Abstract
Objective
Prompt diagnosis of Helicobacter pylori infection is essential for proper treatment and eradication of the pathogen because prolonged infection could lead to gastric cancer. Sensitive and cost effective diagnostic methods are key to guiding treatment options that will reduce mortality. This study was aimed at detecting H. pylori from biopsies of peptic ulcer patients. Real-time PCR using TaqMan and EvaGreen assays targeting 16S rRNA and ureA genes were used to detect H. pylori DNA extracted from 40 biopsy samples comprising 20 biopsies obtained from the antrum and 20 from the corpus of 20 patients undergoing endoscopy for duodenal ulcer investigation in Lagos, Nigeria.
Results
H. pylori was detected in 80% of the biopsy samples by combined cycle threshold (Ct) and melting temperature (Tm) values. Mean Ct value for ureA gene ranged from 21.40 to 37.53 and 22.71 to 35.44 for 16SrRNA gene. Average melting temperatures (Tm) of 81.57 and 82.90 °C among amplicons of ureA and 16S rRNA were observed respectively. H. pylori DNA was generally detected in biopsies collected from antrum and corpus. Real-time PCR in the diagnosis of H. pylori can be considered a simple, low cost and efficient alternative or addition to the gold standard.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13104-021-05505-y.
Keywords: Helicobacter pylori, Diagnosis, Real-time PCR, Biopsy, Ulcer
Introduction
Gastritis, duodenal ulcer, gastric ulcer and in some cases gastric cancers are hallmarks of Helicobacter pylori infection. H. pylori is a Gram negative bacterium that colonize 50% of the stomach of humans globally [1, 2]. H. pylori possess several virulence factors including the production of urease that enable it successfully colonize the stomach where it can persist for a long period of time. The pathogen has been classified as a type 1 carcinogen hence, its persistence in infection without eradication may lead to chronic gastritis, mucosa-associated lymphoid tissue (MALT) lymphoma and gastric cancer [3–5]. H. pylori is a fastidious bacterium making it very delicate to culture. Although non-invasive methods of detecting H. pylori exist [6], culture remains the gold standard which requires competence and a lot of materials making it expensive. Detection of H. pylori DNA directly from biopsies by molecular methods especially PCR have been reported by several workers [7, 8] with excellent sensitivity and specificity. In Nigeria, the use of conventional PCR in the detection of H. pylori DNA from biopsies have been demonstrated [9, 10]. However, the use of real-time PCR in the detection of H. pylori in biopsies have not been explored in the country. The purpose of this study was to detect H. pylori DNA isolated from gastric biopsies (corpus and antrum) obtained from patients in Lagos Nigeria.
Main text
Samples used for this study were biopsies obtained from 20 patients undergoing endoscopy for duodenal ulcer investigation in Lagos, Nigeria. A total of 40 biopsies comprising 20 obtained from the corpus and 20 from the antrum were analysed.
Methodology
DNA was extracted from gastric biopsies (corpus and antrum) and H. pylori reference strain J99 (NCBI:txid85963) with QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions.
Detection of H. pylori was done by singleplex real-time PCR amplifying fragments of 16S rRNA and ureA genes using specific primers and probes listed in Additional file 1: Table S1 with slight modification (quencher of probes was Black Hole Quencher (BHQ) instead of TAMARA). Detection of H. pylori targeting the two set of primers was first validated by using H. pylori DNA extracted from reference H. pylori strain J99 (NCBI:txid85963) and DNA extracted from Salmonella Typhimurium ATCC 14028, Staphylococcus aureus ATCC 29213, Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 29522. A standard curve of the assay was determined as serial dilution of DNA from H. pylori J99 positive control which was prepared with final concentration of 101–105; reactions were ran in triplicate. The curve was determined by plotting threshold cycle (Ct) value against count of log DNA copies from which positive samples were considered as amplification plot with Ct values < 40. Real-time PCR was carried out in a StepOne Real-Time PCR system (Applied Biosystem, Singapore). A 20 µL reaction was used in both the TaqMan and EvaGreen assays which contained 11.2 µL nuclease free water, 4 µL of Solis Biodyne 5 × HOT FIREPol® Probe qPCR Mix Plus (for TaqMan assay) and 5X HOT FIREPol® EvaGreen Supermix (Solis Biodyne Tartu, Estonia), 0.4 µL (10 µM) each of both forward and reverse primers and 4 µL of (20 ng/µL) DNA template. PCR cycling parameters for the EvaGreen assay were an initial denaturation at 95 °C for 12 min and 40 cycles of denaturation at 95 °C for 15 s, annealing for 1 min at 54 °C for 16S rRNA gene, 55 °C annealing temperature for ureA gene, extension at 72 °C for 20 s and a melting step. While PCR cycling parameters for the TaqMan assay were initial denaturation at 95 °C for 12 min, denaturation at 95 °C for 15 s, annealing/elongation at 54 °C for 16S rRNA gene and 55 °C for ureA gene both for 1 min. After which amplification results were analysed with StepOne Software v.23 and GraphPad Prism software version 5 (GraphPad Software, LA Jolla, CA, USA).
Results
Real-time PCR assay for the detection of H. pylori from genomic DNA extracted from biopsies and extracted DNA from H. pylori reference strain J99 (NCBI:txid85963) showed amplification curves for all targeted genes (ureA and 16S rRNA). However, there was no amplification with DNA extracted from other bacterial pathogens (Salmonella Typhimurium ATCC 14028, Staphylococcus aureus ATCC 29,213, Pseudomonas aeruginosa ATCC and Escherichia coli ATCC 29522) as shown in Fig. 1 indicating specificity of primers and probes used.
Fig. 1.
Threshold cycle (ct) value indicated for reference H. pylori (J99) strain on the y-axis. There was no amplification for other bacterial pathogens including S. aureus, S. Typhimurium, P. aeruginosa and E. coli (x-axis) indicating specificity of primer/probe sets targeting 16S rRNA and ureA genes of H. pylori
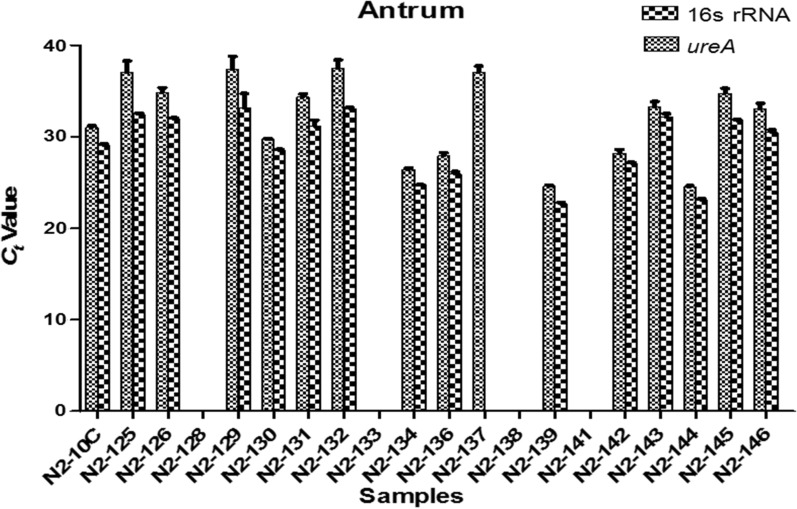
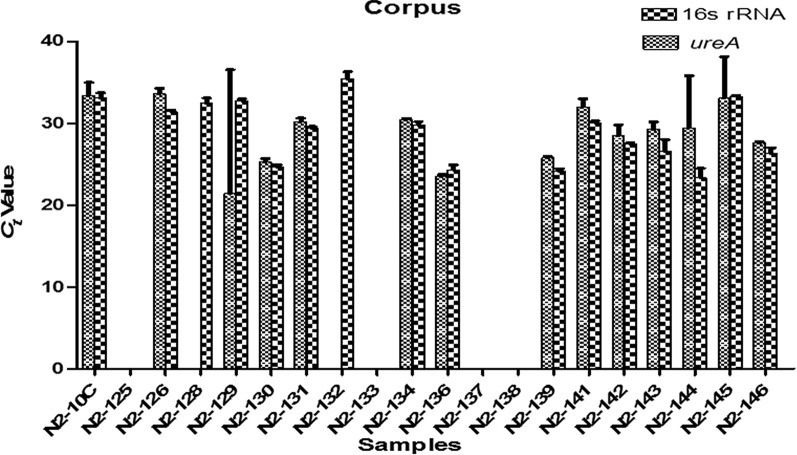
Eighty percent of the samples were positive for H. pylori. However, H. pylori was detected in the antrum of two patients but was not detected in the corpus. The reverse was observed in a third patient in which H. pylori was detected in the corpus but absent in the antrum. The logarithm of the number of H. pylori DNA copies in the samples correlated largely well with the ureA and 16S rRNA threshold cycle (Ct) values which is a representation of the DNA copy number in the PCR reaction. Mean Ct value for ureA gene ranged from Mean ± SD: 21.40 ± 15.14 to 37.53 ± 0.89 and for 16S rRNA gene Mean ± SD: 22.71 ± 0.12 to 35.44 ± 0.87 as shown in Figs. 2 and 3. Correlation coefficient (R2) and amplification efficiency (E) was 0.994/90.35%, 0.997/97.94% for ureA and 16S rRNA respectively.
Fig. 2.
Threshold cycle (Ct) values for ureA and 16S rRNA gene amplification in antrum biopsy samples is indicated on the y-axis. Each value is mean of triplicates. While samples N2-134, N2-136, N2-139 and N2-144 had relatively low Ct values for both genes, there was no amplification in samples N2-128, N2-133, N2-138 and N2-141. Error bar indicates the ± standard deviation (SD)
Fig. 3.
Threshold cycle (Ct) values for ureA and 16S rRNA genes amplification in corpus biopsy samples. Each value is mean of triplicates. Amplification was not observe in N2-125, N2-133, N2-137 and N2-138. Error bar indicates the ± standard deviation (SD)
The intercalating chemistry EvaGreen used showed smooth melting curve (Additional file 2: Figure S1) and average melting temperatures (Tm) of 81.57 and 82.90 °C among amplicons of ureA and 16S rRNA respectively.
Discussion
Helicobacter pylori infection remains a major public health issue around the world. The prevalence of H. pylori infection in Nigeria is estimated at about 87.7% which indicates a high infection burden [1] making prompt, accurate and efficient diagnosis imperative. The PCR results for the detection of H. pylori from biopsies and other bacterial pathogens indicated the reliability of using the H. pylori ureA and 16S rRNA primer set in this study. Eighty percent of the samples were positive for H. pylori with the assay showing great efficiency in detecting small quantities of H. pylori DNA. Correlation coefficient (R2) and amplification efficiency (E) of both ureA (0.994/90.35%) and 16S rRNA (0.997/97.94%) proved to be good. Hence it could be inferred that these targets had great specificity since all H. pylori strains harbour the gene that encodes urease. In the study of Ramı´rez-La´zaro et al. [11], they suggested the combination of both 16S rRNA and ureA genes in the diagnosis of H. pylori from biopsies for better sensitivity. However, in the study by Beer-Davidson et al. [12] in which they reported the detection of H. pylori in stool samples of children in Israel using real-time PCR targeting urease gene, it was observed that the gene gave a clearer amplification curve compared to 16S rRNA gene. In this study, the mean temperature separation between ureA (81.57 °C) and 16S rRNA (82.90 °C) was 1.33 °C. Thus it could be asserted that amplification products of ureA gene could easily be distinguished from 16S rRNA even in a multiplex qPCR reaction. Contreras et al. [13] reported a melting temperature range between 57.0 and 57.4 °C that enabled them detect 16S rRNA single mutation associated with antibiotic resistance in H. pylori strains isolated from biopsies in Venezuela compared to a higher Tm that was observed in wild type strains. The detection of H. pylori in biopsies collected from the antrum and corpus is widely reported. Pichon et al. [14], reported the detection of H. pylori in both biopsies of antrum and corpus obtained from patients with H. pylori infection. However, in this present study there was zero detection of H. pylori in the corpus of two patients in which H. pylori was detected in their antrum biopsy samples. Similarly, in one patient, H. pylori was detected in corpus biopsy but absent in the antrum. Palamides et al. [15] reported similar findings in which H. pylori was detected in biopsies from either antrum or corpus of some patients using conventional PCR. This suggest that there are variations in the distribution of H. pylori in the gut of H. pylori infected patients. Lan et al. [16] reported in their study that corpus biopsy enhances the detection of H. pylori infection. Similarly, Latif et al. [17] opined that in addition to antral biopsy, corpus biopsy increases the sensitivity in the detection of H. pylori infection.
Conclusion
Real-time PCR in the diagnosis of H. pylori can be considered an alternative or in addition to the gold standard and including histology since it relies on the detection of DNA isolated from biopsies and not necessarily viable bacteria coupled with its competitive cost [11]. Furthermore, direct detection of H. pylori from biopsies can circumvent the difficulty and extended time lapse encountered with culture.
Limitation of study
It would be difficult to make far reaching conclusion as the number of samples in this study are few and other diagnostic methods such as histology and culture were not evaluated alongside real-time PCR. Hence, future study should increase the number of samples and evaluate other diagnostic methods alongside.
Supplementary Information
Additional file 1: Table S1. Primers used in the detection of H. pylori by TaqMan and EvaGreen qPCR assay.
Additional file 2: Figure S1. Melting curve of EvaGreen real-time PCR targeting a 16SrRNA b ureA. Melting peaks were derived by the plot of derivative reporter (−R) against temperature (oC).
Acknowledgements
SIS wishes to acknowledge the Alexander von Humboldt Foundation for the donation of the StepOne Real-Time PCR System.
Abbreviations
- ATCC
American Type Culture Collection
- BHQ
Black Hole Quencher
- Ct
Cycle threshold
- MALT
Mucosa-associated Lymphoid Tissue
- Tm
Melting Temperature
Authors' contributions
Conception and design: SSI; Laboratory experiment: AA JT; Result Analysis and manuscript writing/review: AA, JT, SSI. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethical approval and consent to participate
Written consent was obtained from participants before they will recruited for the study and ethical approval was obtained from Institutional Review Board of the Nigerian Institute of Medical Research with number IORG0002656.
Consent for publication
Not applicable.
Competing interests
There is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smith S, Fowora M, Pellicano R. Infections with Helicobacter pylori and challenges encountered in Africa. World J Gastroenterol. 2019;25(25):3183–3195. doi: 10.3748/wjg.v25.i25.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haddadi M-H, Negahdari B, Asadolahi R, Bazargani A. Helicobacter pylori antibiotic resistance and correlation with cagA motifs and homB gene. Postgrad Med. 2020 doi: 10.1080/00325481.2020.1753406. [DOI] [PubMed] [Google Scholar]
- 3.Syahniar R, Wahid MH, Syam AF, Yasmon A. Detecting the Helicobacter pylori 16S rRNA gene in dyspepsia patients using real-time PCR. Acta Med Indones-Indones J Intern Med. 2019;51(1):34–40. [PubMed] [Google Scholar]
- 4.Vazirzadeh J, Falahi J, Moghim S, Narimani T, Rafiei R, Karbasizadeh V. Molecular assessment of resistance to clarithromycin in Helicobacter pylori strains isolated from patients with dyspepsia by fluorescent in situ hybridization in the center of Iran. BioMed Res Int. 2020;2304173:1–7. doi: 10.1155/2020/2304173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamidi S, Badmasti F, Heravi FS, Safapoor MH, Tabrizi AMA, Ghorbani M, et al. Antibiotic resistance and clonal relatedness of Helicobacter pylori strains isolated from stomach biopsy specimens in northeast of Iran. Helicobacter. 2020;00:e12684. doi: 10.1111/hel.12684. [DOI] [PubMed] [Google Scholar]
- 6.Wang YK, Kuo FC, Liu CJ, et al. Diagnosis of Helicobacter pylori infection: current options and developments. World J Gastroenterol. 2015;21(40):11221–11235. doi: 10.3748/wjg.v21.i40.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bénéjat L, Ducournau A, Lehours P, Mégraud F. Real-time PCR for Helicobacter pyloridiagnosis. The best tools available. Helicobacter. 2018 doi: 10.1111/hel.12512. [DOI] [PubMed] [Google Scholar]
- 8.Van den Poel B, Gils S, Micalessi I, Carton S, Christiaens P, Cuyle P-J, Moons V, Van Olmen G, Smismans A, Bourgain C, Bossuyt P, Frans J. Molecular detection of Helicobacter pylori and clarithromycin resistance in gastric biopsies: a prospective evaluation of RIDA®GENE Helicobacter pylori assay. Acta Clin Belg. 2019 doi: 10.1080/17843286.2019.1685741. [DOI] [PubMed] [Google Scholar]
- 9.Smith SI, Oyedeji KS, Arigbabu AO, Cantet F, Megraud F, Ojo OO, Uwaifo AO, et al. Comparison of three PCR methods for detection of Helicobacter pylori DNA and detection of cagA gene in gastric biopsy specimens. World J Gastroenterol. 2004;10(13):1958–1960. doi: 10.3748/wjg.v10.i13.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith SI, Fowora MA, Otegbayo JA, Abdulkareem FB, Omonigbehin EA, Adegboyega A, et al. Comparison of PCR with other diagnostic techniques for the detection of H. pylori infection in patients presenting with gastroduodenal symptons in Nigeria. Int J Mol Epidemiol Genet. 2011;2(2):178–184. [PMC free article] [PubMed] [Google Scholar]
- 11.Ramı’rez-La’’zaro MJ, Lario S, Casalots A, Sanfeliu E, Boix L, Garcıa-Iglesias P, et al. Real-time PCR improves Helicobacter pylori detection in patients with peptic ulcer bleeding. PLoS ONE. 2011;6(5):e20009. doi: 10.1371/journal.pone.0020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beer-Davidson G, Hindiyeh M, Muhsen K. Detection of Helicobacter pyloriin stool samples of young children using real-time polymerase chain reaction. Helicobacter. 2017 doi: 10.1111/hel.12450. [DOI] [PubMed] [Google Scholar]
- 13.Contreras M, Benejat L, Mujica H, Peña J, García-Amado M-A, Michelangeli F, et al. Real-time PCR detection of a 16S rRNA single mutation of Helicobacter pylori isolates associated with reduced susceptibility and resistance to tetracycline in the gastroesophageal mucosa of individual hosts. J Med Microbiol. 2019;68:1287–1291. doi: 10.1099/jmm.0.001051. [DOI] [PubMed] [Google Scholar]
- 14.Pichon M, Tran CT, Motillon G, Debiais C, Gautier S, Aballea M, et al. Where to biopsy to detect Helicobacter pylori and how many biopsies are needed to detect antibiotic resistance in a human stomach. J Clin Med. 2020;9:2812. doi: 10.3390/jcm9092812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palamides P, Jolaiya T, Idowu A, Loell E, Onyekwere C, Ugiagbe R, et al. Helicobacter pylori patient isolates from South Africa and Nigeria differ in virulence factor pathogenicity profile and associated gastric disease outcome. Sci Rep. 2020;10:11409. doi: 10.1038/s41598-020-66128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lan H-C, Chen T-S, Li AF-Y, Chang F-Y, Lin H-C. Additional corpus biopsy enhances the detection of Helicobacter pylori infection in a background of gastritis with atrophy. BMC Gastroenterol. 2012;12:182. doi: 10.1186/1471-230X-12-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latif MEA, Shahin R, Abdrabou RM, Shawqy A, El-Ghadban HM, Arafa M, et al. Value of additional corpus biopsy for diagnosis of Helicobacter pylori in atrophic gastritis. J Gastro Hepato Dis. 2016;2(1):108. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Primers used in the detection of H. pylori by TaqMan and EvaGreen qPCR assay.
Additional file 2: Figure S1. Melting curve of EvaGreen real-time PCR targeting a 16SrRNA b ureA. Melting peaks were derived by the plot of derivative reporter (−R) against temperature (oC).
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.