To the Editor,
Coronavirus disease-19 (COVID-19) patients suffer a high incidence of pulmonary embolisms (PE) [1,2]. For non-COVID-19 patients, D-dimer is commonly used to determine if a computed tomography pulmonary angiogram (CTPA) should be performed. Although in COVID-19 patients, higher D-dimer levels at ICU admission have been linked to increased mortality [3], it is unclear to what extent D-dimer concentrations during ICU-stay relate to the occurrence of PE. Consequently, conflicting suggestions for serial D-dimer determinations were published [4,5]. We prospectively performed serial measurements of D-dimer in a cohort of critically ill COVID-19 patients and related this to the incidence of PE.
We analyzed 76 consecutive COVID-19 patients admitted between March 11th 2020 until April 27th 2020 to our university hospital ICU. All patients were mechanically ventilated. Patients or legal representatives were informed and could decline use of data. D-dimer levels (ug/ml FEU) were measured every other day, using STA liatest (Stago, Asières sur Seine, France). A CTPA was performed on clinicians' discretion based on an increase in D-dimer levels and/or worsening of ventilatory parameters or other clinical suspicion. Patients with a CTPA-confirmed PE were compared to those with no PE and to those in whom no CTPA was performed. A CTPE was performed in 39 out of 76 patients (51%).
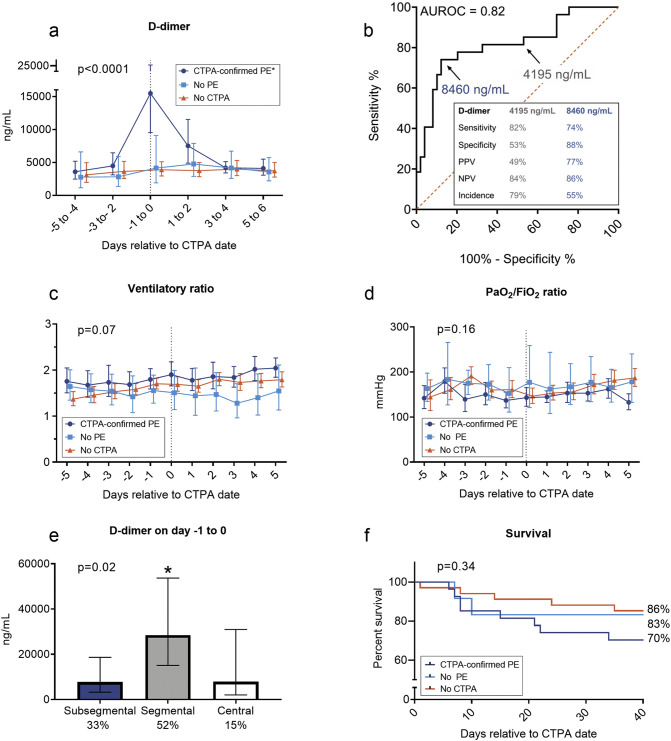
The median [IQR] age of the patients was 64 [57–71] years, 26% was female and hospital mortality 22%. See Table 1 for all patient characteristics. All patients received low molecular weight heparin in prophylactic doses (dalteparin, <100 kg: 5000 IU once a day, >100 kg: 5000 IU twice per day). Twenty-seven patients were diagnosed with a PE during their ICU stay (median [IQR] 6 [3–14] days post-ICU-admission). All patients diagnosed with PE, subsequentially received therapeutic anticoagulant therapy with dalteparin twice daily based on body weight (<55 kg: 5000 IE, 55–85 kg: 7500 IE, >85 kg: 10000 IE). At ICU admission, there were no relevant differences in demographics, comorbidities, or clinical/laboratory parameters between patients with CTPA-confirmed PE and those without PE or in whom no CTPA performed (Table 1). Nevertheless, patients with a PE exhibited a clear increase in D-dimer (median [IQR] 10,960 [1840 to 34,580] ng/mL) one day before/on the day of diagnosis, which was not the case in patients with no PE (350 [−1830 to 3370] ng/mL), or in the group where no CTPA was performed (410 [−931 to 1186] ng/mL, Fig. 1A). As depicted in Fig. 1B, a D-dimer level of 8460 ng/mL, which was found in 55% of patients at any point during their ICU stay and in 17% of all measurements performed in the ICU in all patients, had a sensitivity of 74%, a specificity of 88%, a positive predictive value of 77%, and negative predictive value of 86%. Ventilatory and PaO2/FiO2 ratios were similar between the three groups and showed no relationship with PE (Fig. 1C–D), which was also the case for other parameters (heart rate, minute ventilation, respiratory rate, PaCO2, C-reactive protein, ferritin, procalcitonin, and thrombocyte and leukocyte counts; data not shown). Most patients had a segmental PE, which was also associated with the highest D-dimer levels (Fig. 1E). In surviving patients, time on mechanical ventilation and ICU length of stay after alignment day were prolonged in patients with a CTPA-confirmed PE compared to patients in whom no CTPA was performed (Table 1). Hospital survival appeared worse in patients with a CTPA-confirmed PE than in the other groups but this did not reach statistical significance (Table 1, and see Fig. 1F for survival curves).
Table 1.
Patient characteristics and outcome parameters.
| All patients (N = 76) | CTPA-confirmed PE (N = 27) | No PE (N = 12) | No CTPA (N = 37) | p-Value | |
|---|---|---|---|---|---|
| Characteristics | |||||
| Sex, male | 56 (74%) | 21 (78%) | 11 (92%) | 24 (65%) | 0.16 |
| Age, years | 64 [57–71] | 65 [59–71] | 56 [51–71] | 67 [57–71] | 0.30 |
| BMI, kg/m2 | 28 [25–30] | 27 [25–29] | 26 [24–31] | 28 [24–31] | 0.88 |
| APACHE II score | 15 [12–19] | 15 [12–19] | 12 [9–16] | 16 [13−20] | 0.08 |
| Any comorbidities | |||||
| Diabetes mellitus | 16 (21%) | 5 (19%) | 3 (25%) | 8 (22%) | 0.89 |
| Hypertension | 35 (46%) | 9 (33%) | 7 (58%) | 19 (51%) | 0.23 |
| COPD | 6 (8%) | 1 (4%) | 1 (8%) | 4 (11%) | 0.58 |
| Metastatic neoplasm | 5 (7%) | 1 (4%) | 1 (8%) | 3 (8%) | 0.75 |
| Hematologic malignancy | 4 (5%) | 1 (4%) | 1 (8%) | 2 (5%) | 0.84 |
| Renal insufficiency | 1 (1%) | 0 (0%) | 1 (8%) | 0 (0%) | 0.07 |
| Immunological insufficiency | 9 (12%) | 3 (11%) | 1 (8%) | 5 (14%) | 0.88 |
| Clinical/laboratory parameters | |||||
| PaO2/FiO2 ratio, mmHg | 143 [103–182] | 136 [102–160] | 216 [146–255] | 140 [91–181] | 0.053 |
| <100 mmHg | 16 (21%) | 6 (22%) | 2 (17%) | 8 (22%) | |
| 100–200 mmHg | 43 (57%) | 18 (67%) | 4 (33%) | 21 (57%) | |
| 200–300 mmHg | 17 (22%) | 3 (11%) | 6 (50%) | 8 (22%) | |
| CRP, mg/L | 219 [140–292] | 235 [95–291] | 196 [145–314] | 219 [170–295] | 0.80 |
| Procalcitonin, μg/L | 0.75 [0.36–1.89] | 0.68 [0.24–1.27] | 0.95 [0.53–2.34] | 0.76 [0.41–2/64]] | 0.34 |
| D-dimer, ng/mL | 3180 [1608–5640] | 3190 [1600–11,545] | 2785 [973–3298] | 3430 [1855–5550] | 0.38 |
| Thrombocytes, 109/L | 244 [164–304] | 209 [133–258]* | 284 [195–338] | 264 [179–356] | 0.01 |
| Clinical outcome parameters | |||||
| Time on mechanical ventilation in survived patients, days | 17 [18–25] | 21 [15–29]* | 23 [11–27] | 11 [4–19] | 0.004 |
| ICU length of stay in survived patients, days | 18 [9–27] | 23 [19–35]* | 20 [12–27] | 12 [5–22] | 0.008 |
| Hospital mortality | 15 (20%) | 8 (30%) | 2 (17%) | 5 (14%) | 0.27 |
Data were obtained at ICU admission and are presented as n (%) or median [IQR]. P-values were calculated using Chi-Square or Kruskal-Wallis tests. * indicates p < 0.05 vs. no CTPA calculated using Dunn's post-hoc test.
BMI: body mass index, COPD: Chronic obstructive pulmonary disease, CRP: C-reactive protein, ICU: intensive care unit.
Fig. 1.
A. Serial levels of D-dimer aligned relative to the day that CTPA was performed (n = 39, consisting of CTPA-confirmed PE [n = 27] and no PE [n = 12]). Alignment day was designated day 0. In the group of patients in whom no CTPA was performed (n = 37), the median day of PE diagnosis of the CTPA-confirmed PE group (6 days post-ICU admission) was used as the alignment day. The p-value in the panel was calculated using repeated measures linear mixed model analysis (time*group interaction term). * indicates p < 0.008 vs. no PE group and p < 0.0001 vs. no CTPA groups calculated using pairwise repeated measures linear mixed model analyses (time*group interaction term). B. ROC to illustrate sensitivity and specificity of D-dimer levels to predict PE. Positive predictive value (PPV) and negative predictive value (NPV) are provided for two D-dimer levels. Incidence of patients with these values in serially measured D-dimer levels are also provided. C. Ventilatory Ratio was calculated using the formula: (minute ventilation (ml/min) × PaCO2 (mm Hg))/(predicted body weight × 100 × 37.5). Higher values indicate increased dead alveolar space ventilation or increased CO2 production. Normal values are close to 1. The p-value in the panel was calculated using repeated measures linear mixed model analysis (time*group interaction term). D. PaO2/FiO2 ratio. PaO2 values in mmHg were used for calculations. The p-value in the panel was calculated using repeated measures linear mixed model analysis (time*group interaction term). E. Bar graph of D-dimer levels of the subtypes of PE and their incidence. P-value in panel was calculated using repeated measures linear mixed model analysis. * indicates p = 0.02 vs. subsegmental and p = 0.046 vs. central calculated using False Discovery rate-adjusted post-hoc tests. F. Kaplan-Meier curve of survival from alignment day onwards. The p-value in the panel was calculated using a log-rank test.
In our cohort, more than one-third of critically ill COVID-19 patients developed PE during their stay in the ICU which in is line with other studies [2]. Our data illustrate that a rapid increase in D-dimer levels suggests presence of a PE that warrants further diagnostic follow-up even when clinical parameters such as increased alveolar dead space ventilation (ventilatory ratio), or worse oxygenation (PaO2/FiO2 ratio) are absent. Therefore, serial measurement of D-dimer levels appears to be an important tool to timely detect PE in COVID-19 patients. Our study is limited by the monocentric nature and a relatively low sample size, which, among others, precludes correction for possible confounders and proper assessment of possible between-group differences in clinical outcomes. Because a significant part of our COVID-19 cohort was not scanned, PE may be underdiagnosed although these patients had similar survival compared to the patients in whom PE was excluded. Nevertheless, PE cannot be excluded solely by low D-dimer levels and high clinical suspicion in the presence of low D-dimer levels could still warrant further diagnostic work-up.
As virtually all COVID-19 patients show elevated D-dimer levels, the usual threshold to perform a CTPA should not be used. We report new cut-off values that may warrant further diagnostic work-up. Although critically ill COVID-19 patients represent a relatively homogenous group, showing similar clinical characteristics and incidence of PE in other studies, additional studies are needed to establish the generalizability of our proposed cut-off levels.
In conclusion, by sequential measurements of D-dimer levels, PE can be detected in COVID-19 patients who exhibit no clinical signs of PE thereby facilitating the timely detection of PE in critically ill COVID-19 patients. Future studies should address whether this is cost-effective The cut-off values we report may aid physicians in the decision to perform a CTPA in the context of their available resources.
Ethics approval and consent to participate
The study was carried out in accordance with the applicable rules concerning the review of research ethics committees and informed consent in the Netherlands. All patients or legal representatives were informed about the study details and allowed to abstain from participation.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This study was funded by the department of Intensive Care Medicine.
Author's contributions
MvdB, MK and PP designed the study. MvdB, DW, EK, MK and PP analyzed and interpreted the data. MvdB, DW and EK drafted the manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
Not applicable.
References
- 1.Wichmann D., Sperhake J.P., Lütgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020:M20–2003. doi: 10.7326/M20-2003. published online ahead of print, 2020 May 6. [DOI] [PubMed] [Google Scholar]
- 2.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L., Yan X., Fan Q., et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18(6):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes G.D., Burnett A., Allen A., et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020:1–10. doi: 10.1007/s11239-020-02138-z. published online ahead of print, 2020 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.