Abstract
Patients with rheumatic diseases are often more susceptible to different bacteria and viruses because of immune impairment, but it is not clear whether there is a higher risk of infection and a more serious course of disease for novel coronavirus (SARS-CoV-2). We performed this systematic review and meta analysis to assess the risk and clinical outcomes of COVID-19 in patients with rheumatic diseases compared with the general population. We searched PubMed, EMBASE, Scopus and Web of Science databases from January 1, 2020 to October 20, 2020 to determine epidemiological information related to patients with rheumatic diseases and COVID-19, including clear risk estimate or data that could be converted and extracted. We included 26 observational studies, totaling about 2000 patients with rheumatic diseases of whom were infected with COVID-19. Meta-analysis showed that the risk of COVID-19 infection in rheumatic patients was significantly higher than that in the general population (OR = 1.53, 95% CI 1.24–1.88, P = 0.000). In terms of hospitalization and severe clinical outcomes associated with COVID-19, we found that rheumatic patients showed similar results to the reference population (hospitalization OR = 1.36, 95% CI 0.81–2.29, P = 0.247; admitted to ICU OR = 1.94, 95% CI 0.88–4.27, P = 0.098; death OR = 1.29, 95% CI 0.84–1.97, P = 0.248). The presence of comorbidities, hypertension, lung diseases were significantly associated with the increased risk of COVID-19-related hospitalization in rheumatic patients and anti-TNF drugs were associated with lower hospitalization risk. Older age was related to severe COVID-19. Our meta-analysis indicated that rheumatic patients were at a higher risk of COVID-19 infection but might not lead to a more serious disease process.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00296-021-04803-9.
Keywords: Rheumatic diseases, COVID-19, Risk, Systematic review, Meta analysis
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by highly transmitted novel coronavirus (SARS-CoV-2), has brought great medical challenges and caused tremendous deaths and socioeconomic losses [1]. Recently, a second wave of COVID-19 outbreaks has occurred in some European countries due to imported cases and deregulation [2]. Patients with rheumatic diseases (RD) may have a higher risk of infection due to dysregulated and excessive innate immune responses and disease activity [3–5]. However, it is not clear whether this population has a higher risk of COVID-19 and a more serious disease progression. It is generally believed that RD patients’ risk of COVID-19 and their disease progression are similar to those in the general population [6–8]; however, there is very limited data on this topic, and some results are contradictory due to differences in region and sample size. In addition, individual risk factors that may lead to more severe COVID-19 disease progression have aroused widespread concern among patients and rheumatologists. According to recent data, advanced age, male sex, and comorbidities in hospitalized patients were significant risk factors for severe COVID-19 in the general population. The presence of inflammatory markers was most strongly associated with poor clinical prognosis [9–11]. The purpose of this systematic review and meta-analysis is to integrate data on the epidemiology of COVID-19 in the RD population and to analyze the risk of COVID-19 infection and its complications in RD patients compared with the general population. We also analyzed the impact of individual risk factors associated with severe COVID-19, including age, sex, drug therapy, comorbidities, and different RD categories.
Methods
This meta-analysis was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [12] and has been registered on PROSPERO (CRD: 42020214220).
Search strategy
We conducted a comprehensive search of the PubMed, EMBASE, Web of Science and Scopus databases, including all relevant articles from January 1, 2020 to October 20, 2020 [13]. To supplement any omissions in our online searches, we manually searched the reference lists of related articles, including reviews, guidelines, and statements. Search was limited to articles published in English. The following terms were searched in comprehensive free text. For PubMed: (“Rheumatic Diseases” OR “rheumatic disease” OR “rheumatism” OR “osteoarthritis” OR “arthritis” OR “lupus” OR “systemic sclerosis” OR “vasculitis” OR “ankylosing spondylitis” OR “gout”) AND (“COVID-19” OR “COVID19” OR “coronavirus disease 2019” OR “coronavirus disease-19” OR “2019-nCoV” OR “2019nCoV” OR “2019 novel coronavirus” OR “novel coronavirus pneumonia” OR “SARS-CoV-2” OR “SARSCoV-2” OR “severe acute respiratory syndrome coronavirus 2” OR “corona virus” OR “new coronavirus”). See Supplemental Appendix S1–S4.
Study selection
We adopted the following inclusion criteria: original research describing COVID-19 infection risk in RD patients compared with reference population and research on hospitalization and severe COVID-19 outcomes. From original research, we extracted estimation of unadjusted effect (odds ratios, ORs) and 95% confidence interval (CI) or event number and sample size. Risk factor analysis extracted adjusted ORs and 95% CI from multivariable logistic regression model, and combined them only when the study contained at least two items. There were no restrictions based on gender, age, geographical distribution or study duration. We excluded the following: review articles, case reports, case, studies with sample sizes of less than 10, studies from which data could not be obtained, studies with inconsistent content or without reference population data, and any publications in languages other than English or for which we could not obtain the full text. For studies containing duplicate data from the same database, we chose the study with the largest sample size. When necessary, we contacted authors to obtain as much data as possible from missing reports. For each article, two researchers (QXW and RXS) independently filtered the title and abstract, identified the full text relevant to the study, and further reviewed the study to determine if it met the inclusion criteria. Disagreements were resolved through discussion or negotiation with a third reviewer (XPH).
Data extraction and quality assessment
The data were pre-extracted by two authors (QXW and RXS), and then the calibrated data extraction table was used to extract the data independently. The following data were collected: author, year of publication, country, type of study, sample size, patient gender and age, diagnosis of disease, anti-rheumatic treatment drugs, number of patients diagnosed or suspected COVID-19 and outcome indicators.
The Newcastle–Ottawa Scale (NOS) was applied for assessing the quality of cohort and case–control studies [14]. Using the tool, each study was awarded stars based on 8 items, 1–3 stars were of low quality, 4–6 stars were of moderate quality, and 7 or more stars were classified as high quality [15]. For cross-sectional studys, the Agency for Healthcare Research and Quality (AHRQ) (https://www.ncbi.nlm.nih.gov/books/NBK35156/) was applied. Each item was worth 1 point: 0–3 = low quality; 4–7 = moderate quality; 8–11 = high quality [16]. The JBI key assessment tool was used to evaluate case series studies, which included 10 questions relating to the internal validity and risk of bias [17]. Each question could be answered with “yes”, “no”, “unclear” or “not applicable”, and the answers to “yes” > 7 were regarded as high quality (low risk of bias).
Outcome assessment
The primary outcome of the study was to determine the risk of diagnosed or suspected COVID-19 in RD patients with SARS-COV-2 exposure, relative to the reference population. The “reference population” refers to the non-rheumatic group matched with the rheumatic group according to clinical characteristics, or the general population in the same community. Confirmed patients were positive for SARS-CoV-2 as measured by real-time PCR. Suspected patients met the following conditions: (1) close contact history; (2) symptoms related to COVID-19; (3) with or without typical imaging features of COVID-19; (4) PCR test was negative or not tested. The secondary results were: (1) hospitalization, (2) admission to intensive care unit (ICU), (3) death and (4) analysis of individual risk factors associated with hospitalization and severe COVID-19. “Severe COVID-19” was defined as ICU admission/ mechanical ventilation and death related to COVID-19 infection.
To perform subgroup analyses for the diagnosis of different diseases, we classified RD as follows: (a) inflammatory rheumatic disease included rheumatoid arthritis (RA), psoriatic arthritis (PsA), spondylo arthritis (SpA), juvenile idiopathic arthritis and autoinflammatory syndrome; (b) non-inflammatory rheumatic disease included osteoarthritis, osteoporosis and fibromyalgia. We divided the routine drug use of RD patients prior to COVID-19 diagnosis into the following four categories: (a) conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs); (b) targeted synthetic/biologic DMARDs (ts/bDMARDs); (c) glucocorticoids (GCs); (d) non-steroidal anti-inflammatory drugs (NSAIDs). csDMARDs included methotrexate, cyclophosphamide, azathioprine, cyclosporine, leflunomide, mycophenolate mophetilo, antimalarials (chloroquine and hydroxychloroquine), colchicine, sulfasalazine; ts/bDMARDs included anti-TNF drugs (infliximab, etanercept, adalimumab, golimumab and certolizumab) and non-anti-TNF drugs (anti-IL6, anti- IL-17, anti-IL-17/23, rituximab, belimumab, abatacept, Janus kinase inhibitors).
Statistical analysis
We used Stata software (version15.1, Statacorp, College Station, TX, USA) for statistical analysis of the data. Due to the expected heterogeneity of the included studies, we used a random effects model to pool the data related to infection risk and clinical outcomes of COVID-19 in RD patients. Cochran’s Q test (P value < 0.1 indicates significant) and I2 statistics were used to evaluate the heterogeneity between studies, and I2 value of > 50% indicates significant heterogeneity [18, 19]. Subgroup analyses were performed based on disease diagnosis, geographical distribution and research design, all of which were decided a priori, and subgroups could be further grouped if necessary. When 5 or more studies were included, we used the funnel chart to evaluate potential publication bias. When more than 10 studies were included, we used Begg’s test and Egger’s test to determine the funnel chart asymmetry. Sensitivity analysis detected the stability of the results by removing each study [20]. All statistical tests used a bilateral P value of 0.05 for significance.
Results
Literature search and research features
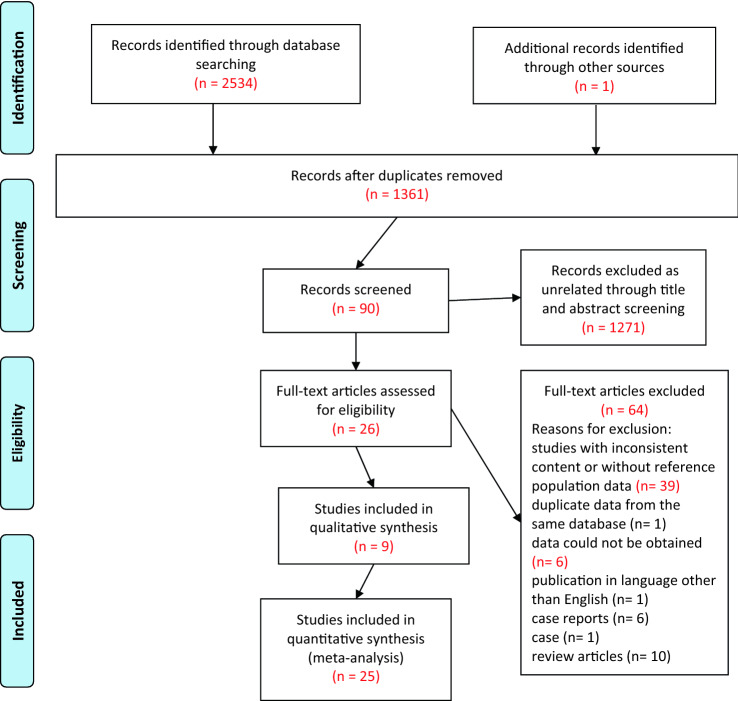
The initial search identified 2534 records, and the manual search identified another one [21]. 1361 articles were obtained once duplicate records were removed. After screening titles and abstracts, 1271 articles were excluded and 90 were further assessed for eligibility. Ultimately, 26 articles met all eligibility criteria (Fig. 1).
Fig. 1.
PRISMA flow diagram of literature search and screening
A total of 26 observational studies were included [21–46], reporting on a total of 101,124 RD patients, including about 2000 of whom were infected with COVID-19. The studies were conducted in China (2), the United States (3), Italy (10), Spain (9), Germany (1), and also included a study with data of the first 600 patients presented to the COVID-19 Global Rheumatology Alliance (C19-GRA) registry [22]. The systematic review and meta-analysis included 4 cohort studies, 1 case–control study, 14 cross-sectional studies and 7 case series studies, of which 5 were of high quality, 11 were of medium quality, and 10 were of low quality. A detailed description of the included studies is presented in Table 1 (see Supplemental Table S1–S3 for quality assessment).
Table 1.
Characteristics of the included studies
| Authors, year | Country | Study type | Sample size | Age (years) Mean ± SD e |
Male (%)e | Disease diagnosis | Anti-rheumatic drugs | Confirmed/suspected COVID-19 casese | Quality grading |
|---|---|---|---|---|---|---|---|---|---|
| Michelena et al., 2020 [26] | Spain | Cross-sectional study | 959 | 45 (30–63)a | 337 (35.1) | IRD | ts/bDMARD | 11/NA | Moderate |
| Pablos et al., 2020 [38] | Spain | Matched cohort study | 228 | 63 (54–78)a | 87 (38.2) | RD | csDMARD + ts/bDMARD | 228/NA | Moderate |
| Nuño et al., 2020 [43] | Spain | Case series | 122 | 58.3 ± 16.3 | 42 (34.4) | RD | csDMARD + ts/bDMARD | 100/22 | Low |
| Benucci et al., 2020 [32] | Italy | Cross-sectional study | 295 | NR | NR | RD | bDMARD | 4/NA | Moderate |
| Haberman et al., 2020 [45] | USA | Case series | 103 | 52.7 (28–88)b | 29 (28.2) | IRD | csDMARD + ts/bDMARD | 80/23 | Low |
| Fredi et al., 2020 [40] | Italy | Matched case–control study | 26 | NR | NR | RD | csDMARD + bDMARD | 26/NA | Moderate |
| Pablos et al., 2020 [31] | Spain | Cross-sectional study | 26,131 | 65 (53–78)a | 11,497 (44.0) | RD | csDMARD + ts/bDMARD | 199/NA | Low |
| Damiani et al., 2020 [34] | Italy | Cross-sectional study | 1193 | 55 ± 12.7 | 811 (68.0) | PsO | bDMARD | 22/NA | Low |
| D'Silva et al., 2020 [39] | USA | Matched cohort study | 52 | 62.5 ± 15.1 | 16 (30.8) | RD | csDMARD + ts/bDMARD | 52/NA | Moderate |
| Scirè et al., 2020 [46] | Italy | Case series | 232 | 62.2 ± 13.9 | 83 (35.8) | RD | csDMARD + ts/bDMARD | 232/NA | High |
| Zen et al., 2020 [29] | Italy | Cross-sectional study | 916 | 53.6 ± 14.3 | 196 (21.4) | RD | csDMARD + bDMARD | 2/NA | Moderate |
| Freites Nuñez et al., 2020 [42] | Spain | Case series | 123 | 59.9 ± 14.9 | 37 (30.1) | RD | csDMARD + ts/bDMARD | 58/65 | Low |
| Quartuccio et al., 2020 [28] | Italy | Cross-sectional study | 1051 | 58.4 ± 14.6 | 348 (33.1) | RD | bDMARD | 4/NA | Moderate |
| Favalli et al., 2020 [25] | Italy | Cross-sectional study | 955 | 53.7 ± 14 | 311 (32.6) | RD | csDMARD + ts/bDMARD | 6/NA | High |
| Aries et al., 2020 [27] | Germany | Cross-sectional study | 11,771 | NR | NR | RD | csDMARD + ts/bDMARD | 30c | Low |
| Jovani et al., 2020 [37] | Spain | Cross-sectional study | 1037 | NR | NR | RD | csDMARD + ts/bDMARD | NR | Low |
| Gianfrancesco et al., 2020 [22] | C19-GRA | Case series | 600 | 56 (45–67)a | 177 (29.5) | RD | csDMARD + ts/bDMARD | 437/163 | Low |
| Montero et al., 2020 [41] | Spain | Case series | 62 | 60.9 ± 13.9 | 36 (58.1) | RD | csDMARD + bDMARD | 51/11 | High |
| Zhong et al., 2020 [24] | China | Retrospective cohort study | 43 | 49·2 ± 11·6 | 10 (23.3) | RD | csDMARD + bDMARD | 20/7 | High |
| Ferri et al., 2020 [23] | Italy | Prospective cohort study | 1641 | 60 ± 13 | 385 (23.5) | RD | csDMARD + ts/bDMARD | 11/NA | Moderate |
| Fernandez-Ruiz et al., 2020 [44] | USA | Case series | 226 | NR | 16 (7.1) | SLE | csDMARD | 41/NA | High |
| Gisondi et al., 2020 [21] | Italy | Cross-sectional study | 5206 | 53.2 ± 11.2 | 2383 (45.8) | PsO | bDMARD | NR | Low |
| So et al., 2020 [33] | China | Cross-sectional study | 39,835 | NR | NR | RD | csDMARD + ts/bDMARD | 5/NA | Moderate |
| Mena Vázquez et al., 2020 [36] | Spain | Cross-sectional study | 5020 | 60.8 ± 13.5 | 7 (30.4) | IRD | csDMARD + ts/bDMARD | 15/NA | Moderate |
| Blanch-Rubió et al., 2020 [30] | Spain | Cross-sectional study | 2102 | 66.4 ± 13.3 | 409 (19.5) | non-IRD | Anti-osteoporosis therapy + DMARD | 109d | Low |
| Salvarani et al., 2020 [35] | Italy | Cross-sectional study | 1195 | NR | 523 (43.8) | RD | ts/bDMARD | 9/NA | Moderate |
COVID-19 coronavirus disease 2019, C19-GRA COVID-19 Global Rheumatism Alliance, RD rheumatic diseases, IRD inflammatory rheumatic disease, non-IRD non-inflammatory rheumatic disease, PsO psoriasis, SLE systemic lupus erythematosus, csDMARD conventional synthetic disease-modifying anti-rheumatic drug, ts/bDMARD targeted synthetic/biologic disease-modifying anti-rheumatic drug, NR not reported, NA not applicable
aRepresented as median (interquartile range)
bRepresented as median (range)
cRepresented as clinical symptoms and SARS- CoV-2 PCR and/or IgG positive
dRepresented as confirmed or highly suspected COVID-19 cases
eUnless otherwise specified
Risk of COVID-19 infection in rheumatic diseases
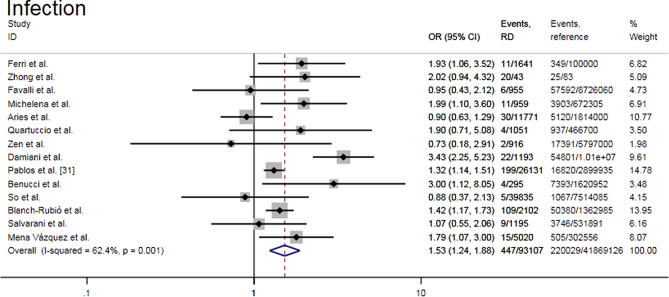
Meta-analysis of fourteen studies [23–36] showed that patients with RD had a significantly higher risk of developing COVID-19 than the general population (OR = 1.53, 95% CI 1.24–1.88, P = 0.000; I2 = 62.4%, P = 0.001) (Fig. 2). Subgroup analysis according to different disease diagnoses showed that each subgroup showed similar results to the whole, and the heterogeneity was not significant (I2 = 0–28.7%). There was a higher risk of COVID-19 infection in inflammatory rheumatic disease subgroup when compared with RD (OR = 1.87, 95% CI 1.27–2.76 vs. OR = 1.28, 95% CI 1.04–1.58) (Supplemental Fig. S1a). Subgroup analysis based on geography showed that each subgroup had moderate heterogeneity (I2 = 48.5% with Asia, and I2 = 66.3% with Europe). Compared with the reference population, European countries had an increased risk of COVID-19 (OR = 1.54, 95% CI 1.24–1.93, P = 0.000), while Asia did not show any remarkable differences (OR = 1.38, 95% CI 0.61–3.09, P = 0.438) (Supplemental Fig. S1b). Further grouping of Europe subgroup according to country showed that Italy had the highest risk of COVID-19 infection (OR = 1.73, 95% CI 1.07–2.79, P = 0.024; I2 = 64.5%, P = 0.010) (Supplemental Fig. S1c). The funnel chart drawn was visually symmetrical, indicating that there was no publication bias. This was supported by Egger’s test (P = 0.470) and Begg’s test (P = 0.913). Sensitivity analysis showed that the combined effect estimate varied between 1.37 and 1.62 when individual studies were removed in turn (Supplemental Fig. S2a–c).
Fig. 2.
Forest plot of the risk of COVID-19 infection in rheumatic patients
Hospitalization risk of COVID-19 in rheumatic diseases
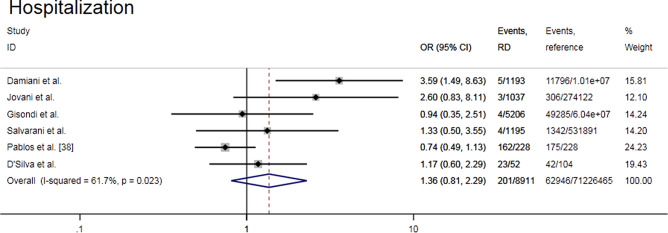
Meta-analysis of six observational studies [21, 34, 35, 37–39] showed that there was no difference in the risk of hospitalization of RD patients due to COVID-19 compared with reference group (OR = 1.36, 95% CI 0.81–2.29, P = 0.247; I2 = 61.7%, P = 0.023) (Fig. 3). The subgroup analysis according to the study design showed the conclusion consistent with the whole and the heterogeneity was not significant (I2 = 20.6% with cohort study, and I2 = 41.3% with cross-sectional study). The funnel plot was not asymmetric. When each of the six included studies was removed in turn, the combined effect estimate remained within the overall confidence interval (OR 1.03–1.63) (Supplemental Fig. S3a–c).
Fig. 3.
Forest plot of the COVID-19-related hospitalization risk in rheumatic patients
ICU admission risk of COVID-19 in patients with rheumatic diseases
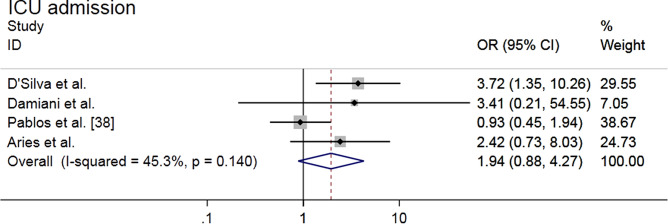
Meta-analysis of four studies [27, 34, 38, 39] showed that there was no higher risk of ICU admission related to COVID-19 in RD population compared with reference population (OR = 1.94, 95% CI 0.88–4.27, P = 0.098; I2 = 45.3%, P = 0.140) (Fig. 4). Sensitivity analysis showed that the effect estimate varied within the overall range when studies were eliminated one by one (Supplemental Fig. S4a).
Fig. 4.
Forest plot of ICU admission risk related to COVID-19 in patients with rheumatic diseases
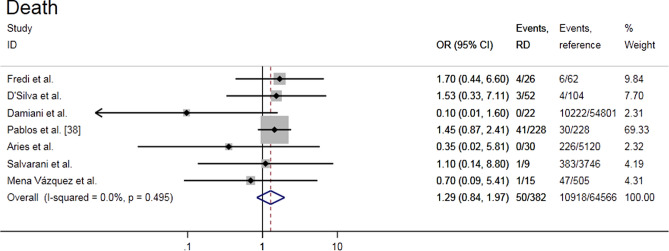
Risk of death from COVID-19 in rheumatic patients
Seven studies assessed the risk of death from COVID-19 in RD patients and included a quantitative synthesis [27, 34–36, 38–40]. The results showed that there was no higher risk of death due to COVID-19 in patients with RD compared with reference population (OR = 1.29, 95% CI 0.84–1.97, P = 0.248; I2 = 0%, P = 0.495) (Fig. 5). The resulting funnel chart was visually asymmetric, indicating the possibility of publication bias. Sensitivity analyses showed that effect estimate ranged from 0.98 to 1.37 upon removal of individual studies (Supplemental Fig. S4b and c).
Fig. 5.
Forest plot of the risk of death from COVID-19 in rheumatic patients
Risk factors for hospital admissions related to COVID-19 in rheumatic patients
From six studies, we extracted twenty-seven variables related to the risk of hospitalization in COVID-19 [22, 41–45], of which ten potential risk factors were identified and were quantitatively described (Table 2, Supplemental Table S4). Seventeen of these were considered routinely collected (Supplemental Table S4). In the meta-analysis, the presence of comorbidities (OR = 2.41, 95% CI 1.04–5.61, P = 0.041), hypertension (OR = 3.69, 95% CI 1.41–9.69, P = 0.008) and lung diseases (OR = 2.93, 95% CI 1.64–5.23, P = 0.000) were significantly associated with the increased risk of hospitalization in patients with RD and COVID-19 and anti-TNF drugs were associated with lower hospitalization risk (OR = 0.38, 95% CI 0.19–0.81, P = 0.005). Heterogeneity was low in each variable (I2 = 0–6.5%). Increased age, female sex, glucocorticoids, NSAIDs, csDMARD and antimalarial drugs had no significant correlation with hospitalization risk, and each variable had a low to considerable heterogeneity (I2 = 0–84.3%). Race (non-white vs. white) (OR = 7.78, 95% CI 1.13–53.58), diabetes (OR = 2.61, 95% CI 1.39–4.88), chronic renal insufficiency/end- stage renal disease (OR = 3.02, 95% CI 1.21–7.54), systemic autoimmune conditions (chronic inflammatory arthritis = reference) (OR = 3.55, 95% CI 1.30–9.67), methotrexate (OR = 2.06, 95% CI 1.01–5.29) and Janus kinase inhibitors (OR = 10.23, 95% CI 1.88–55.51) showed an increased risk of hospitalization but only present in one study each. Another study showed that ts/bDMARD (OR = 0.46, 95% CI 0.22–0.93) was associated with a lower risk of hospitalization. Body mass index (BMI), obesity, smoking, cardiovascular diseases, systemic lupus erythematosus, PsA, SpA, vasculitis, csDMARD + ts/bDMARD and non-anti-TNF drugs were not associated with hospitalization risk in the separate study they included (all P values > 0.05).
Table 2.
Meta-analysis of hospital admission risk factors related to COVID-19 in patients with rheumatic diseases
| Variable | No. of studies | Heterogeneity | Meta-analysis | ||
|---|---|---|---|---|---|
| I2 (%) | P value | OR (95% CI) | P value | ||
| Age (years) | 2 | 84.3 | 0.011 | 1.17 (0.97–1.42) | 0.093 |
| Gender, women | 3 | 63.8 | 0.063 | 0.47 (0.19–1.17) | 0.106 |
| Comorbidities (yes) | 2 | 4.5 | 0.306 | 2.41 (1.04–5.61) | 0.041 |
| Hypertension | 2 | 0 | 0.638 | 3.69 (1.41–9.69) | 0.008 |
| Lung disease | 3 | 6.5 | 0.343 | 2.93 (1.64–5.23) | 0.000 |
| Glucocorticoids | 2 | 82.2 | 0.018 | 6.23 (0.50–77.55) | 0.155 |
| NSAIDs | 2 | 32.9 | 0.222 | 0.79 (0.39–1.64) | 0.532 |
| csDMARD | 2 | 0 | 0.652 | 1.15 (0.71–1.88) | 0.570 |
| Antimalarial drugs | 3 | 51.3 | 0.129 | 1.21 (0.53–2.79) | 0.646 |
| Anti-TNF drugs | 2 | 0 | 0.739 | 0.38 (0.19–0.81) | 0.005 |
COVID-19 coronavirus disease 2019, NSAIDs non-steroidal anti-inflammatory drugs, csDMARD conventional synthetic disease-modifying anti-rheumatic drug
Analysis of risk factors related to severe COVID-19
We included three studies [36, 38, 46], including eleven variables associated with severe COVID-19 (Supplemental Table S4). Not all variables were included in the meta-analysis as not all studies presented data that could be used in models. Two studies described that older age was a risk factor for severe clinical outcomes in patients with RD and COVID-19 (OR = 1.13, 95% CI 1.01–1.27; OR = 4.83, 95% CI 2.78–8.37). One study described that male sex (OR = 1.93, 95% CI 1.21–3.07), connective tissue disease (OR = 1.82, 95% CI 1.00–3.30) and antivirals (OR = 2.05, 95% CI 1.30–3.23) significantly increased the risk of poor prognosis. In two studies, obesity, diabetes, heart failure, ts/bDMARD, csDMARD and csDMARD + ts/bDMARD were not statistically significant risk factors for severe COVID-19 (all P values > 0.05). Two studies reported that use of hormones (any dose) was not associated with poor COVID-19 outcomes (all P values > 0.05).
Discussion
In this systematic review and meta-analysis of 26 observational studies, we showed that patients with RD had an increased risk of developing COVID-19 compared with the general population. This was reflected in the whole and subgroup analysis of European countries and was especially true in Italy. The risk of hospitalization due to COVID-19 among RD patients was similar to that in the control population. We found that the presence of comorbidities, hypertension, and lung diseases were related to the increased risk of hospitalization caused by COVID-19. Anti-TNF might be a protective factor for hospitalization related to COVID-19. In our combined results, age was no longer a statistically significant risk factor for hospitalization. This was similar to the findings from Montero et al. [41] (OR = 2.6, 95% CI 0.42–16.21, P = 0.30) but disagreed with another study [22]. In this study, individuals age > 65 had an increased risk of COVID-19-related hospitalization (OR = 2.56, 95% CI 1.62–4.04, P < 0.01). Our meta-analysis also did not find that hormones increased the risk of hospitalization, which contradicts previously published findings. A study on the C19-GRA registry [22] described that patients taking glucocorticoids ≥ 10 mg/day had a higher risk of hospitalization (OR = 2.05, 95% CI 1.06–3.96, P = 0.03), and Montero et al. [41] also found that glucocorticoids dose ≥ 5 mg/day was associated with increased risk of hospitalization (OR = 5.00, 95% CI 1.08–23.15, P = 0.04). Our meta-analysis showed that the risk of severe COVID-19 in the RD population was similar to that observed in the reference population. Older age might be a risk factor for severe COVID-19 among RD patients. We did not find the role of hormones in the progression of serious disease. For other factors potentially affecting COVID-19 prognosis in RD patients, conclusions could not be drawn due to limited data.
This study is the first comprehensive meta-analysis of COVID-19 risk assessment in the RD population, and it consolidated data from different countries and different time periods. The study also had limitations. On the one hand, the studies were mainly distributed in European countries affected at the early stage of the epidemic, especially in Italy (38.5%) and Spain (34.6%) [47–49]. Therefore, these findings best reflected the European RD population during the COVID-19 pandemic. In addition, many studies occurred in the same city, which made it difficult to rule out the possibility of overlapping patients. On the other hand, the combined results showed significant heterogeneity. Thus, we undertook subgroup analyses to explore possible sources of heterogeneity. The cause of heterogeneity regarding the infection and hospitalization risk results could be potentially explained by different disease diagnoses and study design, respectively. Furthermore, due to shortages of medical resources at the peak of the epidemic, some patients might have been unable to seek medical treatment, resulting in an underestimation of serious patients. Moreover, if patients with RD took preventive measures (masks, hand hygiene) for fear that they had a higher risk of infection, it would lead to an underestimation of the risk of this population. Finally, taking into account the differences in SARS-CoV-2 PCR testing between countries and the scope of availability, as well as the sensitivity of the test, which would also affect the results of the study [50].
Our results showed that people with RD were potentially at high risk of SARS-CoV-2 infection. However, as in previous coronavirus outbreaks, RD patients were not at increased risk of serious complications [5]. We suggest that patients with RD should strengthen their own protection, avoid exposure to SARS-CoV-2, and strictly follow health guidelines such as wearing masks, washing hands, and restricting social distancing. Additionally, the rapid spread of SARS-CoV-2 is closely linked to travel. Therefore, policymakers should monitor incoming travelers and mandate quarantines [47, 51]. Our data were not sufficient to recommend medication for RD patients, but we recommended following the guidelines issued by the European League Against Rheumatism (EULAR), which suggested that patients should continue immunosuppressive therapy and immediately seek further healthcare advice of an expert as COVID-19 symptoms worsened [52]. The data on COVID-19 in RD population were still very limited, so our conclusions were unsafe. We hope to see additional future large-scale population-based studies that integrate multiple data sources to further confirm our conclusions.
Conclusions
This systematic review and meta-analysis showed that RD patients were more likely to be infected with SARS-CoV-2 than the general population, but we did not find that RD patients had higher hospitalization risk or more serious clinical outcomes related to COVID-19. The presence of comorbidities, hypertension, lung diseases were significantly associated with the increased risk of COVID-19-related hospitalization in rheumatic patients and anti-TNF drugs were associated with lower hospitalization risk. Older age was associated with worse clinical prognosis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are thankful to Dr. Yingying Zhao, department of Rheumatology, the Second Affiliated Hospital of Zhengzhou University, Zhengzhou City, Henan Province, China for providing her information consultation related to rheumatism during the manuscript preparation. We also thank Guangzhou Greenwood Culture Communication Co. Ltd,. for medical editing assistance with an earlier version of the manuscript.
Author contributions
QXW designed the study and drafted the original manuscript. JBL assisted in the conception and strictly revised the manuscript. RXS drafted the work and assisted QXW in literature search and screening, and jointly completed data extraction. WJL participated in the drafting of the manuscript and evaluated the quality of the included studies. XPH and CHS assisted in drafting the manuscript and completed the data analysis. All authors approved submission of the final version of the manuscript. All authors agreed to take full responsibility for all aspects of this work.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-for-profit sectors.
Compliance with ethical standards
Conflict of interest
Authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qingxiu Wang, Email: hnzz64@outlook.com.
Jianbo Liu, Email: llshxxn@163.com.
Runxia Shao, Email: shaorunxia@163.com.
Xiaopeng Han, Email: hanxp@foxmail.com.
Chenhao Su, Email: suchenhao@126.com.
Wenjia Lu, Email: 1525121170@qq.com.
References
- 1.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet (London, England) 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun J, Chen X, Zhang Z, et al. Forecasting the long-term trend of COVID-19 epidemic using a dynamic model. Sci Rep. 2020;10(1):21122. doi: 10.1038/s41598-020-78084-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal R, Bulua AC, Nikolov NP, Schwartzberg PL, Siegel RM. Rheumatologic and autoimmune manifestations of primary immunodeficiency disorders. Curr Opin Rheumatol. 2009;21(1):78–84. doi: 10.1097/BOR.0b013e32831cb939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transplant. 2020;26(6):832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 5.Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785–791. doi: 10.1136/ard.2010.128637. [DOI] [PubMed] [Google Scholar]
- 6.Favalli EG, Agape E, Caporali R. Incidence and clinical course of COVID-19 in patients with connective tissue diseases: a descriptive observational analysis. J Rheumatol. 2020;47(8):1296. doi: 10.3899/jrheum.200507. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Piedra C, Diaz-Torne C, Manero J, et al. Clinical features and outcomes of COVID-19 in patients with rheumatic diseases treated with biological and synthetic targeted therapies. Ann Rheum Dis. 2020;79(7):988–990. doi: 10.1136/annrheumdis-2020-217948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serling-Boyd N, D'Silva KM, Hsu TY, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-219279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popov GT, Baymakova M, Vaseva V, Kundurzhiev T, Mutafchiyski V. Clinical characteristics of hospitalized patients with COVID-19 in Sofia, Bulgaria. Vector Borne Zoonotic Dis (Larchmont, NY) 2020;20(12):910–915. doi: 10.1089/vbz.2020.2679. [DOI] [PubMed] [Google Scholar]
- 10.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ (Clinical research ed) 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ (Clinical research ed) 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011;31(11):1409–1417. doi: 10.1007/s00296-011-1999-3. [DOI] [PubMed] [Google Scholar]
- 14.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 20 Nov 2020
- 15.Li J, Huang DQ, Zou B, et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2020 doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shu X, Mai QQ, Blatz M, Price R, Wang XD, Zhao K. Direct and indirect restorations for endodontically treated teeth: a systematic review and meta-analysis, IAAD 2017 Consensus Conference Paper. J Adhes Dent. 2018;20(3):183–194. doi: 10.3290/j.jad.a40762. [DOI] [PubMed] [Google Scholar]
- 17.Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127–2133. doi: 10.11124/JBISRIR-D-19-00099. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins J, Green S (2011) Cochrane Handbook for systematic reviews of interventions, version 5.1. 0. The Cochrane Collaboration, London
- 20.Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 21.Gisondi P, Facheris P, Dapavo P, et al. The impact of the COVID-19 pandemic on patients with chronic plaque psoriasis being treated with biological therapy: the Northern Italy experience. Br J Dermatol. 2020;183(2):373–374. doi: 10.1111/bjd.19158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferri C, Giuggioli D, Raimondo V, et al. COVID-19 and rheumatic autoimmune systemic diseases: report of a large Italian patients series. Clin Rheumatol. 2020;39(11):3195–3204. doi: 10.1007/s10067-020-05334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong J, Shen G, Yang H, et al. COVID-19 in patients with rheumatic disease in Hubei province, China: a multicentre retrospective observational study. Lancet Rheumatol. 2020;2(9):e557–e564. doi: 10.1016/S2665-9913(20)30227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Favalli EG, Monti S, Ingegnoli F, Balduzzi S, Caporali R, Montecucco C. Incidence of COVID-19 in patients with rheumatic diseases treated with targeted immunosuppressive drugs: what can we learn from observational data? Arthritis Rheumatol (Hoboken, NJ) 2020;72(10):1600–1606. doi: 10.1002/art.41388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michelena X, Borrell H, López-Corbeto M, et al. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum. 2020;50(4):564–570. doi: 10.1016/j.semarthrit.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aries P, Iking-Konert C. No increased rate of SARS-CoV-2 infection for patients with inflammatory rheumatic diseases compared with the general population in the city of Hamburg (Germany) Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218400. [DOI] [PubMed] [Google Scholar]
- 28.Quartuccio L, Valent F, Pasut E, Tascini C, De Vita S. Prevalence of COVID-19 among patients with chronic inflammatory rheumatic diseases treated with biologic agents or small molecules: a population-based study in the first two months of COVID-19 outbreak in Italy. Jt Bone Spine. 2020;87(5):439–443. doi: 10.1016/j.jbspin.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zen M, Fuzzi E, Astorri D, et al. SARS-CoV-2 infection in patients with autoimmune rheumatic diseases in northeast Italy: a cross-sectional study on 916 patients. J Autoimmun. 2020;112:102502. doi: 10.1016/j.jaut.2020.102502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanch-Rubió J, Soldevila-Domenech N, Tío L, et al. Influence of anti-osteoporosis treatments on the incidence of COVID-19 in patients with non-inflammatory rheumatic conditions. Aging. 2020;12(20):19923–19937. doi: 10.18632/aging.104117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pablos JL, Abasolo L, Alvaro-Gracia JM, et al. Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann Rheum Dis. 2020;79(9):1170–1173. doi: 10.1136/annrheumdis-2020-217763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benucci M, Damiani A, Giannasi G, et al. Serological tests confirm the low incidence of COVID-19 in chronic rheumatic inflammatory diseases treated with biological DMARD. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218214. [DOI] [PubMed] [Google Scholar]
- 33.So H, Mak JW, So J, et al. Incidence and clinical course of COVID-19 in patients with rheumatologic diseases: a population-based study. Semin Arthritis Rheum. 2020;50(5):885–889. doi: 10.1016/j.semarthrit.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damiani G, Pacifico A, Bragazzi NL, Malagoli P. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33(5):e13475. doi: 10.1111/dth.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvarani C, Bajocchi G, Mancuso P, et al. Susceptibility and severity of COVID-19 in patients treated with bDMARDS and tsDMARDs: a population-based study. Ann Rheum Dis. 2020;79(7):986–988. doi: 10.1136/annrheumdis-2020-217903. [DOI] [PubMed] [Google Scholar]
- 36.Mena Vázquez N, Manrique-Arija S, Cabezudo-García P, et al. Incidence and case fatality rate of COVID-19 in patients with inflammatory articular diseases. Int J Clin Pract. 2020 doi: 10.1111/ijcp.13707. [DOI] [PubMed] [Google Scholar]
- 37.Jovani V, Calabuig I, Peral-Garrido ML, et al. Incidence of severe COVID-19 in a Spanish cohort of 1037 patients with rheumatic diseases treated with biologics and JAK-inhibitors. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218152. [DOI] [PubMed] [Google Scholar]
- 38.Pablos JL, Galindo M, Carmona L, et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. 2020;79(12):1544–1549. doi: 10.1136/annrheumdis-2020-218296. [DOI] [PubMed] [Google Scholar]
- 39.D'Silva KM, Serling-Boyd N, Wallwork R, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US 'hot spot'. Ann Rheum Dis. 2020;79(9):1156–1162. doi: 10.1136/annrheumdis-2020-217888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fredi M, Cavazzana I, Moschetti L, Andreoli L, Franceschini F. COVID-19 in patients with rheumatic diseases in northern Italy: a single-centre observational and case-control study. Lancet Rheumatol. 2020;2(9):e549–e556. doi: 10.1016/S2665-9913(20)30169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montero F, Martínez-Barrio J, Serrano-Benavente B, et al. Coronavirus disease 2019 (COVID-19) in autoimmune and inflammatory conditions: clinical characteristics of poor outcomes. Rheumatol Int. 2020;40(10):1593–1598. doi: 10.1007/s00296-020-04676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freites Nuñez DD, Leon L, Mucientes A, et al. Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79(11):1393–1399. doi: 10.1136/annrheumdis-2020-217984. [DOI] [PubMed] [Google Scholar]
- 43.Nuño L, Novella Navarro M, Bonilla G, et al. Clinical course, severity and mortality in a cohort of patients with COVID-19 with rheumatic diseases. Ann Rheum Dis. 2020;79(12):1659–1661. doi: 10.1136/annrheumdis-2020-218054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernandez-Ruiz R, Masson M, Kim MY, et al. Leveraging the United States epicenter to provide insights on COVID-19 in patients with systemic lupus erythematosus. Arthritis Rheumatol (Hoboken, NJ) 2020;72(12):1971–1980. doi: 10.1002/art.41450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haberman RH, Castillo R, Chen A, et al. COVID-19 in patients with inflammatory arthritis: a prospective study on the effects of comorbidities and disease-modifying antirheumatic drugs on clinical outcomes. Arthritis Rheumatol (Hoboken, NJ) 2020;72(12):1981–1989. doi: 10.1002/art.41456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scirè CA, Carrara G, Zanetti A, et al. COVID-19 in rheumatic diseases in Italy: first results from the Italian registry of the Italian Society for Rheumatology (CONTROL-19) Clin Exp Rheumatol. 2020;38(4):748–753. [PubMed] [Google Scholar]
- 47.Puca E, Čivljak R, Arapović J, et al. Short epidemiological overview of the current situation on COVID-19 pandemic in Southeast European (SEE) countries. Journal of infection in developing countries. 2020;14(5):433–437. doi: 10.3855/jidc.12814. [DOI] [PubMed] [Google Scholar]
- 48.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288(3):335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gianfrancesco M, Yazdany J, Robinson PC. Epidemiology and outcomes of novel coronavirus 2019 in patients with immune-mediated inflammatory diseases. Curr Opin Rheumatol. 2020;32(5):434–440. doi: 10.1097/BOR.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 51.Popescu CP, Marin A, Melinte V, et al. COVID-19 in a tertiary hospital from Romania: epidemiology, preparedness and clinical challenges. Travel Med Infect Dis. 2020;35:101662. doi: 10.1016/j.tmaid.2020.101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landewé RB, Machado PM, Kroon F, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis. 2020;79(7):851–858. doi: 10.1136/annrheumdis-2020-217877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.