Abstract
Osteochondral repair requires the induction of both articular cartilage and subchondral bone development, necessitating the presentation of multiple tissue-specific cues for these highly distinct tissues. To provide a singular hydrogel system for the repair of either tissue type, we have developed biofunctionalized, mesenchymal stem cell-laden hydrogels that can present in situ biochemical cues for either chondrogenesis or osteogenesis by simple click modification of a crosslinker, poly(glycolic acid)-poly(ethylene glycol)-poly(glycolic acid)-di(but-2-yne-1,4-dithiol) (PdBT). After modifying PdBT with either cartilage-specific biomolecules (N-cadherin peptide, chondroitin sulfate) or bone-specific biomolecules (bone marrow homing peptide 1, glycine-histidine-lysine peptide), the biofunctionalized, PdBT-crosslinked hydrogels can selectively promote the desired bone- or cartilage-like matrix synthesis and tissue-specific gene expression, with effects dependent on both biomolecule selection and concentration. Our findings establish the versatility of this click functionalized hydrogel system as well as its ability to promote in vitro development of osteochondral tissue phenotypes.
Keywords: click, bioconjugation, modular, hydrogel, osteochondral
1. Introduction
Articular cartilage repair remains a significant clinical need, with poor patient outcomes from trauma and degenerative disease due to cartilage’s inherently weak capacity for regeneration.1 These injuries typically involve damage to both the cartilage and underlying subchondral bone within the osteochondral unit, which presents a heterogeneous milieu of tissue phenotypes and biochemical cues.1 Thus, it is essential to present multiple tissue-specific cues for the induction of distinct chondrogenic and osteogenic tissue development. Tissue engineers have traditionally created osteochondral constructs by fabricating distinct scaffolds and hydrogels for the bone and cartilage layers,2–4 mimicking gross differences in composition such as mineral content, but have not fully recapitulated the disparities in biochemical microenvironment between bone and cartilage. Furthermore, traditional methods of providing bone- or cartilage-specific biochemical cues such as microparticle-based delivery are prone to loss of spatial localization due to ease of diffusion and resulting off-target effects.5 Bioconjugation strategies, which can utilize highly specific click chemistry, have thus emerged as a means of directly tethering biomolecules of interest to hydrogels for tissue engineering, albeit with limited biomolecule compatibility in many cases.6,7
To address these needs, we developed a modular polymeric crosslinker, known as poly(glycolic acid)-poly(ethylene glycol)-poly(glycolic acid)-di(but-2-yne-1,4-dithiol) (PdBT), that can be conjugated to biomolecules of varied size and chemical character via a simple mixing process at room temperature in water.8 In the present study, we sought to fabricate PdBT-crosslinked, mesenchymal stem cell (MSC)-laden hydrogels that are click functionalized with a variety of in situ biochemical cues for bone and cartilage development, investigating their potential to promote tissue-specific regeneration in vitro (Fig. 1).
Figure 1. Development of biofunctionalized, PdBT-crosslinked hydrogels for osteochondral tissue engineering.

PdBT crosslinkers are first functionalized with cartilage- or bone-specific biochemical cues, then mixed with suspensions of poly(N-isopropylacrylamide-co-glycidyl methacrylate) (P(NiPAAm-co-GMA)) and mesenchymal stem cells to generate cell-laden hydrogels for either cartilage or bone repair.
Specifically, we investigated the promotion of chondrogenesis by PdBT-crosslinked, thermoresponsive poly(N-isopropylacrylamide-co-glycidyl methacrylate) (P(NiPAAm-co-GMA)) hydrogels functionalized with the cartilage-specific biomolecules, chondroitin sulfate (CS) and N-cadherin peptide (NC), as well as the promotion of osteogenesis by hydrogels functionalized with the bone-specific biomolecules, bone marrow homing peptide 1 (BMHP1) and glycine-histidine-lysine (GHK). P(NiPAAm-co-GMA) belongs to a class of thermoresponsive P(NiPAAm)-based polymers which gel above a lower critical solution temperature and have been utilized for bone and cartilage tissue engineering – albeit with the requirement of providing secondary growth factor and biomolecule delivery most often by controlled release.9 In the present study, CS was selected due to being a large sulfated glycosaminoglycan (sGAG) found throughout native cartilage tissue,10 while NC was chosen as a mimic of the N-cadherin protein involved in formation of cell-cell contacts during early chondrogenesis, known as MSC condensation.11 BMHP1, on the other hand, is a chemotactic agent of MSCs that has been utilized to promote bone mineralization,12 while GHK represents a fragment of the bone-specific protein osteonectin which has also been used for bone tissue engineering.13 By functionalizing PdBT-crosslinked hydrogels with varying concentrations of these cartilage- and bone-specific biomolecules, we sought to elucidate the effects of biomolecule presence and concentration on the promotion of chondrogenic and osteogenic activity within this modular hydrogel system.
2. Materials and Methods
2.1. Materials
Fetal bovine serum (FBS) was purchased from Gemini Bio-Products (Sacramento, CA, Lot A65G00J). Low glucose Dulbecco’s modified eagle medium (DMEM), minimum essential medium alpha (α-MEM), antibiotic-antimycotic, Quant-iT PicoGreen dsDNA assay kit, proteinase K, Cytoseal XYL, optimal cutting temperature (OCT) compound, and TRIzol reagent kit were purchased from ThermoFisher Scientific (Waltham, MA). ITS+ Premix was purchased from Corning (Corning, NY). Chloro(pentamethylcyclopentadienyl)(cycloocta-diene)ruthenium(II) (Cp*Ru(cod)Cl), dithiothreitol, phosphate buffered saline, ascorbic acid, dexamethasone, β-glycerol 2-phosphate, pepstatin A, iodacetamide, tris(hydroxymethyl aminomethane), ethylenediaminetetraacetic acid (EDTA), 1,9-dimethyl-methylene blue (DMMB) zinc chloride double salt, 10% neutral buffered formalin, Alcian Blue staining kit, von Kossa staining kit, nuclear fast red staining kit, xylene, ethanol, and chloroform were purchased from MilliporeSigma (St. Louis, MO). Arsenazo III assay kit was purchased from Pointe Scientific (Canton, MI). RNeasy mini kit was purchased from Qiagen (Hilden, Germany). iScript cDNA synthesis kit and iTaq Universal SYBYR Green Supermix were purchased from Bio-Rad (Hercules, CA), and all DNA primers were purchased from Integrated DNA Technologies (Coralville, IA). Ultrapure water was obtained from a Millipore Super-Q water system (Billerica, MA).
2.2. Click Functionalization of Tissue-Specific Crosslinkers
The synthesis and characterization of PdBT and biofunctionalized PdBT crosslinkers were performed as described in prior studies8 and are briefly summarized here. Azide-modified CS was synthesized as described previously by activated ester chemistry.8 NC (“GGGHAVDI”), BMHP1 (“GGGPFSSTKT”), and GHK (“GGGGHKSP”) with N-terminal azides were synthesized by solid phase peptide synthesis as described in detail elsewhere.8,14 To functionalize PdBT for chondrogenesis, CS and NC were conjugated to PdBT by a simple mixing process in water,8 generating CS/PdBT and NC/PdBT. Briefly, the biomolecule of interest was mixed with PdBT in 2:1 molar ratio in water at ambient temperature for 6 hr in the presence of a cytocompatible Cp*RuCl(cod) catalyst and dithiothreitol for disulfide inhibition, followed by purification via dialysis for 24 hr. To functionalize PdBT for osteogenesis, BMHP1 and GHK were conjugated to PdBT by the same process to generate BMHP1/PdBT and GHK/PdBT. As a biomolecule-free control for all studies, PdBT was used without any biomolecule functionalization.
2.3. Mesenchymal Stem Cell (MSC) Harvest and Culture
MSCs were harvested via aspiration from the tibia of anesthetized 6 month old New Zealand White rabbits from Charles River Laboratories (Wilmington, MA), in accordance with protocols approved by the Rice Institutional Animal Care and Use Committee and in agreement with the animal care and use guidelines set forth by the National Institutes of Health.8 The MSCs were cultured in T-225 flasks in DMEM supplemented with 10% v/v FBS and 1% v/v antibiotic-antimycotic inside a humidified incubator at 37 °C and 5% CO2, and cells were used at Passage 3.
2.4. Hydrogel Fabrication and Study Design
P(NiPAAm-co-GMA), a thermogelling polymer with crosslinkable epoxides for PdBT, was synthesized as described elsewhere.15 All polymers were pre-sterilized by UV exposure for 3 hr, while Teflon molds and other materials were sterilized by autoclaving or ethylene oxide exposure. To investigate the effect of conjugated biomolecule concentration on chondrogenic and osteogenic activity, (Low) and (High) crosslinker formulations were designed as shown in Table 1. Hydrogel formulations were optimized and characterized in previous studies, and the total crosslinker concentration in each formulation was set to 25 mM based on the minimum PdBT concentration established in those studies8 and also to maintain equal crosslinking density across conditions. The theoretical crosslinking density was thus equalized across conditions by ensuring that the total molar concentration of PdBT macromers remained constant between each formulation. Furthermore, (Low) and (High) concentrations were designed to represent 50% and 100% molar functionalization of PdBT in hydrogels for all crosslinkers except CS/PdBT, which was limited to a maximum concentration of 2.6 mM due to the solubility limits of CS as a large glycosaminoglycan.
Table 1: Biofunctionalized crosslinker formulations investigated in the present study.
Distinct Low and High formulations represent varying biomolecule concentration. CS/PdBT was limited to a maximum concentration of 2.6 mM due to the solubility limit of CS as a high molecular weight sGAG.
| Crosslinker Formulation | Non-Functionalized PdBT Concentration (mM) | Click-Functionalized PdBT Concentration (mM) |
|---|---|---|
| PdBT | 25 | 0 |
| CS/PdBT (Low) | 23.7 | 1.3 |
| CS/PdBT (High) | 22.4 | 2.6 |
| NC/PdBT (Low) | 12.5 | 12.5 |
| NC/PdBT (High) | 0 | 25 |
| BMHP1/PdBT (Low) | 12.5 | 12.5 |
| BMHP1/PdBT (High) | 0 | 25 |
| GHK/PdBT (Low) | 12.5 | 12.5 |
| GHK/PdBT (High) | 0 | 25 |
To fabricate hydrogels, each crosslinker formulation was mixed on ice with P(NiPAAm-co-GMA) and MSCs in PBS to generate pre-hydrogel suspensions with a final concentration of 10% w/v P(NiPAAm-co-GMA) and 15,000,000 cells/mL. The suspensions were then injected at 30 μL into 6 mm diameter x 1 mm height cylindrical Teflon molds and moved to a humidified incubator at 37 °C and 5% CO2 to undergo gelation and crosslinking for 90 min, forming cell-laden hydrogels with in situ cues for chondrogenesis or osteogenesis.
In parallel studies, hydrogels were cultured in 1 mL of either chondrogenic medium (PdBT, CS/PdBT, NC/PdBT) or complete osteogenic medium (PdBT, BMHP1/PdBT, GHK/PdBT) over 35 days with replacement of media every 3 days. Chondrogenic medium contained DMEM supplemented with 1% v/v ITS+ Premix (6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 μg/mL selenous acid, 5.35 μg/mL linoleic acid and 1.25 μg/mL bovine serum albumin), 50 mg/L ascorbic acid, 10−7 M dexamethasone, and 1% v/v antibiotic-antimycotic,16 while complete osteogenic medium contained α-MEM supplemented with 10% v/v FBS, 50 mg/L ascorbic acid, 10−8 M dexamethasone, 10 mM β-glycerol 2-phosphate, and 1% v/v antibiotic-antimycotic.17 Tissue-specific extracellular matrix (ECM) deposition within the hydrogels was assessed via biochemical assays (n=3) and histological imaging (n=2), while chondrogenic and osteogenic gene expression were assessed by reverse transcription polymerase chain reaction (RT-PCR) (n=3).
2.5. Biochemical Assays
After 0, 3, 7, 14, 28, or 35 days of culture, biochemical assays were used to assess cell viability by DNA content (PicoGreen assay), cartilage-like matrix synthesis by sGAG content (DMMB assay), and bone-like matrix deposition by Ca2+ content (Arsenazo III assay).17,18 All data were normalized to acellular controls cultured under the same conditions and then expressed relative to hydrogel wet mass.16
Hydrogels were washed in PBS for 15 min at 37 °C and transferred to sterilized, pre-weighed polystyrene tubes, each containing a 5 mm stainless steel bead. Each hydrogel was weighed to determine its wet mass and then frozen at −20 °C until ready for characterization. Thawed samples were homogenized in 500 μL of sterile ultrapure H2O using a Qiagen TissueLyser II (Hilden, Germany) at 30 s−1 for 5 min. A 60 μL aliquot of each suspension was then mixed in 1:1 volume ratio with 1M acetic acid and incubated at room temperature for 16 hr to free Ca2+ ions for quantification using the Arsenazo III assay kit.17 The remaining 440 μL of homogenized sample suspension was then mixed in 1:1 volume ratio with an established digestion buffer containing 2 mg/mL proteinase K, 20 μg/mL pepstatin A, and 370 μg/mL iodoacetamide in tris–EDTA solution (12.11 mg/mL tris(hydroxymethyl aminomethane), 0.744 mg/mL EDTA, pH 7.6) to free DNA and sGAG content, and allowed to digest at 60 °C for 16 hr.18 The DNA and sGAG content of the digested samples were then quantified using PicoGreen and DMMB assay kits, respectively.
2.6. RNA Extraction and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
After 7 or 35 days of culture, hydrogels were washed in PBS for 15 min at 37 °C, transferred to sterilized polystyrene tubes, each containing a 5 mm stainless steel bead, and immediately frozen at −80 °C until ready for characterization. RNA was isolated from each hydrogel by a combination of TRIzol extraction and RNeasy mini kit purification.18 Hydrogels were homogenized in 500 μL each of TRIzol reagent using the Qiagen TissueLyser II at 30 s−1 for 5 min and then centrifuged at relative centrifugal force (RCF) of 16,000 g for 3 min to isolate supernatants. The supernatants were then mixed with 100 μL of chloroform and incubated for 2 min at room temperature, followed by centrifugation at RCF of 12,000 g for 15 min at 4 °C to induce phase separation. 250 μL of each aqueous phase containing RNA was then isolated, mixed with 250 μL of ethanol, and transferred to RNeasy spin columns for purification according to kit instructions.
To quantify baseline expression at the point of hydrogel fabrication, 1 μg of sample RNA from PdBT controls at 0 days of culture was reverse transcribed using the iScript cDNA Synthesis Kit. 2 μL of a 1:10 dilution of cDNA were analyzed for gene expression via RT-PCR, with a final primer concentration of 500 nM. Subsequent day 7 and 35 samples were analyzed using the same primer concentration as day 0, but instead using 2 μL of undiluted cDNA to ensure robust gene expression profiles. RT-PCR was performed using a Bio-Rad CFX96 Touch Real-Time PCR Detection System (Hercules, CA). All primers and corresponding genes are listed in Supplemental Table 1. All target gene expression was normalized to GAPDH and expressed as the fold change relative to baseline expression of the PdBT control at 0 days of culture. Fold changes in gene expression at days 7 and 35, representing early- and mid/late stage differentiation, were calculated using the ΔΔCt method.
2.7. Histological Sectioning and Imaging
After 35 days of culture, both acellular and cell-laden hydrogels were sectioned and histologically stained according to established protocols.16 Hydrogels were fixed in 1 mL each of 10% neutral buffered formalin for 24 hr at 37 °C, followed by dehydration in 1 mL of 70% ethanol for 24 hr at room temperature. The dehydrated hydrogels were then transferred to 10 mm x 10 mm x 5 mm cryomolds and immersed in OCT compound for 24 hr at room temperature, followed by freezing overnight at −20 °C. After freezing, a Leica CM1850 cryotome (Wetzlar, Germany) was used to acquire 10 μm sections that were mounted on glass slides and allowed to dry and bind overnight at room temperature. The mounted sections were sequentially stained by von Kossa, Alcian Blue, and Nuclear Fast Red according to kit instructions, followed by dehydration in a standard graded ethanol wash to xylene and attachment of coverslips using Cytoseal XYL. The stained sections were imaged using a Nikon Eclipse Ti2 (Tokyo, Japan) microscope at 10x magnification to show cell content (red/purple), cartilage-like GAG deposition (blue), and mineralization (brown/black).
2.8. Statistical Analysis
Data from biochemical assays and RT-PCR (n=3) were analyzed using two-way analysis of variance with post-hoc testing by Tukey’s honestly significant difference. All tests were performed at α=0.05.
3. Results
3.1. Biochemical Analysis of Tissue-Specific Hydrogels
Biofunctionalized, PdBT-crosslinked, and MSC-laden hydrogels were cultured in vitro for up to 35 days in either chondrogenic or osteogenic conditions and then assessed for their biochemical content to quantify cell viability and tissue-specific ECM deposition (Fig. 2).
Figure 2. Biochemical content of chondrogenic and osteogenic hydrogels cultured for 0–35 days, normalized to hydrogel wet mass.
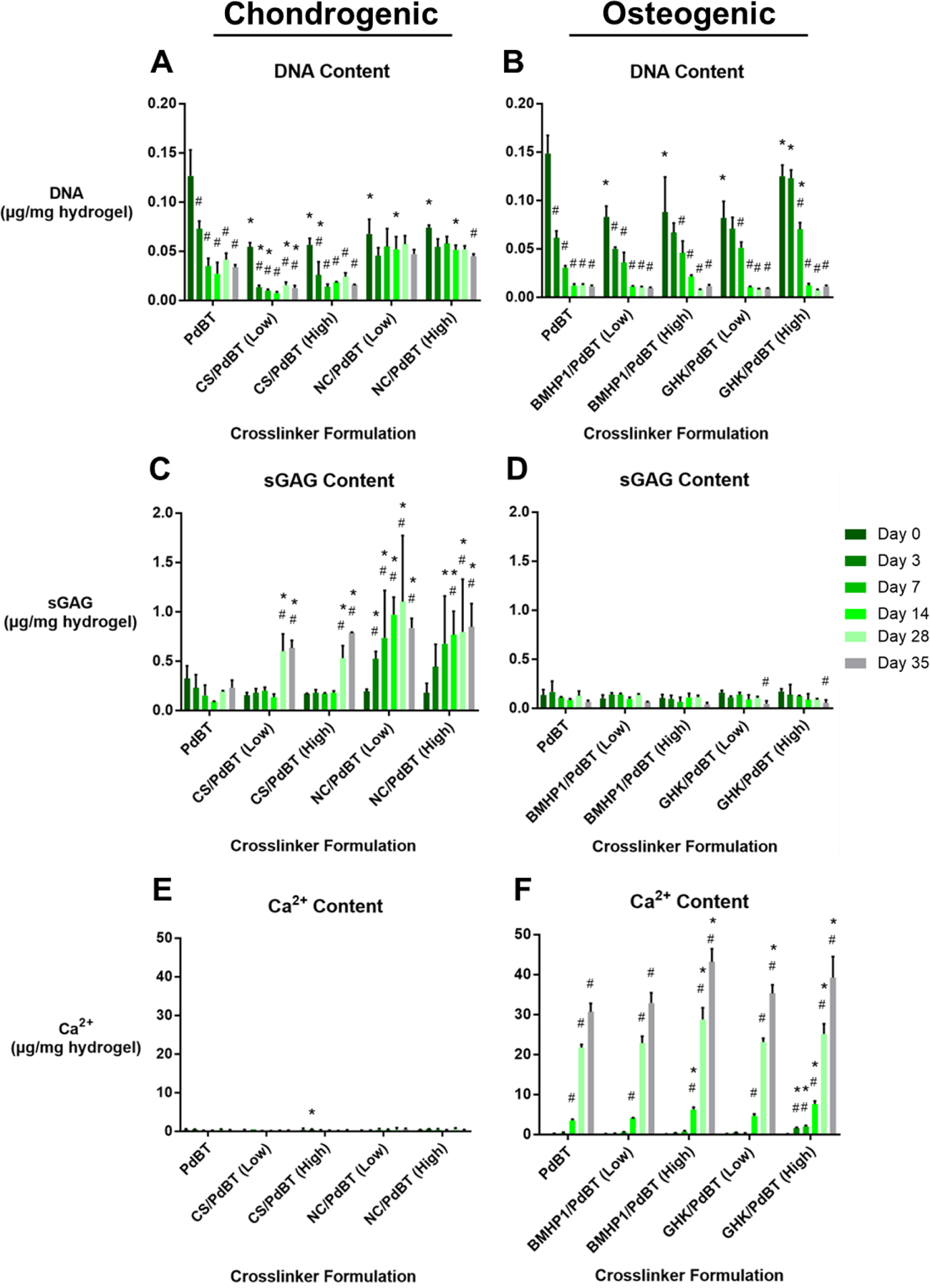
DNA content of (A) chondrogenic and (B) osteogenic hydrogels; sGAG content of (C) chondrogenic and (D) osteogenic hydrogels; and Ca2+ content of (E) chondrogenic and (F) osteogenic hydrogels. All data are reported as means ± standard deviation for a sample size of n=3. * indicates statistical significance compared to the biomolecule-free PdBT control at the same timepoint, # indicates significance compared to Day 0 of the same crosslinker formulation.
By day 7 and day 14 for the chondrogenic and osteogenic studies, respectively, normalized DNA content stabilized for all conditions (Fig. 2A, 2B). NC/PdBT (Low) and NC/PdBT (High), uniquely, showed no decrease in DNA content up to days 35 and 28, respectively, indicating preservation of cell viability and retention of MSCs within these hydrogels (Fig. 2A).
All CS/PdBT and NC/PdBT conditions showed statistically significant sGAG synthesis by day 28, with NC/PdBT (Low) initiating significant sGAG synthesis the earliest at day 3 (Fig. 2C). Both NC/PdBT conditions initiated sGAG synthesis earlier than either CS/PdBT condition as well. CS/PdBT and NC/PdBT hydrogels at all concentrations continued producing sGAG synthesis up to day 35, at which point they showed statistical significance compared to both the PdBT control and day 0 samples. Biomolecule-free PdBT hydrogels failed to produce any statistically significant sGAG deposition by day 35. Additionally, no conditions in the osteogenic study produced statistically significant sGAG deposition (Fig. 2D).
In the chondrogenic study, no conditions produced significant mineralization by day 35 (Fig. 2E), indicating no occurrence of hypertrophic calcification. In the osteogenic study, BMHP1/PdBT (Low) showed no significant differences in Ca2+ content compared to the biomolecule-free PdBT control, while BMHP1/PdBT (High) produced significantly greater Ca2+ deposition than the control starting at day 14 and continuing to day 35 (Fig. 2F). For GHK/PdBT, the (Low) formulation failed to produce significant improvements mineralization until day 35, while the (High) formulation promoted significant early mineralization starting at day 3 and continued enhancement of mineralization up to day 35.
3.2. Chondrogenic and Osteogenic Gene Expression
RT-PCR was used to characterize early (day 7) and mid/late stage (day 35) gene expression by MSCs (Fig. 3), using the early chondrogenic marker CDH2, mid/late stage chondrogenic marker SOX9, early/mid stage osteogenic marker RUNX2, and mid/late stage osteogenic marker OPN.
Figure 3. Fold changes in gene expression of encapsulated MSCs as determined by RT-PCR.

(A) Expression of early chondrogenic marker CDH2 and mid/late stage chondrogenic marker SOX9 by chondrogenic hydrogels. (B) Expression of early/mid stage osteogenic marker RUNX2 and mid/late stage osteogenic marker OPN by chondrogenic hydrogels. (C) Expression of chondrogenic markers by osteogenic hydrogels. (D) Expression of osteogenic markers by osteogenic hydrogels. Conditions with no bars had no detectable signal by 40 cycles of RT-PCR amplification. All data are reported as means ± standard deviation for a sample size of n=3. * indicates statistical significance compared to PdBT control at the same timepoint, # indicates significance between day 7 and day 35 of the same crosslinker.
NC/PdBT (Low) exhibited time-dependent up-regulation of chondrogenic markers, with significantly higher expression of CDH2 at day 7 and significantly higher expression of SOX9 at day 35 compared to the control (Fig. 3A). The PdBT control showed significant up-regulation of early chondrogenic marker CDH2 at day 35. Interestingly, CS/PdBT (Low), CS/PdBT (High), and NC/PdBT (High) exhibited lower expression of SOX9 at day 35 compared to PdBT, while NC/PdBT (High) demonstrated comparable CDH2 expression to PdBT. CS/PdBT and NC/PdBT conditions showed down-regulation of osteogenic markers RUNX2 and OPN at both timepoints, with undetectable RUNX2 signal by 40 cycles of amplification for almost all CS/PdBT and NC/PdBT conditions but not the PdBT control (Fig. 3B).
For the osteogenic hydrogels, all conditions showed comparable expression of chondrogenic markers at day 7, but BMHP1/PdBT (High) and GHK/PdBT (High) showed a significant drop in chondrogenic CDH2 expression at day 35 (Fig. 3C). Additionally, all conditions except for BMHP1/PdBT (High) and GHK/PdBT (High) showed an increase in chondrogenic SOX9 expression at day 35. In terms of osteogenic marker expression, BMHP1/PdBT (High) and both GHK/PdBT formulations showed significantly higher RUNX2 and OPN expression compared to the PdBT control at day 7, but these differences became non-significant at day 35 (Fig. 3D).
3.3. Histological Imaging of Hydrogels
Histological sections of all chondrogenic and osteogenic hydrogels were stained and imaged for cells (red/purple), sGAG deposition (blue), and mineralization (brown/black) after 35 days of culture to visualize ECM and assess tissue-specific matrix deposition (Fig. 4). Acellular hydrogels that were cultured under the same conditions were also stained as controls (Fig. S1), in which CS/PdBT hydrogels stained blue due to the presence of CS in the polymer network. For cell-laden hydrogels, extensive cartilage-like sGAG deposition was seen in all CS/PdBT and NC/PdBT conditions, but not in biomolecule-free PdBT hydrogels (Fig 4A). No visible mineralization was seen in chondrogenic hydrogels. For the osteogenic hydrogels, all sections showed only nominal sGAG staining (Fig. 4B). BMHP1/PdBT (Low) showed comparable mineralization to the PdBT control, with mineral deposition concentrated solely around the perimeter of cells. BMHP1/PdBT (High), GHK/PdBT (Low), and GHK/PdBT (High), on the other hand, showed mineralization distributed throughout the histological sections, with BMHP1/PdBT (High) and GHK/PdBT (High) in particular exhibiting notably dense brown/black staining of mineral content.
Figure 4. Histological imaging of 10 μm sections from MSC-laden hydrogels cultured for 35 days, showing cell content (red/purple), sGAG deposition (blue), and mineralization (brown/black).

(A) Chondrogenic hydrogels. (B) Osteogenic hydrogels. Scale bar represents 50 μm.
4. Discussion
Osteochondral defect repair remains challenging due to the necessity of regenerating bone and cartilage with distinct biochemical microenvironments, as well as the difficulty of repairing avascular and largely acellular cartilage.1 Our goal in this study was thus to develop a modular hydrogel system that could be easily tailored for chondrogenesis or osteogenesis by selection of click conjugated biochemical cues. To achieve this goal, we performed parallel in vitro studies investigating the promotion of chondrogenesis (CS/PdBT, NC/PdBT) and osteogenesis (BMHP1/PdBT, GHK/PdBT).
CS/PdBT and NC/PdBT successfully promoted cartilage-like matrix synthesis over 35 days of culture while avoiding hypertrophic calcification. Interestingly, NC/PdBT (Low), but not NC/PdBT (High), provided the earliest initiation of sGAG synthesis at day 3, which seems to be consistent with prior studies in the literature showing that the NC peptide’s promotion of chondrogenic processes can be inhibited above certain concentrations.11,19 Another positive effect observed with NC/PdBT hydrogels is their robust maintenance of cell viability over 35 days and in effect their ability to inhibit the typical diffusion and release of cells from hydrogel systems,16–18 which can likely be attributed to the cell-cell contact signals provided by the NC peptide.19 CS/PdBT hydrogels, on the other hand, may not support these early chondrogenic processes and cell-cell interactions as directly, and given their delayed onset of sGAG synthesis, may be more effective at promoting mid/late stage chondrogenic matrix deposition. The longer culture time required for promotion of sGAG synthesis by CS/PdBT is also consistent with other CS-based constructs, which traditionally show an onset around 14–28 days of culture.20–22 The lack of hypertrophic calcification within CS/PdBT and NC/PdBT hydrogels is beneficial given that many conventional methods of chondrogenic induction, such as transforming growth factor-β supplementation, produce unwanted conversion of hypertrophic chondrocytes to osteoblasts.23 Furthermore, both CS/PdBT and NC/PdBT inhibited gene expression associated with the osteogenic phenotype, while NC/PdBT (Low) in particular exhibited time-dependent up-regulation of the early and mid/late stage chondrogenic markers. This finding could imply an interesting positive feedback loop in which presentation of an N-cadherin peptide up-regulates expression of the N-cadherin protein (CDH2) itself, promoting early chondrogenic processes.19 CS/PdBT, on the other hand, showed comparable expression of chondrogenic markers to that of the control at day 7 but lower expression at day 35, and it is possible that the presence of concentrated, bulky CS chains may be inhibiting or sterically hindering the processes required for early chondrogenic differentiation such as MSC condensation. In many CS-based constructs, enzymatic digestion of CS is often utilized to reduce average molecular weight prior to usage in tissue engineering constructs,24,25 in contrast with CS used in the present study, which was left undigested at a native molecular weight of ~37 kDa. Optimization of CS molecular weight and concentration are thus needed to improve chondrogenic performance. Ultimately, the synthesis of cartilage-specific ECM by both CS/PdBT and NC/PdBT hydrogels was confirmed by histological imaging, and the overall data suggest that there is an optimum in the NC/PdBT content for promotion of chondrogenesis.
BMHP1/PdBT and GHK/PdBT hydrogels promoted osteogenesis most effectively at higher peptide concentrations. BMHP1/PdBT (Low), for instance, produced essentially no improvements to mineralization compared to the PdBT control over 35 days, as shown by biochemical analysis and histology, while BMHP1/PdBT (High) produced significant improvements to Ca2+ content from days 14 to 35 and visibly dense mineralization throughout histological sections on day 35. A positive correlation of peptide concentration to mineralization density is supported by prior applications of BMHP1, in which higher peptide density on 2D substrates promoted greater mineralization.12 GHK/PdBT demonstrated similar concentration-dependent effects, with the (High) formulation uniquely initiating early mineralization at day 3, in addition to producing greater final mineralization at day 35 and denser mineral staining in histological sections compared to the (Low) formulation. These results represent, to our knowledge, the first demonstration of concentration-dependent effects for the GHK peptide, as its prior utilization in the literature involved a single peptide concentration representing about 25% of the amount used in the GHK/PdBT (Low) formulation.13 Gene expression analysis at day 7 further supported the presence of concentration-dependent effects for the osteogenic hydrogels, with only PdBT and BMHP1/PdBT (Low) demonstrating undetectable early osteogenic RUNX2 expression. All other biofunctionalized conditions, including BMHP1/PdBT (High), produced detectable RUNX2 expression and significantly greater OPN expression compared to the PdBT control. Additionally, the non-detectable RUNX2 expression at day 35 across all conditions is expected given its nature as an earlier osteogenic marker, and the comparable levels of OPN expression across conditions at day 35 indicates that the given duration of culture is likely sufficient to induce commitment to an osteoblast-like phenotype by all MSCs regardless of hydrogel functionalization. Ultimately, PdBT bioconjugation successfully recapitulated the chondrogenic and osteogenic effects associated with each biomolecule.
While the promotion of tissue-specific development by CS, NC, BMHP1, and GHK functionalization was successfully established in the present study, this modular hydrogel system may be less effective for biomolecular cues that require cell internalization, which would require hydrolysis of PdBT to release biomolecules. While the hydrolysis rate of PdBT-crosslinked hydrogels can be modulated by both biomolecule selection and PdBT composition,8 further investigation is needed to assess the release rate and bioactivity of compounds delivered by PdBT network degradation. Furthermore, in vitro studies with culture of the bone- and cartilage-functionalized hydrogels in mixed osteochondral media could help elucidate how the click-conjugated cues influence cell differentiation in the presence of varied biochemical stimuli. A related point to consider is how cartilage- and bone-functionalized hydrogels may influence each other’s tissue development when in spatial proximity, i.e. within the osteochondral defect. In vivo studies of bilayered hydrogels with spatially distinct delivery of chondrogenic and osteogenic growth factors have established that the induction of adjacent cartilage and bone development can synergistically enhance subchondral bone repair, though the converse effects on cartilage growth are less clear.26 It can thus be speculated that the fabrication of bilayered, PdBT-crosslinked hydrogels could produce similar effects through paracrine signaling between bone- and cartilage-specific layers, though future investigations will be required to elucidate how bilayered effects could play out as each layer develops towards its click functionalized tissue type.
5. Conclusion
PdBT-crosslinked, tissue-specific hydrogels functionalized with various in situ biomolecular cues successfully promoted chondrogenesis and osteogenesis as desired. Furthermore, it was shown that the degree of hydrogel biofunctionalization, as determined by conjugated biomolecule concentration, influenced the extent of tissue-specific matrix-deposition and gene expression, with a lower NC concentration group promoting greater chondrogenic activity and higher BMHP1 and GHK concentration groups promoting greater osteogenic activity. Our findings demonstrate the versatility and efficacy of the biofunctionalized PdBT hydrogel system in promoting differentiation of two highly distinct tissues, establishing strong potential for its utilization in the osteochondral unit and other target tissues.
Supplementary Material
Acknowledgments:
We acknowledge support by the National Institutes of Health (R01 AR068073). G.B. acknowledges support by the Cancer Prevention and Research Institute of Texas (CPRIT) (RR14008 and RP170721). B.T.S. and E.W. acknowledge support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (F30 AR071258) and National Institute of Dental and Craniofacial Research (F31 DE027586), respectively.
References:
- (1).Cohen DL; Lipton JI; Bonassar LJ; Lipson H Additive Manufacturing for in Situ Repair of Osteochondral Defects. Biofabrication 2010, 2 (3), 035004. [DOI] [PubMed] [Google Scholar]
- (2).Clearfield D; Nguyen A; Wei M Biomimetic Multidirectional Scaffolds for Zonal Osteochondral Tissue Engineering via a Lyophilization Bonding Approach. Journal of Biomedical Materials Research Part A 2018, 106 (4), 948–958. 10.1002/jbm.a.36288. [DOI] [PubMed] [Google Scholar]
- (3).Jiang L; Wang Y; Liu Z; Ma C; Yan H; Xu N; Gang F; Wang X; Zhao L; Sun X Three-Dimensional Printing and Injectable Conductive Hydrogels for Tissue Engineering Application. Tissue Engineering Part B: Reviews 2019, 25 (5), 398–411. [DOI] [PubMed] [Google Scholar]
- (4).Gang F; Yan H; Ma C; Jiang L; Gu Y; Liu Z; Zhao L; Wang X; Zhang J; Sun X Robust Magnetic Double-Network Hydrogels with Self-Healing, MR Imaging, Cytocompatibility and 3D Printability. Chemical Communications 2019, 55 (66), 9801–9804. [DOI] [PubMed] [Google Scholar]
- (5).Bittner SM; Guo JL; Mikos AG Spatiotemporal Control of Growth Factors in Three-Dimensional Printed Scaffolds. Bioprinting 2018, 12, e00032. 10.1016/j.bprint.2018.e00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Guo JL; Kim YS; Mikos AG Biomacromolecules for Tissue Engineering: Emerging Biomimetic Strategies. Biomacromolecules 2019, 20 (8), 2904–2912. 10.1021/acs.biomac.9b00792. [DOI] [PubMed] [Google Scholar]
- (7).Park SH; Seo JY; Park JY; Ji YB; Kim K; Choi HS; Choi S; Kim JH; Min BH; Kim MS An Injectable, Click-Crosslinked, Cytomodulin-Modified Hyaluronic Acid Hydrogel for Cartilage Tissue Engineering. NPG Asia Mater 2019, 11 (1), 1–16. 10.1038/s41427-019-0130-1. [DOI] [Google Scholar]
- (8).Guo JL; Kim YS; Xie VY; Smith BT; Watson E; Lam J; Pearce HA; Engel PS; Mikos AG Modular, Tissue-Specific, and Biodegradable Hydrogel Cross-Linkers for Tissue Engineering. Science Advances 2019, 5 (6). 10.1126/sciadv.aaw7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Klouda L; Mikos AG Thermoresponsive Hydrogels in Biomedical Applications. European Journal of Pharmaceutics and Biopharmaceutics 2008, 68 (1), 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Muzzarelli RA; Greco F; Busilacchi A; Sollazzo V; Gigante A Chitosan, Hyaluronan and Chondroitin Sulfate in Tissue Engineering for Cartilage Regeneration: A Review. Carbohydrate polymers 2012, 89 (3), 723–739. [DOI] [PubMed] [Google Scholar]
- (11).Bian L; Guvendiren M; Mauck RL; Burdick JA Hydrogels That Mimic Developmentally Relevant Matrix and N-Cadherin Interactions Enhance MSC Chondrogenesis. Proceedings of the National Academy of Sciences 2013, 110 (25), 10117–10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Cao F-Y; Yin W-N; Fan J-X; Zhuo R-X; Zhang X-Z A Novel Function of BMHP1 and CBMHP1 Peptides to Induce the Osteogenic Differentiation of Mesenchymal Stem Cells. Biomaterials science 2015, 3 (2), 345–351. [DOI] [PubMed] [Google Scholar]
- (13).Klontzas ME; Reakasame S; Silva R; Morais JC; Vernardis S; MacFarlane RJ; Heliotis M; Tsiridis E; Panoskaltsis N; Boccaccini AR Oxidized Alginate Hydrogels with the GHK Peptide Enhance Cord Blood Mesenchymal Stem Cell Osteogenesis: A Paradigm for Metabolomics-Based Evaluation of Biomaterial Design. Acta biomaterialia 2019, 88, 224–240. [DOI] [PubMed] [Google Scholar]
- (14).Aulisa L; Dong H; Hartgerink JD Self-Assembly of Multidomain Peptides: Sequence Variation Allows Control over Cross-Linking and Viscoelasticity. Biomacromolecules 2009, 10 (9), 2694–2698. [DOI] [PubMed] [Google Scholar]
- (15).Ekenseair AK; Boere KWM; Tzouanas SN; Vo TN; Kasper FK; Mikos AG Synthesis and Characterization of Thermally and Chemically Gelling Injectable Hydrogels for Tissue Engineering. Biomacromolecules 2012, 13 (6), 1908–1915. 10.1021/bm300429e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lam J; Clark EC; Fong ELS; Lee EJ; Lu S; Tabata Y; Mikos AG Evaluation of Cell-Laden Polyelectrolyte Hydrogels Incorporating Poly(l-Lysine) for Applications in Cartilage Tissue Engineering. Biomaterials 2016, 83, 332–346. 10.1016/j.biomaterials.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Vo TN; Shah SR; Lu S; Tatara AM; Lee EJ; Roh TT; Tabata Y; Mikos AG Injectable Dual-Gelling Cell-Laden Composite Hydrogels for Bone Tissue Engineering. Biomaterials 2016, 83, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Lu S; Lee EJ; Lam J; Tabata Y; Mikos AG Evaluation of Gelatin Microparticles as Adherent-Substrates for Mesenchymal Stem Cells in a Hydrogel Composite. Annals of biomedical engineering 2016, 44 (6), 1894–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).DeLise AM; Tuan RS Alterations in the Spatiotemporal Expression Pattern and Function of N‐cadherin Inhibit Cellular Condensation and Chondrogenesis of Limb Mesenchymal Cells in Vitro. Journal of cellular biochemistry 2002, 87 (3), 342–359. [DOI] [PubMed] [Google Scholar]
- (20).Agrawal P; Pramanik K Enhanced Chondrogenic Differentiation of Human Mesenchymal Stem Cells in Silk Fibroin/Chitosan/Glycosaminoglycan Scaffolds under Dynamic Culture Condition. Differentiation 2019, 110, 36–48. 10.1016/j.diff.2019.09.004. [DOI] [PubMed] [Google Scholar]
- (21).Sawatjui N; Damrongrungruang T; Leeanansaksiri W; Jearanaikoon P; Hongeng S; Limpaiboon T Silk Fibroin/Gelatin–Chondroitin Sulfate–Hyaluronic Acid Effectively Enhances in Vitro Chondrogenesis of Bone Marrow Mesenchymal Stem Cells. Materials Science and Engineering: C 2015, 52, 90–96. 10.1016/j.msec.2015.03.043. [DOI] [PubMed] [Google Scholar]
- (22).Meghdadi M; Pezeshki-Modaress M; Irani S; Atyabi SM; Zandi M Chondroitin Sulfate Immobilized PCL Nanofibers Enhance Chondrogenic Differentiation of Mesenchymal Stem Cells. International Journal of Biological Macromolecules 2019, 136, 616–624. 10.1016/j.ijbiomac.2019.06.061. [DOI] [PubMed] [Google Scholar]
- (23).Deng Y; Lei G; Lin Z; Yang Y; Lin H; Tuan RS Engineering Hyaline Cartilage from Mesenchymal Stem Cells with Low Hypertrophy Potential via Modulation of Culture Conditions and Wnt/β-Catenin Pathway. Biomaterials 2019, 192, 569–578. 10.1016/j.biomaterials.2018.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Chen Y-L; Lee H-P; Chan H-Y; Sung L-Y; Chen H-C; Hu Y-C Composite Chondroitin-6-Sulfate/Dermatan Sulfate/Chitosan Scaffolds for Cartilage Tissue Engineering. Biomaterials 2007, 28 (14), 2294–2305. 10.1016/j.biomaterials.2007.01.027. [DOI] [PubMed] [Google Scholar]
- (25).van Susante JLC; Pieper J; Buma P; van Kuppevelt TH; van Beuningen H; van der Kraan PM; Veerkamp JH; van den Berg WB; Veth RPH Linkage of Chondroitin-Sulfate to Type I Collagen Scaffolds Stimulates the Bioactivity of Seeded Chondrocytes in Vitro. Biomaterials 2001, 22 (17), 2359–2369. 10.1016/S0142-9612(00)00423-3. [DOI] [PubMed] [Google Scholar]
- (26).Lu S; Lam J; Trachtenberg JE; Lee EJ; Seyednejad H; van den Beucken JJJP; Tabata Y; Wong ME; Jansen JA; Mikos AG; et al. Dual Growth Factor Delivery from Bilayered, Biodegradable Hydrogel Composites for Spatially-Guided Osteochondral Tissue Repair. Biomaterials 2014, 35 (31), 8829–8839. 10.1016/j.biomaterials.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Cao L; Yang F; Liu G; Yu D; Li H; Fan Q; Gan Y; Tang T; Dai K The Promotion of Cartilage Defect Repair Using Adenovirus Mediated Sox9 Gene Transfer of Rabbit Bone Marrow Mesenchymal Stem Cells. Biomaterials 2011, 32 (16), 3910–3920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


