenylphosphate substrate. Optical density values for IgG anti-SE36 antibodies were measured at 405 nm. Pooled sera from foster mothers that received PBS and from the pups that suckled them had mean IgG OD values comparable with that of the PBS control (Figure 1C). Pooled sera from foster mothers that received SE36 without adjuvant had a mean IgG OD comparable with pooled sera from foster mothers that received PBS (P = 0.787). By contrast, pooled sera from pups breastfed on foster mothers exposed to SE36 antigen had a significantly higher mean IgG anti-SE36 antibody OD value than pooled sera from mice pups breastfed on foster mothers that received PBS (P = 0.002). Breast milk contains important immunoregulatory molecules which complement deficient immune responses in infants.19 The higher mean IgG anti-SE36 antibody OD of sera of breastfed pups by comparison with that of sera from foster mothers that received the antigen without adjuvant suggests that immunoregulatory molecules in maternal milk acted as natural adjuvants (Figure 1C).
Free antigen in the presence of TGF-β in breast milk induces oral tolerance in mice.16 However, the effect of TGF-β can be neutralized by the coadministration of anti-TGF-β, which prevents the development of oral tolerance.16 In our experiments, mice pups breastfed on dams that received anti-TGF-β and SE36 developed specific antibodies. Our observations confirm a previous report that an allergen presented to lactating mice through an aerosol chamber not only appears in breast milk but also sensitizes breastfed pups born of nonexposed mothers.16
This study has several limitations. First, during the immunization of mice dams with SE36, there was no specificity control consisting of an irrelevant P. falciparum antigen expressed in the same vector as SE36. The possibility of antibody cross-reactivity in pup sera cannot therefore be ruled out. Second, we reported on only IgG antibody ODs in pup sera but did not confirm the functional activity of the antibodies by in vitro growth inhibitory assays. Finally, the effect of immunizing malaria-exposed mice dams was not investigated. This would have been more representative of malaria-experienced mothers in malaria-endemic countries.
In conclusion, we demonstrate for the first time that maternal immunization and breastfeeding is a feasible strategy for inducing antigen-specific antibodies in breastfed mice pups and that oral tolerance can be overcome. Current maternal immunization practices are designed to protect infants by passive transfer of the mother’s immunity.20 Our research demonstrates that maternal immunization strategies can be reengineered to stimulate the development of the infant’s own antibody responses by optimizing antigen transfer to breast milk and circumventing oral tolerance. Malaria blood-stage antigens have been demonstrated in human breast milk, but the mechanism of antigen transport remains unknown.13 The public health significance of malaria antigen shedding in breast milk remains to be investigated in prospective studies of mother–infant pairs. Vaccination against killer diseases by infants nursing on lactating mothers shedding vaccines in milk would be transformative for infant vaccinology if validated and would boost breastfeeding promotions.
ACKNOWLEDGMENTS
We acknowledge the kind gift of recombinant SE36 and mouse polyclonal antisera against SE36 by Professor Toshihori Horii, Osaka University; Research Institute for Microbial Diseases (RIMD), Osaka; and the technical support by veterinarians and animal technicians at the Institute of Primate Research, Nairobi, Kenya.
REFERENCES
- 1.D’Alessandro U, et al. 2012. Malaria in infants aged less than six months-is it an area of unmet medical need? Malar J 11: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ceesay SJ, et al. 2015. Malaria prevalence among young infants in different transmission settings, Africa. Emerg Infect Dis 21: 1114–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mbonye MK, Burnett SM, Naikoba S, Colebunders R, Wouters K, Weaver MR, Van Geertruyden JP, 2015. Malaria care in infants aged under six months in Uganda: an area of unmet needs! PLoS One 10: e0123283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fairhurst RM, Dondorp AM, 2016. Artemisinin-resistant Plasmodium falciparum malaria. Scheld W, Hughes J, Whitley R. (eds), Emerging Infections 10. Washington, DC: ASM Press, 409–429. [Google Scholar]
- 5.Mulamba C, Riveron JM, Ibrahim SS, Irving H, Barnes KG, Mukwaya LG, Birungi J, Wondji CS, 2014. Widespread pyrethroid and DDT resistance in the major malaria vector Anopheles funestus in East Africa is driven by metabolic resistance mechanisms. PLoS One 9: e110058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.RTS,S Clinical Trials Partnership Agnandji ST, et al. 2012. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 367: 2284–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van den Berg M, Ogutu B, Sewankambo NK, Biller-Andorno N, Tanner M, 2019. RTS,S malaria vaccine pilot studies: addressing the human realities in large-scale clinical trials. Trials 20: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrios C, 1996. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur J Immunol 26: 1489–1496. [DOI] [PubMed] [Google Scholar]
- 9.Black A, Bhaumik S, Kirkman RL, Weaver CT, Randolph DA, 2012. Developmental regulation of Th17-cell capacity in human neonates. Eur J Immunol 42: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegrist CA, 2003. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 21: 3406–3412. [DOI] [PubMed] [Google Scholar]
- 11.Mosconi E, et al. 2010. Breast milk immune complexes are potent inducers of oral tolerance in neonates and prevent asthma development. Mucosal Immunol 3: 461–474. [DOI] [PubMed] [Google Scholar]
- 12.Verhasselt V, 2015. Is infant immunization by breastfeeding possible? Philos Trans R Soc Lond B Biol Sci 370: 20140139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Elsen LWJ, Verhasselt V, Egwang T, 2020. Malaria antigen shedding in the breast milk of mothers from a region with endemic malaria. JAMA Pediatr 174: 297–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palacpac NMQ, et al. 2013. Phase 1b randomized trial and follow-up study in Uganda of the blood-stage malaria vaccine candidate BK-SE36. PLoS One 8: e64073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sirima S, Tiono AB, Houard S, Bougouma EC, Coulibaly SA, Leroy O, Palacpac N, Horii T, Ouedraogo IN, 2019. OC 8546 safety and immunogenicity of the malaria vaccine candidate BK-SE36 in young children living in Burkina Faso. BMJ Glob Health 4: A12–A13. [Google Scholar]
- 16.Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D, Glaichenhaus N, Julia V, 2008. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med 14: 170–175. [DOI] [PubMed] [Google Scholar]
- 17.De Peters EJ, Hovey RC, 2009. Methods for collecting milk from mice. J Mammary Gland Biol Neoplasia 14: 397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willingham K, McNulty E, Anderson K, Hayes-Klug J, Nalls A, Mathiason C, 2014. Milk collection methods for mice and Reeves’ muntjac deer. J Vis Exp 89: 51007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Field CJ, 2005. The immunological components of human milk and their effect on immune development in infants. J Nutr 135: 1–4. [DOI] [PubMed] [Google Scholar]
- 20.MacDougall DM, Halperin SA, 2016. Improving rates of maternal immunization: challenges and opportunities. Hum Vaccin Immunother 12: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]