ABSTRACT
Timely diagnosis of group A streptococcal (GAS) sore throat coupled with appropriate antibiotic treatment is necessary to prevent serious post-streptococcal complications, including rheumatic fever (RF) and rheumatic heart disease (RHD). Traditional medicine (TM) is a known common adjunct to formal medical care in sub-Saharan Africa. A better understanding of health-seeking behavior for sore throat both within and outside the formal medical system is critical to improving primary prevention efforts of RF and RHD. A prospective mixed-methods study on the use of TM for sore throat was embedded within a larger epidemiological study of RF in Northern Uganda. Children presenting with symptoms of RF were interviewed about recent TM use as well as health services use for sore throat. One hundred children with a median age of 10 years (interquartile range: 6.8–13 years) completed the TM interview with their parent/guardian as part of a research study of RF. Seventeen, or 17%, accessed a TM provider for sore throat as part of the current illness, and 70% accessed TM for sore throat in the past (73% current or past use). Of the 20 parents who witnessed the TM visit, 100% reported use of crude tonsillectomy. Penicillin was the most frequently prescribed medication by TM providers in 52% of participants who were seen by a TM provider. The use of TM among children presenting with symptoms of sore throat in northern Uganda is common and frequently used in tandem with diagnostic services offered through the formal healthcare system. Engagement with TM practitioners may provide an important avenue for designing effective primary prevention and management strategies of RF and reduce the global burden of RHD.
INTRODUCTION
Among post-streptococcal complications, rheumatic fever (RF) and resulting rheumatic heart disease (RHD) exert the largest global toll on childhood morbidity and mortality. Current estimates place the global burden of RHD at 39 million cases.1 Rheumatic fever and RHD can largely be prevented by timely diagnosis and appropriate antibiotic treatment of group A streptococcal (GAS) pharyngitis.2,3 However, in Uganda and other low- and middle-income settings, RF is rarely diagnosed, and most patients with RHD present only after complications have developed because of advanced heart disease.4 The healthcare-seeking practices of children with sore throat in Uganda remain unknown.
According to the WHO, traditional medicine (TM) refers to “the sum total of the knowledge, skills, and practices based on the theories, beliefs, and experiences indigenous to different cultures, whether explicable or not, used in the maintenance of health as well as in the prevention, diagnosis, improvement, or treatment of physical and mental illness.”5 The use of the word “traditional” suggests these medical practices are informed by core beliefs that are preserved over time and connect with indigeneous peoples’ identity.6 Training of TM healers relies largely on an apprenticeship model.7
Traditional medicine is a common adjunct to standard medical care in sub-Saharan Africa (SSA) and is often used before, or in place of, care within the formal medical system.8 A recent study in East Africa demonstrated 70% of people consult traditional healers before accessing medical care within the formal medical system.9 Traditional medicine is preferred over formal medical care for many reasons, including 1) its availability/accessibility in rural settings, 2) users’ belief in its intrinsic efficacy, 3) perceived barriers to accessing care within the formal medical system, and 4) high cost of biomedical care.10–14 A study of TM use among people with diabetes in eastern Uganda found that patients with chronic diabetes use TM because of community influence, inadequacies in the functionality of the healthcare system, and easy access.11 Partnering with TM providers to improve appropriate healthcare delivery may be more effective than discouraging TM use in certain populations. Engagement with, as well as regulation of, TM practices is also in alignment with the WHO’s TM Strategy: 2014–2023.5
In Uganda, crude tonsillectomy (CT) is often performed by traditional healers.15 Crude tonsillectomy is the incomplete resection of the tonsils through the use of a blunt instrument. Although sore throat can result from a number of environmental, allergic, and infectious processes, GAS sore throat remains a priority as inadequate treatment can lead to serious post-streptococcal complications. However, there is a paucity of published literature on the utilization of TM in the context of sore throat, or the use of TM in patients seeking standard medical care for GAS post-streptococcal complications.
A better understanding of health-seeking behavior for sore throat, both within and outside the formal medical system, is critical to improving primary prevention efforts of RF and RHD. This study examines the use of TM for sore throat among children being evaluated for RF in the first contemporary epidemiological study of RF in SSA. A better understanding of TM use in this population may reveal opportunities to improve proper diagnosis and treatment of sore throat in low-resource settings where TM use is common.
MATERIALS AND METHODS
General methods.
This mixed-methods, prospective, survey-based study was conducted from March to July 2018 at Lira Regional Referral Hospital (LRRH) as part of a larger epidemiological study of RF. Lira Regional Referral Hospital is located in Lira district in the northern region of Uganda (see Figure 1) and is the referral hospital for the districts of Amolatar, Apac, Dokolo, Lira, Kole, and Oyam. Lira is the ninth largest municipality in Uganda (urban population: 99,059).16
Figure 1.
Traditional medicine use questionnaire.
Before this study, a widespread community educational messaging campaign was implemented in Lira district through radio announcements, posters in high-traffic community spaces, and direct visits to schools and health centers throughout the district. The goal of the educational campaign was to raise awareness of signs and symptoms of RF in the community, and inform schools and health centers of the dedicated RF evaluation program at LRRH. Throughout the duration of the study, an educational campaign raised awareness about the signs of RF in the community using radio messaging, village healthcare teams, as well as direct school-based and clinic-based education.
Study population.
The larger RF epidemiological study invited children aged 3–18 years with one of the following inclusion criteria to be evaluated for RF: 1) fever (≥ 48 hours) and joint pain, 2) suspicion of acute rheumatic carditis, or 3) suspicion of Sydenham’s chorea. Children were excluded if there was a known alternate diagnosis responsible for the presenting symptoms (e.g., sickle cell crisis). A convenience sample of the first 100 participants from the larger RF study was chosen.
Data collection.
Within this larger RF epidemiological study, we embedded a prospective, guided interview to describe and quantify the use of TM for sore throat. Use of TM for joint pain, the presenting feature of most children evaluated for RF, was also included.
A guided interview was developed by the research team to capture use of TM for sore throat (during the presenting illness and for past sore throats), or joint pain (Figure 2). If respondents answered “yes” to the question of using TM in the past, the interviewer directed the family to focus on the most recent visit in reference to follow-up questions. Respondents were asked about their healthcare utilization behavior, specifically which type of facility (e.g., district hospital, and health center) they would use in the absence of care offered through the study. Presence of fever during the described illness was captured. Questions on the source of referral to the TM provider (self, friend, or family) were also recorded. Subsequent open-ended qualitative questions explored details of TM visits including completed procedures, adjunct therapies (i.e., medications) provided, and parent’s perception of improvement in symptoms.
Figure 2.
Map of Lira, Uganda source: worldatlas.com. This figure appears in color at www.ajtmh.org.
Before administration, the questionnaire was translated from English into Lango, the local language. Guided interviews were conducted by one of three local Ugandan research nurses with both the parent/guardian and the child in the local language (J. P., I. O., and J. A.). Established positive rapport between research nurses and families in the larger RF study facilitated open dialogue between staff and families regarding the use of TM. During the interview, only the study nurse, child, and parent were present. No repeat interviews were carried out. Transcripts were not returned to families for editing. There were no audio or visual recordings of the interviews.
Definitions.
Sore throat was defined broadly as any of the following: throat pain, difficulty swallowing, inability to eat due to pain, or swollen tonsils. Traditional medicine was defined broadly using the WHO definition as “the knowledge, skills, and practices based on the theories, beliefs, and experiences indigenous to different cultures, used in the maintenance of health and in the prevention, diagnosis, improvement, or treatment of physical and mental illness.”5 In practice, TM in this population refers to the use of healing practices outside of the formal medical system and implemented by TM practitioners in the community.
This study was approved by Makerere University and the Children’s National Hospital Institutional Review Board, as well as the Ugandan National Council of Science and Technology. Written informed consent was signed by a parent/guardian or participant (> 18 years of age), and written informed assent was signed by children ≥ 8 years of age.
Data analysis.
Basic sociodemographic information of participating children was collected as part of the larger RF study. Demographics and coded responses were summarized by median and interquartile range (IQR), or by number and percentage, as appropriate. Open-ended responses were recorded verbatim and later categorized by the research team, and representative quotes were extracted to provide additional data. No formal qualitative analysis was performed.
RESULTS
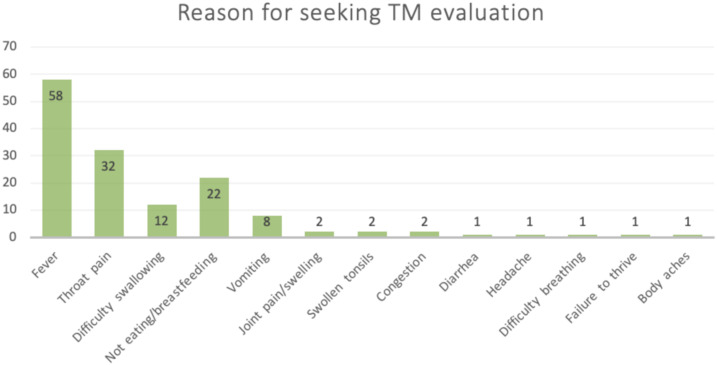
Between March and July 2018, 100 children with a median age of 10 years (IQR: 6.8–13 years) were enrolled in this study. These children presented with symptoms of RF to LRRH for evaluation and completed a questionnaire with their parent/guardian (Table 1). Of the 100 children enrolled, 85 children presented with fever and joint pain (one of whom had suspected chorea), and 15 children presented with suspicion of acute rheumatic carditis. Seventeen of the 100 children (17%) had been taken for TM evaluation for sore throat during the present illness, and 70 of the 100 children (70%) had seen a TM provider in the past for sore throat. Therefore, 73% of surveyed children had seen a TM provider for either the present or a past illness. The most common primary reasons identified for seeking care from a TM provider in the context of sore throat included presence of fever (80%), throat pain (44%), not eating/breastfeeding (30%), difficulty swallowing (16%), and vomiting (11%). Traditional medicine was rarely sought for the primary evaluation of joint pain (3%) (Table 2, Figure 3).
Table 1.
Characteristics of participants
| Median (interquartile range) | |
|---|---|
| Age (years) | 10 (6.8–13) |
| Number of people in household | 6 (5–8) |
| Number of subjects (n = 100) | |
| Gender | |
| Female | 52 |
| Male | 48 |
| Housing | |
| Permanent | 48 |
| Semi-permanent | 52 |
| Symptoms at presentation | |
| Fever and joint pain | 85 |
| Suspicion of RF | 15 |
| Diagnosis of RF by Jones criteria | 30 |
RF = rheumatic fever.
Table 2.
Characteristics of TM use for children with sore throat, past or present
| Use of TM | Number of subjects (%) (n = 73) |
|---|---|
| TM for sore throat, current illness | 17 (23) |
| TM for sore throat, past illness | 70 (95) |
| Mean number of visits (range) | |
| Number of TM encounters for a similar illness in past | 2.45 (1–10) |
| Primary reason(s) for seeking TM evaluation* | Number of subjects (%) (n = 73) |
| Fever | 58 (79) |
| Throat pain | 32 (44) |
| Difficulty swallowing | 12 (16) |
| Not eating/breastfeeding | 22 (30) |
| Vomiting | 8 (11) |
| Joint pain/swelling | 2 (3) |
| Swollen tonsils | 2 (3) |
| Congestion | 2 (3) |
| Diarrhea | 1 (1) |
| Headache | 1 (1) |
| Difficulty breathing | 1 (1) |
| Failure to thrive | 1 (1) |
| Body aches | 1 (1) |
| Reported improved symptoms following TM visit | 65 (89) |
| Consulted multiple traditional healers | 12 (16) |
| Medication prescribed by TM practitioner† | |
| Medication prescribed during visit | 50 (68) |
| Medication not prescribed during visit | 23 (34) |
| Antimicrobials | |
| Penicillin V | 26 (52) |
| Ampicillin–cloxacillin | 4 (8) |
| Amoxicillin–Clavulanate | 1 (2) |
| Tetracycline | 1 (2) |
| Cloxacillin | 1 (2) |
| Analgesics | |
| Paracetamol | 12 (24) |
| Diclofenac | 2 (4) |
| Aspirin | 1 (2) |
| Artenum | 1 (2) |
| Diclofenac | 1 (2) |
| Coartem | 1 (2) |
| Unknown medication | 7 (12) |
TM = traditional medicine.
Some reported multiple reasons (total > 100%).
Some traditional healers prescribed multiple medications (total > 100%).
Figure 3.
Reason for seeking traditional medicine evaluation. This figure appears in color at www.ajtmh.org.
Of the 20 parents who witnessed the TM visit, all (100%) reported the use of CT at the TM visit. Participants uniformly described a tonsillectomy procedure where the traditional healer aggravated the tonsils until blood and pus were extruded. Parents reported variability in CT technique with healers using metal objects (e.g., bicycle spoke, pliers, scissors, and spoons), sticks, hooks, or fingers to apply pressure to the tonsils. One parent described a TM visit where “the healer asked her to kneel, she then washed her hands, donned a plastic glove on her hand, pushed her finger into her mouth, and pressed it against the painful back of throat and sides. She withdrew her hand and it had pus mixed with blood.” Another parent described being “…asked to hold the child between my legs and asked him to open his mouth. The healer then used an improvised long metallic instrument, made out of a bicycle spoke, attached it around the tonsil and pulled once. This was followed by pus and blood from the site. This was followed by pain and bleeding for a few hours.”
Pharmacologic therapy was commonly given by TM providers, with 50% of children surveyed receiving at least one medication from a TM provider. The most commonly provided medication by TM providers was penicillin V, which was prescribed for 26 participants (52% of participants with reported prior TM use). In addition, paracetamol (24%) and ampicillin–cloxacillin (8%) were also frequently prescribed. Information on dosage was not obtained. The vast majority of families who reported current or previous TM use for sore throat perceived improvement in the child’s symptoms following the TM visit (89%). In regard to healthcare utilization, 58% of participants indicated they would seek care for their child at the district hospital in the absence of care offered through the current research study (Table 3).
Table 3.
Preferences for healthcare service delivery
| If care through the study were not offered, where would you seek care for your child’s sore throat? | Number of subjects (%) (n = 100) |
|---|---|
| District hospital | 58 (58) |
| HCIV | 7 (7) |
| Would go to any facility referred 5 | 5 (5) |
| Private clinic | 5 (5) |
| Do not know | 4 (4) |
| Private hospital | 4 (4) |
| HC | 3 (3) |
| HCII | 3 (3) |
| Regional referral hospital | 2 (2) |
| HCIII | 2 (2) |
| Would seek help elsewhere | 1 (1) |
| Pray | 1 (1) |
| National referral hospital | 1 (1) |
| HC for referral | 1 (1) |
| Private clinic or HCIV | 1 (1) |
| Private clinic or district hospital | 1 (1) |
| Private clinic or national hospital | 1 (1) |
HC = health center (levels II through IV).
DISCUSSION
Our data show that children presenting with symptoms of RF frequently access TM for treatment of sore throat: 73% for either a current or past episode. Notably, although TM was commonly used for sore throat in the community, use for evaluation and treatment of joint pain was uncommon, with only 2% of enrolled children reporting joint pain as a reason for TM use. Crude tonsillectomy was the only reported TM intervention, and adjunct prescription of antibiotics by a TM provider, in particular penicillin, was frequently reported. Participants indicated a strong preference for healthcare service delivery at the district hospital (58%) in the absence of care offered through the study, consistent with a recent call to action to support district-level hospitals in low-resource settings.17
Crude tonsillectomy was frequently reported by the parents and children in our study. A previous qualitative study of CT practice in southwestern Uganda found that greater than 50% of pediatric patients admitted for general illness undergo a local CT in the week before or during admission, and admitted patients sometimes leave the hospital setting at night to receive CT.15 That study investigated CT for treatment of a locally defined illness known as gapfura with symptoms that overlap with streptococcal pharyngitis including fever, sore throat, and difficulty swallowing.15 Additional research is needed to understand the choice of CT among TM practitioners, in particular to determine if they are aware of the risk of RF following sore throat, and if there are beliefs that CT modifies this risk. Historically, complete tonsillectomy was thought to reduce risk of RF, but this was later disproven.18–21
We found that TM was often used in tandem with standard medical services offered through the formal healthcare delivery system. Of those enrolled, 17% of kids saw a TM provider before coming to the RF study, and TM providers often prescribed antibiotics and other medications, in addition to using traditional practices such as CT. These findings are similar to data from Tanzania, where TM is used alongside biomedical healthcare delivery.22 To our knowledge, no previous study has reported antibiotic prescription by TM practitioners. Importantly, antibiotics are available over the counter in Uganda.23 Further studies are needed to understand if antibiotics and other medications are being prescribed by traditional healers for appropriate indications, with adequate doses, and correct durations of treatment. Future work to improve medication prescribing practices and antibiotic stewardship among TM practitioners has the potential to greatly improve sore throat treatment in similar community settings.
Parents of children in this study overwhelmingly reported satisfaction with TM treatment, with greater than 89.0% reporting improvement in symptoms. Sore throat due to viral, environmental, allergic, and bacterial causes, including GAS, typically resolve, even without appropriate treatment. In fact, children with GAS pharyngitis who receive oral penicillin recover in similar time to peers who do not receive antibiotics.24 Educational programs need to stress the importance of treatment not for symptom relief but to prevent post-streptococcal complications, including RF and RHD.
Although this study was not designed to evaluate the association between TM use and development of RF or RHD, it is important to recognize that prior studies have found a negative association between previous TM use and disease outcomes. Benzekri et al.25 reported that one-third of surveyed patients with HIV in Senegal reported previous TM use, and those who reported TM use had significantly greater mortality than those who did not. This presents an area of future study with the potential for great impact on RHD-endemic regions. With an improved understanding of the association of TM use and outcomes of sore throat, more specific strategies can be developed to guide appropriate GAS diagnosis and management, and thereby improve RF/RHD prevention in SSA.
Our study had several limitations. We surveyed 100 children who were evaluated at the time they were symptomatic with symptoms of possible RF including fever and joint pain, but only 30 patients within the sample were diagnosed with definite RF. Therefore, the study was not powered to compare healthcare-seeking behaviors of children who develop RF and those who do not, or to determine if interventions (CT or medications) affected the risk of developing RF. Our study did not include children who avoid the formal healthcare center altogether as our population was interviewed at a regional referral hospital. In the design of this study, we were not able to directly interview TM providers. Direct interview would have been necessary to fully understand the rationale for specific practices, such as CT and antibiotic use, and to determine the baseline knowledge of TM practitioners in regard to pharyngitis and RF. Lastly, the study population was taken from Lira district, which is one of the areas most affected by recent conflict and still endures the effects of widespread poverty and migration.26 Therefore, this study may not be generalizable to other districts in Uganda, or to other countries with endemic RF/RHD.
CONCLUSION
This study is the first to provide data on TM healthcare-seeking behaviors of children and parents in northern Uganda. Our findings help characterize the intersection of TM and pharyngitis, which is the major driver of RF and RHD around the globe. In line with the WHO TM strategy, we have demonstrated that TM practitioners are critical to local disease management strategies for GAS and prevention of RHD. Future research is needed to determine whether it is possible for biomedical personnel to engage and collaborate with traditional healers to prevent complications from GAS.
ACKNOWLEDGMENTS
We would like to thank our dedicated acute rheumatic fever study staff in Lira and the children and families who participated in this study. This project was only possible because of the trusting relationships that the ARF team developed with patients and families.
REFERENCES
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators , 2018. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffey PM, Ralph AP, Krause VL, 2018. The role of social determinants of health in the risk and prevention of group A streptococcal infection, acute rheumatic fever and rheumatic heart disease: a systematic review. PLoS Negl Trop Dis 12: e0006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yacoub M, Mayosi B, ElGuindy A, Carpentier A, Yusuf S, 2017. Eliminating acute rheumatic fever and rheumatic heart disease. Lancet (London, England) 390: 212–213. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Mondo C, Okello E, Musoke C, Kakande B, Nyakoojo W, Kayima J, Freers J, 2013. Presenting features of newly diagnosed rheumatic heart disease patients in Mulago Hospital: a pilot study. Cardiovasc J Afr 24: 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO , 2013. WHO Traditional Medicine Strategy: 2014–2023. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 6.Appelbaum Belisle H, Hennink M, Ordóñez CE, John S, Ngubane-Joye E, Hampton J, Sunpath H, Preston-Whyte E, Marconi VC, 2015. Concurrent use of traditional medicine and ART: perspectives of patients, providers and traditional healers in Durban, South Africa. Glob Public Health 10: 71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nompumelelo M, Gomo E, Gqaleni N, Ngcobo M, 2019. Core competencies acquired in indigenous training of traditional health practitioners in Kwazulu-Natal. Afr Health Sci 19: 3100–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasilo OM, Trapsida JM; Regional Off’ce for Africa, Brazzaville ; World Health Organization , 2010. Regulation of traditional medicine in the WHO African region. Afr Health Monitor (Spec Iss): 25–31. [Google Scholar]
- 9.Maurice J, 2015. Mounting cancer burden tests Africa’s health resources. Lancet 385: 2564–2565. [DOI] [PubMed] [Google Scholar]
- 10.de-Graft Aikins A, 2005. Healer shopping in Africa: new evidence from rural-urban qualitative study of Ghanaian diabetes experiences. BMJ 331: 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutebemberwa E, Lubega M, Katureebe SK, Oundo A, Kiweewa F, Mukanga D, 2013. Use of traditional medicine for the treatment of diabetes in eastern Uganda: a qualitative exploration of reasons for choice. BMC Int Health Hum Rights 13: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbo C, Ekblad S, Waako P, Okello E, Musisi S, 2009. The prevalence and severity of mental illnesses handled by traditional healers in two districts in Uganda. Afr Health Sci 9 (Suppl 1): S16–S22. [PMC free article] [PubMed] [Google Scholar]
- 13.Mwaka AD, Okello ES, Orach CG, 2015. Barriers to biomedical care and use of traditional medicines for treatment of cervical cancer: an exploratory qualitative study in northern Uganda. Eur J Cancer Care (Engl) 24: 503–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuwaha F, Musinguzi G, 2013. Use of alternative medicine for hypertension in Buikwe and Mukono districts of Uganda: a cross sectional study. BMC Complement Altern Med 13: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao-Cohen ML, Miller JS, Baganizi M, Musominali S, Burton WB, Paccione GA, 2013. Crude tonsillectomy and local concepts of illness in Kisoro, Uganda: community perception of gapfura and its treatment. Glob Public Health 8: 298–311. [DOI] [PubMed] [Google Scholar]
- 16.Uganda Bureau of Statistics , 2014. National Population and Housing Census 2014. Kampala, Uganda: Uganda Bureau of Statistics. [Google Scholar]
- 17.Rajbhandari R, McMahon DE, Rhatigan JJ, Farmer PE, 2020. The neglected hospital - the district hospital’s central role in global health care delivery. N Engl J Med 382: 397–400. [DOI] [PubMed] [Google Scholar]
- 18.Feinstein AR, Levitt M, 1970. The role of tonsils in predisposing to streptococcal infections and recurrences of rheumatic fever. N Engl J Med 282: 285–291. [DOI] [PubMed] [Google Scholar]
- 19.Macbeth RG, 1950. The tonsil problem. Proc R Soc Med 43: 324–328. [PubMed] [Google Scholar]
- 20.Chamovitz R, Rammelkamp CH, Wannamaker LW, Denny FW, 1960. The effect of tonsillectomy on the incidence of streptococcal respiratory disease and its complications. Pediatrics 26: 355–367. [PubMed] [Google Scholar]
- 21.Matanoski GM, 1972. The role of the tonsils in streptococcal infections: a comparison of tonsillectomized children and sibling controls. Am J Epidemiol 95: 278–291. [DOI] [PubMed] [Google Scholar]
- 22.Stanifer JW, et al. Comprehensive Kidney Disease Assessment for Risk Factors, Epidemiology , Knowledge, and Attitudes (CKD AFRIKA) Study , 2015. The determinants of traditional medicine use in northern Tanzania: a mixed-methods study. PLoS One 10: e0122638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mbonye AK, Buregyeya E, Rutebemberwa E, Clarke SE, Lal S, Hansen KS, Magnussen P, LaRussa P, 2016. Prescription for antibiotics at drug shops and strategies to improve quality of care and patient safety: a cross-sectional survey in the private sector in Uganda. BMJ Open 6: e010632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zwart S, Sachs AP, Ruijs GJ, Gubbels JW, Hoes AW, de Melker RA, 2000. Penicillin for acute sore throat: randomised double blind trial of seven days versus three days treatment or placebo in adults. BMJ 320: 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benzekri NA, et al. 2019. Traditional healers, HIV outcomes, and mortality among people living with HIV in Senegal, West Africa. AIDS (London, England) 33: 1521–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen K, Marshak A, Ofori-Adjei A, Kembabazi J, 2006. IDP livelihoods: using microenterprise interventions to support the livelihoods of forcibly displaced people: the impact of a microcredit program in IDP camps in Lira, northern Uganda. Refugee Surv Q 25: 23–39. [Google Scholar]