Abstract
Purpose:
The aim of this study was to analyze the impact on vision due to delay in presentation of patients requiring intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections, consequent to COVID-19-related travel restrictions.
Methods:
Data were collected retrospectively of patients who received anti-VEGF injections during four months of the COVID-19 pandemic. Visual acuities, indication for treatment were noted along with basic demographic characteristics.
Results:
Data were analyzed for 303 eyes of 263 patients. The indication for treatment was age-related macular degeneration (AMD) in 60 eyes (19.8%), while 162 eyes (53.5%) had Diabetic Macular Edema, 71 eyes (23.4%) had Retinal Vein Occlusion and 10 eyes (3.3%) had other diagnosis. The visual acuity in the treatment naïve eyes (Group A, n = 168) was significantly worse (P < 0.001) than those who presented for retreatment (Group B, n = 135). In Group B, there was a significant decline in vision for the entire cohort (P = 0.009) and those with AMD (P = 0.036). Those in Group B presented at a mean interval of 19.1 ± 10.6 (range, 4–64) weeks for retreatment.
Conclusion:
The COVID-19 pandemic has led to a delay in patients receiving anti-VEGF injections. The visual acuity is worse in both treatment naïve as well as those requiring retreatment. This could have long-term impact on vision of patients requiring this vision preserving treatment.
Keywords: Age-related macular degeneration, anti-VEGF, COVID-19, diabetic macular edema, retinal vein occlusion
The impact of the COVID-19 pandemic reaches far beyond that of a respiratory illness. To curb transmission of this contagious virus, the Government of India ordered a total lockdown in the country from March 25 to May 17, followed by various phases of unlock. As a result, ophthalmic practice was severely affected, with total cessation of work in some private clinics to only emergency work in institutes.[1] Lack of public transport facilities, restricted movement and fear of infection led to delay in presentation to the hospital.[2] Detailed guidelines on preferred practice for ophthalmology[3] as well as vitreo-retinal subspecialty have been laid down.[4]
Intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy is an established treatment modality for various retinal diseases like age-related macular degeneration (AMD), polypoidal choroidal vasculopathy, diabetic macular edema (DME), macular edema due to retinal vein occlusions (RVO) etc.[5] The outcomes in these diseases is optimal with early institution of treatment as well as regular follow-up with retreatments. Administration of anti-VEGF injections is considered as essential medical treatment that needs to be continued during the COVID-19 pandemic.[3,4,6]
In this study we analyze the impact on vision due to delay in presentation of patients requiring intravitreal anti-VEGF injections secondary to COVID-19-related travel restrictions.
Methods
This retrospective observation study included patients who were administered intravitreal anti-VEGF injections (bevacizumab, ranibizumab and aflibercept) from April 1, 2020 to July 31, 2020 during COVID-19 lockdown and unlock phase 1. Institutional ethics committee approval was obtained and the study adhered to the declaration of Helsinki. The patients who received other drugs intravitreally (steroids or antibiotics) during this time period were excluded. We considered only the first injection during this time frame (and not the subsequent visits) for the analysis. The data recorded included age, gender, diagnosis and number of eyes that were treatment naïve (Group A) or had received prior injections (Group B). Best corrected visual acuity (BCVA) was recorded in LogMAR, at current visit for both groups, along with BCVA at baseline (at first visit when treatment was initiated) and prior visit (defined as the clinical evaluation visit immediately prior to the one in study period) for Group B. The duration between prior and current injection for Group B was noted.
The primary outcome measure was visual acuity of patients who presented for retreatment (Group B) during this time period. The secondary outcome measure was presenting visual acuity of those who were treatment naïve (Group A).
Statistical analysis
Continuous variables were presented as mean with standard deviation or median with interquartile range (IQR) and categorical variables were presented as proportions (n, %). Shapiro Wilk test was used to check the normality of the data. Mann–Whitney U test/t test was used to find out the significance between continuous variables. Paired t test/Wilcoxon signed rank test was used to find out the significance between paired data. A value of P < 0.05 was considered as statistically significant. All statistical analysis was done by using statistical software STATA 14.2 (Texas, Illinois).
Results
Of a total of 457 intravitreal anti-VEGF injections administered in the above specified time period, 303 eyes of 263 patients were eligible for inclusion in the study. Of 263 patients, 183 (69.6%) were males and 80 (30.4%) were females. 223 (84.8%) patients were administered intravitreal injection in one eye whereas 40 (15.2%) patients were injected bilaterally. Laterality was equally distributed between right eye (n = 150, 49.5%) and left eye (n = 153, 50.5%). Sixty eyes (19.8%) were diagnosed to have AMD, 162 eyes (53.5%) had DME, 71 eyes (23.4%) had RVO and 10 eyes (3.3%) had other diagnosis. Other diagnosis included choroidal neovascular membranes (CNVM) in three eyes with MacTel type 2; two eyes each with myopic CNVM, CNVM secondary to central serous chorioretinopathy and uveitis-related CNVM; and CNVM with angioid streaks in one eye. Those in AMD group were oldest in the cohort [Table 1].
Table 1.
Age-wise distribution of patients across different diagnosis
| Diagnosis | Age in years | |
|---|---|---|
| Mean (SD) | Range | |
| AMD | 65.7 (11.9) | 41-86 |
| DME | 56.1 (8.9) | 35-75 |
| RVO | 58.0 (12.6) | 30-80 |
| Others | 51 (13.5) | 19-61 |
| Overall | 58.3 (11.3) | 19-86 |
Of the 303 eyes, 168 (55.5%) eyes were treatment naïve (Group A) and 135 (44.5%) had received prior injections and reported for retreatment (Group B). Diagnosis-wise distribution of the eyes in Group A and Group B is shown in Fig. 1. The mean interval between prior and current injection for Group B eyes with AMD was 20.6 ± 12.6 (range, 8–64) weeks, with DME was 19.9 ± 9.5 (range, 4–52) weeks, with RVO was 15.8 ± 9.9 (range, 4–45) weeks and overall was 19.1 ± 10.6 (range, 4–64) weeks. Ten patients were lost to follow-up before the lockdown period (defined as prior visit >6 months before current visit) and came for consultation during the study period and were advised repeat injection.
Figure 1.
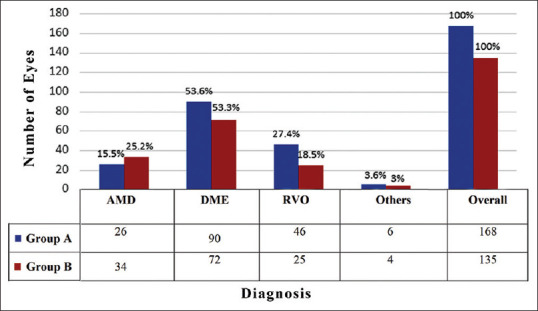
Bar diagram illustrating the number of eyes in Group A (treatment naïve) and those in Group B (for retreatment) across various disease categories
Visual acuities of both groups are reported in Table 2. There was a significant worsening of BCVA in Group B eyes at current visit when compared to prior visit, in the entire cohort (P = 0.009) and those with AMD (P = 0.036). On comparing BCVA between Group A with baseline BCVA of Group B, it was noted that the vision was significantly worse in Group A for the entire cohort (P <0.001), those with AMD (P = 0.0002), DME (P = 0.005) and RVO (P = 0.007).
Table 2.
Comparison of Best corrected visual acuity of eyes receiving anti-VEGF
| Parameters | AMD | DME | RVO | Others | Overall |
|---|---|---|---|---|---|
| BCVA Group A | |||||
| Median | 0.8 (6/38) | 0.5 (6/19) | 0.6 (6/24) | 0.8 (6/38) | 0.6 (6/24) |
| IQR | 0.5-1 | 0.3-0.8 | 0.3-1 | 0.2-1 | 0.3-1 |
| Baseline BCVA Group B | |||||
| Median | 0.3 (6/12) | 0.3 (6/12) | 0.3 (6/12) | 0.9 (6/60) | 0.3 (6/12) |
| IQR | 0.2-0.6 | 0.2-0.6 | 0.2-0.6 | 0.7-1 | 0.2-0.6 |
| BCVA at prior visit Group B | |||||
| Median | 0.4 (6/15) | 0.3 (6/12) | 0.5 (6/19) | 0.9 (6/48) | 0.3 (6/12) |
| IQR | 0.2-0.6 | 0.2-0.6 | 0.2-1 | 0.8-1 | 0.2-0.6 |
| BCVA at current visit Group B | |||||
| Median | 0.5 (6/19) | 0.3 (6/12) | 0.5 (6/19) | 1 (6/60) | 0.5 (6/19) |
| IQR | 0.3-0.9 | 0.3-0.6 | 0.3-0.8 | 0.9-1 | 0.3-0.8 |
| Comparison of vision Group B at prior and current visit | 0.036* | 0.113* | 0.447* | 0.391# | 0.009* |
| Comparison of vision between Group A and Group B at baseline | 0.0002@ | 0.005@ | 0.007@ | 0.564$ | <0.001@ |
*Wilcoxon signed rank test; #paired t-test; S - Significant; @Mann-Whitney U test; $Independent; t-test; IQR - Inter quartile Range. Group A - Treatment Naïve eyes. Group B - Eyes that had received prior treatment and now reported for retreatment. All visual acuities are reported in LogMAR
Discussion
Anti-VEGF injections form a major part of the treatment armamentarium of vitreo-retinal practice. They need to be administered repeatedly, necessitating numerous hospital visits by the patients. Patient care during the COVID-19 pandemic requires many modifications in patient care protocols.[3,4] Our institution has incorporated all the recommendations to include PPE and respirators for all medical personnel, 3-ply mask use by all patients and attenders, with slit lamp and indirect ophthalmoscope shields to minimize spread of virus. Temperature screening for employees and patients, along with COVID-19 declaration, minimizing consultation time by history on phone, social distancing in all waiting areas and staggering patient appointments are strictly followed. To enhance safety during evaluation, the vitreo-retina clinic fast tracks the cases that require intravitreal anti-VEGF injections by identifying them at entry and performing an OCT scan before consult with the ophthalmologist. The OCT machine is placed in separate area with an acrylic sheet separating the technician and patient [Fig. 2]. Patients are counselled to undergo injections on the same day, reducing need for further travel. In the operating room, injections are staggered with a time gap of 5-10 minutes between consecutive patients. The operating table is cleaned with chlorhexidine solution and the floor is mopped with Lysol surface disinfectant.[7]
Figure 2.
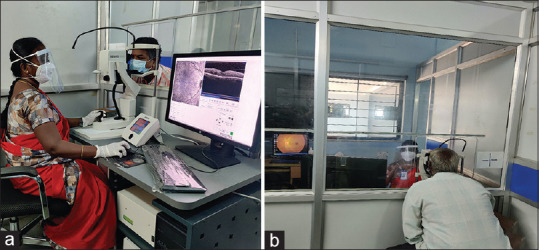
Specialized cubicle for optical coherence tomography. The technician and patient sit on two sides separated by a partition, the upper part of which is made of a transparent acrylic sheet to facilitate communication. (a) view from technician side. (b) view from patient side
Most of the patients requiring anti-VEGF injections are older, and have systemic co-morbidities like diabetes and hypertension. In this report, we found that those with AMD were amongst the older group, while the maximum anti-VEGF injections were administered for DME. These patients are more susceptible to COVID-19 infection and might face a more fulminant course of disease.[8] This has reflected in reduction of these patients seeking care for their ocular conditions.
The mean interval between prior and current injection in Group B was found to be 19.1 ± 10.6 weeks. There is a delay in follow-up in these patients, possibly due to due to travel restrictions during the pandemic. Possibly other factors like non- availability of attendant, monetary issues and old age could also have influenced the delay.[9] There is a decrease in vision of the patients that need re-injection, when evaluated post delay. The drop in vision is significant for the entire cohort and for those with AMD. As AMD is a progressive disease, patients need continuous monitoring with customized retreatment criteria when considering treat and extend.[7,10] Outer retinal edema in patients with AMD can cause rapid loss of photoreceptors with decrease in vision.[7] Those with DME and RVO maintained vision, even with missed injections. Visual gains in DME are noted to be stable even with limitation of retreatment or in presence of chronic DME. The initial visual gains from anti-VEGF treatment are sustained later on, and patients are seen to require fewer retreatments.[11,12] Retinal vein occlusions are inner retinal diseases, where the accumulated fluid can be cleared by an active retinal pigment epithelium pump. Early treatment with anti-VEGF can have sustained effect later, as was seen in the CRUISE and BRAVO trials, where patients maintained vision in the observation period of pro-re-nata treatment.[13,14]
An alarming trend that was seen in this study was the significantly poorer visual acuity of those who were treatment naïve across the entire cohort; as well as for those with AMD, DME and RVO. It is possible that the delay in presentation due to fear in visiting a hospital as well as travel restrictions has resulted in a poorer baseline vision. In AMD, early institution of treatment with better baseline visual acuities has better outcomes, before chronicity can set in.[7,12,15] In the open label extension of RISE and RIDE trials, those with shorter duration of DME required fewer injections and had better visual outcomes.[12] In eyes with central or branch vein occlusion, those who receive treatment late, do show improved vision and decreased central macular thickness, but never achieve same outcomes as those treated early.[13,14] Moreover, when baseline vision is better at initiation of treatment, visual gain is faster and more sustained.[16]
The inclusion of a survey questionnaire, eliciting reason for delayed presentation and COVID-19-related issues faced by the patients would have made the study more robust, and is the major limitation here.
Conclusion
To conclude, travel restrictions and fear of contracting COVID-19 have resulted in a delay in patients receiving essential vision preserving treatment like anti-VEGF injections. There is a significant reduction of visual acuities in those who need retreatment, while the treatment naïve are presenting late with worse baseline vision. This could lead to prolonged impact with a large subset of population having lifelong reduced visual potential. The COVID-19 pandemic has presented added challenges in managing retinal diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Ms. Iswarya Mani, MSc Biostatistics, Dept. of Biostatistics, Aravind Eye Hospital, Madurai for statistical analysis of our data.
References
- 1.Nair A, Gandhi R, Natarajan S. Effect of COVID-19 related lockdown on ophthalmic practice and patient care in India: Results of a survey. Indian J Ophthalmol. 2020;68:725–30. doi: 10.4103/ijo.IJO_797_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babu N, Kohli P, Mishra C, Sen S, Arthur D, Chhablani D, et al. To evaluate the effect of COVID-19 pandemic and national lockdown on patient care at a Tertiary-care ophthalmology institute. Indian J Ophthalmol. 2020;68:1540–4. doi: 10.4103/ijo.IJO_1673_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sengupta S, Honavar S, Sachdev M, Sharma N, Kumar A, Ram J, et al. All India ophthalmological society – Indian journal of ophthalmology consensus statement on preferred practices during the COVID-19 pandemic. Indian J Ophthalmol. 2020;68:711–24. doi: 10.4103/ijo.IJO_871_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta V, Rajendran A, Narayanan R, Chawla S, Kumar A, Palanivelu M, et al. Evolving consensus on managing vitreo-retina and uvea practice in post-COVID-19 pandemic era. Indian J Ophthalmol. 2020;68:962–73. doi: 10.4103/ijo.IJO_1404_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornel S, Adriana ID, Mihaela TC, Speranta S, Simone D, Mehdi B, et al. Anti-vascular endothelial growth factor indications in ocular disease. Rom J Ophthalmol. 2015;59:235–42. [PMC free article] [PubMed] [Google Scholar]
- 6.Korobelnik J-F, Loewenstein A, Eldem B, Joussen AM, Koh A, Lambrou GN, et al. Guidance for anti-VEGF intravitreal injections during the COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2020;258:1149–56. doi: 10.1007/s00417-020-04703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer MA, Awh CC, Sadda S, Freeman WR, Antoszyk AN, Wong P, et al. HORIZON: An open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119:1175–83. doi: 10.1016/j.ophtha.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Coronavirus Disease 2019 [Internet] Centers for Disease Control and Prevention. 2020. Last cited on 2020 Aug 27. Available from: https://www.cdc.gov/media/releases/2020/p0625-update-expands-covid-19.html .
- 9.Vengadesan N, Ahmad M, Sindal M, Sengupta S. Delayed follow-up in patients with diabetic retinopathy in South India: Social factors and impact on disease progression. Indian J Ophthalmol. 2017;65:376–84. doi: 10.4103/ijo.IJO_620_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E, Schlingemann R, et al. Guidelines for the management of neovascular age-related macular degeneration by the European society of retina specialists (EURETINA) Br J Ophthalmol. 2014;98:1144–67. doi: 10.1136/bjophthalmol-2014-305702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bressler NM, Beaulieu WT, Glassman AR, Blinder KJ, Bressler SB, Jampol LM, et al. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: A secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;136:257–69. doi: 10.1001/jamaophthalmol.2017.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wykoff CC, Elman MJ, Regillo CD, Ding B, Lu N, Stoilov I. Predictors of diabetic macular edema treatment frequency with ranibizumab during the open-label extension of the RIDE and RISE trials. Ophthalmology. 2016;123:1716–21. doi: 10.1016/j.ophtha.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Brown DM, Campochiaro PA, Bhisitkul RB, Ho AC, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118:1594–602. doi: 10.1016/j.ophtha.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: Twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041–9. doi: 10.1016/j.ophtha.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen A, Brandi S, Fuchs J, Hansen LH, Lund-Andersen H, Sander B, et al. Visual outcomes in relation to time to treatment in neovascular age-related macular degeneration. Acta Ophthalmol (Copenh) 2015;93:616–20. doi: 10.1111/aos.12781. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd Clark W, Liu M, Kitchens J, Wang P, Haskova Z. Baseline characteristics associated with early visual acuity gains after ranibizumab treatment for retinal vein occlusion. BMC Ophthalmol. 2019;19:11. doi: 10.1186/s12886-018-1012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


