Abstract
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had health implications of unprecedented magnitude. The infection can range from asymptomatic, mild to life threatening respiratory distress. It can affect almost every organ of the body. Ophthalmologists world over are reporting various manifestations of the infection in the eye. This review was undertaken to help ophthalmologists recognize the possible manifestations and the stage of the viral disease when they commonly appear. Literature search was performed for the publications on ophthalmic manifestations of coronavirus disease-19 (COVID-19) between January 1, 2020 and January 31, 2021. 46 case reports, 8 case series, 11 cross sectional/cohort observational studies, 5 prospective interventional studies, 3 animal models/autopsy studies and 6 reviews/meta-analysis were included. Conjunctivitis is the most common manifestation and can develop at any stage of the disease. Direct effect due to virus, immune mediated tissue damage, activation of the coagulation cascade and prothrombotic state induced by the viral infection, the associated comorbidities and drugs used in the management are responsible for the findings in the eye. The viral ribonucleic acid (RNA) has been isolated from ocular tissues but the role of eye as a route for infection is yet to be substantiated. Ophthalmic manifestations may be the presenting feature of COVID-19 infection or they may develop several weeks after recovery. Ophthalmologists should be aware of the possible associations of ocular diseases with SARS-CoV-2 in order to ask relevant history, look for specific signs, advise appropriate tests and thereby mitigate the spread of infection as well as diagnose and initiate early treatment for life and vision threatening complications.
Keywords: COVID-19, ophthalmic manifestations, SARS-CoV-2, follicular conjunctivitis, central retinal vein occlusion, central retinal artery occlusion, mucormycosis, optic neuritis, cranial nerve palsy
The coronavirus global pandemic has had far-reaching and lasting consequences. The full spectrum of the disease is yet to be unraveled. The permanent sequelae to different organs, the multitude of presentations, the theories of pathogenesis, and the true associations with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are being reported and studied at a rate that may only be described as a scientifically demanded frenzy. A simple search of 'COVID-19' in search engines throws up about a lakh of articles. If we refine it with the addition of the word 'Ophthalmology', the results are less staggering but none the less large with over 1000 publications. To bring down the numbers to more conquerable values, 'ophthalmic manifestations' has just over 100 publications. We believe it is important for ophthalmologists to have knowledge about the ophthalmic manifestations of the novel viral infection in order to suspect, diagnose, refer and treat the conditions with skills, machinery, and drugs that we already possess. This article gives an overview of the ophthalmic conditions that have been associated with the virus, directly or indirectly. We have also tried to categorize the manifestations into the phase of the coronavirus disease-19 (COVID-19) when they are most likely to present.
Methods
Literature search was performed in PubMed for 'COVID-19', 'SARS-CoV-2', 'ophthalmology', 'ophthalmic manifestations', 'anterior segment', 'conjunctiva', 'ocular surface', 'retina', 'choroid', 'uveitis', 'neuro-ophthalmology', 'cranial nerve palsy', and 'orbit'. Articles in the English language, published between January 1, 2020 to January 31, 2020, were included to formulate the description of the current understanding of the ophthalmic manifestations of SAR-CoV-2 virus. While the search is not exhaustive, we have tried to include the important and unique articles. We have also included the observations and cases seen by the authors in the relevant sections. 46 case reports, 8 case series, 11 cross-sectional/cohort observational studies, 5 prospective interventional studies, 3 animal models/autopsy studies, and 6 reviews/meta-analysis were included. Severity of COVID-19 disease was considered as per the description in the article, if mentioned, or based on the symptoms and management described. All the cases were diagnosed as COVID-19 based on nasopharyngeal or oropharyngeal swabs or antibody titers. The statistical analysis was done using Microsoft Excel.
Eyelid, Ocular Surface and Anterior Segment Manifestations of COVID-19
Ocular surface and cornea
In our review of literature, there were 120 patients with ocular surface and corneal symptoms and signs. The mean age was 45± 15.3 (range 24-72, median 46.9) years. The median gap between COVID-19 symptom/diagnosis and ophthalmic findings was 8.5 (mean 11.1 ± 8.8, 2–32) days. But it was the initial or concurrent presentation in 12/26 published articles. Table 1 shows the case reports, case series, and cross-sectional studies that have reported ocular surface findings.
Table 1.
Review of literature of ocular surface and corneal manifestations of COVID-19
| Study | Type | Location | Sample (ocular manifestation/ total sample studied) | Age (mean/ median) (years) | Duration between COVID-19 symptoms/diagnosis and ophthalmic symptoms (days) | Diagnosis |
|---|---|---|---|---|---|---|
| Sindhuja et al.[1] | Retrospective cross sectional | India | 11/127 | 38.8 (median) | Mean 9.4 Initial in one | Conjunctival congestion, lid edema, hyperaemia, watering |
| Nayak et al.[4] | Case | India | 1 | 65 | 32 | Follicular conjunctivitis |
| Wu et al.[13] | Retrospective case series | China | 12/38 | 67 (mean) | NA | Conjunctival chemosis, epiphora, hyperaemia, secretions |
| Chen et al.[2] | Cross sectional study | China | 27/535 | 44 (median) | NA | Conjunctival congestion |
| Scalinci et al.[29] | Case series | Italy | 5 | 46.8 (mean) | Initial | Conjunctival chemosis, epiphora |
| Chen et al.[3] | Case | China | 1 | 30 | 13 | Follicular conjunctivitis |
| Guan et al.[14] | Retrospective cohort | China | 9/1099 | 47 (median) | NA | Conjunctivitis |
| Colavita et al.[31] | Case | Italy | 1 | 65 | Initial | Conjunctivitis |
| Daruich et al.[32] | Case | Argentina | 1 | 27 | Initial | Conjunctival hyperaemia, eyelid edema |
| Khavandi et al.[33] | Case | Iran | 1 | 65 | Initial | Follicular conjunctivitis |
| Salducci et al.[34] | Case | Italy | 1 | 72 | Concurrent | Conjunctivitis |
| Zhou et al.[15] | Retrospective cohort | China | 1/67 | 35.7 | Initial | Conjunctivitis |
| Xia et al.[16] | Prospective interventional | China | 1/30 | 53 | 3 | Conjunctivitis |
| Atum et al.[17] | Prospective interventional | Turkey | 10/40 | 41.3 (mean) | NA | Conjunctivitis |
| Zhang et al.[18] | Cross sectional | China | 2/72 | NA | Initial in one | Conjunctivitis |
| Lan et al.[19] | Cross sectional | China | 3/81 | Na | 16 | Dryness, conjunctival chemosis, swelling, itching |
| Karimi et al.[20] | Cross sectional | Iran | 2/43 | NA | NA | Foreign body sensation, follicular conjunctivitis |
| Hong et al.[21] | Cross sectional | China | 15/56 | 50 (mean) | Initial in 6 | Redness, dryness FB sensation, pain |
| Seah et al.[22] | Prospective case series | Singapore | 1/17 | NA | 17 | Conjunctival injection and chemosis |
| Zhou et al.[23] | Cross sectional | China | 8/121 | NA | NA | Itching, redness, tearing, foreign body sensation |
| Goel et al.[30] | Letter to editor | India | 2 | 38 (mean) | Initial | Conjunctivitis |
| Cheema et al.[5] | Case | Canada | 1 | 29 | Initial | Keratoconjunctivitis |
| Guo et al.[6] | Case | China | 1 | 53 | 10 | Relapsing viral keratoconjunctivitis |
| Navel et al.[7] | Case | France | 1 | 63 | 19 | Haemorrhagic, pseudomembranous conjunctivitis |
| Otaif et al.[10] | Case | Saudi Arabia | 1 | 29 | Initial | Episcleritis |
| Mangana et al.[11] | Letter to editor | Spain | 1 | 31 | 7 | Episcleritis |
FB: Foreign body
-
Follicular conjunctivitis
Conjunctivitis is the most common ophthalmic manifestation documented in COVID-19 patients. In a large series of cases with mild COVID-19 infection, Sindhuja et al. reported that 11/127 (8.66%) patients had conjunctivitis. All symptomatic patients gave a history of redness of one or both eyes. Presence of respiratory tract symptoms were associated with conjunctival congestion. A positive history of hand-eye contact was elucidated in four patients; however, this did not attain clinical significance as a risk factor.[1] This was different from the results of a cross-sectional study performed by Chen et al. in 535 cases of COVID-19 patients which showed that hand-eye contact was independently correlated to the presence of conjunctival congestion amongst patients.[2]
Chen et al. suggested that ocular manifestations are more common in the middle phase of the disease based on their findings of bilateral acute follicular conjunctivitis in a patient on the 13th day of the illness. [Fig. 1] The conjunctival swab remained positive for five days, though with progressively increasing cycle threshold (Ct) values.[3] Nayak et al. reported delayed onset of follicular conjunctivitis four weeks after severe COVID-19 infection in a 65-year-old male with diabetes, hypertension, and asthma. The conjunctival swab did not reveal any bacterial or fungal infection. The conjunctivitis resolved in two weeks with lubricants and preservative-free moxifloxacin eye drops. The authors also concluded that virus shedding in the conjunctiva may persist even after the nasopharyngeal swab becomes negative for SAR-CoV virus.[4]
-
Viral keratoconjunctivitis
Keratoconjunctivitis as the initial presentation in a patient with mild respiratory symptoms has been reported by Cheema et al. The patient presented with redness, discharge, and photophobia and was treated as herpetic keratoconjunctivitis, and later, as epidemic keratoconjunctivitis with oral valacyclovir and topical moxifloxacin. SARS-CoV-2 testing was done only in view of updated guidelines for testing patients with travel history in Canada. The nasopharyngeal and conjunctival swabs both turned out to be positive. This case highlights the importance of considering conjunctivitis as a presenting symptom of COVID-19.[5]
In a case report from China, Guo et al. reported a patient with moderate-severe COVID-19 infection with left eye conjunctivitis developing ten days after COVID-19 symptoms. In the first episode, the cornea was clear, and patient had viscous discharge. Conjunctival swab was positive for SARS-CoV-2 virus ribonucleic acid (RNA) by reverse transcriptase polymerase chain reaction (RT-PCR) but not for herpes simplex virus (HSV) or adenovirus and this was repeated daily. On the second day after initiation of treatment with topical levofloxacin and sodium hyaluronate, the swab became negative. Patient recovered well within a week but presented with a relapse and peripheral corneal staining in both eyes after five days. This time the conjunctival swab was negative for both SARS-CoV-2 and HSV. However, the interleukin-6 (IL-6) levels showed ten-fold elevation in the left eye. With an immune-mediated pathogenesis in mind, topical fluoromethalone was started and patient responded well with complete resolution. Since SAR-CoV-2 virus was detected in the conjunctiva, the first episode of conjunctivitis was attributed to local invasion and inflammation of the ocular surface caused by the virus. It was localized to the left eye with resolution within a week. The relapse, with more widespread bilateral manifestation, on the other hand, was presumed to be due to a cytokine surge caused by an autoimmune response mediated by the virus. A longer follow-up with proper use of topical glucocorticoid is recommended by some to diminish the risk of immune-mediated keratoconjunctivitis.[6]
-
Hemorrhagic and pseudomembranous conjunctivitis
Navel et al. in France reported a case of a 63-year-old male patient with severe COVID-19 infection, admitted in intensive care unit (ICU), developing hemorrhagic and pseudomembranous conjunctivitis 19 days after the onset of systemic symptoms. Treatment was with azithromycin and dexamethasone drops and daily debridement of pseudomembrane.[7]
-
Conjunctivitis in children
A 30 fold increase in the incidence of Kawasaki disease-like condition has been reported in children in some parts of Italy with strong association with COVID-19. This atypical presentation is known as multisystem inflammatory syndrome in children (MIS-C).[8] Kawasaki disease, a form of self-limiting vasculitis, is associated with iridocyclitis, punctate keratitis, vitreous opacities, papilloedema, subconjunctival hemorrhage and conjunctival injection.[9] In the literature available on MIS-C, the ophthalmic manifestations have mainly been in the form of conjunctivitis.[8] MIS-C is commonly being noted to have serological positivity for SARS-CoV-2 than on nasopharyngeal swab indicating it to be a manifestation of delayed immunological response to COVID-19. Treatment is directed towards suppressing the systemic inflammation. Corticosteroids, intravenous immunoglobulin (IVIG) and aspirin have been used in the cases reported.[8]
-
Episcleritis
A case of episcleritis as the initial manifestation of COVID-19 has been described in a 29-year old male by Otaif et al. Patient had history of foreign body sensation in the left eye and examination revealed nasal conjunctival and episcleral congestion with blanching with phenylephrine. He developed mild viral infection with symptoms appearing three days after the ocular signs.[10] Managna et al. reported another case of episcleritis which developed seven days after the onset of symptoms of COVID-19 infection. Most cases of episcleritis are idiopathic and self-limiting. About a third of them may be associated with viral infections including ebola, HSV and hepatitis C and now possibly, SARS-CoV-2 virus.[11]
Figure 1.
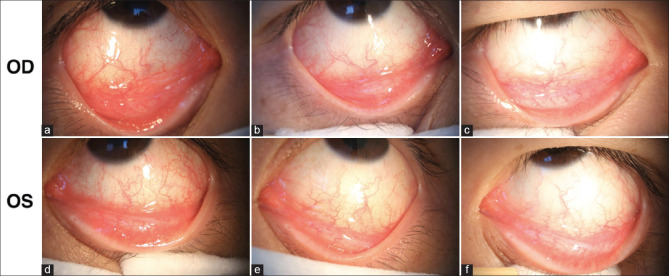
Follicular conjunctivitis following COVID-19: A 30-year-old man developed bilateral follicular conjunctivitis 13 days after mild COVID-19 infection. Slit lamp examinations showed evidence of acute viral conjunctivitis. (a and d)The examination on illness day 13 showed moderate conjunctival injection and inferior palpebral conjunctival follicles. (b and e) Examinations on illness day 17 and (c and f) illness on day 19 demonstrated that treatment with ribavirin eye-drops gradually improved the patient's symptoms. (Reproduced with permission from Chen L, Liu M, Zhang Z, Qiao K, Huang T, Chen M, Xin N, Huang Z, Liu L, Zhang G, Wang J. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol. 2020;104:748-51)
Eyelid
Eyelid manifestations in the form of meibomian orifice abnormalities and lid margin hyperemia/telangiectasia was found in 11/27 (38%) patients in the study by Meduri et al. in Italy. Blepharitis positively correlated with the COVID-19 disease duration.[12] It may develop as late manifestation of the disease and the incidence is also expected to rise in the post-pandemic era especially in patients with pre-existing ocular surface alteration.
Table 2 lists the reported signs and symptoms commonly seen in the patients with their incidence. The prevalence of eyelid, ocular surface and anterior segment manifestations in different studies has varied from 0.81% to 34.5%.[1,2,12,13,14,15,16,17,18,19,20,21,22,23] The basic problem in defining the prevalence is that the studies have been conducted at various stages of the disease, in patients with different severity with lack of uniformity in the examination method and collection of data.[1] A review and meta-analysis performed by Agarwal et al. with 16 studies, of 2347 confirmed cases, showed a prevalence of 11.6%. Ocular symptoms were seen in 6.9% of the patients with severe pneumonia while 4.13% of mild to moderate cases had ocular features.[24] Wu et al. have shown that ocular manifestations are seen more commonly in patients with higher white blood cell (WBC) count, C-reactive protein (CRP), procalcitonin and lactate dehydrogenase (LDH) levels.[13] A study in Italy showed that conjunctival findings were significantly correlated with the severity of the disease but not the duration.[12] A cross-sectional study conducted in Turkey showed that older age, high fever, increased neutrophils/lymphocyte ratio and high levels of acute-phase reactants were risk factors for development of ocular surface and anterior segment manifestations.[25] A cause of ambiguity is that dry eyes, pain, conjunctival redness, and chemosis may be present in severely ill patients (60% of those admitted in ICU) with renal failure, cardiopulmonary failure, and carbon dioxide retention and may be unrelated to the COVID-19 infection.[12,24]
Table 2.
| Symptoms and signs | Incidence |
|---|---|
| Dryness | 6.9-37% |
| Pain | 10.3-31.2% |
| Discharge | 6.9-29.6% |
| Redness | 10.8-24.1% |
| Tearing | 9.7-22.2% |
| Foreign body sensation | 6-18.5% |
| Photophobia | 2.6-16.1% |
| Itchiness | 9.6-15.7% |
| Blurred vision | 4.8-12.8% |
| Burning sensation | 8.4% |
| Lid margin hyperaemia | 34.5% |
| Crusted eyelashes | 24.1% |
| Meibomian orifices abnormality | 20.7% |
| Follicular conjunctivitis | 7.7-8.6% |
| Chemosis | 3.4% |
| Episcleritis | 2.2% |
Deng et al. demonstrated that SARS-CoV-2 can infect rhesus macaques through the conjunctiva.[26] Zhou et al. successfully demonstrated the presence of viral RNA in the conjunctival sac of COVID-19 patients. Detection of viral RNA in conjunctival swabs with RT-PCR gives positive results in only 3.5-5.2% of the cases subject to timing of collection during the course of disease and method of collection.[13] In a meta-analysis performed by Ulhaq et al., the pooled specificity was 100% but the pooled sensitivity of ocular tissues/fluid for detecting SAR-CoV-2 was extremely low (0.6%).[27] What is more interesting is that the viral RNA may be detected in conjunctival swabs even in patients without ocular manifestations as reported by Zhou et al.[15] But it is unclear if the viral RNA in ocular fluids has infectious potential. Till date, the virus has not been isolated in culture or had any cytopathic effect on Vero-E6 cell lines indicating that it does not replicate in the conjunctiva.[24] Viral genomic and subgenomic RNA of SARS-CoV-2 have been detected in cornea of deceased COVID-19 patients but further studies are required to determine the transmission of the infection following corneal transplant.[28]
Ocular surface manifestations of COVID-19 can be acute, (within a week) or delayed (after a week). Although diffuse follicular conjunctivitis may be found in both types, immune response is considered to play a major role in the delayed development of signs. It is much more diffuse, presents with corneal involvement, and responds well to steroids. The recurrent and delayed cases also had more severe forms of the disease as compared to isolated acute conjunctivitis.[6] Conjunctival congestion may be an early sign of COVID-19 infection even before the development of systemic symptoms in 2.26% of the patients.[24] About half the articles presented in Table 2 had conjunctivitis as the presenting feature of the viral infection. A series of five cases of COVID-19 reported by Scalinci et al. from Italy and two cases reported by Goel et al from India showed that conjunctivitis was the sole manifestation of COVID-19 infection.[29,30] Treatment involves mainly lubricants and the disease is self-limiting. Topical antibiotics may be added to prevent bacterial superinfection. Ribavirin has been used in some cases.[3] Topical steroids have a role in immune-mediated keratoconjunctivitis and episcleritis. Long term sequelae are yet to be studied.
For ophthalmologists, the important consideration is that, in the current scenario, one should have a high index of suspicion for COVID-19 in patients presenting with conjunctivitis. Thorough ophthalmic examination using all safety measures should be done to rule out more common causes like bacterial, chlamydial, adenoviral or microsporidial diseases. Patients with conjunctivitis should be specifically asked about COVID-19-related symptoms and advised to get tested if present. Further studies are necessary to determine if the conjunctiva can be a portal of entry and reservoir of the virus.
Posterior Segment Manifestations of COVID-19
Posterior segment involvement has varied manifestation and are actually vascular, inflammatory, and neuronal changes triggered by the viral infection but not specific to COVID-19. The literature review showed that the mean age of the patients was 47.4 ± 14.8 (median 50, 17-75) years. The median duration between appearance of ophthalmic symptoms and the COVID-19 symptoms /diagnosis was 12 (17.6 ± 13.1, 4–55) days. About 50% (14/23) were male and eight had no associated systemic comorbidity. [Table 3]
Table 3.
Review of literature of posterior segment manifestations of COVID-19
| Study | Type | Location | Age (years) | Sex | Duration between COVID-19 symptoms/ diagnosis and ophthalmic symptoms (days) | Covid illness | Signs | Diagnosis | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Invernizzi et al.[35] | Case | Italy | 54 | F | 9 | Mild | OD Va 20/40, RAPD, retinal hemorrhages, venular tortuosity, diffuse fern like retinal whitening | Impending CRVO | Oral prednisolone in tapering doses | 1 weekcomplete resolution |
| Walinjkar et al.[36] | Case | India | 17 | F | 21 | Mild | OD 6/24, disc edema, splinter hemorrhages, flame shaped, blot hemorrhages in all quadrants | CRVO | Intravitreal ranibizumab x 3 doses | 3 months, vision improved 6/12 |
| Sheth et al.[37] | Case | India | 52 | M | 10 | Moderate- severe | 6/60, inferior hemiretinal vein occlusion with SN BRVO, macular edema. | Vasculitic RVO | Intravitreal ranibizumab biosimilar, oral steroids | 1 month- 6/9, resolution of macular edema, SMD. Resolving DRIL. Loss of EZ and ELM. |
| Gaba et al.[38] | Case | UAE | 40 | M | 4 | Severe | OD 6/9, OS 6/18. OU dilated tortuous retinal veins, cotton wool spots, dot blot intraretinal hemorrhages, optic disc edema | Bilateral CRVO | LMWH, rivaroxaban | 2 weeksrecovered |
| Acharya et al.[39] | Case | USA | 60 | M | 12 | Severe | Optic disc- indistinct margin, cherry red spot, retinal whitening | CRAO | ||
| Dumitrascu[40] | Case | USA | 48 | M | 21 | Severe | OD no PL, optic disc edema, retinal whitening, retinal exudates, attenuated vessels. Retained perfusion in IT peripapillary area | Incomplete OAO | Enoxaparin | |
| Gascon et al.[41] | Case | France | 53 | M | Presented with ocular complaints | Mild | 20/60, deep retinal hemorrhages in posterior pole, Roth spots, subtle whitish parafoveal lesions | AMN, PAMM | 2 weeks- Vision improved OCT- resolution of SRF and hyperreflectivity. OPL/ONL/INL thinning | |
| Zamani et al.[42] | Letter to editor | Iran | 35 | F | Presented with ocular complaints | Severe | OU Multiple hemorrhages with Roth’s spots around optic disc and vascular arcades | AMN | 6 days- Expired | |
| Virgo et al.[43] | Letter to editor | London | 37 | F | 35 | Mild | No clinical findings. OCT changes | PAMM | ||
| 32 | M | 16 | NA | OCT findings | AMN | |||||
| Filho et al. [44] | Letter to editor | Brazil | 57 | F | 12 | Mild | OD 20/25, OS 20/20. conjunctival hyperaemia,. Vitreous opacities+1. Yellowish lesion in the macular area | Vitritis with outer retinal abnormalities | 2 monthsreduction in vitritis, decrease in reflectivity on OCT | |
| Gupta et al.[45] | Case | London | 75 | F | Presented with ocular complaints | Mild | OD 6/12, superior peripheral retinitis with minimal anterior or vitreous inflammation. OS FC, panuveitis- stellate diffuse KPs, AC cells, vitritis, extensive peripheral and midperipheral necrotising retinitis. | VZV associated Acute retinal necrosis triggered by COVID-19 | OS- intravitreal foscarnet, oral valacyclovir | 2 monthsimprovement in OD. OS- retinal thinning |
| Providencia et al.[47] | Case | Portugal | 41 | F | 28 | Mild | OS FC 2m. multiple peripapillary atrophic lesions, larger diffuse, yellow-whitish deep amoeboid patch with indistinct margins extending to fovea | Reactivation of serpiginous choroiditis | IVMP x 3 days, methotrexate weekly | 1 month- partial resolution of active lesions, decrease in choroidal thickness |
| Pereira et al.[46] | Cross sectional study | Brazil | 62.5 (median) | |||||||
| 40s | M | 7 | Severe | Peripheral retinal hemorrhages | ||||||
| 60s | M | 11 | Severe | Macular RPE hyperplasia | ||||||
| 60s | F | 6 | Severe | Retinal sectoral pallor at LTA | ||||||
| 60s | M | 55 | Severe | Peripheral retinal hemorrhage | ||||||
| 60s | M | 10 | Severe | Macular hemorrhage and hard exudates, peripapillary flame shaped hemorrhages | ||||||
| 70s | M | 12 | Severe | Cotton wool spots UTA | ||||||
| 50s | M | 12 | Severe | Peripapillary flame shaped hemorrhages | ||||||
| 50s | M | 15 | Severe | Cotton wool spots UTA | ||||||
| 60s | F | 39 | Severe | Asteroid hyalosis, cotton wool spots, flame shaped hemorrhages LTA | ||||||
| 40s | M | Concurrent | Severe | Peripapillary flame shaped hemorrhages |
AC: anterior chamber, AMN: acute macular neuroretinopathy, ARN: acute retinal necrosis, CRAO: central retinal artery occlusion, CRVO: Central retinal vein occlusion, DRIL: disorganization of retinal-inner-layers, ELM: external limiting membrane, EZ: ellipsoid zone, F: female, FC: finger counting, INL: inner nuclear layer, IT: inferotemporal, IVMP: intravenous methylprednisolone, IZ: interdigitation zone, KP: keratic precipitates, LMWH: low molecular weight heparin, LTA: lower temporal arcade, M: male, OAO: ophthalmic artery occlusion, OCT: optical coherence tomography, OD: Right eye, OPL: outer plexiform layer, ONL: outer nuclear layer, OS: Left eye, OU: bilateral, PAMM: paracentral acute middle maculopathy, RAPD: relative afferent pupillary defect, RPE: retinal pigment epithelium, SMD: serous macular detachment, SN: superonasal, SRF: subretinal fluid, UTA: upper temporal arcade, Va: visual acuity, VZV: varicella zoster virus
Retinal vascular occlusions
-
Central retinal vein occlusion (CRVO)
CRVO is one of the many vascular manifestations of COVID-19. In the published reports, only one patient suffered from hypertension and morbid obesity. It is not possible to correlate the development with the severity of COVID-19 disease. Investigations in these cases, like fluorescein angiography (FA) and optical coherence tomography (OCT) demonstrate features not different from CRVO from non-COVID-19-related causes. [Fig. 2] Patients of COVID-19 are in a procoagulant state evident by elevated D-dimer, prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, and cytokines even in the absence of common systemic conditions like hypertension, diabetes or dyslipidemia. Additionally, intermittent hypoxia in patients with pneumonia can induce the endothelial cells to release tissue factor and trigger the extrinsic coagulation cascade. In the impending stage, high dose steroids may help to normalize the inflammatory markers and coagulation indices. Management is with anti-vascular endothelial growth factor (anti-VEGF) in the established phase.[35,36,37,38] In patients with systemic comorbidities with severe COVID-19 infection, early anticoagulant prophylaxis should be considered.
-
Central retinal artery occlusion (CRAO)
Sudden onset of painless vision loss can herald the occlusion of central retinal artery with grave visual prognosis. Both patients, in the reported cases, had elevated inflammatory markers including IL-6, CRP, ferritin, fibrinogen and D-dimer as a result of severe COVID-19 infection, possibly resulting in the vascular occlusion.[39,40] In the case reported by Dumitrascu et al., incomplete ophthalmic artery occlusion developed despite the patient being on enoxaparin for deep vein thrombosis.[40] Combined retinal vein and artery occlusion has also been seen (unpublished) in a patient following COVID-19 infection [Fig. 3].
-
Acute macular neuroretinopathy (AMN), paracentral acute middle maculopathy (PAMM)
AMN is a rare condition with unknown etiology but, about 50% have been shown to be associated with respiratory or influenza-like illness. Ischemic mechanism involving the deep capillary plexus has been proposed. Cases of AMN and PAMM have been reported following/concurrently with COVID-19 diagnosis.[41,42,43] In a yet unpublished report from India, a 28-year-old healthy woman was diagnosed with AMN 1 week after recovery from mild COVID-19 infection. [Fig. 4] In the case reported by Zamani et al. from Iran, the patient was recently diagnosed with acute myeloid leukemia (AML) and was on chemotherapy. It is difficult to conclude with certainty if the retinal findings in this case were a manifestation of AML or if the viral infection predisposed the patient to AMN.[42] Acute painless diminution of vision, faintly colorful paracentral scotoma, and dyschromatopsia are the common symptoms. Fundus examination may not reveal any obvious abnormality, although retinal hemorrhages with Roth spots and a wedge-shaped reddish-brown lesion directed towards fovea have been described. Spectral-domain OCT (SD-OCT) is invaluable in detecting hyperreflectivity at the level of outer plexiform layer (OPL), outer nuclear layer (ONL) or between outer plexiform layer (OPL) and inner nuclear layer (INL). Disruption of ellipsoid zone (EZ), interdigitation zone (IZ), and loss of INL volume have also been described [Fig. 5]. OCT-angiography (OCT-A) in PAMM shows reduced flow in intermediate, deep and superficial capillary plexus, and in AMN, there is reduced flow in the deep plexus.
Figure 2.
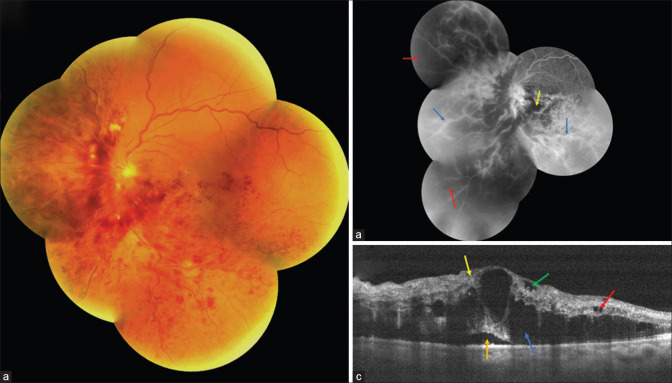
Vasculitic retinal vein occlusion as a manifestation of COVID-19: A 52-year-old patient presented with the diminution of vision in the left eye 10 days after he tested positive for SARS-CoV-2. (a) Fundus photograph demonstrating inferior hemiretinal vein occlusion with superonasal branch retinal vein occlusion. (b) Fundus fluorescein angiogram showing the presence of dilated tortuous vein in inferior and superonasal quadrants with late phases showing staining and leakage from the vessel walls (Blue arrow), multiple areas of hypofluorescence corresponding to retinal hemorrhages clinically, suggestive of blocked fluorescence (Yellow arrow) and areas of hypofluorescence suggestive of capillary nonperfusion (Blue arrow) in involved quadrants. The macular region and optic disc also showed hyperfluorescence in late phases suggestive of leakage. (c) Spectral domain optical coherence tomography illustrating the presence of serous macular detachment (Orange arrow), cystoid macular edema, cysts located in outer nuclear layer (Blue arrow), inner nuclear layer (Red arrow) and ganglion cell layer (Green arrow) and disorganization of retinal inner layers (Yellow arrow) (Reproduced with permission from Sheth JU, Narayanan R, Goyal J, Goyal V. Retinal vein occlusion in COVID-19: A novel entity. Ind J Ophthalmol 2020;68:2291-3).
Figure 3.
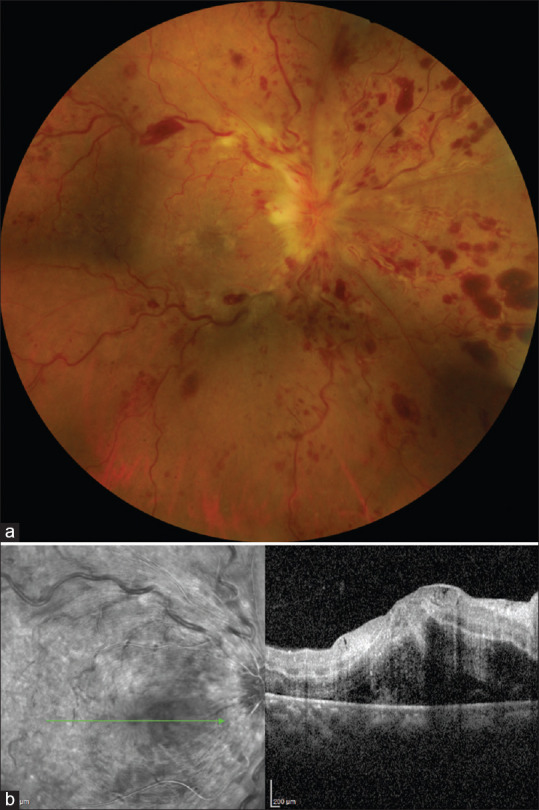
Combined central retinal artery and vein occlusion following COVID-19: A 32-year-old lady, known hypertensive with past history of COVID-19, presented with sudden onset, painless diminution of vision in the right eye. Examination showed right eye visual acuity of finger counting at 50cm and RAPD. (a) Fundus photograph showing retinal hemorrhages in all quadrants, dilated tortuous vessels and optic disc edema. (b) SD-OCT showing neurosensory detachment with intraretinal fluid and hyper-reflectivity of inner retinal layers. (Contributed by Rajashree Salvi and Shrinivas Joshi, M M Joshi Eye Institute, Hubli, India)
Figure 4.
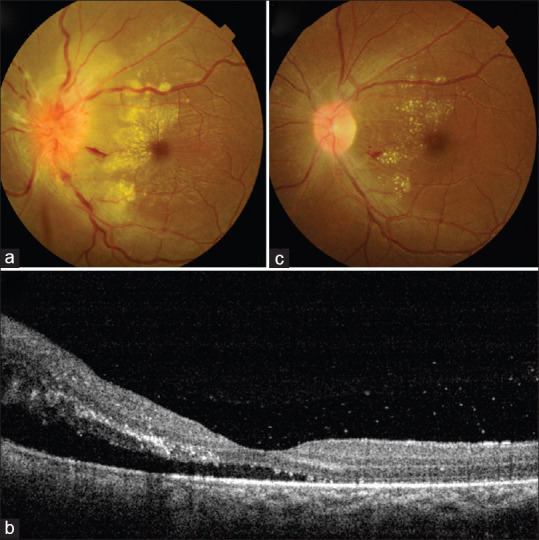
Acute macular neuroretinopathy following COVID-19: A 28-year-old woman presented with diminution of vision in left eye seven days after recovering from a mild COVID-19 infection. Vision was 6/36 in left eye with RAPD. (a) Fundus examination showed vitritis 1+, blurred disc margins, hard exudates over macular area and internal limiting membrane folds. (b) SD-OCT showed neurosensory detachment and outer retinal hyperreflective foci. She was managed with tapering doses of oral steroids and topical steroid and homatropine. (c) After 1 month, vision had recovered, disc edema had subsided with resolving exudates. (Contributed by Debdulal Chakraborty, Vitreoretina Services, Disha Eye Hospitals, Kolkata, India
Figure 5.
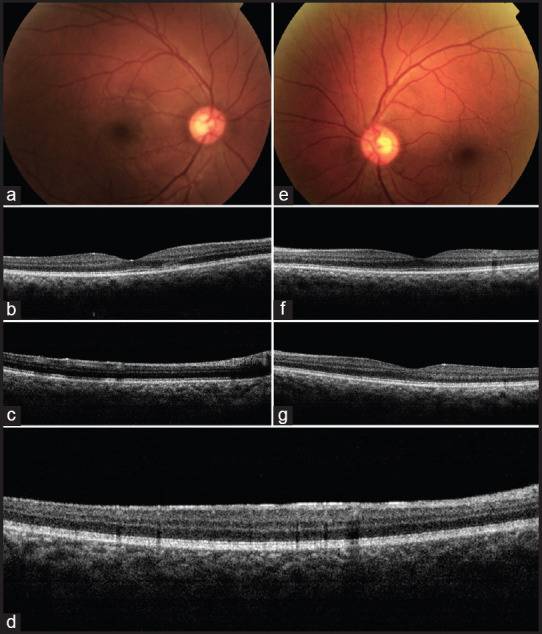
Acute macular neuroretinitis (AMN) and paracentral acute middle maculopathy (PAMM) as a manifestation of COVID-19: A 32-yearold male presented with abrupt onset paracentral triangular negative scotoma in his right eye noted below and to the right side of the centre of his visual field. History was significant for recovery from COVID-19 infection recently. Left eye was asymptomatic. (a) Fundus evaluation of the right eye revealed a triangular greyish-white lesion in deeper retina superonasal to the macular centre. (b) Optical coherence tomography (OCT) revealed corresponding areas of disruption in the outer retinal layers in addition to (c) other hyper-reflective lesions in more superficial retinal layers causing shadowing in underlying deeper retina. (d)There was hyper-reflectivity of the entire inner retinal surface just inferior to foveal centre. (e)Left eye fundus examination revealed a small whitish lesion nasal to foveal centre and multiple smaller lesions inferonasal and temporal to centre; (e) OCT revealed a single hyper-reflective lesion in the superficial retina with shadowing temporal to centre and (g) hyperreflectivity of the entire inner retinal surface nasal to centre. These findings suggested post-COVID-19 right eye symptomatic AMN and bilateral asymptomatic PAMM. (Contributed by Mallika Goyal, Retina-Vitreous Service, Apollo Eye Institute, Apollo Hospitals, Hyderabad, India)
Retina
-
Vitritis and outer retinal abnormalities
The presenting complaint in this case was bilateral redness in eyes. SD-OCT showed hyperreflectivity at the level of posterior vitreous hyaloid corresponding to the vitritis. Hyperreflectivity was also present at the level of inner plexiform layer (IPL), ganglion cell layer (GCL) with disruption of EZ. FA showed corresponding hyperfluorescence. It is important to rule out other infectious causes of vitritis like HSV, cytomegalovirus (CMV), syphilis, bartonella, toxoplasma, borrelia, toxocara, and inflammatory diseases which can cause uveitis. In the absence of any of these, COVID-19 was presumed to have led to the development of abnormalities detected on OCT.[44]
-
Acute retinal necrosis (ARN)
The reported patient was immunocompromised with relapsed diffuse large B cell lymphoma and had completed chemotherapy two months ago. A known case of systemic lupus erythematosus (SLE), she presented with ocular complaints of floaters and reduced vision. Intravitreal specimen tested positive for varicella-zoster virus (VZV) but not for COVID-19.[45] ARN is not common in immunosuppressed states, neither is the amount of inflammation seen in this patient which led to the presumption that COVID-19 had a role to play in triggering the VZV-related ARN by its effect on the immune system. It is possible that SARS-CoV-2 may compromise the blood-retinal barrier allowing a heightened inflammatory response.
-
Other retinal findings seen in patients with COVID-19
Pereira et al., from Brazil, reported retinal findings in patients admitted with severe COVID-19. The cross-sectional study showed retinal changes in ten patients (55.6%) and included peripheral retinal hemorrhages, macular hyperpigmentation, retinal sectoral pallor, peripapillary flame-shaped hemorrhages, hard exudates, and cotton wool spots. All the patients were on prophylactic or full intensity anticoagulants to counter the prothrombotic condition in severe cases of COVID-19. But the superadded or primary effect of pre-existing comorbidities, ICU admission, and vasoactive pharmacological support was not taken into account. The retinal findings, thus, cannot be solely attributed to the viral infection.[46]
Uvea
Serpiginous choroiditis
Reactivation of serpiginous choroiditis following COVID-19 infection was reported by Providencia et al. This patient had older pictures of prior retinal examination which showed evidence of atrophic lesions on FA indicative of previous episode of choroiditis.[47] There are unpublished cases of multifocal or serpiginous choroiditis presenting in patients with a history of SARS-CoV-2 infection. It is difficult to determine whether these are new onset or reactivation of inflammation. Autoimmunity activated by SARS-CoV-2 is believed to play a role in this. Tests for tuberculosis (TB), Hepatitis B and C (HBV, HCV), human immunodeficiency virus (HIV), borrelia, and syphilis should be done to diagnose serpiginous like choroiditis and before starting immunomodulatory therapy.[47]
SARS-CoV-2 RNA has been detected in the retina of patients diagnosed with COVID-19 in a study by Casagrande et al. in Germany. Three of the fourteen eyes enucleated on autopsy showed the presence of all three gene sequences on RT-PCR- RdRp-gene, E-gene, Orfl. The authors rightly state that the actual rate of RNA detection in retina may be much more because it depends on the post-mortem interval for the collection of the specimen, the Ct values and the biopsy size.[48] In animal models, retinitis and uveitis have been shown to develop. Angiotensin converting enzyme 2 (ACE-2) receptors have been detected in the retina. But none of the studies have answered the question of viral replication within ocular structures. Many of the manifestations are a result of predisposition to arterial and venous thrombosis in patients with the novel coronavirus infection. Cavalcanti et al. reported three patients, younger than 41 years with cerebral venous thrombosis.[49] Pulmonary embolism, stroke, disseminated intravascular coagulation (DIC), limb, and digit infarcts are also seen in these patients. Venous thromboembolism is seen in as many as 19-25% of the COVID-19 patients in ICU and on anticoagulants. Thus, development of retinal venous or arterial occlusion is not surprising. But what is surprising is, their development even in patients with mild to moderate symptoms.[35,36] It can develop within a few days to almost three weeks after the onset of COVID-19 symptoms. Patients presenting with CRVO to an ophthalmologist could have undiagnosed active or past infection with COVID-19. In the panel of investigations for a patient with retinal vascular occlusion, COVID-19 should now find a place. In the absence of comorbidities and in young adults, vasculitis can produce retinal vascular occlusion. The delayed onset can be explained by the immune complex deposition as a part of type 3 hypersensitivity reaction producing a pro-inflammatory state with cytokine storm. This is similar to the pathogenesis of vasculitis in other viral infections like chikungunya and dengue and systemic vasculitis.
In a correspondence, Marinho et al. in May 2020 discussed about retinal findings on OCT in 12 patients with COVID-19. All patients showed hyperreflective lesion at GCL and IPL prominently at the papillomacular bundle. The affinity for ganglion cells and plexiform layer may explain the associated central nervous system (CNS) manifestations as well.[50] In a case-control study from Spain, COVID-19 patients with moderate to severe disease had consistently decreased central vascular density on OCT-A as compared to patients with mild disease or controls without the viral infection. The immune cells recruited by the virus in the vessel walls are believed to produce endothelial cellular edema. Indirectly, the viral infection can induce an immune response with endothelial dysfunction associated with apoptosis. Central vascular density is considered a biomarker for several diseases like diabetes, chronic kidney disease, inflammatory bowel disease and Alzheimer's and has a potential to become a biomarker for microvascular damage in COVID-19 patients, though larger, population-based studies are essential.[51]
Neuro-ophthalmic Manifestations of COVID-19
Neuro-ophthalmic manifestations are not common and at present, isolated case reports are all that we have to build a foundation.
The mean age of the patients with neuro-ophthalmic manifestations was 42.3 ± 16.2 (median 43, 6–71) years. Of the 19 cases reported, 13 were males while only seven had systemic comorbidity in the form of hypertension and diabetes. One patient had SLE with advanced chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD). Patients presented with ophthalmic complaints either concurrently or within a few days of onset of systemic symptoms of COVID-19. The median gap from COVID-19 to development of ophthalmic symptoms was 5 (mean 11.3 ± 13.3, 0–42) days. [Table 4]
Table 4.
Review of literature of neuro-ophthalmic manifestations of COVID-19
| Study | Type | Location | Age (years) | Sex | Duration between COVID-19 symptoms/diagnosis and ophthalmic symptoms (days) | Covid illness | Signs | Diagnosis | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Insausti- Garcia et al.[52] | Case | Spain | 40 | M | 42 | Mild | Va- 20/200. Dilated tortuous retinal vessels, disc edema, superficial retinal hemorrhages, cotton wool spot, macular edema | Papillophelbitis | Intravitreal dexamethasone implant | Decreased disc and macular edema, Va- 20/40 |
| Sawalha[54] | Case | USA | 44 | M | 7 | Mild | OD 20/200, OS 20/20, OD RAPD, superior arcuate VF defect | OU optic neuritis | IVMP 1 g daily for 5 days, followed by oral in tapering doses | Remarkable improvement in Va in OD, complete recovery in OS |
| Zhou[55] | Case | USA | 26 | M | Concurrent | Mild | OU vision loss, OD HM, OS 20/250, disc edema, retinal haemorrhage | MOG-Ab associated ON in the setting of COVID19- parainfectious demyelinating | IVMP, oral steroids | 3 weeksdramatic improvement in vision, resolution of disc edema |
| Mendez Guerrero et al.[56] | Case | Spain | 58 | M | 36 | Severe | Roving eye movements, opsoclonus, ‘round the house’ sign | Acute hypokinetic rigid syndrome | Nil | Spontaneous improvement |
| Ortiz-Seller et al.[57] | Case | Spain | 51 | F | 2 | Mild | OU- 20/25, OU poorly reactive pupil. Pupillary dilatation in bright illumination, light near dissociation. OU hypersensitive response with pupillary constriction to 0.1% pilocarpine. Fundus- multiple, white yellowish placoid lesions in posterior pole and mid peripheral retina. | OU inflammary chorioretinal disease with Adie’s syndrome possibly associated with COVID-19 | Oral prednisolone | Full visual, anatomical and functional recovery in first week. 3 months- BCVA- 20/20 |
| Dinkin et al.[59] | Case | USA | 36 | M | 4 | Mild | OS partial 3rd nerve palsy, lower limb hyporeflexia, gait ataxia, right abduction defect, lower limb paraesthesia, areflexia | MFS | IVIG | Significant improvement |
| Gutierrez- Ortiz[60] | Case | Spain | 50 | M | 5 | Mild | Vertical diplopia, broad based gait, absent deep tendon reflexes, right hypertropia, limitation of ocular movements, right internuclear ophthalmoplegia, right fascicular oculomotor nerve palsy | MFS | IVIG | Significant improvement |
| Greer[61] | Case | USA | 43 | F | 3 | Mild | Horizontal diplopia | 6th nerve palsy | ||
| Greer et al.[61] | Study | USA | 52 | M | Concurrent | Mild | Horizontal diplopia | 6th nerve palsy | Resolved in 6 days | |
| Dinkin et al.[59] | Case | USA | 71 | F | Concurrent | Moderate, hospital admission | Abduction limitation of OD | Cranial nerve palsy | HCQ | 2 weeks- gradual improvement |
| Gutierrez-Ortiz et al.[60] | case | Spain | 39 | M | 3 | Mild | OU 6th nerve palsy, absent deep tendon reflexes | Multiple cranial nerve palsy | 2 weeks- recovered | |
| Falcone et al.[62] | case | USA | 32 | M | 3 | Severe | Horizontal diplopia | 6th nerve palsy | HCQ | Persistent limitation of abduction |
| Belghmaidi et al.[63] | case | Morocco | 24 | F | 1 | Mild | Diplopia, OS restricted upgaze, adduction and downgaze | Incomplete 3rd nerve palsy, pupil sparing | HCQ, azithromycin | 6th day- complete recovery |
| Theophanous et al.[64] | Case | USA | 6 | M | Initial | Mild | Lagophthalmos | Bell’s palsy | IV acyclovir, IVIG, lubricating eye drops, oral steroids | 3 weeks- improvement |
| Assini et al.[65] | Letter to editor | Italy | 55 | M | 20 | Severe | Bilateral ptosis, dysphagia, dysphonia, bilateral paralysis of 12th nerve, hyporeflexia | Bilateral ptosis with GBS | IVIG | 5th day onwards- complete remission |
| Huber et al.[66] | Case | Germany | 21 | F | 21 | Mild | Intermittent diplopia, OD ptosis, limited upgaze, Cogan’s lid twitch. ice test negative, Tensilon positive | Ocular MG | IVIG, oral pyridostigmine | Improvement |
| Cyr et al.[67] | Case | USA | 61 | M | 5 | Mild | OU no PL | Acute OU occipital territorial ischemic infarct | Expired- 3 days | |
| 34 | 7 | Severe | PL+, OU pallor of optic disc | Occlusion of right MCA, CVA | ||||||
| Yang et al.[68] | Case | UK | 60 | M | Not specified, delayed | Mildmoderate | OD HMCF. Diplopia, vertical gaze palsy central and superior field loss, vertigo, unsteady gait | Bilateral supranuclear gaze palsy, left paramedian midbrain infarct, OD BRAO | High dose aspirin, apixaban, antibiotic for bacterial endocarditis | Gradual improvement |
anti-MOG: anti-myelin oligodendrocyte glycoprotein, BCVA: best corrected visual acuity, BRAO: branch retinal artery occlusion, CKD: chronic kidney disease, COPD: chronic obstructive pulmonary disease, CVAcerebrovascular accident, F: female, FC: finger counting, GBS: Guillain-Barré syndrome, HM: hand movement, IVIG- intravenous immunoglobulin, IVMP: intravenous methylprednisolone, M: male, MCA: middle cerebral artery infarct, MFS: Miller Fischer syndrome, MG: myasthenia gravis, OD: Right eye, OPL: outer plexiform layer, OS: Left eye, OU: bilateral, PL: perception of light, RAPD: relative afferent pupillary defect, SLE: systemic lupus erythematosus, Va: visual acuity, VF: visual fields
-
Papillophlebitis
Papillophlebitis is an uncommon condition seen in healthy, young adults and one such case has been reported in a COVID-19 patient. There is painless, unilateral, slight diminution of vision. Visual fields show an enlarged blind spot. Ophthalmic findings include dilated, tortuous retinal vessels, disc edema, superficial retinal hemorrhages, cotton wool spot with or without macular edema. FA shows discrete venous staining and leakage, late staining of optic disc but no evidence of ischemia or peripheral vasculitis. [Fig. 6] While the final visual prognosis is quite favorable, about 30% of the cases develop vision-threatening ischemic venous occlusion with consequent neovascular glaucoma and macular edema. Systemic evaluation for hypercoagulable state, vasculitis syndromes, hyperviscosity, and vascular inflammatory disorders should be done to determine the possible etiology that could result in inflammation of retinal vasculature and capillaries of the disc. The role of COVID-19 as a possible cause comes in view of its association with coagulopathy and disproportionate inflammatory response or cytokine storm.[52]
-
Optic neuritis
In humans, neurological manifestations have been documented in almost 36% of the cases.[53] These were anosmia, headache, dizziness, hypogeusia, Guillain-Barré syndrome (GBS), and ischemic stroke. Ischemic stroke has been particularly noted in the younger adults similar to the average age of the reported patients with neuro-ophthalmic features.[48] The SARS-CoV-2 virus has been shown to cause optic neuritis is animal models. Neurotropism of the virus has been proposed as one of the mechanisms for the neurological and neuro-ophthalmic manifestations. [Fig. 7] shows a case of bilateral optic neuritis in a healthy young female two weeks after a mild COVID-19 infection. In a case report by Sawalha et al., bilateral optic neuritis followed within a week of COVID-19 symptoms.[54] Similarly, another case of optic neuritis developing within a few days of COVID-19 was reported by Zhou et al.[55] Patients presented with painful vision loss, relative afferent pupillary defect (RAPD) in the more severely affected eye with visual field defects and optic nerve enhancement on magnetic resonance imaging (MRI). Both cases had anti-myelin oligodendrocyte glycoprotein (MOG) antibodies. Cerebrospinal fluid (CSF) examination, immunological profile, viral panel and MRI brain did not reveal any other underlying etiology. Treatment was on the same lines as a typical case of optic neuritis with intravenous methylprednisolone (IVMP) followed by oral prednisolone leading to visual recovery and resolution of disc edema. MOG-antibody associated optic neuritis in the setting of COVID-19 is a parainfectious demyelinating syndrome with a viral prodrome. The virus has not been isolated from the CSF of the patients indicating that the virus may not be directly involved, rather it may be an immune-mediated insult. It is possible that in future, a spike in demyelinating neurological conditions may be seen, triggered by the viral infection.[53]
A case of acute hypokinetic rigid syndrome with transient opsoclonus was reported in a patient admitted for severe COVID-19 infection. In this case as well, a parainfectious immune-mediated midbrain affliction was the suggested mechanism.[56]
-
Adie's tonic pupil
Adie's tonic pupil can result from systemic conditions like diabetes or other viral infections. Development of tonic pupil in the patient after COVID-19 onset made the authors consider the association. The patient was a health care worker who gave a history of retro-ocular pain and reading difficulty two days after the onset of systemic COVID-19 symptoms. Pupillary hypersensitivity to 0.1% pilocarpine confirmed the diagnosis of Adie's tonic pupil. The short duration between COVID-19 symptoms and ocular features points towards the direct role of the virus itself on the nerves.[57] The functional receptor for the virus, ACE-2 receptor, has been identified in both brain and the basal layer of nasal epithelium. It has been suggested that the virus can enter the brain from the nasal epithelium via the olfactory bulb. Countering this theory, others have suggested that olfactory sensory neuron does not contain ACE-2 receptor and transmembrane protease serine 2 (TMPRSS2). However, radiological changes have been shown in the olfactory bulb and gyri recti.[58] The patient also had bilateral chorioretinopathy. The systemic evaluation for autoimmune and infectious causes were all negative. The etiology of spectrum of white dot syndrome remains unknown though it follows autoimmune diseases and viral infections. Systemic oral steroids led to full anatomical and functional recovery, further favoring the role of autoimmune factors mediated by COVID-19 in the development of both chorioretinopathy and Adie's tonic pupil.
-
Miller Fisher Syndrome (MFS) and cranial nerve palsy
MFS with acute onset ataxia, loss of tendon reflexes, and ophthalmoplegia and cases of cranial nerve palsies have been reported in several patients recently diagnosed with COVID-19.[59,60,61,62,63,64] Patients give history of acute onset of diplopia as the ocular complaint. 6th nerve was most commonly involved followed by oculomotor nerve [Fig. 8]. A case of right-sided facial nerve palsy has been reported in a child with agammaglobulinaemia and hyper IgM syndrome, asthma, and obstructive sleep apnoea in the USA.[64] RT-PCR was positive for SARS-CoV-2 but not for HSV and VZV. Cases of MFS responded well to intravenous immunoglobulin (IVIG) while cranial nerve palsies resolved spontaneously in most cases in 2-6 weeks. In these cases, again a misdirected immune system triggered by the viral infection is believed to be at fault.
-
Neurogenic ptosis
Acute onset of bilateral ptosis with other neurological signs of GBS was reported by Assini et al. from Italy.[65] Symptoms developed almost 20 days after severe COVID-19 infection. CSF examination showed oligoclonal bands with increased IgG/albumin ratio. No SARS-CoV-2 virus was detected in the CSF. GBS with cranial nerve involvement can thus be a late manifestation of severe COVID-19 infection. Good response to immunoglobulin supports the immune-mediated pathogenesis.
Delayed onset of ocular myasthenia gravis was reported by Huber et al. in a 21-year-old healthy woman.[66] She gave history of mild flu-like symptoms a month ago. Her antibody titers were suggestive of past infection with SARS-CoV-2. Acetylcholine receptor antibodies were positive. In view of rapid worsening of symptoms, she was treated with IVIG with gradually increasing dose of pyridostigmine. It is likely that COVID-19 infection can potentially trigger or exacerbate autoimmune diseases.
-
Cerebrovascular accident (CVA) with vision loss
Acute vision loss following CVA can also result from the procoagulant state in COVID-19 infection. Pre-existing endothelial dysfunction may make patients more susceptible. In the two cases reported, one had diabetes mellitus and the other patient had SLE with end-stage kidney disease and COPD with a prior history of CVA. Acute onset of bilateral, painless vision loss should prompt the treating physicians to advise an urgent imaging of the brain with angiography.[67]
Yang et al. described the development of bilateral supranuclear gaze palsy with right branch retinal artery occlusion in a 60-year old patient with a history of atrial fibrillation, COPD, bladder carcinoma on chemotherapy and bacterial endocarditis. Diffusion-weighted MRI revealed an infarct in left paramedian midbrain. In this case as well, COVID-19 possibly aggravated the procoagulant state of the patient.[68]
Figure 6.
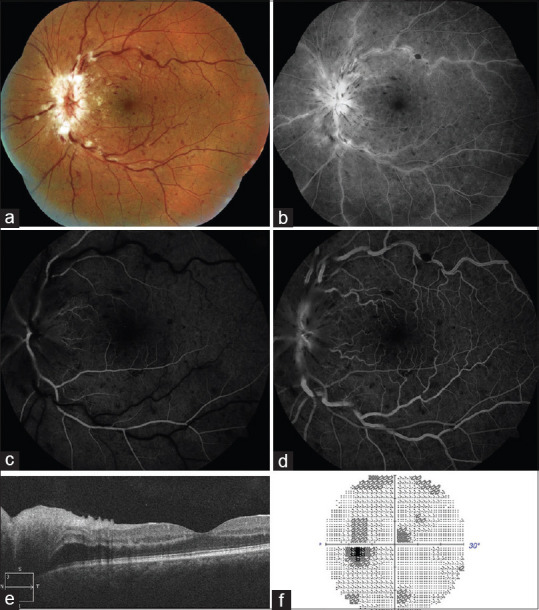
Papillophlebitis as a manifestation of COVID-19: A 40-year-old patient developed diminution of vision in left eye 6 weeks after a mild COVID-19 infection. (a) Fundus photograph and (b) red free retinography showing inflammation of the optic disc, retinal venous vasodilatation and tortuosity, and superficial hemorrhages in all four quadrants. (c) Early and (d) late arteriovenous phase FA showing discrete venous staining and leakage, in addition to leakage and late staining from the optic disc. (e) OCT showing optic disc edema without macular edema. (f) Visual field with slight central scotoma and a slight to moderate increase in the blind spot. (Reproduced with permission from Insausti-García A, Reche-Sainz JA, Ruiz-Arranz C, Vázquez ÁL, Ferro-Osuna M. Papillophlebitis in a COVID-19 patient: Inflammation and hypercoagulable state. Eur J Ophthalmol 2020 Jul 30.)
Figure 7.
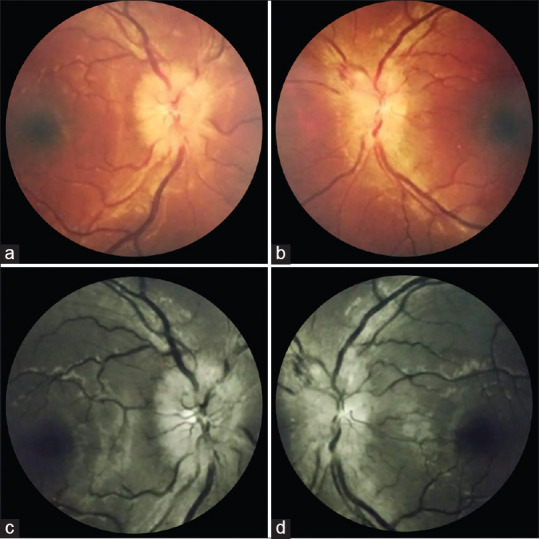
Bilateral atypical optic neuritis after a mild COVID-19 infection: A 34-year-old female presented with complaints of gradual blurring of vision in right eye with pain on eye movements since 1 week and history of a similar episode 3 weeks back in left eye, which improved spontaneously. She had recovered from a mild COVID-19 infection 2 weeks before the onset of ocular symptoms. On examination, her uncorrected visual acuity was 20/200, N24 in right eye, and 20/25, N6 in left eye. Pupil examination revealed a Grade III RAPD in right eye. (a and b) Fundus photograph and (c and d) red-free imaging showing bilateral disc oedema, more in the right eye. (Contributed by Rachna Vinaya Kumar, Paediatric ophthalmology, Neuro ophthalmology and Adult Strabismus Services, Apollo Eye Institute, Apollo Hospitals, Hyderabad, India)
Figure 8.
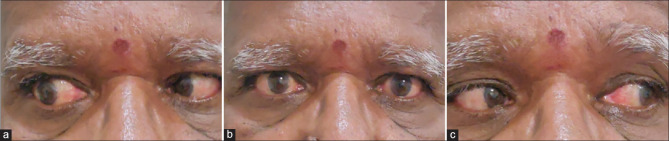
Sixth nerve palsy after COVID-19: A 64-year-old male presented with acute onset diplopia. (a) On examination there was right abduction limitation with (b) orthophoria in primary gaze and (c) normal adduction of right eye. (Contributed by Rachna Vinaya Kumar, Paediatric ophthalmology, Neuro-ophthalmology and Adult Strabismus Services, Apollo Eye Institute, Apollo Hospitals, Hyderabad, India)
Based on the above findings, it is important that physicians ask leading questions about double vision, decreased vision, pain with eye movements, gait abnormalities, or other neurological conditions while screening patients with COVID-19 symptoms. In patients presenting with these complaints, COVID-19 testing may be prudent while advising tests to determine the etiology. Treating doctors should also do a quick assessment of visual acuity, pupillary response, ocular motility, ptosis, optic disc, and reflexes since majority of these conditions occur in the early phase of the disease. Neuroimaging with angiography with attention to cranial nerves for any abnormal enhancement or cerebral infarcts can be advised based on the assessment.
Orbital Manifestations of COVID-19
There are not many orbital manifestations described but it is expected that their incidence will rise considering the interplay of comorbidities and treatment along with the infection itself. The case reports and series published show patients with a mean age of 50.2 ± 43 (median 60, 12-76) years. 12/14 patients were males with nine being diabetic and six hypertensive patients. Asthma was notably present in eight patients. Five of these patients presented either with ophthalmic symptoms and were tested for COVID-19 on screening or presented concurrently with systemic symptoms of viral infection. The median time of presentation from the development of COVID-19 symptoms was 12 (mean 15.8 ± 13.8, 2-42) days. 10/14 patients had moderate to severe disease. [Table 5]
Table 5.
Review of literature of orbital manifestations of COVID-19
| Study | Type | Location | Age (years) | Sex | Duration between COVID-19 symptoms/diagnosis and ophthalmic symptoms (days) | Covid illness | Signs | Diagnosis | Management | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Diaz et al.[69] | Case | London | 22 | M | Presented with ophthalmic symptoms | Nil | Va- OD 20/25, mild anisocoria, sluggish pupillary response, UL edema, ptosis, chemosis, conjunctival injection, mild proptosis, limitation of abduction and elevation, diplopia. No lymphadenopathy | Acute dacryoadenitis | Oral amoxicillin-clavulinic acid, NSAID. Worsening of painful ophthalmoplegia, increased periocular inflammation after 1 day- oral prednisolone | Improvement in 2 days. 6 monthscomplete resolution |
| Ruiy et al.[70] | Case | Taiwan | 62 | F | Presented with ophthalmic symptoms | Mild | - | Bilateral retro-orbital pain related to COVID-19 | HCQ, lopinavir-ritonavir | Subsided from day 7 |
| Turbin et al.[71] | Case | USA | 12 | M | Presented with ophthalmic symptoms | Mild | Va normal, RAPD, UL, LL edema, erythema, mild-mod conjunctival chemosis, 3-4 mm proptosis, limited movements. Ipsilateral pansinusitis, extraconal subperiosteal fluid collection on CT, dural enhancement on MRI | Orbital cellulitis | Vancomycin, ceftriaxone. Irrigation with bacitracin/ polymyxin. Superior orbitotomy with drainage of subperiosteal mucopyocoele. Post operative- parenteral vancomycin, ceftriaxone, metronidazole, fluticasone and nasal spray of oxymetazoline | 1.5 monthsresolved. |
| 15 | M | Concurrent | Moderate to severe | Normal vision, no RAPD, UL, LL edema, scant discharge, mild non chemotic conjunctival hyperaemia, moderate ocular movement limitation, 3-4 mm proptosis. Pansinusitis, thrombophlebitis of SOV extending to facial vein on CT. MRI- pachymeningitis, epidural abscess | Orbital cellulitis | Endoscopic frontal sinusotomy, total ethmoidectomy, maxillary antrostomy, intraoperative thickened mucosa. Stain- gram positive cocci. Parenteral vancomycin, ceftriaxone, metronidazole, enoxaparin HCQ, zinc, vitamin C, thiamine | Resolution | |||
| Shires et al.[73] | Case | USA | 76 | M | NA | NA | Proptosis, palpable abscess, dilated pupil. Intraoperatively- avascular nasal cavity. Later- spontaneous drainage, perforation into globe | Sinusitis with orbital abscess with MRSA, Peptinophilus indolicus | Endoscopic left middle turbinate reduction, maxillary antrostomy, anterior ethmoidectomy, orbitotomy with abscess drainage. Later, enucleation | On antibiotics |
| Ehrenreich et al.[74] | Case | USA | 33 | F | 2 | Severe | 1 cm proptosis, fixed dilated pupil, complete ophthalmoplegia, elevated IOP. Palate- brown secretion. DKA, ICE on MRI | ROC mucormycosis | Vancomycin+piperacillin tazobactam. Amphotericin B added for coverage of possible mucormycosis. Sinus debridement. | Expired- day 26 |
| Mehta et al.[75] | Case | India | 60 | M | 10 | Severe | OD proptosis, periorbital edema, soft tissue necrosis around lids, exposure keratitis. OS- fixed, dilated non- reactive pupil- Cavernous sinus extension or COVID-19 coagulopathy. Pansinusitis on MRI | ROC mucormycosis | IV meropenem, oral oseltamivir, IVMP, insulin, sc enoxaparin. Amphotericin B added and corticosteroids stopped after ophthalmic symptoms developed. | Expired day 6 of admission |
| Mekonnen et al.[76] | Case | USA | 60 | M | 8 | Severe | OD proptosis, erythema, edema of lids. Dilated fixed pupil- 3rd nerve palsy. Pansinusitis | RO mucormycosis | Remdesivir. Vancomycin, cefepime, liposomal Amphotericin B. Caspofungin added. 3 day retrobulbar injection of Amphotericin B. Concurrently started on dexamethasone 6 mg daily and convalescent plasma for COVID-19. Steroids worsened hyperglycemia. Endoscopic sinus debridement done on day 10. Posaconazole later | Expired day 31 |
| Sen et al.[77] | Case series | India | 46.2 | M | Concurrent | Moderate to severe | OD no PL, total ophthalmoplegia, no view of pupil, ptosis, 5 mm proptosis, conjunctival chemosis, nasal black discharge | ROC mucormycosis | Liposomal Amphotericin B, posaconazole, sinus debridement, exenteration | Life salvage |
| 60.9 | M | 17 | Moderatesevere | OS 6/60, total ophthalmoplegia, pupil reacting to light, ptosis, 7 mm proptosis, conjunctival chemosis, disc pallor, NPDR | RO mucormycosis | IV methylprednisolone, oral prednisolone for COVID-19. Sinus debridement, liposomal Amphotericin B, posaconazole | Life, eye salvage | |||
| 73.9 | M | 30 | Moderatesevere | OS no PL, total ophthalmoplegia, RAPD, ptosis, 4 mm proptosis, disc hyperaemic | ROC mucormycosis | IV dexamethasone, oral prednisolone for COVID-19. Sinus debridement, liposomal Amphotericin B, posaconazole | Life, eye salvage | |||
| 72.9 | M | 14 | Moderatesevere | OD no PL, recovered movements, fixed pupil, resolved ptosis, proptosis, disc pallor | ROC mucormycosis | Oral prednisolone for COVID-19. sinus debridement, liposomal Amphotericin B, posaconazole | Life, eye salvage | |||
| 62 | M | 42 | Moderatesevere | OS no PL, total ophthalmoplegia, fixed pupil, ptosis, 4 mm proptosis, conjunctiva chemosis, nasal black discharge, palatal eschar | ROC mucormycosis | IV dexamethasone for COVID-19. Sinus debridement, exenteration, liposomal Amphotericin B, posaconazole | Life salvage | |||
| Sen et al.[77] | Case series | India | 47 | M | 3 | Moderatesevere | OD no PL, total ophthalmoplegia, pupil no view, ptosis, 3 mm proptosis, conjunctiva chemosis, nasal black discharge, palatal eschar | ROC mucormycosis | IV dexamethasone for COVID-19. Sinus debridement, liposomal Amphotericin B | Life, eye salvage |
CT: computed tomography, DKA- diabetic ketoacidosis, F: female, FC: finger counting, HCQ- hydroxychloroquine, ICE- intracranial extension, IOP: intraocular pressure, IV- intravenous, LL- lower lid, M: male, MRI: magnetic resonance imaging, MRSA- methicillin resistant Staphylococcus aureus, NPDR: non-proliferative diabetic retinopathy, NSAID- non-steroidal anti-inflammatory drug, OD: Right eye, OS: Left eye, OU: bilateral, PL- perception of light, RAPD: relative afferent pupillary defect, ROC- rhino-orbito-cerebral, sc: subcutaneous, SOV- superior ophthalmic vein, USA: United States of America, UL: upper lid, Va: visual acuity
-
Dacryoadenitis
Dacryoadenitis is the most common cause of a painful lacrimal gland mass in a healthy young adult and the most common cause of dacryoadenitis is viral infection. [Fig. 9] In the only reported case, the patient had a four-day history of eyelid swelling and pain. The patient had history of contact with COVID-19 infected patients and his antibody tests for IgM, IgG were positive. Other tests for autoimmune conditions, infectious diseases particularly TB, mumps, adenovirus, Epstein-Barr virus (EBV), HSV, and Herpes zoster virus (HZV) were all negative. A diagnosis of acute dacryoadenitis as a late complication of SARS-CoV-2 virus was made.[69] In the early stages of the disease, the virus can travel to the lacrimal gland via the lacrimal ductules or by direct hematogenous spread. Later, immunological response incited by the virus may affect the lacrimal gland producing inflammation. Acute dacryoadenitis responds well to systemic steroids.
-
Retro-orbital pain
Bilateral retro-orbital pain may be a prominent and even presenting symptom in patients with COVID-19 and can mimic conditions like dengue.[70] This case highlights the important fact that COVID-19 has features that are highly non-specific and can simulate a lot of other common conditions.
-
Orbital cellulitis and sinusitis
In the two cases reported by Turbin et al., two adolescent boys developed acute onset unilateral, progressive, painful orbital swelling.[71] RT-PCR for COVID-19 was done as a preoperative investigation. There were no symptoms of chronic sinus disease. Suggested mechanism is that COVID-19 induced upper respiratory congestion can compromise mucociliary clearance with secondary sinus obstruction and bacterial infection. Children have a relatively indolent course with 56% of them being asymptomatic or having mild symptoms.[72] The superior ophthalmic vein thrombosis with facial vein extension may be a thrombotic complication of SARS-CoV-2.
In another case reported by Shires et al., a 76-year-old man, diabetic, hypertensive with testicular cancer and COVID-19 developed a spontaneously-draining orbital abscess and globe perforation necessitating enucleation with sinus debridement. Cultures grew methicillin resistant Staphylococcus aureus (MRSA), Streptococcus constellatus, and Peptoniphilus indolicus with negative blood cultures. Intraoperatively, an unusual finding was a highly avascular nasal mucosa. COVID-19 may predispose a patient to infection by bacteria not known to be found in the orbit like Peptoniphilus indolicus which is present in vagina and stomach. Orbital infection with this bacteria has not been reported previously. The avascularity was most likely because of thromboembolic complications of COVID-19.[73]
-
Mucormycosis
Mucormycosis is a life-threatening, opportunistic infection and patients with moderate to severe COVID-19 are more susceptible to it because of the compromised immune system with decreased CD4+ and CD8+ lymphocytes, associated comorbidities such as diabetes mellitus which potentiates both the conditions, decompensated pulmonary functions, and the use of immunosuppressive therapy (corticosteroids) for the management. Literature shows that rhino-orbital cerebral (ROC) mucormycosis can present concurrently with COVID-19 infection in patients under treatment or diagnosed on preoperative evaluation.[74,75,76] Mortality rate is as high as 50% even with treatment. In the series by the authors, all, except one patient, presented after recovering from COVID-19.[77] 5/6 cases had received intravenous and/or oral steroids and all were diabetics. Almost 70% of rhino-orbital-cerebral mucormycosis is seen in patients with uncontrolled diabetes and most of them have ketoacidosis at the time of presentation. What is interesting to note in this series is that symptoms of rhino-orbital mucormycosis developed as late as 30-42 days after the diagnosis of COVID-19. High index of suspicion, early diagnosis with histopathological and microbiological evidence, appropriate management with antifungals and aggressive surgical debridement (functional endoscopic sinus surgery and orbital exenteration) can improve survival. The signs and symptoms of orbital mucormycosis are not different from those of mucormycosis in non-COVID-19 patients. [Fig. 10] Simple tests like vision, pupil, ocular motility and sinus tenderness can be part of routine physical evaluation of a COVID-19 patient hospitalized with moderate to severe infection or diabetics with COVID-19 or those receiving systemic corticosteroids. A nasal swab for KOH mount and culture is a bedside procedure. Orbital exenteration for life-threatening infection is triaged as an urgent condition requiring surgery within 4-72 hours. Thus, appropriate surgery has to be undertaken with full personal protective equipment. Intravenous liposomal amphotericin B is started based on clinical suspicion or results of deep nasal swab. MRI is very useful to determine the extent of the disease and intracranial extension. Patients should also be made aware about the risks involved with the treatment of COVID-19 and the need for strict glycemic control. Development of unilateral facial or orbital pain, headache, periocular swelling or double vision or diminution of vision should prompt even the COVID-19 recovered patients to seek immediate medical attention. Since majority of the patients developed symptoms of mucormycosis after recovering from COVID-19, follow-up of high-risk COVID-19 patients for sequelae is imperative.[77]
-
Orbital histiocytic lesion
The authors have seen a case (unpublished) of a 77-year-old man with bilateral proptosis, eyelid swelling, enlarged lacrimal glands, orbital mass, and cervical, axillary and mediastinal lymphadenopathy with history of COVID-19 infection six months ago. Incisional biopsy was done and histopathology with immunohistochemistry was suggestive of a benign histiocytic proliferative lesion, possibly Rosai-Dorfman disease. This is very unusual in an elderly individual and the infection with SARS-CoV-2 may have a role in with its influence on the immune system of the body.[78]
Figure 9.
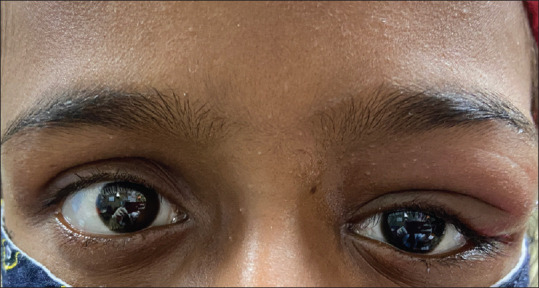
Acute dacryoadenitis manifesting with COVID-19: A 10-year-old girl developed painful, progressive left eyelid swelling and lacrimal gland mass concurrently with a mild COVID-19 infection. (Contributed by Ayushi Agarwal, Guru Nanak Eye Center, New Delhi, India)
Figure 10.
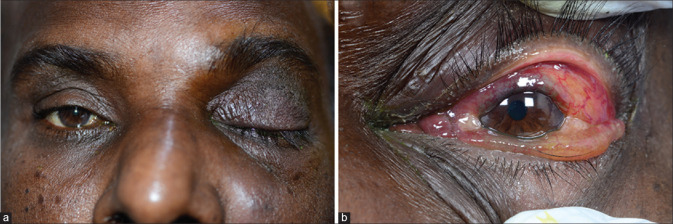
Rhino-orbito-cerebral mucormycosis following COVID-19 infection: (a) Clinical picture of a 61-year-old, diabetic, male who developed left eye periocular edema, complete ptosis, ophthalmoplegia, (b) proptosis, conjunctival congestion, and severe chemosis 17 days after moderate to severe COVID-19 infection treated with steroids. (Reproduced with permission from Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a viral land: A tale of two pathogens. Ind J Ophthalmol 2021;69:244-52.)
The orbital manifestations of COVID-19 can vary from intense retro-orbital pain to life-threatening invasive mucormycosis. Orbital emphysema is seen as a complication in intubated patients receiving positive end expiratory pressure ventilation. As with other ophthalmic manifestations, direct effect of the virus, altered immune status, proinflammatory milieu and escalated coagulative profile play variable role in the pathogenesis.
Discussion
COVID-19, first reported in Wuhan in China in December 2019, spread to all parts of the world to the proportion of a pandemic by March 2020. SARS-CoV-2 is a member of the coronaviridae family, Betacoronavirus genus and is an enveloped single-stranded RNA virus. The COVID-19 illness can range from asymptomatic or mild flu-like symptoms to severe respiratory distress. It is now known that it can have effects on almost all organs of the body including the cardiovascular, neurological, and gastrointestinal systems. An ophthalmologist was the first to report the virus in Wuhan and himself contracted and succumbed to the disease while treating a patient for glaucoma. Ophthalmic manifestations are varied in terms of presentation, severity, and timing. Wu et al. suggested that ophthalmic manifestations are more common in patients with severe systemic disease with abnormal blood and inflammatory parameters.[13] Based on the findings in the eyes of the patients, it has been suggested that exposure of unprotected eyes can also lead to infection with SAR-CoV-2 virus.[18] The theories about routes of transmission of the virus to the eyes include direct inoculation of conjunctiva by droplets, migration of upper respiratory tract infection through the nasolacrimal duct or lacrimal gland involvement by the hematogenous route.[79] Samples collected with Schirmer strips and conjunctival swabs have detected the viral RNA in very few patients. Low sensitivity of RT-PCR, significantly lower viral load in conjunctival samples as compared to nasopharynx, and sampling timing related to disease can account for the low yield. A negative result does not exclude the possibility of the virus being present in tears or ocular surface and the presence of the virus in ocular samples does not imply an infection. As discussed before, there is no evidence of viral replication in ocular tissues.[79] Keeping in mind that the conclusions of different studies are still blurred, it is advisable to use goggles, slit lamp breath shields, and sanitization techniques while examining patients.
The review of available literature suggests that there is very low risk of transmission through the ocular surface. This could be because of the very fact that although ACE-2 receptors and TMPRSS2 have been demonstrated on conjunctival and corneal epithelia, the number of these receptors is very low as compared to the respiratory tissues. The binding capacity of the virus to the receptors on ocular surface also appears to be low, mediated by the lactoferrin in tears which prevents attachment of the virus to heparan sulfate proteoglycans which helps in its subsequent binding to ACE-2 receptor. Serum IgA may also play a protective role.[79]
The medications that have been used to treat COVID-19 also have ocular toxicities. Long-term use of chloroquine and hydroxychloroquine can lead to retinal toxicity but it is not expected or seen with the brief period of use for COVID-19. Lopinavir/Ritonavir may cause reactivation of autoimmune conditions. Ribavirin has not been used much for COVID-19 but is known to cause retinopathy, retinal vein occlusion, serous retinal detachment, non-arteritic ischemic optic neuropathy and Vogt-Koyanagi-Harada (VKH) disease. Interferon has been associated with retinopathy, VKH, conjunctivitis, uveitis, optic neuropathy, corneal ulcers, epithelial defects and Sjogren's syndrome. Tocilizumab has been reported to produce cotton wool spots and retinal hemorrhages. Systemic corticosteroids are known to cause cataract, glaucoma and central serous chorioretinopathy. The risk of life-threatening fungal infection in predisposed individuals cannot be overemphasized. Central retinal vein occlusion has been reported in patients receiving IVIG. These points should be kept in mind by an ophthalmologist during the history and examination of patients.[80]
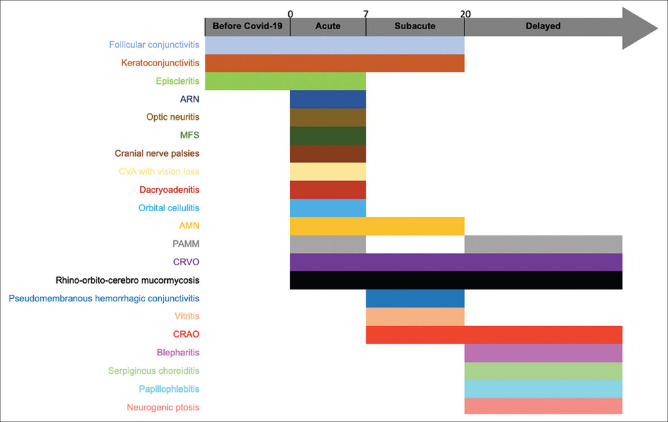
The ophthalmic features can develop at any point in the disease course. The median time of appearance from the time of development of COVID-19 symptoms/diagnosis of neuro-ophthalmic features is 5 days, of ocular surface and anterior segment manifestations is 8.5 days and posterior segment and orbital pathology is 12 days. Fig. 11 provides a simplified timeline to help ophthalmologists and treating physicians to look for specific features depending on the stage of the disease.
Figure 11.
A broad timeline of the different ophthalmic manifestations of COVID-19. They can be divided into those which present with ocular symptoms initially (before COVID-19), within the first week of infection (Acute, Day 0-day 7), between the second and third week since the onset of COVID-19 symptoms (Subacute, day 7-day 20) and those which present as late sequelae of the infection (Delayed, after 20 days)
Future Directions and Conclusion
The prevalence of ophthalmic manifestations among COVID-19 patients ranges from 2-32%.[3] The causal relation with SARS-CoV-2 is yet to be established with certainty for any of these conditions. Whether they are a result of pre-existing systemic condition, whether the virus has, in fact, aggravated the underlying condition, whether the virus causes direct damage to the nerves, vessels, and other structures or whether it is ultimately the body's own immune system responsible for the pathology, are some of the unanswered questions which would take larger population-based studies with standardized methods of examination, investigations and data collection to resolve. While the viral RNA has been identified in different parts of the eye, its replication and infectivity is not established. The transmission of the virus via eye secretions is being actively investigated. There is an imminent need for establishing evidence-based guidelines for prophylactic use of antifungals in patients with high risk of rhino-orbito-cerebral mucormycosis diagnosed with COVID-19 who require corticosteroids. Thromboembolic complications are well established. Studies to establish risk factors for ophthalmic vascular occlusions in COVID-19 patients followed by development of anticoagulation prophylaxis regimen keeping ophthalmic implications in mind are also required. As we enter the phase of vaccination, a substantial proportion of the population has already been exposed to the SARS-CoV-2 virus, either in the form of overt clinical disease or contact with a patient diagnosed with COVID-19 with subclinical illness. Several countries of the world are experiencing resurgence of cases with mutated strains. We can definitely expect to see more manifestations of the disease in the eye and even clusters of similar cases. For now, we have tried to present a broad overview of the various possible features that have been published till date from around the world and the stage of the disease when they can be expected to help ophthalmologists keep in mind the importance of asking specific history about COVID-19 infection, contact with infected person or related symptoms. COVID-19 should be included in the lists of causes of common ophthalmic pathologies elucidated above. It should also be suspected when there is unusual presentation of a disease in an age group or population phenotype where it is not expected like histiocytic lesion in an elderly individual. Knowing that many of these manifestations can be the presenting feature can help diagnose the infection early and limit the disease transmission. Tests like nasopharyngeal swab for RT-PCR, antibody titers for previous infection for patients with ophthalmic complaints or computed tomography of the paranasal sinuses to look for sinusitis along with a scan for the chest in high-risk patients by physicians treating COVID-19 cases need to be advised conscientiously and logically. Ophthalmologists are also encouraged to report cases seen in association with COVID-19 to add to the pool of knowledge on a global level.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sindhuja K, Lomi N, Asif MI, Tandon R. Clinical profile and prevalence of conjunctivitis in mild COVID-19 patients in a tertiary care COVID-19 hospital: A retrospective cross-sectional study. Indian J Ophthalmol. 2020;68:1546–50. doi: 10.4103/ijo.IJO_1319_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Deng C, Chen X, Zhang X, Chen B, Yu H, et al. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China: A cross-sectional study. Acta Ophthalmol. 2020;98:e951–9. doi: 10.1111/aos.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Liu M, Zhang Z, Qiao K, Huang T, Chen M, et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol. 2020;104:748–51. doi: 10.1136/bjophthalmol-2020-316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nayak B, Poddar C, Panigrahi MK, Tripathy S, Mishra B. Late manifestation of follicular conjunctivitis in ventilated patient following COVID-19 positive severe pneumonia. Indian J Ophthalmol. 2020;68:1675–7. doi: 10.4103/ijo.IJO_1682_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheema M, Aghazadeh H, Nazarali S, Ting A, Hodges J, McFarlane A, et al. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19) Can J Ophthalmol. 2020;55:e125–9. doi: 10.1016/j.jcjo.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo D, Xia J, Wang Y, Zhang X, Shen Y, Tong JP. Relapsing viral keratoconjunctivitis in COVID-19: A case report. Virol J. 2020;17:1–7. doi: 10.1186/s12985-020-01370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navel V, Chiambaretta F, Dutheil F. Haemorrhagic conjunctivitis with pseudomembranous related to SARS-CoV-2. Am J Ophthalmol Case Rep. 2020;19:100735. doi: 10.1016/j.ajoc.2020.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danthuluri V, Grant MB. Update and recommendations for ocular manifestations of COVID-19 in adults and children: A narrative review. Ophthalmol Ther. 2020;9:853–75. doi: 10.1007/s40123-020-00310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertoli F, Veritti D, Danese C, Samassa F, Sarao V, Rassu N, et al. Ocular findings in COVID-19 patients: A review of direct manifestations and indirect effects on the eye? J Ophthalmol. 2020;2020:4827304. doi: 10.1155/2020/4827304. doi: 10.1155/2020/4827304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otaif W, Al Somali AI, Al Habash A. Episcleritis as a possible presenting sign of the novel coronavirus disease: A case report? Am J Ophthalmol Case Rep. 2020;20:100917. doi: 10.1016/j.ajoc.2020.100917. doi: 10.1016/j.ajoc.2020.100917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangana CM, Kargacin AB, Barraquer RI. Episcleritis as an ocular manifestation in a patient with COVID-19. Acta Ophthalmol. 2020 doi: 10.1111/aos.14484. doi: 10.1111/aos.14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meduri A, Oliverio GW, Mancuso G, Giuffrida A, Guarneri C, Rullo EV, et al. Ocular surface manifestation of COVID-19 and tear film analysis. Sci Rep. 2020;10:1–7. doi: 10.1038/s41598-020-77194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–8. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Zeng Y, Tong Y, Chen C. Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva. MedRxiv. 2020 doi: 10.1101/2020.02.11.20021956. [Google Scholar]
- 16.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92:589–94. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atum M, Boz AA, Çakir B, Karabay O, Köroglu M, Ögütlü A, et al. Evaluation of conjunctival swab PCR results in patients with SARS-CoV-2 infection. Ocul Immunol Inflamm. 2020;28:745–8. doi: 10.1080/09273948.2020.1775261. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Chen X, Chen L, Deng C, Zou X, Liu W, et al. The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf. 2020;18:360–2. doi: 10.1016/j.jtos.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lan QQ, Zeng SM, Liao X, Xu F, Qi H, Li M. Screening for novel coronavirus related conjunctivitis among the patients with corona virus disease-19. [Zhonghua yan ke za zhi] Chin J Ophthalmol. 2020;56:E009. doi: 10.3760/cma.j.cn112142-20200322-00213. [DOI] [PubMed] [Google Scholar]
- 20.Karimi S, Arabi A, Shahraki T, Safi S. Detection of severe acute respiratory syndrome Coronavirus-2 in the tears of patients with Coronavirus disease 2019. Eye. 2020;34:1220–3. doi: 10.1038/s41433-020-0965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong N, Yu W, Xia J, Shen Y, Yap M, Han W. Evaluation of ocular symptoms and tropism of SARS-CoV-2 in patients confirmed with COVID-19. Acta Ophthalmol. 2020;98:e649–55. doi: 10.1111/aos.14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seah IY, Anderson DE, Kang AE, Wang L, Rao P, Young BE, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020;127:977–9. doi: 10.1016/j.ophtha.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y, Duan C, Zeng Y, Tong Y, Nie Y, Yang Y, et al. Ocular findings and proportion with conjunctival SARS-COV-2 in COVID-19 patients. Ophthalmology. 2020;127:982–3. doi: 10.1016/j.ophtha.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aggarwal K, Agarwal A, Jaiswal N, Dahiya N, Ahuja A, Mahajan S, et al. Ocular surface manifestations of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. PLoS One. 2020;15:e0241661. doi: 10.1371/journal.pone.0241661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceran BB, Ozates S. Ocular manifestations of coronavirus disease 2019. Graefes Arch Clin Exp Ophthalmol. 2020;258:1959–63. doi: 10.1007/s00417-020-04777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng W, Bao L, Gao H, Xiang Z, Qu Y, Song Z, et al. Ocular conjunctival inoculation of SARS-CoV-2 can cause mild COVID-19 in rhesus macaques. Nat Commun. 2020;11:1–7. doi: 10.1038/s41467-020-18149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulhaq ZS, Soraya GV. The prevalence of ophthalmic manifestations in COVID-19 and the diagnostic value of ocular tissue/fluid. Graefes Arch Clin Exp Ophthalmol. 2020;258:1351–2. doi: 10.1007/s00417-020-04695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casagrande M, Fitzek A, Spitzer MS, Püschel K, Glatzel M, Krasemann S, et al. Presence of SARS-CoV-2 RNA in the cornea of viremic patients with COVID-19. JAMA Ophthalmol. 2021:e206339. doi: 10.1001/jamaophthalmol.2020.6339. doi: 10.1001/jamaophthalmol.2020.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scalinci SZ, Battagliola ET. Conjunctivitis can be the only presenting sign and symptom of COVID-19. IDCases. 2020;20:e00774. doi: 10.1016/j.idcr.2020.e00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goel R, Arora R, Chhabra M, Kumar S. Viral shedding in tears of COVID-19 cases presenting as conjunctivitis. Indian Journal of Ophthalmology. 2020;68:2308. doi: 10.4103/ijo.IJO_2567_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colavita F, Lapa D, Carletti F, Lalle E, Bordi L, Marsella P, et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann Intern Med. 2020;173:242–3. doi: 10.7326/M20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daruich A, Martin D, Bremond-Gignac D. Unilateral conjunctivitis as first presentation of Coronavirus Disease 2019 (COVID-19): A telemedicine diagnosis. J Fr Ophtalmol. 2020;43:e167–8. doi: 10.1016/j.jfo.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khavandi S, Tabibzadeh E, Naderan M, Shoar S. Corona virus disease-19 (COVID-19) presenting as conjunctivitis: Atypically high-risk during a pandemic. Cont Lens Anterior Eye. 2020;43:211–2. doi: 10.1016/j.clae.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salducci M, La Torre G. COVID-19 emergency in the cruise's ship: A case report of conjunctivitis. Clin Ter. 2020;171:e189–91. doi: 10.7417/CT.2020.2212. [DOI] [PubMed] [Google Scholar]
- 35.Invernizzi A, Pellegrini M, Messenio D, Cereda M, Olivieri P, Brambilla AM, et al. Impending central retinal vein occlusion in a patient with coronavirus disease 2019 (COVID-19) Ocul Immunol Inflamm. 2020;28:1290–2. doi: 10.1080/09273948.2020.1807023. [DOI] [PubMed] [Google Scholar]
- 36.Walinjkar JA, Makhija SC, Sharma HR, Morekar SR, Natarajan S. Central retinal vein occlusion with COVID-19 infection as the presumptive etiology. Indian J Ophthalmol. 2020;68:2572–4. doi: 10.4103/ijo.IJO_2575_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheth JU, Narayanan R, Goyal J, Goyal V. Retinal vein occlusion in COVID-19: A novel entity. Indian J Ophthalmol. 2020;68:2291–3. doi: 10.4103/ijo.IJO_2380_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaba WH, Ahmed D, Al Nuaimi RK, Al Dhahani AA, Eatmadi H. Bilateral central retinal vein occlusion in a 40-year-old man with severe coronavirus disease 2019 (COVID-19) pneumonia. Am J Case Rep. 2020;21:e927691. doi: 10.12659/AJCR.927691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acharya S, Diamond M, Anwar S, Glaser A, Tyagi P. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases. 2020;21:e00867. doi: 10.1016/j.idcr.2020.e00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dumitrascu OM, Volod O, Bose S, Wang Y, Biousse V, Lyden PD. Acute ophthalmic artery occlusion in a COVID-19 patient on apixaban. J Stroke Cerebrovasc Dis. 2020;29:104982. doi: 10.1016/j.jstrokecerebrovasdis.2020.104982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gascon P, Briantais A, Bertrand E, Ramtohul P, Comet A, Beylerian M, et al. Covid-19-associated retinopathy: A case report. Ocul Immunol Inflamm. 2020;28:1293–7. doi: 10.1080/09273948.2020.1825751. [DOI] [PubMed] [Google Scholar]
- 42.Zamani G, Azimi SA, Aminizadeh A, Abadi ES, Kamandi M, Mortazi H, et al. Acute macular neuroretinopathy in a patient with acute myeloid leukemia and deceased by COVID-19: A case report. J Ophthal Inflamm Infect. 2020;10:1–5. doi: 10.1186/s12348-020-00231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Virgo J, Mohamed M. Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye. 2020;34:2352–3. doi: 10.1038/s41433-020-1069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zago Filho LA, Lima LH, Melo GB, Zett C, Farah ME. Vitritis and outer retinal abnormalities in a patient with COVID-19. Ocul Immunol Inflamm. 2020;28:1298–300. doi: 10.1080/09273948.2020.1821898. [DOI] [PubMed] [Google Scholar]
- 45.Gupta A, Dixit B, Stamoulas K, Akshikar R. Atypical bilateral acute retinal necrosis in a coronavirus disease 2019 positive immunosuppressed patient. Eur J Ophthalmol. 2020 doi: 10.1177/1120672120974941. 1120672120974941. doi: 10.1177/1120672120974941. [DOI] [PubMed] [Google Scholar]
- 46.Pereira LA, Soares LCM, Nascimento PA. al Retinal findings in hospitalised patients with severe COVID-19 British Journal of Ophthalmology Published Online First. 2020 Oct 16; doi: 10.1136/bjophthalmol-2020-317576. doi: 10.1136/bjophthalmol-2020-317576. [DOI] [PubMed] [Google Scholar]
- 47.Providência J, Fonseca C, Henriques F, Proença R. Serpiginous choroiditis presenting after SARS-CoV-2 infection: A new immunological trigger.?? Eur J Ophthalmol. 2020 doi: 10.1177/1120672120977817. 1120672120977817. doi: 10.1177/1120672120977817. [DOI] [PubMed] [Google Scholar]
- 48.Casagrande M, Fitzek A, Püschel K, Aleshcheva G, Schultheiss HP, Berneking L, et al. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm. 2020;28:721–5. doi: 10.1080/09273948.2020.1770301. [DOI] [PubMed] [Google Scholar]
- 49.Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K, et al. Cerebral venous thrombosis associated with COVID-19. Am J Neuroradiol. 2020;41:1370–6. doi: 10.3174/ajnr.A6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marinho PM, Marcos AA, Romano AC, Nascimento H, Belfort R. Retinal findings in patients with COVID-19. Lancet. 2020;395:1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zapata MÁ, García SB, Sánchez A, Falcó A, Otero-Romero S, Arcos G, et al. Retinal microvascular abnormalities in patients after COVID-19 depending on disease severity. Br J Ophthalmol. 2020 doi: 10.1136/bjophthalmol-2020-317953. bjophthalmol-2020-317953. doi: 10.1136/bjophthalmol-2020-317953. [DOI] [PubMed] [Google Scholar]
- 52.Insausti-García A, Reche-Sainz JA, Ruiz-Arranz C, Vázquez ÁL, Ferro-Osuna M. Papillophlebitis in a COVID-19 patient: Inflammation and hypercoagulable state. Eur J Ophthalmol. 2020 doi: 10.1177/1120672120947591. 1120672120947591. doi: 10.1177/1120672120947591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tisdale AK, Chwalisz BK. Neuro-ophthalmic manifestations of coronavirus disease 19. Curr Opin Ophthalmol. 2020;31:489–94. doi: 10.1097/ICU.0000000000000707. [DOI] [PubMed] [Google Scholar]
- 54.Sawalha K, Adeodokun S, Kamoga GR. COVID-19-induced acute bilateral optic neuritis? J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620976018. 2324709620976018. doi: 10.1177/2324709620976018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin oligodendrocyte glycoprotein antibody–associated optic neuritis and myelitis in COVID-19. J Neuroophthalmol. 2020 doi: 10.1097/WNO.0000000000001049. 10.1097/WNO.0000000000001049. doi: 10.1097/WNO.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Méndez-Guerrero A, Laespada-García MI, Gómez-Grande A, Ruiz-Ortiz M, Blanco-Palmero VA, Azcarate-Diaz FJ, et al. Acute hypokinetic-rigid syndrome following SARS-CoV-2 infection. Neurology. 2020;95:e2109–18. doi: 10.1212/WNL.0000000000010282. [DOI] [PubMed] [Google Scholar]
- 57.Ortiz-Seller A, Martínez Costa L, Hernández-Pons A, Valls Pascual E, Solves Alemany A, Albert-Fort M. Ophthalmic and neuro-ophthalmic manifestations of coronavirus disease 2019 (COVID-19) Ocul Immunol Inflamm. 2020;28:1285–9. doi: 10.1080/09273948.2020.1817497. [DOI] [PubMed] [Google Scholar]
- 58.Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol. 2020;77:1028–9. doi: 10.1001/jamaneurol.2020.2125. [DOI] [PubMed] [Google Scholar]
- 59.Dinkin M, Gao V, Kahan J, Bobker S, Simonetto M, Wechsler P, et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020;95:221–3. doi: 10.1212/WNL.0000000000009700. [DOI] [PubMed] [Google Scholar]
- 60.Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Mañas R, et al. Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–5. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 61.Greer CE, Bhatt JM, Oliveira CA, Dinkin MJ. Isolated cranial nerve 6 palsy in 6 patients with COVID-19 infection. J Neuroophthalmol. 2020;40:520–2. doi: 10.1097/WNO.0000000000001146. [DOI] [PubMed] [Google Scholar]
- 62.Falcone MM, Rong AJ, Salazar H, Redick DW, Falcone S, Cavuoto KM. Acute abducens nerve palsy in a patient with the novel coronavirus disease (COVID-19) J AAPOS? 2020;24(4):216–217. doi: 10.1016/j.jaapos.2020.06.001. doi:10.1016/j.jaapos.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belghmaidi S, Nassih H, Boutgayout S, El Fakiri K, El Qadiri R, Hajji I, et al. Third cranial nerve palsy presenting with unilateral diplopia and strabismus in a 24-year-old woman with COVID-19? Am J Case Rep. 2020;21:e925897. doi: 10.12659/AJCR.925897. doi: 10.12659/AJCR.925897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Theophanous C, Santoro JD, Itani R. Bell's palsy in a pediatric patient with hyper IgM syndrome and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Brain Dev. 2021;43:357–9. doi: 10.1016/j.braindev.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Assini A, Benedetti L, Di Maio S, Schirinzi E, Del Sette M. New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: Two Italian cases. Neurol Sci. 2020;41:1657–8. doi: 10.1007/s10072-020-04484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huber M, Rogozinski S, Puppe W, Framme C, Höglinger G, Hufendiek K, et al. Postinfectious onset of myasthenia gravis in a COVID-19 patient? Front Neurol. 2020;11:576153. doi: 10.3389/fneur.2020.576153. doi: 10.3389/fneur.2020.576153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cyr DG, Vicidomini CM, Siu NY, Elmann SE. Severe bilateral vision loss in 2 patients with coronavirus disease 2019. J Neuroophthalmol. 2020;40:403–5. doi: 10.1097/WNO.0000000000001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Y, Qidwai U, Burton BJ, Canepa C. Bilateral, vertical supranuclear gaze palsy following unilateral midbrain infarct. BMJ Case Reports. 2020;13:e238422. doi: 10.1136/bcr-2020-238422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martínez Díaz M, Copete Piqueras S, Blanco Marchite C, Vahdani K. Acute dacryoadenitis in a patient with SARS-CoV-2 infection? Orbit. 2021:1–4. doi: 10.1080/01676830.2020.1867193. doi: 10.1080/01676830.2020.1867193. [DOI] [PubMed] [Google Scholar]
- 70.Ruiy W, Hsu SY, Tsai HL, Chen CT, Tseng CP, Chen WT. COVID-19 mimicking dengue fever with the initial manifestation of retro-orbital pain–A rare case. J Formos Med Assoc. 2020;119:1715–6. doi: 10.1016/j.jfma.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turbin RE, Wawrzusin PJ, Sakla NM, Traba CM, Wong KG, Mirani N, et al. Orbital cellulitis, sinusitis and intracranial abnormalities in two adolescents with COVID-19. Orbit. 2020;39:305–10. doi: 10.1080/01676830.2020.1768560. [DOI] [PubMed] [Google Scholar]
- 72.Dong Y, Mo XI, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020;145:e20200702. [Google Scholar]
- 73.Shires CB, Klug T, Dryden S, Ford J. Unusual cause of acute sinusitis and orbital abscess in COVID-19 positive patient: Case report. Int J Surg Case Rep. 2021;79:164–8. doi: 10.1016/j.ijscr.2021.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.09.032. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12:e10726. doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mekonnen ZK, Ashraf DC, Jankowski T, Grob SR, Vagefi MR, Kersten RC, et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg. 2020 doi: 10.1097/IOP.0000000000001889. doi: 10.1097/IOP. 0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a viral land: A tale of two pathogens. Indian J Ophthalmol. 2021;69:244–52. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vemuganti GK, Naik MN, Honavar SG. Rosai dorfman disease of the orbit. J Hematol Oncol. 2008;1:1–7. doi: 10.1186/1756-8722-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ali MJ. The SARS-CoV-2, tears, and ocular surface debate: What we know and what we need to know. Indian J Ophthalmol. 2020;68:1245–6. doi: 10.4103/ijo.IJO_1881_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Douglas KA, Douglas VP, Moschos MM. Ocular manifestations of COVID-19 (SARS-CoV-2): A critical review of current literature. In Vivo. 2020;34(3 Suppl):1619–28. doi: 10.21873/invivo.11952. [DOI] [PMC free article] [PubMed] [Google Scholar]