Abstract
Background
Coronavirus disease 2019 (COVID-19) has been associated with coagulation disorders, in particular high concentrations of D-dimer, and increased frequency of venous thromboembolism.
Aim
To explore the association between D-dimer at admission and in-hospital mortality in patients hospitalised for COVID-19, with or without symptomatic venous thromboembolism.
Methods
From 26 February to 20 April 2020, D-dimer concentration at admission and outcomes (in-hospital mortality and venous thromboembolism) of patients hospitalised for COVID-19 in medical wards were retrospectively analysed in a multicenter study in 24 French hospitals.
Results
Among 2878 patients enrolled in the study, 1154 (40.1%) patients had D-dimer measurement at admission. Receiver operating characteristic curve analysis identified a D-dimer concentration > 1128 ng/mL as the best cut-off value for in-hospital mortality (area under the curve 64.9%, 95% confidence interval [CI] 60–69), with a sensitivity of 71.1% (95% CI 62–78) and a specificity of 55.6% (95% CI 52–58), which did not differ in the subgroup of patients with venous thromboembolism during hospitalisation. Among 545 (47.2%) patients with D-dimer concentration > 1128 ng/mL at admission, 86 (15.8%) deaths occurred during hospitalisation. After adjustment, in Cox proportional hazards and logistic regression models, D-dimer concentration > 1128 ng/mL at admission was also associated with a worse prognosis, with an odds ratio of 3.07 (95% CI 2.05–4.69; P < 0.001) and an adjusted hazard ratio of 2.11 (95% CI 1.31–3.4; P < 0.01).
Conclusions
D-dimer concentration > 1128 ng/mL is a relevant predictive factor for in-hospital mortality in patients hospitalised for COVID-19 in a medical ward, regardless of the occurrence of venous thromboembolism during hospitalisation.
Keywords: COVID-19, D-dimer, Microvascular thrombosis, Pulmonary embolism, Deep venous thrombosis
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; ICU, intensive care unit; ISTH, International Society onThrombosis and Haemostasis; ROC, receiver operating characteristic; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VTE, venous thromboembolism
Résumé
Contexte
La COVID-19 a été associée à des troubles de la coagulation, en particulier des niveaux élevés de D-dimères, et une fréquence accrue d’évènement thromboembolique veineux (ETEV).
Objectif
Nous avons étudié l’association entre les D-dimères à l’admission au service d’accueil des urgences (SAU) et la mortalité chez les patients hospitalisés pour COVID-19 avec ou sans ETEV symptomatique.
Méthodes
Du 26 février au 20 avril 2020, pour les patients COVID-19 hospitalisés dans un service de médecine, le taux de D-dimères à l’admission et les critères de jugement (décès et ETEV) ont été rétrospectivement analysés dans une étude multicentrique dans 24 hôpitaux français. Les analyses statistiques comprenaient une courbe ROC, des régressions logistiques et des modèles de Cox. Cette recherche a été réalisée dans le respect de la réglementation sur la recherche.
Résultats
Parmi 2878 patients inclus dans la cohorte, 1154 (40,1 %) patients avaient bénéficié d’un dosage des D-dimères à l’admission au SAU. La courbe ROC a identifié une valeur de D-dimères supérieure à 1128 ng/mL comme valeur seuil optimale pour prédire la mortalité à l’hôpital (AUC 64,9 %; IC à 95 % 60–69) avec une sensibilité de 71,1 % (IC à 95 % 62–78) et une spécificité de 55,6 % (IC à 95 % 52–58). Dans l’analyse de sensibilité, ce seuil était similaire dans le sous-groupe de patients atteints de ETEV pendant l’hospitalisation. Parmi 545 (47.2 %) patients avec un taux de D-dimères > 1128 ng/mL à l’admission, 86 (15,8 %) décès étaient survenus pendant l’hospitalisation. Après ajustement, les modèles de régression logistique et de Cox ont confirmé qu’un taux de D-dimères > 1128 ng/mL à l’admission était associé à un mauvais pronostic avec un OR à 3,07 (IC à 95 % 2,05–4,69 ; p < 0,001) et un HR de 2,11 (IC à 95 % 1,31–3,4 ; p < 0,01).
Conclusions
Un taux de D-dimères > 1128 ng/mL est un facteur prédictif pertinent de la mortalité chez les patients COVID-19 avec critère d’hospitalisation, quelle que soit la survenue d' ETEV pendant l’hospitalisation.
Mots clés: COVID-19, D-dimères, Microthrombose, Embolie pulmonaire, Thrombose veineuse profonde
Background
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with various clinical respiratory syndromes, ranging from mild upper airway symptoms to progressive life-threatening viral pneumopathy [1], [2]. Patients with severe coronavirus disease 2019 (COVID-19) have progressive hypoxaemia, inducing the need for mechanical ventilatory support. One specific feature of COVID-19 is induced vascular disease. Ackermann et al. examined the morphological and molecular features of lungs obtained during autopsies of patients who died from COVID-19, and evidenced an abnormal angiogenic process inside the lungs, in contrast to lungs from patients who died from influenza or age-matched and uninfected control lungs [3]. COVID-19-induced vascular disease is also associated with an increased level of circulating endothelial cells [4]. Moreover, plasma biomarkers of endothelial lesions are also predictive factors for future referral to an intensive care unit (ICU), reinforcing the hypothesis of COVID-19-associated vascular injury [5]. The SARS-CoV-2 virus has been shown to infect blood vessels and to induce vascular damage [6], and fibrin deposits have been found in vascular beds in the lungs, but also in the kidneys.
A high prevalence of venous thromboembolism (VTE) – particularly pulmonary embolism–has been observed in patients hospitalised for COVID-19 [6], [7], [8]. However, more than these macrothrombotic events, microvascular thrombosis in the lungs has been reported following autopsies, suggesting acute respiratory distress syndrome in COVID-19 [9], [10], [11]. A thromboinflammatory process in the pulmonary capillary vessels is probably the main cause of microthrombosis in the lung capillaries, inducing COVID-19-associated coagulopathy [12], which is characterised by an increase in procoagulant factors, such as fibrinogen, together with a strong increase in D-dimer at admission [2], [10]. D-dimer concentration at admission has been associated with in-hospital mortality in several studies [2], [10], [11], although the cut-off allowing discrimination between patients with favourable and poor outcomes is still a matter of debate.
Using data from a large multicentre French case series, we aimed to identify a D-dimer cut-off at admission that could be a clear independent predictor of in-hospital mortality.
Methods
Study settings and population
From 26 February to 20 April 2020, all consecutive adult patients admitted to hospital with a diagnosis of SARS-CoV-2 infection were included in a retrospective multicentre (24 centres) observational study, which was initiated by the French society of cardiology, and named the Critical COVID-19 France study (ClinicalTrials.gov Identifier: NCT04344327) [7]. Following World Health Organisation criteria, SARS-CoV-2 infection was determined by positive results from real-time reverse transcriptase-polymerase chain reaction tests of nasal and pharyngeal swabs or lower respiratory tract aspirates (confirmed case), or by typical imaging characteristics on chest computed tomography scan when laboratory testing was inconclusive (probable case) [9].
Ethics approval and consent to participate
The critical COVID-19 France study was declared and authorised by the French data protection committee (Authorization No. 2207326v0), and was conducted in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its later amendments.
Data collection
All data were collected by local investigators in an electronic case report form via REDCap software (Research Electronic Data Capture; Vanderbilt University, Nashville, TN, USA), hosted by a secured server from the French institute of health and medical research at the Paris cardiovascular research centre. Patient baseline information included demographic characteristics, coexisting medical conditions, cardiovascular comorbidities and chronic medications. Clinical variables and biological findings were recorded at admission. On the chest computed tomography scan, the degree of pulmonary lesions with ground-glass opacities and areas of consolidation was categorised as low/moderate (< 50% involvement) or severe (> 50% involvement). The oral anticoagulation regimen at admission was categorised into two groups:
-
•
No anticoagulation;
-
•
And oral anticoagulant therapy with vitamin K antagonists or non-vitamin K antagonist oral anticoagulants.
The occurrence of symptomatic VTE during hospitalisation included pulmonary embolism and/or deep vein thrombosis.
Outcomes
The primary outcome was the time from diagnosis to death, to assess the predictive performance of D-dimer concentration at admission in patients with COVID-19. Outcomes were assessed using the electronic medical records.
Statistical analysis
Continuous data are expressed as means ± standard deviations and categorical data as proportions. Continuous variables were compared using the Mann–Whitney test, and categorical variables were compared using Fisher's exact test [13]. We generated D-dimer concentration at admission receiver operating characteristic (ROC) curves for in-hospital mortality. We identified the optimal threshold of D-dimer concentration at admission using Youden's J statistic. In the univariate analysis, patients were compared according to the optimal threshold of D-dimer at admission. In the multivariable analysis, we used logistic regression to assess the association between the concentration of D-dimer (as a categorical dependent variable dichotomised according to the optimal threshold) and platelet count, leukocyte count or in-hospital mortality [14], [15]. The model included as covariates: sex; age; cardiovascular comorbidities, such as history of high blood pressure; history of malignancy (cancer in remission or active cancer); plasma creatinine concentration (dichotomised according to the normal value of 107 μmol/L); C-reactive protein (mg/L); the degree of pulmonary lesions with ground-glass opacities and areas of consolidation (dichotomised < or > 50%); the use of oral anticoagulant therapy; and the occurrence of VTE during hospitalisation. A Cox proportional hazards model with length of stay (in days) as a time scale was used to investigate the relationship between the concentration of D-dimer (as a categorical dependent variable dichotomised according to the optimal threshold) and in-hospital mortality. The model was adjusted for the same potential confounders included in the logistic regression model. The Kaplan–Meier method was used to represent the Cox proportional hazards model results according to the concentration of D-dimer (as a categorical dependent variable dichotomised according to the optimal threshold). We used the log-rank test to compare the survival distributions according to the optimal threshold of D-dimer. We performed three sensitivity analysis:
-
•
To take into account the retrospective design and to avoid bias caused by censored data (n=268/1154, 23.2%), we performed the same multivariable analysis in the population of patients who were discharged alive from hospital or who died in hospital (total patients analysed, n=886/1154, 76.8%), and thus we excluded patients with a censored outcome;
-
•
We generated the D-dimer concentration at admission ROC curve only in the subgroup of patients with VTE during hospitalisation (n = 127), and compared the area under the curve of the two ROC curves using Delong's test;
-
•
To adjust for bias caused by non-random allocation of potential covariates, we performed a propensity-matched analysis [16] of patients who had VTE during hospitalisation for COVID-19 compared with those who did not have VTE, and repeated the Cox proportional hazards model adjusted only on plasma creatinine concentration (> 107 μmol/L), the use of oral anticoagulant therapy, VTE occurrence during hospitalisation, fraction of inspired oxygen and the degree of pulmonary lesions with ground-glass opacities and areas of consolidation.
All analyses were two-sided, and a P value < 0.05 was considered statistically significant. Statistical analysis was performed using R studio software (R Development Core Team [2019]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria).
Results
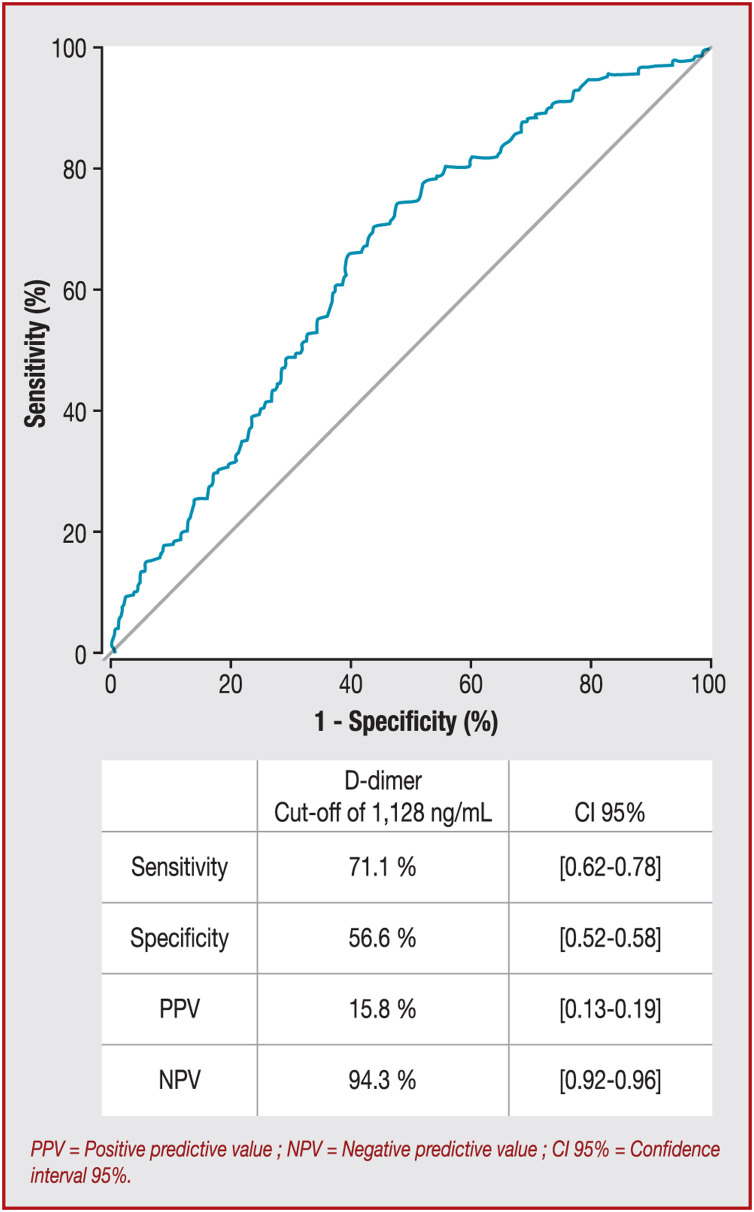
During the study period, a total of 2878 consecutive patients who were hospitalised in a medical ward for SARS-CoV-2 infection were included. At admission, 1154/2878 (40.1%) patients had D-dimer measurement [mean age 64.35 ± 16.63 years; 59.8% (690/1154) male, Table 1 ]. The optimum cut-off value for D-dimer at admission with the best prognostic ability of in-hospital mortality was 1128 ng/mL according to the ROC curve (Fig. 1 ), with a sensitivity of 71.1% (95% confidence interval [CI] 62–78), a specificity of 55.6% (95% CI 52–58), a positive predictive value of 15.8% (95% CI 13–19) and a negative predictive value of 94.3% (95% CI 92–96). The area under the curve for in-hospital mortality was 64.9% (95% CI 60–69). Listed in Table 1 are the initial clinical, biological and radiological characteristics and outcomes of the patients above and beyond the D-dimer cut-off of 1128 ng/mL. We also explored the prognostic performance of D-dimer thresholds proposed previously, and a D-dimer concentration at admission > 1128 ng/mL remained the best threshold (Table 2 ). At admission, 609/1154 (52.8%) patients had D-dimer concentrations ≤ 1128 ng/mL and 545/1154 (47.2%) had D-dimer concentrations > 1128 ng/mL. Compared with patients with D-dimer concentrations ≤ 1128 ng/mL, patients with D-dimer concentrations > 1128 ng/mL were older, and more frequently had high blood pressure and chronic kidney disease. These patients had higher concentrations of creatinine, C-reactive protein and fibrinogen, higher platelet and leukocyte counts and a higher rate of severe parenchymal involvement on chest computed tomography scan. Moreover, those patients had a lower haemoglobin concentration and prothrombin ratio. The in-hospital mortality rate (15.8% vs. 5.7%) and the mean duration of hospitalisation (10.25 ± 6.47 days vs. 8.75 ± 5.83 days) were significantly greater for patients with COVID-19 with a D-dimer concentration > 1128 ng/mL at admission (Table 1).
Table 1.
Clinical and biological characteristics and outcomes according to optimal threshold of D-dimers at admission (≤ or > 1128 ng/mL).
| Overall population | D-dimers ≤ 1128 ng/mL | D-dimers > 1128 ng/mL | P | |
|---|---|---|---|---|
| (n = 1154) | (n = 609) | (n = 545) | ||
| Age (years) | 64.35 ± 16.63 | 61.02 ± 15.97 | 68.06 ± 16.59 | < 0.001 |
| Age range | ||||
| 0–50 years | 232 (20.1) | 153 (25.1) | 79 (14.5) | < 0.001 |
| 50–60 years | 210 (18.2) | 137 (22.5) | 73 (13.4) | |
| 60–70 years | 263 (22.8) | 133 (21.8) | 130 (23.9) | |
| 70–80 years | 224 (19.4) | 106 (17.4) | 118 (21.7) | |
| 80–90 years | 157 (13.6) | 61 (10.0) | 96 (17.6) | |
| 90–110 years | 65 (5.6) | 17 (2.8) | 48 (8.8) | |
| Male sex | 690 (59.8) | 348 (57.1) | 342 (62.8) | 0.06 |
| BMI (kg/m2) | 28.24 ± 6.21 | 28.46 ± 5.76 | 28.00 ± 6.67 | 0.24 |
| BMI range | ||||
| 0–25 kg/m2 | 313 (27.1) | 149 (24.5) | 164 (30.1) | 0.20 |
| 25–30 kg/m2 | 349 (30.2) | 193 (31.7) | 156 (28.6) | |
| 30–66 kg/m2 | 320 (27.7) | 174 (28.6) | 146 (26.8) | |
| Time from illness onset to hospitalisation (days) | 7.12 ± 4.76 | 7.14 ± 4.61 | 7.10 ± 4.92 | 0.90 |
| Comorbidities | ||||
| High blood pressure | 557 (48.3) | 254 (41.7) | 303 (55.6) | < 0.001 |
| Diabetes | 259 (22.4) | 126 (20.7) | 133 (24.4) | 0.32 |
| Dyslipidaemia | 314 (27.2) | 152 (25.0) | 162 (29.7) | 0.08 |
| History of stroke | 91 (7.9) | 46 (7.6) | 45 (8.3) | 0.35 |
| Chronic kidney disease | 150 (13.0) | 59 (9.7) | 91 (16.7) | < 0.001 |
| Malignancy | ||||
| No cancer | 987 (85.5) | 544 (89.3) | 443 (81.3) | < 0.001 |
| Cancer in remission | 97 (8.4) | 40 (6.6) | 57 (10.5) | |
| Active cancer | 70 (6.1) | 25 (4.1) | 45 (8.3) | |
| Current smoker | 155 (13.4) | 82 (13.5) | 73 (13.4) | 0.79 |
| Atrial fibrillation | 129 (11.2) | 71 (11.7) | 58 (10.6) | 0.86 |
| Type of anticoagulation used at admission | ||||
| No use of anticoagulation | 1025 (88.8) | 539 (88.5) | 486 (89.2) | 0.98 |
| NOAC | 74 (6.4) | 40 (6.6) | 34 (6.2) | |
| VKA | 50 (4.3) | 27 (4.4) | 23 (4.2) | |
| Unfractionated heparin | 5 (0.4) | 3 (0.5) | 2 (0.4) | |
| Use of oral anticoagulation (NOAC or VKA) | ||||
| Yes | 124 (10.7) | 67 (11.0) | 57 (10.5) | 0.91 |
| No | 1025 (88.8) | 539 (88.5) | 486 (89.2) | |
| In-hospital exploration | ||||
| Haemoglobin (g/dL) | 13.21 ± 1.96 | 13.57 ± 1.75 | 12.80 ± 2.10 | < 0.001 |
| Platelets (×109/L) | 222.46 ± 100.28 | 208.34 ± 80.98 | 238.31 ± 116.31 | < 0.001 |
| Plasma creatinine (μmol/L) | 98.61 ± 99.85 | 87.87 ± 79.77 | 110.60 ± 117.22 | < 0.001 |
| Aspartate aminotransferase (IU/L) | 56.18 ± 83.32 | 51.53 ± 64.91 | 61.37 ± 99.74 | 0.050 |
| Leucocytes (×109/L) | 7.54 ± 5.98 | 6.61 ± 3.24 | 8.58 ± 7.89 | < 0.001 |
| Lymphocytes (×109/L) | 1.31 ± 3.76 | 1.21 ± 1.30 | 1.41 ± 5.31 | 0.37 |
| C-reactive protein (mg/L) | 91.52 ± 76.14 | 74.57 ± 68.24 | 110.40 ± 80.00 | < 0.001 |
| Fibrinogen (g/L) | 6.00 ± 1.66 | 5.76 ± 1.57 | 6.24 ± 1.71 | < 0.001 |
| Ferritin (μg/L) | 1063.80 ± 1508.13 | 1000.28 ± 1504.82 | 1121.25 ± 1512.83 | 0.45 |
| Prothrombin ratio (%) | 85.47 ± 18.16 | 87.40 ± 18.67 | 83.36 ± 17.37 | < 0.001 |
| aPTT ratio | 1.15 ± 0.31 | 1.15 ± 0.32 | 1.15 ± 0.30 | 0.86 |
| Abnormalities on chest CT scan | ||||
| Parenchymal involvement low or moderate (< 50%) | 762 (66.0) | 436 (71.6) | 326 (59.8) | < 0.001 |
| Parenchymal involvement severe (> 50%) | 201 (17.4) | 80 (13.1) | 121 (22.2) | |
| No chest CT scan | 191 (16.6) | 93 (15.3) | 98 (18.0) | |
| Outcomes | ||||
| Duration of length of stay (days) | 9.36 ± 6.14 | 8.75 ± 5.83 | 10.25 ± 6.47 | 0.001 |
| Time from admission to in-hospital death (days) | 15.22 ± 10.29 | 16.6 ± 7.82 | 13.7 ± 9.19 | 0.001 |
| In-hospital death | 121 (10.5) | 35 (5.7) | 86 (15.8) | < 0.001 |
Data are expressed as mean ± standard deviation or number (%). aPTT: activated partial thromboplastin time; BMI: body mass index; CT: computed tomography; NOAC: non-vitamin K antagonist oral anticoagulant; VKA: vitamin K antagonist.
Figure 1.
D-dimer concentration at admission receiver operating characteristic curve for in-hospital mortality. Area under the curve = 64.9% (95% CI 60–69.7%). A D-dimer concentration at admission of > 1128 ng/mL represents an optimal threshold using Youden's J statistic. CI: confidence interval; NPV: negative predictive value; PPV: positive predictive value.
Table 2.
Diagnostic performance of different D-dimer thresholds for in-hospital mortality.
| D-dimer threshold (ng/mL) |
|||||||
|---|---|---|---|---|---|---|---|
| > 500 | > 1000 | > 1128 | > 1500 | > 2000 | > 2500 | > 3000 | |
| Sensitivity | 95.1 (89.1–97.9) | 74.3 (65.5–81.6) | 71.1 (62.5–78.6) | 52.9 (43.6–61.9) | 35.5 (27.2–44.9) | 50 (42.2–57.7) | 23 (16.8–32.8) |
| Specificity | 17.6 (15.3–20.1) | 48.9 (45.9–52.1) | 55.6 (52.5–58.1) | 66.6 (63.6–69.5) | 76.9 (74.2–79.5) | 82.2 (79.8–84.5) | 85.9 (83.7–87.9) |
| PPV | 11.9 (9.9–14.1) | 14.6 (11.9–17.7) | 15.8 (12.9–19.7) | 15.6 (12.3–19.7) | 15.3 (11.4–20.2) | 31.7 (26.3–37.7) | 16.7 (11.7–23.2) |
| NPV | 96.8 (92.8–98.7) | 94.2 (91.8–95.9) | 94.3 (91.9–95.9) | 92.4 (90.1–94.1) | 91.1 (88.9–92.8) | 90.9 (88.8–92.6) | 90.6 (88.6–92.3) |
Data are expressed as % (95% confidence interval). NPV: negative predictive value; PPV: positive predictive value.
We also evaluated D-dimer concentration at admission in the subgroup of patients who developed VTE during hospitalisation (n = 127). In this subgroup, the optimum cut-off value for D-dimer at admission was 1202 ng/mL using the ROC curve, with a sensitivity of 61% (95% CI 17–92), a specificity of 25.3% (95% CI 12–58), a positive predictive value of 5.8% (95% CI 1–16) and a negative predictive value of 95.3% (95% CI 84–98). The area under the curve for in-hospital mortality was 63.7% (95% CI 37–90). This cut-off value of 1202 ng/mL did not differ significantly from that of the whole study population (P = 0.92).
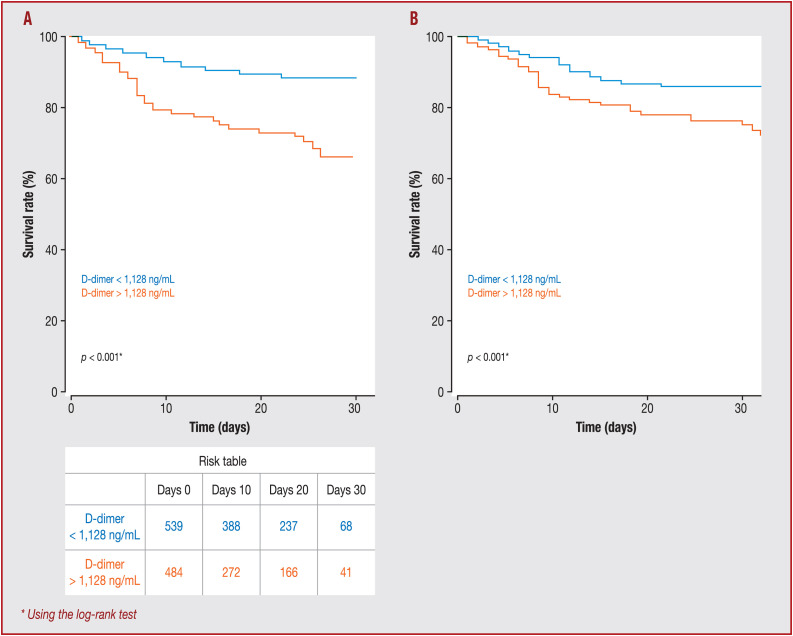
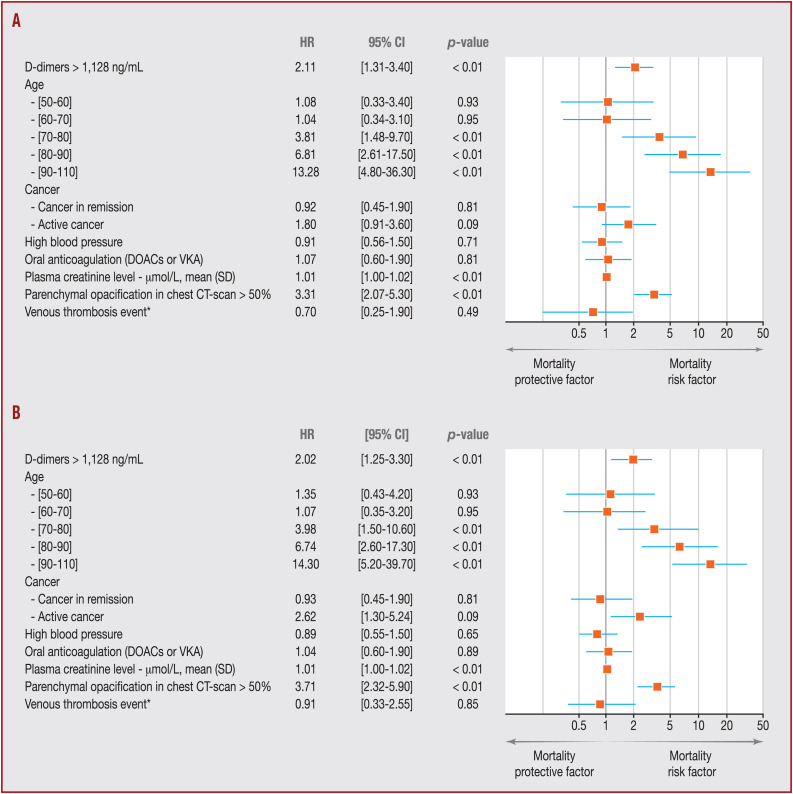
Kaplan–Meier survival curves for D-dimer concentration showed that a concentration > 1128 ng/mL at admission was a significant predictor of in-hospital mortality (P < 0.001; Fig. 2 A). Statistical significance of separation between the two groups was achieved at 9 days. As shown in Table 3 , D-dimer concentration > 1128 ng/mL was significantly associated with higher in-hospital mortality (odds ratio 2.08, 95% CI 1.24–3.54; P = 0.006) in the logistic regression. In the same way, Cox proportional hazards analysis showed that D-dimer concentration > 1128 ng/mL at admission was also a significant determinant for worse prognosis (hazard ratio 2.11, 95% CI 1.31–3.4; P < 0.01) after adjustment (Figure 2, Figure 3 ).
Figure 2.
A. Kaplan–Meier survival curves, illustrating the prognostic impact of the D-dimer threshold (1128 ng/mL) at admission. B. Adjusted Kaplan–Meier survival curves for Cox proportional hazards model that included age, history of malignancy, history of high blood pressure, the use of oral anticoagulation before COVID-19, the concentration of plasma creatinine, abnormalities on chest computed tomography scan (< or > 50% of parenchymental involvement) and the occurrence of a venous thrombosis event (deep vein thrombosis and/or pulmonary embolism). Adjusted survival curves show how a D-dimer threshold at admission of 1128 ng/mL influenced survival estimated from the Cox proportional hazards model. *Using the log-rank test.
Table 3.
Association between D-dimer cut-off of 1128 ng/mL and in-hospital mortality using logistic regression.
| Alive | In-hospital death | Univariate |
Multivariable |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |||
| D-dimer > 1128 ng/mL | 459 (44.4) | 86 (71.1) | 3.07 (2.05–4.69) | < 0.001 | 2.08 (1.24–3.54) | 0.006 |
| Age | ||||||
| 50–60 years | 467 (18.6) | 15 (4.2) | 1.90 (0.82–4.75) | 0.15 | 0.96 (0.28–3.19) | 0.94 |
| 60–70 years | 577 (23.0) | 45 (12.5) | 4.60 (2.27–10.63) | < 0.001 | 0.88 (0.28–2.84) | 0.82 |
| 70–80 years | 498 (19.8) | 77 (21.4) | 9.12 (4.63–20.70) | < 0.001 | 3.49 (1.40–9.99) | 0.011 |
| 80–90 years | 361 (14.4) | 135 (37.5) | 22.06 (11.38–49.57) | < 0.001 | 9.74 (3.81–28.59) | < 0.001 |
| 90–110 years | 138 (5.5) | 80 (22.2) | 34.20 (17.12–78.40) | < 0.001 | 14.94 (5.23–47.60) | < 0.001 |
| Cancer | ||||||
| Cancer in remission | 183 (7.3) | 43 (11.9) | 1.87 (1.30–2.64) | 0.001 | 0.80 (0.33–1.76) | 0.56 |
| Active cancer | 146 (5.8) | 43 (11.9) | 2.34 (1.61–3.34) | < 0.001 | 1.84 (0.77–4.11) | 0.15 |
| High blood pressure | 1191 (47.6) | 262 (73.0) | 2.97 (2.33–3.81) | < 0.001 | 0.97 (0.56–1.69) | 0.92 |
| Oral anticoagulation (NOAC or VKA) | 298 (12.0) | 84 (23.5) | 2.26 (1.72–2.96) | < 0.001 | 1.08 (0.53–2.10) | 0.82 |
| Plasma creatinine (μmol/L) | 92.3 ± 86.4 | 139.6 ± 137.5 | 1.00 (1.00–1.00) | < 0.001 | 1.00 (1.00–1.00) | 0.001 |
| Parenchymal opacification in chest CT scan > 50% | 356 (17.8) | 74 (30.0) | 1.98 (1.46–2.64) | < 0.001 | 2.00 (1.16–3.42) | 0.012 |
| Venous thrombosis eventa | 116 (4.6) | 11 (3.0) | 0.65 (0.33–1.17) | 0.18 | 0.72 (0.20–1.98) | 0.56 |
Data are expressed as number (%) or mean ± standard deviation, unless otherwise indicated. CI: confidence interval; CT: computed tomography; NOAC: non-vitamin K antagonist oral anticoagulant; OR: odds ratio; VKA: vitamin K antagonist.
Venous thrombosis event included deep vein thrombosis and pulmonary embolism.
Figure 3.
A. Forest plot of Cox proportional hazards model for in-hospital mortality. B. Forest plot of Cox proportional hazards model for in-hospital mortality in the population without censored outcome (n = 886). CI: confidence interval; CT: computed tomography; HR: hazard ratio; NOAC: non-vitamin K antagonist oral anticoagulant; VKA: vitamin K antagonist. *Venous thrombosis event included deep vein thrombosis and pulmonary embolism.
In the sensitivity analysis, the D-dimer concentration at admission ROC curve for in-hospital mortality in the subgroup of patients with VTE during hospitalisation (n = 127) was similar. Based on the matched and balanced dataset (Table A.1), we performed two sensitivity analyses. Firstly, we performed a univariate comparison according to VTE occurrence during hospitalisation, and observed that in-hospital mortality was not different between patients with VTE and those without VTE (respectively 8.8% [33/381] vs. 7.1% [9/127]; P = 0.72). Secondly, we repeated the same Cox proportional hazards model adjusted, and observed a significant association between concentration of D-dimer > 1128 ng/mL at admission and in-hospital mortality, with a hazard ratio of 3.11 (95% CI 1.26–7.80; P = 0.014). According to the prediction (hazard ratio) for in-hospital mortality, after adjustment, the best predictor remained > 1128 ng/mL, with the higher prognostic ability (Table A.2). Moreover, when the analysis was restricted to patients without censored outcome (n = 1886), the level of association between D-dimer concentration > 1128 ng/mL and in-hospital mortality remained similar, with an odds ratio of 1.88 (95% CI 1.08–3.31; P = 0.02) and a hazard ratio of 2.20 (95% CI 1.25–3.3; P < 0.01) (Table 4 and Fig. 3B).
Table 4.
Association between D-dimer cut-off of 1128 ng/mL and in-hospital mortality using logistic regression in the selected population of patients without censored outcome.
| Alive | In-hospital death | Univariate |
Multivariable |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |||
| D-dimer > 1128 ng/mL | 313 (40.9) | 86 (71.1) | 3.55 (2.35–5.45) | < 0.001 | 1.88 (1.08–3.30) | 0.026 |
| Age | ||||||
| 50–60 years | 157 (20.5) | 7 (5.8) | 1.23 (0.41–3.66) | 0.71 | 1.07 (0.32–3.61) | 0.91 |
| 60–70 years | 168 (22.0) | 19 (15.8) | 3.12 (1.33–8.15) | 0.012 | 1.03 (0.32–3.41) | 0.95 |
| 70–80 years | 133 (17.4) | 25 (20.8) | 5.18 (2.29–13.30) | < 0.001 | 4.47 (1.73–13.17) | 0.003 |
| 80–90 years | 86 (11.3) | 40 (33.3) | 12.82 (5.86–32.33) | < 0.001 | 9.70 (3.66–29.38) | < 0.001 |
| 90–110 years | 27 (3.5) | 22 (18.3) | 22.47 (9.17–61.57) | < 0.001 | 18.04 (5.78–62.28) | < 0.001 |
| Cancer | ||||||
| Cancer in remission | 67 (8.8) | 12 (9.9) | 1.25 (0.62–2.31) | 0.51 | 0.80 (0.32–1.84) | 0.62 |
| Active cancer | 36 (4.7) | 14 (11.6) | 2.71 (1.37–5.11) | 0.003 | 2.80 (1.06–6.99) | 0.031 |
| High blood pressure | 333 (43.8) | 80 (66.7) | 2.56 (1.72–3.88) | < 0.001 | 0.92 (0.51–1.64) | 0.77 |
| Oral anticoagulation (NOAC or VKA) | 64 (8.4) | 23 (19.0) | 2.56 (1.50–4.26) | < 0.001 | 1.46 (0.68–3.01) | 0.32 |
| Plasma creatinine (μmol/L) | 86.6 (63.0) | 139.2 (135.4) | 1.01 (1.00–1.01) | < 0.001 | 1.01 (1.00–1.01) | < 0.001 |
| Parenchymal opacification in chest CT scan > 50% | 98 (15.0) | 30 (32.6) | 2.74 (1.67–4.42) | < 0.001 | 3.01 (1.64–5.49) | < 0.001 |
| Venous thromboemboliceventa | 43 (5.6) | 5 (4.1) | 0.72 (0.25–1.70) | 0.50 | 1.05 (0.29–3.07) | 0.93 |
Data are expressed as number (%) or mean ± standard deviation, unless otherwise indicated. CI: confidence interval; CT: computed tomography; NOAC: non-vitamin K antagonist oral anticoagulant; OR: odds ratio; VKA: vitamin K antagonist.
Venous thromboembolic event included deep vein thrombosis and pulmonary embolism.
Discussion
The main finding of this retrospective study is that D-dimer concentration at admission > 1128 ng/mL is an independent predictor of in-hospital mortality for patients with COVID-19. This multicentre French study of patients hospitalised for COVID-19 is the largest non-monocentric study to date of patients hospitalised in a medical ward to provide evidence that initial D-dimer concentration could be a valuable tool to predict further in-hospital mortality. Moreover, to the best of our knowledge, we show for the first time that VTE occurrence during hospitalisation does not interfere with the predictive value of D-dimers for in-hospital mortality.
High D-dimer concentration has been widely reported to be one of the most common laboratory findings reported in patients with COVID-19 at hospital admission. We previously demonstrated that D-dimer measurement at admission is a discriminant factor during COVID-19 suspicion. Indeed, adding a D-dimer cut-off beyond 500 ng/mL to female sex and absence of pneumonia on computed tomography scan could exclude a COVID-19 diagnosis with high sensitivity and specificity [4]. Moreover, we and others showed that D-dimer concentration at admission was higher in patients who needed ICU referral compared with those who did not [5], [17]. Moreover, several reports have described that increased D-dimer concentrations were related to in-hospital mortality [10], [18], [19]. Only one study provided a well-evaluated cut-off for D-dimer [11] (2000 ng/mL) for a relationship with in-hospital mortality in 343 patients. However, this study did not specify whether patients were hospitalised in a medical ward or if they were directly hospitalised in an ICU, making proper and accurate use of this cut-off difficult for clinicians. Our study only included patients with COVID-19 admitted to a medical ward; some were subsequently referred to an ICU, but none was directly hospitalised in an ICU. Our results propose COVID-19-increased D-dimer concentration as a clear consequence of respiratory disease through the development of capillary microthrombosis, as observed in post-mortem studies [20], [21], and attributed to vascular thickening or vascular congestion [22]. Recently, we evidenced D-dimer involvement in the pathophysiology of COVID-19, and correlation with right ventricular dysfunction, which allows us to confirm pulmonary vascular obstruction as a site of coagulopathy and a source of circulating D-dimer [23]. Thus, in COVID-19, the hypothesis of microthrombosis is proposed in lung, but also in kidney, as the elevation of serum creatinine was associated with higher concentrations of D-dimer (> 500 ng/mL) [1], [2]. The SARS-CoV-2 receptor (angiotensin-converting enzyme 2) is strongly expressed in endothelial cells [24]. Infection of endothelial cells could therefore induce endothelial lesions, triggering massive activation of coagulation and diffuse microthrombotic process, impairing renal function and respiratory gas exchanges. We previously described increased numbers of circulating endothelial cells in patients with COVID-19 [4] and an association between circulating biomarkers of endothelial activation in COVID-19 and ICU admission [5]. Angiopoietin-2 was also inversely correlated with respiratory system compliance in this study, paving the way for a relationship between endothelial dysfunction and pulmonary disease severity. Integrity of endothelial cells provides an antithrombotic environment that is reversed during COVID-19 upon the burst of inflammation related to interleukin-6. Therefore, SARS-CoV-2 infection induces a disruption of the endothelial thromboprotective barrier that leads to this coagulopathy and increased D-dimer. In the present cohort, patients were at the same stage of disease according to the time to onset of symptoms of disease; so endothelial-induced coagulopathy reflected by D-dimer could be a consequence of viral loading phase and severity of viral infection. The importance of the viral loading hypothesis needs to be confirmed, with association between D-dimer and viraemia quantified with sensitive tests.
A major confounding factor for D-dimer increase could be macrothrombosis, as a high incidence of VTE (pulmonary embolism or deep vein thrombosis) [7], [8], [25] has been described in COVID-19. In clinical practice, D-dimer measurements have been used only to exclude VTE. Indeed, no such D-dimer-based strategy has been described during COVID-19-associated coagulopathy in patients with a high concentration of D-dimer. Even if increased D-dimer concentrations at admission have been associated with VTE during follow-up in patients with COVID-19 [26], no threshold is currently available to diagnose VTE. Furthermore, the international society on thrombosis and haemostasis (ISTH) does not recommend routine screening for VTE based on elevated D-dimer concentrations in patients with COVID-19 [27]. However, we demonstrate here that a D-dimer cut-off of 1128 ng/mL at admission is independently correlated with in-hospital mortality, regardless of VTE occurrence during hospitalisation. Moreover, we identified several other predictors of in-hospital mortality, such as renal function impairment, age and lung damage extent > 50%. Even after adjustment for those risk factors, D-dimer cut-off at admission remains independently correlated with in-hospital mortality. D-dimer might be used to monitor COVID-19 worsening [28]. Indeed, previous studies have observed that a progressive increase in D-dimer was observed in non-survivors of COVID-19 [11].
Study limitations
Our study has several limitations. Firstly, in this multicentre study, we could not identify the manufacturer or type of D-dimer assay used for all tested D-dimer, as suggested by ISTH [7]. It is well recognised by experts in the field that all D-dimer assays are not the same – they use different detection antibodies, different detection methods and often different calibrators [29]. Indeed, different D-dimer assays vary in their specificity against degradation products, resulting substantial variability between D-dimer assay kits. This technical point is a limitation to multicentre studies. This limitation reduces the generalisability of the use of optimal D-dimer thresholds. Secondly, we did not have the delay from COVID-19 admission to VTE onset during hospitalisation. Thirdly, serial D-dimer monitoring has been suggested by ISTH [5], [30] as being helpful in determining prognosis in patients with COVID-19. Indeed, a peak of D-dimers has been found to be associated with VTE in COVID-19 [31], [32], but in the present study, we only assessed D-dimer at admission. However, as VTE occurrence did not modify in-hospital mortality in the present study, this lack of continuous monitoring of D-dimer is unlikely to modify the results.
Conclusions
This multicentre retrospective study suggests that D-dimer concentration at admission could be a valuable biomarker to predict mortality related to COVID-19, independent of VTE occurrence during hospitalisation. The determined cut-off at 1128 ng/mL could be a valuable tool to guide anticoagulation intensity in patients with COVID-19. Further prospective studies are necessary to confirm whether this D-dimer threshold reflects COVID-19 worsening.
Sources of funding
David M. Smadja’COVID team has been funded with grants from the French national agency for research ANR SARCODO (Fondation de France) and Mécénat Covid AP-HP.
Disclosure of interest
R.C. receives consultant fees from the company Aspen, without any relation to the current manuscript. NG Consultant and lecture fees or travel awards from the companies Aspen,Bayer, Alliance BMS-Pfizer, LEO-Pharma and Boehringer Ingelheim, without any relation to the current manuscript. A.C. receives research grant from RESICARD (research nurses), consultant and lecture fees from the companies Amgen, AstraZeneca, Bayer Pharma, Alliance BMS-Pfizer, Novartis and Sanofi-Aventis, without any relation to the current manuscript. D.M.S. receives consultant and lecture fees or travel awards from the companies Aspen, Bayer, Carmat, Alliance BMS-Pfizer, LEO-Pharma and Boehringer Ingelheim, without any relation to the current manuscript. The other authors declare that they have no competing interest.
Acknowledgements
A complete list of the Critical COVID-19 France Investigators is provided in the Appendix.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.acvd.2021.02.003.
Online Supplement. Supplementary data
References
- 1.Debuc B., Smadja D.M. Is COVID-19 a new hematologic disease? Stem Cell Rev Rep. 2020 doi: 10.1007/s12015-020-09987-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khider L., Gendron N., Goudot G., et al. Curative anticoagulation prevents endothelial lesion in COVID-19 patients. J Thromb Haemost. 2020;18:2391–2399. doi: 10.1111/jth.14968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smadja D.M., Guerin C.L., Chocron R., et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 2020;23:611–620. doi: 10.1007/s10456-020-09730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fauvel C., Weizman O., Trimaille A., et al. Pulmonary embolism in COVID-19 patients: a French multicentre cohort study. Eur Heart J. 2020;41:3058–3068. doi: 10.1093/eurheartj/ehaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poissy J., Goutay J., Caplan M., et al. Pulmonary embolism in patients with COVID-19: awareness of an increased prevalence. Circulation. 2020;142:184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 9.Revel M.P., Parkar A.P., Prosch H., et al. COVID-19 patients and the radiology department – advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI) Eur Radiol. 2020;30:4903–4909. doi: 10.1007/s00330-020-06865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L., Yan X., Fan Q., et al. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bewick V., Cheek L., Ball J. Statistics review 10: further non-parametric methods. Crit Care. 2004;8:196–199. doi: 10.1186/cc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reichenheim M.E., Coutinho E.S. Measures and models for causal inference in cross-sectional studies: arguments for the appropriateness of the prevalence odds ratio and related logistic regression. BMC Med Res Methodol. 2010;10:66. doi: 10.1186/1471-2288-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sedgwick P. Bias in observational study designs: cross sectional studies. BMJ. 2015;350:h1286. doi: 10.1136/bmj.h1286. [DOI] [PubMed] [Google Scholar]
- 16.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fei Y., Tang N., Liu H., Cao W. Coagulation dysfunction. Arch Pathol Lab Med. 2020;144:1223–1229. doi: 10.5858/arpa.2020-0324-SA. [DOI] [PubMed] [Google Scholar]
- 19.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of Coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magro C., Mulvey J.J., Berlin D., et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toraih E.A., Elshazli R.M., Hussein M.H., et al. Association of cardiac biomarkers and comorbidities with increased mortality, severity, and cardiac injury in COVID-19 patients: a meta-regression and decision tree analysis. J Med Virol. 2020;92:2473–2488. doi: 10.1002/jmv.26166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goudot G., Chocron R., Augy J.L., et al. Predictive factor for COVID-19 worsening: insights for high-sensitivity troponin and D-dimer and correlation with right ventricular afterload. Front Med (Lausanne) 2020;7:586307. doi: 10.3389/fmed.2020.586307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrario C.M., Jessup J., Chappell M.C., et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 25.Helms J., Severac F., Merdji H., Angles-Cano E., Meziani F. Prothrombotic phenotype in COVID-19 severe patients. Intensive Care Med. 2020;46:1502–1503. doi: 10.1007/s00134-020-06082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalised patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spyropoulos A.C., Levy J.H., Ageno W., et al. Scientific and standardisation committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalised patients with COVID-19. J Thromb Haemost. 2020;18:1859–1865. doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mouhat B., Besutti M., Bouiller K., et al. Elevated D-dimers and lack of anticoagulation predict PE in severe COVID-19 patients. Eur Respir J. 2020;56:2001811. doi: 10.1183/13993003.01811-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longstaff C., Adcock D., Olson J.D., et al. Harmonisation of D-dimer – A call for action. Thromb Res. 2016;137:219–220. doi: 10.1016/j.thromres.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 30.Thachil J., Tang N., Gando S., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faggiano P., Bonelli A., Paris S., et al. Acute pulmonary embolism in COVID-19 disease: preliminary report on seven patients. Int J Cardiol. 2020;313:129–131. doi: 10.1016/j.ijcard.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maatman T.K., Jalali F., Feizpour C., et al. Routine venous thromboembolism prophylaxis may be inadequate in the hypercoagulable state of severe Coronavirus disease 2019. Crit Care Med. 2020;48:e783–e790. doi: 10.1097/CCM.0000000000004466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.