Abstract
Stress is a risk factor that plays a considerable role in the development and maintenance of alcohol (ethanol) abuse and relapse. Preclinical studies examining ethanol-stress interactions have demonstrated elevated ethanol drinking, cognitive deficits, and negative affective behaviors in mice. However, the neural adaptations in prefrontal cortical regions that drive these aberrant behaviors produced by ethanol-stress interactions are unknown. In this study, male C57BL/6J mice were exposed to chronic intermittent ethanol (CIE) and repeated forced swim stress (FSS). After two cycles of CIE × FSS, brain slices containing the prelimbic (PrL) and infralimbic (IfL) cortex were prepared for analysis of adaptations in dendritic spines and synaptic plasticity. In the PrL cortex, total spine density was increased in mice exposed to CIE. Immediately following induction of long-term potentiation (LTP), the fEPSP slope was increased in the PrL of CIE × FSS treated mice, indicative of a presynaptic adaptation on post-tetanic potentiation (PTP). In the IfL cortex, CIE exposure regardless of FSS experience resulted in an increase in spine density. FSS alone or when combined with CIE exposure increased PTP following LTP induction. Repeated FSS episodes increased IfL cortical paired-pulse facilitation, a second measure of presynaptic plasticity. In summary, CIE exposure resulted in structural adaptations while repeated stress exposure drove metaplastic changes in presynaptic function, demonstrating distinct morphological and functional changes in PrL and IfL cortical neurons. Thus, the structural and functional adaptations may be one mechanism underlying the development of excessive drinking and cognitive deficits associated with ethanol-stress interactions.
Keywords: chronic ethanol, stress, prelimbic, infralimbic, cortex, plasticity, spines
1. Introduction
There is a complex and bidirectional interaction between stressful life experiences, alcohol (ethanol) drinking, and the risk for developing an alcohol use disorder (AUD). General life stressors, such as losing a job, have mixed influences on alcohol drinking (Thomas et al., 2003; Park et al., 2004; Keyes et al., 2012). Experiencing more severe forms of stress and trauma (e.g., war combat, childhood maltreatment) have been reported to increase problem drinking and the risk of developing AUD (Keyes et al., 2012; Ramchandani et al., 2018). Moreover, alcohol consumption may be used as a coping strategy for individuals faced with chronic stressors, and experiencing a stressor is considered an important trigger for alcohol relapse (Keyes et al., 2012; Ramchandani et al., 2018). Further complicating this interaction, alcohol may acutely activate the hypothalamic-pituitary-adrenal (HPA) axis (Keyes et al., 2012; Becker, 2017), and heavy alcohol drinking causes adaptations in HPA axis function and stress-related brain systems and neurocircuitry (Stephens and Wand, 2012; Lu and Richardson, 2014; Blaine and Sinha, 2017; Weera and Gilpin, 2019). Given the complex nature of the relationship between alcohol and stress, it is imperative to identify the neurobiological adaptations produced by co-occurring external stressors that are associated with alcohol drinking and relapse. A better understanding the neurobiological adaptations that underlie alcohol and stress interactions might lead to effective strategies for treating individuals with AUD.
The medial prefrontal cortex (mPFC) governs executive function and is critical for the regulation of behavioral flexibility and appetitive behaviors (Koechlin, 2016; Kamigaki, 2019). In the rodent, there are distinct subregions along the dorsal-ventral axis of the mPFC that differ in function and anatomical projections (Vertes, 2004, 2006). Two of these subregions, the prelimbic (PrL) and infralimbic (IfL) cortex, are susceptible to maladaptations caused by both chronic ethanol exposure and repeated stress experiences. Indeed, acute stress or recovery from stress has been reported to enhance synaptic plasticity in the mPFC and improve working memory (Goldwater et al., 2009; Yuen et al., 2009; Yuen et al., 2011), whereas chronic stressors generally reduce functional plasticity and dendritic arborization within the mPFC (Holmes and Wellman, 2009; Ito et al., 2010; Wellman and Moench, 2019; Wellman et al., 2020). Stress-induced changes in mPFC structure and function have been linked to impaired cognitive function and regulation of mPFC-dependent behaviors (Cerqueira et al., 2007; Arnsten, 2015; Wellman et al., 2020). In comparison, a history of chronic ethanol exposure enhances synaptic plasticity and has been linked to altered structure and function of mPFC neurons (Holmes et al., 2012; Kroener et al., 2012; Lu and Richardson, 2014; Pleil et al., 2015; Mulholland et al., 2016; McGuier et al., 2018; Varodayan et al., 2018; Cannady et al., 2020; Kim and Martin-Fardon, 2020). These aberrant neuroadaptations in the PrL and IfL cortex are thought to negatively influence cognitive function and mPFC-dependent behaviors (George et al., 2012; Holmes et al., 2012; Kroener et al., 2012; Trantham-Davidson et al., 2014) and these mPFC subregions are important for driving excessive and compulsive alcohol drinking (Meinhardt et al., 2013; Lu and Richardson, 2014; Pfarr et al., 2015; Siciliano et al., 2019; Kim and Martin-Fardon, 2020). While adaptations in stress-related signaling in the mPFC has been linked to compulsive and binge-like drinking and ethanol-induced cognitive impairments (Jacquot et al., 2008; George et al., 2012; Vendruscolo et al., 2012; Lu and Richardson, 2014; Robinson et al., 2019), the molecular, functional, and structural adaptations that occur in response to chronic ethanol and stress co-exposure are largely unexplored and warrant further investigation.
While animal models are useful for determining the mechanisms and key biological substrates driving ethanol and stress interactions, preclinical experimental outcomes examining the relationship between ethanol and stress are inconsistent and often depend upon species, sex, age, and the type and timing of the stressor, among other critical variables (Becker et al., 2011; Noori et al., 2014; Spanagel et al., 2014; Becker, 2017; Weera and Gilpin, 2019). While there are a number of studies that report decreased ethanol intake in rodents following an acute stressor (Becker et al., 2011; Lopez et al., 2016), additional studies report increased drinking and relapse in stressed rodents (Becker et al., 2011; Spanagel et al., 2014; Becker, 2017; Weera and Gilpin, 2019). In rats and mice, social defeat stress, footshock, exposure to predator odor, and forced swim stress (FSS) have been reported to increase operant ethanol self-administration, ethanol-seeking behaviors, and two-bottle choice home cage drinking (Molander et al., 2012; Edwards et al., 2013; Meyer et al., 2013; Norman et al., 2015; Lopez et al., 2016; Manjoch et al., 2016; King and Becker, 2019). In the CIE+FSS model of escalated drinking developed by the INIAstress Consortium (www.iniastress.org), mice establish baseline ethanol drinking in their home age prior to chronic intermittent ethanol (CIE) exposure in vapor inhalation chambers (Anderson et al., 2016b, a; Lopez et al., 2016). During the post-CIE test drinking weeks, mice experience 10-min episodes of FSS four hours prior to access to ethanol or water. When combined with CIE exposure, FSS mice drink significantly more ethanol than mice in the other groups after 1–2 cycles of CIE and FSS (Anderson et al., 2016b, a; Lopez et al., 2016; Rodberg et al., 2017; Padula et al., 2019; den Hartog et al., 2020). Not only does this model generate excessive drinking in mice that experience CIE+FSS, it also produces cognitive deficits on mPFC-dependent tasks (Rodberg et al., 2017) and negative affective behaviors (Padula et al., 2019; den Hartog et al., 2020), demonstrating that the CIE+FSS model recapitulates a number of key phenotypes in individuals with AUD.
To address the gap in our understanding of the maladaptive plasticity changes that occur in response to chronic ethanol and repeated stress experiences, the current study examined functional and structural adaptations in the mPFC following CIE exposure and repeated episodes of FSS in male C57BL/6J mice (Anderson et al., 2016a, b; Lopez et al., 2016; Rodberg et al., 2017; Padula et al., 2019). Because the PrL and IfL subdivisions are thought to have distinct functional roles in regulating alcohol-seeking behavior and alcohol-associated memories (Gass and Chandler, 2013; Pfarr et al., 2015; Cannady et al., 2017), we explored whether CIE exposure and repeated FSS episodes would differentially alter synaptic and morphological plasticity within PrL and IfL cortical subregions. Specifically, we assessed changes in induction of long-term potential (LTP) and dendritic spine density in mice treated with CIE+FSS.
2. Materials and Methods
2.1. Animals
Adult, male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME; N = 140) were 8–10 weeks of age at the start of each experiment and were individually housed in an AAALAC-accredited temperature- and humidity-controlled vivarium for one week prior to the start of experimentation. Mice were kept on a 12 h reverse light/dark cycle (lights off at 9 am) with ad libitum access to food (Harlan Teklad Diet 2918) and water throughout the study. These studies were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina and conducted according to the requirements of the NIH Guide for the Care and Use of Laboratory Animals (2011).
2.2. Chronic Intermittent Ethanol and Force Swim Stress Exposure
In the CIE-FSS model, mice were pseudo-randomly divided into Air (control) or ethanol vapor groups before exposure to a cycle of CIE vapor or Air in inhalation chambers. Air or CIE exposure was delivered 16 h/day for four consecutive days starting on Monday afternoon (Becker and Lopez, 2004; Lopez and Becker, 2005; Padula et al., 2015). Before entry into the ethanol chambers for each exposure period, intoxication was initiated by administration of ethanol (1.6 g/kg; 8% w/v in sterile saline; 20 ml/kg injection volume; IP) and pyrazole (1 mmol/kg), an alcohol dehydrogenase inhibitor. Air-exposed mice were handled similarly, but received pyrazole (1 mmol/kg; IP) without ethanol in sterile saline. Mice were exposed to ethanol vapor at concentrations that yield blood ethanol concentrations (BECs) in the range of 175–225 mg/dL. BECs in samples taken from the retroorbital sinus were determined using an Analox Analyzer (Analox Instruments, Stourbridge, UK) as described previously (Lopez and Becker, 2005).
After a 72 h period, half of the Air and CIE exposed mice were subjected to a 10-min forced swim stress (FSS) test once a day for 5 days (Monday through Friday). The FSS test was performed in a glass cylinder (20 cm diameter × 40 cm high) that was half-filled with 23–25°C tap water following previously reported methods (Anderson et al., 2016b, a; Lopez et al., 2016; Padula et al., 2019). Mice were acclimated to the behavioral testing room for one hour prior to the FSS test, and after removal from the swim test cylinders, mice were hand-dried and placed on a heating pad for 5–10 min before they were returned to the vivarium. This exposure paradigm of CIE and FSS was repeated for one more cycle. During the final week of testing, mice were exposed to two to four FSS sessions, and brains were prepared 24 h after their final FSS session. The CIE and FSS testing model is presented in Fig. 1A, and there were four treatment groups: Air controls, FSS, CIE, and CIE+FSS. To determine a potential influence of repeated pyrazole treatment on functional plasticity in the IfL cortex, separate groups of mice were treated with 7–9 sessions of FSS without pyrazole injections and were not exposed to CIE in vapor inhalation chambers.
Fig. 1.

Schematic of the experimental timeline and medial prefrontal cortex subregions that were the focus of analysis using the INIAstress Consortium model of CIE-FSS co-exposure. (A) Timeline showing the 2 (air vs CIE exposure) × 2 (no forced swim stress (FSS) vs FSS) experimental design. Brains were prepared for analysis of morphological or functional plasticity 24 h after the last FSS episode during the second cycle. (B) Schematic of the medial aspect of the prefrontal cortex. The prelimbic (PrL) and infralimbic (IfL) cortex were selected for analysis in these studies. fEPSP, field excitatory postsynaptic potentials; PPF, paired-pulse facilitation.
2.3. Dendritic spine labeling, morphological classification, and analysis
Neuronal labeling and morphological classification of pyramidal neuron dendritic spines in the PrL and IfL cortex (Fig. 1B) were carried out using previously reported methods (McGuier et al., 2015; Uys et al., 2016). At 24 h after the last stress exposure (see Fig. 1A), mice (N = 3–8 mice/group; 44 total mice; 4493 total spines across 122 dendritic sections) were anesthetized with urethane (1.5 g/kg, IP) and perfused with 0.1 M phosphate buffer (PB) followed by 1.5% paraformaldehyde (PFA) in PB. Brains were blocked and post-fixed for 30 min in 1.5% PFA. Next, 150 μm thick coronal slices were prepared using a vibratome. DiI-coated (1,1’-dioctadecyl-3,3,3’3’-tetramethylindo-carbocyanine perchlorate) tungsten particles (1.3 μm diameter; BD Biosciences; San Jose, CA) were delivered to the slices using a modified Helio Gene Gun (Bio-Rad; Hercules, CA) fitted with a polycarbonate filter (3.0 μm pore size; BD Biosciences; San Jose, CA). Slices were left overnight at 4°C in PB to allow the DiI to completely diffuse through labeled neurons and then were post-fixed in 4% PFA for 1 h at room temperature. After mounting with Prolong Gold Antifade mounting media (Life Technologies; Carlsbad, CA), images of second order distal dendrites (~50–60 μm in length) that started >75 μm from the soma were collected from pyramidal neurons in the IfL and PrL cortex. Slices containing the IfL cortex were imaged (voxel size: 47 × 47 × 100 nm; 4 frame average; 2.5X zoom) using a Zeiss LSM 510 confocal microscope fitted with a 63x oil immersion objective (Plan-Apochromat, Carl Zeiss, NA = 1.4, working distance = 190 μm). Because of an equipment upgrade, dendritic sections in the PrL cortex were acquired using a Zeiss LSM 880 confocal microscope (voxel size: 45 × 45 × 130 nm; 2 frame average; 2.5X zoom) equipped with an Airyscan detector and a 63x oil immersion objective (Plan-Apochromat, Carl Zeiss, NA = 1.4, working distance = 190 μm). Images of dendritic sections from the IfL cortex were deconvolved using AutoQuant (Media Cybernetics; Rockville, MD), and raw Airyscan images from the PrL cortex were post-processed (Wiener filter-based deconvolution with pixel reassignment) in ZEN Black Software (version 14; Carl Zeiss) using the automatic filter strength setting. Imaris XT (Bitplane; Zurich, Switzerland) was used to generate a filament of the dendritic shaft and spines. Regardless of image acquisition, dendritic spines in images from the PrL and IfL cortex were identified using Imaris software and the same classification algorithm. Spines were classified into 4 categories (stubby, long, filopodia, and mushroom) based on the spine length and the width of the spine head and neck, where L is spine length, DH is spine head diameter, and DN is spine neck diameter. Long spines were identified as having a L ≥ 0.75 μm and < 3 μm, mushroom spines had a L < 3.5 μm, DH > 0.35 μm and a DH > DN, stubby spines had a L < 0.75 μm, and filopodia were identified as having a L ≥ 3 μm. Data on dendritic spine parameters were averaged for each dendritic section and were collated from the Imaris output via custom scripts written in Python.
2.4. Extracellular Field Potential Recordings
Extracellular recordings of field excitatory post-synaptic potentials (fEPSPs) were completed as previously described (Cannady et al., 2017). Mice (N = 42 for PrL and 54 for IfL recordings) were deeply anesthetized with isoflurane gas and decapitated 24 h after the last FSS session. Brains were quickly removed and submerged into an ice-cold dissection solution consisting of (in mM) 200 sucrose, 1.9 KCl, 1.2 NaH2PO4, 6.0 MgCl2, 0.5 CaCl2, 0.4 ascorbate, 10 glucose, and 25 NaHCO3. The brains were then sectioned in ice-cold dissection solution into coronal slices (350 μm) containing the PrL and IfL cortex using a Leica VT1200S vibratome (Leica Biosystems). Slices were transferred to an incubation chamber filled with artificial cerebrospinal fluid solution (ACSF), which contained (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1.3 MgCl2, 2.5 CaCl2, 0.4 ascorbate, 10 glucose, and 25 NaHCO3 at 37°C for 30 minutes and then at room temperature for at least another 30 minutes before recording. The pH of all solutions listed was adjusted to 7.4, and the osmolarity was measured to be ~300 mOsm. All external buffers were continuously aerated with 5% carbon dioxide/95% oxygen.
ACSF-filled theta-glass stimulating pipettes were inserted into layer II/III, while ACSF-filled recording pipettes were inserted into layer V of the PrL and IfL cortex (See Fig 3A and 5A). fEPSPs were recorded in ACSF at 30°C. Recordings were acquired with a Multiclamp 700B amplifier (Molecular Devices) and analyzed using AxoGraphX software (AxoGraph Scientific, New South Wales, Australia). All recordings were band-pass filtered (0.2–1.2 kHz) and digitized at 10 kHz using an ITC-18 data acquisition interface (InstruTECH Corp., Great Neck, N.Y., USA). Input/output curves were generated to determine the maximum stimulation intensity for each recording, and stimulation was adjusted to ~50% maximum intensity for the duration of each recording. During a 10-minute baseline period, slices were stimulated at a rate of 0.02 Hz. High-frequency stimulation (HFS) at 50 Hz (2-second duration repeated 5 times with 30-second intervals) was used to induce LTP and was followed by a 60 minute post-HFS stimulation phase at a rate of 0.02 Hz, as described previously (Moussawi et al., 2009; Cannady et al., 2017). Synaptic strength was measured as the change in the initial fEPSP slope after HFS, relative to the normalized 10-minute baseline slope. To determine changes in paired-pulse facilitation (PPF), fEPSPs were evoked by two consecutive stimulating pulses separated by 50 ms in layer II/III of the mPFC and were recorded in layer V. To avoid population spike artifacts on amplitude measurements (Hanson et al., 2010), the PPF ratio was calculated by dividing the second fEPSP slope by the first fEPSP slope of the paired pulses.
Fig. 3.
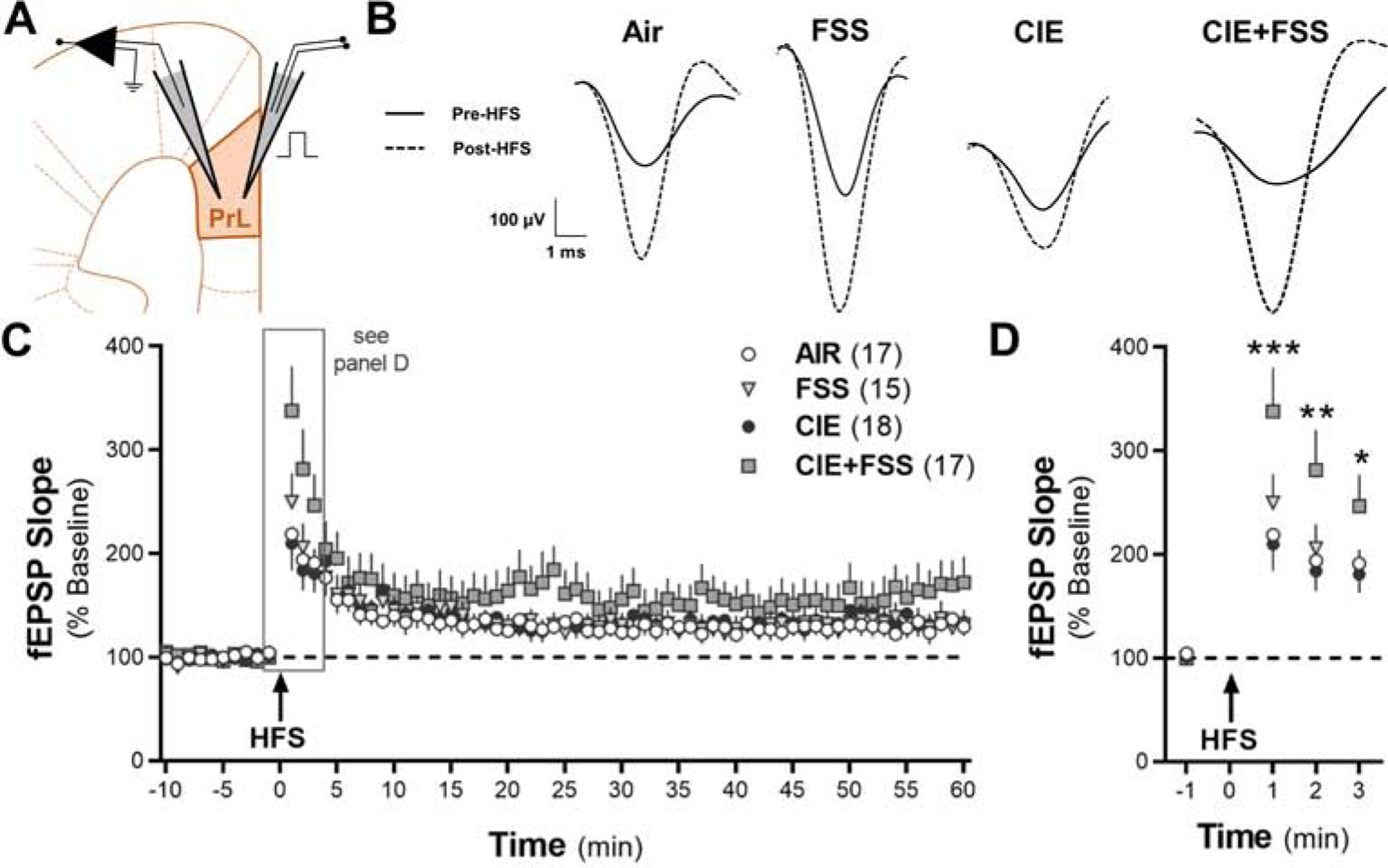
Long-term potentiation of synaptic activity induced by high-frequency stimulation (HFS) in the prelimbic cortex of mice exposed to CIE and FSS. (A) Section of the mouse brain at Bregma 1.78 mm and typical placement of the stimulating and recording electrodes in layers II/III and V, respectively, in the prelimbic cortex. (B) Representative traces of normalized fEPSPs. Slices were stimulated in layer II/III and recorded once per minute in layer V of the PrL cortex for 60 min post-HFS. (C) Mean normalized slope of the fEPSPs before and following HFS (n values for the individual groups are shown in parentheses). (D) Mean normalized slope of the fEPSPs one min before and three min after HFS to highlight the significant interaction between CIE+FSS. *p < 0.05, **p < 0.01, and *** p < 0.001 in CIE+FSS slices vs other three experimental groups.
Fig. 5.

Long-term potentiation of synaptic activity induced by high-frequency stimulation (HFS) in the infralimbic cortex of mice exposed to CIE and FSS. (A) Section of the mouse brain at Bregma 1.78 mm and typical arrangement of the stimulating and recording electrodes in layers II/III and V, respectively, in the infralimbic cortex. (B) Representative traces of normalized fEPSPs. (C) Mean normalized slope of the fEPSPs ten min before and 60 min after HFS. (D) Mean normalized slope of the fEPSPs one min before and one min after HFS to show the complex and significant interaction between CIE and FSS. *p < 0.05 for CIE+FSS vs air controls, **p < 0.01 for FSS vs CIE, and *** p < 0.001 for CIE+FSS vs CIE.
2.5. Statistical Analysis
Dendritic spine data were analyzed as a general linear mixed model (SAS PROC MIXED; version 9.4) using Tukey post-hoc tests, when applicable, following our previous methods (McGuier et al., 2015; Mulholland et al., 2018). This statistical approach was selected because of its capacity to analyze unbalanced and complex repeated-measures data and the ability to model the variance and correlation structure of repeated-measures experimental designs (Littell et al., 1998). The dendritic spine variables were nested within each mouse and were further nested across the sequential slices. Electrophysiological data were analyzed in GraphPad Prism (version 8.4.3) using one-sample or unpaired t-tests, repeated-measures one- or two-way ANOVAs with multiple comparisons post-hoc tests, when appropriate. In all cases, data are reported as mean ± SEM and statistical significance was established with α = 0.05.
3. Results
3.1. Prelimbic cortex and plasticity
We previously reported that CIE exposure increased the density of mature, mushroom spines and spike-timing-dependent plasticity in the PrL cortex (Kroener et al., 2012). To determine if ethanol-stress interactions produce adaptations in structural and functional plasticity, we examined dendritic spine changes (Fig. 2) and the expression of LTP (Fig. 3) in the PrL cortex of mice exposed to the CIE-FSS model. Brain slices were prepared on the last day of cycle 2 for spine analysis or the final 3 days of cycle 2 for functional measurements (see Fig. 1A). Notably, brain preparation occurred 24 h after the last FSS exposure for both measures. For analysis of dendritic spines, there was a significant CIE by FSS interaction for total spine density on distal dendrites (> 75 μm away from the soma) of PrL pyramidal neurons (two-factor, mixed linear model: F1,19 = 4.60, p = 0.045; N = 3–8 mice/group and 43 total dendritic sections; Fig. 2C). Tukey’s post-hoc analysis revealed that CIE-exposed mice had significantly higher total spine density compared with Air control mice (p = 0.043). We then examined if there were changes in the density of dendritic spine subclasses. While CIE and FSS exposure did not affect density of long (two-factor, mixed linear model: F1,19 = 0.17, p = 0.684; Fig. 2D) or mushroom (two-factor, mixed linear model: F1,19 = 0.01, p = 0.929; Fig. 2E) spines, there was a main effect of CIE exposure on stubby spines (two-factor, mixed linear model: F1,19 = 6.32, p = 0.02; Fig. 2F) and a significant interaction on filopodia density (two-factor, mixed linear model: F1,19 = 11.42, p = 0.003) with Tukey’s post-hoc analysis showing that FSS mice had a higher density of filopodia compared with the other 3 groups (p < 0.01; Fig. 2G).
Fig. 2.
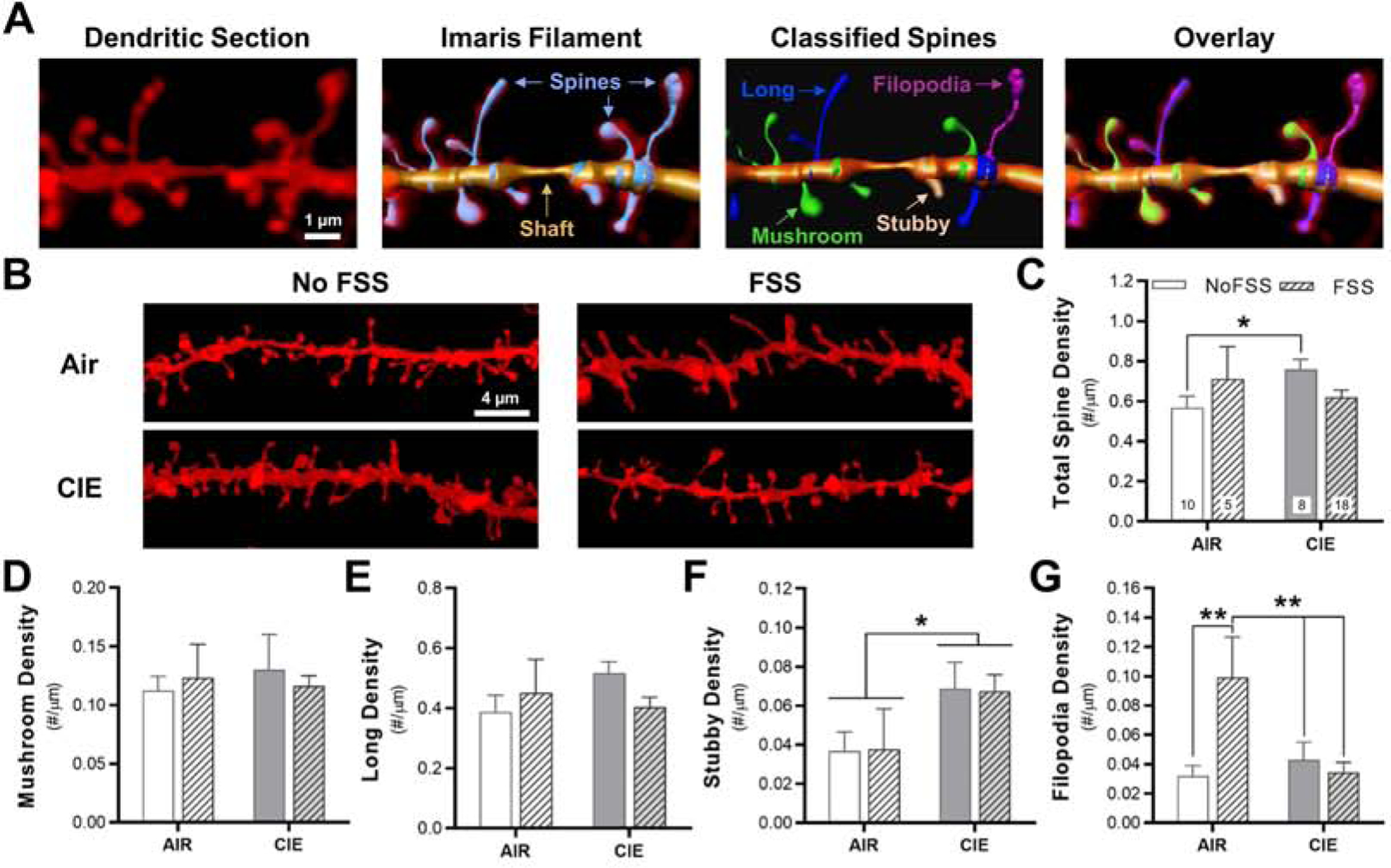
Interaction between chronic intermittent ethanol exposure and repeated forced swim stress episodes on dendritic spine density in the prelimbic cortex. (A) Representative fluorescent labeling of a dendritic section in the prelimbic cortex acquired using a super-resolution confocal microscope. Imaris software was used to generate a 3D reconstruction of the dendritic shaft and spines from the fluorescent signal using the Filament function. Spines were then classified into 4 distinct morphological subtypes: mushroom spines, long spines, stubby spines, and filopodia. (B) Representative images of sections of dendrites that were used for spine analysis in the four experimental groups. (C) Total spine density (total image number for the individual treatment groups is shown each bar) and (D-F) spine density in the four spine subclasses. *p < 0.05, **p < 0.01.
Next, we determined whether CIE and FSS altered the expression of synaptic plasticity in the PrL cortex. To increase throughput for the LTP studies, brain slices were prepared 24 h after the last FSS exposure during the final 3 days of cycle 2. To determine if HFS produces LTP, we first analyzed the fEPSP slope in the Air control mice. A repeated-measures one-way ANOVA revealed a significant effect of HFS on the slope of the fEPSPs (F4.364, 69.82 = 8.42, p < 0.0001; N = 18 recordings from 8 mice). Dunnett’s post-hoc comparisons demonstrated that HFS increased the fEPSP slope at minute 1 (p < 0.003) and minutes 2–6, 9, 18, 21, 24, 26, 41, 56, and 58–59 (p < 0.05) post-stimulation compared with the baseline in the Air controls. In mice exposed to the CIE-FSS model, there was a significant interaction between CIE exposure and FSS treatment (two-way RM ANOVA: F207, 4347 = 1.18, p = 0.04; Fig. 3B,C). Tukey’s post-hoc comparisons indicated that the CIE+FSS group had increased fEPSP slope in comparison with the Air controls, FSS mice, and CIE mice at minutes 1 (p < 0.001), 2 (p < 0.01), and 3 (p < 0.05) post-HFS, but not at the other time points after HFS. The first 3 minutes after HFS are shown in Fig. 3D to highlight the significant interaction of CIE+FSS exposure on fEPSP slope in the PrL cortex.
3.2. Infralimbic cortex and plasticity
Because the IfL cortex is a critical brain region that controls excessive ethanol drinking (Meinhardt et al., 2013; Pfarr et al., 2015), we wanted to determine the morphological and functional adaptations that occur in this structure as a comparison with our findings in the PrL cortex. There was a main effect of CIE treatment on total dendritic spine density of distal dendrites of pyramidal neurons in the IfL cortex (two-factor, mixed linear model: F1,16 = 6.02, p = 0.03; N = 5–8 mice/group; 78 total dendritic sections; Fig. 4A,B). The main effect of FSS (F1,16 = 0.37, p = 0.5495) and the interaction between ethanol and stress (F1,16 = 0.00, p = 0.95) were not significant. Because there was a main effect of CIE exposure on total spine density, the spine data were first collapse across treatments. A treatment (Air vs CIE) by spine subclass analysis demonstrated that there was a main effect of CIE exposure on spine subclass density (two-factor, mixed linear model: F1,18 = 5.60, p = 0.0294; Fig. 4C). In addition, there was also a main effect across the density of spine subclass (two-factor, mixed linear model: F3, 54 = 203.50, p < 0.001), where Tukey’s post-hoc comparisons revealed that the density of long spines > mushroom spines > stubby spines = filopodia. Next, we analyzed the density within each of the four spine subclasses. The density of mushroom (two-factor, mixed linear model: F1,16 = 1.72, p = 0.209; Fig. 4D), long (two-factor, mixed linear model: F1,16 = 0.02, p = 0.886; Fig. 4E), and stubby spines (two-factor, mixed linear model: F1,16 = 0.19, p = 0.668; Fig. 4F) were not significantly changed by CIE, FSS, or CIE+FSS. In contrast, analysis revealed a main effect of CIE exposure on the density of filopodia (two-factor, mixed linear model: F1,16 = 4.80, p = 0.0437; Fig. 4G).
Fig. 4.
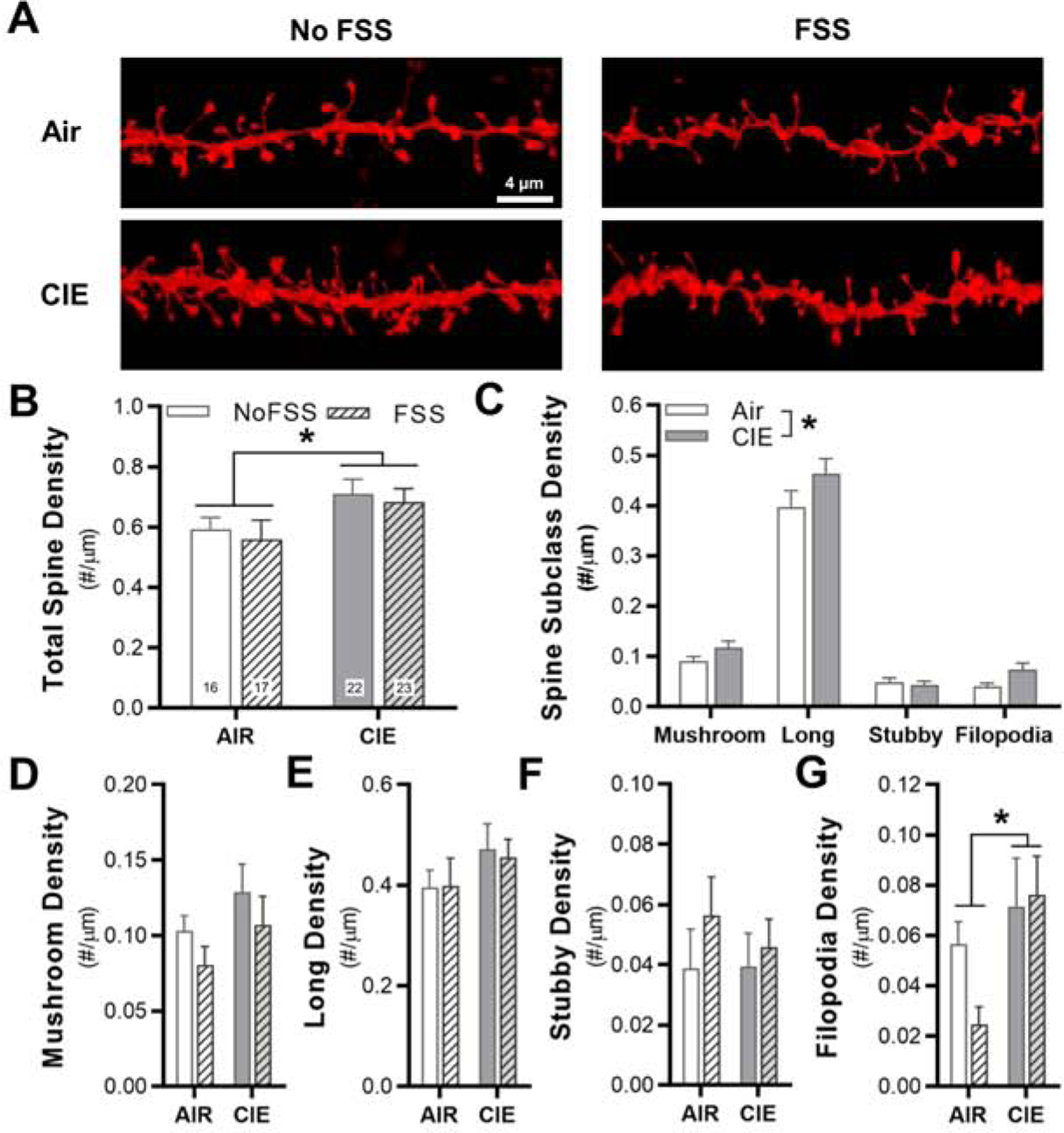
Chronic intermittent ethanol exposure increases dendritic spine density in the IfL cortex, regardless of stress experience. (A) Representative images of sections of dendrites that were used for spine analysis in the four experimental groups. (B) Total spine density across the four treatment groups (total image number for the individual treatment groups is shown each bar), (C) spine density when collapses across FSS history, and (D-G) spine density in the four spine subclasses. *p < 0.05 for CIE vs Air.
LTP induction in the IfL cortex followed the methods described for the functional plasticity measurement in the PrL cortex. In Air controls, a repeated-measures one-way ANOVA indicated that HFS significantly increased the fEPSP slope post-stimulation (F3.121, 53.06 = 16.71; p < 0.0001; N = 18 recordings in 8 mice). Dunnett’s post-hoc comparisons showed that HFS increased the slope of the fEPSP across the entire 60-min post-HFS recording period (p < 0.001 for minutes 1, 2, 8, 12, 33, and 42 vs baseline, p < 0.05 for the remaining minutes vs baseline). In the CIE-FSS model, there was a significant interaction (two-way RM ANOVA: F207, 3726 = 1.586, p < 0.0001; N = 10–18 recordings from 7–8 mice/group; Fig. 5B,C). Bonferroni’s multiple comparison analysis revealed that mice in the CIE+FSS group had increased fEPSP slope in IfL slices when compared to Air controls and CIE exposed mice at post-HFS minutes 1, 2, and 5 (p < 0.05). Mice in the CIE+FSS group also showed increased fEPSP slope at post-HFS minutes 4, 7, and 8 compared with CIE mice (p < 0.05). Lastly, the fEPSP slope in mice exposed to FSS was significantly higher than CIE mice at post-HFS minute 1 (p = 0.002). Treatment groups were not significantly different from each other at all other time points (p > 0.05). To highlight the complex relationship between CIE and FSS exposure on the early phase after HFS, Fig. 5D shows the fEPSP slope data for minute 1 post-HFS compared with baseline, where post-hoc analyses revealed significant differences between treatment groups.
3.3. Pyrazole-naïve experiments
In the previous experiments, mice received repeated injections of pyrazole, an alcohol dehydrogenase inhibitor, to stabilize blood ethanol concentrations. Alcohol dehydrogenase inhibitors have been shown to affect NMDA receptor function in glutamatergic neurons (Pereira et al., 1992). Although acute bath application of an alcohol dehydrogenase inhibitor did not affect LTP in hippocampus (Tokuda et al., 2013), it is possible that chronic pyrazole treatment interacts with FSS to alter the induction of LTP in the IfL cortex. To exclude this possibility, we treated a separate group of mice with or without chronic FSS in the absence of the pyrazole injections that would normally occur during the week of CIE exposure. In the pyrazole-naïve control mice, a repeated-measures one-way ANOVA indicated that HFS significantly increased the fEPSP slope in IfL slices post-stimulation (F2.471, 54.36 = 16.44; N = 23 recordings from 11 mice for controls), with Dunnett’s post-hoc comparisons showing that HFS increased the slope of the fEPSP across the entire 60-min post-HFS recording period (p < 0.001 for minutes 1 – 5 vs baseline, p < 0.01 for minutes 6 – 20 vs baseline, and p < 0.05 for minutes 21 – 60 vs baseline). Analysis of pyrazole-naïve control and FSS mice demonstrated a significant FSS x time interaction (two-way RM ANOVA: F69, 2898 = 2.54, p < 0.0001; N = 23 recordings from 11 mice for controls and 21 recordings from 12 mice for FSS; Fig. 6A,B). Bonferroni’s post-hoc comparisons showed that the fEPSP slope was increased in the FSS mice compared with pyrazole-naïve control mice at 1 min-post HFS (p = 0.002).
Fig. 6.

Long-term potentiation and paired-pulse facilitation of synaptic activity in the infralimbic of pyrazole-naïve mice that experienced repeated FSS episodes. (A) Representative traces of normalized fEPSPs. (B) Mean normalized slope of the fEPSPs ten min before and 60 min after HFS in control and FSS mice. (C) Representative traces of paired-pulse stimulations in the IfL cortex. fEPSPs were stimulated at 50 ms intervals and stimulus artifacts were removed for clarity. (D) The paired-pulse facilitation ratio was significantly increased in mice treated with repeated FSS episodes compared to controls. **p < 0.01 for FSS vs control.
3.4. Infralimbic cortex and paired-pulse facilitation
Functional measures in the current study suggest that regardless of CIE exposure, chronic FSS can produce an increase in fEPSP slope during the early induction phase of long-term potentiation, known as post-tetanic potentiation (PTP). PTP is a transient increase in presynaptic response following sustained HFS that can last for seconds to minutes depending on stimulation strength (Zucker and Regehr, 2002; Regehr, 2012). To explore if FSS affects another form of short-term presynaptic plasticity, a separate group of pyrazole-naïve mice were treated with chronic FSS prior to analysis of PPF in the IfL cortex. In pyrazole-naïve mice, a one-sample t-test revealed a significant increase in the PPF ratio above a hypothetical value of 1 (t8 = 3.01, p = 0.01, N = 9 recordings from 4 mice). When comparing the two treatment groups, an unpaired t-test showed that the PPF ratio was significantly increased in the FSS mice compared with pyrazole-naïve controls (t18 = 3.60, p = 0.002, N = 9 – 11 recordings from 4–6 mice/group; Fig. 6C,D).
4. Discussion
Episodes of excessive drinking and relapse are heavily influenced by stressful and traumatic life events (Sinha et al., 2009; Ramchandani et al., 2018). While it is known that stress or chronic ethanol can disrupt the capability of the brain to reorganize and adapt to new information and environmental factors (Holmes and Wellman, 2009; Kroener et al., 2012; Arnsten, 2015), the underlying cellular mechanisms of combined chronic ethanol- and stress-induced neuroadaptations are unclear. Using a mouse model of repeated CIE and FSS co-exposure that escalates ethanol intake and produces cognitive deficits (Anderson et al., 2016b, a; Lopez et al., 2016; Rodberg et al., 2017; Padula et al., 2019), the current study examined CIE+FSS interactions on synaptic and structural plasticity within the PrL and IfL subdivisions of the mPFC that are known for their role in modulating drug-seeking, decision-making, and associative learning. Specifically, the morphological adaptations in spine density in the PrL and IfL cortex were primarily driven by CIE exposure, whereas the functional changes in these subregions required an interaction between CIE and FSS or were primarily attributed to FSS exposure. Interestingly, these functional adaptations reflected a metaplastic shift in mechanisms that control short-term presynaptic plasticity. Taken together, the results of this study demonstrate that CIE+FSS interactions produce distinct and complex forms of structural and functional plasticity in the PrL and IfL cortex in male C57BL/6J mice.
Our investigation into synaptic plasticity revealed that regardless of mPFC subregion, chronic FSS resulted in an increase in the initial phase of long-term potentiation (i.e., PTP) in CIE-exposed mice. In the IfL cortex, we also demonstrated that repeated FSS alone increased PTP in ethanol- and pyrazole-naïve mice. These data demonstrating a metaplastic shift in presynaptic short-term plasticity are surprising given reports of enhanced spike-timing-dependent synaptic plasticity in the PrL and orbitofrontal cortex of CIE exposed mice (Kroener et al., 2012; Nimitvilai et al., 2016) and general reductions in plasticity observed in other preclinical models of chronic stress (Chattarji et al., 2015). However, the effects of stress on the direction of synaptic plasticity may be region dependent, as one study reported enhanced LTP in the anterior cingulate cortex of mice exposed to chronic restraint stress (Ito et al., 2010). In the current study, LTP was not changed relative to control mice following FSS or CIE and suggest that CIE+FSS interactions produce specific neuroadaptations that control short-term presynaptic plasticity. These observed changes in PTP after CIE+FSS exposure or FSS alone are important since forms of short-term plasticity are implicated in several cognitive processes including short-term or working memory formation (Powell, 2006) and in modulating circuit function during computations that drive cognitive function (Deng and Klyachko, 2011; Deng et al., 2011). Using the same model as in the present study, Vazey and colleagues reported deficits in contextual learning and extradimensional set-shifting in the mice exposed to CIE+FSS (Rodberg et al., 2017). Given the role of these regions in controlling behavioral flexibility and excessive ethanol drinking, it is possible that the functional adaptations in presynaptic plasticity in the PrL and IfL cortex may be important mechanisms that are responsible for both the elevated drinking and cognitive impairments that result from this CIE+FSS co-exposure model. It will be important to determine which inputs into the Prl and IfL are important mediators of altered functional plasticity and the corresponding behavioral consequences of these morphological and functional changes in mice treated with repeated CIE and FSS. Moreover, as sex is an important variable for the development of an AUD and the motivation to drink alcohol in excess (Zywiak et al., 2006; Diehl et al., 2007; Ehlers et al., 2010; Slade et al., 2016), future studies should examine how sex influences measures of synaptic plasticity, spine morphology, and alcohol-related behaviors in CIE+FSS treated mice.
In addition to the increase in PTP, another intriguing finding from the current study is that repeated FSS episodes increased PPF in the IfL cortex, providing further evidence that FSS induced metaplastic changes in mechanisms that regulate presynaptic function. Similarly, mice treated with chronic restraint stress showed an increase in PPF and LTP in the anterior cingulate cortex (Ito et al., 2010). There are multiple forms of short-term presynaptic facilitation that are differentiated based on the time scale following stimulation. Facilitation and augmentation last for tens of milliseconds or a few seconds, respectively (Thomson, 2000; Regehr, 2012). Potentiation is a third form of presynaptic plasticity that has a decay of tens of seconds to minutes, which is within the time scale of the increased PTP that we observed in the FSS and CIE+FSS mice. However, we also observed increases in PPF where the dual stimulations occurred on a faster time scale (i.e., 50 ms), suggesting that FSS and the interaction between CIE and FSS affect multiple forms of presynaptic plasticity in the IfL cortex, although these two forms of short-term plasticity may be difficult to resolve. While we did not measure the magnitude of PTP and PPF in the same slices, there is evidence linking PPF with PTP. In line with the present studies, inputs with high PPF also exhibited increased LTP during the early PTP phase in rat cortical and hippocampal slices (Son and Carpenter, 1996; Volgushev et al., 1997). Thus, convergence of our findings implicates IfL cortex layer II/III neurons or other axonal terminals that innervate layer V neurons (i.e., thalamic inputs) as being sensitive to repeated FSS.
While the present study showed that CIE+FSS exposure produced short-term metaplasticity, we also observed dendritic spine remodeling in the PrL and IfL cortex. Interestingly, spine changes induced by CIE and FSS co-exposure did not parallel those identified in our functional studies, likely due to the effects of FSS and CIE+FSS interactions on presynaptic forms of functional plasticity. The adaptations in dendritic spine density across both subregions were largely driven by CIE exposure with some exceptions in the PrL cortex that showed a complex interaction between CIE and FSS. The CIE effects on increased spine density in cortical subregions are consistent with previous evidence in chronic ethanol exposure models (Kroener et al., 2012; Kim et al., 2015; McGuier et al., 2015; Klenowski et al., 2016; Mulholland et al., 2016; McGuier et al., 2018; Varodayan et al., 2018; Frost et al., 2019; den Hartog et al., 2020). Models used to study chronic stress and negative affective behaviors have reported dendritic retraction and reductions in dendritic spine density in the PrL and IfL cortex (Moench and Wellman, 2015; Qiao et al., 2016). In the present study, we did not observe reductions in spine density in mice exposed to repeated FSS tests as would be expected from the existing literature. In contrast, there was a marked increase in filopodia density in the PrL cortex of FSS mice. While the repeated FSS model is not typically used to study behavioral, morphological, and functional adaptations, one study reported dendritic retraction of IfL, but not PrL, neurons 72 h after one and three FSS sessions (Izquierdo et al., 2006). Note that alterations in dendritic spine density were not reported in that study. Given the sensitivity to dendritic remodeling after acute FSS, it is possible that dendritic spine changes in the PrL and IfL occur acutely and return to basal levels after additional swim sessions. An alternative possibility to habituation of spine adaptations is that changes in PrL and IfL dendritic spine density are insensitive to this model of repeated FSS. Additional studies would be required to test these possibilities, especially as morphological adaptations translate into shifts in cortical metaplasticity and altered behaviors, such as escalated drinking and cognitive impairments in CIE+FSS exposed male mice.
Although the mechanisms underlying the presynaptic metaplasticity caused by FSS and CIE+FSS interactions are unclear, there is evidence in the PFC that presynaptic signaling proteins, including SNARE complex proteins that regulate synaptic vesicle trafficking (e.g., syntaxin 1A, SNAP-25), are sensitive to acute and repeated stressors (Musazzi et al., 2010; Muller et al., 2011; Yuen et al., 2017). In addition, studies focused on the cortex reported that CIE exposure in mice and long-term ethanol drinking in non-human primates increased use-dependent presynaptic glutamate release and elevated expression of proteins that regulate synaptic vesicle trafficking, respectively (Hu et al., 2015; Nimitvilai et al., 2017). Munc13–2 and synaptogyrin-1 are two abundant presynaptic proteins that control forms of short-term synaptic function and are also sensitive to ethanol (Janz et al., 1999; Gioia et al., 2017; Gioia and McCool, 2017; Nimitvilai et al., 2017; Raja et al., 2019). Thus, adaptations in these presynaptic proteins may drive the short-term metaplasticity that we observed in FSS mice, and they should be the target of future studies to determine their role in the increased PTP and PPF in the PrL and IfL cortex.
5. Summary
In conclusion, we have shown distinct morphological and functional responses to CIE and repeated FSS exposure within the PrL and IfL cortex. While the structural adaptations were largely driven by CIE exposure in both regions, we observed a metaplastic shift in two forms of short-term presynaptic function in FSS and CIE+FSS mice in two mPFC subregions of male C57BL/6J mice. Given that CIE+FSS interactions produce cognitive deficits and increased drinking (Anderson et al., 2016b, a; Lopez et al., 2016; Rodberg et al., 2017; Padula et al., 2019; den Hartog et al., 2020), our results suggest that structural and functional neuroadaptations in the PrL and IfL cortex may underlie deficits in control over ethanol drinking and cognitive performance.
Highlights.
Chronic ethanol and repeated stress exposure produced distinct morphological and functional changes in the medial prefrontal cortex
Chronic intermittent ethanol exposure increased dendritic spine density
Repeated stress exposure increased the early phase of synaptic plasticity
Chronic stress episodes produced metaplastic changes in presynaptic function
Acknowledgements
This work was supported by NIH grants AA020930 (PJM), OD021532 (PJM), AA026642 (RC), AA020929 (MFL), and AA014095 (HCB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Anderson RI, Lopez MF, Becker HC (2016a) Forced swim stress increases ethanol consumption in C57BL/6J mice with a history of chronic intermittent ethanol exposure. Psychopharmacology (Berl) 233:2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, Becker HC (2016b) Stress-Induced Enhancement of Ethanol Intake in C57BL/6J Mice with a History of Chronic Ethanol Exposure: Involvement of Kappa Opioid Receptors. Front Cell Neurosci 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF (2015) Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci 18:1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC (2017) Influence of stress associated with chronic alcohol exposure on drinking. Neuropharmacology 122:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF (2004) Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res 28:1829–1838. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL (2011) Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 218:131–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Sinha R (2017) Alcohol, stress, and glucocorticoids: From risk to dependence and relapse in alcohol use disorders. Neuropharmacology 122:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, Nimitvilai-Roberts S, Jennings SD, Woodward JJ, Mulholland PJ (2020) Distinct Region- and Time-Dependent Functional Cortical Adaptations in C57BL/6J Mice after Short and Prolonged Alcohol Drinking. eNeuro 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, McGonigal JT, Newsom RJ, Woodward JJ, Mulholland PJ, Gass JT (2017) Prefrontal Cortex KCa2 Channels Regulate mGlu5-Dependent Plasticity and Extinction of Alcohol-Seeking Behavior. J Neurosci 37:4359–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N (2007) The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci 27:2781–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahman MM (2015) Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci 18:1364–1375. [DOI] [PubMed] [Google Scholar]
- den Hartog CR, Blandino KL, Nash ML, Sjogren ER, Grampetro MA, Moorman DE, Vazey EM (2020) Noradrenergic tone mediates marble burying behavior after chronic stress and ethanol. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Klyachko VA (2011) The diverse functions of short-term plasticity components in synaptic computations. Commun Integr Biol 4:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Sojka D, Klyachko VA (2011) Abnormal presynaptic short-term plasticity and information processing in a mouse model of fragile X syndrome. J Neurosci 31:10971–10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl A, Croissant B, Batra A, Mundle G, Nakovics H, Mann K (2007) Alcoholism in women: is it different in onset and outcome compared to men? Eur Arch Psychiatry Clin Neurosci 257:344–351. [DOI] [PubMed] [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW (2013) Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl Psychiatry 3:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, Vieten C, Gilder A, Gilder DA, Stouffer GM, Lau P, Wilhelmsen KC (2010) Age at regular drinking, clinical course, and heritability of alcohol dependence in the San Francisco family study: a gender analysis. Am J Addict 19:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost ME, Peterson VL, Bird CW, McCool B, Hamilton DA (2019) Effects of Ethanol Exposure and Withdrawal on Neuronal Morphology in the Agranular Insular and Prelimbic Cortices: Relationship with Withdrawal-Related Structural Plasticity in the Nucleus Accumbens. Brain Sci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Chandler LJ (2013) The Plasticity of Extinction: Contribution of the Prefrontal Cortex in Treating Addiction through Inhibitory Learning. Front Psychiatry 4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF (2012) Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A 109:18156–18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia DA, McCool B (2017) Strain-Dependent Effects of Acute Alcohol on Synaptic Vesicle Recycling and Post-Tetanic Potentiation in Medial Glutamate Inputs to the Mouse Basolateral Amygdala. Alcohol Clin Exp Res 41:735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia DA, Alexander N, McCool BA (2017) Ethanol Mediated Inhibition of Synaptic Vesicle Recycling at Amygdala Glutamate Synapses Is Dependent upon Munc13–2. Front Neurosci 11:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH (2009) Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience 164:798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JE, Orr AL, Madison DV (2010) Altered hippocampal synaptic physiology in aged parkin-deficient mice. Neuromolecular Med 12:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL (2009) Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev 33:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M (2012) Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci 15:1359–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Morris B, Carrasco A, Kroener S (2015) Effects of acamprosate on attentional set-shifting and cellular function in the prefrontal cortex of chronic alcohol-exposed mice. Alcohol Clin Exp Res 39:953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Nagano M, Suzuki H, Murakoshi T (2010) Chronic stress enhances synaptic plasticity due to disinhibition in the anterior cingulate cortex and induces hyper-locomotion in mice. Neuropharmacology 58:746–757. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A (2006) Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci 26:5733–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquot C, Croft AP, Prendergast MA, Mulholland P, Shaw SG, Little HJ (2008) Effects of the glucocorticoid antagonist, mifepristone, on the consequences of withdrawal from long term alcohol consumption. Alcohol Clin Exp Res 32:2107–2116. [DOI] [PubMed] [Google Scholar]
- Janz R, Sudhof TC, Hammer RE, Unni V, Siegelbaum SA, Bolshakov VY (1999) Essential roles in synaptic plasticity for synaptogyrin I and synaptophysin I. Neuron 24:687–700. [DOI] [PubMed] [Google Scholar]
- Kamigaki T (2019) Prefrontal circuit organization for executive control. Neurosci Res 140:23–36. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, Grant BF, Hasin DS (2012) Stress and alcohol: epidemiologic evidence. Alcohol Res 34:391–400. [PMC free article] [PubMed] [Google Scholar]
- Kim A, Zamora-Martinez ER, Edwards S, Mandyam CD (2015) Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain Struct Funct 220:1705–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Martin-Fardon R (2020) Possible Role of CRF-Hcrt Interaction in the Infralimbic Cortex in the Emergence and Maintenance of Compulsive Alcohol-Seeking Behavior. Alcohol Clin Exp Res 44:354–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Becker HC (2019) Oxytocin attenuates stress-induced reinstatement of alcohol seeking behavior in male and female mice. Psychopharmacology (Berl) 236:2613–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenowski PM, Fogarty MJ, Shariff M, Belmer A, Bellingham MC, Bartlett SE (2016) Increased Synaptic Excitation and Abnormal Dendritic Structure of Prefrontal Cortex Layer V Pyramidal Neurons following Prolonged Binge-Like Consumption of Ethanol. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E (2016) Prefrontal executive function and adaptive behavior in complex environments. Curr Opin Neurobiol 37:1–6. [DOI] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ (2012) Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One 7:e37541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell RC, Henry PR, Ammerman CB (1998) Statistical analysis of repeated measures data using SAS procedures. Journal of Animal Science 76:1216 – 1231. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC (2005) Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 181:688–696. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, Becker HC (2016) Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent C57BL/6J mice. Alcohol 51:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YL, Richardson HN (2014) Alcohol, stress hormones, and the prefrontal cortex: a proposed pathway to the dark side of addiction. Neuroscience 277:139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjoch H, Vainer E, Matar M, Ifergane G, Zohar J, Kaplan Z, Cohen H (2016) Predator-scent stress, ethanol consumption and the opioid system in an animal model of PTSD. Behav Brain Res 306:91–105. [DOI] [PubMed] [Google Scholar]
- McGuier NS, Uys JD, Mulholland PJ (2018) Neural morphology and addiction. In: Neural mechanisms of addiction (Torregrossa MM, ed): Elsevier, Amsterdam, The Netherlands. [Google Scholar]
- McGuier NS, Padula AE, Lopez MF, Woodward JJ, Mulholland PJ (2015) Withdrawal from chronic intermittent alcohol exposure increases dendritic spine density in the lateral orbitofrontal cortex of mice. Alcohol 49:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stahlin O, Heilig M, Harper C, Drescher KU, Spanagel R, Sommer WH (2013) Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci 33:2794–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS, Spigelman I (2013) Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcohol Clin Exp Res 37:566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moench KM, Wellman CL (2015) Stress-induced alterations in prefrontal dendritic spines: Implications for posttraumatic stress disorder. Neurosci Lett 601:41–45. [DOI] [PubMed] [Google Scholar]
- Molander A, Vengeliene V, Heilig M, Wurst W, Deussing JM, Spanagel R (2012) Brain-specific inactivation of the Crhr1 gene inhibits post-dependent and stress-induced alcohol intake, but does not affect relapse-like drinking. Neuropsychopharmacology 37:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW (2009) N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci 12:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Chandler LJ, Kalivas PW (2016) Signals from the Fourth Dimension Regulate Drug Relapse. Trends Neurosci 39:472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Teppen TL, Miller KM, Sexton HG, Pandey SC, Swartzwelder HS (2018) Donepezil Reverses Dendritic Spine Morphology Adaptations and Fmr1 Epigenetic Modifications in Hippocampus of Adult Rats After Adolescent Alcohol Exposure. Alcohol Clin Exp Res 42:706–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HK, Wegener G, Popoli M, Elfving B (2011) Differential expression of synaptic proteins after chronic restraint stress in rat prefrontal cortex and hippocampus. Brain Res 1385:26–37. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Milanese M, Farisello P, Zappettini S, Tardito D, Barbiero VS, Bonifacino T, Mallei A, Baldelli P, Racagni G, Raiteri M, Benfenati F, Bonanno G, Popoli M (2010) Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One 5:e8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, Lopez MF, Mulholland PJ, Woodward JJ (2016) Chronic Intermittent Ethanol Exposure Enhances the Excitability and Synaptic Plasticity of Lateral Orbitofrontal Cortex Neurons and Induces a Tolerance to the Acute Inhibitory Actions of Ethanol. Neuropsychopharmacology 41:1112–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, Uys JD, Woodward JJ, Randall PK, Ball LE, Williams RW, Jones BC, Lu L, Grant KA, Mulholland PJ (2017) Orbitofrontal Neuroadaptations and Cross-Species Synaptic Biomarkers in Heavy-Drinking Macaques. J Neurosci 37:3646–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori HR, Helinski S, Spanagel R (2014) Cluster and meta-analyses on factors influencing stress-induced alcohol drinking and relapse in rodents. Addict Biol 19:225–232. [DOI] [PubMed] [Google Scholar]
- Norman KJ, Seiden JA, Klickstein JA, Han X, Hwa LS, DeBold JF, Miczek KA (2015) Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology (Berl) 232:991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula AE, Rinker JA, Khan F, Lopez MF, Mulligan MK, Williams RW, Becker HC, Mulholland PJ (2019) Kcnn3 as a target for treating aberrant behaviors in stressed, ethanol-dependent mice. BioRxiv. [Google Scholar]
- Padula AE, Griffin WC 3rd, Lopez MF, Nimitvilai S, Cannady R, McGuier NS, Chesler EJ, Miles MF, Williams RW, Randall PK, Woodward JJ, Becker HC, Mulholland PJ (2015) KCNN Genes that Encode Small-Conductance Ca2+-Activated K+ Channels Influence Alcohol and Drug Addiction. Neuropsychopharmacology 40:1928–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CL, Armeli S, Tennen H (2004) The daily stress and coping process and alcohol use among college students. J Stud Alcohol 65:126–135. [DOI] [PubMed] [Google Scholar]
- Pereira EF, Aracava Y, Aronstam RS, Barreiro EJ, Albuquerque EX (1992) Pyrazole, an alcohol dehydrogenase inhibitor, has dual effects on N-methyl-D-aspartate receptors of hippocampal pyramidal cells: agonist and noncompetitive antagonist. J Pharmacol Exp Ther 261:331–340. [PubMed] [Google Scholar]
- Pfarr S, Meinhardt MW, Klee ML, Hansson AC, Vengeliene V, Schonig K, Bartsch D, Hope BT, Spanagel R, Sommer WH (2015) Losing Control: Excessive Alcohol Seeking after Selective Inactivation of Cue-Responsive Neurons in the Infralimbic Cortex. J Neurosci 35:10750–10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, McCall NM, Maldonado-Devincci AM, Morrow AL, Jones SR, Kash TL (2015) Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology 99:735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CM (2006) Gene targeting of presynaptic proteins in synaptic plasticity and memory: across the great divide. Neurobiol Learn Mem 85:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Li MX, Xu C, Chen HB, An SC, Ma XM (2016) Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plast 2016:8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja MK, Preobraschenski J, Del Olmo-Cabrera S, Martinez-Turrillas R, Jahn R, Perez-Otano I, Wesseling JF (2019) Elevated synaptic vesicle release probability in synaptophysin/gyrin family quadruple knockouts. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Stangl BL, Blaine SK, Plawecki MH, Schwandt ML, Kwako LE, Sinha R, Cyders MA, O’Connor S, Zakhari S (2018) Stress vulnerability and alcohol use and consequences: From human laboratory studies to clinical outcomes. Alcohol 72:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG (2012) Short-term presynaptic plasticity. Cold Spring Harb Perspect Biol 4:a005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SL, Marrero IM, Perez-Heydrich CA, Sepulveda-Orengo MT, Reissner KJ, Thiele TE (2019) Medial prefrontal cortex neuropeptide Y modulates binge-like ethanol consumption in C57BL/6J mice. Neuropsychopharmacology 44:1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodberg EM, den Hartog CR, Anderson RI, Becker HC, Moorman DE, Vazey EM (2017) Stress Facilitates the Development of Cognitive Dysfunction After Chronic Ethanol Exposure. Alcohol Clin Exp Res 41:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Noamany H, Chang CJ, Brown AR, Chen X, Leible D, Lee JJ, Wang J, Vernon AN, Vander Weele CM, Kimchi EY, Heiman M, Tye KM (2019) A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science 366:1008–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM (2009) Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology 34:1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade T, Chapman C, Swift W, Keyes K, Tonks Z, Teesson M (2016) Birth cohort trends in the global epidemiology of alcohol use and alcohol-related harms in men and women: systematic review and metaregression. BMJ Open 6:e011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H, Carpenter DO (1996) Interactions among paired-pulse facilitation and post-tetanic and long-term potentiation in the mossy fiber-CA3 pathway in rat hippocampus. Synapse 23:302–311. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Noori HR, Heilig M (2014) Stress and alcohol interactions: animal studies and clinical significance. Trends Neurosci 37:219–227. [DOI] [PubMed] [Google Scholar]
- Stephens MA, Wand G (2012) Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res 34:468–483. [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Randall CL, Carrigan MH (2003) Drinking to cope in socially anxious individuals: a controlled study. Alcohol Clin Exp Res 27:1937–1943. [DOI] [PubMed] [Google Scholar]
- Thomson AM (2000) Facilitation, augmentation and potentiation at central synapses. Trends Neurosci 23:305–312. [DOI] [PubMed] [Google Scholar]
- Tokuda K, Izumi Y, Zorumski CF (2013) Locally-generated acetaldehyde is involved in ethanol-mediated LTP inhibition in the hippocampus. Neurosci Lett 537:40–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, Floresco SB, Chandler LJ (2014) Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J Neurosci 34:3706–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uys JD, McGuier NS, Gass JT, Griffin WC 3rd, Ball LE, Mulholland PJ (2016) Chronic intermittent ethanol exposure and withdrawal leads to adaptations in nucleus accumbens core postsynaptic density proteome and dendritic spines. Addict Biol 21:560–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Sidhu H, Kreifeldt M, Roberto M, Contet C (2018) Morphological and functional evidence of increased excitatory signaling in the prelimbic cortex during ethanol withdrawal. Neuropharmacology 133:470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW Jr., Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF (2012) Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci 32:7563–7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2004) Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58. [DOI] [PubMed] [Google Scholar]
- Vertes RP (2006) Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142:1–20. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Voronin LL, Chistiakova M, Singer W (1997) Relations between long-term synaptic modifications and paired-pulse interactions in the rat neocortex. Eur J Neurosci 9:1656–1665. [DOI] [PubMed] [Google Scholar]
- Weera MM, Gilpin NW (2019) Biobehavioral Interactions Between Stress and Alcohol. Alcohol Res 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL, Moench KM (2019) Preclinical studies of stress, extinction, and prefrontal cortex: intriguing leads and pressing questions. Psychopharmacology (Berl) 236:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL, Bollinger JL, Moench KM (2020) Effects of stress on the structure and function of the medial prefrontal cortex: Insights from animal models. Int Rev Neurobiol 150:129–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Yan Z (2017) Molecular and Epigenetic Mechanisms for the Complex Effects of Stress on Synaptic Physiology and Cognitive Functions. Int J Neuropsychopharmacol 20:948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z (2009) Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci U S A 106:14075–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z (2011) Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry 16:156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG (2002) Short-term synaptic plasticity. Annu Rev Physiol 64:355–405. [DOI] [PubMed] [Google Scholar]
- Zywiak WH, Stout RL, Trefry WB, Glasser I, Connors GJ, Maisto SA, Westerberg VS (2006) Alcohol relapse repetition, gender, and predictive validity. J Subst Abuse Treat 30:349–353. [DOI] [PubMed] [Google Scholar]


