Abstract
BACKGROUND
Liver injury is common and also can be fatal, particularly in severe or critical patients with coronavirus disease 2019 (COVID-19).
AIM
To conduct an in-depth investigation into the risk factors for liver injury and into the effective measures to prevent subsequent mortality risk.
METHODS
A retrospective cohort study was performed on 440 consecutive patients with relatively severe COVID-19 between January 28 and March 9, 2020 at Tongji Hospital, Wuhan, China. Data on clinical features, laboratory parameters, medications, and prognosis were collected.
RESULTS
COVID-19-associated liver injury more frequently occurred in patients aged ≥ 65 years, female patients, or those with other comorbidities, decreased lymphocyte count, or elevated D-dimer or serum ferritin (P < 0.05). The disease severity of COVID-19 was an independent risk factor for liver injury (severe patients: Odds ratio [OR] = 2.86, 95% confidence interval [CI]: 1.78-4.59; critical patients: OR = 13.44, 95%CI: 7.21-25.97). The elevated levels of on-admission aspartate aminotransferase and total bilirubin indicated an increased mortality risk (P < 0.001). Using intravenous nutrition or antibiotics increased the risk of COVID-19-associated liver injury. Hepatoprotective drugs tended to be of assistance to treat the liver injury and improve the prognosis of patients with COVID-19-associated liver injury.
CONCLUSION
More intensive monitoring of aspartate aminotransferase or total bilirubin is recommended for COVID-19 patients, especially patients aged ≥ 65 years, female patients, or those with other comorbidities. Drug hepatotoxicity of antibiotics and intravenous nutrition should be alert for COVID-19 patients.
Keywords: COVID-19, Liver injury, Prognosis, Risk factors, Drugs, Alanine aminotransferase
Core Tip: The prevalence of liver injury in hospitalized patients with coronavirus disease 2019 (COVID-19) is high and also can be fatal. Therefore, high-risk population, especially patients aged ≥ 65 years, female patients, or patients with other comorbidities should be intensively monitored. On-admission total bilirubin has the strongest correlation with the prognosis of COVID-19 patients, which can be used for monitoring of COVID-19 patients at risk of liver injury. Intravenous nutrition and antibiotics are associated with abnormal liver biochemistry; these drugs should be given with caution. Hepatoprotective drugs are favorable for patients with liver injury.
INTRODUCTION
The World Health Organization declared the coronavirus disease 2019 (COVID-19) outbreak on March 11, 2020, and the numbers of cases and deaths of COVID-19 globally are soaring[1]. As of March 2, 2021, more than 114000000 confirmed cases of COVID-19 and more than 2500000 deaths had been reported worldwide. The global spread of COVID-19 is an enormous threat to humanity.
Liver injury has been reported as a common clinical manifestation in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection[2,3]. Between 14% and 53% of patients have elevated levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST)[4-7], and a recent study reported that patients with abnormal liver tests were more likely to progress to severe disease[8]. It becomes extremely emergent to treat severe patients and reduce mortality. Some studies have focused on severe cases with liver injury and their survival outcome, but the proportion of severe and critical cases is low.
Comprehensive and detailed studies on the clinical features, risk factors, and prognosis of patients with liver injury are still lacking, and it is urgent to clarify predictive factors and therapeutic approaches for liver injury.
Here we conducted a retrospective cohort study to identify the risk factors related to liver injury in COVID-19 patients, to evaluate the impact of liver injury on prognosis and to determine whether it is a reliable and independent predictor of disease prognosis, and to investigate the efficacy of hepatoprotective drugs in patients with liver injury. Our results will provide relatively comprehensive and reliable references for clinical decisions, and are expected to improve the outcomes of COVID-19 patients.
MATERIALS AND METHODS
Study participants
This study was a retrospective observational study. All patients with COVID-19 consecutively admitted to six wards at the Sino-French New City Branch of Tongji Hospital, Huazhong University of Science and Technology from January 28 to March 9, 2020 were enrolled. Tongji Hospital was designated to treat severe and critical COVID-19 patients by the Wuhan government in January 2020. Follow-up of the clinical outcomes of all patients was censored on March 27, 2020.
Data collection
Sociodemographic characteristics, clinical symptoms, laboratory parameters, chest computed tomography (CT) features, outcomes, and medications were extracted from the electronic medical records. We followed laboratory-specific thresholds for all laboratory parameters and categorized each into two or three strata. To quantify the severity of pneumonia, we utilized two scores, i.e., confusion, uremia, respiratory rate, blood pressure, and age ≥ 65 (CURB-65)[9], and the quick Sequential Organ Failure Assessment (qSOFA)[10].
Definition
COVID-19 patients and disease severity: We included all patients with confirmed COVID-19 according to “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia” (trial seventh edition) by the Chinese National Health Commission[11]. Severity of COVID-19 was categorized into three stages: Severe cases were defined as (1) respiratory rate ≥ 30 breaths/min; (2) oxygen saturation ≤ 93%; or (3) PaO2/FiO2 ratio ≤ 300 mmHg. Critical cases were defined as including one or more of the following criteria: Shock, respiratory failure requiring mechanical ventilation, in combination with other organ failures, or admission to intensive care unit. Or else, other COVID patients were general cases. In-hospital disease severity was the severest grade during hospitalization.
Liver biochemistry abnormality or injury and acute liver injury: COVID-19-related liver injury was divided into three stages as per the Chinese Guideline for COVID-19-related liver injury[12]. Stage 0, normal liver biochemistry (LB), was defined as markers indicating liver function, i.e., ALT, AST, and total bilirubin (TBIL), within normal limits. Stage 1, LB abnormality (LBA), was defined as liver function markers outside the normal limits but not reaching the thresholds for stage 2. Stage 2, acute liver injury (ALI), was defined as an ALT or AST increase of ≥ 3 times the upper limit of normal, or TBIL increase of ≥ 2 times the upper limit of normal. In our study, LB abnormality or injury (LBAI) was defined as either stage 1 or stage 2. Worsening of LB was defined as any stage elevation during hospitalization. Cases were excluded from the study if no data on ALT, AST and TBIL were available.
Other terms: We discriminated liver injury associated with COVID-19 by temporal patterns, e.g., admission to the hospital, in-hospital, and cumulative incidence that took both time points into account. We also linked the time of illness onset that refers to the onset of symptoms. Hepatoprotective drugs used in the study included magnesium isoglycyrrhizinate, diammonium glycyrrhizinate, and polyene pho-sphatidylcholine.
Ethics approval
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and the Harmonized Tripartite Guideline for Good Clinical Practice from the International Conference on Harmonization. The study was approved by the ethics committee of Peking University Third Hospital (IRB00006761-M2020060).
Statistical analysis
Categorical variables are summarized as counts (percentages) and continuous variables are presented as the median with interquartile range (IQR). Univariate binary logistic regression was used to explore the factors correlating with cumulative LBAI. The cumulative incidence rates of mortality were assessed using the Kaplan-Meier method and significant differences between the subgroups were determined using the standard log-rank test. Univariable and multivariable Cox proportional hazard regression was used for the survival analysis associated with liver injury markers and other risk factors for mortality. All descriptive statistical analyses were conducted with SPSS version 23.0 (IBM Corp, United States) and analytical analyses were done using R version 4.0.0 (R Foundation for Statistical Computing, Austria). A two-sided P value less than 0.05 was considered statistically significant.
RESULTS
Demographic and clinical characteristics associated with LBAI
A total of 440 patients with COVID-19 were included in the final analysis. The median age was 63 years (IQR 18) and 233 (53%) were female. Median follow-up time was 17 d (IQR 21). The total incidence rate of cumulative LBAI was 57.7%, including 15.7% with ALI. The different incidence rates of cumulative LBAI stratified by sociodemographic and clinical characteristics are shown in Table 1. The likelihood of cumulative LBAI increased with the age of patients, with a 2-fold risk in patients aged ≥ 65 years compared with those aged 50 years or younger [odds ratio (OR) = 1.83; 95% confidence interval (CI): 1.03 to 3.26]. Female patients were at a higher risk of cumulative LBAI (OR = 2.09; 95%CI: 1.46 to 3.06) than males.
Table 1.
Demographics, clinical characteristics, laboratory parameters, and computed tomography imaging features of 440 patients with coronavirus disease 2019, stratified by severity of cumulative liver abnormality or injury
|
|
All patients (n = 440)
|
Normal cumulative LB (n = 186)
|
Cumulative LBA (n = 185)
|
Cumulative ALI (n = 69)
|
Cumulative LBAI (n = 254)
|
OR (95%CI)1
|
P
value2
|
| Characteristics | |||||||
| Age, yr, median (IQR) | 63 (25) | 61 (18) | 63 (23) | 65 (17) | 64 (18) | 2.536 | 0.012 |
| < 50 | 65 (14.8) | 33 (50.8) | 23 (35.4) | 9 (13.8) | 32 (49.2) | Ref | Ref |
| 50-64 | 203 (46.1) | 91 (44.8) | 85 (41.9) | 27 (13.3) | 112 (55.2) | 1.27 (0.73 to 2.22) | 0.404 |
| ≥ 65 | 172 (39.1) | 62 (36.0) | 77 (44.8) | 33 (19.2) | 110 (64.0) | 1.83 (1.03 to 3.26) | 0.040 |
| Sex, female | 233 (53.0) | 79 (33.9) | 109 (46.8) | 45 (19.3) | 154 (66.1) | 2.09 (1.42 to 3.06) | < 0.0001 |
| BMI, ≥ 24 kg/m2 | 124 (49.8) | 63 (50.8) | 45 (36.3) | 16 (12.9) | 61 (49.2) | 0.95 (0.58 to 1.57) | 0.849 |
| Epidemiological exposure3 | 268 (60.9) | 123 (45.9) | 104 (38.8) | 41 (15.3) | 145 (54.1) | 0.68 (0.46 to 1.01) | 0.055 |
| Smoking history | 62 (20.9) | 21 (33.9) | 31 (50.0) | 10 (16.1) | 41 (61.1) | 1.48 (0.84 to 2.61) | 0.176 |
| Comorbidities4 | 285 (64.8) | 109 (38.2) | 123 (43.2) | 53 (18.6) | 176 (61.8) | 1.59 (1.07 to 2.37) | 0.021 |
| Hypertension | 181 (41.1) | 69 (38.1) | 83 (45.9) | 29 (16.0) | 112 (61.9) | 1.34 (0.91 to 1.97) | 0.141 |
| Diabetes | 136 (30.9) | 48 (35.3) | 53 (39.0) | 35 (25.7) | 88 (64.7) | 1.52 (1.00 to 2.31) | 0.048 |
| Cardiovascular disease | 56 (12.7) | 22 (39.3) | 19 (33.9) | 15 (26.8) | 34 (60.7) | 1.15 (0.65 to 2.04) | 0.628 |
| Cerebrovascular disease | 16 (3.6) | 4 (25.0) | 8 (50.0) | 4 (25.0) | 12 (75.0) | 2.26 (0.72 to 7.11) | 0.165 |
| Chronic renal disease | 21 (4.8) | 11 (52.4) | 9 (42.9) | 1 (4.8) | 10 (47.6) | 0.65 (0.27 to 1.57) | 0.340 |
| Chronic liver disease | 12 (2.7) | 4 (33.3) | 7 (58.3) | 1 (8.3) | 8 (66.7) | 1.48 (0.44 to 4.99) | 0.527 |
| Chronic hepatitis B | 8 (1.8) | 2 (25.0) | 5 (62.5) | 1 (12.5) | 6 (75.0) | 2.23 (0.44 to 11.15) | 0.331 |
| Other chronic liver disease5 | 4 (0.9) | 2 (50.0) | 2 (50.0) | 0 | 2 (50.0) | 0.73 (0.10 to 5.23) | 0.754 |
| Chronic respiratory disease | 31 (7.0) | 16 (51.6) | 9 (29.0) | 6 (19.4) | 15 (48.4) | 0.67 (0.32 to 1.39) | 0.278 |
| Signs and symptoms at disease onset | |||||||
| Fever (temperature ≥ 37.3 °C) | 369 (85.8) | 149 (40.4) | 159 (43.1) | 61 (16.5) | 220 (59.6) | 1.63 (0.95 to 2.81) | 0.079 |
| Sputum | 236 (53.6) | 93 (39.4) | 104 (44.1) | 39 (16.5) | 143 (60.6) | 1.29 (0.88 to 1.88) | 0.191 |
| Dyspnoea | 243 (55.2) | 82 (33.7) | 116 (47.7) | 45 (18.5) | 161 (66.3) | 2.2 (1.49 to 3.23) | < 0.0001 |
| Haemoptysis | 63 (14.3) | 25 (39.7) | 26 (41.3) | 12 (19.0) | 38 (60.3) | 1.13 (0.66 to 1.95) | 0.653 |
| Chest pain/tightness | 227 (51.6) | 88 (38.8) | 100 (44.1) | 39 (17.2) | 139 (61.2) | 1.35 (0.92 to 1.97) | 0.125 |
| Pharyngalgia/nasal congestion/running | 94 (21.4) | 47 (50.0) | 33 (35.1) | 14 (14.9) | 47 (50.0) | 0.67 (0.42 to 1.06) | 0.088 |
| Headaches/dizziness | 135 (30.7) | 57 (42.2) | 54 (40.0) | 24 (17.8) | 78 (57.8) | 1.00 (0.67 to 1.51) | 0.989 |
| Myalgia/fatigue | 289 (65.7) | 122 (42.2) | 120 (41.5) | 47 (16.3) | 167 (57.8) | 1.01 (0.68 to 1.50) | 0.973 |
| Nausea/vomiting | 133 (30.2) | 53 (39.8) | 59 (44.4) | 21 (15.8) | 80 (60.2) | 1.15 (0.76 to 1.75) | 0.498 |
| Abdominal pain | 70 (15.9) | 29 (41.4) | 26 (37.1) | 15 (21.4) | 41 (58.6) | 1.04 (0.62 to 1.75) | 0.876 |
| Diarrhea | 185 (42.0) | 78 (42.2) | 83 (44.9) | 24 (13.0) | 107 (57.8) | 1.01 (0.69 to 1.48) | 0.968 |
| On-admission vital signs | |||||||
| Temperature, ≥ 37.3 °C | 103 (23.8) | 26 (25.2) | 50 (48.5) | 27 (26.2) | 77 (74.8) | 2.74 (1.67 to 4.49) | < 0.0001 |
| Respiratory rate, ≥ 30 breaths per min | 53 (12.1) | 13 (24.5) | 21 (39.6) | 19 (35.8) | 40 (75.5) | 2.50 (1.30 to 4.82) | 0.006 |
| Pulse oximeter O2 saturation, ≤ 93% | 146 (35.0) | 25 (17.1) | 82 (56.2) | 39 (26.7) | 121 (82.9) | 6.37 (3.89 to 10.43) | < 0.0001 |
| Systolic blood pressure, < 90 mmHg | 4 (0.9) | 2 (50.0) | 2 (50.0) | 0 | 2 (50.0) | 0.74 (0.10 to 5.27) | 0.760 |
| Heart rate, > 125 beats per min | 23 (5.2) | 5 (21.7) | 10 (43.5) | 8 (34.8) | 18 (78.3) | 2.77 (1.01 to 7.61) | 0.048 |
| Severity of illness | |||||||
| In-hospital disease severity status, general | 128 (29.1) | 88 (68.8) | 37 (28.9) | 3 (2.3) | 40 (31.3) | Ref | Ref |
| Severe | 184 (41.8) | 80 (43.5) | 84 (45.7) | 20 (10.9) | 104 (56.5) | 2.86 (1.78 to 4.59) | < 0.0001 |
| Critical | 128 (29.1) | 18 (14.1) | 64 (50.0) | 46 (35.9) | 110 (85.9) | 13.44 (7.21 to 25.07) | < 0.0001 |
| qSOFA score, 0 | 231 (52.7) | 114 (49.4) | 95 (41.1) | 22 (9.5) | 117 (50.6) | Ref | Ref |
| 1 | 179 (40.9) | 59 (33.0) | 80 (44.7) | 40 (22.3) | 120 (67.0) | 1.98 (1.32 to 2.97) | < 0.0001 |
| 2-3 | 28 (6.4) | 13 (46.4) | 9 (32.1) | 6 (21.4) | 15 (53.6) | 1.12 (0.51 to 2.47) | 0.770 |
| CURB-65 score, 0-1 | 330 (75.3) | 159 (48.2) | 132 (40.0) | 39 (11.8) | 171 (51.8) | Ref | |
| 2 | 71 (16.2) | 17 (23.9) | 41 (57.7) | 13 (18.3) | 54 (76.1) | 2.95 (1.64 to 5.31) | < 0.0001 |
| 3-5 | 37 (8.4) | 10 (27.0) | 11 (29.7) | 16 (43.2) | 27 (73.0) | 2.51 (1.18 to 5.35) | 0.017 |
| Routine blood results | |||||||
| White blood cell count, < 3.5 × 109/L | 44 (10.0) | 17 (38.6) | 19 (43.2) | 8 (18.2) | 27 (61.4) | 0.68 (0.35 to 1.29) | 0.234 |
| 3.5-9.5 | 313 (71.3) | 151 (48.2) | 126 (40.3) | 36 (11.5) | 162 (51.8) | Ref | Ref |
| > 9.5 | 82 (18.7) | 18 (22.0) | 40 (48.8) | 24 (29.3) | 64 (78.0) | 2.24 (1.00 to 4.99) | 0.049 |
| Lymphocyte count, < 1.1 × 109/L | 232 (52.8) | 62 (26.7) | 115 (49.6) | 55 (23.7) | 170 (73.3) | 4.1 (2.74 to 6.12) | < 0.0001 |
| Neutrophil count, > 6.3 × 109/L | 149 (33.9) | 44 (29.5) | 73 (49.0) | 32 (21.5) | 105 (70.5) | 2.29 (1.50 to 3.49) | < 0.0001 |
| Hemoglobin, < 130 g/L for male; < 115 g/L for female | 194 (44.4) | 85 (43.8) | 76 (39.2) | 33 (17.0) | 109 (56.2) | 0.90 (0.61 to 1.31) | 0.576 |
| Platelet count, < 125 × 109/L | 59 (13.5) | 17 (28.8) | 31 (52.5) | 11 (18.6) | 42 (71.2) | 1.98 (1.09 to 3.6) | 0.026 |
| Biochemical results-basic values | |||||||
| Albumin, < 35 g/L | 232 (52.7) | 58 (25.0) | 118 (50.9) | 56 (24.1) | 174 (75.0) | 4.80 (3.19 to 7.22) | < 0.0001 |
| ALT, > 41 U/L for male; > 33 U/L for female | 113 (25.7) | 0 | 77 (68.1) | 36 (31.9) | 113 (100.0%) | - | - |
| AST, > 40 U/L for males; > 32 U/L for females | 144 (32.7) | 0 | 91 (63.2) | 53 (36.8) | 144 (100.0) | - | - |
| Total bilirubin, > 26 mmol/L for males; > 21 mmol/L for females | 25 (5.7) | 0 | 12 (48.0) | 13 (52.0) | 25 (100.0) | - | - |
| Direct bilirubin, > 8.0 mmol/L | 61 (13.9) | 4 (6.6) | 29 (47.5) | 28 (45.9) | 57 (93.4) | 13.16 (4.68 to 37.01) | < 0.0001 |
| Lactate dehydrogenase, > 225 U/L for males; > 214 U/L for females | 332 (75.5) | 105 (31.6) | 162 (48.8) | 65 (19.6) | 227 (68.4) | 6.49 (3.96 to 10.62) | < 0.0001 |
| γ-Glutamyl transferase, > 71 U/L for males; > 42 U/L for females | 115 (26.4) | 20 (17.4) | 62 (53.9) | 33 (28.7) | 95 (82.6) | 5.12 (3.01 to 8.7) | < 0.0001 |
| Creatinine, > 104 μmol/L for males; > 84 μmol/L for females | 70 (16.0) | 22 (31.4) | 31 (44.3) | 17 (24.3) | 48 (68.6) | 1.73 (1.01 to 2.99) | 0.048 |
| C-reactive protein, ≥ 1 mg/L | 369 (87.6) | 145 (39.3) | 161 (43.6) | 63 (17.1) | 224 (60.7) | 2.92 (1.59 to 5.36) | 0.0010 |
| Coagulation and inflammation index results | |||||||
| Prothrombin time, ≤ 14.5 s | 289 (66.3) | 144 (49.8) | 116 (40.1) | 29 (10.0) | 145 (50.2) | 0.35 (0.23 to 0.54) | < 0.0001 |
| Prothrombin activity, < 75% | 82 (18.8) | 14 (17.1) | 43 (52.4) | 25 (30.5) | 68 (82.9) | 4.39 (2.38 to 8.09) | < 0.0001 |
| Activated partial thromboplastin time, > 42.0 s | 166 (38.2) | 46 (27.7) | 89 (53.6) | 31 (18.7) | 120 (72.3) | 2.69 (1.77 to 4.07) | < 0.0001 |
| D-dimer, > 0.5 μg/mL | 295 (68.4) | 87 (29.5) | 145 (49.2) | 63 (21.4) | 208 (70.5) | 5.17 (3.33 to 8.03) | < 0.0001 |
| Serum ferritin, > 400 μg/L for males; > 150 μg/L for females | 319 (81.0) | 111 (34.8) | 153 (48.0) | 55 (17.2) | 208 (65.2) | 6.91 (3.80 to 12.57) | < 0.0001 |
| Interleukin-6, ≥ 7 pg/mL | 255 (62.3) | 73 (28.6) | 123 (48.2) | 59 (23.1) | 182 (71.4) | 4.62 (3.01 to 7.08) | < 0.0001 |
| Interleukin-10, ≥ 5 pg/mL | 177 (44.1) | 41 (23.2) | 90 (50.8) | 46 (26.0) | 136 (76.8) | 4.67 (3.01 to 7.25) | < 0.0001 |
| Procalcitonin, ≥ 0.5 ng/mL | 57 (13.6) | 10 (17.5) | 29 (50.9) | 18 (31.6) | 47 (82.5) | 3.76 (1.84 to 7.68) | < 0.0001 |
| Imaging features | |||||||
| Bilateral | 381 (93.2) | 155 (40.7) | 162 (42.5) | 64 (16.8) | 226 (59.3) | 2.62 (1.18 to 5.84) | 0.018 |
| Ground-glass opacity | 306 (74.8) | 119 (38.9) | 131 (42.8) | 56 (18.3) | 187 (61.1) | 1.73 (1.10 to 2.72) | 0.017 |
| Consolidation | 89 (21.8) | 29 (32.6) | 36 (40.4) | 24 (27.0) | 60 (67.4) | 1.69 (1.03 to 2.78) | 0.037 |
| Pleural effusion | 47 (11.5) | 6 (12.8) | 25 (53.2) | 16 (34.0) | 41 (87.2) | 5.85 (2.42 to 14.13) | < 0.0001 |
Data are presented as medians (interquartile range) or n (%) according to the corresponding conditions.
ORs were calculated using univariate unconditional logistic regression.
All P values were calculated using univariate unconditional logistic regression for the characteristics between the patients with normal cumulative liver biochemistry and those with cumulative liver biochemical abnormality or injury, but two independent samples (unpaired) Student's t-test was used to compare the median ages.
Referring to exposure to confirmed coronavirus disease 2019 patients.
Comorbidities were defined as history of at least one disease out of the following: Hypertension, diabetes, cardiovascular disease, cerebrovascular disease, chronic renal disease, chronic respiratory disease, and chronic liver disease.
Four patients reported history of either fatty liver or cirrhosis. “-“: It is not applicable due to the no individuals with abnormal alanine aminotransferase or aspartate aminotransferase or total bilirubin in normal cumulative liver biochemistry group. COVID-19: Coronavirus disease 2019; LB: Liver biochemistry; LBA: Liver biochemical abnormality; ALI: Acute liver injury; LBAI: Liver biochemical abnormality or injury; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CURB-65: Confusion, uremia, respiratory rate, blood pressure, and age ≥ 65; IQR: Interquartile range; OR: Odds ratio; CI: Confidence interval; qSOFA: Quick Sequential Organ Failure Assessment; BMI: Body mass index.
Additional significant associated risk factors with LBAI included comorbidities, especially diabetes, vital signs on admission (including temperature, respiratory rate, pulse oximeter O2 saturation, and heart rate), and severity of illness (qSOFA and CURB-65 scores). Compared to general COVID-19 patients, LBAI occurred more often in severe and critical patients with a 2-fold (OR = 2.86; 95%CI: 1.78 to 4.59) and 13-fold (OR = 13.44; 95%CI: 7.21 to 25.07) higher likelihood, respectively.
Laboratory and imaging features associated with LBAI
The likelihood of cumulative LBAI among COVID-19 patients varied with the laboratory parameters and chest CT imaging features, as shown in Table 1. Patients with abnormal counts of blood cells at admission were more prone to later develop cumulative LBAI, especially decreased lymphocyte count (OR = 4.10; 95%CI: 2.74 to 6.12) and elevated neutrophil count (OR = 2.29; 95%CI: 1.50 to 3.49). Most biochemical indices at admission indicated the subsequent incidence of cumulative LBAI, such as elevated γ-glutamyl transferase (GGT), decreased albumin, and elevated C-reactive protein. Abnormal coagulation parameters were strongly associated with cumulative LBAI, especially the increase of D-dimer levels, which suggested a 5-fold increase in the likelihood of cumulative LBAI. Serum ferritin, an index of inflammation, also showed a positive association with the cumulative incidence of LBAI (OR = 6.91; 95%CI: 3.8 to 12.57). The presence of bilateral lesions, ground-glass shadows, consolidation, or pleural effusion on CT images all suggested an increased risk of cumulative LBAI (P < 0.05).
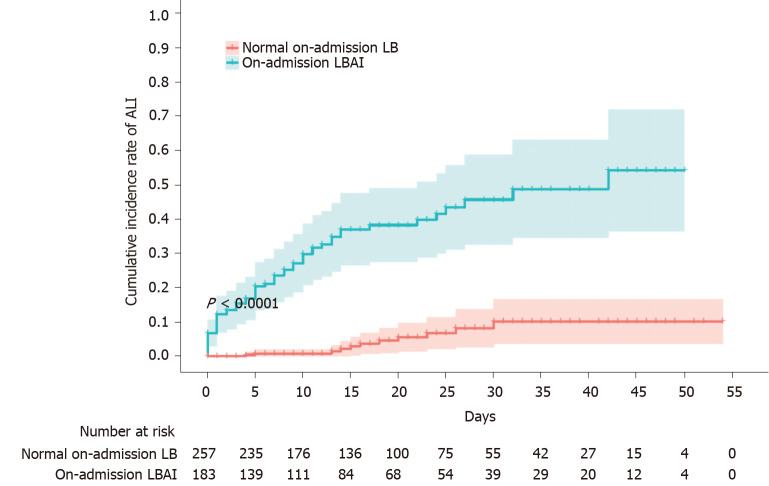
On-admission LBAI is associated with cumulative ALI
Liver function on admission predicted the subsequent risk of cumulative occurrence of ALI among COVID-19 patients to a great extent, as shown in Figure 1. The cumulative incidence of ALI over 2 mo was 41.9% (95%CI: 31.4 to 51.9) in patients with on-admission LBAI, dramatically higher than the 9.6% (95%CI: 4.7 to 16.5) in patients with on-admission normal LB (P < 0.0001); the risk was 8-fold higher in the LBAI group than in the normal LB group [hazard ratio (HR) = 8.07; 95%CI: 4.23 to 5.37]. We also observed similar patterns albeit with a moderately reduced effect (HR = 4.83; 95%CI: 2.45 to 9.54) when on-admission LBA associated with the subsequent risk of in-hospital incidence of ALI was analyzed (Supplementary Figure 1).
Figure 1.
Cumulative incidence rate of acute liver injury stratified by on-admission liver biochemistry. Shadows indicate the 95% confidence intervals of the corresponding estimates cumulative incidence rate. ALI: Acute liver injury; LB: Liver biochemistry; LBAI: Liver biochemical abnormality or injury.
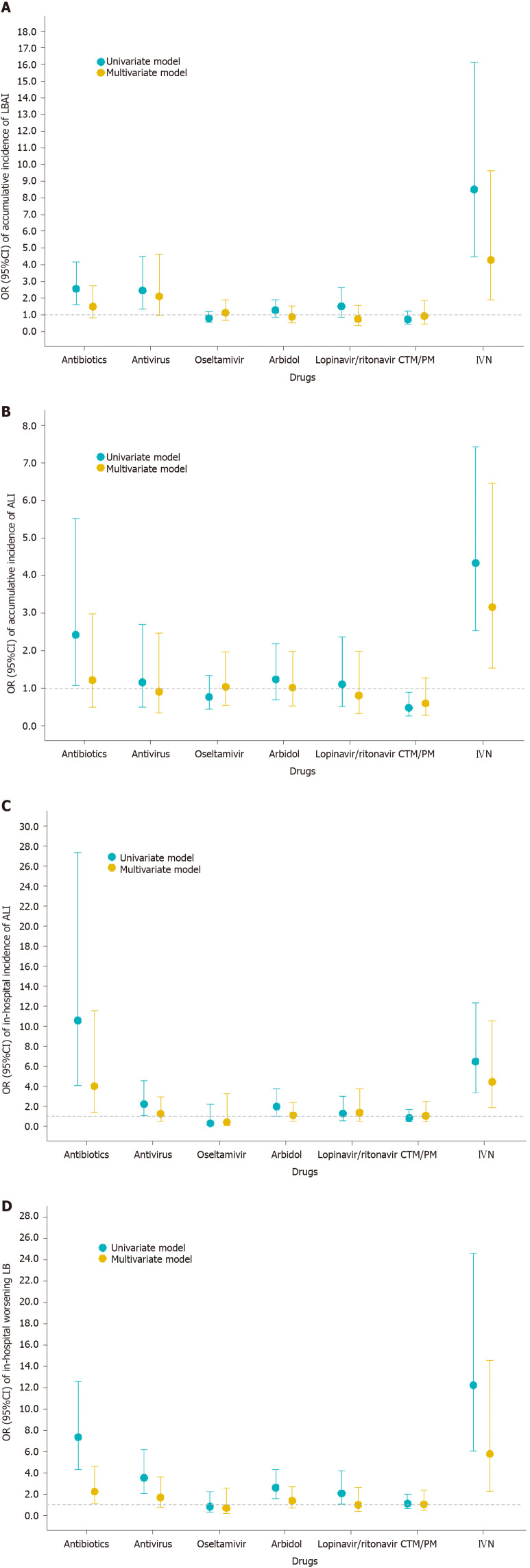
Drugs and treatment associated with LBAI
To address the issue of whether liver dysfunction was caused by drugs commonly used to treat COVID-19, such as antibiotics, antivirus, traditional Chinese medicine/Chinese patent drug, and intravenous nutrition (IVN), we analyzed the association of drugs with subsequent incidence of ALI or LBAI or worsening LB (Figure 2 and Supplementary Figure 2). The results showed that patients who received IVN either on admission or in hospital were associated with cumulative LBAI or ALI; a similar association was found between in-hospital use of antibiotics and subsequent ALI or worsening LB.
Figure 2.
Association between drugs and liver injury. A: Cumulative incidence of liver biochemical abnormality or injury and drugs used during the whole course; B: Cumulative incidence of acute liver injury and drugs used during the whole course; C: In-hospital incidence of acute liver injury and drugs used after admission; D: In-hospital worsening liver biochemistry and drugs used after admission. Univariate model refers to univariate binary logistic regression model. Multivariate model refers to multivariate binary logistic regression model adjusted by age, sex, comorbidities (defined as history of at least one disease out of hypertension, diabetes, cardiovascular disease, cerebrovascular disease, chronic renal disease, chronic respiratory disease, and chronic liver disease), in-hospital disease severity status, lymphocyte count, D-dimer, and serum ferritin. Antivirals included oseltamivir, arbidol, lopinavir/ritonavir, and some other uncommonly used antiviral drugs. OR: Odds ratio; CI: Confidence interval; ALI: Acute liver injury; LB: Liver biochemistry; LBAI: Liver biochemical abnormality or injury.
Treatment with oxygen therapy by mask, high-flow nasal cannula, or mechanical ventilation were used approximately 6-fold more in patients with cumulative LBAI than those with normal cumulative LB, indicating that hypoxia was an important risk factor for LBAI (Supplementary Table 1).
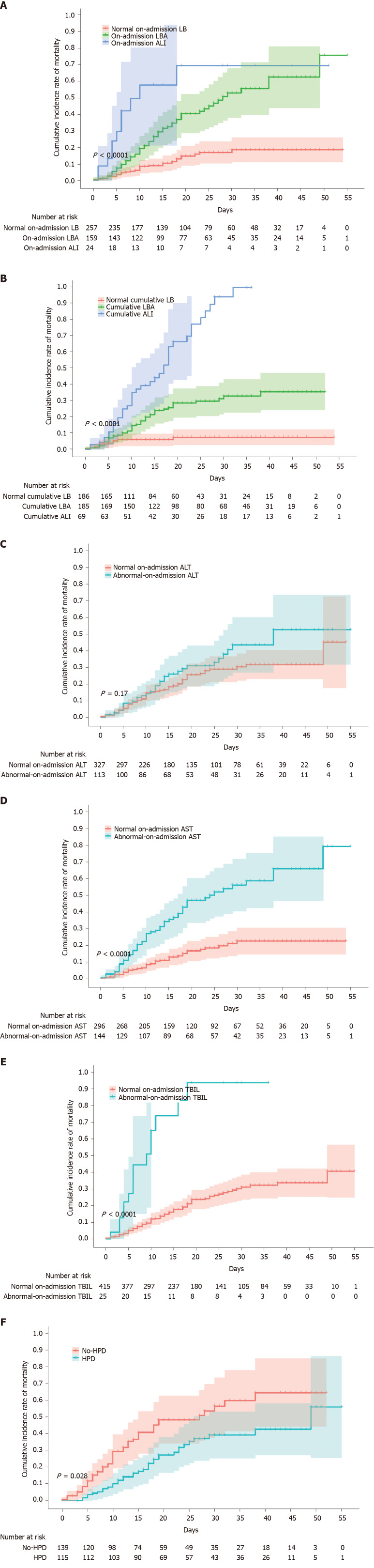
On-admission and cumulative LBAI is associated with increased risk of in-hospital mortality
In this cohort predominated by severe or critical COVID patients, a mortality of 22.3% (98/440) was observed from admission to thereafter 2 mo with a substantial difference in mortality between patients with cumulative LBAI and normal cumulative LB (34.3% vs 5.9%, P < 0.0001) (Table 2). With the worsening status of liver function on-admission, the mortality risk significantly increased from double in on-admission LBA (adjusted HR = 1.78; 95%CI: 1.03 to 3.06) to up to four times the risk in on-admission ALI (adjusted HR = 4.00; 95%CI: 1.68 to 9.50; Figure 3A and Supplementary Table 2). An even stronger association was noticed when cumulative liver injury instead of on-admission liver injury was used (Figure 3B).
Table 2.
Outcomes for 440 patients of coronavirus disease 2019, stratified by severity of cumulative liver abnormality or injury
|
|
All patients (n = 440)
|
Normal cumulative LB (n = 186)
|
Cumulative LBA (n = 185)
|
Cumulative ALI (n = 69)
|
Cumulative LBAI (n = 254)
|
P
value1
|
| Outcomes | ||||||
| Discharge | 304 (69.1) | 166 (54.6) | 117 (38.5) | 21 (6.9) | 138 (45.4) | < 0.00012 |
| Death | 98 (22.3) | 11 (11.2) | 45 (45.9) | 42 (42.9) | 87 (88.8) | |
| Still in hospital | 38 (8.6) | 9 (23.7) | 23 (60.5) | 6 (15.8) | 29 (76.3) | |
| ICU admission | 91 (20.7) | 11 (12.1) | 45 (49.5) | 35 (38.5) | 80 (88.0) | < 0.0001 |
| Time from illness onset to hospital admission (median, IQR), d | 438 | 19 (11 to 35) | 13 (9 to 19) | 11 (8 to 16) | 12 (9 to 18) | < 0.0001 |
| Hospital stay time (median, IQR), d | 402 | 12 (6 to 22) | 19 (11 to 29) | 17 (8 to 28) | 19 (10 to 29) | < 0.0001 |
| Time from illness onset to death (median, IQR), d | 93 | 19 (9 to 26) | 23 (16 to 33) | 28 (18 to 33) | 25 (17 to 33) | 0.037 |
| Time from illness onset to discharge (median, IQR), d | 303 | 40 (32 to 49) | 40 (34 to 51) | 42 (31 to 51) | 40 (34 to 50) | 0.088 |
| Time from illness onset to last day of follow-up (median, IQR), d | 37 | 61 (52 to 66) | 59 (49 to 64) | 58 (52 to 67) | 59 (51 to 65) | 0.689 |
| Time from illness onset to ICU admission (median, IQR), d | 90 | 15 (13 to 25) | 18 (11 to 30) | 19 (14 to 26) | 18 (12 to 26) | 0.478 |
| Duration of viral shedding after illness onset (median, IQR), d | 298 | 33 (24 to 42) | 30 (25 to 38) | 26 (21 to 36) | 30 (24 to 38) | 0.172 |
Data are presented as n (%).
Comparison of the characteristics between normal cumulative liver biochemistry and cumulative liver biochemical abnormality or injury was calculated by Pearson's chi-square test for outcomes and a nonparametric Mann-Whitney U test was used for time.
Comparison of the distribution of disease outcomes (discharge, death, and still in hospital) between normal cumulative liver biochemistry and cumulative liver biochemical abnormality or injury. COVID-19: Coronavirus disease 2019; LB: Liver biochemistry; LBA: Liver biochemical abnormality; LBAI: Liver biochemical abnormality or injury; ALI: Acute liver injury; ICU: Intensive care unit; IQR: Interquartile.
Figure 3.
Cumulative incidence of in-hospital mortality of patients with coronavirus disease 2019, stratified by liver disease indicators or hepatoprotective drugs. A-F: Shadows indicate the 95% confidence intervals of the corresponding estimates: Stages of on-admission liver injury (A), stages of cumulative liver injury (B), on-admission alanine aminotransferase (C), on-admission aspartate aminotransferase (D), on-admission total bilirubin (E), and hepatoprotective drug uses in patients with abnormal liver function (F). LB: Liver biochemistry; LBA: Liver biochemical abnormality; ALI: Acute liver injury; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TBIL: Total bilirubin; HPD: Hepatoprotective drugs.
In-depth analysis into single markers indicating liver function revealed that the levels of AST and TBIL but not ALT at admission or at peak were strong risk indicators of mortality (Figure 3C-E, Supplementary Figure 3, and Supplementary Table 2). To clarify the potential impact of existing chronic liver disease on the prognosis, such as hepatitis, fatty liver, or cirrhosis, we conducted a sensitivity analysis by removing 12 cases with chronic liver diseases, and found similar results (Supplementary Table 2). Meanwhile, to clarify the effects of 38 censored patients on the survival analysis, we also conducted another sensitivity analysis and confirmed similar conclusions (Supplementary Table 2).
In addition, shorter intervals between illness onset and hospital admission (12 d vs 19 d, P < 0.0001), longer hospital stays (19 d vs 12 d, P < 0.0010), and prolonged time from illness onset to death (25 d vs 19 d, P = 0.037) were observed in the patients with cumulative LBAI compared to the patients with normal cumulative LB (Table 2).
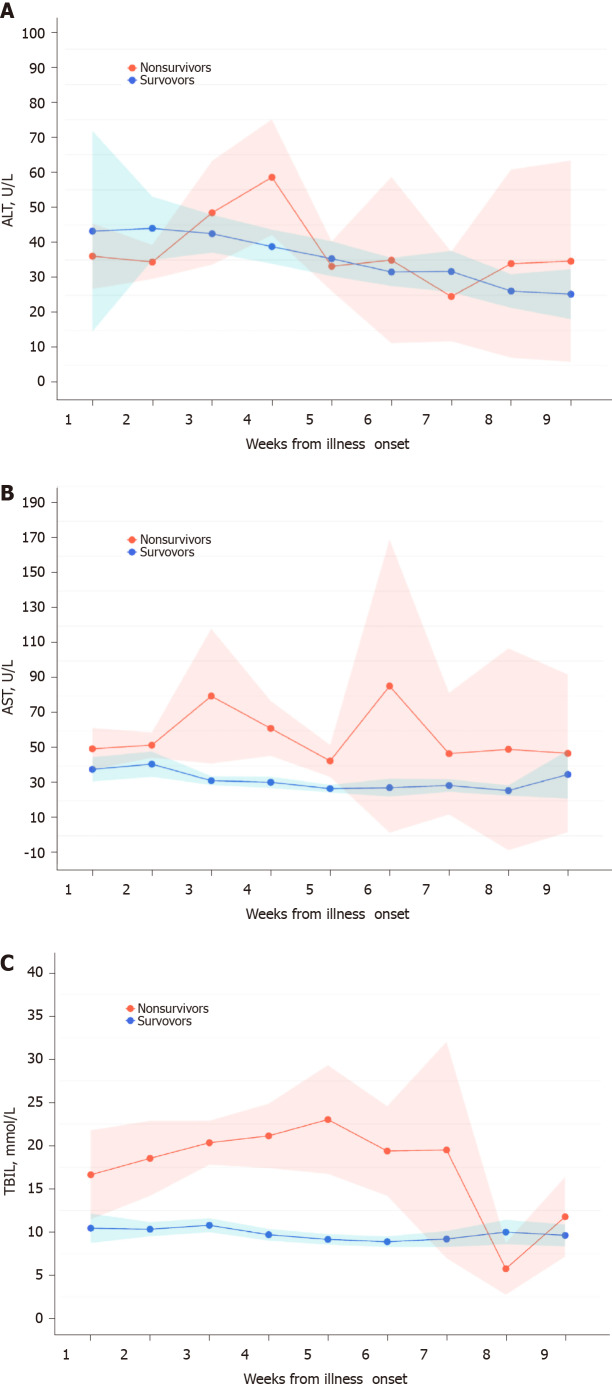
Dynamic variations of liver function monitoring of the risk of in-hospital mortality
The temporal patterns of LB parameters over time from illness onset differed by different survival outcomes of COVID-19 patients (Figure 4). In general, the levels of ALT, AST, and TBIL varied in a wider range over time in the non-survivors than in the survivors. Although no pronounced elevation of ALT levels was observed especially at the time of admission, there was a smooth decrease over time in the survivors compared to a sharp elevation at the fourth week in the non-survivors. AST and TBIL levels at the time of admission tended to be higher in the non-survivors and showed a sharp elevation at the fourth and sixth weeks or continuous elevation until the fifth week, respectively, in contrast with almost no variation over time in survivors (Figure 4). The peak of ALT, AST, and TBIL happened at the fourth, sixth, and fifth weeks from illness onset, respectively. Additional LB markers such as GGT, direct bilirubin, and albumin also presented different time-varying tendency in the non-survivors from that of survivors (Supplementary Figure 4).
Figure 4.
Dynamic variations of liver biochemistry from illness onset. A-C: Shadows indicate the 95% confidence intervals of the corresponding estimates: Alanine aminotransferase (A), aspartate aminotransferase (B), and total bilirubin (C). TBIL: Total bilirubin.
Hepatoprotective drugs associated with a reduced risk of in-hospital mortality
The use of hepatoprotective drugs in the patients with cumulative LBAI tended to attenuate the risk of in-hospital mortality by 38% in the univariate model (crude HR = 0.62; 95%CI: 0.41 to 0.96; Figure 3F and Supplementary Table 2) compared to their normal LB counterparts, although this association was not significant after adjustment for confounding risk factors such as age, sex, comorbidities, disease severity, lymphocyte count, D-dimer, and serum ferritin (adjusted HR = 0.67; 95%CI: 0.4 to 1.08).
DISCUSSION
In the present retrospective cohort study, we observed a high LBAI prevalence of up to 57.7% and a high mortality of 22.3% in hospitalized patients with relatively severe COVID-19. Some key demographics, clinical features, and lab parameters, e.g., age ≥ 65 years, female gender, comorbidities, the severity of disease, depleted lymphocyte count, abnormal D-dimer, and elevated serum ferritin levels, were found to be significantly associated with liver injury. On-admission liver function is a reliable predictor of subsequent liver injury. Patients with liver injury had up to four times the risk of mortality. AST and TBIL were identified to be factors that were most strongly related to prognosis and therefore could aid in prognosis monitoring. The use of antibiotics and IVN were associated with liver injury and therefore should be used with caution among COIVD-19 patients. Hepatoprotective drugs tended to favor survival, and their uses deserve recommendations for patients with abnormal liver function.
In some recent studies, elevated levels of ALT and AST were reported in patients with COVID-19, with rates ranging from 14% to 53%[4-7]. The prevalence of liver injury in the present study was higher, partly due to the high proportion of severe and critical COVID-19. Previous studies found that liver injury was common in critically ill patients with COVID-19[4,6]. Several indices of liver function, including ALT, AST, and TBIL, were significantly elevated in severe COVID-19 cases compared with mild cases[1]. These results were coincident with ours. Besides this, we found some key demographics, e.g., age 65 years or above, female gender, and comorbidities, were significant indicators of liver injury. These demographic characteristics and comorbidities are wind vanes for liver injury and are cue signals which are helpful for intensive care and individualized tailored surveillance.
Multiple explanations for the mechanism of liver injury have been proposed to date[13]. One hypothesis is that COVID-19 itself is a likely cause of liver injury. Liver biopsy specimens of patients who died from COVID-19 show degeneration of hepatocytes and focal necrosis[8]. Abundant SARS-CoV-2 viral particles were observed in hepatocytes[14]. Angiotensin-converting enzyme 2 (ACE2) receptor is the cell entry receptor of SARS-CoV-2, and according to a recent study using single-cell RNA sequencing, ACE2 was highly expressed not only in type II alveolar epithelial cells, but also in bile duct cells[15]. Hence, it is hypothesized that SARS-CoV-2 may infect the liver and cause abnormal liver function in these patients[16]. Our findings that the elevated GGT, a cholangiocyte-related enzyme, was strongly associated the increased risk of liver injury may provide supportive evidence for the hypothesis.
Other causes of ALI may be systemic inflammatory response, hypoxemia, and drug usage[17]. During the course of COVID-19, immune cells can release a group of inflammatory cytokines[18], which cause systemic inflammatory response syndrome (SIRS) and acute respiratory distress syndrome (ARDS). SIRS and ARDS can lead to more cell damage and necrosis. This vicious cycle can lead not only to lung damage but also to liver damage[19]. Our study observed that depleted lymphocytes, elevated procalcitonin, elevated interleukin-6, and elevated serum ferritin levels are significant indicators of liver injury, and these markers should be monitored. Many COVID-19 patients who experience different degrees of hypoxemia need to receive oxygen therapy. In theory, hypoxemia in severe COVID-19 pneumonia may lead to hypoxia of liver tissue and abnormalities of liver function[5].
Many COVID-19 patients, especially those who are severe and critically severe, are treated with multiple drugs. Liver injury emerging during the course of disease could be the side effect of drugs or drug interactions. In the present study, we explored the effect of drugs on liver function, and found two commonly used drugs, IVN and antibiotics, related with increased risk of LBAI. This indicated that IVN and antibiotics should be given with great caution in patients with COVID-19.
The patients included in the present study were severely ill and the overall mortality was high. The studied wards were rebuilt during the epidemic for severe or critical patients, although in the later part of the study period, we received some general patients. In addition, patients were sometimes transferred to the studied wards late in their illness, contributing to the high rate of poor clinical outcomes. Protecting high-risk individuals to reduce the mortality of COVID-19 patients is a fundamental challenge in managing the pandemic. In addition to old age and underlying comorbidities as the well-established predictors of high risk[20], we found that patients with liver abnormalities were at high risk. In particular, acute liver injury was an independent risk factor with a mortality four times higher than normal liver function. Moreover, progressive liver injury, indicated by liver function markers which were continuously abnormal over the course of disease, strongly predicted mortality in COVID-19. Our findings were supported by a recent study in which AST abnormality was found to be strongly associated with risk of mortality[21]. However, the design of our study, which included more complete baseline clinical characteristics to effectively adjust for bias, leads to more extensive findings than that of previous studies. For instance, TBIL abnormality, not a significant indicator in the earlier study at admission or peak, sensitively indicated subsequent mortality risk in our study; liver injury panel results rather than single indices such as AST were more informative for evaluating both the severity of liver injury and the mortality risk.
To manage the liver injury and thereafter reduce the mortality risk, several tips might be of great assistance. First, the underlying liver diseases should be treated properly. Second, the therapeutic regimen, e.g., abovementioned antibiotics, with respect to the varieties, doses, and duration should be used cautiously and moderately for reducing the risk of drug-induced liver injury. Third, all COVID-19 patients, particularly at severe or critical stage, were advised to receive meticulous monitoring of liver associated biochemical indicators, such as AST and TBIL, so as to catch liver injury in time. Fourth, for COVID-19 patients, any prophylactic use of drugs to protect the liver is not mandatory. But for patients with LBAI, as the evidence of this present study, the commonly used hepatoprotective drugs could improve survival to some degree.
Our study, which is among the first studies of survival analysis associated with liver injury in COVID-19, provided valuable information on the effective prevention, intensive monitoring, and individualized treatment for COVID-19 patients. However, there are several limitations to be addressed. First, any retrospective single-center study could have selection biases in identifying and recruiting participants. Second, the population size was relatively small. Third, we found that comorbidities were related to liver injury, but a small fraction of patients had various degrees of consciousness disorders at admission which made it difficult to collect detailed medical history. Fourth, we did not have enough medical resource to assess clinical-relevant injury such as ascites and encephalopathy.
CONCLUSION
In conclusion, liver injury is highly prevalent among COVID-19 patients and also can be fatal, particularly in severe or critical patients. More intensive monitoring of AST or TBIL and individualized tailored therapeutics are highly recommended for COVID-19 patients with liver injury, especially in patients aged ≥ 65 years, female patients, or those with other comorbidities. Drug hepatotoxicity should be alert for the use of antibiotics and IVN for COVID-19 patients. When severe liver damage occurs, liver protective drugs are favorable for improved prognosis. Further research should focus on the mechanism of liver injury in COVID-19 and more effective measures to prevent progressive liver injury and mortality risks of COVID-19.
ARTICLE HIGHLIGHTS
Research background
The prevalence of liver injury in hospitalized patients with coronavirus disease 2019 (COVID-19) is high.
Research motivation
Comprehensive and detailed studies on the clinical features, risk factors, and prognosis of patients with liver injury are still lacking, and it is urgent to clarify predictive factors and therapeutic approaches for liver injury.
Research objectives
We aimed to conduct an in-depth investigation into the risk factors of liver injury and into the effective measures to prevent subsequent mortality risk.
Research methods
A retrospective cohort study was performed on 440 consecutive patients with relatively severe COVID-19 between January 28 and March 9, 2020 at Tongji Hospital, Wuhan, China. Data on clinical features, laboratory parameters, medications, and prognosis were collected.
Research results
High-risk population, especially patients aged ≥ 65 years, female patients, or those with other comorbidities should be intensively monitored. On-admission total bilirubin has the strongest correlation with the prognosis of COVID-19 patients, which can be used for monitoring of COVID-19 patients at risk of liver injury. Intravenous nutrition (IVN) and antibiotics are associated with abnormal liver biochemistry; these drugs should be given with caution. Hepatoprotective drugs are favorable for patients with liver injury.
Research conclusions
More intensive monitoring of aspartate aminotransferase or total bilirubin is recommended for COVID-19 patients, especially patients aged ≥ 65 years, female patients, or those with other comorbidities. Drug hepatotoxicity of antibiotics and IVN should be alert for COVID-19 patients.
Research perspectives
IVN and antibiotics are associated with abnormal liver biochemistry; these drugs should be given with caution. Hepatoprotective drugs are favorable for patients with liver injury.
ACKNOWLEDGEMENTS
We acknowledge the clinicians and staff of Aiding Teams for Hubei Province of Peking Union Medical College Hospital, Peking University First Hospital, Peking University People’s Hospital, Peking University Third Hospital, Henan, and Jilin. Tongji Hospital, Huazhong University of Science and Technology offered much help. We thank Peking University Third Hospital medical aid team, especially the sixth group, for spiritual support. We are also grateful to the doctors, nurses, and many other front-line workers for their dedication to the fight against COVID-19 at the risk of their lives. They were not compensated for their contributions.
Footnotes
Institutional review board statement: The study was approved by the Ethics Committee of Peking University Third Hospital (IRB00006761-M2020060).
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment. Due to the particularity of the COVID-19 epidemic, all the data of patients could not be taken out and were sealed in place.
Conflict-of-interest statement: There are no conflicts of interest to report.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Peer-review started: November 21, 2020
First decision: December 27, 2020
Article in press: February 4, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen VL, Sipahi A S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Ma YJ
Contributor Information
Shui-Sheng Zhang, Department of General Surgery, Peking University Third Hospital, Beijing 100191, China.
Li Dong, Institutes of Biomedical Sciences, Shanxi University, Taiyuan 030006, Shanxi Province, China.
Gao-Ming Wang, Department of General Surgery, Peking University Third Hospital, Beijing 100191, China.
Yuan Tian, Department of Radiotherapy Oncology, Shandong Provincial Qianfoshan Hospital, The First Hospital Affiliated with Shandong First Medical University, Jinan 250014, Shandong Province, China.
Xiao-Fang Ye, Department of Respiratory and Critical Medicine, Beijing Anzhen Hospital, Capital Medical University, Beijing 100029, China.
Yue Zhao, Department of General Surgery, Peking University Third Hospital, Beijing 100191, China.
Zheng-Yin Liu, Department of Infectious Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing 100005, China.
Jia-Yu Zhai, Department of Rheumatology, Peking University Third Hospital, Beijing 100191, China.
Zhi-Ling Zhao, Department of Critical Care Medicine, Peking University Third Hospital, Beijing 100191, China.
Jun-Hong Wang, Department of Emergency Medicine, Peking University Third Hospital, Beijing 100191, China.
Hui-Min Zhang, Department of Cardiac Surgery, Peking University Third Hospital, Beijing 100191, China.
Xiao-Long Li, Department of Urology, Peking University Third Hospital, Beijing 100191, China.
Chang-Xin Wu, Institutes of Biomedical Sciences, Shanxi University, Taiyuan 030006, Shanxi Province, China.
Cai-Ting Yang, Institutes of Biomedical Sciences, Shanxi University, Taiyuan 030006, Shanxi Province, China.
Li-Juan Yang, Department of Gynecology, Peking University Third Hospital, Beijing 100191, China.
Hai-Xia Du, Department of Rehabilitation, Peking University Third Hospital, Beijing 100191, China.
Hui Wang, Department of Gastroenterology, Peking University Third Hospital, Beijing 100191, China.
Qing-Gang Ge, Department of Critical Care Medicine, Peking University Third Hospital, Beijing 100191, China.
Dian-Rong Xiu, Department of General Surgery, Peking University Third Hospital, Beijing 100191, China. xiudianrong@yeah.net.
Ning Shen, Department of Respiratory and Critical Medicine, Peking University Third Hospital, Beijing 100191, China.
Data sharing statement
No additional data are available.
References
- 1.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 2.Humar A, McGilvray I, Phillips MJ, Levy GA. Severe acute respiratory syndrome and the liver. Hepatology. 2004;39:291–294. doi: 10.1002/hep.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan HL, Leung WK, To KF, Chan PK, Lee N, Wu A, Tam JS, Sung JJ. Retrospective analysis of liver function derangement in severe acute respiratory syndrome. Am J Med. 2004;116:566–567. doi: 10.1016/j.amjmed.2003.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murillo-Zamora E, Medina-González A, Zamora-Pérez L, Vázquez-Yáñez A, Guzmán-Esquivel J, Trujillo-Hernández B. Performance of the PSI and CURB-65 scoring systems in predicting 30-day mortality in healthcare-associated pneumonia. Med Clin (Barc) 2018;150:99–103. doi: 10.1016/j.medcli.2017.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, Pilcher DV Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resource Evaluation (CORE) Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults With Suspected Infection Admitted to the Intensive Care Unit. JAMA. 2017;317:290–300. doi: 10.1001/jama.2016.20328. [DOI] [PubMed] [Google Scholar]
- 11.National Health Commission of the People's Republic of China. Chinese management guideline for COVID-19 (trial version 7.0). [cited Mar 3, 2020]. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf .
- 12.China Digestion Association; Chinese Medical Doctor Association. The protocal for prevention, diagnosis and treatment of liver injury in coronavirus disease 2019. Zhonghua Ganzang Bing Zazhi. 2020;28:E004–E004. doi: 10.3760/cma.j.cn501113-20200309-00095. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Fan J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. 2020 Preprint. Available from: bioRxiv.
- 16.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, Wu F, Song ZG, Huang W, Chen J, Hu BJ, Wang S, Mao EQ, Zhu L, Zhang WH, Lu HZ. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright DJM. Prevention of the cytokine storm in COVID-19. Lancet Infect Dis. 2021;21:25–26. doi: 10.1016/S1473-3099(20)30376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu LL, Wang WJ, Zhu QJ, Yang L. [Novel coronavirus pneumonia-related liver injury: etiological analysis and treatment strategy] Zhonghua Gan Zang Bing Za Zhi. 2020;28:97–99. doi: 10.3760/cma.j.issn.1007-3418.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Zhou J, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu L, Chen G, Li H, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.