Abstract
Coronavirus disease 2019 (COVID-19) has become a global pandemic and garnered international attention. The causative pathogen of COVID-19 is severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel, highly contagious coronavirus. Numerous studies have reported that liver injury is quite common in patients with COVID-19. Hepatitis B has a worldwide distribution as well as in China. At present, hepatitis B virus (HBV) remains a leading cause of cirrhosis, liver failure, and hepatocellular carcinoma. Because both viruses challenge liver physiology, it raises questions as to how coinfection with HBV and SARS-CoV-2 affect disease progression and mortality. Is there an increased risk of COVID-19 in patients with HBV infection? In this review, we summarize the current reports of SARS-CoV-2 and HBV coinfection and elaborate the interaction of the two diseases. The emphasis was placed on evaluating the impact of HBV infection on disease severity and clinical outcomes in patients with COVID-19 and discussing the potential mechanism behind this effect.
Keywords: COVID-19, Hepatitis B virus, Liver injury, SARS-CoV-2, Coinfection, Immune exhaustion
Core Tip: Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has become a global public health crisis. Liver impairment is frequent in COVID-19 regardless of whether it is combined with hepatitis B virus (HBV) infection. Currently, there is no evidence to suggest that HBV increases susceptibility to SARS-CoV-2. HBV and SARS-CoV-2 coinfection does not increase the risk of severity and outcome of COVID-19. Nucleoside analogs are recommended due to the risk of HBV reactivation in COVID-19.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It has become a global pandemic and a major public health threat[1-3]. The disease mainly involves the respiratory system, causing flu-like symptoms such as fever, dry cough, and dyspnea, and severe cases may deteriorate to acute respiratory distress syndrome[4]. Apart from respiratory disorder, SARS-CoV-2 can also contribute to multiorgan dysfunction such as acute cardiac injury, acute renal insufficiency, and liver damage[5-7]. According to the previous reports, the incidence of abnormal liver function ranges from 14.8% to 53% in patients with COVID-19[8-10].
Hepatitis B virus (HBV), a prototypical member of the Hepadnaviridae family, has a worldwide distribution, especially in China[11]. Currently, 3.5% of the global population is chronically infected with HBV and 5%-6% (70 million) of the Chinese population are carriers of hepatitis B surface antigen (HBsAg)[12-14]. Although new infection with HBV is decreasing due to vaccination, HBV is still the primary cause of liver cirrhosis and hepatocellular carcinoma (HCC), resulting in many deaths each year[15,16].
Because SARS-CoV-2 and HBV can both cause abnormal liver function, one of the major concerns is whether people with pre-existing HBV infection have increased susceptibility to and severity of COVID-19, thus leading to a worse prognosis. Another concern is whether SARS-CoV-2 infection accelerates the course of hepatitis B progression and leads to active viral replication. It is important to figure out the interaction between the two diseases. To elucidate this complexity, we summarize the limited clinical research to compare the severity of organ injury and clinical outcome between coinfected patients and those with COVID-19 alone, to provide insights into early risk stratification, and follow-up disease management.
IMPACT OF HBV ON COVID-19
Liver injury
According to the data from two large cohorts, which enrolled 5700 and 1099 COVID-19 patients, respectively, 0.1%-2.1% of patients had HBV coinfection[6,17]. The first issue that attracts widespread attention is whether there is more serious liver damage in chronic hepatitis B patients after coinfection with SARS-CoV-2.
Based on the currently available research, the pattern and degree of liver injury in patients with HBV and SARS-CoV-2 coinfection are like those with SARS-CoV-2 alone. We know from previous studies that liver injury has a prevalence of 14.8%-53.0% in COVID-19. The characteristics of liver injury mainly manifest as different degrees of elevation in alanine aminotransferase (ALT, 2.5%-50.0%), aspartate aminotransferase (AST, 2.5%-61.1%), γ-glutamyl transferase, and total bilirubin (0%-35.3%), and patients with severe disease may also show reduced albumin[8,10,18-20]. In a retrospective study by Zou et al[21], 105 patients with COVID-19 and HBV coinfection were studied. Liver injury was observed in a small proportion of patients (14, 13.33%), which is within the range of incidence in patients with COVID-19 alone, and four (28.57%) patients with liver dysfunction progressed to acute-on-chronic liver failure. Moreover, other retrospective studies have shown that liver injury in COVID-19 patients with HBV coinfection also presents with varying degrees of elevated transaminases (such as ALT, AST, γ-glutamyl transferase, and total bilirubin), but most studies have found no significant difference in the degree of liver damage compared to that in patients with COVID-19 alone[22-26]. The characteristics of liver injury in patients with SARS-CoV-2 and HBV coinfection are listed in Table 1.
Table 1.
Characteristics of liver injury in patients with coronavirus disease 2019 and hepatitis B virus coinfection
|
Reference
|
Number of analyzed cases
|
HBV cases, n (%)
|
HBV status
|
Anti-HBV therapy, n (%)
|
ALT (U/L)1
|
AST (U/L)1
|
TBil (μmol/L)1
|
GGT (U/L)1
|
Note
|
| Zou et al[21] | 105 | 105 (100) | HBsAg(+), 94% HBeAg (–) | 13 (12.38); entecavir (9, 8.75); tenofovir (3, 2.86); lamivudine/defovir (1, 0.95) | 23 (15-33) | 28 (19-43) | 8.3 (6.6-12.8) | 24 (16-36) | Four patients developed ACLF and liver injury was associated with disease severity and worse prognosis |
| Chen et al[22] | 326 | 20 (6.1) | HBsAg(+); HBeAg(–); HBV DNA < 100 IU/mL | NA | 28.00 (16.25-42.25) | 27.50 (22.00-42.25) | 10.55 (6.83-15.73) | 23.50 (15.50-35.25) | No differences in the level of liver function (HBV vs non-HBV) |
| Liu et al[23] | 347 | 21 (6.4) | HBsAg(+); 95% HBeAg(–) | 1 (4.8) tenofovir | 30.40 (22.00-36.85) | 34.15 (27.00-39.58 | 12.60 (10.50-16.43) | 28.50 (17.25-43.42) | Three patients had HBV reactivation |
| Li et al[24] | 342 | 7 (2) | HBsAg(+); 14% HBeAg(+) | 2 (28.6) | 31 (29-38) | 31 (29-38) | 12.7 (11.1-16.6) | NA | Liver injury was common but mild with no severe liver-related complications |
| 2Chen et al[39] | 123 | 15 (12.2) | HBsAg(+) 6.7% HBeAg(+); 67% HBV DNA; > 20 IU/mL | 3 (20) entecavir | 25 (16-44) | 28 (19-58) | 13.2 (10.0-17.4) | 20 (14-28) | The level of TBil was higher in patients with HBV infection (P < 0.05) |
| Wu et al[40] | 620 | 70 (11.3) | NA | NA | 50 (28-69) | 40 (25-54) | NA | NA | 33% of patients had abnormal ALT and AST; ALT/AST levels were higher in patients with HBV (P < 0.05) |
| He et al[25] | 571 | 15 (2.63) | NA | 3 (20) entecavir | NA | NA | NA | NA | HBV infection was observed to have a lower risk of severe events (P < 0.05) |
| Zhang et al[26] | 23 | 23 (100) | 65.2% HBV carriers; 30.4% CHB; 4.3% cirrhosis | NA | 38.6 (17.0-42.0) | 31.6 (15.0-36.8) | 24.9 (7.2-13.9) | 32.3 (13.5-41.0) | 26% of patients had abnormal liver function test results at admission |
Data are expressed as median and interval interquartile.
Another person whose family name is Chen. ACLF: Acute-on-chronic liver failure; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CHB: Chronic hepatitis B; GGT: Gamma-glutamyl transferase; HBeAg: Hepatitis B e antigen; HBsAg: Hepatitis B surface antigen; HBV: Hepatitis B virus; NA: Not available; TBil: Total bilirubin.
Although liver injury is common in COVID-19, severe liver damage is rare. With recovery of the disease, liver function in most patients gradually recover back to normal. Guo et al[27] recently showed that males, COVID-19 severity, low liver computed tomography density, and medication are risk factors closely related to liver injury. The possible mechanism of liver injury for individuals infected with SARS-CoV-2 includes the following: (1) Direct damage caused by SARS-CoV-2 particles; (2) Immune-mediated organ damage; (3) Hypoxic-ischemic liver injury; (4) Drug-induced liver injury; and (5) Reactivation of pre-existing liver disease[28-31]. These factors may participate together to cause abnormal physiological function of the liver.
Disease severity and clinical outcome
A meta-analysis reported that 3% of patients with COVID-19 have underlying chronic liver disease (CLD)[32]. In addition, disease progression is higher in COVID-19 patients with CLD[33]. Metabolic-associated fatty liver disease, one of the etiologies of CLD, has been reported to increase the severity of COVID-19[34-37]. Although most studies have shown that HBV coinfection does not aggravate the liver injury, whether it affects disease severity and outcomes remains controversial.
Some studies have suggested that HBV coinfection does not aggravate the disease in patients with COVID-19. In an analysis of several large studies[4-6,38], the major complications in COVID-19 included acute respiratory distress syndrome (3.4%-29.0%), acute cardiac injury (4%-12%), acute kidney injury (0.5%-7.0%), and shock (1.1%-8.7%). Zou et al[21] and Zhang et al[26] showed that 8.7%-44.8% of patients had acute respiratory distress syndrome, 13.3% of patients had an acute cardiac injury, 3.81%-4.30% of patients had acute kidney injury, 2.81% patients had a shock, and 4.3% had deep venous thrombosis and upper gastrointestinal hemorrhage in patients with SARS-CoV-2 and HBV coinfection. According to these data, the percentage of organ injury in patients with HBV coinfection was roughly parallel to that in patients with COVID-19 alone. It seems that SARS-CoV-2 and HBV coinfection does not exacerbate organ impairment in COVID-19.
Additionally, in a cohort of 326 confirmed COVID-19 patients, of which 20 (6.1%) had HBV coinfection, Chen et al[22] reported that there were no differences in discharge rate and length of stay between the two groups. HBV coinfection did not affect the course and prognosis of COVID-19. In another study by Liu et al[23], 21 (6.4%) patients with COVID-19 and HBV coinfection were included, and 51 matched COVID-19 patients without HBV were used for comparison. They explored the independent impact of chronic HBV infection on the progression to severe COVID-19 and found that HBV did not delay SARS-CoV-2 shedding and did not increase the risk of progression and poor outcomes related to SARS-CoV-2. Similarly, Li et al[24] and He et al[25] enrolled seven (2%) and fifteen (2.6%) patients with HBV infection out of 342 and 571 COVID-19 patients, respectively. They found that chronic HBV coinfection was not associated with disease severity or poor prognosis.
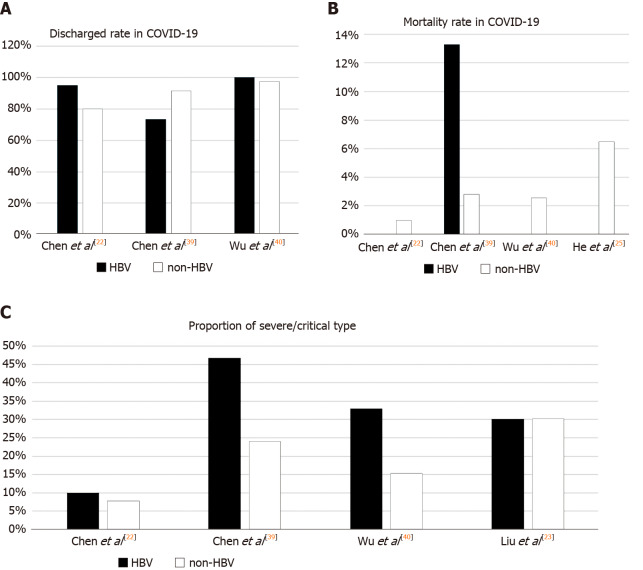
However, a few studies have reported conflicting results. A study of 15 (12.2%) patients with chronic hepatitis B and COVID-19 found that they had a more severe disease course and higher mortality rate (13.3% vs 2.8%) compared with those without HBV infection, suggesting that HBV coinfection may facilitate the development of liver injury, which is associated with adverse outcomes[39]. Another recent study involving 70 cases of coinfection by Wu et al[40] indicated that ALT, AST, and activated partial thromboplastin time were significantly higher in patients with COVID-19 and HBV coinfection. The proportion of severe/critically ill patients was also higher than that in the non-HBV infection group (32.86% vs 15.27%). Despite this, all patients with HBV coinfection in the study of Wu et al[40] were discharged, and the length of hospital stay and negative nucleic acid tests were both consistent with those without HBV coinfection, indicating no differences in clinical outcomes between the two groups. Comparison of disease severity and clinical outcome (discharge rate and mortality rate) in the above studies are shown in Figure 1. These studies appeared to suggest that in most cases, chronic HBV infection did not increase the risk of disease severity or lead to a worse prognosis in COVID-19.
Figure 1.
Comparison of clinical outcomes of coronavirus disease 2019 in hepatitis B virus and non-hepatitis B virus groups. A: Discharge rate; B: Mortality rate; C: Proportion of severe/critically ill patients. The data were collected from different clinical studies. COVID-19: Coronavirus disease 2019; HBV: Hepatitis B virus.
Cirrhosis and HCC
Clinicians may be concerned about whether HBV-related cirrhosis and carcinoma are associated with poor outcomes in COVID-19. Data on this issue are currently scarce. Zhang et al[26] compared the impact of different hepatitis B status (HBV carrier group, hepatitis B/cirrhosis group) on COVID-19. Most HBV carriers do not develop severe or critical illness, and no significant differences were found in the length of hospital stay, disease severity, and prognosis between the two groups. It is worth mentioning that only one patient was cirrhotic in this study, although there may have been biasing in the results.
A large cohort[41] enrolled 745 CLD patients from 29 countries, of whom 386 had cirrhosis and 359 did not, and mortality was significantly higher in the cirrhotic patients (32% vs 8%). Mortality increased with Child-Turcotte-Pugh class, which showed for the first time that the stage of liver disease is strongly associated with COVID-19 mortality. The data from some other multicenter retrospective studies also supported the conclusion that patients with liver cirrhosis in COVID-19 had higher mortality and worse prognosis compared with patients without cirrhosis[42-45].
HBV-related cirrhosis only accounted for a small proportion of patients, and most cases of cirrhosis were attributed to nonalcoholic fatty liver disease (24%-32.5%), alcohol-related liver disease (4.6%-24%), and chronic hepatitis C virus infection (24%)[44,46]. More importantly, HBV accounted for the lowest proportion of severe cases and deaths compared with other etiologies. Alcohol-related liver disease rather than HBV was an independent risk factor associated with the outcome of COVID-19. Although the severity of cirrhosis is closely related to mortality and prognosis in COVID-19, the limited data about HBV-related cirrhosis are insufficient to confirm that HBV worsens the clinical outcome.
As for patients with HCC, they usually have a higher risk of infection and poor outcome due to their immunocompromised condition. Much of the research has revealed that individuals with cancer are more vulnerable to SARS-CoV-2 and have an increased risk of mortality[47,48]. However, there is no available data about HBV-related HCC in patients with COVID-19. Therefore, reducing exposure and preventing SARS-CoV-2 infection is important for these patients.
Interpretation of results
According to the limited research, HBV infection is not associated with the clinical outcome of COVID-19, although some patients may have a higher level of liver enzymes. We analyzed the underlying reasons behind this phenomenon.
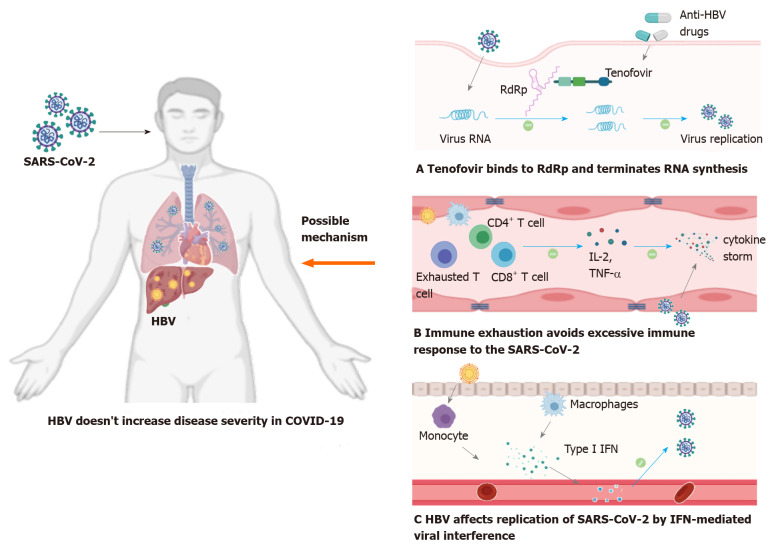
(1) Some of the hepatitis B patients included in the study received nucleoside analogs as anti-HBV therapy (entecavir, tenofovir, etc.) in the long term, which may play a role in combating SARS-CoV-2 to some extent. Tenofovir tightly binds to SARS-CoV RNA-dependent RNA polymerase (RdRp) and terminates RNA synthesis catalyzed by SARS-CoV-2 RNA-dependent RNA polymerase[49,50]. These results provide a molecular basis for these nucleotide analogs to be viewed as a potential therapy for COVID-19. Additionally, a large cohort study in Spain found that the incidence of SARS-CoV-2 infection was low (0.4%, 8/1764) in patients with chronic hepatitis B who took tenofovir as anti-HBV therapy, which indirectly reflected that nucleoside analogs have a positive effect on resisting the novel coronavirus[51].
(2) Immune dysfunction caused by chronic HBV infection may play a crucial role in disease progression in COVID-19. Studies have proved that chronic HBV infection is associated with exhaustion of virus specific CD4+ and CD8+ T cells due to persisting viral antigens[52,53]. HBV-specific exhausted T cells lead to impaired secretion of cytokines, especially interleukin (IL) 2 and tumor necrosis factor-alpha, which is accompanied by progressive reduced antiviral function[54]. To our knowledge, the excessive immune response to SARS-CoV-2 infection (cytokine storm) results in overproduction of proinflammatory cytokines (such as IL-2, IL-6, and tumor necrosis factor-alpha), which is a critical factor associated with disease severity and mortality[55]. Under this circumstance, it is plausible that the exhaustion of HBV-specific T lymphocytes and the status of immunosuppression may avoid an overactive immune response to the novel coronavirus and reduce the cytokine storm, resulting in milder disease.
(3) Viral interference, which is defined as one virus in the host competitively suppressing the replication of a second coinfecting virus, probably participates in the disease outcome in SARS-CoV-2 and HBV coinfection. Several studies have found that impaired type I interferon activity is a major feature in severe COVID-19 patients, which is associated with autoantibodies and genetic defects[56-58]. Viral interference can suppress coinfected viruses by enhancing type I interferon signaling[59]. Prior studies have proved that viral interference can occur in influenza virus, hepatitis virus, and human immunodeficiency virus[59-61]. For example, hepatitis C virus infection can limit the replication of HBV, and GB virus C and human immunodeficiency virus coinfection can reduce viral loads and prolong survival compared with patients with human immunodeficiency virus-1 infection. These observations support the hypothesis that HBV coinfection can affect replication and proliferation of SARS-CoV-2 by interferon-mediated viral interference.
(4) The number of patients with HBV coinfection in the published retrospective studies was small, which may have influenced the results. Almost all available studies about SARS-CoV-2 and HBV coinfection did not describe the baseline characteristics of HBV infection well, so the clinical stage of the patients could not be determined clearly. Hence, these results should be interpreted with caution and further conclusive research is needed.
IMPACT OF SARS-COV-2 ON HBV
For severe COVID-19 patients with HBV coinfection, there is a risk of HBV reactivation. There has been little consensus about the standardized definition of HBV reactivation. Primarily reactivation is defined as a sudden and rapid increase in HBV DNA levels in individuals with previously detectable HBV DNA or reappearance of HBV DNA viremia in individuals without detectable viral DNA[62]. HBV reactivation is usually associated with immunosuppressive therapy such as IL-6 receptor antagonists (tocilizumab and siltuximab), IL-1 receptor antagonists (anakinra), and high-dose corticosteroids[63-65]. In severe COVID-19 patients, these therapies may be used to control the cytokine storm, thus reducing the immune-mediated multiorgan injury.
In a retrospective study[23] of 21 patients with SARS-CoV-2 and HBV coinfection, 19 patients were tested for HBV DNA viral load at least twice during hospitalization. Of the 19 patients, three patients developed HBV reactivation and manifested as a rapid increase in HBV DNA viral load from undetectable to a high level. These three patients were negative for hepatitis B e antigen and did not receive any anti-HBV treatment before admission. During the hospitalization, two of the three patients received methylprednisolone, which may account for the reactivation, and one did not receive any corticosteroids. Another case report[66] showed that one patient with COVID-19 had acute HBV infection, and laboratory results showed AST (4933 U/L), ALT (4758 U/L), total bilirubin (183.9 mmol/L), HBsAg (+), hepatitis B core antibody immunoglobulin M (+), hepatitis B e antigen (–), hepatitis B e antibody (+), and HBV DNA viral load was 2490 IU/mL. The patient did not receive any immunosuppressive therapy. Regardless whether corticosteroids were used, the patient could have a risk of HBV reactivation.
The mechanisms of HBV reactivation following infection with SARS-CoV-2 are primarily due to a broken balance between the host’s immune state and viral replication. In addition to the host baseline virological indicators, the intensity of glucocorticoids or immunosuppression therapies is a primary risk factor for reactivation of HBV during treatment of COVID-19[11,62].
Although infection with SARS-CoV-2 has a risk of HBV reactivation, the overall risk is low. One prospective study[67] evaluated the risk of HBV reactivation in 61 patients with severe COVID-19 and resolved HBV infection (HBsAg-negative, anti-hepatitis B core antibody-positive) undergoing immunosuppressive therapy. After at least 1 mo of follow-up, they found no cases develop HBsAg seroconversion and only two (3%) patients had detectable serum HBV DNA (< 15 IU/mL). Therefore, for patients with severe COVID-19 and coexistent HBV infection, corticosteroids and immu-nosuppressants can be selected clinically.
Given the risk of reactivation, the American Association for the Study of Liver Diseases guidelines strongly recommend that anti-HBV treatment should be initiated or continued once COVID-19 was diagnosed[68]. At the same time, routine HBV virologic indicators and liver-injury related indicators should be closely monitored during the disease.
CONCLUSION
In this review, we summarized reports about SARS-CoV-2 and HBV coinfection and explored the interaction between chronic hepatitis B and COVID-19. The limited clinical evidence reflects that chronic HBV infection does not increase the severity and outcome of COVID-19 in most cases (Figure 2). Given that the stages in patients with chronic hepatitis B are ambiguous (immune tolerance or low viral replication), these findings need to be confirmed in further studies. HBV reactivation is possible in the course of the disease. Therefore, liver function and hepatitis-B-related indicators should be monitored regularly.
Figure 2.
Graphical abstract. COVID-19: Coronavirus disease 2019; HBV: Hepatitis B virus; IFN: Interferon; IL: Interleukin; RdRp: RNA-dependent RNA polymerase; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; TNF-α: Tumor necrosis factor-alpha.
ACKNOWLEDGEMENTS
We would like to express our sincere thanks to all those who have assisted while writing this review.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interest for this article.
Manuscript source: Invited manuscript
Peer-review started: December 8, 2020
First decision: December 31, 2020
Article in press: February 1, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liang K S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
Contributor Information
Tian-Dan Xiang, Department of Infectious Diseases, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, Hubei Province, China.
Xin Zheng, Department of Infectious Diseases, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, Hubei Province, China. xin11@hotmail.com.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Zheng M. Hepatitis B virus persistence and reactivation. BMJ. 2020;370:m2200. doi: 10.1136/bmj.m2200. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Zhang S, Wang Q, Shen H, Zhang M, Zhang Y, Yan D, Liu M. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21-49 years in rural China: a population-based, cross-sectional study. Lancet Infect Dis. 2016;16:80–86. doi: 10.1016/S1473-3099(15)00218-2. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ. 2019;97:230–238. doi: 10.2471/BLT.18.219469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuen MF, Chen DS, Dusheiko GM, Janssen HLA, Lau DTY, Locarnini SA, Peters MG, Lai CL. Hepatitis B virus infection. Nat Rev Dis Primers. 2018;4:18035. doi: 10.1038/nrdp.2018.35. [DOI] [PubMed] [Google Scholar]
- 15.Cooke GS, Andrieux-Meyer I, Applegate TL, Atun R, Burry JR, Cheinquer H, Dusheiko G, Feld JJ, Gore C, Griswold MG, Hamid S, Hellard ME, Hou J, Howell J, Jia J, Kravchenko N, Lazarus JV, Lemoine M, Lesi OA, Maistat L, McMahon BJ, Razavi H, Roberts T, Simmons B, Sonderup MW, Spearman CW, Taylor BE, Thomas DL, Waked I, Ward JW, Wiktor SZ Lancet Gastroenterology & Hepatology Commissioners. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2019;4:135–184. doi: 10.1016/S2468-1253(18)30270-X. [DOI] [PubMed] [Google Scholar]
- 16.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321–1326. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H, Chen S, Li H, Zhou XL, Dai Y, Wu J, Zhang J, Shao L, Yan R, Wang M, Wang J, Tu Y, Ge M. The association between markers of liver injury and clinical outcomes in patients with COVID-19 in Wuhan. Aliment Pharmacol Ther. 2020;52:1051–1059. doi: 10.1111/apt.15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrido I, Liberal R, Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52:267–275. doi: 10.1111/apt.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou X, Fang M, Li S, Wu L, Gao B, Gao H, Ran X, Bian Y, Li R, ShanshanYu , Ling J, Li D, Tian D, Huang J. Characteristics of Liver Function in Patients With SARS-CoV-2 and Chronic HBV Coinfection. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L, Huang S, Yang J, Cheng X, Shang Z, Lu H, Cheng J. Clinical characteristics in patients with SARS-CoV-2/HBV co-infection. J Viral Hepat. 2020;27:1504–1507. doi: 10.1111/jvh.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Wang T, Cai Q, Sun L, Huang D, Zhou G, He Q, Wang FS, Liu L, Chen J. Longitudinal changes of liver function and hepatitis B reactivation in COVID-19 patients with pre-existing chronic hepatitis B virus infection. Hepatol Res. 2020;50:1211–1221. doi: 10.1111/hepr.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Li C, Wang J, Zhu C, Zhu L, Ji F, Liu L, Xu T, Zhang B, Xue L, Yan X, Huang R, Wu C, Yan X. A case series of COVID-19 patients with chronic hepatitis B virus infection. J Med Virol. 2020;92:2785–2791. doi: 10.1002/jmv.26201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Q, Zhang G, Gu Y, Wang J, Tang Q, Jiang Z, Shao C, Zhang H, Chen Z, Ma B, Liu D, Xie G, Xu D, Huang Y, Zhang H, Liang M, Huang H, Wang Y, Liu H, Yang J, Pan H, Zou S, Li F, Wang F, Liu C, Wang W, Xiong B, Li X, Liu L, Yang J, Qi X. Clinical Characteristics of COVID-19 Patients With Pre-existing Hepatitis B Virus Infection: A Multicenter Report. Am J Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B, Huang W, Zhang S. Clinical Features and Outcomes of Coronavirus Disease 2019 (COVID-19) Patients With Chronic Hepatitis B Virus Infection. Clin Gastroenterol Hepatol. 2020;18:2633–2637. doi: 10.1016/j.cgh.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo H, Zhang Z, Zhang Y, Liu Y, Wang J, Qian Z, Zou Y, Lu H. Analysis of liver injury factors in 332 patients with COVID-19 in Shanghai, China. Aging (Albany NY) 2020;12:18844–18852. doi: 10.18632/aging.103860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. :e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: Two intersecting pandemics. Eur J Clin Invest. 2020;50:e13338. doi: 10.1111/eci.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.APASL Covid-19 Task Force , Lau G, Sharma M. Clinical practice guidance for hepatology and liver transplant providers during the COVID-19 pandemic: APASL expert panel consensus recommendations. Hepatol Int. 2020;14:415–428. doi: 10.1007/s12072-020-10054-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020;40:1316–1320. doi: 10.1111/liv.14465. [DOI] [PubMed] [Google Scholar]
- 33.Ji D, Zhang D, Yang T, Mu J, Zhao P, Xu J, Li C, Cheng G, Wang Y, Chen Z, Qin E, Lau G. Effect of COVID-19 on patients with compensated chronic liver diseases. Hepatol Int. 2020;14:701–710. doi: 10.1007/s12072-020-10058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545–1547. doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 36.Zhou YJ, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J Hepatol. 2020;73:719–721. doi: 10.1016/j.jhep.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao F, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Chen YP, George J, Zheng MH. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol. 2021;36:204–207. doi: 10.1111/jgh.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Jiang Q, Ma Z, Ling J, Hu W, Cao Q, Mo P, Yao L, Yang R, Gao S, Gui X, Hou W, Xiong Y, Li J, Zhang Y. Clinical Characteristics of Hospitalized Patients with SARS-CoV-2 and Hepatitis B Virus Co-infection. Virol Sin. 2020;35:842–845. doi: 10.1007/s12250-020-00276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J, Yu J, Shi X, Li W, Song S, Zhao L, Zhao X, Liu J, Wang D, Liu C, Huang B, Meng Y, Jiang B, Deng Y, Cao H, Li L. Epidemiological and clinical characteristics of 70 cases of coronavirus disease and concomitant hepatitis B virus infection: A multicentre descriptive study. J Viral Hepat. 2021;28:80–88. doi: 10.1111/jvh.13404. [DOI] [PubMed] [Google Scholar]
- 41.Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, Genescà J, Gill US, James TW, Jones PD, Marshall A, Mells G, Perumalswami PV, Qi X, Su F, Ufere NN, Barnes E, Barritt AS, Marjot T. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol. 2020;73:705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M APASL COVID Task Force; APASL COVID Liver Injury Spectrum Study (APCOLIS Study- NCT 04345640) Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study) Hepatol Int. 2020;14:690–700. doi: 10.1007/s12072-020-10072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen V, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin K, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch A, Viveiros K, Chan W, Chascsa D, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients with Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2020 doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan SL, Kudo M. Impacts of COVID-19 on Liver Cancers: During and after the Pandemic. Liver Cancer. 2020;9:491–502. doi: 10.1159/000510765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, Chackathayil J, Cheng VW, Curley HM, Fittall MW, Freeman-Mills L, Gennatas S, Goel A, Hartley S, Hughes DJ, Kerr D, Lee AJ, Lee RJ, McGrath SE, Middleton CP, Murugaesu N, Newsom-Davis T, Okines AF, Olsson-Brown AC, Palles C, Pan Y, Pettengell R, Powles T, Protheroe EA, Purshouse K, Sharma-Oates A, Sivakumar S, Smith AJ, Starkey T, Turnbull CD, Várnai C, Yousaf N UK Coronavirus Monitoring Project Team; Kerr R; Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jockusch S, Tao C, Li X, Anderson TK, Chien M, Kumar S, Russo JJ, Kirchdoerfer RN, Ju J. A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID-19. Antiviral Res. 2020;180:104857. doi: 10.1016/j.antiviral.2020.104857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elfiky AA. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lens S, Miquel M, Mateos-Muñoz B, García-Samaniego J, Forns X. SARS-CoV-2 in patients on antiviral HBV and HCV therapy in Spain. J Hepatol. 2020;73:1262–1263. doi: 10.1016/j.jhep.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rehermann B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat Med. 2013;19:859–868. doi: 10.1038/nm.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anugwom CM, Aby ES, Debes JD. Inverse Association Between Chronic Hepatitis B Infection and Coronavirus Disease 2019 (COVID-19): Immune Exhaustion or Coincidence? Clin Infect Dis. 2021;72:180–182. doi: 10.1093/cid/ciaa592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, Péré H, Charbit B, Bondet V, Chenevier-Gobeaux C, Breillat P, Carlier N, Gauzit R, Morbieu C, Pène F, Marin N, Roche N, Szwebel TA, Merkling SH, Treluyer JM, Veyer D, Mouthon L, Blanc C, Tharaux PL, Rozenberg F, Fischer A, Duffy D, Rieux-Laucat F, Kernéis S, Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, Dorgham K, Philippot Q, Rosain J, Béziat V, Manry J, Shaw E, Haljasmägi L, Peterson P, Lorenzo L, Bizien L, Trouillet-Assant S, Dobbs K, de Jesus AA, Belot A, Kallaste A, Catherinot E, Tandjaoui-Lambiotte Y, Le Pen J, Kerner G, Bigio B, Seeleuthner Y, Yang R, Bolze A, Spaan AN, Delmonte OM, Abers MS, Aiuti A, Casari G, Lampasona V, Piemonti L, Ciceri F, Bilguvar K, Lifton RP, Vasse M, Smadja DM, Migaud M, Hadjadj J, Terrier B, Duffy D, Quintana-Murci L, van de Beek D, Roussel L, Vinh DC, Tangye SG, Haerynck F, Dalmau D, Martinez-Picado J, Brodin P, Nussenzweig MC, Boisson-Dupuis S, Rodríguez-Gallego C, Vogt G, Mogensen TH, Oler AJ, Gu J, Burbelo PD, Cohen JI, Biondi A, Bettini LR, D'Angio M, Bonfanti P, Rossignol P, Mayaux J, Rieux-Laucat F, Husebye ES, Fusco F, Ursini MV, Imberti L, Sottini A, Paghera S, Quiros-Roldan E, Rossi C, Castagnoli R, Montagna D, Licari A, Marseglia GL, Duval X, Ghosn J; HGID Lab; NIAID-USUHS Immune Response to COVID Group; COVID Clinicians; COVID-STORM Clinicians; Imagine COVID Group; French COVID Cohort Study Group; Milieu Intérieur Consortium; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort, Tsang JS, Goldbach-Mansky R, Kisand K, Lionakis MS, Puel A, Zhang SY, Holland SM, Gorochov G, Jouanguy E, Rice CM, Cobat A, Notarangelo LD, Abel L, Su HC, Casanova JL. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, Ogishi M, Sabli IKD, Hodeib S, Korol C, Rosain J, Bilguvar K, Ye J, Bolze A, Bigio B, Yang R, Arias AA, Zhou Q, Zhang Y, Onodi F, Korniotis S, Karpf L, Philippot Q, Chbihi M, Bonnet-Madin L, Dorgham K, Smith N, Schneider WM, Razooky BS, Hoffmann HH, Michailidis E, Moens L, Han JE, Lorenzo L, Bizien L, Meade P, Neehus AL, Ugurbil AC, Corneau A, Kerner G, Zhang P, Rapaport F, Seeleuthner Y, Manry J, Masson C, Schmitt Y, Schlüter A, Le Voyer T, Khan T, Li J, Fellay J, Roussel L, Shahrooei M, Alosaimi MF, Mansouri D, Al-Saud H, Al-Mulla F, Almourfi F, Al-Muhsen SZ, Alsohime F, Al Turki S, Hasanato R, van de Beek D, Biondi A, Bettini LR, D'Angio' M, Bonfanti P, Imberti L, Sottini A, Paghera S, Quiros-Roldan E, Rossi C, Oler AJ, Tompkins MF, Alba C, Vandernoot I, Goffard JC, Smits G, Migeotte I, Haerynck F, Soler-Palacin P, Martin-Nalda A, Colobran R, Morange PE, Keles S, Çölkesen F, Ozcelik T, Yasar KK, Senoglu S, Karabela ŞN, Rodríguez-Gallego C, Novelli G, Hraiech S, Tandjaoui-Lambiotte Y, Duval X, Laouénan C; COVID-STORM Clinicians; COVID Clinicians; Imagine COVID Group; French COVID Cohort Study Group; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort; NIAID-USUHS/TAGC COVID Immunity Group, Snow AL, Dalgard CL, Milner JD, Vinh DC, Mogensen TH, Marr N, Spaan AN, Boisson B, Boisson-Dupuis S, Bustamante J, Puel A, Ciancanelli MJ, Meyts I, Maniatis T, Soumelis V, Amara A, Nussenzweig M, García-Sastre A, Krammer F, Pujol A, Duffy D, Lifton RP, Zhang SY, Gorochov G, Béziat V, Jouanguy E, Sancho-Shimizu V, Rice CM, Abel L, Notarangelo LD, Cobat A, Su HC, Casanova JL. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li N, Ma WT, Pang M, Fan QL, Hua JL. The Commensal Microbiota and Viral Infection: A Comprehensive Review. Front Immunol. 2019;10:1551. doi: 10.3389/fimmu.2019.01551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kovesdi I, Bakacs T. Therapeutic Exploitation of Viral Interference. Infect Disord Drug Targets. 2020;20:423–432. doi: 10.2174/1871526519666190405140858. [DOI] [PubMed] [Google Scholar]
- 61.Makoti P, Fielding BC. HIV and Human Coronavirus Coinfections: A Historical Perspective. Viruses. 2020;12 doi: 10.3390/v12090937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loomba R, Liang TJ. Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology. 2017;152:1297–1309. doi: 10.1053/j.gastro.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sonneveld MJ, Murad SD, van der Eijk AA, de Man RA. Fulminant Liver Failure due to Hepatitis B Reactivation During Treatment With Tocilizumab. ACG Case Rep J. 2019;6:e00243. doi: 10.14309/crj.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen LF, Mo YQ, Jing J, Ma JD, Zheng DH, Dai L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: a prospective clinical observation. Int J Rheum Dis. 2017;20:859–869. doi: 10.1111/1756-185X.13010. [DOI] [PubMed] [Google Scholar]
- 65.Wong GL, Wong VW, Yuen BW, Tse YK, Yip TC, Luk HW, Lui GC, Chan HL. Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J Hepatol. 2020;72:57–66. doi: 10.1016/j.jhep.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 66.Aldhaleei WA, Alnuaimi A, Bhagavathula AS. COVID-19 Induced Hepatitis B Virus Reactivation: A Novel Case From the United Arab Emirates. Cureus. 2020;12:e8645. doi: 10.7759/cureus.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodríguez-Tajes S, Miralpeix A, Costa J, López-Suñé E, Laguno M, Pocurull A, Lens S, Mariño Z, Forns X. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J Viral Hepat. 2021;28:89–94. doi: 10.1111/jvh.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reddy KR. SARS-CoV-2 and the Liver: Considerations in Hepatitis B and Hepatitis C Infections. Clin Liver Dis (Hoboken) 2020;15:191–194. doi: 10.1002/cld.970. [DOI] [PMC free article] [PubMed] [Google Scholar]