Abstract
Proteins are naturally occurring functional building blocks that are useful for the fabrication of materials. Naturally-occurring proteins are biodegradable and most are biocompatible and non-toxic, making them attractive for the fabrication of biomaterials. Moreover, the fabrication of protein-based materials can be conducted in a green and sustainable manner due to their high aqueous solubility. Consequently, the applicability of protein-based materials is limited by their aqueous and mechanical instability. This review summarizes strategies for the stabilization of protein films, highlighting their salient features and potential limitations. Applications of protein films ranging from food packaging materials, tissue engineering scaffolds, antimicrobial coatings etc. are also discussed. Finally, the need for robust and efficient fabrication strategies for translation to commercial applications as well as potential applications of protein films in the field of sensing, diagnostics and controlled release systems are discussed.
Keywords: Protein films, biomaterials, biomedical applications, antimicrobial coatings, tissue engineering
Graphical Abstract
Proteins are ideal for biomaterial fabrication due to their sustainability and biocompatibility. However, materials applications of proteins can be limited due to their aqueous instability. This review discusses commonly utilized strategies to stabilize protein films while highlighting their salient features, potential limitations and biomaterial applications.

1. Introduction
Proteins are sustainable, biocompatible and biodegradable building blocks for designing materials.[1, 2] Proteins naturally feature a variety of functionalities - for instance, structural proteins like collagen are widely used in biomedical applications due to their abundance in the extracellular matrix.[3] Silk fibroin and soy protein isolate films have been used as food packaging materials[4, 5] as well as in the fabrication of medical devices,[6, 7] due to their ready availability, efficient processability, biodegradability and biocompatibility.[8, 9] Furthermore, the process of isolating proteins and fabricating protein-based materials can be conducted with limited use of organic solvents, as most proteins are water soluble.[10, 11, 12] Therefore, protein are an attractive approach for designing sustainable materials for biomedical applications.
Proteins are widely to generate materials including coatings,[13] hydrogels,[14] 3D scaffolds[15] and adhesives.[16] However, the use of proteins in these materials is complicated by their instability in aqueous media, which results in degradation and loss of structure in materials.[17, 18] Therefore, current technologies have been focused on improving the aqueous stability of protein-based materials, while maintaining desirable properties such as biocompatibility, biodegradability and native functionality.
This review focuses on strategies for generating protein films and protein-based coatings, as they are one of the most widely synthesized protein-based material. Currently, three main strategies have been employed, as illustrated in Figure 1 – 1. Naturally self-assembling proteins such as silk fibroin self-assemble into water-stable films upon during; [19] 2. Crosslinking of proteins through physical or chemical approaches;[20, 21] and 3. Thermal treatment of proteins to initiate reorganization of proteins, including recent approaches that retain native structure and properties.[22, 23] Additionally, post-functionalization of the surface[24] or incorporation of additives[25] can be used to enhance native properties of the film or impart new characteristics. Protein films are widely used as food packaging materials,[5] tissue engineering scaffolds,[7] and coatings for specific biomedical applications such as anti-fouling coatings, cytophilic coatings etc.[22] Previous reviews on this subject have focused on either biomedical applications of a particular protein,[26, 27] or on the different types of protein films utilized for a particular application.[28, 29] This progress report serves as a guide to the common methods of protein film fabrication, their application as biomaterials and potential limitations of these approaches.
Figure 1.

Schematic depiction of strategies of fabrication of protein films and examples of biomaterial applications. Stable protein films can be fabricated through self-assembly of proteins, physical or chemical crosslinking, and thermal treatment. Applications of protein films include tissue engineering scaffolds, drug eluting coatings and antimicrobial surfaces.
2. Strategies for fabrication of protein films
2.1. Structural proteins for self-assembled films
Structural proteins with highly repetitive amino acid sequences such as silk, collagen, keratin, elastin etc. naturally self-assemble into water-stable protein films upon processing through the methods described in Section III of Figure 2. [19, 30, 31] Protein films can be efficiently fabricated using these naturally self-assembling proteins to form robust, biocompatible and biodegradable coatings. For instance, silk fibroin protein has been used to fabricate protein films for biomedical applications due to the biocompatibility, slow degradation and robust mechanical properties of the resulting films.[32, 33, 34, 35] Silk fibroin self-assembles into β-sheets that result in water-stable films. Film properties such as mechanical strength and biodegradability can be enhanced by controlling β-sheet percentage through different strategies such water annealing, methanol annealing and drying speed.[36, 37] Figure 2 summarizes different strategies for the preparation of silk protein solution, processing methods for film formation, and strategies for the manipulation of film properties.
Figure 2.
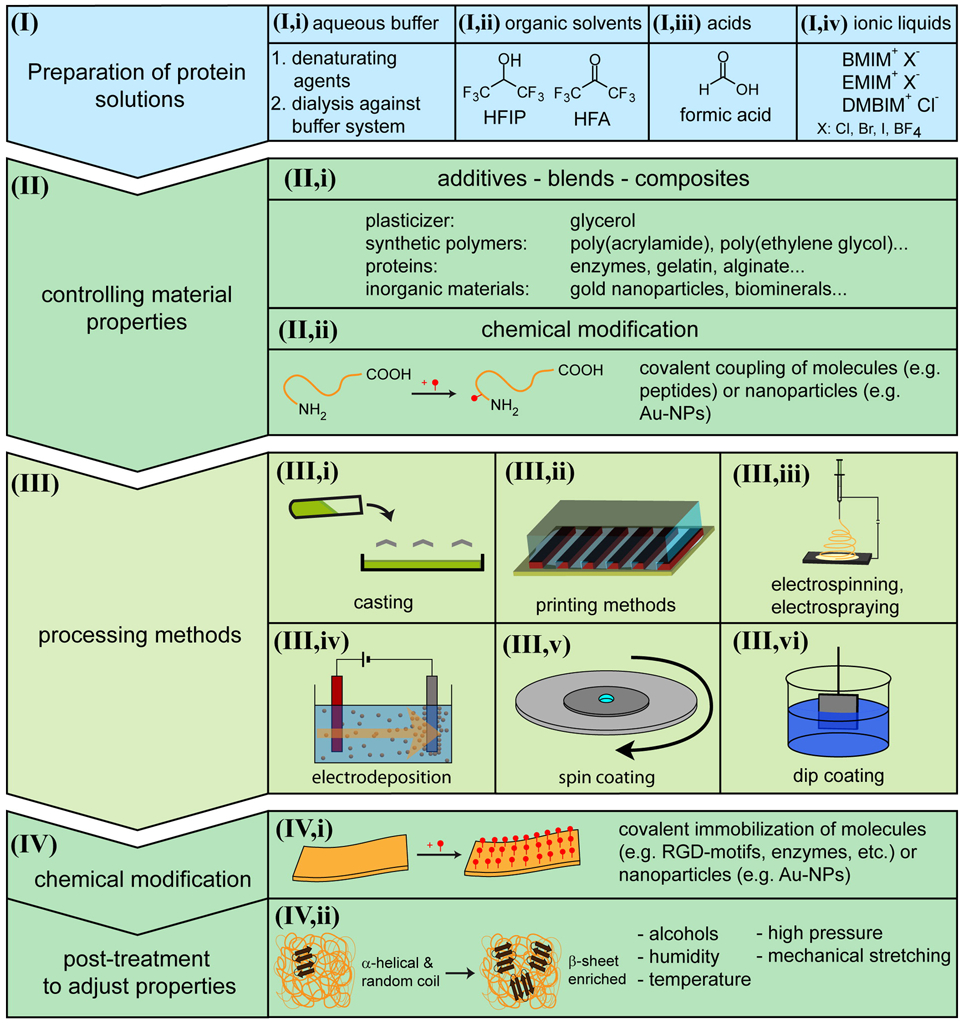
Schematic overview of the strategies of fabrication of silk films and coatings. (I) summarizes strategies for preparation of silk solutions and (III) summarizes methods of fabrication of silk films. (II) and (IV) summarize strategies for manipulation of film properties either before (II) or after (IV) film formation. Reproduced with permission. [19] Copyright 2014, American Chemical Society.
Collagen is a naturally self-assembling protein abundant in the extracellular matrix.[3] It supports cell attachment, migration and proliferation. It consists of three parallel polypeptide-α chains in a right-handed triple-helical structure that self-associates to form highly ordered cross-linked fibrils. [38] The triple helix structure of collagen and its fibrous structure make collagen films and coatings insoluble in water. For this reason, it is widely used in biomedical applications such as tissue engineering scaffolds, wound healing patches etc.[39, 40] Collagen is usually purified and can be dissolved in acid solutions to form films[41] or alternatively collagen fibers are directly used to prepare films.[42]
Self-assembly is an excellent approach to utilize the natural ability of some proteins to self-assemble through non-covalent crosslinking into water-stable films, without manipulating their native protein primary structure. The resultant protein films retain most of their native protein properties. However, there are a limited number of naturally-occurring structural proteins that exhibit this behavior. Therefore, various physical or chemical treatments must be employed to obtain varied functionalities in these self-assembled protein films, which may affect the native protein structure by causing denaturation, increase cytotoxicity of the film, or affect the functional properties of the protein film.
2.2. Crosslinked protein films
Self-assembling structural proteins offer an efficient and additive-free approach for fabricating protein films. However, there are a limited number of these proteins and they often need to be treated through chemical or physical treatment strategies to suit biomedical applications. Consequently, crosslinkers offer an alternative approach to utilize a wider array of proteins to generate water-stable films while also enhancing film properties. Crosslinking is typically conducted through (1) physical strategies that utilize non-covalent interactions between two proteins such as ionic interactions and hydrogen bonding, or (2) chemical strategies that result in covalent bonding between proteins accomplished through irradiation, sulfur vulcanization and chemical reagents. [20, 21, 43, 44]
Significant research has been conducted in the development of composite protein films fabricated through physical crosslinking strategies, often with other proteins or biopolymers. This approach allows for efficient manipulation of biophysical properties of protein films such as strength, elasticity, roughness, degradability and biocompatibility. For instance, the physical properties of natively self-assembling silk fibroin films and scaffolds were modulated through blending with biopolymers such as hyaluronic acid (HA) using a freezing-annealing technique to induce self-assembly of stable SF/HA scaffolds. Figure 3(a) demonstrates the freezing-annealing technique – SF chains for silk 1 form crystal networks upon freezing at −80 °C, and upon annealing at 3-5 °C silk crystals trap HA.[45] Increasing HA increases water solubility, indicating that SF crystals are unable to effectively trap the high number of HA chains (Figure 3b). Surface morphologies observed through SEM imaging (Figures 3(c) - (f)) show interconnected microporous morphologies with a typical irregular fusiform shape. Increased HA content did not change the morphology significantly, though the average pore size gradually decreased. HA, being a component of the extracellular matrix, enhances cell growth in the composite protein films, while SF provides mechanical stability. [46]
Figure 3.

(a) Schematic depiction of freezing-annealing technique for the crosslinking of silk-fibroin (SF) and hyaluronic acid (HA) fibers (b) % Water solubility with respect to increase in % HA ratio. (c)-(f) Surface morphology of SF/HA scaffolds with varying wt% of HA obtained through SEM imaging. Scale bars are 500 μm. Adapted with permission.41 Copyright 2020, Elsevier.
Chemical crosslinking is another strategy to fabricate water-stable protein films. Utilizing chemical crosslinkers allows for precise manipulation of film properties such as mechanical strength, flexibility and rate of degradation. Aldehydes with moderate reactivity such as glutaraldehyde and formaldehyde have been widely utilized as crosslinkers for protein films used in food packaging applications to improve their mechanical properties.[47, 48, 49] However, the use of small molecule aldehydes in biomedical applications is limited due to their high cytotoxicity and environmental hazards.[50] Consequently, biopolymer-based dialdehydes have been developed as non-toxic and biocompatible crosslinkers for protein films. Alginate dialdehyde (AD), synthesized through peroxidate oxidation of sodium alginate, has been used for crosslinking of casein (CAS), a milk protein, as shown in Figure 4(a).[51] Alginate is a non-toxic and biocompatible polysaccharide biopolymer widely used in wound dressing materials.[52] These dialdehyde alginates crosslinked casein (AD-X-CAS), a protein with an overall negative charge due to the presence of COO− groups. These films are frequently used for loading and controlled release of cationic drugs such as gentamicin sulfate (GS), an antibiotic. Figure 4(b) and 4(c) shows the surface morphology of AD-X-CAS films and GS-loaded AD-X-CAS films, where GS crystals are clearly observed in the latter. Antimicrobial activity of GS-loaded AD-X-CAS was observed through a disk diffusion assay (Figure 4(d)) where diffusion of GS inhibited growth of E. coli. Similarly, soy protein isolate films have also been generated using dialdehyde carboxymethyl cellulose as a crosslinker, to fabricate water-stable SPI films with significantly improved tensile strength and thermal stability. [53]
Figure 4.

(a) Synthesis scheme for fabrication of AD-X-CAS (dialdehyde sodium alginate crosslinked Casein). Schematic representation and SEM image of (b) AD-X-CAS and (c) GS-loaded AD-X-CAS. (d) Disk diffusion assay depicting antimicrobial activity of GS-loaded AD-X-CAS against E. coli. Diffusion of GS inhibits bacterial growth around loaded AD-X-CAS as compared to control. Scale bars are 50 μm. Adapted with permission.[47] Copyright 2016, Informa UK Limited, trading as Taylor and Francis Group.
Enzymatic chemical crosslinking is another natural, non-toxic strategy for fabrication of protein films. Enzymes that characterize covalent bond formation between amino acid residues (e.g. sulfhydryl oxidases that catalyze formation of disulfide bonds, peroxidases that catalyze formation of radicals, and transglutaminase (TGase) that catalyzes acyl-transfer reactions between protein residues) are ideal enzymes for this strategy.[54] For instance, TGase has been utilized for the crosslinking of collagen fibers by catalyzing intra- and interchain bonds between lysine and glutamine residues.[55] TGase crosslinking when combined with thermal treatment notably improved crosslinking efficiency, stabilized film thickness, and improved mechanical and thermal stability.
Covalent crosslinking strategies are applicable to a number of proteins, and therefor offer a versatile approach for fabricating protein films. However, a concern with crosslinking strategies is increased cytotoxicity either from the crosslinkers themselves or from the resultant by-products. While this review highlights some progress in the development of non-toxic and biodegradable crosslinking strategies, further research on the scalability of these approaches is desired.
2.3. Thermal Treatment Strategies
Thermal treatment of protein films is a promising additive-free strategy to generate water stable films. High temperatures utilized in thermal treatment cause reorganization of native proteins resulting in physical crosslinking, thereby generating water-stable films.[56] Heat-cured films have been generated using plant-isolated proteins such as canola,[57] soy,[58] vicilin,[59] and whey protein.[60] Several reports also indicate that heat-curing improved the mechanical stability in addition to water stability.[61, 62, 63, 64] Furthermore, aging treatments that control both the relative humidity and temperature during treatment have been used to modulate mechanical properties such as tensile strength of collagen films.[65]
One of the main limitations of thermal treatment is the loss of protein structure due to heat-induced denaturation. Loss of protein structure often results in loss of protein properties (e.g. surface charge, hydrophilicity), thereby limiting the applicability of films in functional materials. A recently developed thermal treatment strategy using Nanoimprint lithography (NIL) preserves the structure and surface functionality of native proteins while generating water-stable films. [66] As depicted in Figure 5(a), a fluorosilane coated silicon mold was used to apply pressure during heat curing of spin-casted protein films. Treatment at 180 °C at 3 MPa results in stable, hydrophilic protein films (Figure 5(b). Circular dichroism (CD) spectra (Figure 5(c)) revealed that most of the secondary structure was retained, while Kelvin Probe Force Microscopy (KPFM) demonstrated retention of the protein native charge in the film. Positive, negative and neutral films were generated using bovine serum albumin (BSA; pI = 4.8), hemoglobin (Hemo; pI = 6.8), and lysozyme (Lyso; pI = 11). Charged protein films were utilized to dictate cell adhesion on the substrate. As seen in Figure 5(d) anionic and neutral protein films generated using BSA and Hemo result in cytophobic films that prevent cell adhesion, while cationic Lyso results in cytophilic films that enhance cell adhesion. NIL also facilitated fabrication of patterned protein films that dictated cell alignment.
Figure 5.

(a) Schematic depiction of the NIL treatment of protein films using flat or patterned mold. (b) Heat map depicting the correlation between treatment conditions and stability of the films. Treatment at 180 °C and 3 MPa resulted in stable BSA, Hemo and Lyso films. (c) CD spectra of 1. Spin-coated protein films 2. NIL films and 3. Protein solution (0.1 mg/ml in 5 mM PB) depicting that treated protein films retained most of their secondary structure. (d) Cell adhesion on BSA, Hemo and Lyso films treated at 180 °C. BSA and Hemo show minimal cell adhesion while Lyso shows enhanced cell adhesion. Scale bars are 100 μm. Adapted with permission.[62] Copyright 2015, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
While NIL successfully translates native protein properties such as charge into the protein films, one major limitation of this approach is its restriction to 2D substrates. Experiments revealed that the pressure applied during NIL was not the factor that minimized denaturation, as protein films generated in a pressure chamber show significant denaturation.[22] It was hypothesized that the fluorous environment provided by the fluoro-silane mold minimized protein rearrangement at the interface, thereby preserving protein structure. Consequently, fluorous-cured protein films were generated by heating protein film in a fluorous solvent PFHP (perfluoroperhydrophenanthrene), as shown in Figure 6(a). [22] This resulted in water-stable protein films that are hydrophilic as compared to heat-cured (HC) protein films both treated at 180 °C for 15 min, as shown in Figure 6(b). PFHP-treated films retain most of their secondary structure as determined by CD spectra (Figure 6(c)). They also retain their surface charge, as evidenced by KPFM measurements of the surface potential shown in Figure 6(d), where the potential difference between HC-BSA and HC-Lyso films is significantly lesser than PFHP-BSA and PFHP-Lyso. This indicates that distinct surface charges of BSA (−) and Lyso (+) is retained during PFHP treatment. Furthermore, this strategy was successfully utilized to generate conformal protein coatings on 3D substrates, as seen in Figure 6(e) by brilliant blue staining and SEM images of protein coatings on dental screws – a model medical implant. As shown in Figure 6(f), negatively charged BSA protein was used to fabricate anti-fouling protein coatings on medical implants, using this strategy.
Figure 6.
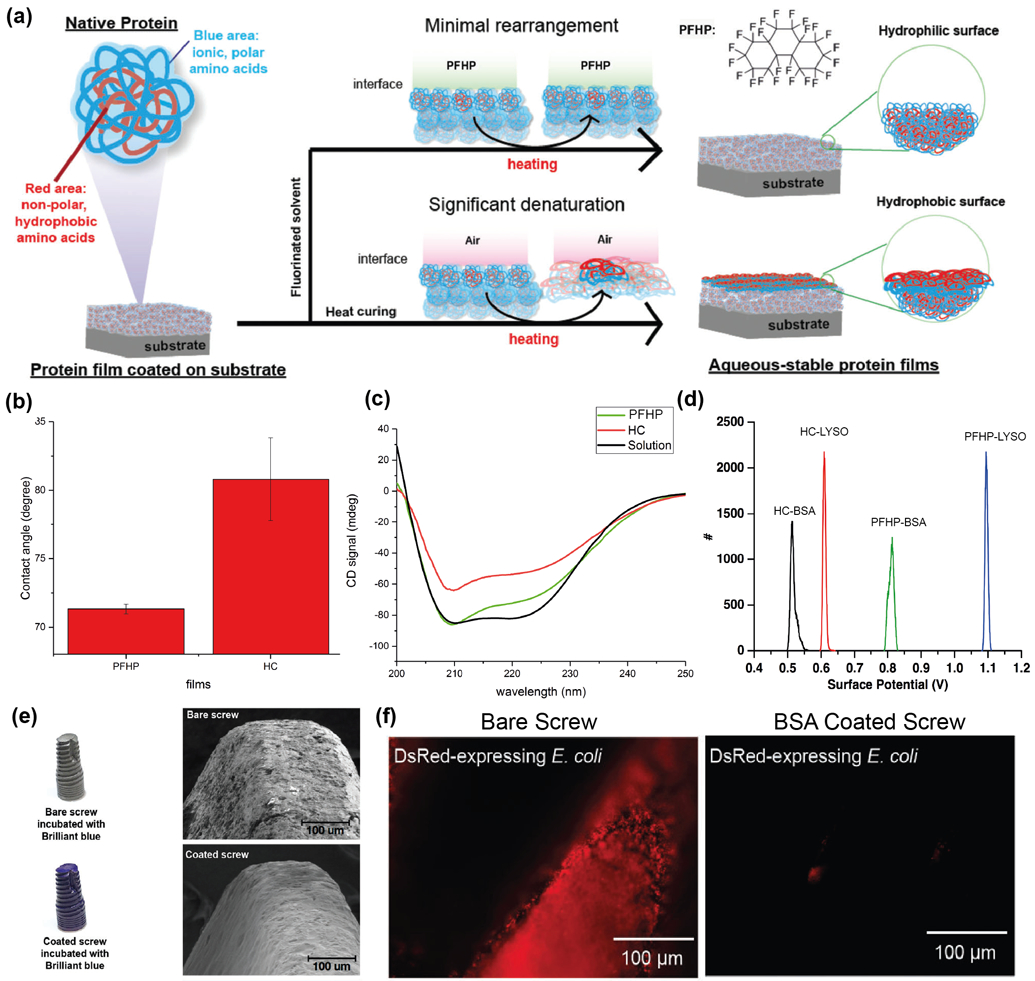
(a) Schematic depiction of fluorous treatment using PFHP, showing minimal rearrangement at interface of PFHP as compared to air. (b) Water contact angle measurement through static sessile drop method indicating PFHP treatment results in hydrophilic films as compared to heat-cured films. (c) CD spectra of PFHP treated films (green), heat-cured films (red) and BSA solution (black) shows minimal denaturation of protein due to thermal treatment in the case of PFHP as compared to traditional heat-curing. (d) Surface potential measured by KPFM (Kelvin Probe Force Microscopy) indicating significant potential difference between PFHP-treated BSA (−) and Lyso (+) as compared to heat-cured films. This suggests that PFHP-treated proteins retain their native charges. (e) Bare and BSA coated dental screws stained with Brilliant Blue protein stain. Coated screw shows uniformly dispersed blue color indicative of conformal coating. (f) Conformal coating of BSA on dental screw verified using SEM. BSA forms smooth, uniform coating on the screw. (g) Anti-fouling activity of BSA coating demonstrated through challenge of E. coli ds Red biofilm. No bacteria adhered to BSA coated screw while the bare screw has been completely contaminated. Adapted with permission.[22] Copyright 2018, The Royal Society of Chemistry.
Thermal treatment is also widely utilized to improve mechanical properties of natively self-assembled protein films.[57, 58, 59, 60] As-prepared collagen films are prone to mechanical damage due to wear-and-tear and tend to swell in aqueous media thereby resulting in loss of structural features, and are prone to rapid degradation due to proteolysis. [67] Thermal treatment of collagen has been shown to induce tighter packing of collagen fibers, thereby improving mechanical and structural properties.[68, 69] However, thermal treatment results in denaturation and oxidation of collagen fibers.[70] In a recent study, the effect of fluorous-based thermal stabilization on collagen fibers was studied (Figure 7). [23] Fluorous-curing (FC) and air-curing (AC) both result in a reduction of thickness proportional to the treatment temperature due to tighter packing of collagen fibers (Figure 7(a)). FC collagen films, however, showed a lower degree of swelling as compared to HC films, as seen in Figure 7(b), indicating higher structural stability of FC films in aqueous media. This stability was substantiated through CD and FTIR measurements, which indicated that heat-curing results in degradation of collagen fibers into a gelatin-like structure which is prevented by fluorous-curing. FC films also showed improved mechanical strength (Figure 7(c)) and enzymatic stability (Figure 7(d)), as compared to spin-coated and HC collagen films.
Figure 7.
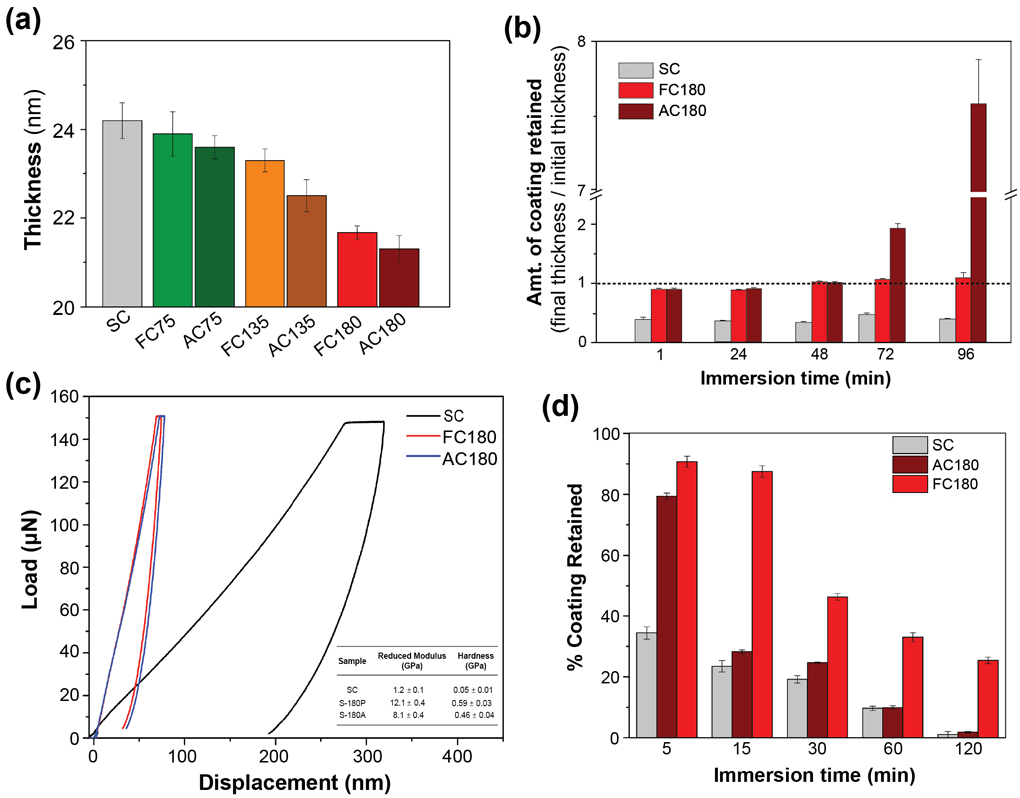
(a) Thickness of spin-coated (SC) collagen films and fluorous-cured and air-cured films (FCT and ACT, where T represents treatment temperature), obtained through Ellipsometry. Thickness of the film decreases with increased temperature due to tighter packing of collagen fibers. (b) Structural stability measurements through incubation of films in aqueous media for different durations of time. Increased thickness of AC films at 72 and 96 h indicates structural instability due to swelling. (c) Load-displacement curves of SC, FC180 and AC180 and (inset) reduced modulus and hardness measurements. FC180 shows higher reduced modulus and hardness. (d) Enzymatic stability measured through incubation in protease (trypsin) for different durations of time. FC180 shows slower degradation in enzyme as compared to AC180 and SC. Adapted with permission.[23] Copyright 2020, American Chemical Society.
Thermal treatment offers additive-free approaches for fabricating water-stable protein films. However, the high temperatures utilized in most thermal treatment strategies can cause uncontrolled protein denaturation and consequently loss of protein functionality/properties. These losses may be minimized by utilizing the NIL strategy summarized above however, the application of NIL is limited to flat, two-dimensional substrates. Fluorous-curing offers an alternative approach to the NIL-treated protein films by demonstrating similar robustness to the NIL approach. Additionally, PFHP-based fluorous curing has also shown to enhance mechanical and enzymatic stability in addition to aqueous stability. However, this PFHP-based thermal treatment approach is recent and its generalizability to different types of proteins remains untested.
2.4. Modification through surface functionalization or incorporation of additives
Modification strategies, either through surface functionalization or incorporation of additives into protein films, offer new areas of film application by endowing them with unique properties. Surface modifications are often conducted to increase either the hydrophilicity or hydrophobicity of protein films, impart specific chemical functionality to the film surface, or enhance biological properties such as cell adhesion. For instance, mussel-inspired dopamine chemistry has been utilized for the surface modification of silk fibroin used to fortify soy-protein adhesives.[24] Silk fibroin films have also been treated with ethanol to induce conformational and morphological modifications in the SF fibers, thereby increasing crystallinity, mechanical strength and water stability.[71] Superhydrophobic and self-cleaning films have also been developed by creating hierarchical micro-/nano-structures on soy protein film surfaces with hydrophobic nanoparticles.[72, 73] Adjusting the precursor concentration and crystal growth time constructed hierarchical micro-/nano-crystals on the soy protein film surface forming a biomimetic film with potential application as water harvesting materials. Gao et al. developed a strategy for grafting different functional groups on lysozyme protein films to impart different properties to the films. [74] Figure 8(a) illustrates superhydrophobic and superhydrophilic modifications on phase-transitioned lysozyme (PTL) films. Superhydrophobic coatings were generated through covalent attachment of perfluorooctanyl chloride on the surface, while superhydrophilic films were generated through the grafting of hydrophilic polymer POEGMA. (Figures 8(b) and (c)). Thermal stability of the superhydrophobic PTL films was evaluated by subjecting modified films to 200 °C and −196 °C, followed by recording SEM images of the surface morphology and the contact angle. As seen in Figures 8(d) and (e), no changes due to heating or cooling were observed. Mechanical stability was evaluated through the scotch tape test, shown in Figure 8(f). No peeling or significant change in contact angle was observed. PTL films have also been utilized to fabricate conformal and transparent coatings on different types of substrates as well as free-floating films with large areas.[75] This strategy of self-assembled phase transitioned proteins to generate amyloid-like aggregates is generalizable to other proteins such as BSA, [76] and has also been utilized to incorporate functional peptides on the protein film surface.[77] Wang et al. have utilized this approach to fabricate amelogenin mimics for controlling enamel remineralization by incorporating amelogenin into PTL films.[77]
Figure 8.
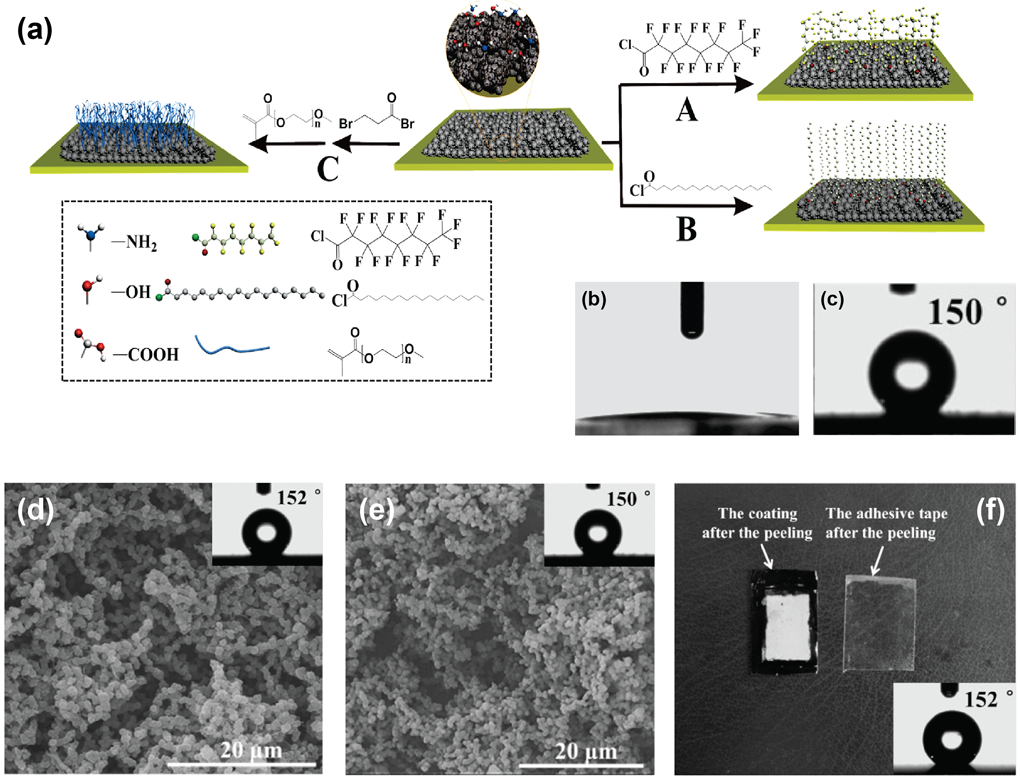
(a) Schematic depiction of the surface modification strategies to prepare A. superhydrophobic films, B. Hydrophobic films and C. superhydrophilic films. Contact angle measurements of (b) superhydrophilic film and (c) superhydrophobic film. Thermal stability observed through SEM images and contact angle measurements (inset) at (d) high temperature of 200 °C and (e) low temperature of −196 °C. No significant change due to temperature was observed. (f) Mechanical stability evaluated through scotch-tape test. No significant changes were observed. Adapted with permission.[70] Copyright 2015, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Incorporation of additives is another common approach to impart specific useful properties to protein films. Carbon nanotubes (CNTs) have been incorporated into different types of protein films to impart mechanical and electric properties to protein films. CNT/silk-elastin-like protein composites showed enhanced mechanical and piezoelectric properties, where mechanical deformation of film correlated with electrical conductivity;[78] Collagen-CNT composites enhanced stability and electrical conductivity, imparted electromagnetic shielding membrane;[79, 80] Hemoglobin-collagen-CNT composites have also been employed to fabricate a hydrogen peroxide biosensor.[81] Multifunctional films have been made using lysozyme and polyphosphate-based composites. Lysozyme, an antimicrobial enzyme, imparts antimicrobial activity to the film as well as an overall positive charge that enhances cell adhesion, while polyphosphate improves propensity for osteoblast proliferation and bone formation. These composite films provided titanium implant coatings to enhance bone-regenerative properties while preventing infections.[82]
Modification strategies offer efficient and robust methods to obtain protein films with a variety of functionalities. Surface modifications enable the fabrication of multifunctional films, while utilizing additives help overcome inherent limitations of native protein films such as rigidity, mechanical instability etc. A major limitation of both approaches is the cytotoxicity arising from the reagents utilized for modifications or the resultant by-products. Furthermore, often organic solvents must be utilized for some modification strategies, which may result in protein denaturation and loss of function.
3. Applications of protein coatings
Protein films are already widely used in biomedical and food packaging applications. Broadly, the applications of protein films may be categorized into the following - tissue engineering and wound-repair scaffolds, antimicrobial coatings, sensing and diagnostic devices, and food-packaging applications.
Tissue-engineering scaffolds must provide a cytophilic surface to enhance cell adhesion, provide support to the cells by mimicking stiffness of natural tissue and degrade at a rate proportional to cell growth.[83] For this reason, materials chosen for fabricating tissue engineering and wound-repair scaffolds must be biocompatible, have modulable mechanical properties and show enzymatic stability. Silk fibroin films are often employed as scaffolds for tissue engineering, [84] wound repair coatings,[85] and bioelectronic devices such as electrodes.[6, 86] Collagen is another protein that is extensively applied to tissue engineering and wound-healing scaffolds, as it is the dominant protein in the extracellular matrix. [87] Various crosslinking, thermal treatment and additive-based strategies have been utilized to enhance the mechanical and enzymatic stability of collagen films.
Another avenue for the application of protein films is as antimicrobial coatings. Antimicrobial coatings prevent the bacterial contamination of medical devices and consequent infections. Antimicrobial protein coatings are designed by three main strategies – anti-fouling coatings that prevent bacteria adhesion on the surface; contact-killing wherein antimicrobial agent incorporated within protein film kills bacteria adhered to the surface; controlled-release systems wherein the protein film acts as a scaffold to load and release antimicrobial agents. Properties of the native proteins have been translated into the protein film to design anti-fouling[22, 66] and antimicrobial (contact-killing) coatings.[75] Grafting of antimicrobial or anti-fouling polymers onto the surface is another approach to design contact killing systems.[88] Controlled-release coatings have also be prepared through the incorporation of antimicrobial agents such as chlorine,[89] antibiotics[51,90] and phytochemicals[4, 91] within the protein film.
Protein films are potential candidates for sensing and diagnostic tools owing to their varied functionalities. Smart, responsive films that respond to external stimuli have been fabricated using gelatin.[92] Silk fibroin films have been used for immunosensing applications through the incorporation of antibodies within the protein film.[93] Additionally, silk fibroin films have also been used to fabrication of flexible and wearable electronic skins for monitoring human physiological responses.[94, 95] Recently, wearable electronic devices have been developed using protein nanowire films for humidity sensing.[96] Composite materials of amyloid aggregates and graphene have exhibited shape memory and enzyme sensing properties.[97]
Additionally, protein coatings have been widely used in food packaging applications. Gelatin coatings are commonly used to enhance the shelf-life of vegetables;[98] gelatin,[99] whey[100] and soy[101] protein isolate films are used in meat packaging, while zein[102] and wheat[103] protein films have been used in the packaging of dairy products.
4. Conclusions and Perspectives
This review discusses strategies for the fabrication of stable protein films, and the application of these biomaterials. Proteins are naturally occurring functional building blocks that are water-processible, making them ideal for designing sustainable and biodegradable materials. Furthermore, the choice of protein can be used to dictate properties such as biocompatibility, cytophilicity, anti-fouling and antimicrobial behavior, all of which are especially attractive for biomedical applications. Protein film stabilization strategies predominantly use one of the three different strategies discussed in this review – (1) using self-assembling structural proteins (2) using chemical or physical crosslinking strategies and (3) using thermal treatment strategies. Additionally, strategies employing post-functional modifications and additives to enhance properties of protein films are frequently utilized in combination with the above approaches.
Protein films have long been used in applications such as edible coatings for food packaging,[104] surface patterning strategies,[105] biodegradable coatings[106] and plastic alternatives,[107] and tissue engineering scaffolds.[108] More recently, fabrication strategies have been developed that greatly expand the range of properties accessible with these materials. Protein properties have been translated into films to fabricate anti-fouling and bactericidal coatings, [ 22, 66, 82, 87] and cargo-loaded protein films have been fabricated and applied as biodegradable antimicrobial coatings. [4, 51, 89, 90, 91]
Looking forward, the development of scalable and cost-effective fabrication strategies will allow for the translation of more sophisticated protein-based materials into commercial applications. Development of 2D and 3D patterning strategies will enable complex designs that will facilitate the creation of tissue engineering scaffolds that mimic native tissue properties. Furthermore, the ability to harness native protein properties such as stimuli-responsiveness (enzymes) and binding specificity (receptors) will provide new responsive materials. These active materials will open up numerous applications including bio-sensing, point-of-care diagnostics and controlled/triggered release systems.
Acknowledgements
The support of the NIH (AI134770) is gratefully acknowledged.
Biography
Sanjana Gopalakrishnan
Sanjana Gopalakrishnan received her B.S. in chemistry from the Indian Institute of Technology, Kanpur in 2016, where she studied the application of AFM in the characterization of biomaterials under Professor T. G. Gopakumar. She is currently a Ph.D. candidate at the Chemistry Department in University of Massachusetts, Amherst under the supervision of Professor Vincent M. Rotello. Her current research is focused on developing functional biomaterials for applications such as anti-fouling surfaces and antimicrobial systems.

Jinlong Xu
Jinlong Xu received his B.S. in food science from Henan Agricultural University, China in 2013. He then received his M. S. degree in food science from Hefei University of Technology, China in 2016, where he studied the natural macromolecular polysaccharides under the supervision of Professor Junhui Wang. He is currently a Ph.D. candidate at the School of Food Science and Technology, Jiangnan University, China under the supervision of Professor Fang Zhong. His current research is focused on the natural food packaging materials.

Prof. Vincent M. Rotello
Vincent Rotello is the Charles A. Goessmann Professor of Chemistry and a University Distinguished Professor at the University of Massachusetts at Amherst. He received his B.S. in 1985 from Illinois Institute of Technology, his Ph. D. in 1990 from Yale University, and was an NSF postdoctoral fellow at M.I.T. from 1990-1993, joining the faculty at the University of Massachusetts in 1993. He is the Editor in Chief of Bioconjugate Chemistry and is on the Editorial Board of 14 other journals. His research spans the areas of devices, polymers, and nanotechnology/bionanotechnology, with over 590 peer-reviewed papers published to date.

References
- [1].DeFrates KG, Moore R, Borgesi J, Lin G, Mulderig T, Beachley V, Hu X, Nanomaterials 2018, 8, 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang L-S, Gopalakrishnan S, Rotello VM, Langmuir 2018, 35, 10993. [DOI] [PubMed] [Google Scholar]
- [3].Andonegi M, Peñalba M, de la Caba K, Guerrero P, Materials Science and Engineering: C 2020, 108, 110394. [DOI] [PubMed] [Google Scholar]
- [4].Lin L, Liao X, Cui H, Food Packaging and Shelf Life 2019, 21, 100337. [Google Scholar]
- [5].Bruni GP, de Oliveira JP, Gómez-Mascaraque LG, Fabra MJ, Martins VG, da Rosa Zavareze E, López-Rubio A, Food Packaging and Shelf Life 2020, 23, 100426. [Google Scholar]
- [6].Holland C, Numata K, Rnjak-Kovacina J, Seib FP, Adv. Healthc. Mater 2019, 8, 1800465. [DOI] [PubMed] [Google Scholar]
- [7].Goder D, Matsliah L, Giladi S, Reshef-Steinberger L, Zin I, Shaul A, Zilberman M, International Journal of Polymeric Materials and Polymeric Biomaterials 2020, 1. [Google Scholar]
- [8].Valentini L, Bittolo Bon S, Pugno NM, Nanomaterials 2018, 8, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kuruwita DP, Jiang X, Darby D, Sharp JL, Fraser AM, Food Control 2020, 112, 107153. [Google Scholar]
- [10].Tao H, Kaplan DL, Omenetto FG, Adv. Mater 2012, 24, 2824. [DOI] [PubMed] [Google Scholar]
- [11].Arora A, Padua G, J. Food Sci 2010, 75, R43. [DOI] [PubMed] [Google Scholar]
- [12].Zhao S, Wang Z, Kang H, Li J, Zhang S, Han C, Huang A, Ind. Crops Prod 2018, 122, 366. [Google Scholar]
- [13].Hassan B, Chatha SAS, Hussain AI, Zia KM, Akhtar N, Int. J. Biol. Macromol 2018, 109, 1095. [DOI] [PubMed] [Google Scholar]
- [14].Abaee A, Mohammadian M, Jafari SM, Trends Food Sci. Technol 2017, 70, 69. [Google Scholar]
- [15].Perez-Puyana V, Jiménez-Rosado M, Romero A, Guerrero A, J. Appl. Polym. Sci 2019, 136, 47954. [Google Scholar]
- [16].Kord Forooshani P, Lee BP, J. Polym. Sci., Part A: Polym. Chem 2017, 55, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhu L, Yin P, Xie T, Liu X, Yang L, Wang S, Li J, Liu H, Colloids Surf. B. Biointerfaces 2020, 186, 110707. [DOI] [PubMed] [Google Scholar]
- [18].Zhao S, Wang Z, Pang H, Li Z, Zhang W, Zhang S, Li J, Li L, Macromol. Mater. Eng 2020, 305, 1900462. [Google Scholar]
- [19].Borkner CB, Elsner MB, Scheibel T, ACS Appl. Mater. Interfaces 2014, 6, 15611. [DOI] [PubMed] [Google Scholar]
- [20].Hu X, Cebe P, Weiss AS, Omenetto F, Kaplan DL, Mater. Today 2012, 15, 208. [Google Scholar]
- [21].Azeredo HMC, Waldron KW, Trends Food Sci. Technol 2016, 52, 109. [Google Scholar]
- [22].Wang L-S, Gopalakrishnan S, Lee Y-W, Zhu J, Nonnenmann SS, Rotello VM, Materials Horizons 2018, 5, 268. [Google Scholar]
- [23].Zhang L, Gopalakrishnan S, Li K, Wang L.-s., Han Y, Rotello VM, ACS Appl. Mater. Interfaces 2020, 12, 6590. [DOI] [PubMed] [Google Scholar]
- [24].Pang H, Zhao S, Mo L, Wang Z, Zhang W, Huang A, Zhang S, Li J, J Appl. Polym. Sci 2020, 137, 48785. [Google Scholar]
- [25].Zhang S, Xia C, Dong Y, Yan Y, Li J, Shi SQ, Cai L, Ind. Crops Prod 2016, 80, 207. [Google Scholar]
- [26].Huang W, Ling S, Li C, Omenetto F, Kaplan DL, Chem. Soc. Rev 2018, 47, 6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Feroz S, Muhammad N, Ratnayake J, Dias G, Bioact. Mater 2020, 5, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zubair M, Ullah A, Critical Reviews in Food Science and Nutrition 2020, 60, 406. [DOI] [PubMed] [Google Scholar]
- [29].Avramescu SM, Butean C, Popa CV, Ortan A, Moraru I, Temocico G, Coatings 2020, 10, 687. [Google Scholar]
- [30].Shavandi A, Silva TH, Bekhit AA, Bekhit AE-DA, Biomaterials science 2017, 5, 1699. [DOI] [PubMed] [Google Scholar]
- [31].Bax DV, Smalley HE, Farndale RW, Best SM, Cameron RE, Acta Biomater. 2019, 86, 158. [DOI] [PubMed] [Google Scholar]
- [32].Chen X, Knight DP, Shao Z, Vollrath F, Biochemistry 2002, 41, 14944. [DOI] [PubMed] [Google Scholar]
- [33].Jiang C, Wang X, Gunawidjaja R, Lin YH, Gupta MK, Kaplan DL, Naik RR, Tsukruk VV, Adv. Funct. Mater 2007, 17, 2229. [Google Scholar]
- [34].You R, Zhang J, Gu S, Zhou Y, Li X, Ye D, Xu W, Materials Science and Engineering: C 2017, 79, 430. [DOI] [PubMed] [Google Scholar]
- [35].Liu H, Fan H, Wang Y, Toh SL, Goh JC, Biomaterials 2008, 29, 662. [DOI] [PubMed] [Google Scholar]
- [36].Jin HJ, Park J, Karageorgiou V, Kim UJ, Valluzzi R, Cebe P, Kaplan DL, Adv. Funct. Mater 2005, 15, 1241. [Google Scholar]
- [37].Lu Q, Hu X, Wang X, Kluge JA, Lu S, Cebe P, Kaplan DL, Acta Biomater. 2010, 6, 1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xu J, Liu F, Goff HD, Zhong F, Food Hydrocoll 2019, 105326. [Google Scholar]
- [39].Diaz Quiroz JF, Rodriguez PD, Erndt-Marino JD, Guiza V, Balouch B, Graf T, Reichert WM, Russell B, Höök M, Hahn MS, ACS Biomaterials Science & Engineering 2018, 4, 2934. [DOI] [PubMed] [Google Scholar]
- [40].Chattopadhyay S, Raines RT, Biopolymers 2014, 101, 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].McManamon C, Cameron A, de Silva JP, Daly R, O’Brien FJ, Cross GL, Acta Biomater 2020. [DOI] [PubMed] [Google Scholar]
- [42].Xu J, Liu F, Wang T, Goff HD, Zhong F, Food Hydrocoll 2020, 106016. [Google Scholar]
- [43].Costa MJ, Marques AM, Pastrana LM, Teixeira JA, Sillankorva SM, Cerqueira MA, Food Hydrocoll 2018, 81, 442. [Google Scholar]
- [44].Ostrowska-Czubenko J, Gierszewska-Drużyńska M, Carbohydr. Polym 2009, 77, 590. [DOI] [PubMed] [Google Scholar]
- [45].Guan Y, You H, Cai J, Zhang Q, Yan S, You R, Carbohydr. Polym 2020, 116232. [DOI] [PubMed] [Google Scholar]
- [46].Stern R, Maibach HI, Clin. Dermatol 2008, 26, 106. [DOI] [PubMed] [Google Scholar]
- [47].Cheung DT, Nimni ME, Connect. Tissue Res 1982, 10, 187. [DOI] [PubMed] [Google Scholar]
- [48].Chen X, Zhou L, Xu H, Yamamoto M, Shinoda M, Tada I, Minami S, Urayama K, Yamane H, Int. J. Biol. Macromol 2019, 135, 959. [DOI] [PubMed] [Google Scholar]
- [49].Iahnke A. O. e. S., Stoll L, Bellé AS, Hertz PF, Rios A. d. O., Rahier H, Flôres SH, Packag. Technol. Sci 2020, 33, 15 [Google Scholar]
- [50].Mi X, Chang Y, Xu H, Yang Y, Food Chem. 2019, 300, 125181. [DOI] [PubMed] [Google Scholar]
- [51[.Bajpai S, Shah FF, Bajpai M, Designed monomers and polymers 2017, 20, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sarheed O, Rasool BKA, Abu-Gharbieh E, Aziz US, AAPS Pharm. Sci. Tech 2015, 16, 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zheng T, Yu X, Pilla S, Carbohydr. Polym 2017, 157, 1333. [DOI] [PubMed] [Google Scholar]
- [54].Buchert J, Ercili Cura D, Ma H, Gasparetti C, Monogioudi E, Faccio G, Mattinen M, Boer H, Partanen R, Selinheimo E, Lantto R, Kruus K, Annu. Rev. Food Sci. Technol 2010, 1, 113. [DOI] [PubMed] [Google Scholar]
- [55].Cheng S, Wang W, Li Y, Gao G, Zhang K, Zhou J, Wu Z, Food Chem. 2019, 271, 527. [DOI] [PubMed] [Google Scholar]
- [56].Kchaou H, Benbettaieb N, Jridi M, Nasri M, Debeaufort F, Food Hydrocoll. 2019, 97, 105196. [Google Scholar]
- [57].Li S, Donner E, Thompson M, Zhang Y, Rempel C, Liu Q, Eur. Polym. J 2017, 89, 419. [Google Scholar]
- [58].Rhim J-W, Gennadios A, Lee J-J, Weller CL, Hanna MA, Food Sci Biotechnol 2003, 12, 96. [Google Scholar]
- [59].Olsson E, Hedenqvist MS, Johansson C, Järnström L, Carbohydr. Polym 2013, 94, [DOI] [PubMed] [Google Scholar]
- [60].Amin S, Ustunol Z, Int. J. Dairy Technol 2007, 60, 149. [Google Scholar]
- [61].Rhim J, J. Agric. Food Chem 2000, 48, 4937. [DOI] [PubMed] [Google Scholar]
- [62].Micard V, Belamri R, Morel M-H, Guilbert S, J. Agric. Food Chem 2000, 48, 2948. [DOI] [PubMed] [Google Scholar]
- [63].Ali Y, Ghorpade VM, Hanna MA, Ind. Crops Prod 1997, 6, 177. [Google Scholar]
- [64].Neo YP, Perera CO, Nieuwoudt MK, Zujovic Z, Jin J, Ray S, Gizdavic-Nikolaidis M, J. Agric. Food Chem 2014, 62, 5163. [DOI] [PubMed] [Google Scholar]
- [65].Shi D, Liu F, Yu Z, Chang B, Goff HD, Zhong F, Food Hydrocoll. 2019, 87, 436. [Google Scholar]
- [66].Jeoung E, Duncan B, Wang LS, Saha K, Subramani C, Wang P, Yeh YC, Kushida T, Engel Y, Barnes MD, Rotello VM Adv. Mater 2015, 27, 6251. [DOI] [PubMed] [Google Scholar]
- [67].Sun L, Li B, Yao D, Song W, Hou H, J. Mech. Behav. Biomed. Mater 2018, 80, 51. [DOI] [PubMed] [Google Scholar]
- [68].Koide M, Osaki K, Konishi J, Oyamada K, Katakura T, Takahashi A, Yoshizato K, J. Biomed. Mater. Res 1993, 27, 79. [DOI] [PubMed] [Google Scholar]
- [69].Sionkowska A, Skopinska-Wisniewska J, Gawron M, Kozlowska J, Planecka A, Int. J. Biol. Macromol 2010, 47, 570. [DOI] [PubMed] [Google Scholar]
- [70].Samouillan V, Delaunay F, Dandurand J, Merbahi N, Gardou J-P, Yousfi M, Gandaglia A, Spina M, Lacabanne C, J. Funct. Biomater 2011, 2, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Puerta M, Arango MC, Jaramillo-Quiceno N, Álvarez-López C, Restrepo-Osorio A, SN Applied Sciences 2019, 1, 1443. [Google Scholar]
- [72].Xie W-Y, Wang F, Xu C, Song F, Wang X-L, Wang Y-Z, Chem. Eng. J 2017, 326, 436. [Google Scholar]
- [73].Liu, Xie W-Y, Song F, Wang X-L, Wang Y-Z, Chem. Eng. J 2019, 369, 1040. [Google Scholar]
- [74].Gao A, Wu Q, Wang D, Ha Y, Chen Z, Yang P, Adv. Mater 2016, 28, 579. [DOI] [PubMed] [Google Scholar]
- [75].Wang D, Ha Y, Li Q, Zhang L, Yang P, Adv. Mater 2016, 28, 7414. [DOI] [PubMed] [Google Scholar]
- [76].Hu X, Tian J, Li C, SU=u H, Qin R, Wang Y, Cao X, Yang P, Adv. Mater 2020, 32, 2000128. [DOI] [PubMed] [Google Scholar]
- [77].Wang D, Deng J, Deng X, Fang C, Zhang X, Yang P, Adv. Mater 2020, 32, 2002080. [DOI] [PubMed] [Google Scholar]
- [78].Liu C, Ye X, Wang X, Liao X, Huang X, Shi B, Ind. Eng. Chem. Res 2017, 56, 8553. [Google Scholar]
- [79].Meiyazhagan A, Thangavel S, Palanisamy T, Mater. Chem. Phys 2015, 157, 8. [Google Scholar]
- [80].Wang J, He C, Cheng N, Yang Q, Chen M, You L, Zhang Q, J Nanosci Nanotechnol 2015, 15, 4844. [DOI] [PubMed] [Google Scholar]
- [81].Li J, Mei H, Zheng W, Pan P, Sun X, Li F, Guo F, Zhou H, Ma J, Xu X, Colloids Surf. B. Biointerfaces 2014, 118, 77. [DOI] [PubMed] [Google Scholar]
- [82].Xu X, Zhang D, Gao S, Shiba T, Yuan Q, Cheng K, Tan H, Li J, Biomacromolecules 2018, 19, 1979. [DOI] [PubMed] [Google Scholar]
- [83].Xing H, Lee H, Luo L, Kyriakides TR, Biotech. Adv 2020, 42, 107421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Keirouz A, Zakharova M, Kwon J, Robert C, Koutsos V, Callanan A, Chen X, Fortunato G, Radacsi N, Materials Science and Engineering: C 2020, 110939. [DOI] [PubMed] [Google Scholar]
- [85].Arthe R, Arivuoli D, Ravi V, Int. J. Biol. Macromol 2019. [DOI] [PubMed] [Google Scholar]
- [86].Chen G, Matsuhisa N, Liu Z, Qi D, Cai P, Jiang Y, Wan C, Cui Y, Leow WR, Liu Z, Adv. Mater 2018, 30, 1800129. [DOI] [PubMed] [Google Scholar]
- [87].Pereda M, Ponce A, Marcovich N, Ruseckaite R, Martucci J, Food Hydrocoll. 2011, 25, 1372. [Google Scholar]
- [88].Li S, Donner E, Xiao H, Thompson M, Zhang Y, Rempel C, Liu Q, Materials Science and Engineering: C 2016, 69, 947. [DOI] [PubMed] [Google Scholar]
- [89].Wang L-S, Gupta A, Duncan B, Ramanathan R, Yazdani M, Rotello VM, ACS Biomaterials Science & Engineering 2016, 2, 1862. [DOI] [PubMed] [Google Scholar]
- [90].Egozi D, Baranes-Zeevi M, Ullmann Y, Gilhar A, Keren A, Matanes E, Berdicevsky I, Krivoy N, Zilberman M, Burns 2015, 41, 1459. [DOI] [PubMed] [Google Scholar]
- [91].Vahedikia N, Garavand F, Tajeddin B, Cacciotti I, Jafari SM, Omidi T, Zahedi Z, Colloids Surf. B. Biointerfaces 2019, 177, 25. [DOI] [PubMed] [Google Scholar]
- [92].Musso YS, Salgado PR, Mauri AN, Food Hyd. 2017, 66, 8. [Google Scholar]
- [93].Moraes ML, Lima LR, Vincentini-Oliveira JC, de Souza AVG, Oliveira ON, Deffune E, Ribero SJL, J. Nanosci. Nanotechnol 2019, 19, 3772. [DOI] [PubMed] [Google Scholar]
- [94].Wang X, Gu Y, Xiong Z, Cui Z, Zhang T, Adv. Mater 2013, 26, 1336. [DOI] [PubMed] [Google Scholar]
- [95].Liu J, Chen Q, Liu Q, Zhao B, Ling S, Yao J, Shao Z, Chen X, Adv. Mater. Tech 2020, 5, 2000430. [Google Scholar]
- [96].Liu X, Fu T, Ward J, Gao H, Yin B, Woodard T, Lovley D, Yao J, Adv. Elec. Mater 2020, 2000721. [Google Scholar]
- [97].Li C, Adamcik J, Mezzenga R, Nature Nanotech. 7, 421. [DOI] [PubMed] [Google Scholar]
- [98].Wang X, Kong D, Ma Z, Zhao R, Irish Journal of Agricultural and Food Research 2015, 54, 64. [Google Scholar]
- [99].Kodal Coşkun B, Çalikoğlu E, Karagöz Emiroğlu Z, Candoğan K, J. Food Qual 2014, 37, 203. [Google Scholar]
- [100].Badr K, Ahmed ZS, El Gamal M, Int. J. Agric. Res 2014, 9, 242. [Google Scholar]
- [101].Thaker M, Hanjabam MD, Gudipati V, Kannuchamy N, J. Food Process. Eng 2017, 40, e12270. [Google Scholar]
- [102].Di Pierro P, Sorrentino A, Mariniello L, Giosafatto CVL, Porta R, LWT-Food science and technology 2011, 44, 2324. [Google Scholar]
- [103].Ünalan İU, Arcan I, Korel F, Yemenicioğlu A, Innov. Food Sci. Emerg. Technol 2013, 20, 208. [Google Scholar]
- [104].Su JF, Huang Z, Yuan XY, Wang XY, Li M, Carb. Pol 2010. 79, 1, 145–153 [Google Scholar]
- [105].Clemmens J, Hess H, Lipscomb R, Hanein Y, Bohringer KF, Matzke CM, Bachand GD, Bunker BC, Vogel V Langmuir 2003. 19, 26, 10967–10974 [Google Scholar]
- [106].Gilbert V, Rouabhia M, Wang HX, Arnould AL, Remondetto G, Subirade M Biomaterials 2005. 77, 1, 101–104 [DOI] [PubMed] [Google Scholar]
- [107].Chen Y, Zhang L, Lu Y, Ye C, Du L, J. Appl. Polym. Sci, 2003. 90, 3790–3796 [Google Scholar]
- [108].Levesque SG, Lim RM, Shoichet MS Biomaterials, 2005. 26, 35, 7436–7446 [DOI] [PubMed] [Google Scholar]


