Abstract
Purpose:
To describe a series of sight-threatening ocular and adnexal involvement associated with dengue hemorrhagic fever and their treatment options.
Methods:
Retrospective, interventional, non-comparative case series. Medical records of patients who presented with ocular symptoms attributed to dengue hemorrhagic fever were reviewed. Demography, presenting features, and treatment outcomes were recorded. Outcome measures assessed included globe salvage, vision salvage, and visual improvement. The outcome was deemed as favorable if vision salvage was possible and unfavorable if the vision was lost.
Results:
Twenty-nine eyes of 23 patients were included. Bilateral ocular involvement was seen in 6 (26%) patients. The mean age was 37.62 ± 18.68 years (range: 14 to 81 years). Coexistent diabetes mellitus and enteric fever were present in three patients. History of blood transfusion was present in nine (40%) and thrombocytopenia in eight (35%) patients at the time of presentation with ocular complaints. Presenting features included endophthalmitis in 11 (38%), panophthalmitis in 10 (35%), orbital cellulitis with panophthalmitis in four (14%), isolated corneal or scleral melt in three (10%), and orbital hemorrhage with panophthalmitis in one (3%) patient. Globe salvage was achieved in 21/29 eyes (72.4%), vision salvage in 6/29 eyes (20.68%), and improvement in visual acuity was noted in 5/29 eyes (17.24%). Logistic regression analysis revealed no significant effect of any clinical-microbiological factors on globe salvage, vision salvage, and visual improvement. However, visual improvement and globe salvage were possible in eyes that underwent early endoscopic vitrectomy.
Conclusion:
Dengue fever can present with sight-threatening ocular and adnexal inflammation resulting in endophthalmitis and panophthalmitis, orbital cellulitis, corneal and scleral melt, and orbital hemorrhage. Early vitrectomy may improve vision and globe salvage in cases with significant vitritis.
Keywords: Dengue, necrosis, panophthalmitis, phthisis bulbi, scleral melt
Dengue fever is caused by a flavirius, spread by the Aedes mosquitoes. Four serotypes numbered 1 to 4 are prevalent in India. Dengue is an important health problem in India with the numbers on the rise.[1,2,3,4,5,6,7,8] Ophthalmic complications have earlier been known to occur in the form of anterior uveitis, panuveitis, retinitis, sub-hyaloid hemorrhage, retinal hemorrhages, maculopathy, neuororetinitis, optic neuropathy, and corneal epitheliopathy.[9,10,11,12,13,14,15,16,17,18] Notably, over the past decade, the disease has manifested in a severe form of ocular and orbital involvement with the scleral melt, necrotizing scleritis, globe rupture, endophthalmitis, and panophthalmitis.[19,20,21,22,23,24,25,26,27] However, the existing literature is limited to the case report and short case series. In the current communication, we report the presentations and management outcomes of cases with various presentations of severe ocular and adnexal involvement in dengue hemorrhagic fever. We also discuss the management approaches to such cases.
Methods
The electronic medical records of patients who were diagnosed with severe ocular manifestations and with a positive history of dengue fever, presenting to a tertiary eye care center from January 2015 to November 2019 were reviewed. Patients with a systemic diagnosis of dengue infection either based on typical clinical features or positive serology for antibody to NS1 within 3 months of ocular involvement were included. Patients with non-sight threatening complaints but with a recent history of dengue fever and normal ocular examination were excluded. Lack of adequate documentation of dengue fever in cases of post-fever viral retinitis and uveitis were excluded. Demographic details such as age, gender, details of systemic comorbidities, duration between onset of systemic to ocular symptoms, records of systemic and prior ocular treatment, laterality of ocular involvement, ocular symptoms and signs including visual acuity, anterior segment, posterior segment, and adnexal involvement were noted. Intraoperative details of the ocular and periocular tissue, details of medical and surgical treatment, ocular and body fluid cultures, and hemogram parameters were recorded. Samples for microbiological culture and sensitivity were inoculated under aseptic precautions onto sterile culture plates for bacterial and microbiological culture. The identification of microorganisms was performed using VITEK 2 compact identification system (Biomerieux, NC, Marcy l'Etoile, France). The primary outcome measure was globe salvage and secondary outcome measures were vision salvage and visual improvement at the final follow-up visit. The outcome was deemed as favorable if vision salvage was possible and unfavorable if the vision was lost.
Statistical analysis
Data was entered in numbers (version 6.2, Apple Inc.) with descriptive data expressed as mean ± SD, median, range, and percentage. The analysis was performed using statistical package for the social sciences (SPSS) (version 23, IBM statistics) using Levene's test for normality, student t-test, Chi-square test, Fischer's exact test, and logistic regression analysis as appropriate. The effects of predictors such as age, presence of systemic comorbidities, history of blood transfusion, thrombocytopenia, general condition at presentation, duration between onset of systemic and ocular symptoms, the severity of ocular inflammation, isolation of microorganism from ocular tissue and body fluids on outcomes measures i.e., globe salvage, vision salvage, and visual improvement were assessed by logistic regression analysis. A P value of <0.05 was considered statistically significant.
Results
Demographics
A total of 23 patients presented with a systemic diagnosis of dengue fever before or at the time of onset of ocular symptoms. Eighteen patients were males. The mean age at presentation was 37.62 ± 18.68 years (range: 14 to 81 years). The number of cases each year showed an increasing trend over 2015 (n = 1), 2016 (n = 2), 2017 (n = 4), 2018 (n = 6), and 2019 (n = 10 till November 2019).
Systemic status
Systemic comorbidities, unrelated to dengue such as diabetes mellitus were present in two patients and one patient was recovering from enteric fever when he contracted dengue infection. Systemic complications resulting from dengue such as acute renal injury, polyserositis, bedsores from prolonged hospitalization, and ascites were seen in seven (30%) patients. H/o blood transfusion was present in nine (40%) patients. Thrombocytopenia was documented in eight (35%) patients.
Ocular involvement
Table 1 describes the demography, presenting visual acuity and clinical features in this cohort of dengue patients. Bilateral ocular involvement was seen in six (26%) of the patients [Fig. 1a]. Among the unilateral ocular presentations (n = 17), right eye involvement was seen in seven (41%) and left eye in 10 (59%) patients. Visual acuity at presentation ranged from no perception of light to 0.06 LogMAR. Ocular symptoms started after a mean of 9 ± 20.9 days (median 3, range 0-111) days following the onset of systemic symptoms. Presenting features included decreased vision, eye pain, redness, discharge, bleeding from the eye, corneal clouding, proptosis, limitation of extraocular motility [Fig. 1b], focal [Fig. 1b], and diffuse scleral melt. Posterior segment involvement was manifest as exudates in the vitreous cavity [Fig. 1c], retinal detachment, vitreous hemorrhage, and presence of fluid in sub-tenon's space in advanced cases. Intraocular pressure (IOP) was recordable in six patients and it ranged from 4 to 62 mmHg. Clinical evaluation in 11 patients revealed proptosis. On computed tomography (CT) scan imaging, proptosis was confirmed with fat stranding and straightening of the optic nerve. The diagnostic spectrum included endophthalmitis (n = 11), panophthalmitis (n = 10), panophthalmitis with orbital cellulitis (n = 4), isolated corneal melt (n = 2), scleral melt with vitreous hemorrhage (n = 1), and panophthalmitis with orbital hematoma (n = 1). None of the cases had sub-hyaloid hemorrhage visible clinically. However, since sub-hyaloid hemorrhage is a relatively common occurrence in ocular affection due to dengue, clinically this may have been missed due to the presence of corneal edema at a presentation in most of the cases in our subset (7/11).
Table 1.
Demography and clinical features of ocular and adnexal involvement in dengue hemorrhagic fever
| Demographic variable | N | % |
|---|---|---|
| Demographics Mean age: 37.6 years (range: 14-81) | ||
| Gender | ||
| Male | 18 | 78.3 |
| Female | 5 | 21.7 |
| Systemic condition | ||
| Hypertension | 2 | 8.7 |
| Diabetes mellitus | 2 | 8.7 |
| Typhoid | 1 | 4.3 |
| Dengue related complications | ||
| Acute kidney injury | ||
| Polyserositis | 1 | 4.3 |
| Ascites | 1 | 4.3 |
| Bed sore | 1 | 4.3 |
| Thrombocytopenia | 8 | 34.8 |
| Blood transfusion | 9 | 39.1 |
| Ocular involvement | ||
| Unilateral | 17 | 73.9 |
| Bilateral | 6 | 26.1 |
| Visual acuity Range: NPL to 20/320 (n=29) | n | % |
| >20/1200 | 1 | 3.4 |
| 20/1200 to CFCF | 2 | 6.9 |
| HM | 2 | 6.9 |
| PL + | 7 | 24.1 |
| NPL | 17 | 58.6 |
| Clinical features | ||
| Presenting symptoms | n | % |
| Decreased vision | 29 | 100 |
| Eye pain | 22 | 75.9 |
| Redness | 21 | 72.4 |
| Watering and discharge | 13 | 44.8 |
| Bleeding from eye | 2 | 6.9 |
| Presenting signs | n | % |
| EOM limitation | 12 | 41.3 |
| Corneal infiltrate/ulceration | 6 | 20.7 |
| Proptosis | 7 | 24.1 |
| Scleral melt | 4 | 13.8 |
| Anterior uveitis | 14 | 48.2 |
| Cataract | 4 | 13.8 |
| Retinal hemorrhages, vitreous exudates | 19 | 66 |
| Retinal detachment | 1 | 3.4 |
| Sub tenon fluid | 14 | 48.3 |
n: Number of patients, n: Number of eyes
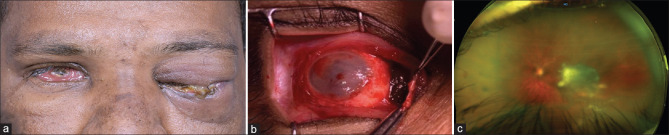
Figure 1.
(a) External photograph of a 34-year-old male at first presentation with severe bilateral orbital inflammation. (b) Intraoperative image a 21-year-old male who presented with panophthalmitis in whom scleral melt was noted intraoperatively. (c) Postoperative fundus photograph of the left eye of a 55-year old gentleman showing resolving subretinal exudate after pars plana vitrectomy
Clinical course and management outcomes
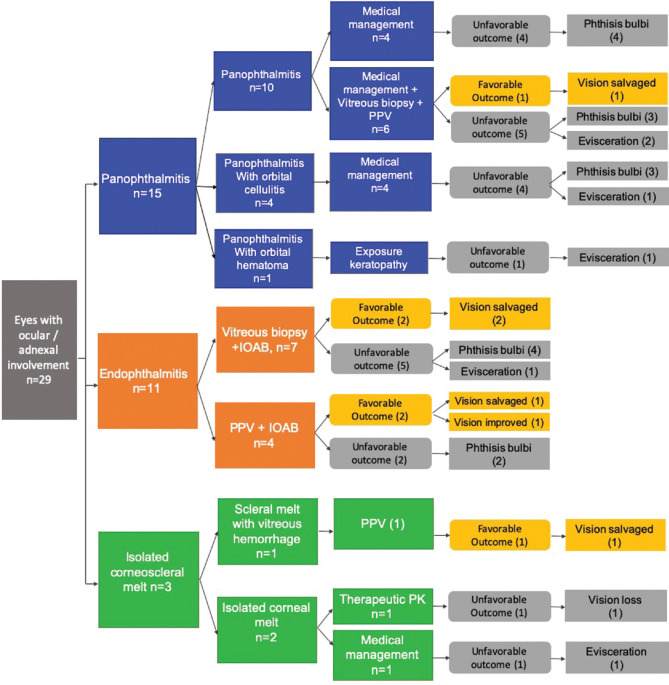
The clinical course and management outcomes are highlighted in Fig. 2. Four of the 11 eyes (36%) with endophthalmitis had a favorable outcome. Among the 11 eyes with endophthalmitis, seven underwent vitreous biopsy and injection of intraocular antibiotics (IOAB) and four underwent pars plana vitrectomy (PPV) with IOAB. In the group that underwent only a vitreous biopsy, the vision was salvaged in two (29%) eyes. Four eyes progressed to phthisis bulbi and one needed evisceration for severe intraocular infection. In the group that underwent a PPV with IOAB, two of the four culminated in the phthisis bulbi. One eye underwent therapeutic keratoplasty for coexisting microbial keratitis and showed visual improvement. Endoscopic PPV was performed in one eye which had a good visual outcome.
Figure 2.
Flow chart diagram showing the clinical course and management outcomes of the dengue cases
Among the 15 eyes with panophthalmitis, a favorable outcome was observed in one eye (7%). Ten eyes presented with panophthalmitis, four had panophthalmitis with evidence of orbital cellulitis on CT orbit, and one patient developed an orbital hematoma with exposure keratopathy and subsequent panophthalmitis requiring evisceration [Fig. 3]. Among the 10 patients presented with panophthalmitis, four were treated with systemic antibiotics followed by systemic steroids that were started after 48 h of induction with antibiotics. All the four eyes developed phthisis bulbi. Six eyes were treated with systemic antibiotics along with intraocular antibiotics. Two of these eyes progressed to phthisis bulbi and one eye required evisceration. The remaining three eyes were considered for endoscopic PPV. One of the three eyes that underwent PPV developed corneal infiltrates and required therapeutic keratoplasty following which vison was maintained and the remaining two eyes went into phthisis bulbi. In the subset that developed orbital cellulitis along with panophthalmitis, all four eyes had an unfavorable response. One eye needed evisceration for a progressive scleral melt and the other three developed phthisis bulbi.
Figure 3.
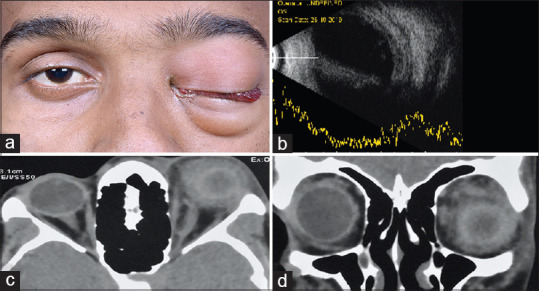
(a) External photograph of a 17-year-old male who presented with panuveitis. (b) Slit-lamp photograph showing a focal area of scleral thinning with the uveal show. (c) Ultrasonography B scan showing dot and membranous low echoes. (d) Ultrasound biomicroscopy scan through the superonasal quadrant showed no intraocular mass lesion
One patient presented with a focal scleral melt [Fig. 4] and vitreous hemorrhage and underwent PPV. The vision improved and the globe was salvaged. Both the eyes presenting with corneal melt showed an unfavorable response. One eye needed evisceration and therapeutic penetrating keratoplasty was performed in others, however, the vision was lost.
Figure 4.
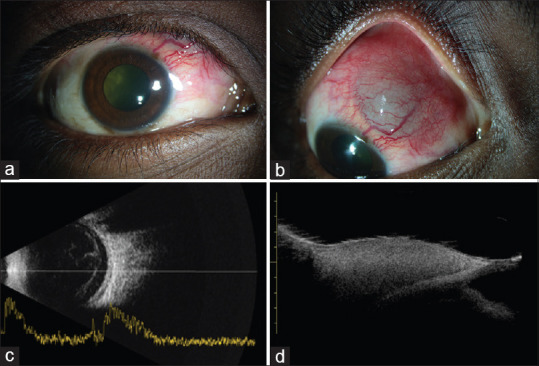
(a) External photograph of a 21-year-old-male with suspected left eye retrobulbar hemorrhage and visible vitreous hemorrhage. (b) Ultrasonography B scan showing linear and cluster echoes suggestive of intraocular hemorrhage. (c and d) Axial and coronal sections of computed tomography scans of the orbits showing ill-defined hyperdensities within the left orbit and straightening of the optic nerve
Microbiological work-up
Ocular samples sent for culture included vitreous biopsy (8), corneal scraping (2), corneal button (1), AC tap (1), eviscerated contents (7), and pus from eyelid (1).
Microorganisms isolated included bacteria such as Escherichia coli, Corynebacterium amycolatum, Acinetobacter iwoffii, and fungi such as Aspergillus and Fusarium.
Outcomes
The ocular outcomes are presented in Table 2. Sixteen eyes progressed to phthisis bulbi, six eyes required evisceration, one eye lost vision, and the vision was salvaged in six eyes. Vision salvage was possible in six (21%) (one focal scleral melt, four endophthalmites, one panophthalmitis) eyes, and visual improvement was noted in one (one endophthalmitis) eye. Visual acuity of 20/50 was achieved in a patient who presented with 20/320 vision and underwent early endoscopic PPV. Globe salvage was achieved in 21 eyes (72%). A favorable outcome was observed in six (21%) of our patients and an unfavorable outcome was seen in 23 (79%).
Table 2.
Functional outcome of ocular and adnexal involvement in dengue hemorrhagic fever. The outcomes are classified into visual improvement, vision salvage, globe salvage, and evisceration
| Clinical presentation | Number of cases | Visual improvement | Vision salvage | Globe salvage | Eviscerated |
|---|---|---|---|---|---|
| Focal scleral melt | n=1 | 0 | 1 | 1 | 0 |
| Corneal melt | n=2 | 0 | 0 | 1 | 1 |
| Orbital hemorrhage with panophthalmitis | n=1 | 0 | 0 | 0 | 1 |
| Orbital cellulitis with panophthalmitis | n=4 | 0 | 0 | 3 | 1 |
| Panophthalmitis | n=10 | 0 | 1 | 8 | 2 |
| Endophthalmitis | n=11 | 1 | 4 | 10 | 1 |
| Total | n=29 | 1 | 6 | 23 | 6 |
n=number of cases
Logistic regression analysis to study the effects of predictors such as age, presence of systemic comorbidities, history of blood transfusion, thrombocytopenia, anemia, general condition at presentation, duration between onset of systemic and ocular symptoms, the severity of ocular inflammation, microbiological isolation of organisms from ocular samples and body fluids on outcomes measured i.e., globe salvage, vision salvage, and visual improvement revealed no statistically significant correlation.
Discussion
The dengue virus belongs to the family Flaviviridae and is an arthropod-borne virus spread by Aedes mosquitoes.[1,2,3,4] Described as “water poison” by the Chinese as early as 265 AD, dengue fever has caused several epidemics over the centuries across the world. Setting its foot in the Indian subcontinent in the 1940s, the epidemiology and serotypes of dengue virus in India seem to be constantly evolving posing a threat to the community and a challenge to the medical fraternity.[1,2,3,4,5,6,7]
Existing literature about ophthalmic complications secondary to dengue describes uveitis, retinitis, sub-hyaloid hemorrhage, retinal hemorrhage maculopathy, neuororetinitis, panophthalmitis, and optic neuropathy.[8,9,10,11,12,13,14,15,16] Anecdotal reports of corneal epitheliopathy exist.[17] With medical management, good visual recovery has been documented, and vision loss is considered rare.[18,19] World Health Organization in 2011, coined the term expanded dengue syndrome for cases which do not fall under dengue shock syndrome or dengue hemorrhagic fever and have atypical manifestations with multisystem involvement including the liver, heart, kidney, the central nervous system alerting the physicians to evaluate for dengue in presence of atypical clinical features.[6,7] Ophthalmic manifestations included in this entity were macular hemorrhage, impaired visual acuity, and optic neuritis.[6] Interestingly, the same time frame marked the emergence of an unusually severe and fulminant orbital inflammation, intense tissue destruction with a dismal visual prognosis which in some cases warranted removal of the eye.[20,21,22,23,24,25,26,27,28] This is in stark contrast to the benign course of the ophthalmic disease reported earlier. Isolated reports have shown ophthalmic involvement in its most severe form causing scleral melt, necrotizing scleritis, globe rupture, endophthalmitis, and panophthalmitis, at times bilateral involvement described.[20,21,22,23,24,25,26,27,28]
An interesting finding noted in the present study which reviewed records over 5 years shows a linear increase in the number of cases with a steeper slope over the past year. The cause for this temporal pattern could be directly related to the increase in overall cases of dengue infection in the community.[29] All the 26 patients in this series had florid scleral and orbital involvement, severe enough to cause extensive tissue destruction and necrosis. The visual recovery was dismal. Patients developed these ocular manifestations from few days to weeks after the onset of illness. Unusual manifestation in our series was the localized scleral melt with uveal show [Fig. 3] raising the suspicion of a masquerader and rapid involvement of a completely healthy contralateral eye with 20/20 vision in a few days [Fig. 4]. This could not be attributed to any particular systemic factor. Whether the varied ocular features constitute a part of the multisystem expanded dengue syndrome needs to be relooked at.
A possible inciting factor could be the transfusion of blood products. Ramananda et al.[26] described three cases of transfusion-related endophthalmitis and panophthalmitis in dengue fever and emphasized that prophylactic platelet transfusion is to be avoided given secondary complications. However, more than half of our patients did not receive platelet transfusion and developed symptoms despite it. Besides, there was no correlation between disease severity and transfusion in this series. Some of the patients developed ocular involvement within a day of onset of systemic symptoms. These observations downplay the role of blood transfusion as an inciting factor. A third notable factor postulated in literature is the antibody-mediated destruction of endothelial cells resulting in septicemia, endogenous endophthalmitis, and subsequently panophthalmitis.[28] Bacterial and fungal isolates were seen in ocular samples of five patients and urine culture in three patients in the present series. No microorganisms were isolated from blood cultures.
Concerning ocular treatment, amongst four patients who underwent early PPV, three showed remarkable improvement and the fourth patient developed microbial keratitis warranting a therapeutic keratoplasty. Vitrectomy as the first line of management in these cases is a novel approach, but early vitrectomy appears to have the potential to restore visual acuity in less severe cases, therefore, the threshold for surgical intervention may be kept low in these cases. In cases where globe salvage cannot be achieved, due emphasis has to be laid on counseling and cosmetic rehabilitation considering the young age of these patients and the rapid downhill course of the disease.
This study is not without limitations. Patients presented to us were at different stages of dengue fever, and no particular pattern of development and resolution of ocular involvement concerning the phase of systemic illness could be identified. Secondly, though this study shows an increasing trend in the number of cases with dengue-related florid orbital inflammation, being a tertiary care center-based study, what fraction of patients afflicted with dengue fever develop this manifestation could not be estimated. Dengue is a multisystem illness. Evaluation and management are required for the non-ophthalmic features as well. Our institute is a stand-alone ophthalmic care institute. All the patients mentioned in this study were diagnosed with dengue fever elsewhere and were having systemic management for the same done elsewhere too. Due to this, we did not have access to the patient charts and hence details of systemic management could not be elaborated in this report. This is another limitation of the current study.
Conclusion
To conclude, dengue fever can present with devastating sight-threatening ocular and adnexal inflammation. The mechanism of dengue-related ocular complications remains unclear and less well understood than the systemic manifestations of the disease. Further research is warranted to elaborate on the pathogenesis of the disease. At this point, it can only be hypothesized that the fulminant inflammation is a complex interplay between the virus-mediated cellular destruction, hemodynamic alternations as well as the host's immune response with a possible component of superimposed microbial infection. Treating internists, as well as ophthalmologists, need to be aware of this entity to allow early diagnosis and prompt referral. Early vitrectomy, seems to have the potential for vision and globe salvage, hence recommended in select cases of vitritis, offering maximum benefit to the patients who present in the early stage of disease with less severe ocular manifestations.
Financial support and sponsorship
This study is supported by a research grant from the Hyderabad Eye Research Foundation.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dengue and severe dengue. World Health Organization. [Internet] 2019. Nov, [[Last accessed on 2020 Aug 02]]. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue .
- 2.Ganeshkumar P, Murhekar MV, Poornima V, Saravanakumar V, Sukumaran K, Anandaselvasankar A, et al. Dengue infection in India: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12:e0006618. doi: 10.1371/journal.pntd.0006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karamchandani PV. Dengue group of fevers in India. Lancet. 1946;1:92. doi: 10.1016/s0140-6736(46)91229-9. [DOI] [PubMed] [Google Scholar]
- 4.Gupta N, Srivastava S, Jain A, Chaturvedi UC. Dengue in India. Indian J Med Res. 2012;136:373–90. [PMC free article] [PubMed] [Google Scholar]
- 5.Dhar SK, Samant S, Tudu PK, Tripathy D, K VR, Prasad KRC. Clinical spectrum of dengue at a tertiary care hospital in eastern India. Int J Adv Med. 2019;6:1554–8. [Google Scholar]
- 6.World Health Organization, Regional Office for South-East Asia. Comprehensive guideline for prevention and control of dengue and dengue haemorrhagic fever. revised and expanded edition. WHO Regional Office for South-East Asia. World Health Organization. [Internet] 2011. [[Last accessed on 2020 Aug 02]]. Available from: https://apps.who.int/iris/handle/10665/204894 .
- 7.Mohanty B, Sunder A, Pathak S. Clinico laboratory profile of expanded dengue syndrome - Our experience in a teaching hospital. J Family Med Prim Care. 2019;8:1022–7. doi: 10.4103/jfmpc.jfmpc_12_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vivien CH, Srinivasan S, Yan TK. Ophthalmic complications of dengue fever: A Systematic review. Ophthalmol Ther. 2012;1:2. doi: 10.1007/s40123-012-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussain I, Afzal F, Shabbir A, Adil A, Zahid A, Tayyib M. Ophthalmic manifestation of dengue fever. Ophthalmol Update. 2012;10:93–6. [Google Scholar]
- 10.Chan DP, Teoh SC, Tan CSH, Nah GK, Rajagopalan R, Prabhakaragupta MK, et al. Ophthalmic complications of dengue. Emerg Infect Dis. 2006;12:285–9. doi: 10.3201/eid1202.050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chlebicki MP, Ang B, Barkham T, Laude A. Retinal hemorrhages in 4 patients with dengue fever. Emerg Infect Dis. 2005;11:770–2. doi: 10.3201/eid1105.040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta A, Srinivasan R, Setia S, Soundravally R, Pandian DG. Uveitis following dengue fever. Eye (Lond) 2009;23:873–6. doi: 10.1038/eye.2008.124. [DOI] [PubMed] [Google Scholar]
- 13.Khairallah M, Chee SP, Rathinam SR, Attia S, Nadella V. Novel infectious agents causing uveitis. Int Ophthalmol. 2010;30:465–83. doi: 10.1007/s10792-009-9319-6. [DOI] [PubMed] [Google Scholar]
- 14.Ooi KG, Inglis H, Paramanathan N, Downie JA, Hennessy MP. Dengue Fever-Associated Maculopathy and Panuveitis in Australia. Case Rep Ophthalmol Med. 2016;2016:5704695. doi: 10.1155/2016/5704695. doi:10.1155/2016/5704695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawali A, Mahendradas P, Ashwin Mohan, Mallavarapu M, Shetty B. Epidemic retinitis. Ocul Immunol Inflamm. 2019;27:571–7. doi: 10.1080/09273948.2017.1421670. [DOI] [PubMed] [Google Scholar]
- 16.Haritoglou C, Dotse SD, Rudolph G, Stephan CM, Thurau SR, Klauss V. A tourist with dengue fever and visual loss. Lancet. 2002;360:1070. doi: 10.1016/s0140-6736(02)11145-7. [DOI] [PubMed] [Google Scholar]
- 17.Chhavi N, Venkatesh C, Soundararajan P, Gunasekaran D. Unusual ocular manifestations of dengue fever in a young girl. Indian J Pediatr. 2013;80:522–3. doi: 10.1007/s12098-012-0871-0. [DOI] [PubMed] [Google Scholar]
- 18.Lim WK, Mathur R, Koh A, Yeoh R, Chee SP. Ocular manifestations of dengue fever. Ophthalmology. 2004;111:2057–64. doi: 10.1016/j.ophtha.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 19.Somkijrungroj T, Kongwattananon W. Ocular manifestations of dengue. Curr Opin Ophthalmol. 2019;30:500–5. doi: 10.1097/ICU.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 20.Saranappa SB, Sowbhagya HN. Panophthalmitis in dengue fever. Indian Pediatr. 2012;49:760. doi: 10.1007/s13312-012-0142-1. [DOI] [PubMed] [Google Scholar]
- 21.Nagaraj KB, Jayadev C, Yajmaan S, Prakash S. An unusual ocular emergency in severe dengue. Middle East Afr J Ophthalmol. 2014;21:347–9. doi: 10.4103/0974-9233.142276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sriram S, Kavalakatt JA, Pereira Ad, Murty S. Bilateral panophthalmitis in dengue fever. Ann Trop Med Public Health. 2015;8:217–8. [Google Scholar]
- 23.Kamoi K, Mochizuki M, Ohno-Matsui K. Dengue fever-associated necrotizing scleritis: A case report with long-term follow-up. Medicine (Baltimore) 2018;97:e11875. doi: 10.1097/MD.0000000000011875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jyothi PT, Farseenamol AP, Subi AS, George AE. Spontaneous globe rupture in dengue: A case series. Kerala J Ophthalmol. 2018;30:117–20. [Google Scholar]
- 25.Arya D, Das S, Shah G, Gandhi A. Panophthalmitis associated with scleral necrosis in dengue hemorrhagic fever. Indian J Ophthalmol. 2019;67:1775–7. doi: 10.4103/ijo.IJO_2050_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padmanaban S, Jeevakala C, Saravanan J, Shalini G. Sight threatening ocular complications in dengue fever - A prospective study. Indian J Basic Appl Med Res. 2018;7:635–40. [Google Scholar]
- 27.Ramananda K, Sundar MD, Mandal S, Ravani R, Kumar V. Platelet transfusion related panophthalmitis and endophthalmitis in patients with dengue hemorrhagic fever. Am J Trop Med Hyg. 2018;99:1053–4. doi: 10.4269/ajtmh.18-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamal R, Shah, Sharma S, Janani MK, Kar A, Saurabh K, et al. Culture-positive unilateral panophthalmitis in a serology-positive case of dengue hemorrhagic fever. Indian J Ophthalmol. 2018;66:1017–9. doi: 10.4103/ijo.IJO_113_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mutheneni SR, Morse AP, Caminade C, Upadhyayula SM. Dengue burden in India: Recent trends and importance of climatic parameters. Emerg Microbes Infect. 2017;6:e70. doi: 10.1038/emi.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]