Abstract
Specular microscopy is a noninvasive diagnostic tool that allows for in vivo evaluation of corneal endothelium in health and various diseased states. Endothelial imaging helps in the diagnosis and management of several endothelial disorders. The review focuses on the principles of specular microscopy, limitations of endothelial imaging, and its interpretation in common conditions seen in the clinical practice. A thorough PubMed search was done using the keywords specular microscopy, corneal endothelium, and endothelial imaging.
Keywords: Cornea, corneal endothelium, endothelial dystrophy, endothelial keratoplasty, keratoplasty, penetrating keratoplasty, Specular microscopy
Specular microscopy is a diagnostic modality for imaging the corneal endothelium that allows for direct observation of the endothelial cell morphological characteristics either in a clinic or eye bank setting. Endothelial imaging using a specular microscope is routinely used in the assessment of endothelial health in various endothelial diseases, evaluation of the donor cornea prior to keratoplasty and postoperative follow-up after keratoplasty. A PubMed search was done using the keywords specular microscopy, corneal endothelium and endothelial imaging and appropriate references were included in the citation.
Specular Microscopes - Principles and Types
The specular light reflex with the slit lamp is a routine method of evaluating corneal endothelium in the clinics. The term 'Specular reflection' refers to a situation, where the angle of the reflected beam of light makes an equal angle with that of the incident light.[1] The endothelial cells have a refractive index greater than 1.336 value for the aqueous humor, and hence can be imaged because the endothelial layer––aqueous interface reflects 0.022% of the projected light.[2]
The clinical specular microscopes are all designed from the original specular microscope introduced by Maurice[3] for laboratory use. The specular microscope is an optical reflection microscope where a slit of light is focussed on the corneal endothelial surface and specularly (mirror-like) reflected light rays are focussed onto film plane for viewing on a real-time monitor. By virtue of its design, the specular microscope does not allow non specular light rays to be observed. The light that is reflected from the endothelial surface is collected by the same objective lens and focussed onto a film plane or a video monitor screen for examination [Fig. 1].
Figure 1.
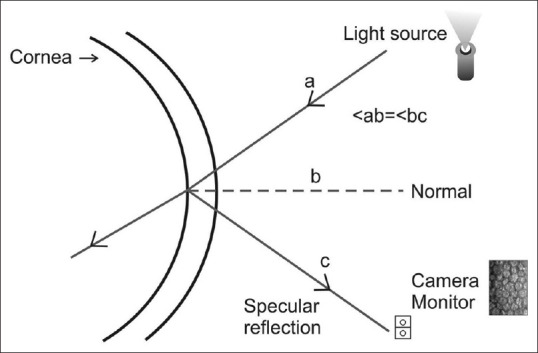
Schematic representation of principle of specular microscopy: A slit of light is focused on the endothelial surface. Specularly reflected light rays are focused onto a camera monitor to capture the image of endothelial cells. (a-incident light ray, b-normal, c-reflected light ray, ab- angle of incident light, bc- angle of the reflected light)
The surface area of the specular reflex image is dependent on the curvature of the reflecting surface.[4] There are many types of specular microscope which can be divided into horizontal (clinical use) and upright (used in the eye banks). The presently available instruments for use in clinics are of two types––corneal epithelial contact and noncontact models, that capture the image and analyze the endothelial cell morphology.[3] The contact instrument has an objective lens that applanates the corneal surface. During applanation, the cornea is flattened and hence the image is enlarged. The noncontact instruments (Examples: Konan CellChek, Nidek CM 530, Tomey EM 4000) use automatic image focusing technology. As the specular reflex area comes from a curved surface, the specular reflex area is smaller than the contact method.
Human Corneal Endothelial Characteristics
The knowledge of human corneal endothelial characteristics is important in the interpretation of specular microscopy. The endothelial monolayer comprises of cells that are hexagonal. Six-sided cellular arrangement of the cells is the most energy-efficient polygonal geometric shape as this confers the advantage of the greatest surface area relative to its perimeter. The endothelial cells are arrested in the G1 phase of cell cycle[3] and there is no evidence that endothelial cells divide in vivo under any normal conditions.[5,6]
The corneal endothelial cell density declines throughout life at an average rate of 0.6%/year.[7] This typically involves two phases: a rapid and a slow component.[8] At birth, human endothelial cell density is approximately 5000-6000 cells/mm2, but gradually decreases to about 3500 cells/mm2 by 5 years of age, 3000 cells/mm2 by the age 14-20 years and 2500 cells/mm2 in late adulthood. This is due to an age-related increase in the corneal dimensions and normal senescence of the endothelial cells. Racial and geographical differences along with environmental factors are known to influence the rate of decrease in endothelial cell density.[9,10,11]
The human corneal endothelium does not regenerate. Hence, any focal endothelial injury/loss of endothelial cell is repaired by maintaining its continuity by migration and expansion of surviving cells. The endothelial health is interpreted with parameters such as percentage of hexagonal endothelial cells, coefficient of variation of cell area, and endothelial cell density. An increase in the variability of cell area is termed as polymegathism. A deviation from hexagonality is referred to as pleomorphism. The percentage of hexagonal cells (pleomorphism) and the coefficient of variation of cell area increases (polymegathism) with age and endothelial cell attrition due to various causes. A perfect 100% hexagonality is not possible due to age-related senescence and environmental stressors; however, a healthy cornea can be expected to have 60% of the hexagonal endothelial cells. The coefficient of variation of mean cell area is the most sensitive index of corneal endothelial dysfunction, whereas hexagonality is a good index of progress of endothelial wound healing.[12]
Endothelial Cell Morphology Analysis
Fig. 2a and b shows representative specular microscopy imaging of a 12-year-old and 40-year-old normal eye. The readability of the images is ascertained by the distinctly visible endothelial cells in the image frame.
Figure 2.
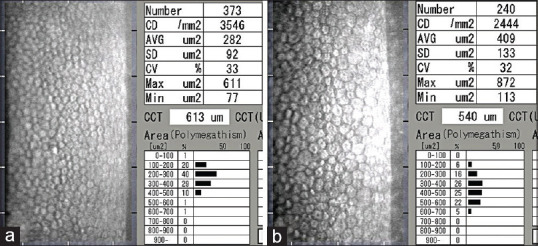
Representative specular microscopy images of the right eye of a 12-year-old (a) and a 40-year-old (b) male. Notice the difference in the mean cell area (282 versus 409 μm2) and the age related decline in endothelial cell density
Endothelial cell morphology analysis includes
Cell area ± SD (square micrometers, μm2)
Cell density (cells/mm2)
Polymegathism (CV)
Pleomorphism (percentage of hexagonal cells).
The cell density is determined by the following equation:
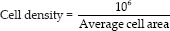
The coefficient of variation (CV) is derived by the equation:
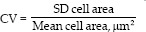
Techniques of Specular Imaging and Determining Endothelial Cell Density
There are several methods of obtaining quantitative information about the corneal endothelium such as frame method (fixed or variable), center to center method, flex center method, corner method, and comparison method. The frame method provides a quantitative assessment of the cell density by counting the number of cells within a frame. The corner method is performed by locating the intersecting sides on the endothelial image frame. In the center method, the central dot of the endothelial cell is identified locating the nearby cells around this cell. The comparison method provides a subjective cell density value by a visual comparison of the image to a known set of hexagonal patterns for various cell densities.
Errors can arise in the quantitative evaluation of corneal endothelium. The fixed frame method can have large errors in the presence of a higher number of border cells (endothelial cells cut by one border of the frame). The border errors are eliminated by the variable frame cell counting method and hence are preferred over fixed-frame method. Another source of error is subjective decisions of determining the cellular boundaries in the center-to-center method and cell border intersections in corner method. Irrespective of the strategy of the technique used, the accuracy of assessment is dependent on the quality of the endothelial image obtained on specular microscopy.[3]
As the endothelial imaging is based on specular reflex, any optical hindrances in front of the endothelial monolayer will affect the quality of the image to delineate the endothelial cells, Various conditions that interfere with the quality of endothelial imaging are a poor ocular surface tear film, epithelial haze, stromal scarring, and alterations in the Descemet's membrane (excrescence/guttae). Based on the endothelial image quality procured on specular microscopy, it can be graded as good, fair, poor, or impossible to analyze.[3]
The specular microscopy gives the endothelial cell analysis from the cellular boundaries that are automatically detected. The automatic method of analysis is fairly accurate when the individual cell borders are well delineated. However, in those eyes with fair quality of endothelial images (where valid outlines of cell borders are not very clearly imaged due to optical hindrances during the acquisition of endothelial images), it is preferable to perform a manual counting method by a technician well trained in performing specular microscopy. The interobserver variabilities in cell density analysis is determined to be around 0-6% for excellent to good quality endothelial images, and 6%–11% for fair quality images.[3,13]
Specular Microscopy in Various Endothelial Disorders
The specular microscopy imaging shows a distinctive pattern in various endothelial diseases. The imaging characteristics and its clinical applications in primary endotheliopathies and secondary corneal endotheliopathies is described here.
-
Primary Endothelial Disorders
-
1) Fuchs endothelial corneal Dystrophy (FECD)FECD is characterized by the formation of Descemet's membrane excrescences/guttae that are focal thickening and projections in the otherwise homogenous Descemet's membrane.[14] The correlation between the presence of guttae with visual acuity and endothelial functions is not perfect. Guttae can be fine or coarse, confluent or nonconfluent and few to numerous. Based upon their nature, endothelial imaging using specular microscopy can be varied. Guttae lead to drop out areas on the endothelial image. In those with discrete/nonconfluent/fewer guttae, the endothelial cells in between the drop out areas (corresponding to guttae) can be made out to allow for readability of such images (as seen in the Fig. 3a-d). However, in the eyes with confluent/numerous guttae, endothelial cells cannot be imaged and hence the specular microscopy images are nonreadable for meaningful analysis (as seen in the Fig. 3e and f) in these eyes. Inability to image the endothelial cells in these cases does not necessarily mean absence of endothelial cells or its functionality. Hence, specular microscopy imaging in FECD should be corelated to nature of the clinical condition and the limitations of imaging in FECD should be understood. The 'true' nature and functionality of endothelium in these eyes is assessed primarily by the clinical signs and symptoms such as early morning blurry vision, subepithelial haze, and central versus peripheral pachymetry and not entirely upon the specular microscopyFECD typically begins with changes in the central corneal endothelial cells, then progressing to peripheral endothelial cells in the later stages. An alternative method of management of some cases is by performing Descemet rhexis without endothelial keratoplasty (DWEK).[15] Specular microscopy imaging in different gazes to image the peripheral corneal endothelial health can help in preoperative evaluation and decision making of those cases that may be considered for DWEK over conventional management with endothelial keratoplasty [Fig. 3 g-i]. The pre-requisite for DWEK is peripheral endothelial cell density above 1000 cells/mm2The decision making of cataract surgery with or without endothelial keratoplasty in early stages of FECD is mainly based on the patient's symptoms of early morning blurring of vision, pachymetry, and clinical features suggestive of subepithelial changes. A readable specular imaging with a good endothelial cell density and fewer guttae can be managed with cataract surgery alone with an endothelial protective viscoelastic and careful surgical maneuver during surgery.[16] However, the risk of early or late corneal decompensation should be explained in those eyes were the central ECD is less than 1000 cells/mm[2,17]The guttae of FECD should be differentiated from other conditions which on endothelial imaging also shows 'drop out' areas. The pigments on the endothelial surface as in anterior uveitis [Fig. 4] can lead to nonimaging of the endothelial cells in that area and appear as dark lesions similar to guttae as seen in FECD. The 'Pseudo-guttae' or secondary guttae are caused by swelling of endothelial cells in conditions such as infection, inflammation, and uveitis. The endothelial cell edema is seen as 'dark areas' on the specular imaging. The pseudo-guttae are transient and disappear on reversal of endothelial edema once the primary pathology is treated and does not involve the descemet membrane. Hence, the interpretation of specular imaging should be corelated with the clinical features on the slit-lamp examination.
-
2) Posterior Polymorphous Endothelial Dystrophy.PPCD is characterized by vesicles, bands, and placoid lesions on endothelial surface. The dystrophy can exist in unilateral or bilateral form.[18] In many eyes, the endothelial changes can be subtle and easily overlooked. Unilateral forms may have amblyopia as the affected cornea may have a steep curvature.[19] Many cases get misdiagnosed as keratoconus in view of corneal steepening. Specular microscopy helps in making an appropriate clinical diagnosis. The endothelial imaging in this condition typically shows lower endothelial cell density and increase in average cell area when compared to age-matched normal eyes. In the area of vesicles and bands, endothelial cells are not visualized [Fig. 5 a-c].
-
3) Congenital Hereditary Endothelial DystrophyMajority of the patients with CHED are seen to have a significant degree of stromal haze. Hence, specular microscopy fails to capture endothelial images.
-
4) Irido-corneal Endothelial (ICE) syndromeICE syndrome comprises of three variants of the disease––Chandler syndrome, Progressive iris atrophy, and Cogan Reese syndrome. The specular microscopy shows distinct morphological changes in the endothelial cells. Two grading systems based on Specular Microscopy have been used to describe and grade the endothelial changes––Hirst's grading system and Sherrard's grading system.[20,21] The morphological changes evident on specular microscopy are––rounding of the cell borders, increased intracellular blackout areas, and dark- light reversal pattern (the endothelial cell border is seen in white instead of black, and inside of the cell seen as black instead of white) of the normal endothelium [Fig. 6 a-d].
-
-
Secondary Corneal Endothelial Disorders
-
1) Endothelial changes after surgical procedureA variable degree of endothelial cell loss happens after intraocular surgeries such as cataract extraction and phakic intraocular lens implantation.[22,23] As a result of this, endothelial cell density is lower and mean cell area larger in pseudophakic eye versus the normal age-matched cell density or the opposite eye without any intraocular interventionThe endothelial changes or cell loss after keratoplasty is studied extensively. The endothelial cell loss happens at a fairly rapid pace in the initial years after penetrating keratoplasty. Armitage et al.[24] reported a biexponential cell loss after keratoplasty. Bourne et al.[25] reported an endothelial cell density decline of 7.8%/year between 3 and 5 years postkeratoplasty, and 4.2%/year between 5 and 10 years. The mean 5-year endothelial cell loss after endothelial keratoplasty is 47%–48% (comparable between Descemet stripping endothelial keratoplasty and Descemet membrane endothelial keratoplasty).[26,27] Fig. 3 show the endothelial imaging after penetrating and endothelial keratoplasty. The endothelial imaging in post keratoplasty eyes helps in the monitoring and assessment of the graft health [Figs. 7-9]
-
2) Endothelial changes in PseudoexfoliationPseudoexfoliation is a disorder known to be associated with endothelial alterations.[28] The endothelial changes seen are guttae and reduced cell density.
-
3) Systemic conditions and medicationsDiabetes and chronic kidney disease patients especially those on hemodialysis have been reported to have endothelial abnormalities.[29,30] Endothelial imaging should be considered in the pre-operative evaluation when planning for cataract surgery in such patientsCertain systemic medications have been implicated in causing gradual loss of endothelial cells that eventually can lead to bilateral corneal edema.[31] The medications known to cause endothelial cell loss are memantine, medications prescribed for movement disorders/Parkinsonism such as amantadine [Fig. 10]. It is important to recognize medication history in those presenting with bilateral corneal edema as the continuation of the medications can affect the endothelial health of the transplanted cornea in such patients[32]
-
4) Forceps injuryA rupture of the Descemet membrane during birth trauma leads to corneal edema at birth. The healing of the tear and resolution of corneal edema happens with the migration of the endothelial cells to cover the defect. As a result of this, the endothelial cell density is lower in these eyes compared to the normal eye [Fig. 11 d-f]. A similar healing process happens after acute hydrops in ectatic corneal conditions such as Keratoconus and Pellucid marginal corneal degeneration.
-
5) Endothelial changes in Macular Corneal DystrophyMacular Corneal dystrophy is characterized by the deposition of glycosaminoglycans in the stroma and corneal endothelium. The affected posterior membrane (Descemet-endothelial complex) in these patients show guttae similar to that seen in FECD.[33,34] Fig. 12 shows the specular microscopy after uneventful Deep anterior lamellar keratoplasty in three patients with Macular corneal dystrophy. Serial endothelial imaging helps in evaluating the progression of endothelial involvement in macular corneal dystrophy. Progressive worsening of the endothelial changes and cell density are important considerations when cataract surgery is needed in these eyes.
-
6) Endothelial alterations in Uveitis/EndotheliitisAnterior segment inflammatory conditions affect the corneal endothelium and can lead to endothelial cell loss. Lowered endothelial cell density and increased mean cell area are well documented endothelial changes after uveitis of various causes[35,36] and immune-mediated endothelitis [Fig. 11 a-c].[37] The endothelial pigments and pseudoguttae in active uveitis stages can lead to dark lesions on endothelial imaging [Fig. 2].
-
7) Endothelial cell response and changes after contact lens wearContact lens-induced hypoxia can produce acute and chronic morphological changes in the corneal endothelium.[38] It is more commonly associated with contact lenses with low oxygen permeability. Contact lens-related endotheliopathy is characterized by increased polymegathism and pleomorphism. Discontinuing contact lens wear does not reverse the morphological changes rapidly. However, some degree of recovery is possible over several years if contact lens wear is discontinued.[39]
-
8) Miscellaneous
-
Figure 3.
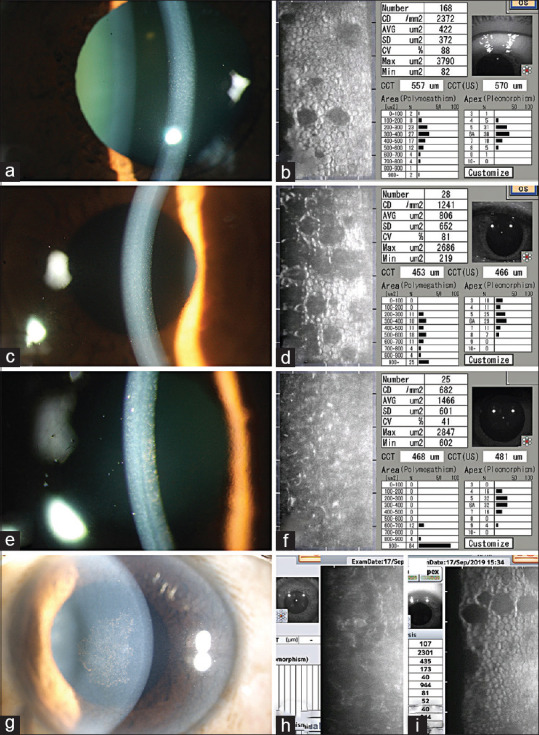
Specular Microscopy in Fuchs endothelial corneal dystrophy: (a and b) Specular microscopy (b) showing drop out areas corresponding to nonconfluent guttae in a 50 year old patient with FECD with nonconfluent guttae (a), normal endothelial cells are seen in most areas of the image frame. (c and d) Specular microscopy (d) showing more numerous drop out areas in the endothelial image frame of a 53 year old patient with FECD (c). Notice that the endothelial cells have larger mean cell area (806 μm2 vs 422 μm2) when compared to that in image b. (b and d are in the same scale). (e and f) Specular microscopy (e) in a 49 year old patient with confluent guttae in central cornea (d), fails to capture a readable image. The quantitative parameters such as ECD, mean cell area depicted in the image are erroneous values in view of the fact that the cell analysis was done in automatic mode. This image is 'non analysable' as the individual cells are not captured due to confluent guttae. Note that the patient has a well-functioning endothelium despite confluent guttae, cornea is noticeably clear without evidence of subepithelial scarring, and pachymetry of 481 μm. (g-i) Slit lamp photograph (g) of a patient with confluent guttae; specular microscopy image from the central cornea is 'non analysable'(h); the specular microscopy from the mid peripheral cornea shows a readable image with few guttae, reasonably good endothelial cell density of 2301 cells/mm2(i)
Figure 4.
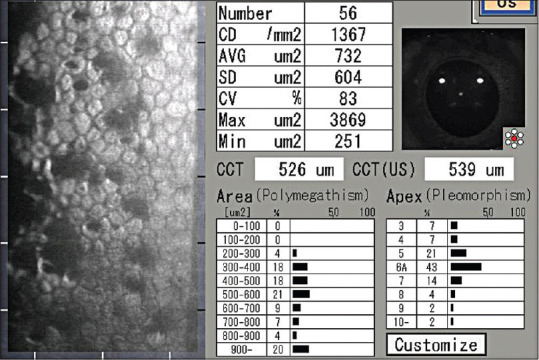
Specular microscopy imaging in a pseudophakic eye with anterior uveitis showing dark lesions corresponding to endothelial pigments and swollen endothelial cells (pseudoguttae)
Figure 5.
Specular microscopy images in PPCD: (a-c) Slit lamp photograph (a) of right of a 25 year old male showing serpentine bands at posterior membrane level; the specular microscopy of the same eye (b) shows the bands, reduced endothelial cell density (1176 cells/mm2), increased mean cell area (851 μm) when compared to the specular microscopy image of the normal left eye of the patient. (Images b and c have the same scale)
Figure 6.
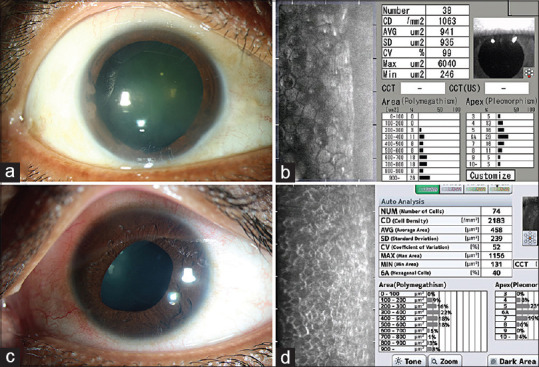
Specular microscopy in ICE syndrome: (a and b) Slit lamp photograph (a) of a 45 year old patient with ICE syndrome showing broad peripheral synechiae, central to paracentral corneal haze in the inferotemporal quadrant; the specular image (b) from the superior mid peripheral cornea (clear area of the cornea) shows enlarged endothelial cells, rounding of the cellular boundaries and increased black out areas within the cells. (c and d) Slit lamp photograph (c) of a 25 year old patient with ICE syndrome; the specular microscopy (d) shows the characteristic dark-light reversal pattern
Figure 7.
Specular microscopy after therapeutic penetrating keratoplasty: (a-c) Slit lamp photograph (a) of a 36 year old patient who had a therapeutic penetrating keratoplasty in the right eye 11 years ago; the specular image of the right eye (b) shows a lower endothelial cell density, increased mean cell area when compared to the specular image of the normal left eye (c). (Images b and c are in same scale)
Figure 9.
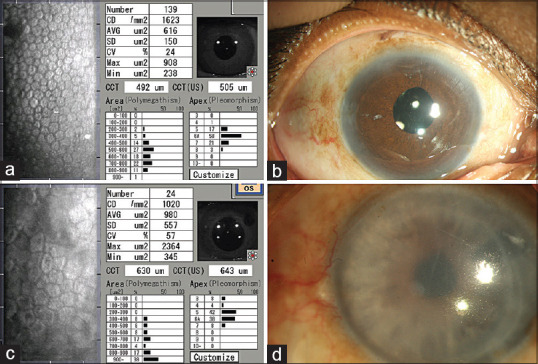
Specular Microscopy after Descemet stripping endothelial keratoplasty: (a and b) Specular microscopy (a) of a patient who had Descemet stripping endothelial keratoplasty 11 years ago, the endothelial cell density is 1623 cells/mm2. The posterior lamellar graft is compact and cornea is clear (b). (c and d) Specular microscopy of another patient who had a Descemet stripping endothelial keratoplasty 11 years ago. Notice the increased mean cell area, lowered cell density in this specular image compared to that in (c), suggesting the likelihood of an imminent endothelial failure. This patient as anticipated, presented a year later with decrease in vision and secondary graft failure as seen in (d)
Figure 10.

Specular Microscopy of the right (a) and left eye (b) of a patient who was on Amantadine treatment for a movement disorder. Both eyes have abnormal endothelial cell morphology at the visit images were captured. Patient eventually presented with bilateral corneal edema
Figure 11.
Specular microscopy in secondary endothelial disorders: (a and b) Slit lamp photograph (a) of a patient who had recurrent episodes of endothelitis and immune stromal keratitis in the left eye; the specular microscopy (b) of the left eye shows a reduced cell counts compared to the normal right eye image (c).[Images b and c have the same scale]. (d-f) Slit lamp photograph (d) of a patient who had a forceps injury in the right eye; the specular microscopy of the right eye (e) shows a reduced cell density compared to the normal left eye (f). [Images e and f have the same scale]
Figure 12.
Specular microscopy after Deep anterior lamellar keratoplasty (DALK) in Macular Corneal Dystrophy. Specular microscopy images (1c, 2c and 3c) showing the drop out areas/guttae similar to that is seen in FECD. Pre-operative endothelial imaging is not possible due to stromal deposits, but after DALK, restoration of stromal clarity helps in capturing the endothelial images. Notice the severe Descemet membrane changes (3c) in the third patient compared to the first (1a-c) and the second patient (3a-c).
Figure 8.
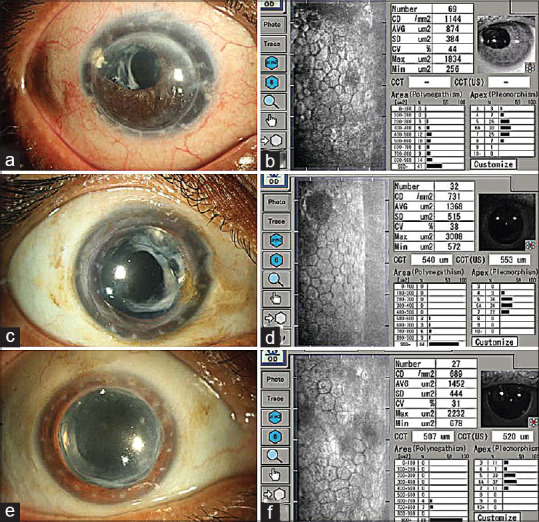
Endothelial imaging in 30-year-old penetrating keratoplasty grafts: (a-f) Slit lamp photographs (a-e) of 3 patients who had penetrating keratoplasty 30 years ago. All the grafts were clear and their corresponding specular images (b, d and f) show the endothelial cell densities (1144, 731, 689 cells/mm2)
Specular Microscopy in Eye Banks
Specular microscopy of donor corneas is a standard practice in the evaluation of donor corneas and assessing the suitability for various types of keratoplasty. Standard eye bank specular microscopy allows evaluation of magnified view of central endothelial cells. A wide-field ex vivo dual imaging specular microscope (Ex. CellChek, Konan Medical) can enable assessment of a larger area of the endothelial surface. The donor corneas must be warmed to 25°C before evaluation. The warming time required to obtain a good endothelial imaging can range for 45 min to over 2 h.[1] As per the donor evaluation criteria followed by most eye banks and corneal surgeons, the donor cornea is suitable for penetrating keratoplasty and endothelial keratoplasty if ECD is above 2000 and 2200 cells/mm2, respectively.
Future Prospects
The commercially available specular microscopy models are expensive. The other limitations are that these cannot be used to study endothelial parameters in the community and in pediatric eyes examined under anesthesia in supine position. Smartphone-based endothelial imaging is evolving to overcome these limitations.[42,43] Further, the current imaging devices capture the endothelium reliably in the central cornea. Although mid-peripheral cornea can be imaged by shifting the gaze, the far peripheral cornea cannot be imaged easily. The feasibility and accuracy of specular microscopy in- vivo imaging from different areas of the posterior endothelial layer will enhance the understanding and management of various clinical conditions.
Conclusion
Specular microscopy is a simple and valuable tool for the evaluation of corneal endothelium in normal and diseased eyes. It is critical to perform the specular microscopy as per the standard guidelines and interpreted with a clear understanding of its limitations in various situations. Clinico-specular correlation helps in making an accurate diagnosis of a condition, assessing the functional reserve of the cornea and pre-operative evaluation in surgical management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sayegh RR, Benetz BA, Lass JH. Specular microscopy. In: Mannis MJ, Holland EJ, editors. Cornea. 4th ed. Mosby: Elsevier Health Sciences; 2017. pp. 160–79. [Google Scholar]
- 2.Laing RA, Sandstorm MM, Leibowitz HM. Clinical specular microscopy. I. Optical principles. Arch Ophthalmol. 1979;97:1714–9. doi: 10.1001/archopht.1979.01020020282021. [DOI] [PubMed] [Google Scholar]
- 3.Maurice DM. Cellular membrane activity in the corneal endothelium of intact eye. Experientia. 1968;24:1094–5. doi: 10.1007/BF02147776. [DOI] [PubMed] [Google Scholar]
- 4.McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea. 2008;27:1–16. doi: 10.1097/ICO.0b013e31815892da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joyce NC, Navon SE, Roy S, Zieske JD. Expression of cell cycle associated proteins in human and rabbit corneal endothelium in situ. Invest Ophthalmol Vis Sci. 1996;37:1566–75. [PubMed] [Google Scholar]
- 6.Senoo T, Joyce NC. Cell cycle kinetics in corneal endothelium from old and young donors. Invest Ophthalmol Vis Sci. 2000;41:660–7. [PubMed] [Google Scholar]
- 7.Bourne WM. Biology of the corneal endothelium in health and disease. Eye. 2003;17:912–8. doi: 10.1038/sj.eye.6700559. [DOI] [PubMed] [Google Scholar]
- 8.Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997;38:779–82. [PubMed] [Google Scholar]
- 9.Dawson DG, Geroski DH, Edelhauser HF. Corneal endothelium: Structure and function in health and disease. In: Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea. 3rd ed. Mosby: Elsevier Health Sciences; 2010. pp. 57–70. [Google Scholar]
- 10.Matsuda M, Yee RW, Edelhauser HF. Comparison of the corneal endothelium in an American and a Japanese population. Arch Ophthalmol. 1985;103:68–70. doi: 10.1001/archopht.1985.01050010072023. [DOI] [PubMed] [Google Scholar]
- 11.Rao SK, Sen PR, Fogla R, Gangadharan S, Padmanabhan P, Badrinath SS. Corneal endothelial cell density and morphology in normal Indian eyes. Cornea. 2000;19:820–3. doi: 10.1097/00003226-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Nishida T, Saiko S, Morishige N. Cornea and sclera: Anatomy and physiology. In: Mannis MJ, Holland EJ, editors. Cornea. 4th ed. Mosby: Elsevier Health Sciences; 2017. pp. 1–22. [Google Scholar]
- 13.Lass JH, Gal RL, Ruedy KJ, Benetz BA, Beck RW, Baratz KH, et al. Cornea donor study group. An evaluation of image quality and accuracy of eye bank measurement of donor cornea endothelial cell density in the Specular Microscopy Ancillary Study. Ophthalmology. 2005;112:431–40. doi: 10.1016/j.ophtha.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 14.Vedana G, Villarreal G, Jr, Jun AS. Fuchs endothelial corneal dystrophy: Current perspectives. Clin Ophthalmol. 2016;10:321–30. doi: 10.2147/OPTH.S83467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iovieno A, Neri A, Soldani AM, Adani C, Fontana L. Descemetorhexis without graft placement for the treatment of fuchs endothelial dystrophy: Preliminary results and review of the literature. Cornea. 2017;36:637–41. doi: 10.1097/ICO.0000000000001202. [DOI] [PubMed] [Google Scholar]
- 16.Traish AS, Colby KA. Approaching cataract surgery in patients with fuchs' endothelial dystrophy. Int Ophthalmol Clin. 2010;50:1–11. doi: 10.1097/IIO.0b013e3181c5728f. [DOI] [PubMed] [Google Scholar]
- 17.Liesegang TJ, Skuta GL, Cantor LB. External Disease and Cornea. San Francisco (USA): American Academy Ophthalmology; 2005. [Google Scholar]
- 18.Chaurasia S, Mittal R, Bichappa G, Ramappa M, Murthy SI. Clinical characterization of posterior polymorphous corneal dystrophy in patients of Indian ethnicity. Int Ophthalmol. 2017;37:945–52. doi: 10.1007/s10792-016-0360-y. [DOI] [PubMed] [Google Scholar]
- 19.Ahn YJ, Choi SI, Yum HR, Shin SY, Park SH. Clinical features in children with posterior polymorphous corneal dystrophy. Optom Vis Sci. 2017;94:476–81. doi: 10.1097/OPX.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 20.Hirst LW, Quigley HA, Stark WJ, Shields NB. Specular microscopy of irido-corneal endothelial syndrome. Aust J Ophthalmol. 1980;8:139–46. doi: 10.1111/j.1442-9071.1980.tb01672.x. [DOI] [PubMed] [Google Scholar]
- 21.Sherrard ES, Frangoulis MA, Muir MG, Buckley RJ. The posterior surface of the cornea in the irido-corneal endothelial syndrome: A specular microscopical study. Trans Ophthalmol Soc U K. 1985;104:766–74. [PubMed] [Google Scholar]
- 22.Bourne RR, Minnassian DC, Dart JK, Rosen P, Kaushal S, Wingate N. Effect of cataract surgery on the corneal endothelium. Ophthalmology. 2004;111:679–85. doi: 10.1016/j.ophtha.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Goukon H, Kamiya K, Shimizu K, Igarashi A. Comparison of corneal endothelial cell density mad morphology after posterior chamber phakic intraocular lens implantation with and without a central hole. Br J Opthalmol. 2017;101:1461–5. doi: 10.1136/bjophthalmol-2016-309363. [DOI] [PubMed] [Google Scholar]
- 24.Armitage WJ, Dick AD, Bourne WM. Predicting endothelial cell loss and long-term corneal graft survival. Invest Ophthalmol Vis Sci. 2003;44:3326–31. doi: 10.1167/iovs.02-1255. [DOI] [PubMed] [Google Scholar]
- 25.Ing JJ, Ing HH, Nelson LR, Hodge DO, Bourne WM. Ten-year post-operative results of penetrating keratoplasty. Ophthalmology. 1998;105:1855–65. doi: 10.1016/S0161-6420(98)91030-2. [DOI] [PubMed] [Google Scholar]
- 26.Price MO, Calhoun P, Kollman C, Price FW, Jr, Lass JH. Descemet stripping endothelial keratoplasty: Ten-year endothelial cell loss compared with penetrating keratoplasty. Ophthalmology. 2016;123:1421–7. doi: 10.1016/j.ophtha.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Pavlovic I, Shajari M, Herrmann E, Schmack I, Lencova A, Kohnen T. Meta-analysis of postoperative outcome parameters comparing descemet membrane endothelial keratoplasty versus descemet stripping automated endothelial keratoplasty. Cornea. 2017;36:1445–51. doi: 10.1097/ICO.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 28.Palko JR, Qi O, Sheybani A. Corneal alterations associated with pseudoexfoliation syndrome and glaucoma: A literature review. J Ophthalmic Vis Res. 2017;12:312–24. doi: 10.4103/jovr.jovr_28_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein AS, Janson BJ, Skeie JM, Ling JJ, Greiner MA. The effects of diabetes mellitus on the corneal endothelium: A review. Surv Ophthalmol. 2020;65:438–50. doi: 10.1016/j.survophthal.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Sati A, Jha A, Moulick PS, Shankar S, Gupta S, Khan MA, et al. Corneal endothelial alterations in chronic renal failure. Cornea. 2016;35:1320–5. doi: 10.1097/ICO.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 31.Mancera N, Wadia HP. Corneal edema associated with systemic dopaminergic agents. Cornea. 2019;38:1040–2. doi: 10.1097/ICO.0000000000001941. [DOI] [PubMed] [Google Scholar]
- 32.Feng MT, Price FW, Jr, McKee Y, Price MO. Memantine-associated corneal endothelial dysfunction. JAMA Ophthalmol. 2015;133:1218–20. doi: 10.1001/jamaophthalmol.2015.2476. [DOI] [PubMed] [Google Scholar]
- 33.Snip RC, Kenyon KR, Green WR. Macular corneal dystrophy: Ultrastructural pathology of corneal endothelium and Descemet's membrane. Invest Ophthalmol Vis Sci. 1972;12:88–97. [PubMed] [Google Scholar]
- 34.Chaurasia S, Ramappa M, Murthy S, Garg P. Primary graft failure after big bubble deep anterior lamellar keratoplasty in macular corneal dystrophy. Indian J Ophthalmol. 2018;66:1196–7. doi: 10.4103/ijo.IJO_220_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alfawaz AM, Holland GN, Yu F, Margolis MS, Giaconi JA, Aldave AJ. Corneal endothelium in patients with anterior uveitis. Ophthalmology. 2016;123:1637–45. doi: 10.1016/j.ophtha.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 36.Sravani NG, Mohamed A, Chaurasia S, Durgam SS, Murthy SI. Corneal endothelium in unilateral Fuchs heterochromic iridocyclitis. Indian J Ophthalmol. 2020;68:447–9. doi: 10.4103/ijo.IJO_869_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hillenaar T, Weenen C, Wubbels RJ, Remeijer L. Endothelial involvement in herpes simplex virus keratitis: An in vivo confocal microscopy study. Ophthalmology. 2009;116:2077–86. doi: 10.1016/j.ophtha.2009.04.022. e1-2. [DOI] [PubMed] [Google Scholar]
- 38.Liesegang TJ. Physiologic changes of the cornea with contact lens wear. CLAO J. 2002;28:12–27. [PubMed] [Google Scholar]
- 39.Sibug ME, Datiles MB, Kashima K. Specular microscopy studies on the corneal endothelium after cessation of contact lens wear. Cornea. 1991;10:395–401. doi: 10.1097/00003226-199109000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Dhakal R, Mohamed A, Chaurasia S, Ramappa M, Jalali S. Corneal endothelial cell density in uveal coloboma associated with microcornea. Cornea. 2019;38:74–7. doi: 10.1097/ICO.0000000000001755. [DOI] [PubMed] [Google Scholar]
- 41.Chaurasia S, Mulay K, Ramappa M, Sangwan V, Murthy S, Nair R, et al. Corneal changes in xeroderma pigmentosum: A clinicopathologic report. Am J Ophthalmol. 2014;157:495–500e2. doi: 10.1016/j.ajo.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Toslak D, Thapa D, Erol MK, Chen Y, Yao X. Smartphone-based imaging of the corneal endothelium at sub-cellular resolution. J Mod Opt. 2017;64:1229–32. doi: 10.1080/09500340.2016.1267815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fliotsos MJ, Deljookorani S, Dzhaber D, Chandan S, Ighani M, Eghrari AO. Qualitative and quantitative analysis of the corneal endothelium with smartphone specular microscopy. Cornea. 2020;39:924–9. doi: 10.1097/ICO.0000000000002277. [DOI] [PMC free article] [PubMed] [Google Scholar]






