Abstract
Cardiovascular stents are life-saving devices and one of the top 10 medical breakthroughs of the 21st century. Decades of research and clinical trials have taught us about the effects of material (metal or polymer), design (geometry, strut thickness, and the number of connectors), and drug-elution on vasculature mechanics, hemocompatibility, biocompatibility, and patient health. Recently developed novel bioresorbable stents are intended to overcome common issues of chronic inflammation, in-stent restenosis, and stent thrombosis associated with permanent stents, but there is still much to learn. Increased knowledge and advanced methods in material processing have led to new stent formulations aimed at improving the performance of their predecessors but often comes with potential tradeoffs. This review aims to discuss the advantages and disadvantages of stent material interactions with the host within five areas of contrasting characteristics, such as 1) metal or polymer, 2) bioresorbable or permanent, 3) drug elution or no drug elution, 4) bare or surface-modified, and 5) self-expanding or balloon-expanding perspectives, as they relate to pre-clinical and clinical outcomes and concludes with directions for future studies.
Keywords: Stent, Bare-metal, Drug-eluting, Bioresorbable, Surface-functionalized, Self-expanding
Graphical Abstract
As materials processing and knowledge increase, newer stents have been developed to meet the needs of patient health. A contrast of these materials shows bare-metal stents suffer from restenosis, DES suffer from strut protrusion/malapposition, bioresorbable materials can suffer from weak mechanical properties, surface-functionalization may risk late complications from permanent caging, and self-expanding stents are not suitable for heavily calcified plaques.

1. Introduction
One of the greatest medical breakthroughs in recent history is the development of the stent to treat coronary artery disease. This deadly disease claims the life of every 3 out of 10 people in the US and this number continues to rise, making it the leading cause of death.[1] The vascular disease culminates in a buildup of fatty plaque, thickening the vessel which reduces blood flow upstream of the constriction. Charles Dotter pioneered the invention of balloon angioplasty, and Andreas Grüntzig expanded this treatment to include very narrow arteries. Percutaneous coronary intervention (PCI) combines balloon angioplasty and stenting as a primary treatment for vascular disease; Julio Palmaz had the idea to fit a stent over a balloon catheter which is then moved percutaneously through the vascular system to the site of the blockage. Once inside the blockage, the stent is deployed by inflating the balloon. The expanded stent locks in place, forming a scaffold to support the local endothelium, the lining of the blood vessel. Jacques Puel was the first to implant a stent in man in 1986. Figure 1 shows five different categories of stents and their respective implications. Initial stents from the 1980s and 1990s were primarily bare-metal stents (BMS) composed of stainless steel (SS) or cobalt-chromium (CoCr) alloys typically. Due to their increased strength, the CoCr alloys were able to reduce strut thickness. While both alloy systems provided robust mechanical support, a new problem of neointimal hyperplasia emerged (Figure 1a).[2] To overcome this issue, Patrick Serruys developed the first generation drug-eluting stents (DES) which reduced in-stent restenosis (ISR) by approximately 60–80%.[2] The eluting drug was an anti-mitotic agent that inhibited the proliferation of smooth muscle cells (SMCs). An inadvertent issue with these new stents was impaired endothelial regeneration and vasomotion, which increased the occurrence of late stent thrombosis (ST) (Figure 1b).[3] Second-generation DES resulted in reduced strut thickness, offered greater flexibility/deliverability, and exhibited better polymer biocompatibility;[4–5] however, long-term chronic inflammation and very late ST remains a serious concern.
Figure 1.
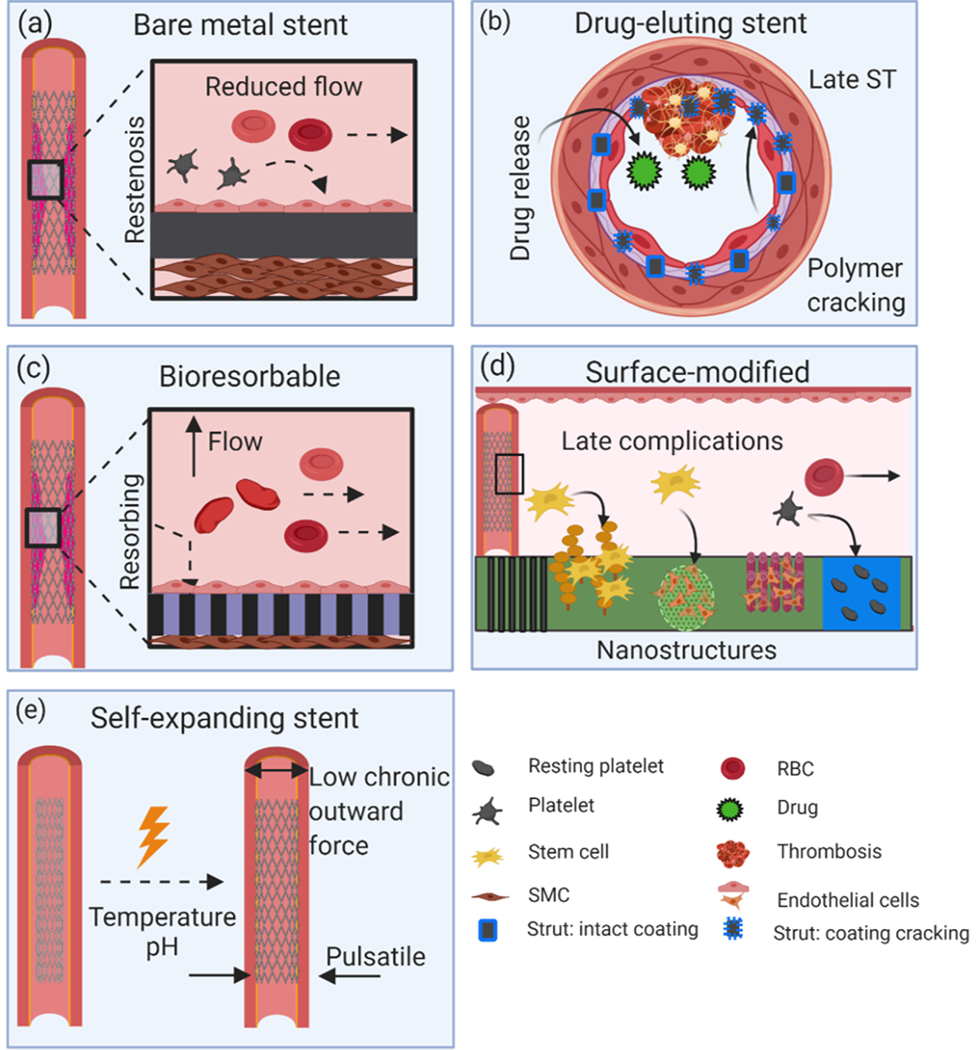
Mechanisms underlying the in vivo vascular responses of balloon-expanding and self-expanding stents. (a) Neointima hyperplasia response of BMS, (b) problems of protrusion, malapposition, and late ST of DES, (c) vascular healing from implantation of a bioresorbable stent as a result of its disappearance, (d) versatile responses of surface-modified stents, and (e) application of self-expanding stents suitable for peripheral or cerebrovascular areas due to their highly tortuous path and pulsatile forces.
To solve problems associated with BMS and DES, bioresorbable polymeric and metallic stents have recently emerged. Again, Patrick Serruys pioneered the invention of the bioresorbable stent (BRS). The premise of a BRS is that the material will be resorbed over 6 months to 2 years, eliminating long-term chronic inflammation and enabling endothelial regeneration (Figure 1c). Ideally, the material will maintain its mechanical properties for at least 6 months. Bioresorbable polymers derived from lactic acid and metals such as Fe, Mg, and Zn have been proposed as potential candidates for this task. Several clinical stents have received Conformité Européenne (CE) approval such as the Absorb bioresorbable vascular scaffolds (BVS) from Abbot Vascular in 2011, the DESolve stent from Elixir Medical Corporation in 2013, and Biotronik’s Mg-based Magmaris stent in 2016, in addition to the peripheral Igaki-Tamari stent developed by Kyoto Medical Planning, Co, Ltd.[6] The Food and Drug Administration (FDA) approved the Absorb BVS in 2016; however, recent reports from the AIDA trial and several ABSORB trials indicated a higher incidence of device thrombosis which led to several safety warnings.[6] In 2017 Abbott Vascular announced that it would stop sales of this stent due to low usage rates and sales. Surface-modified stents, including surface coatings, nano/micro texturing, patterned arrays, or other methods which alter the surface chemistry, can be either permanent where the presence of a permanent metallic cage can create late complications, or bioresorbable which can initiate an early healing response and gradually disappear over time (Figure 1d). Self-expanding stents are better for peripheral or cerebrovascular areas where the vessel path to route the stent is highly tortuous and subjected to large pulsatile forces which require much less stent chronic outward force (Figure 1e).
Several differences exist between permanent metallic, drug-eluting, and BRS. Each type has its advantages and disadvantages. This review is divided into several sections. Firstly, the mechanical properties of each type will be discussed as they relate to stent design and geometry. Secondly, permanent stents will be compared to BRS in terms of tissue reactions and endothelial restoration. Thirdly, the concept of drug-eluting and non-eluting stents will be contrasted. Fourthly, surface-functionalization to improve the integration of the stent with the endothelium will be discussed, and finally, the review will conclude with the benefits of self-expanding (SX) shape memory alloys (SMAs) vs balloon-expanding (BX) stents.
2. Metallic or polymeric stents: mechanical considerations
There are minimal mechanical requirements to be met which serve to support the endothelium during tissue healing while preventing elastic recoil during balloon expansion. The 316LSS is the benchmark standard by which other materials are compared. Generally, a relatively low yield strength (YS) of ~200 MPa, high ultimate tensile strength (UTS) > 300 MPa, and high Young’s modulus (E) are needed to minimize elastic recoil to below < 4% on expansion.[2, 7] Elongation is recommended to be between 15–18%, although even higher is preferred; however, this increase in elongation usually comes with a tradeoff in reduction of YS and UTS. Therefore, a balance is typically necessary. Attempts to achieve these properties while improving stent deliverability, which is dependent on a low-profile design, stent flexibility, radial strength, open/closed cells, and longitudinal forces,[8] have led to some complications. For example, reducing strut thickness and connector links while adopting a helical arrangement improves flexibility and allows longitudinal stent segments to change their length and adjust to the tortuous blood vessel path;[9] however, this flexibility increases susceptibility to deformation when forces are applied longitudinally.[9] Increased susceptibility, which initiates deformation, can lead to strut overlap and separation, malapposition, and even vessel obstruction.[10]
2.1. Permanent metallics
Figure 2 highlights several factors that need to be considered when designing a stent such as material, geometry, and metal microstructure (Figure 2a) and examples of mechanical data taken from different studies. Metals are inherently stronger than polymers so thinner struts can be used while maximizing mechanical strength,[11] as demonstrated with many CoCr alloy stents.[12] A list of permanent and bioresorbable stents that advanced to the clinical level or in pre-clinical stages are provided in Table 1. Thinner struts also induce less tissue injury and improve stent integration.[11]
Figure 2.
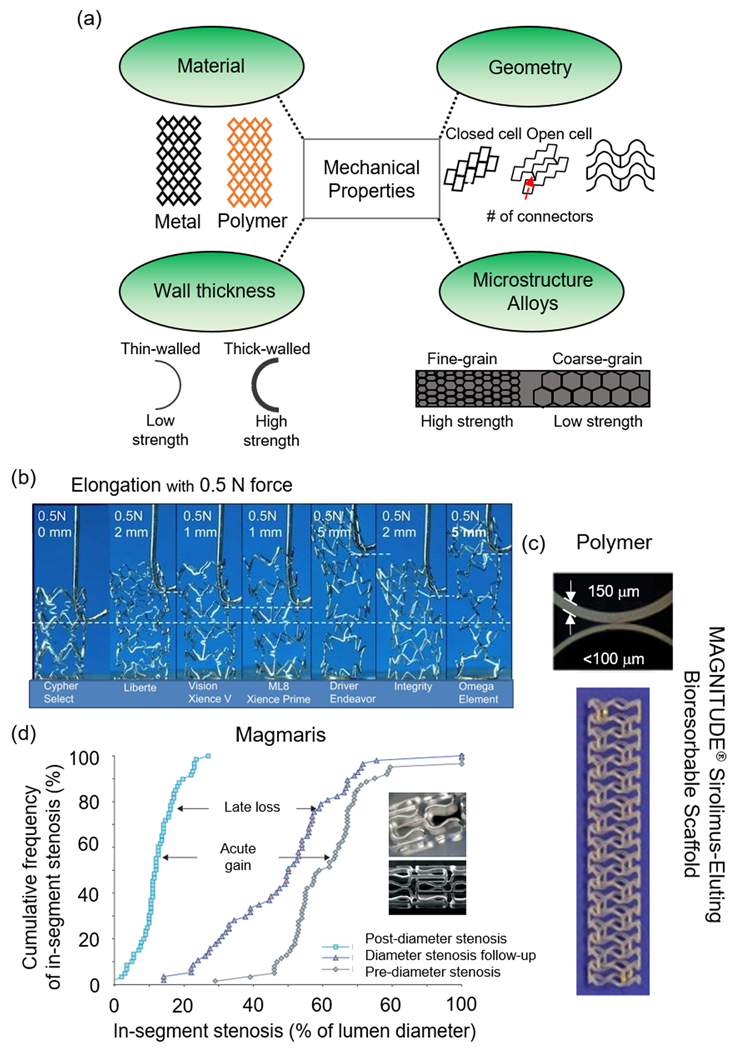
Mechanical properties of PM, polymer, and bioresorbable metal stents. (a) Material, geometric, and microstructural factors to consider when designing stents, (b) elongation testing mechanism of several clinical stents,[10] (c) thin-walled Magnitude stent vs. a thicker-walled polymer stent,[54] and (d) in-segment stenosis of the Magmaris stent.[22] (b) Reproduced with permission.[10] Copyright 2011, Elsevier. (c) Adapted with permission.[54] Copyright 2019, Elsevier. (d) Adapted with permission.[22] Copyright 2007, Elsevier.
Table 1.
| Stent | Clinical Study | Manufacturer | Platform Material | Strut thickness (μm) | Coating | Drug | Late lumen loss (mm) | Resorption time (month) |
|---|---|---|---|---|---|---|---|---|
| Metallic | ||||||||
| Cypher | SIRIUS[15] | Cordis | Bx-Velocity SS | 140 | PEVA/PMBA | Sirolimus | 0.17 | - |
| Taxus Express | TAXUS-IV[16] | Boston Scientific | Express/SS | 132 | SIBS | Paclitaxel | 0.39 | - |
| Promus Element | EVOLVE[17] | Boston Scientific | Omega/PtCr | 81 | PBMA, PVDF-HFP | Everolimus | 0.15 | - |
| Resolute | Resolute All Comers[18] | Medtronic | Driver/ MP35N® CoCr | 91 | BioLinx | Zotarolimus | 0.27 | - |
| BioMatrix | [19] | Biosensors | Gazelle/SS | 112 | PLA | Biolimus | 0.13 | - |
| Endeavor | ENDEAVOR II[20] | Medtronic | MP35N® CoCr | 91 | PC | Zotarolimus | 0.61 | - |
| Xience-V | SPIRIT III[21] | Abbot Vascular | Vision/ L605® CoCr | 81 | PBMA, PVDF-HFP | Everolimus | 0.16 | - |
| Bioresorbable | ||||||||
| AMS 1.0 | PROGRESS-AIMS[22] | Biotronik | Mg alloy | 165 | None | None | 1.08 | <4 |
| DREAMS-1 | BIOSOLVE-I[23] | Biotronik | Mg alloy | 120 | PLGA | Paclitaxel | 0.64 | 9 |
| DREAMS-2 | BIOSOLVE-2[24] | Biotronik | Mg alloy | 150 | PLLA | Sirolimus | 0.39 | 9 |
| Unity | Pre-clinical | QualiMed | Mg | 140 | PLLA | Sirolimus | - | 12–24 |
(PEVA) polyethylene-co-vinyl acetate; (PMBA) poly (n-butyl methacrylate); (SIBS) Poly (styrene-b-isobutylene-b-styrene); (PVDF) poly-vinylidene fluoride; (HFP) hexafluoropropylene; (PC) phosphorylcholine; (PLLA) poly L-lactic acid; (PLGA) poly(lactic-co-glycolic acid)
In one study, the Cypher Select (Cordis), Liberte (Boston Scientific), Vision (Abbott Vascular), Multilink 8 (Abbott Vascular), Driver (Medtronic), Integrity (Medtronic), and the Omega (Boston Scientific), seven clinically successful stents, were compared for longitudinal deformations via compression and extension experiments (Figure 2b).[10] Their results concluded that stents with two connectors (Omega and Driver) required significantly less force to compress and extend leading to longitudinal stent deformation, which can be confused with stent fracture.[10] In a separate study, a custom CoCr alloy stent, denoted as the Chonnan National University Hospital (CNUH), was developed through manipulation of stent geometry and compared to the PROMUS Element™, the Cypher®, Resolute Integrity, and the Xience PRIME.[25] The CNUH stent showed the lowest radial force (2.40 N) by flat plate and segmental compression as compared to the PROMUS Element™ (2.89 N), the Cypher® (3.89 N), Resolute Integrity (3.71 N), and the Xience PRIME (2.73 N), but exhibited the greatest flexibility during 3-point bending (0.47 N to initiate bending), due to the addition of two struts to enhance flexibility and inhibit shear stress.[25] Similarly, elastic recoil was lower in the CNUH stent as compared to the commercial stents.[25]
Early stage 316L SS stents suffered from low radiopacity when thinner struts (130–140 μm) were deemed necessary to overcome the problems of restenosis. This led to several CoCr alloy formulations such as the L605 (Co-20Cr-15W-10Ni) and the MP35N® (Co-20Cr-35Ni-10Mo).[26] However, some groups sought to improve the radiopacity of 316SS through the incorporation of Pt and new alloy formulations. The incorporation of 33 wt. % Pt to 316L (replacing primarily the Fe and Ni content) increased its tensile strength from 595 MPa to 834 MPa and exhibited greater YS (480 MPa) than 316L (275 MPa) and MP35N (414 MPa) but similar to L605 (500 MPa).[26] However, density increased to 9.9 g cm−3 which is reflected in higher radiopacity, suggesting the 33 wt. % Pt 316L alloy is better overall, though more expensive.[26] Another group has created a Ni-free Co alloy (Co-20Cr-12Fe-18Mn-2Mo-4W-N) with substitute additions of Fe and Mn with improved YS (577.3 MPa) and elongation (55.8%) as compared to commercial L605;[27] furthermore, the incorporation of 0.41 wt. % N improved corrosion resistance.[27] A series of Nb-xTa-2Zr (x = 30, 40, 50, 60, and 70 wt. %) alloys were created to improve magnetic susceptibility, a common problem encountered in traditional CoCr alloy and 316LSS stents. The increase in Ta content produced lower volume magnetic susceptibility but increased E linearly and YS (330 MPa) with little change in elongation (24%).[28]
2.2. Polymers
There is no question permanent metallic implants provide ideal mechanical support but clinical problems of in-stent restenosis, neointimal hyperplasia, and ST are serious concerns.[1–2, 29] These dire problems have directed researchers toward alternative materials such as polymers. Several polymer-based stents have been studied and over the years, many advanced to the clinical level such as the Absorb BVS and the Igaki-Tamai®, both made from poly L-lactic acid (PLLA).
Three primary shortcomings of polymer-based stents are their lack or radiopacity, reduced radial force, and lack of material plasticity.[30] A list of polymeric stents which advanced to the clinical setting or are in pre-clinical stages are provided in Table 2. Because polymers are so much weaker than metals, they lack the necessary radial strength and inevitably undergo elastic recoil following balloon expansion, so strut thickness must be increased and geometry must be optimized.[31] To further exacerbate this issue, polymer chains under hydrolysis and cleavage reduces the molecular weight and strength. Stent manufacturers have dealt with this issue by increasing the molecular weight on the luminal layer with a gradual decrease to the abluminal side, resulting in increased degradation kinetics from luminal to abluminal layers.[1] The degradation rate for PLLA is on the order of ~2–3 years.[1–2] Ultimately, the degradation products (e.g. lactic acid) can be metabolized by the Kreb’s cycle with the release of water H2O and carbon dioxide CO2.[30]
Table 2.
| Stent | Clinical Study | Manufacturer | Platform Material | Strut thickness (μm) | Coating | Drug | Late lumen loss (mm) | Resorption time (month) |
|---|---|---|---|---|---|---|---|---|
| Igaki-Tamai | Igaki-Tamai[33] | Kyoto Medical | PLLA | 170 | None | None | 0.48 | 24 |
| BVS 1.0 | FIM Cohort A[34] | Abbot Vascular | PLLA | 150 | PDLLA | Everolimus | 0.44 | 18–24 |
| BVS 1.1 | ABSORB Cohort B[35] | Abbot Vascular | PLLA | 150 | PDLLA | Everolimus | 0.19 | 18–24 |
| DESolve | DESolve 1[36] | Elixir | PLLA | 150 | None | Myolimus | 0.19 | 12–24 |
| DESolve NX[37] | Elixir | PLLA | 150 | None | Novolimus | 0.20 | 12–24 | |
| Xinsorb | FIM[38] | Huaan Biotech | PLLA | 160 | - | Sirolimus | 0.18 | - |
| ART 18AZ | ARTDIVA[39] | ART | PDLLA | 170 | None | None | - | 3–6 |
| REVA | RESORB[40] | REVA Medical | PTD-PC | 200 | None | None | 1.81 | 24 |
| ReZolve | RESTORE[41] | REVA Medical | PTD-PC | 115–230 | None | Sirolimus | 0.29 | 4–6 |
| Fantom | FANTOM 1[42] | REVA Medical | PTD-PC | 114–128 | None | Sirolimus | 0.21 | 24 |
| Fantom II | FANTOM II[43] | REVA Medical | PTD-PC | 125 | None | Sirolimus | 0.25 | 36–48 |
| IDEAL BTI | WHISPER[44] | Xenogenics | Polylactide & salicylates | 200 | Salicylate | Sirolimus | - | 6–9 |
| Ideal BioStent | Pre-clinical[45] | Xenogenics | SA/AA | 175 | Salicylate | Sirolimus | - | 12 |
| Fortitude | Pre-clinical[46] | Amaranth | PLLA | 150–200 | None | None | - | 3–6 |
| Mirage | FIM[47] | Manli Cardiology | PLLA | 125–150 | PLLA | Sirolimus | 0.37 | 14 |
| MeRes100 | MeRes-1 FIM[48] | Meril Life Sciences Pvt. | PLLA | 100 | PDLLA | Sirolimus | 0.13 | 24–36 |
(PLLA) poly L-lactic acid; (PDLLA) poly DL-lactic acid; (SA) salicylic acid; (AA) adipic acid; (PTD-PC) poly-tyrosine-derived polycarbonate
A recent study fabricated an electrospun composite fiber coating of PLA, chitosan, and paclitaxel (PTX) on a PLA stent, and the tensile strength of the optimized drug concentrations (PTX 40- and PTX-60%) reached 7–10 MPa with elongations between 52–65%. Interestingly, PTX increased the stiffness of the stent but reduced elasticity.[49] In a separate study, Novosorb™ polyurethane (PU) block polymers were added to polyester in increasing amounts (60–100 wt. %), and the mechanical analysis showed increases in tensile strength (22 to 53 MPa), YS (12 to 35 MPa), and modulus (652 to 1504 MPa) alongside PU concentration.[50] Another group constructed polymer films composed of PLLA, poly(3-hydroxybutyrate; P(3HB)), poly(4-hydroxybutyrate; P(4HB)), and a PLLA/P(4HB) 78/22% (w/w) blend and looked at cellular responses which are mentioned in section 3.2.[3] Unfortunately, no mechanical data was investigated. Smart stents which utilize microelectromechanical systems (MEMS) wireless pressure sensors derived from polycaprolactone (PCL) have also been described.[51–53] Similar to metals, increases in strength can be obtained by increasing strut thickness but risks of adverse clinical outcomes become more prevalent; however, the Magnitude-BRS with a wall thickness of 98 μm displayed superior biomechanical performance as compared to other leading high-wall thickness polymer-based stents like the Fortitude, Aptitude, and the Absorb BVS (Figure 2c).[54] Reva Medical’s newest Fantom Encore stent reduced the strut thickness and crossing profile from 125 μm and 1.35 mm on the Fantom to 95 μm and 1.3 mm, while maintaining elastic recoil of 2% and radial strength of 0.22 N mm−2.[55]
2.3. Bioresorbable metals
Similar to polymer-based stents, a stent can also be made from bioresorbable metals such as Fe, Mg, and Zn. The appeal for bioresorbable metals is their enhanced strength, and they are essential for the human body, serving hundreds of enzymes and assisting in cellular functions throughout the body, unlike permanent metallic and polymer-based stents. Due to a lack of pre-existing and well-characterized FDA-approved materials, preclinical/clinical progress in this area has been relatively slow, though new materials are being developed quickly. Important considerations here are mechanical properties and corrosion rate. Unlike the simple hydrolysis of polymers, metals can corrode via complex mechanisms, producing a wide variety of products which differ from in vitro and in vivo conditions.[56] In terms of degradation rates, the standard electrode potential of Mg (−2.37 V SCE−1) is much lower than Zn (−0.76 V SCE−1) and Fe (−0.44 V SCE−1).[57–58] This translates to the rapid degradation of Mg, slower degradation of Fe, and intermediate corrosion of Zn. Like polymers, metals can be strengthened through a variety of mechanisms, but the most common method of strengthening is by alloying with small amounts of other bioresorbable elements and/or by forming processes such as forging, rolling, and/or extrusion.
Decades of early research investigated the potential of Fe-based stents, where several reports indicated no significant inflammatory responses, neointimal hyperplasia or toxicity,[59–61] but the resorption period was too slow and hard-to-eliminate voluminous corrosion products which interfere with neighboring cells and matrix production;[56, 62] however, the corrosion rate and strength can be manipulated through grain size refinement,[63] alloying with Mn (Fe-35Mn),[64] and additions of Ag to Fe-30Mn alloys (Fe-30Mn-XAg, X = 1 or 3).[65] Furthermore, a 70 μm strut, nitrided iron stent (0.074 wt% N) exhibited a radial strength of 171 kPa with 2.21 % recoil, surpassing the clinical benchmarks of the XIENCE Prime™, Absorb GT1™, and Magmaris.[66]
To date, the only reported cases of bioresorbable metals advancing to clinical trials are of Mg-based alloys fabricated by Biotronik. Their initial stent (AMS 1.0) degraded within 60 days and was 93 wt. % Mg and ~7 wt. % rare earth elements.[13] The PROGRESS-AMS trial reduced diameter stenosis from 61.5% to 12.5% with an acute gain of 1.41 mm and late loss of 1.08 mm; no myocardial infarction (MI), death, or subacute or late ST occurred (Figure 2d).[22] AMS 1.0 was replaced by DREAMS-1, which exhibited a reduced strut thickness of 120 μm, an altered surface which incorporated PTX, a different alloy composition, and a different strut geometry.[13] DREAMS-2 is an improved version of DREAMS-1 incorporating a thin PLLA layer which releases sirolimus and extends the degradation time to 9 months.[13] In addition to clinical stents, several alloys have been constructed such as AM60B,[67] AZ91D,[67–68] AZ31,[68–69] ZK60,[68] ZK40,[70] AM50,[68] WE43,[68] and Mg-Sr.[71] Additions of Ca to the Mg-Sr (Mg-0.3Sr-0.3Ca) stent provides enhanced corrosion resistance in vitro and in vivo due to the formation of Sr-substituted hydroxyapatite (HA).[69] Other groups have reported Mg-2.5Nd-0.21Zn-0.44Zr (wt. %), which exhibits uniform corrosion with a rate comparable to Biotronik’s Magmaris® Mg alloy (WE43) and similar mechanical YS but with enhanced elongation of 26%.[72–73]
Zn has recently emerged as a relatively new biometal. It is essential for human development, but its mechanical properties are below the minimum benchmark for a stent; however, Zn alone suffers from unacceptably low TS (120 MPa),[2] so alloying has been a common practice to increase its mechanical properties. Several papers have reported on Zn-Mg,[74–75] Zn-Al,[75] Zn-Ag,[76] Zn-Mg-Ca,[74] Zn-Li,[77–78] Zn-1X (X = Mg, Ca, Sr),[79] and Zn-Cu [80] alloys, but only few have surpassed the minimum benchmarks for mechanical stent acceptance. A comprehensive investigation was performed on binary Zn alloys with Ca, Mg, Mn, Cu, Fe, Sr, Ag, and Li, where results showed Li and Mg exhibited the highest strengthening effects.[81] Extruded Zn-0.8Mn-0.4Cu achieved a YS of 191 MPa, UTS of 308 MPa, and elongation of 38.9%,[82] while Zn-0.08Mg (wt. %) had a YS and UTS of 200–300 MPa and 300–400 MPa (dependent on shelf-life storage conditions), respectively, with elongations > 30%.[83] Zn-1.5Sr/Mg (at. %) exhibited a YS and UTS >220 MPa with elongation beyond 15%.[84] Despite these mechanical weaknesses, pure Zn implanted in vivo has proven it can maintain mechanical integrity for 6 months.[29]
2.4. Summary
Mechanical aspects of each material need to be carefully considered to meet the functional demands of a promising stent. Alterations to the material type, geometry, strut thickness, and microstructure, whether of a metallic alloy or polymer composition, can have profound impacts on the mechanical properties (Figure 2). Without a doubt, permanent metallic stents and their respective metals set a precedent and benchmark for polymer and biodegradable materials to meet. MP35N® and L605 can have both good radiopacity and very thin struts without the loss of mechanical strength or structural stability. Also, alloying with Pt to 316L results in large increases in strength with increased radiopacity. Ni-free Co alloys like the L605 can substitute Ni for Fe and Mn with small additions of N to minimize allergic responses and improve corrosion resistance.
Polymer stent design should aim to increase strength by balancing strut thickness and polymer crystallinity. A practical target is to decrease strut thickness, which will minimize the surface area that blood cells contact, while also increasing the molecular weight. This target can likely be achieved by focusing on the processing methods.
Biodegradable metals will benefit from a focus on tailoring the microstructure and mechanical properties through alloying elements and processing, to ensure they meet the minimum benchmarks while also controlling the degradation rate; early dismantling of the stent can lead to ST. Current literature suggest Zn is befitting for this task as Mg degrades too fast and Fe degrades too slow. The goal should be to increase the mechanical strength of Zn alloys.
3. Permanent or bioresorbable stents: clinical findings and cytocompatibility
While stents have a long-standing overall success in the field of interventional cardiology saving millions of patients, the problem of in-stent restenosis (occurring in 30–40% of lesions),[85] neointimal hyperplasia, and ST have never really gone away,[1–2, 29, 85] only minimized with each new generation of a stent. Figure 3 provides simplified models to illustrate the tissue restoration process in both permanent and bioresorbable cases and some affiliated scientific data. While many studies show good biocompatibility in in vitro conditions, in vivo conditions differ significantly, and successful delivery and integration of the stent are multi-faceted.
Figure 3.
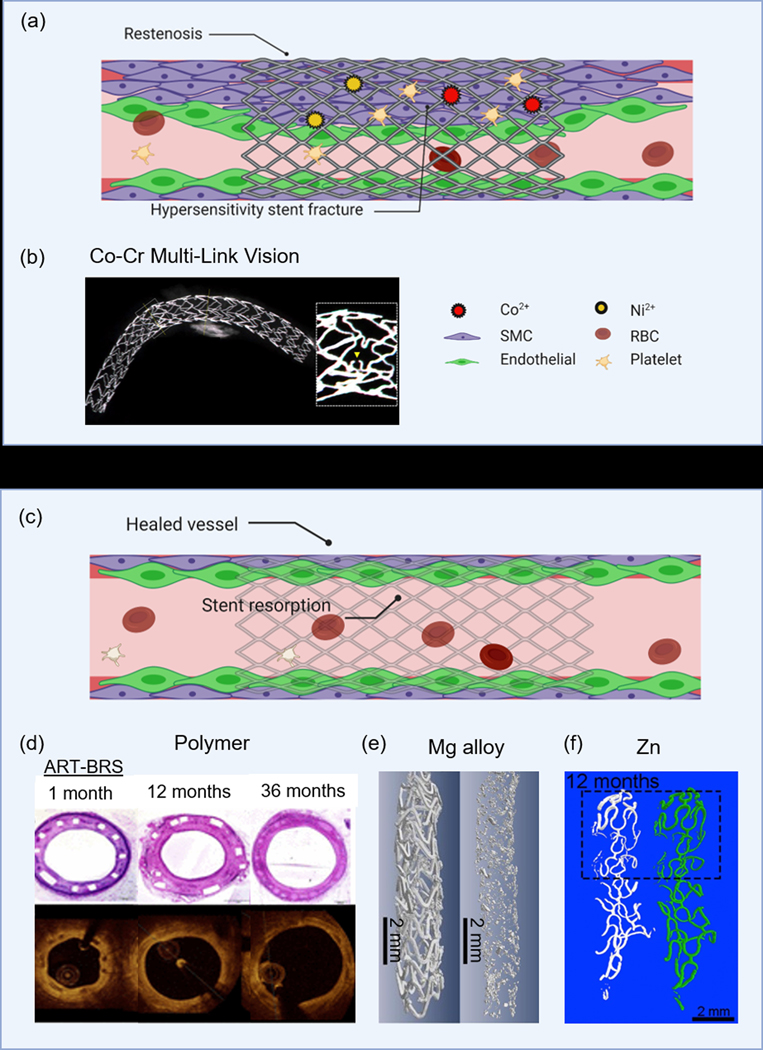
Comparison of clinical findings of permanent or bioresorbable stents. (a) Molecular-level interactions which show BMS-related restenosis and potential for metallic ions to induce hypersensitivity stent fracture, (b) stent fracture of the MULTI-LINK VISION associated with hypersensitivity to metal ions,[91] (c) vessel healing as a result of implanting bioresorbable materials, (d) in vivo histology and OCT images of the polymer-based ART-BRS,[104] (e) micro-CT images at 1 and 20-months implantation time of a JDBM stent,[112] and (f) micro-CT images of pure Zn stent implants over 12-months.[29] (b) Adapted with permission.[91] Copyright 2017, Elsevier. (d) Adapted with permission.[104] Copyright 2017, Wiley. Permission granted from © 2017 Yahagi K., et al. Published under CC BY-NC 4.0 license. Available from: https://doi.org/10.1161/JAHA.117.005693. (e) Adapted with permission.[112] Copyright 2018, Elsevier. (f) Adapted with permission.[29] Copyright 2018, Elsevier.
3.1. Permanent metallics
Without a doubt, first-generation BMS were innovative but suffered paramount complications such as restenosis and hypersensitivity responses to metallic ions (Figure 3a). The permanent presence of a foreign material serves as a site for platelet adhesion and activation of the coagulation cascade.[2] Furthermore, concerns over metallic ions such as Ni, Mo, and chromate release from 316LSS and CoCr alloys have been suspected for quite some time due to localized pitting, crevice, and stress corrosion cracking.[86] The concentration of Ni in 316L is 12–15% and there are reports of Ni-induced mutagenic and genotoxic effects[87] as well as allergic responses[88] due to high levels of Ni ions in tissues.[86] Subcutaneous implants in rats of Ni wire or NiCr alloy resulted in a gradient of Ni concentration up to 5 mm away from the implants with some mild to severe localized inflammation.[89] In-stent restenosis has also been attributed to Ni release from stents in patients with positive allergic patch test reactions to Ni and Mo.[90] Furthermore, hypersensitivity to the modern MULTI-LINK VISION CoCr stent has been associated with in-stent restenosis and stent fracture (Figure 3b).[91] Bare CoCr is also highly prothrombic and proinflammatory, switching endothelial cells from an anticoagulant to a procoagulant phenotype, even if initial endothelialization is achieved.[92] Other groups have shown pitting corrosion and thus Ni release from SMA NiTi is highly dependent on oxide layer formation and thickness[93] and high levels of Ni can be cytotoxic.[94] Recently, researchers are advising to restrict Ni from biomedical implants, and new high nitrogen Ni-free (HNNF) CoCr alloys are being developed with enhanced corrosion resistance and biocompatibility.[27, 95] Similarly, HNNF SS alloys are under development with bimodal cellular responses, encouraging endothelial growth[96] while limiting SMC proliferation compared to conventional 316L.[97]
3.2. Polymers
As mentioned previously, the promise of a BRS stent is you get all of the benefits of tissue healing such as reendothelialization and arterial wall stabilization without all of the long-term problems like chronic inflammation and late-ST (Figure 3c). Degradation products typically include CO2 and water which can be safely eliminated. However, intermediate degradation products may reduce pH, causing excessive inflammation, and stent expansion may require heating of the balloon which may further cause injury to the vessel.[98] Several stents derived from polymers have seen some clinical success, partly because they are well-characterized and FDA-approved materials readily available. The first polymer stent to reach the clinical level was the Igaki-Tamai stent, and in the first-in-man (FIM) study (15 patients), there were no major adverse cardiac events (MACE) or ST within 30 days, but there was 1 PCI at 6-months.[30] In a second larger study of 50 patients, the longest study of BRS to date, 10-year follow-up survival rates free from death, cardiac death, MACE, and TLR were 89%, 98%, 60%, and 76%, respectively;[30, 99] however, poor tissue remodeling was found as the area occupied by the PLLA stent was filled with proteoglycan.[2, 99] The recent ABSORB III trial which compared Abbotts’s PLLA stent to the XIENCE stent found higher rates of adverse events, particularly target vessel myocardial infarction (TVMI) and device thrombosis.[100] Underlying mechanisms on the shortcomings of the Absorb BVS 1.1 were primarily attributed to discontinuity (42.1%) malapposition (18.4%), neoatherosclerosis (18.4%), and stent recoil (10.5%), which suggests unfavorable degradation processes.[101] The non-drug-eluting Reva stent (Reva Medical, Inc.) constructed from absorbable tyrosine-derived polycarbonate impregnated with iodine was fully radio-opaque.[102] The degradation rate could be modified which metabolized into carbon dioxide, ethanol, and amino acids. The FIM RESORB trial of 27 patients observed no vessel recoil, but disappointingly, 2 patients experienced MI at 3-months and another patient with TLR.[102] Arterial Remodeling Technologies, Inc. also developed a non-drug-eluting ART Pure stent composed of 98% L-lactic acid and 2% D-lactic acid stereocopolymers designed to degrade at 3-months with full resorption within 2 years.[103] In mini swine, the stent showed some promising results with complete endothelialization at 1 month, positive arterial remodeling beginning from 3–6 months (which peaked at 18 months), and the lumen area remained the same without vessel shrinkage through 36 months (Figure 3d).[104] In comparing two biostable polymers, PEVA and PBMA, to bioresorbable PLLA, P(3HB), P(4HB), and a 78/22 % (w w−1) PLLA/P(4HB) polymeric blend, the bioresorbable polymers fared better.[3] Cellular behaviors and antigen expression, including cell proliferation, production of nitric oxide synthase (eNOS), and platelet endothelial cell adhesion molecule (PECAM) were upregulated on the PLLA/P(4HB), whereas the thrombotic potential was also lower.
3.3. Bioresorbable metals
Bioresorbable metals represent the next generation in resorbable stents. They possess better mechanical properties – radial strength, elongation, YS, YM, etc... – than polymer-based stents, comparable to BMS, but avoid long-term complications such as ST, in-stent restenosis, and chronic inflammation. Furthermore, the corrosion products evolved during the degradation process are bioactive and possess anti-mitotic potential, inhibiting smooth muscle cell growth.[105] Biotronik’s latest stent, the Magmaris BRS, is the first bio-corrodible stent which received CE approval in 2016.[106] The corrosion process takes about 1 year, generating magnesium hydroxide (MgOH2) which is converted to amorphous calcium phosphate.[106] Corrosion is not expected to be harmful as Mg has well-documented antiarrhythmic properties,[107] can restore ischemia-reperfusion injuries,[108] and inhibits endothelin 1-induced vasoconstriction.[109] Mg has also been shown to promote endothelial cell proliferation while halting SMC proliferation.[110] Mg-4wt.%Zn- 0.5wt.%Zr (ZK40),[70] Mg-0.3Sr-0.3Ca (wt.%),[69] and Mg-0.3–2.5wt.% Sr [71] have also been described with some success in vivo. Other groups have extensively studied a new Mg-based alloy (Mg-2.50Nd-0.44Zr-0.21Zn wt.%), known as JDBM, in vitro and in vivo.[72, 111] A 20-month stent implantation of the common carotid artery in rabbits found complete reendothelialization of the vessel within 28 days with the struts being replaced by degradation products in 4 months and elimination of degradation products, eliminating concerns of toxicity and calcification.[112] By 20 months, the stent struts were largely degraded (Figure 3e).[112] In another study, the JDBM stent implanted in the rabbit abdominal aorta for 16 weeks showed good apposition to the vessel wall with no obvious occlusion or neointimal proliferation.[72]
Iron was one of the first biodegradable metals to be studied for temporary stenting purposes. Earlier studies of Fe stents implanted into porcine descending aortas for up to 360 days showed no signs of local or systemic toxicity, but degradation products were found adjacent to stent struts and within the adventitia accompanied by macrophages.[2, 60] Another study in pigs demonstrated Fe stents implanted into coronary arteries for 28 days are very safe with no sign of particle embolization, thrombosis, or excessive inflammation.[61] Fe stents implanted into descending aortas of rabbits also showed no signs of thrombogenicity, systemic toxicity, or neointimal proliferation.[113] Despite these promising results, Fe stents degrade too slow for bioresorbable stents with substantial voluminous corrosion products retained within the vessel wall which may interfere with tissue restoration.[62] Several groups have aimed to improve the degradation rate of Fe through alloying. A nitrided Fe stent (Fe-0.074wt.% N) exhibits enhanced degradation rate and mass loss of ~76% compared to ~44.2% at 36 months in rabbit abdominal aortas.[66] In the same study, 53-month data showed corrosion products were taken up by macrophages and eliminated through the lymphatic system with the expected degradation timeline on the order or 4 ~ 5 years.[66]
Zinc is appealing because it is one of the most abundant metals in the human body, important for hundreds of enzymatic reactions,[114] and it exerts specific cardioprotective roles against atherosclerosis. Additionally, its degradation rate matches the healing period of the endothelium.[57] Several reports have shown that Zn has promising potential as a cardiovascular stent. Many studies have reported in vitro data, but these analyses cannot accurately predict how properties change under the influence of dynamic blood flow, adhesion of proteins or platelets, or interaction with ions and small organics. Therefore, demonstrating patency, no restenosis, ideal degradation profiles, and minimal inflammation is essential for successful Zn stent design. Of the in vivo studies, results are promising. Pure Zn stents implanted in rabbit abdominal aortas for up to 12 months showed no signs of severe inflammation, platelet aggregation, neointimal hyperplasia, or thromboses (Figure 3f).[29] H&E staining revealed rapid endothelialization at 1 month which slowly increased over time. SEM images observed a smooth, oval, and spindle-shaped confluent monolayer of endothelial cells in the direction of blood flow, similar to that of native arteries. Degradation was shown to be beneficial for arterial remodeling and tissue healing. Zn-0.1wt.%Li wires implanted into abdominal aortas of rats for 2–12 months showed good biocompatibility but moderate inflammation and some medial tissue deterioration was present [78]. In a similar study, Zn-xMg (x = 0.002, 0.005, and 0.08 wt.%) wires exhibited minor inflammation and neointimal formation.[83]
3.4. Summary
The permanent presence of bare-metal metallic stents poses serious hemocompatibility concerns. Furthermore, their release of toxic elements has profound inflammatory reactions; Ni, Mo, and chromate release can elicit hypersensitivity reactions, ISR, and even stent fracture in some cases. These results are often overlooked in a petri dish due to a lack of co-culture systems which account for the immune responses seen in vivo. These materials need to be gradually phased out with the introduction of more biologically sensible stents.
Polymers are generally regarded as biocompatible and hemocompatible as their degradation products are safely metabolized and cell responses are favorable. As mentioned in section 3.2, some degradation products may lower the pH, causing localized inflammatory responses which hinder the regeneration of the vessel. A recent study found proteoglycan within the footprint of a PLLA stent which may indicate poor tissue remodeling.[99] A possible explanation may stem from its bulk erosion properties, allowing premature infiltration of connective tissue. Further evaluation of tissue properties needs to be carefully studied following dissolution of struts.
Biodegradable metals have shown promising results in vivo. Mg and Zn are essential cell nutrients which facilitate tissue regeneration and have anti-mitotic potential to limit SMC growth. Unlike the bulk-erosion of PLLA, both undergo surface corrosion, so the tissue front follows the dissolving stent strut. This may explain why proteoglycan was found in the polymer study vs. reendothelialized tissue in the case of biometals. On the other hand, mixed studies have been found with Fe. Generally, the scientific community agrees the degradation rate is too slow and the corrosion products are difficult to eliminate, so attempts to accelerate its corrosion rate have been undertaken with some promising results.
Taken together, polymer or bioresorbable metals surpass the necessary biocompatibility and tissue restorative properties of bare-metal stents. Future research should 1) continue to eliminate and substitute inflammatory-inducing elements like Ni, Co, Cr, and Mo for biologically safe elements, 2) investigate the tissue restorative properties of polymers, and 3) ensure new alloys of bioresorbable metals continue to facilitate tissue regeneration.
4. DES or no drug-eluting
Initial attempts to avert BMS-related restenosis which occurred in 20–30% of patients included taking oral drugs for systemic delivery. Unfortunately, these were not effective which led to applying endoluminal drug coatings to stent surfaces. [115] These new DES reduced restenosis by approximately 60–80% through inhibition of SMC proliferation, resulting in widespread commercialization of DES. Figure 4 highlights the effects of drug elution on tissue healing in both permanent and bioresorbable instances. Late ST and strut malapposition (Figure 4b) are serious concerns and mixed results have been reported: some clinical data and meta-analysis show DES surpass the performance of BMS,[116] eliminating the need for repeat interventions[117] and lowering MI and death[118] as compared to BMS, whereas other data have found higher rates of cardiac death[119] and nonfatal MI[120] and adverse events of very late ST compared to BMS.[5, 121] Several studies have shown incomplete stent apposition (ISA) – lack of contact with one stent strut and the underlying intimal tissue – also known as stent malappostition, is more common in DES with clinical adverse outcomes.[122] While not the focus here, one group has developed sophisticated techniques to model stent drug release and reversible binding of both free and bound drug to the arterial wall receptors and extracellular matrix (ECM).[123–125] Second-generation DES stents performed better than first-generation DES, so design considerations are an important aspect that must be taken into account including deliverability, efficacy, safety,[126] and subsequent treatment of the occluded vessel. Deliverability was discussed previously but efficacy refers to uniform drug delivery and disease-specific treatment,[126] both related to the selection of the drug, binding to the polymer carrier, and release of the drug. Safety refers to the biocompatibility of the polymer as it should be non-thrombogenic, non-inflammatory, and not toxic while minimizing neointimal growth.[126] First-generation DES consisted of SS platform which released sirolimus (Cypher) or paclitaxel (Taxus)-eluting stent (PES) but was later replaced by second-generation zotarolimus (ZES) and everolimus-eluting (EES) DES bonded to thin-strut CoCr stent.[126] The selection of the polymer is vitally important to achieving the desired host response. The polymer coating should avoid cracking, flaking, and delaminating upon balloon insufflation and must be able to regulate drug release in a controlled and predictable fashion.[126] Permanent polymers were routinely applied to first-generation stents but are now being replaced by advanced cell phospholipid-mimicking polymers such as phosphorylcholine and even biodegradable polymers such as PLLA. A wide variety of drugs in use are classified as anti-inflammatory (Biolimus, Sirolimus, Dexamethasone), anti-thrombogenic (typically dual antiplatelet treatment (DAPT) but can include antibodies to inhibit platelet aggregation), antiproliferative (PTX, Zotarolimus, everolimus, Actinomycin D), and immunosuppressive (Sirolimus, Tacrolimus),[126] which have been reviewed extensively.[127] While DES have dramatically reduced, if not eliminated ISR, several safety concerns over late ST and elevated mortality have risen.
Figure 4.
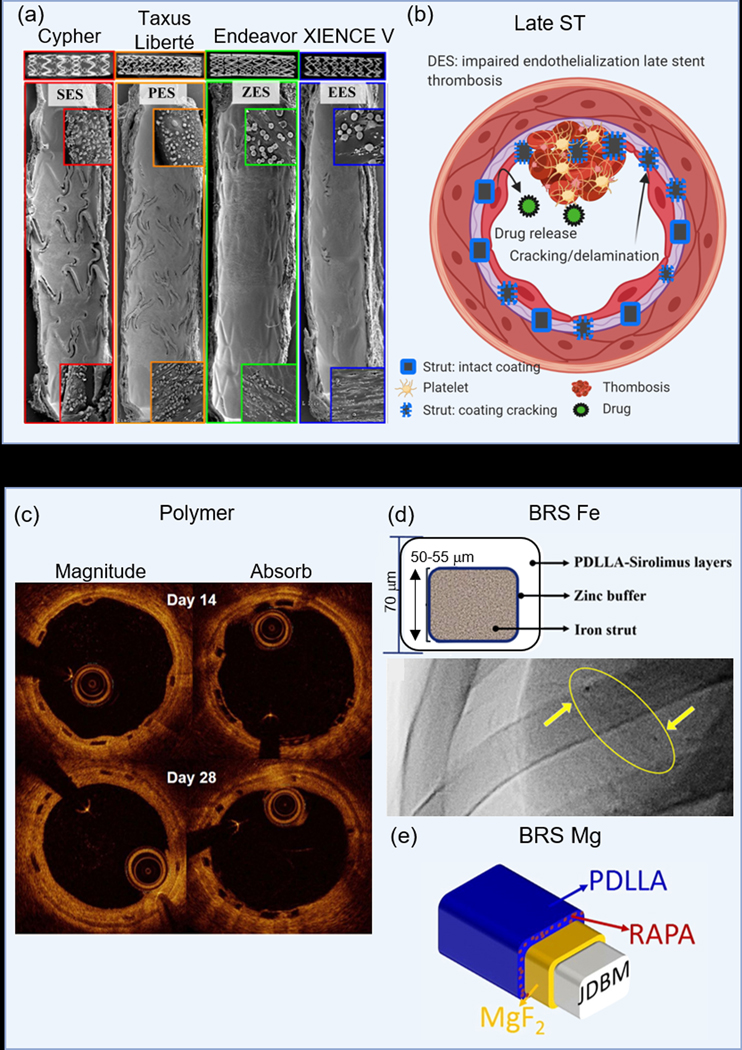
Permanent and bioresorbable-based DES. (a) Comparison of SEM images obtained in vivo of PM-DES which highlight EES over its competitors,[129] (b) molecular-level interactions showing strut malapposition with consequences of late ST, (c) OCT images over 28 days which show higher % of embedded and lower % protruding and % uncovered struts of the Magnitude-BRS as compared to the Absorb-BVS,[54] (d) a nitrided-FE DES,[151] and (e) a PDLLA-carrying Rapamycin coated Mg-JDBM stent.[153] (a) Adapted with permission.[129] Copyright 2008, Elsevier. (c) Adapted with permission.[54] Copyright 2019, Elsevier. (d) Adapted with permission.[151] Copyright 2019, Elsevier. (e) Adapted with permission.[153] Copyright 2017, Elsevier.
4.1. Permanent metallics
Metal-based DES have undergone extensive investigations, including trials at the clinical level. Many commercial companies such as Cordis, Boston Scientific, Medtronic, and Abbott Vascular helped make this a reality as they led the way in taking these stents from research level to becoming clinical benchmarks in the field of interventional cardiology. Medtronic offers two formulations of the MP35N® CoCr stent, the Endeavor with a PC polymer and the Resolute with a BioLinx polymer, which is a mixture of three polymers.[13, 128] Compared with no drug and polymer coating, the PC Endeavor stent performed better in a clinical trial, reducing target vessel failure, MACE, and TLR.[20] Newer formulations include the Integrity™ BMS and Resolute ONYX™ Integrity™. Clinical results of Boston Scientific’s Taxus Express, a PTX-eluting SS with express platform, and the Synergy™ stent, a bioabsorbable PLGA everolimus-eluting stent,[13] demonstrated the PTX-eluting Taxus stent reduced TLR and restenosis at 9 months as compared to its bare-metal counterpart,[16] and the Synergy stent performs comparably to the durable Promus Element stent in the EVOLVE trial.[17] Abbot Vascular’s L605® CoCr Xience-V everolimus-eluting stent releases 80% of the drug in four weeks with enhanced 1-year follow-up data in the SPIRIT III trial.[21] Similarly, Biosensors’ BioMatrix, eluting Biolimus from a biodegradable PLA polymer, faired comparable to Cordis’ sirolimus-eluting stent with durable polymer, the Cypher SELECT, across a primary endpoint composite of cardiac death, MI, and TLR.[19] A comparison of endothelial cell recovery on leading clinical stents revealed significant improvements of reendothelialization on EES as compared to SES, PES, and ZES (Figure 4a).[129]
Several studies have shown the drug or the polymeric coatings may be inhibiting endothelial growth, causing delayed and aberrant vascular healing,[130–132] which are key factors in minimizing inflammation, thrombus formation, and late ST. Several other trends have emerged within the research realm with new therapies to overcome these challenges while combatting more complex disease sites. Poly(n-butylmethacrylate) (PBMA) has been applied to DES but exhibits poor elastic response during stent deformation; the addition of tacrolimus-eluting dextran (DEX-PBMA) helps maintain elasticity and has shown some promise on CoCr stents.[131] The direct coating of antiplatelet drugs with PDLLA to CoCr has also been described.[133] More natural approaches include vitamin-C coated CoCr, hyaluronic acid dopamine/abluminal sirolimus-PDLLA coatings,[134] platelet glycoprotein IIb/IIIa antibodies on 316L stents,[135] and human CREG protein applied to 316L.[136] Polymer-free DES using ceramics to bind drugs such as N-doped TiO2 and alumina (Al2O3) on NiTi are yet another avenue to mitigate drug release and improve biomaterial-tissue responses.
4.2. Polymers
Even though polymer-based stents will undergo complete dissolution, the degradation products can lead to localized acidification of the tissues,[31] generating some cytotoxic response. There is also a growing concern DES are contributing to very late ST. As such, the need to accelerate reendothelialization while halting neointimal tissue growth is a primary focus that can be provided through additional molecular therapy of localized drug release. Several commercially available stents, including those in clinical testing, employ an elution drug. The well known and most studied polymer stent is the Absorb from Abbott Vascular which employs everolimus as its drug of choice; drug release is based on diffusion.[137] Three renditions have been produced culminating in a total of 5 clinical trials that have been reviewed extensively, with only one receiving CE approval.[137–138] Compared to the XIENCE V stent, MACE rates for the Absorb BVS did not differ much at 400 days follow-up.[139] The 2–4 μm thick everolimus-eluting PDLLA polymer coating of the Absorb BVS releases 80% of the drug within 30 days and is an improvement from the Absorb 1.0, which included changes in polymer processing whereby polymer chains were aligned to slow the hydrolysis rate.[140] A comparison of the Absorb BVS and Magnitude-BRS revealed a lower % of embedded struts and higher % protruding and % uncovered struts on the Absorbs BVS (Figure 4c).[54] While several other polymer-based stents are undergoing clinical testing, only three have received CE approval as of April 2018 which includes the ART-BRS, DESolve, and the Fantom from Reva Medical.[137] The ART-BRS is a bare polymer stent with no coating or drug. The DESolve stent incorporates a proprietary spray technique that attaches the polymer-drug matrix to the stent at a thickness of <3 μm and a concentration of 3 μg/mm.[137] Approximately, >85% of the novolimus is released over the first four weeks. Clinical results from the DESolve NX trial which enrolled a total of 122 patients have been promising as 5-year follow-ups reported no scaffolds thrombosis and only 4 cardiac deaths and TLRs.[141] Most notably, the new DESolve Cx trial reported no cardiac death, MI, TLR, or scaffold thrombosis at 1-year implantation.[137, 142] Reva Medical, Inc. has come out with several stent renditions that featured a “slide and lock” mechanism such as the ReZolve and ReZolve 2 – an improvement from the ReZolve which reduced its undeployed profile and increased radial strength by 30%.[140] Reva also introduced the fully radiopaque Fantom stent which is composed of a proprietary Tyrocore™ desaminotyrosine polycarbonate (PTD-PC) polymer which incorporates a PTD-PC-sirolimus matrix for drug release that amounts to 115 μg for a given 3.0 х 18.0 mm stent.[43, 137] Clinical reports of tissue vasomotion restoration after 12 months have been reported.[143] Degradation of this scaffold takes place over 36 months with >80 % mass loss in the first year. The 2-year follow-up data from the FANTOM II trial reported low rates of cardiac death, MI, and TLR.[144] Reva’s newest Fantom Encore stent employs the same fully radiopaque Tyrocore™/sirolimus drug coating.[55]
A recent report comparing a PTX-eluting PLLA coated Igaki-Tamai to a bare Igaki-Tamai stent demonstrated a significantly lower neointimal thickening at 1 month (17.8% vs. 34.9%) and 3 months (27.7% vs. 41.4%).[145] Amaranth Medical, Inc. had released the Fortitude, Aptitude, and most recently, the Magnitude stents which underwent testing in the Renascent, Renascent II, and Renascent III clinical trials; resorption times are 3–6 months while releasing the drug sirolimus.[140] The Huaan Biotechnology Group Co., Ltd. manufactured the PLLA Xinsorb stent coated with a PDLLA/PLLA mixture which releases 80% of the drug sirolimus over 28 days ex vivo;[146] porcine neointimal responses of the Xinsorb compared to a conventional metallic DES were comparable at 3 months [147] and no differences were observed in late lumen loss, device area, neointimal hyperplasia, or area stenosis at 180 days [148]. A 6-month study in humans found similar results that demonstrated both safety and effectiveness for the Xinsorb stent.[38]
4.3. Bioresorbable metals
Degradation products of metallic resorbable stents possess anti-mitotic properties; however, their corrosion products are highly variable and dependent on the experimental conditions and their release are dependent on the metal’s corrosion rate.[2] These products vary significantly from in vitro to in vivo conditions, making translational research from the bench-top to the hands of clinicians quite challenging.
Several groups have adopted new Fe alloys to increase its corrosion rate and are employing Fe-DES in hopes to achieve better stent integration with the host tissue. A recent report of PLGA-coated, rapamycin-loaded nanotube arrays on pure Fe showed low burst release of the drug and extended-release to 30 days.[149] The corrosion rate was faster than pristine Fe, endothelial cell growth was favored over SMCs, and hemocompatibility was very good.[149] Unfortunately, there was no in vivo data. A novel design of an ultrathin (53 μm) zinc-coated, nitrided-Fe stent which incorporated a PDLLA-carrying sirolimus coating showed excellent degradability and biocompatibility in vivo up to 13-months.[150] Interestingly, their PDLLA-sirolimus coating created local acidification which increased the corrosion rate of the nitrided-Fe stent.[150] A follow-up study in swine found higher endothelialization at 14 days, comparable fibrin scores at 90 and 180 days, and no differences in stenosis or ST as compared to a XIENCE Prime-EES (Figure 4d).[151]
In contrast to pure Fe, pure Mg degrades rapidly, so alloying and surface coatings are being used to slow the rate. Given that DES outperformed BMS, it was only natural to apply the same methodology to Mg-based alloy stents. The best examples of Mg-DES are the stents developed by Biotronik. DREAMS-1®, which replaced the AMS-1®, eluted 0.07 μg mm−2 PTX for 3 months and was tested in the BIOSOLVE-1 trial; results were favorable with good efficacy and safety at 12 months with only 1 case of MI and no deaths or ST.[23] DREAMS-1 was optimized to produce the DREAMS-2, which adopted a thin PLLA-based sirolimus-eluting anti-mitotic drug and was tested in the BIOSOLVE-II trial. TLF (6.8%) and TLR (4.3%) were relatively low and comparable to 2nd generation DES out to 36 months; furthermore, no definite or probable ST and LLL was attributed to disease progression rather than the Magmaris resorption time.[152] The BIOSOLVE-III trial was a 36 month pooled analysis from the BIOSOLVE-II and BIOSOLVE-III. 12-month follow-up results from the ongoing BIOSOLVE-IV trial showed low (4.3%) TLF rates at 12 months with 97.3% device and 98.9% procedural success rates.[152] A non-clinical Mg-based alloy (JDBM) which contained a rapamycin-eluting PDLLA coating exhibited promising 3-month results in porcine coronary arteries, which revealed similar neointimal proliferation of the DES Firebird 2 and no adverse complications (Figure 4e).[153]
4.4. Summary
Strut malappoistion and late ST are common dilemmas encompassing the use of DES. Reendothelialization is significantly lowered due to confounding factors surrounding deliverability and the effects of the eluting drug on the endothelium. As a result, struts remain malapposed within the lumen exposed to blood and blood cells. Use of second-generation drugs on permament metallic stents like ZES and EES should continue to be the pillar of drug release while newer cell-mimicking coatings are developed. Furthermore, new drugs which do not affect endothelial growth are essential.
As polymer degradation time is upwards of 1 to 3 years, advances need to be made to extend drug release. If the polymer degrades too fast, releasing the drug too early, we can expect to see similar results of late ST as in the case of permanent metallic stents. The drugs should promote rapid endothelialzation of the stent without stimulating neointimal thickening.
Bioresorbable metals can take advantage of drug-elution in several ways. As mentioned above, Fe-based stents need to employ endothelium-conscience drugs which actually promote resorption while Mg-based stents need to continue using coatings and drugs to slow down the corrosion rate. There have yet to be any Zn-based DES reported in the literature, so it will be interesting to see some investigaters move into this new area. As the corrosion products are anti-mitotic, the drug-of-choice should target other pathways such as anti-thrombogenicity, anti-inflammation, or immunosuppresion.
5. Bare or surface-modified stents
Vascular injury during coronary stent intervention gives rise to inflammatory responses with the migration of monocytes and SMC which can lead to neointimal hyperplasia. Furthermore, extensive research and clinical trials on BMS surfaces have shown the necessity of some form of surface functionalization due to adhesion of platelets and activation of the coagulation cascade. Figure 5 depicts potential applications of surface modifications and relevant scientific data. Functionalization can include polymeric or oxide coatings or surface patterning and texturing techniques at the nano-to-micro scale (Figure 5a).
Figure 5.
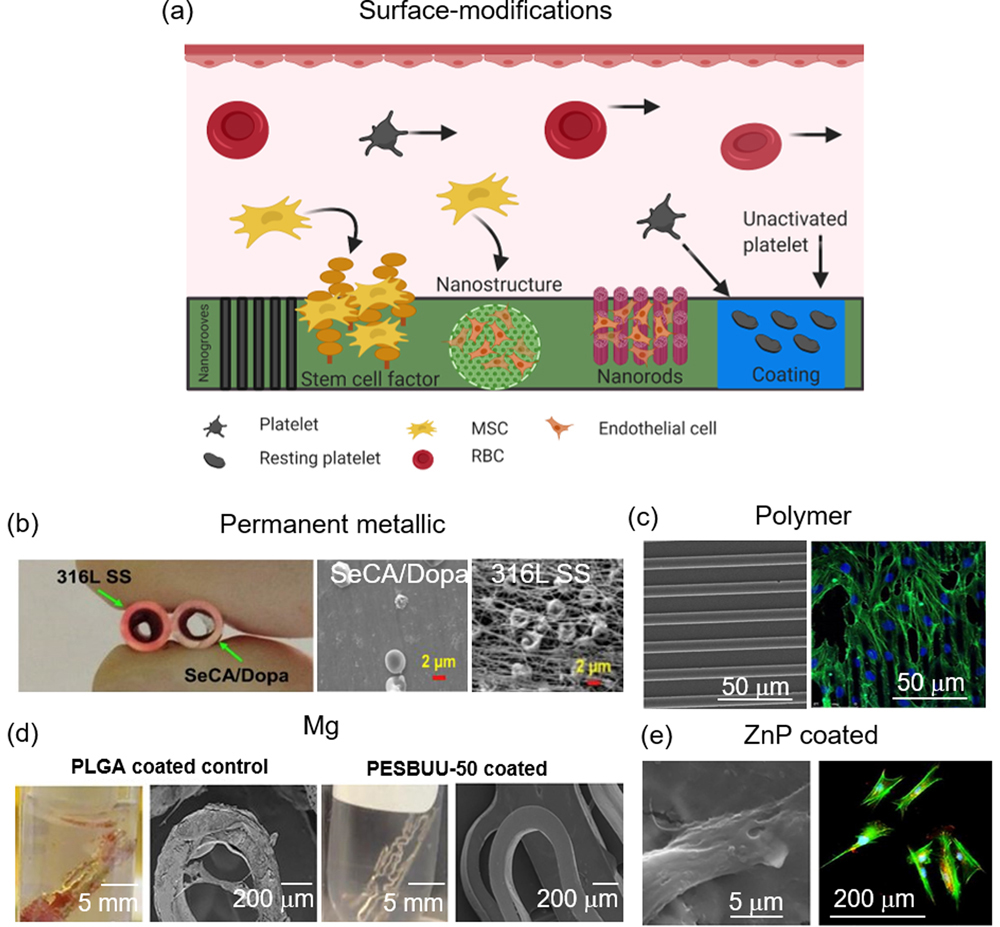
Surface-modifications applied to PM-, polymer-, and bioresorbable-based materials. (a) Potential surface modifications which elicit differential biological activities, (b) comparison of 316L SS and SeCA/DOPA-coated 316L SS stents and their hemocompatibility obtained in vivo,[172] (c) microtopography-guided cell alignment and proliferation on PDMS,[177] (d) zwitterionic polymer coatings on a Mg-based AZ31 stent and SEM images after contact with fresh ovine blood for 3-hours,[185] and (e) endothelial cell attachment on ZnP-coated Zn.[202] (b) Adapted with permission.[172] Copyright 2018, Elsevier. (c) Adapted with permission.[177] Copyright 2017, Scientific Research. Permission granted from © 2017 Wang D., et al. Published under CC BY 4.0 license. Available from: https://doi.org/10.4236/jbnb.2017.83013. (d) Adapted with permission.[185] Copyright 2019, American Chemical Society. (e) Adapted with permission. [202] Copyright 2019, Elsevier.
5.1. Permanent Metallics
The problem of ST with BMS was serious early on. Advances in materials engineering led to a widespread search for the optimal stent surface which eliminated problems associated with BMS and DES such as ST and impaired endothelial regeneration, respectively.
As mentioned previously, it had long been considered metal ion release from implants contributed to inflammatory responses and potentially in-stent restenosis, and materials with corrosion-resistant coatings improved biocompatibility by minimizing this release.[12] In light of this, researchers began applying oxide layers to stents. Diamond-like carbon (DLC) coated SS (BioDiamond, Mainz, Germany) showed reduced metal ion release and platelet activation in vitro compared to controls but this did not translate to improved long-term biocompatibility.[154] Similarly, the Diamond Flex AS and Phytis™ stents (Phytis Medical Devices GmbH, Berlin, Germany) compared against bare SS and Multi-Link Penta™ SS, respectively, showed no significant differences in angiographic restenosis rates.[12, 155–156] Titanium-nitride-oxide (TiNOX) coatings have also been developed with the hope that nitrogen in the oxide layer will be a source to facilitate the evolution of nitric oxide (NO), which is known to support endothelial function and improve vasodilation.[12] Several clinical studies have shown that these titanium-nitride-oxide stents reduce restenosis and MACE,[157–158] even when compared against DES.[159] Iridium-oxide coated stents used in the MOONLIGHT trial employed a layer of iridium oxide that covered a gold-plated SS stent, reducing restenosis rates to 13.8%.[160] More recent advancements include graphene sheets, chitosan, and TiO2 nanoparticles applied to SS,[161] and electropolymerized poly(ethylene glycol) dimethacrylate (PEGDMA) coated on SS.[162] Laser-Induced Periodic Surface Structures (LIPSS)-induced nanotopography has been applied to 316L SS and Pt:SS and demonstrates changes in monocyte and fibroblast cell attachment, favorable for stent applications.[163] In a similar study, femtosecond laser [164](FSL)-generated SMC-bionic surface features from AFM scans were created on 316L SS which enhanced endothelial attachment and migration.[165] Surface pre-treatments of electropolishing, thermal oxidation, plasma immersion ion implantation (PIII) on L605 CoCr has also been studied.[166]
Some research on surface functionalization on NiTi exists in the literature as well.[167–168] For example, Ta coating deposited on nano-roughened NiTi through ion plasma sputtering showed enhanced radiopacity and biocompatibility,[169] and further coating the NiTi stent with polytetrafluoroethylene (PTFE) to achieve a highly lubricious surface with enhanced mechanical stability has been described.[170] Some research on surface functionalization on NiTi exists in the literature as well.
Recently, the research paradigm has shifted to more multifaceted and multifunctional platforms to address common issues such as restenosis, platelet activation, inflammation, while capturing endothelial cells for reendothelialization.[171] For example, two separate peptide amphiphiles (PA), endothelial cell ligand (YIGSR) and NO donor polylysine, both linked to matrix metalloproteinase −2 (MMP-2) degradable sequences, were mixed and reacted to form NO and coated on SS; the stent constructs enhanced endothelial cell attachment and migration, discouraged SMC adhesion and proliferation, and decreased both platelet and monocyte adhesion in vitro.[171] In another study, selenocystamine and dopamine coated on 316L SS, NiTi, and CoCr could elicit sustained NO generation through reaction with endogenous S-nitrothiol in blood, which combined with inhibition of SMC proliferation, reduced platelet activation, and favorable endothelial cell interactions, the stents promoted rapid reendothelialization, reducing in-stent restenosis and neointimal hyperplasia (Figure 5b).[172] Fibroblast-derived extracellular matrix (FDM) proteins (collagen I, fibronectin, and laminin) immobilized on CoCr alloy is superior to bare CoCr in capturing endothelial progenitor cells (EPCs) and umbilical vein cord endothelial cells in vitro,[173] and biofunctionalization of a CoCr surface with REDV-modified, elastin-like recombiners (ELR) improves human umbilical vein endothelial cells (HUVECs) interactions with CoCr.[174]
5.2. Polymers
In addition to bioresorbable metals and permanent metallic materials, surface-functionalized polymer-based systems have been studied extensively. Anti-proliferative drugs induce adverse and inhibitory effects on endothelial cells which impair the regeneration process, thus contributing to late ST and MI.[132, 175] Multiple safety issues have been identified[115] which have directed research efforts to functionalized surfaces that encourage endothelial cell adhesion, proliferation, and migration, all while inhibiting adverse effects of SMC proliferation and platelet adhesion. Picosecond laser ablation of varying laser depth, width, and spacing has been applied to PEG-modified PLLA with success.[176] Patterned surfaces enhanced endothelial adhesion and proliferation three-fold with 20 to 25 μm wide and 6 to 7 μm deep layouts providing the best results overall, but treated surfaces also enhanced platelet adhesion.[176] Microgrooves applied to poly(dimethylsiloxane) (PDMS) membranes also encourages HUVEC alignment with elongated and bipolar morphology and upregulation of several microRNAs responsible for cell proliferation, while inhibiting vascular cell adhesion molecule-1 (VCAM1) (Figure 5c).[177] Similar results were discovered on polyurethane-based shape memory polymers (SMPs) where microgrooves were found to increase surface hydrophobicity which helped to align endothelial cells along the microchannels.[178] PIII is another surface modification technique where ion-induced carbonization and oxidation helps to covalently immobilize biomolecules to the surface.[179] PIII applied to polyurethane enhanced surface wettability and promoted saturation of collagen attachment.[179] The same group also did subcutaneous implantation studies of the same material and found significantly lower acute/subacute inflammatory responses in the PIII-collagen group.[180]
5.3. Bioresorbable metals
Given the relatively new advent of bioresorbable metal stents, much of the research has focused on creating the ideal alloy. Fe and Zn have fairly stable corrosion rates as opposed to Mg, which degrades very fast. The rapid degradation in physiological environments proceeds according to the reaction: Mg + 2H2O → Mg(OH)2 +H2. The corrosion products are loose and porous and do not protect the base metal. Degradation is on the order of weeks to several months, depending on the type of alloy, which can seriously compromise implant integrity. Intense efforts, culminating in a comprehensive list of studies,[181] have looked at the interface between Mg and the tissue[182] and controlling the corrosion rate through surface functionalization.[183] Super-hydrophobic surfaces have been generated on Mg alloy AZ31 with the expectation of repelling water molecules from the bulk metal — slowing the reaction presented above — and proteins and platelets.[184] Methods to enhance corrosion resistance while improving biocompatibility include zwitterionic polymer coatings (Figure 5d),[185] which also introduce non-thrombogenic surfaces, PLGA coatings (better HUVEC adhesion and spreading),[98] spin-coated PLLA and PCL-Mg,[186] and silk fibroin coupled with vacuum ultraviolet-ozone activation treatment to a MgCaZn alloy, resulting in 1 8−1 slower degradation rate as compared to an uncoated alloy.[187] Biofunctionalization with poly(ethylene glycol) (PEG), endogenous protein fibronectin, or fibronectin/heparin also slows corrosion rates and improves blood biocompatibility.[188] Surface modification with plasma polymeric allylamine (PPAam) has also been shown to improve corrosion resistance, lowering the corrosion rate of MgZnMn alloy from 2.30 mm yr−1 to 0.373 mm yr−1 for PPAam-MgZnMn, with improved spreading and migration of endothelial cells.[189] In another example, AZ31 coated with ZnO nanoparticles embedded in a PLA matrix exhibited good adhesion with minimal crack formation during immersion in Hank’s solution and enhanced osteoblast cell growth and antibacterial efficacy.[190]
While Mg degrades rapidly and efforts have been made to reduce corrosion, Fe degrades much slower, so converse efforts to speed up the degradation rate have been undertaken. This has primarily been done by alloying but there are a few reports on surface treatments and functionalization. Such reports include sandblasting,[191] Zn ion implantation,[192] Ag ion implantation,[193] and micro/nanostructured arrays such as Pt,[194] Au,[195] and iron oxide.[196] Increased dislocation density and high surface roughness on Fe generated through sandblasting cannot offer corrosion resistance afforded by the corrosion product layer due to cracks with irregular shape and constant exposure to the electrolyte.[191] Zn ion implantation increases the corrosion rate but at the expense of enhanced platelet adhesion and activation and reduced, but acceptable, cell viabilities of L-929 fibroblasts, EA.hy-926 umbilical vein endothelial cells, and VSMCs ― considered advantageous for stenting applications.[192] Similar results were found with Ag ion implantation.[193] Micro-patterned arrays with noble elements introduce galvanic coupling effects that undergo uniform corrosion and enhance the degradation rate as was demonstrated with Au[195] and Pt[194] on Fe. In contrast to Zn and Ag ion implantation, Pt micro-disks discs exhibited almost no cytotoxicity and reduced number of adhered platelets.[194]
The corrosion rate of Zn matches closely to the wound-healing pace of cardiovascular and bone tissue,[197] so most of the focus has been directed away from altering the degradation profile, though this is highly dependent on the nature of oxide film and cracks present,[198] and aimed at improving cell-implant interactions.[199–200] For example, in vitro direct cell culture assays and Zn ion release have shown a slight cytotoxic response suspected to be related to Zn ion release. Several groups have focused on improving cell behavior. ZnO flowers have been electrodeposited on Zn-1Mg and Zn-2Mg alloys which upon contact with simulated body fluid (SBF) resulted in the production of several known inorganic compounds including HA.[201] Zn phosphate (ZnP) coatings applied to Zn rods have shown superior corrosion resistance and enhanced cytocompatibility in vitro.[202] Pre-osteoblast and endothelial cells cultured on ZnP displayed elongated and spread-out morphology as compared to pure Zn and collagen coated-Zn (Figure 5e).[201] A breakthrough study performed on bone cells and macrophages showed cell function is far superior when cultured directly on Zn samples of varying topographies as opposed to culturing in material extracts.[199]
5.4. Summary
Surface-functionalization is a technique with potentially promising results. For example, the surface can be used to increase migration or attachment of endothelial cells. It may also promote cell growth and attract bone marrow endothelial precursors to the surface. Bare-metal stents can attract and mobilize endothelial cells in the initial phase of healing, but they still suffer from metallic ion-induced inflammatory responses at the later stages of healing, which may lead to late ST. This poses serious consequences which may never be resolved due to the lifelong presence of the foreign material.
Unlike permanent metallic stents, bioresorbable polymers may utilize the surface texturing phenomenon during the acute phase of healing. Utilization of micron sized channels attracts and mobilizes endothelial cells to promote rapid reendothelialization, eliminating protruding and malapposed struts from occluding the lumen as observed in the case of DES. As the material degrades and is eliminated, long-term clinical issues of late ST are averted. PIII should continue to be investigated in conjunction with micropatterning to optimize the host response and integration.
Applying surface texturing methods to bioresorbable metals should be an area of intense efforts to improve material integrations with the host tissue. Methods to slow the corrosion rate of Mg using superhydrophobic surfaces, zwitterionic coatings, or alternative treatments should be the primary focus on Mg moving forward. Conversely, ion implantation or patterned arrays should be applied to Fe to increase the corrosion rate. Perhaps, the best known cytocompatibility reports of cells cultured on Zn utilized ZnP coatings[201] or micro-topographies.[199] Whereas the ZnP significantly slowed the corrosion rate, the micro-topography did not alter the corrosion rate; this may be advantageous for a Zn stent and should necessitate further study.
6. Self-expanding or balloon-expanding stents
Shape memory alloys can recover their original shape after large deformations through mechanical loading (pseudoelasticity) or thermally-induced phase transformations, also known as the shape memory effect.[203] Figure 6 highlights the shape memory process with applicable data taken from polymer and bioresorbable metal cases. This biphasic material response results from an austenite-to-martensite phase transformation and vice versa.[203] In the case of a stent, the stent is produced in the open state and is “shape set” at room temperature. It is further crimped or “deformed” onto the guidewire. When the stent is inserted into the vessel, cold saline solution prevents the stent from undergoing self-expansion since its temperature is below the austenite finish (Af) temperature, which is close to normal body temperature. Once the stent is in the correct location, the wire and saline are removed, the material’s temperature increases above Af, where the material experiences a thermally induced phase transformation back to its original “set shape” (Figure 6a).
Figure 6.

Self-expanding stents which include metallic SMAs, polymer SMPs, and bioresorbable SMAs. (a) Mechanism for shape-setting and stent expansion which illustrates the mechanical processes and change in atomic structure,[204] (b) direct-write 4D scaffold, showing its shape recovery process,[215] (c) mechanical properties of a Fe30Mn6Si bioresorbable SMA,[218] and (d) cyclic stress-strain curves of a Mg- 20.5 at% Sc alloy obtained at 20 °C, −50 °C, −100 °C, and −150 °C.[219] (a) Adapted with permission.[204] Copyright 2004, Elsevier. (b) Adapted with permission.[215] Copyright 2017, American Chemical Society. (c) Adapted with permission.[218] Copyright 2011, Elsevier. (d) Adapted with permission.[219] Copyright 2016, American Association for the Advancement of Science.
6.1. Permanent shape memory alloys
Nickel-titanium, commonly referred to as Nitinol, is perhaps the best-known shape memory alloy with an equiatomic percentage of Ni and Ti.[204] Due to Nitinol’s properties of superelasticity and thermally-induced shape memory,[205–206] an implanted stent can adapt to a long and tortuous vessel during implantation without vascular scarring or vessel trauma and return to its original shape because it’s Af temperature is close to the human body. Balloon-expandable stents do not exhibit superelasticity, so during expansion, the stent may force the vessel to straighten and induce vascular trauma.[207] While balloon-expandable stents are rigid and resist compressive vessel forces, nitinol stents exert very low chronic outward forces in their set shape.[205] During implantation, nitinol stents conform to the vessel while maintaining constant force do their stress/strain behavior.[204] Given these properties, Nitinol stents are preferred as biliary, peripheral, and cerebrovascular stents where their pseudoelastic properties are important during squeezing muscle contractions.[203] A comparison of four commercially available, endovascular coil embolization SX stents such as the Neuroform EZ (Stryker), LEO (Balt), LVIS (Microvention), and Enterprise (Codman Neurovascular) showed different morphological and physical characteristics.[208] Unfortunately, SX stents are not well suited to heavily calcified plaques as they cannot return the vessel to its original patency.[203] In the randomized ICE trial, SX and BX stents for treatment of external iliac artery restenosis were compared, and the SX nitinol stent had lower 12-month restenosis rates and reduced TLR.[209] However, the earlier CRISP-US trial found no significant differences in nitinol and the stainless steel Wallstent.[210] Treatment of intracranial aneurysms found better results when using SX nitinol stents.[211] The properties of NiTi can be adjusted by varying the atomic percentages of Ni or Ti[212] or by the addition of other elements such as Hf[213]
6.2. Polymers
Shape memory polymers are similar to metals in that they can be fixed in temporary shapes and return to their original shape, but the transition is not based on phase transformations. Instead, polymers are activated by heat, light, pH, moisture, magnetic field, etc.[214] The first implanted self-expanding stent in a human was the Igaki Tamai stent made of PLLA. Self-expansion was induced by heating the material beyond its glass transition (Tg) temperature of ~70 °C using a heated balloon during implantation. The 10-year clinical trial results were promising as acceptable rates of MACE and scaffold thrombosis were reported.[99] Polyurethane-iron oxide nanocomposite SX stents triggered by an external magnetic field have been described which causes the stent’s temperature to rise to its activation temperature of 40 °C.[214] This process enables more time for stent placement during the operation and showed acceptable in vitro cytotoxicity but relatively low mechanical strengths for stents.[214] Similarly, additive manufacturing methods applied to the printing of UV-activated tert-Butyl acrylate/1, 6-hexanediol diacrylate (tBa/HDDA), and UV- and remote magnetic field-activation of PLA have been described (Figure 6b).[215] In another study, anthracene substitution of PEG segments of PLA-PEG copolymers was found to act as a light-induced SMP;[216] adjusting the ratio of PU/PCL blends was found to manipulate the melting behavior, and shape recovery with the 70/30 blend was most ideal for body temperature shape recovery.[217]
6.3. Bioresorbable shape memory alloys
Much of the research on biodegradable metals has centered on optimizing mechanical properties, corrosion rates, and in vitro and in vivo biocompatibility. There are only a couple of reports to our knowledge of bioresorbable SMAs, which we are terming “BSMAs”. The iron-manganese-silicon (Fe30Mn6Si) alloy consisted of austenite and martensite phases at room temperature, better mechanical properties than pure Fe, and an accelerated corrosion rate, but showed reduced cell viabilities in vitro (Figure 6c).[218]
The second example is of a Mg-scandium (Mg- 20.5 at% Sc) SMA.[219] This particular alloy exhibited a thermoelastic martensitic transformation and superelastic recoverable strains of 4.4% at −150 °C which recovered its set shape upon heating (Figure 6d).[219] While the temperature is not compatible with experiments conducted in the body and Sc can be considered cytotoxic, this example demonstrates that other potential and bioresorbable SMAs may exist. As such, continued research on bioresorbable SMAs would likely benefit by focusing on known bioresorbable metals such as Mg and Zn.
6.4. Summary
SX stents are necessary for treating biliary, peripheral, and cerebrovascular occlusions, but unfortunately, they are not well fit for heavily calcified plaques. As NiTi-based alloys are the primary alloys used in SX stents, these alloys contain a relatively large percentage of Ni (~50 at.%). As mentioned previously, Ni is especially cytotoxic, genotoxic, and mutagenic and can elicit severe inflammatory reactions several mm away from the implantation site.[87–89] Release of the Ni in NiTi-based alloys can be prevented or minimized by controlling the thickness of TiO2 passivating oxide layer which can be formed from Ti present in these alloys.[204, 220–222] Though it is relatively stable when bonded to Ti, studies should continue to monitor its biocompatibility as these alloys are under constant stress and the oxide layer must be highly stable and resistant to cracking. While NiTi-based alloys are the current standard and serve an instrumental purpose, this material should be gradually phased out with the introduction of more advanced bioresorbable SX stents.
The activation temperature of polymer self-expansion is critical. The time taken for the polymer to undergo self-expansion should exceed the operation time. Otherwise, the stent may be deployed prematurely, and such an event would be detrimental. Future research should continue to increase polymer strength through polymer composition while optimizing self-expansion through potentially alternate means such as UV or magnetic field.
A plethora of SX alloys can be developed within the realm of bioresorbable metals as this is a relatively untapped field. These newly developed BSMAs could phase out and potentially eliminate the classic NiTi-based stents, solving any cytotoxic and immunological host responses.
7. Conclusions and perspectives
Given the steady rise in mortality due to atherosclerosis and cardiovascular disease, the demand for stent interventions has increased remarkably and this trend continues. Over the last two decades, we have learned much about new stent materials and their effects on the vasculature and patient health. This new knowledge has not led to significant paradigm shifts yet as the most popular permanent metallic materials are CoCr, Ti, and NiCr, with SS being the least popular. Stent struts have become thinner and thinner to reduce the material mass and exposed surface areas which act as sites to facilitate foreign body reactions and thrombosis. The major players of permanent metallic stents are Boston Scientific, Medtronic, and Abbot Vascular. Nevertheless, with each new stent generation, we find ways to improve upon previous weaknesses, creating a more robust stent. However, there is always a tradeoff. For example, BMS provide excellent strength, but they are rigid and can induce vascular trauma during implantation and cannot prevent SMC overgrowth which leads to restenosis. DES tackled this issue through the release of antimitotic drugs to prevent SMC overgrowth, significantly lowering ISR. But they cause late ST, and new data shows conflicting evidence of their long-term safety for patients. Moreover, the difference in mechanical properties of the stent and the polymer coating can lead to cracking or delamination of the polymer reservoir. New polymer-based stents are generally biodegradable and break down into metabolites easily dealt with by the human body, avoiding long-term chronic inflammation associated with permanent stents, but they lack radio-opacity and material plasticity and have reduced radial forces which causes too much elastic recoil following balloon expansion. SX stents such as NiTi conform to the blood vessel and induce less vascular trauma, but they are not appropriate for heavily calcified areas. Newer biodegradable metal stents such as Fe-, Mg-, and Zn-based alloys may incorporate the benefits of both BMS and DES as they can be designed and manufactured with the ideal mechanical properties and release corrosion products to modulate cellular responses. Additionally, the ions of these metals are naturally existing in the human body and are necessary for numerous physiological and cellular functions. While not the focus of this review, novel biosensor stents were briefly mentioned. They are being developed with MEMS technology and open doors for monitoring patients without the need for radiographic or MRI interventions. Post PCI, patients may experience ISR which may go undetected for several months. MEMS biosensor stents would be a platform for patients to self-monitor and doctors to remotely monitor critically important parameters such as blood pressure, blood flow, and even the cell behavior and adhesion of various cell types to the stent and lesion site. Key review papers on this subject have been well described.[223–224]
The ongoing basic requirements for a stent include 1) maintain enough mechanical strength and radial force to obtain vessel patency for the desired time, 2) have anti-stenosis properties through drug-elution or other means, 3) have a controlled degradation process (for bioresorbable materials) that allows for tissue healing before resorption begins, and 4) be compatible with imaging, specifically radio-visibility during placement and follow-up. Looking forward, we expect to see more research into bioresorbable metals and alloys as the new generation of stents. They have the potential to eliminate chronic long-term complications and avoid secondary surgeries, in addition to eliminating life-long medication. There are already commercialized bioresorbable stents and we can expect this trend to continue to rise over the years. The production of advanced bioresorbable Zn- and Mg-based SMAs (BSMAs) would be of particular interest in the coming years. These BSMAs may have superior mechanical properties early on and capable of larger strain and recovery deformations, which would also apply to cerebral and peripheral stent applications. Nevertheless, the search for the ideal stent with advanced and functional properties for various biomedical applications continues.
Supplementary Material
Acknowledgment and Funding Sources
We thank Dr. Robert T. Pyo, MD, (Director, Interventional Cardiology, Stony Brook Medicine; Director, Cardiac Catheterization Laboratories, Stony Brook University Hospital; Director, Structural Heart Interventions) for his invaluable input associated with practical aspects of biocompatibility and biofunctionality of stents. This work was supported by the National Institutes of Health (Grant number R01HL140562 to D. Zhu).
Acronyms:
- PCI
Percutaneous coronary intervention
- BMS
bare-metal stents
- SS
stainless steel
- 316SS
stainless steel grade
- CoCr
cobalt-chromium
- DES
drug-eluting stents
- ISR
in-stent restenosis
- SMCs
smooth muscle cells
- ST
stent thrombosis
- BRS
bioresorbable stent
- CE
Conformité Européenne
- BVS
bioresorbable vascular scaffolds
- FDA
The Food and Drug Administration
- SX
self-expanding
- SMA
shape memory alloy
- BX
balloon-expanding
- YS
yield strength
- UTS
ultimate tensile strength
- E
Young’s modulus
- CNUH
Chonnan National University Hospital
- PLLA
poly L-lactic acid
- PLGA
poly(lactic-co-glycolic acid)
- PDLLA
poly DL-lactic acid
- SA
salicylic acid
- AA
adipic acid
- PTX
paclitaxel
- PU
polyurethane
- HB
hydroxybutyrate
- PCL
polycaprolactone
- PEVA
polyethylene-co-vinyl acetate
- PMBA
poly (n-butyl methacrylate)
- SIBS
Poly (styrene-b-isobutylene-b-styrene)
- PVDF
poly-vinylidene fluoride
- HFP
hexafluoropropylene
- PC
phosphorylcholine
- HNNF
high nitrogen Ni-free
- FIM
first-in-man
- MACE
major adverse cardiac events
- TVMI
target vessel myocardial infarction
- MgOH2
magnesium hydroxide
- MI
myocardial infarction
- ISA
incomplete stent apposition
- PES
paclitaxel-eluting stent
- ZES
zotarolimus-eluting stent
- EES
everolimus-eluting stent
- DAPT
dual antiplatelet treatment
- PBMA
poly(n-butylmethacrylate)
- Al2O3
alumina
- PTD-PC
Proprietary Tyrocore™ desaminotyrosine polycarbonate
- DLC
diamond-like carbon
- TiNOX
titanium-nitride-oxide
- PEGDMA
poly(ethylene glycol) dimethacrylate
- LIPSS
Laser-Induced Periodic Surface Structures
- FSL
femtosecond laser
- PIII
plasma immersion ion implantation
- PTFE
polytetrafluoroethylene
- PA
peptide amphiphiles
- NO
nitric oxide
- MMP-2
matrix metalloproteinase-2
- ECM
extracellular matrix
- FDM
fibroblast-derived extracellular matrix
- EPC
endothelial progenitor cells
- ELR
elastin-like recombiners
- HUVECs
human umbilical vein endothelial cells
- PDMS
poly(dimethylsiloxane)
- VCAM1
vascular cell adhesion molecule-1
- SMP
shape memory polymers
- PEG
poly(ethylene glycol)
- PPAam
plasma polymeric allylamine
- SBF
simulated body fluid
- HA
hydroxyapatite
- ZnP
Zn phosphate
- Af
austenite finish
- Tg
glass transition
- tBa/HDDA
tert-Butyl acrylate/1, 6-hexanediol diacrylate
- BSMAs
bioresorbable SMAs
Footnotes
Conflict of Interest
Authors declare no conflict of interest.
References
- [1].Im SH, Jung Y, Kim SH, Acta Biomater. 2017, 60, 3. [DOI] [PubMed] [Google Scholar]
- [2].Bowen PK, Shearier ER, Zhao S, Guillory RJ, Zhao F, Goldman J, Drelich JW, Adv. Healthcare Mater 2016, 5, 1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Busch R, Strohbach A, Rethfeldt S, Walz S, Busch M, Petersen S, Felix S, Sternberg K, Acta Biomater. 2014, 10, 688. [DOI] [PubMed] [Google Scholar]
- [4].Marta Francesca Brancati FB, Trani Carlo, Leonzi Ornella, Cuccia Claudio, Crea[h Filippo, Pragmatic and Observational Research 2017, 2018, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marta Francesca Brancati FB, Trani Carlo, Leonzi Ornella, Cuccia Claudio, Crea[h Filippo, Pragmatic Obs. Res 2017, DOI: 10.2147/POR.S132439137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Testa L, Latib A, Montone Rocco A, Colombo A, Bedogni F, Circ. Cardiovasc. Interv 2016, 9, e003978. [DOI] [PubMed] [Google Scholar]
- [7].Michael Heiden EW, and Lia Stanciu J. Biotechnol. Biomater 2015, 5. [Google Scholar]
- [8].Sakamoto A, Jinnouchi H, Torii S, Virmani R, Finn Aloke V, Bioengineering 2018, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Foin N, Di Mario C, Francis DP, Davies JE, Int. J. Cardiol 2013, 164, 259. [DOI] [PubMed] [Google Scholar]
- [10].Ormiston JA, Webber B, Webster MWI, JACC Cardiovasc. Interv 2011, 4, 1310. [DOI] [PubMed] [Google Scholar]
- [11].Foin N, Lee RD, Torii R, Guitierrez-Chico JL, Mattesini A, Nijjer S, Sen S, Petraco R, Davies JE, Di Mario C, Joner M, Virmani R, Wong P, Int. J. Cardiol 2014, 177, 800. [DOI] [PubMed] [Google Scholar]
- [12].O’Brien B, Carroll W, Acta Biomater. 2009, 5, 945. [DOI] [PubMed] [Google Scholar]
- [13].Serruys PW, Iqbal J, Gunn J, Br. Med. Bull 2013, 106, 193. [DOI] [PubMed] [Google Scholar]
- [14].Iqbal J, Onuma Y, Ormiston J, Abizaid A, Waksman R, Serruys P, Eur. Heart J 2013, 35, 765. [DOI] [PubMed] [Google Scholar]
- [15].Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE, Engl N. J. Med 2003, 349, 1315. [DOI] [PubMed] [Google Scholar]
- [16].Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, Popma JJ, Russell ME, Engl N. J. Med 2004, 350, 221. [DOI] [PubMed] [Google Scholar]
- [17].Meredith IT, Verheye S, Dubois CL, Dens J, Fajadet J, Carrié D, Walsh S, Oldroyd KG, Varenne O, El-Jack S, Moreno R, Joshi AA, Allocco DJ, Dawkins KD, J. Am. Coll. Cardiol 2012, 59, 1362. [DOI] [PubMed] [Google Scholar]
- [18].Serruys PW, Silber S, Garg S, van Geuns RJ, Richardt G, Buszman PE, Kelbæk H, van Boven AJ, Hofma SH, Linke A, Klauss V, Wijns W, Macaya C, Garot P, DiMario C, Manoharan G, Kornowski R, Ischinger T, Bartorelli A, Ronden J, Bressers M, Gobbens P, Negoita M, van Leeuwen F, Windecker S, Engl N. J. Med 2010, 363, 136. [DOI] [PubMed] [Google Scholar]
- [19].Windecker S, Serruys PW, Wandel S, Buszman P, Trznadel S, Linke A, Lenk K, Ischinger T, Klauss V, Eberli F, Corti R, Wijns W, Morice M-C, di Mario C, Davies S, van Geuns R-J, Eerdmans P, van Es G-A, Meier B, Jüni P, The Lancet 2008, 372, 1163. [DOI] [PubMed] [Google Scholar]
- [20].Fajadet J, Wijns W, Laarman G-J, Kuck K-H, Ormiston J, Münzel T, Popma JJ, Fitzgerald PJ, Bonan R, Kuntz RE, Circulation 2006, 114, 798. [DOI] [PubMed] [Google Scholar]
- [21].Stone GW, Midei M, Newman W, Sanz M, Hermiller JB, Williams J, Farhat N, Mahaffey KW, Cutlip DE, Fitzgerald PJ, Sood P, Su X, Lansky AJ, f. t. SPIRIT III Investigators, JAMA 2008, 299, 1903.18430909 [Google Scholar]
- [22].Erbel R, Di Mario C, Bartunek J, Bonnier J, de Bruyne B, Eberli FR, Erne P, Haude M, Heublein B, Horrigan M, Ilsley C, Böse D, Koolen J, Lüscher TF, Weissman N, Waksman R, The Lancet 2007, 369, 1869. [DOI] [PubMed] [Google Scholar]
- [23].Haude M, Erbel R, Erne P, Verheye S, Degen H, Böse D, Vermeersch P, Wijnbergen I, Weissman N, Prati F, Waksman R, Koolen J, The Lancet 2013, 381, 836. [DOI] [PubMed] [Google Scholar]
- [24].Haude M, Ince H, Abizaid A, Toelg R, Lemos PA, von Birgelen C, Christiansen EH, Wijns W, Neumann F-J, Kaiser C, Eeckhout E, Lim ST, Escaned J, Onuma Y, Garcia-Garcia HM, Waksman R, Eur. Heart J 2016, 37, 2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bae I-H, Lim K-S, Park J-K, Park D-S, Lee S-Y, Jang E-J, Ji MS, Sim DS, Hong YJ, Ahn Y, Park JC, Cho JG, Kang JC, Kim I-S, Nah J-W, Jeong M-H, J. Ind. Eng. Chem 2015, 21, 1295. [Google Scholar]
- [26].O’Brien BJ, Stinson JS, Larsen SR, Eppihimer MJ, Carroll WM, Biomaterials 2010, 31, 3755. [DOI] [PubMed] [Google Scholar]
- [27].Wang Q, Ren Y, Babar Shahzad M, Zhang W, Pan X, Zhang S, Zhang D, Mater. Sci. Eng., C 2017, 77, 565. [DOI] [PubMed] [Google Scholar]
- [28].Li H-Z, Xu J, J. Mech. Behav. Biomed. Mater 2014, 32, 166. [DOI] [PubMed] [Google Scholar]
- [29].Yang H, Wang C, Liu C, Chen H, Wu Y, Han J, Jia Z, Lin W, Zhang D, Li W, Yuan W, Guo H, Li H, Yang G, Kong D, Zhu D, Takashima K, Ruan L, Nie J, Li X, Zheng Y, Biomaterials 2017, 145, 92. [DOI] [PubMed] [Google Scholar]
- [30].Garg S, Serruys PW, J. Am. Coll. Cardiol 2010, 56, S43. [Google Scholar]
- [31].Hu T, Yang C, Lin S, Yu Q, Wang G, Mater. Sci. Eng., C 2018, 91, 163. [DOI] [PubMed] [Google Scholar]
- [32].Hytönen JP, Taavitsainen J, Tarvainen S, Ylä-Herttuala S, Cardiovasc. Res 2018, 114, 1063. [DOI] [PubMed] [Google Scholar]
- [33].Yoshinobu Onuma SG, Okamura Takayuki, Ligthart Jurgen, van Geuns Robert Jan, de Feyter Pim J, Serruys Patrick W, Tamai Hideo, EuroIntervention 2009, 5. [DOI] [PubMed] [Google Scholar]
- [34].Ormiston JA, Serruys PW, Regar E, Dudek D, Thuesen L, Webster MWI, Onuma Y, Garcia-Garcia HM, McGreevy R, Veldhof S, The Lancet 2008, 371, 899. [DOI] [PubMed] [Google Scholar]
- [35].Ormiston JA, Serruys PW, Onuma Y, Geuns R.-J. v., Bruyne B. d., Dudek D, Thuesen L, Smits PC, Chevalier B, McClean D, Koolen J, Windecker S, Whitbourn R, Meredith I, Dorange C, Veldhof S, Hebert KM, Rapoza R, Garcia-Garcia HM, Circ. Cardiovasc. Interv 2012, 5, 620. [DOI] [PubMed] [Google Scholar]
- [36].Verheye S, Ormiston JA, Stewart J, Webster M, Sanidas E, Costa R, Costa JR, Chamie D, Abizaid AS, Pinto I, Morrison L, Toyloy S, Bhat V, Yan J, Abizaid A, JACC Cardiovasc. Interv 2014, 7, 89. [DOI] [PubMed] [Google Scholar]
- [37].Abizaid A, Costa RA, Schofer J, Ormiston J, Maeng M, Witzenbichler B, Botelho RV, Costa JR, Chamié D, Abizaid AS, Castro JP, Morrison L, Toyloy S, Bhat V, Yan J, Verheye S, JACC Cardiovasc. Interv 2016, 9, 565. [DOI] [PubMed] [Google Scholar]
- [38].Wu Y, Shen L, Ge L, Wang Q, Qian J, Zhang F, Yao K, Huang D, Chen Y, Ge J, Catheter. Cardiovasc. Interv 2016, 87, 630. [DOI] [PubMed] [Google Scholar]
- [39].Lafont A, Presented in BRS 2014 Boston, MA, USA, July 6th 2014. [Google Scholar]
- [40].Tenekecioglu E, Farooq V, Bourantas CV, Silva RC, Onuma Y, Yılmaz M, Serruys PW, BMC Cardiovasc. Disord 2016, 16, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Costa R, Transcatheter Cardiovascular Therapeutics 2012, Miami Beach, FL. [Google Scholar]
- [42].Costa J. d. R., Abizaid A, Chamie D, Lansky A, Kochman J, Koltowski L, J. Am. Coll. Cardiol 2016, 67, 232.26791070 [Google Scholar]
- [43].Abizaid A, Carrié D, Frey N, Lutz M, Weber-Albers J, Dudek D, Chevalier B, Weng S-C, Costa RA, Anderson J, Stone GW, JACC Cardiovasc. Interv 2017, 10, 1832. [DOI] [PubMed] [Google Scholar]
- [44].Jabara R, Pendyala L, Geva S, Chen J, Chronos N, Robinson K, EuroIntervention 2009, 5 Suppl F, F58. [DOI] [PubMed] [Google Scholar]
- [45].R J, Bio-mechanical properties and ABC of bioresorption of adipic acid, 2012PCR focus group on bioresorbable vascular scaffolds Rotterdam, The Netherlands. [Google Scholar]
- [46].J G, The Amaranth Stent: Design and Future Directions, 2012TCTMiami, FL. [Google Scholar]
- [47].Tenekecioglu E, Serruys PW, Onuma Y, Costa R, Chamié D, Sotomi Y, Yu T-B, Abizaid A, Liew H-B, Santoso T, JACC Cardiovasc. Interv 2017, 10, 1115. [DOI] [PubMed] [Google Scholar]
- [48].Seth A, Onuma Y, Chandra P, Bahl VK, Manjunath CN, Mahajan AU, Kumar V, Goel PK, Wander GS, Kaul U, Ajit Kumar VK, Abizaid A, Serruys PW, EuroIntervention 2019, 15, 607. [DOI] [PubMed] [Google Scholar]
- [49].Khashi M, Hassanajili S, Golestaneh SI, Fibers Polym. 2018, 19, 1444. [Google Scholar]
- [50].Sgarioto M, Adhikari R, Gunatillake PA, Moore T, Malherbe F, Nagel M-D, Patterson J, J. Biomed. Mater. Res., Part B 2014, 102, 1711. [DOI] [PubMed] [Google Scholar]
- [51].Park J, Kim J-K, Park SA, Lee D-W, Microelectron. Eng 2019, 206, 1. [Google Scholar]
- [52].Park J, Kim J-K, Kim D-S, Shanmugasundaram A, Park SA, Kang S, Kim S-H, Jeong MH, Lee D-W, Sens. Actuators, B 2019, 280, 201. [Google Scholar]
- [53].Park J, Kim J, Patil SJ, Park J, Park S, Lee D, Sensors 2016, 16, 1. [Google Scholar]
- [54].Cheng Y, Gasior P, Ramzipoor K, Lee C, McGregor JC, Conditt GB, McAndrew T, Kaluza GL, Granada JF, Int. J. Cardiol 2019, 286, 21. [DOI] [PubMed] [Google Scholar]
- [55].FANTOM encore: Tyrocore Bioresorbable Scaffold, https://www.revamedical.com/wp-content/uploads/2018/10/fantom-encore-datasheet.pdf, accessed: 2020.
- [56].Bowen PK, Guillory RJ 2nd, Shearier ER, Seitz J-M, Drelich J, Bocks M, Zhao F, Goldman J, Mater. Sci. Eng. C Mater. Biol. Appl 2015, 56, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Su Y, Cockerill I, Wang Y, Qin Y-X, Chang L, Zheng Y, Zhu D, Trends Biotechnol. 2019, 37, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Carluccio D, Demir AG, Bermingham MJ, Dargusch MS, Metallurgical and Materials Transactions A 2020, DOI: 10.1007/s11661-020-05829-7. [DOI] [Google Scholar]
- [59].Peuster M, Wohlsein P, Brügmann M, Ehlerding M, Seidler K, Fink C, Brauer H, Fischer A, Hausdorf G, Heart (British Cardiac Society) 2001, 86, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Peuster M, Hesse C, Schloo T, Fink C, Beerbaum P, von Schnakenburg C, Biomaterials 2006, 27, 4955. [DOI] [PubMed] [Google Scholar]
- [61].WAKSMAN R, PAKALA R, BAFFOUR R, SEABRON R, HELLINGA D, TIO FO, J. Interv. Cardiol 2008, 21, 15. [DOI] [PubMed] [Google Scholar]
- [62].Pierson D, Edick J, Tauscher A, Pokorney E, Bowen P, Gelbaugh J, Stinson J, Getty H, Lee CH, Drelich J, Goldman J, J. Biomed. Mater. Res., Part B 2012, 100B, 58. [DOI] [PubMed] [Google Scholar]
- [63].Obayi CS, Tolouei R, Mostavan A, Paternoster C, Turgeon S, Okorie BA, Obikwelu DO, Mantovani D, Biomatter 2016, 6, e959874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hermawan H, Mantovani D, Acta Biomater. 2013, 9, 8585. [DOI] [PubMed] [Google Scholar]
- [65].Sotoudeh Bagha P, Khakbiz M, Sheibani S, Hermawan H, J. Alloys Compd 2018, 767, 955. [Google Scholar]
- [66].Lin W, Qin L, Qi H, Zhang D, Zhang G, Gao R, Qiu H, Xia Y, Cao P, Wang X, Zheng W, Acta Biomater. 2017, 54, 454. [DOI] [PubMed] [Google Scholar]
- [67].Hermawan H, Moravej M, Dubé D, Fiset M, Mantovani D, Adv. Mater. Res 2007, 15–17, 113. [Google Scholar]
- [68].Gu XN, Li N, Zheng YF, Ruan L, J. Mater. Sci. Eng. B 2011, 176, 1778. [Google Scholar]
- [69].Bornapour M, Mahjoubi H, Vali H, Shum-Tim D, Cerruti M, Pekguleryuz M, Mater. Sci. Eng., C 2016, 67, 72. [DOI] [PubMed] [Google Scholar]
- [70].Hong D, Saha P, Chou D-T, Lee B, Collins BE, Tan Z, Dong Z, Kumta PN, Acta Biomater. 2013, 9, 8534. [DOI] [PubMed] [Google Scholar]
- [71].Bornapour M, Muja N, Shum-Tim D, Cerruti M, Pekguleryuz M, Acta Biomater. 2013, 9, 5319. [DOI] [PubMed] [Google Scholar]
- [72].Mao L, Shen L, Niu J, Zhang J, Ding W, Wu Y, Fan R, Yuan G, Nanoscale 2013, 5, 9517. [DOI] [PubMed] [Google Scholar]
- [73].Liu F, Chen C, Niu J, Pei J, Zhang H, Huang H, Yuan G, Mater. Sci. Eng., C 2015, 48, 400. [DOI] [PubMed] [Google Scholar]
- [74].Katarivas Levy G, Leon A, Kafri A, Ventura Y, Drelich JW, Goldman J, Vago R, Aghion E, J. Mater. Sci.: Mater. Med 2017, 28, 174. [DOI] [PubMed] [Google Scholar]
- [75].Mostaed E, Sikora-Jasinska M, Mostaed A, Loffredo S, Demir AG, Previtali B, Mantovani D, Beanland R, Vedani M, J. Mech. Behav. Biomed. Mater 2016, 60, 581. [DOI] [PubMed] [Google Scholar]
- [76].Sikora-Jasinska M, Mostaed E, Mostaed A, Beanland R, Mantovani D, Vedani M, Mater. Sci. Eng., C 2017, 77, 1170. [DOI] [PubMed] [Google Scholar]
- [77].Zhao S, McNamara CT, Bowen PK, Verhun N, Braykovich JP, Goldman J, Drelich JW, Metall. Mater. Trans. A 2017, 48, 1204. [Google Scholar]
- [78].Zhao S, Seitz J-M, Eifler R, Maier HJ, Guillory RJ, Earley EJ, Drelich A, Goldman J, Drelich JW, Mater. Sci. Eng., C 2017, 76, 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Li HF, Xie XH, Zheng YF, Cong Y, Zhou FY, Qiu KJ, Wang X, Chen SH, Huang L, Tian L, Qin L, Sci. Rep 2015, 5, 10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tang Z, Niu J, Huang H, Zhang H, Pei J, Ou J, Yuan G, J. Mech. Behav. Biomed. Mater 2017, 72, 182. [DOI] [PubMed] [Google Scholar]
- [81].Yang H, Jia B, Zhang Z, Qu X, Li G, Lin W, Zhu D, Dai K, Zheng Y, Nat. Commun 2020, 11, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Shi Z-Z, Yu J, Liu X-F, Zhang H-J, Zhang D-W, Yin Y-X, Wang L-N, Mater. Sci. Eng., C 2019, 99, 969. [DOI] [PubMed] [Google Scholar]
- [83].Jin H, Zhao S, Guillory R, Bowen PK, Yin Z, Griebel A, Schaffer J, Earley EJ, Goldman J, Drelich JW, Mater. Sci. Eng., C 2018, 84, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhu D, Cockerill I, Su Y, Zhang Z, Fu J, Lee K-W, Ma J, Okpokwasili C, Tang L, Zheng Y, Qin Y-X, Wang Y, ACS Appl. Mater. Interfaces 2019, DOI: 10.1021/acsami.8b20634. [DOI] [PubMed] [Google Scholar]
- [85].Onuma Y, Ormiston J, Serruys PW, Circ. J 2011, 75, 509. [DOI] [PubMed] [Google Scholar]
- [86].Talha M, Behera CK, Sinha OP, Mater. Sci. Eng., C 2013, 33, 3563. [DOI] [PubMed] [Google Scholar]
- [87].Bal W, Kozłowski H, Kasprzak KS, J. Inorg. Biochem 2000, 79, 213. [DOI] [PubMed] [Google Scholar]
- [88].Vahter M, Berglund M, Åkesson A, Lidén C, Environ. Res 2002, 88, 145. [DOI] [PubMed] [Google Scholar]
- [89].Wataha JC, O’Dell NL, Singh BB, Ghazi M, Whitford GM, Lockwood PE, J. Biomed. Mater. Res 2001, 58, 537. [DOI] [PubMed] [Google Scholar]
- [90].Köster R, Vieluf D, Kiehn M, Sommerauer M, Kähler J, Baldus S, Meinertz T, Hamm CW, The Lancet 2000, 356, 1895. [DOI] [PubMed] [Google Scholar]
- [91].Mori H, Kutys R, Romero M, Virmani R, Finn AV, JACC Cardiovasc. Interv 2017, 10, 1175. [DOI] [PubMed] [Google Scholar]
- [92].Ollivier V, Roques C, Receveur N, Gratz M, Feldman L, Letourneur D, Gachet C, Mangin PH, Jandrot-Perrus M, Platelets 2017, 28, 529. [DOI] [PubMed] [Google Scholar]
- [93].Sullivan SJL, Dreher ML, Zheng J, Chen L, Madamba D, Miyashiro K, Trépanier C, Nagaraja S, Shape Memory and Superelasticity 2015, 1, 319. [Google Scholar]
- [94].Shih C-C, Lin S-J, Chen Y-L, Su Y-Y, Lai S-T, Wu GJ, Kwok C-F, Chung K-H, J. Biomed. Mater. Res 2000, 52, 395. [DOI] [PubMed] [Google Scholar]
- [95].Wang J, Song C, Xiao Y, Liu B, J. Biomater. Appl 2018, 33, 64. [DOI] [PubMed] [Google Scholar]
- [96].Li L, Pan S, Zhou X, Meng X, Han X, Ren Y, Yang K, Guan Y, PLoS One 2013, 8, e62193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Li L, An L, Zhou X, Pan S, Meng X, Ren Y, Yang K, Guan Y, Sci. Rep 2016, 6, 18762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jiang W, Tian Q, Vuong T, Shashaty M, Gopez C, Sanders T, Liu H, ACS Biomater. Sci. Eng 2017, 3, 936. [DOI] [PubMed] [Google Scholar]
- [99].Nishio S, Kosuga K, Igaki K, Okada M, Kyo E, Tsuji T, Takeuchi E, Inuzuka Y, Takeda S, Hata T, Takeuchi Y, Kawada Y, Harita T, Seki J, Akamatsu S, Hasegawa S, Bruining N, Brugaletta S, Winter S. d., Muramatsu T, Onuma Y, Serruys PW, Ikeguchi S, Circulation 2012, 125, 2343. [DOI] [PubMed] [Google Scholar]
- [100].Kereiakes DJ, Ellis SG, Metzger C, Caputo RP, Rizik DG, Teirstein PS, Litt MR, Kini A, Kabour A, Marx SO, Popma JJ, McGreevy R, Zhang Z, Simonton C, Stone GW, J. Am. Coll. Cardiol 2017, DOI: 10.1016/j.jacc.2017.10.01024342. [DOI] [PubMed] [Google Scholar]
- [101].Yamaji K, Ueki Y, Souteyrand G, Daemen J, Wiebe J, Nef H, Adriaenssens T, Loh JP, Lattuca B, Wykrzykowska JJ, Gomez-Lara J, Timmers L, Motreff P, Hoppmann P, Abdel-Wahab M, Byrne RA, Meincke F, Boeder N, Honton B, O’Sullivan CJ, Ielasi A, Delarche N, Christ G, Lee JKT, Lee M, Amabile N, Karagiannis A, Windecker S, Räber L, J. Am. Coll. Cardiol 2017, 70, 2330. [DOI] [PubMed] [Google Scholar]
- [102].Ormiston JA, Serruys PWS, Circ. Cardiovasc. Interv 2009, 2, 255. [DOI] [PubMed] [Google Scholar]
- [103].Durand E, Sharkawi T, Leclerc G, Raveleau M, Leest M. v. d., Vert M, Lafont A, Circ. Cardiovasc. Interv 2014, 7, 70. [DOI] [PubMed] [Google Scholar]
- [104].Yahagi K, Yang Y, Torii S, Mensah J, White RM, Mathieu M, Pacheco E, Nakano M, Barakat A, Sharkawi T, Vert M, Joner M, Finn AV, Virmani R, Lafont A, Am J. Heart Assoc. 2017, 6, e005693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].P. K. Bowen, E. R. Shearier, S. Zhao, R. J. Guillory, F. Zhao, J. Goldman, J. W. Drelich, 2016, 5, 1121. [DOI] [PMC free article] [PubMed]
- [106].Rapetto C, Leoncini M, J. Thorac. Dis 2017, S903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Christensen CW, Rieder MA, Silverstein EL, Gencheff NE, Circulation 1995, 92, 2617. [DOI] [PubMed] [Google Scholar]
- [108].Kemp PA, Gardiner SM, March JE, Rubin PC, Bennett T, Br J Pharmacol 1999, 126, 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Berthon N, Laurant P, Fellmann D, Berthelot A, J. Cardiovasc. Pharmacol 2003, 42, 24. [DOI] [PubMed] [Google Scholar]
- [110].Sternberg K, Gratz M, Koeck K, Mostertz J, Begunk R, Loebler M, Semmling B, Seidlitz A, Hildebrandt P, Homuth G, Grabow N, Tuemmler C, Weitschies W, Schmitz K-P, Kroemer HK, J. Biomed. Mater. Res., Part B 2012, 100B, 41. [DOI] [PubMed] [Google Scholar]
- [111].Zhang J, Kong N, Shi Y, Niu J, Mao L, Li H, Xiong M, Yuan G, Corros. Sci 2014, 85, 477. [Google Scholar]
- [112].Zhang J, Li H, Wang W, Huang H, Pei J, Qu H, Yuan G, Li Y, Acta Biomater. 2018, 69, 372. [DOI] [PubMed] [Google Scholar]
- [113].Peuster M, Wohlsein P, Brugmann M, Ehlerding M, Seidler K, Fink C, Brauer H, Fischer A, Hausdorf G, Heart 2001, 86, 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Haase H, Rink L, Biofactors 2013, 40, 27. [DOI] [PubMed] [Google Scholar]
- [115].Khan W, Farah S, Domb AJ, J. of Controlled Release 2012, 161, 703. [DOI] [PubMed] [Google Scholar]
- [116].Martin DM, Boyle FJ, Med. Eng. Phys 2011, 33, 148. [DOI] [PubMed] [Google Scholar]
- [117].Mauri L, Silbaugh TS, Garg P, Wolf RE, Zelevinsky K, Lovett A, Varma MR, Zhou Z, Normand S-LT, N. Engl. J. Med 2008, 359, 1330. [DOI] [PubMed] [Google Scholar]
- [118].Kirtane AJ, Gupta A, Iyengar S, Moses JW, Leon MB, Applegate R, Brodie B, Hannan E, Harjai K, Jensen LO, Park S-J, Perry R, Racz M, Saia F, Tu JV, Waksman R, Lansky AJ, Mehran R, Stone GW, Circulation 2009, 119, 3198. [DOI] [PubMed] [Google Scholar]
- [119].Camenzind E, Steg PG, Wijns W, Circulation 2007, 115, 1440. [DOI] [PubMed] [Google Scholar]
- [120].Pfisterer M, Brunner-La Rocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, Jeger R, Bader F, Osswald S, Kaiser C, J. Am. Coll. Cardiol 2006, 48, 2584. [DOI] [PubMed] [Google Scholar]
- [121].Wenaweser P, Rey C, Eberli FR, Togni M, Tuller D, Locher S, Remondino A, Seiler C, Hess OM, Meier B, Windecker S, Eur. Heart J 2005, 26, 1180. [DOI] [PubMed] [Google Scholar]
- [122].Attizzani GF, Capodanno D, Ohno Y, Tamburino C, J. Am. Coll. Cardiol 2014, 63, 1355. [DOI] [PubMed] [Google Scholar]
- [123].McGinty S, Pontrelli G, J. Controlled Release 2015, 217, 327. [DOI] [PubMed] [Google Scholar]
- [124].McGinty S, Pontrelli G, J. Math. Chem 2016, 54, 967. [Google Scholar]
- [125].McGinty S, McKee S, McCormick C, Wheel M, Mathematical Medicine and Biology: A Journal of the IMA 2015, 32, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ako J, Bonneau HN, Honda Y, Fitzgerald PJ, Am. J. Cardiol 2007, 100, 3m. [DOI] [PubMed] [Google Scholar]
- [127].Huang Y, Ng HCA, Ng XW, Subbu V, J. of Controlled Release 2014, 193, 188. [DOI] [PubMed] [Google Scholar]
- [128].Rizas KD, Mehilli J, Circ. Cardiovasc. Interv 2016, 9, e002943. [DOI] [PubMed] [Google Scholar]
- [129].Joner M, Nakazawa G, Finn AV, Quee SC, Coleman L, Acampado E, Wilson PS, Skorija K, Cheng Q, Xu X, Gold HK, Kolodgie FD, Virmani R, J. Am. Coll. Cardiol 2008, 52, 333. [DOI] [PubMed] [Google Scholar]
- [130].Hu T, Yang J, Cui K, Rao Q, Yin T, Tan L, Zhang Y, Li Z, Wang G, ACS Appl. Mater. Interfaces 2015, 7, 11695. [DOI] [PubMed] [Google Scholar]
- [131].Delattre C, Velazquez D, Roques C, Pavon-Djavid G, Ollivier V, Lokajczyk A, Avramoglou T, Gueguen V, Louedec L, Caligiuri G, Jandrot-Perrus M, Boisson-Vidal C, Letourneur D, Meddahi-Pelle A, Bioimpacts 2019, 9, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R, Arterioscler. Thromb. Vasc. Biol 2007, 27, 1500. [DOI] [PubMed] [Google Scholar]
- [133].Lih E, Jung JW, Joung YK, Ahn DJ, Han DK, Colloids Surf., B 2016, 140, 353. [DOI] [PubMed] [Google Scholar]
- [134].Kim SM, Park KS, Lih E, Hong YJ, Kang JH, Kim IH, Jeong MH, Joung YK, Han DK, Acta Biomater. 2016, 38, 143. [DOI] [PubMed] [Google Scholar]
- [135].Du R, Wang Y, Huang Y, Zhao Y, Zhang D, Du D, Zhang Y, Li Z, McGinty S, Pontrelli G, Yin T, Wang G, NPG Asia Mater. 2018, 10, 642. [Google Scholar]
- [136].Deng J, Han Y, Sun M, Tao J, Yan C, Kang J, Li S, PLoS One 2013, 8, e60735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Jinnouchi H, Torii S, Sakamoto A, Kolodgie FD, Virmani R, Finn AV, Nat. Rev. Cardiol 2019, 16, 286. [DOI] [PubMed] [Google Scholar]
- [138].Indolfi C, De Rosa S, Colombo A, Nat. Rev. Cardiol 2016, 13, 719. [DOI] [PubMed] [Google Scholar]
- [139].Orlik B, Milewski K, Derbisz K, Jelonek M, Chrząszcz P, Beil S, Młodziankowski A, Picheta W, Buszman PP, Buszman PE, Postepy w kardiologii interwencyjnej 2018, 14, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].McMahon S, Bertollo N, Cearbhaill EDO, Salber J, Pierucci L, Duffy P, Dürig T, Bi V, Wang W, Prog. Polym. Sci 2018, 83, 79. [Google Scholar]
- [141].Verheye S, TCT 16: Prospective, Multicenter Evaluation of the DESolve Novolimus-Eluting Bioresorbable Coronary Scaffold - Imaging Outcomes and Five-Year Clinical and Imaging Results, https://www.tctmd.com/slide/tct-16-prospective-multicenter-evaluation-desolve-novolimus-eluting-bioresorbable-coronary, accessed.
- [142].Verheye S, Desolve Nx, Cx, and Amity: A Family of Progressively Thinner-Strut PLLA-Based BRS With Novel Properties, https://www.tctmd.com/slide/desolve-nx-cx-and-amity-family-progressively-thinner-strut-plla-based-brs-novel-properties, accessed. [Google Scholar]
- [143].Regazzoli D, Leone PP, Colombo A, Latib A, J. Thorac. Dis 2017, 9, S979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Abizaid A, New 24-month data from the FANTOM II clinical trial and initial results from Fantom BRS expansion trials, https://www.revamedical.com/wp-content/uploads/2018/05/europcr_2018_abizaid_final.pdf, accessed.
- [145].Kuwabara K, Zen K, Ito Y, Kadoya Y, Wakana N, Yanishi K, Yamada H, Igaki K, Matoba S, Circulation 2018, 138, A14381. [Google Scholar]
- [146].Wu Y, Shen L, Wang Q, Ge L, Xie J, Hu X, Sun A, Qian J, Ge J, J. Biomed. Biotechnol 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Shen L, Wang Q, Wu Y, Hu X, Xie J, Ge J, Polym. Bull 2012, 68, 1171. [Google Scholar]
- [148].Shen L, Wu Y, Ge L, Zhang Y, Wang Q, Qian J, Qiu Z, Ge J, The International Journal of Cardiovascular Imaging 2017, 33, 1473. [DOI] [PubMed] [Google Scholar]
- [149].Li M, Xu X, Jia Z, Shi Y, Cheng Y, Zheng Y, J. Mater. Chem. B 2017, 5, 1182. [DOI] [PubMed] [Google Scholar]
- [150].Lin W-J, Zhang D-Y, Zhang G, Sun H-T, Qi H-P, Chen L-P, Liu Z-Q, Gao R-L, Zheng W, Mater. Des 2016, 91, 72. [Google Scholar]
- [151].Zheng J-F, Qiu H, Tian Y, Hu X-Y, Luo T, Wu C, Tian Y, Tang Y, Song L-F, Li L, Xu L, Xu B, Gao R-L, JACC Cardiovasc. Interv 2019, 12, 245. [DOI] [PubMed] [Google Scholar]
- [152].Biotronik, https://www.magmaris.com/en/clinical-studies/BIOSOLVE-II, accessed.
- [153].Shi Y, Zhang L, Chen J, Zhang J, Yuan F, Shen L, Chen C, Pei J, Li Z, Tan J, Yuan G, Mater. Sci. Eng., C 2017, 80, 1. [DOI] [PubMed] [Google Scholar]
- [154].Gutensohn K, Beythien C, Bau J, Fenner T, Grewe P, Koester R, Padmanaban K, Kuehnl P, Thromb. Res 2000, 99, 577. [DOI] [PubMed] [Google Scholar]
- [155].Airoldi F, Colombo A, Tavano D, Stankovic G, Klugmann S, Paolillo V, Bonizzoni E, Briguori C, Carlino M, Montorfano M, Liistro F, Castelli A, Ferrari A, Sgura F, Di Mario C, Am. J. Cardiol 2004, 93, 474. [DOI] [PubMed] [Google Scholar]
- [156].Meireles GC, de Abreu LM, Forte AA, Sumita MK, Sumita JH, Aliaga Jdel C, Arq. Bras. Cardiol 2007, 88, 390. [DOI] [PubMed] [Google Scholar]
- [157].Windecker S, Simon R, Lins M, Klauss V, Eberli FR, Roffi M, Pedrazzini G, Moccetti T, Wenaweser P, Togni M, Tuller D, Zbinden R, Seiler C, Mehilli J, Kastrati A, Meier B, Hess OM, Circulation 2005, 111, 2617. [DOI] [PubMed] [Google Scholar]
- [158].Karjalainen PP, Ylitalo AS, Juhani Airaksinen KE, EuroIntervention 2006, 2, 187. [PubMed] [Google Scholar]
- [159].Karjalainen PP, Ylitalo A, Airaksinen JK, Invasive Cardiol J. 2006, 18, 462. [PubMed] [Google Scholar]
- [160].Di Mario C, Grube E, Nisanci Y, Reifart N, Colombo A, Rodermann J, Muller R, Umman S, Liistro F, Montorfano M, Alt E, Int. J. Cardiol 2004, 95, 329. [DOI] [PubMed] [Google Scholar]
- [161].ElSawy AM, Attia NF, Mohamed HI, Mohsen M, Talaat MH, Mater. Sci. Eng., C 2019, 96, 708. [DOI] [PubMed] [Google Scholar]
- [162].Trzaskowska PA, Kuzminska A, Butruk-Raszeja B, Rybak E, Ciach T, Colloids Surf., B 2018, 167, 499. [DOI] [PubMed] [Google Scholar]
- [163].McDaniel C, Gladkovskaya O, Flanagan A, Rochev Y, O’Connor GM, RSC Adv. 2015, 5, 42548. [Google Scholar]
- [164].Velmurugan C, Senthilkumar V, Dinesh S, Arulkirubakaran D, Mach. Sci. Technol 2018, 22, 355. [Google Scholar]
- [165].Liang C, Hu Y, Wang H, Xia D, Li Q, Zhang J, Yang J, Li B, Li H, Han D, Dong M, Biomaterials 2016, 103, 170. [DOI] [PubMed] [Google Scholar]
- [166].Diaz-Rodriguez S, Chevallier P, Paternoster C, Montaño-Machado V, Noël C, Houssiau L, Mantovani D, RSC Adv. 2019, 9, 2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Bai J, Xu Y, He X, Li C, Zhu M, Zhongguo xiu fu chong jian wai ke za zhi = Zhongguo xiufu chongjian waike zazhi = Chinese journal of reparative and reconstructive surgery 2018, 32, 1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Yang B, Zhang Y, Xuan F-Z, Xiao B, He L, Gao Y, Adv. Compos. Mater 2018, 27, 331. [Google Scholar]
- [169].Park C, Kim S, Kim H-E, Jang T-S, Surf. Coat. Technol 2016, 305, 139. [Google Scholar]
- [170].Lee M-K, Park C, Jang T-S, Kim H-E, Jeong S-H, Mater. Lett 2018, 216, 12. [Google Scholar]
- [171].Alexander GC, Hwang PTJ, Chen J, Kim J, Brott BC, Yoon YS, Jun HW, ACS Biomater. Sci. Eng 2018, 4, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Yang Z, Yang Y, Zhang L, Xiong K, Li X, Zhang F, Wang J, Zhao X, Huang N, Biomaterials 2018, 178, 1. [DOI] [PubMed] [Google Scholar]
- [173].Bedair TM, Min IJ, Park W, Park BJ, Joung YK, Han DK, J. Ind. Eng. Chem 2019, 70, 385. [Google Scholar]
- [174].Castellanos MI, Zenses AS, Grau A, Rodriguez-Cabello JC, Gil FJ, Manero JM, Pegueroles M, Colloids Surf., B 2015, 127, 22. [DOI] [PubMed] [Google Scholar]
- [175].Pacharra S, Ortiz R, McMahon S, Wang W, Viebahn R, Salber J, Quintana I, J. Biomed. Mater. Res. B Appl. Biomater 2019, 107, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Pacharra S, Ortiz R, McMahon S, Wang W, Viebahn R, Salber J, Quintana I, Journal of Biomedical Materials Research Part B: Applied Biomaterials 2019, 107, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Wang D, Liu M, Gu S, Zhou Y, Li S, J. Biomater. Nanobiotechnol 2017, 8, 189. [Google Scholar]
- [178].Govindarajan T, Shandas R, ACS Appl. Bio Mater 2019, DOI: 10.1021/acsabm.8b00833. [DOI] [PubMed] [Google Scholar]
- [179].Cheng X, Kondyurin A, Bao S, Bilek MMM, Ye L, Appl. Surf. Sci 2017, 416, 686. [Google Scholar]
- [180].Cheng X, Fei J, Kondyurin A, Fu K, Ye L, Bilek MMM, Bao S, Mater. Sci. Eng., C 2019, 99, 863. [DOI] [PubMed] [Google Scholar]
- [181].Luthringer B, Feyerabend F, Willumeit R, Magnesium research : official organ of the International Society for the Development of Research on Magnesium 2014, 27, 142. [DOI] [PubMed] [Google Scholar]
- [182].Willumeit-Römer R, JOM 2019, 71, 1447. [Google Scholar]
- [183].Willumeit-Römer R, Ahmad Agha N, Luthringer B, Cham 2018. [Google Scholar]
- [184].Wan P, Wu J, Tan L, Zhang B, Yang K, Mater. Sci. Eng., C 2013, 33, 2885. [DOI] [PubMed] [Google Scholar]
- [185].Ye S-H, Chen Y, Mao Z, Gu X, Shankarraman V, Hong Y, Shanov V, Wagner WR, Langmuir 2019, 35, 1421. [DOI] [PubMed] [Google Scholar]
- [186].Xu L, Yamamoto A, Colloids Surf., B 2012, 93, 67. [DOI] [PubMed] [Google Scholar]
- [187].Wang C, Fang H, Qi X, Hang C, Sun Y, Peng Z, Wei W, Wang Y, Acta Biomater. 2019, DOI: 10.1016/j.actbio.2019.04.048. [DOI] [PubMed] [Google Scholar]
- [188].Zhang L-C, Xu M, Hu Y-D, Gao F, Gong T, Liu T, Li X, Pan C-J, Appl. Surf. Sci 2018, 451, 20. [Google Scholar]
- [189].Qi P, Yang Y, Zhao S, Wang J, Li X, Tu Q, Yang Z, Huang N, Mater. Des 2016, 96, 341. [Google Scholar]
- [190].Mousa HM, Abdal-hay A, Bartnikowski M, Mohamed IMA, Yasin AS, Ivanovski S, Park CH, Kim CS, ACS Biomater. Sci. Eng 2018, 4, 2169. [DOI] [PubMed] [Google Scholar]
- [191].Zhou J, Yang Y, Alonso Frank M, Detsch R, Boccaccini AR, Virtanen S, ACS Appl Mater Interfaces 2016, 8, 26482. [DOI] [PubMed] [Google Scholar]
- [192].Huang T, Zheng Y, Han Y, Regener. Biomater 2016, 3, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Huang T, Cheng Y, Zheng Y, Colloids Surf., B 2016, 142, 20. [DOI] [PubMed] [Google Scholar]
- [194].Huang T, Zheng Y, Sci. Rep 2016, 6, 23627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Cheng J, Huang T, Zheng YF, Mater. Sci. Eng., C 2015, 48, 679. [DOI] [PubMed] [Google Scholar]
- [196].Yang Y, Zhou J, Detsch R, Taccardi N, Heise S, Virtanen S, Boccaccini AR, Mater. Sci. Eng., C 2018, 85, 203. [DOI] [PubMed] [Google Scholar]
- [197].Santos C, Alves M, Montemor M, Carmezim M, in Advanced Materials and their Applications - Micro to nano scale (Eds: Ahmad I, Sia PD, Raza R), One Central Press (OCP) 2017, p. 219. [Google Scholar]
- [198].Drelich A, Bowen P, LaLonde L, Goldman J, Drelich J, Importance of Oxide Film in Endovascular Biodegradable Zinc Stents, 2016. [Google Scholar]
- [199].Cockerill I, Su Y, Lee JH, Berman D, Young ML, Zheng Y, Zhu D, Nano Lett. 2020, 20, 4594. [DOI] [PubMed] [Google Scholar]
- [200].Cockerill I, Su Y, Bitten R, Cloarec B, Aouadi S, Zhu D, Young ML, JOM 2020, 72, 1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Alves MM, Prosek T, Santos CF, Montemor MF, Mater. Sci. Eng. C Mater. Biol. Appl 2017, 70, 112. [DOI] [PubMed] [Google Scholar]
- [202].Su Y, Wang K, Gao J, Yang Y, Qin Y-X, Zheng Y, Zhu D, Acta Biomater. 2019, DOI: 10.1016/j.actbio.2019.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Petrini L, Migliavacca F, J. Metall 2011, 2011. [Google Scholar]
- [204].Morgan NB, J. Mater. Sci. Eng. A 2004, 378, 16. [Google Scholar]
- [205].Bekken JA, Jongsma H, de Vries J-PP, Fioole B, Medical devices (Auckland, N.Z.) 2014, 7, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Gupta S, Pelton AR, Weaver JD, Gong X-Y, Nagaraja S, J. Mech. Behav. Biomed. Mater 2015, 44, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Duda SH, Wiskirchen J, Tepe G, Bitzer M, Kaulich TW, Stoeckel D, Claussen CD, J. Vasc. Interv. Radiol 2000, 11, 645. [DOI] [PubMed] [Google Scholar]
- [208].Cho S-H, Jo W-I, Jo Y-E, Yang KH, Park JC, Lee DH, Neurointervention 2017, 12, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Krankenberg H, Zeller T, Ingwersen M, Schmalstieg J, Gissler HM, Nikol S, Baumgartner I, Diehm N, Nickling E, Muller-Hulsbeck S, Schmiedel R, Torsello G, Hochholzer W, Stelzner C, Brechtel K, Ito W, Kickuth R, Blessing E, Thieme M, Nakonieczny J, Nolte T, Gareis R, Boden H, Sixt S, JACC Cardiovasc. Interv 2017, 10, 1694. [DOI] [PubMed] [Google Scholar]
- [210].Ponec D, Jaff MR, Swischuk J, Feiring A, Laird J, Mehra M, Popma JJ, Donohoe D, Firth B, Keim E, Snead D, J. Vasc. Interv. Radiol 2004, 15, 911. [DOI] [PubMed] [Google Scholar]
- [211].Szikora I, Berentei Z, Kulcsar Z, Barath K, Berez A, Bose A, Nyary I, Acta Neurochir. (Wien.) 2006, 148, 711. [DOI] [PubMed] [Google Scholar]
- [212].Shamimi A, Amin-Ahmadi B, Stebner A, Duerig T, Shape Memory and Superelasticity 2018, 4, 417. [Google Scholar]
- [213].Amin-Ahmadi B, Gallmeyer T, Pauza JG, Duerig TW, Noebe RD, Stebner AP, Scripta Mater. 2018, 147, 11. [Google Scholar]
- [214].Gu S-Y, Chang K, Jin S-P, J. Appl. Polym. Sci 2018, 135, 45686. [Google Scholar]
- [215].Wei H, Zhang Q, Yao Y, Liu L, Liu Y, Leng J, ACS Appl. Mater. Interfaces 2017, 9, 876. [DOI] [PubMed] [Google Scholar]
- [216].Xie H, He MJ, Deng XY, Du L, Fan CJ, Yang KK, Wang YZ, ACS Appl Mater Interfaces 2016, 8, 9431. [DOI] [PubMed] [Google Scholar]
- [217].Ajili SH, Ebrahimi NG, Soleimani M, Acta Biomater. 2009, 5, 1519. [DOI] [PubMed] [Google Scholar]
- [218].Liu B, Zheng YF, Ruan L, Mater. Lett 2011, 65, 540. [Google Scholar]
- [219].Ogawa Y, Ando D, Sutou Y, Koike J, Science 2016, 353, 368. [DOI] [PubMed] [Google Scholar]
- [220].Duerig T, Pelton A, Stöckel D, J. Mater. Sci. Eng. A 1999, 273–275, 149. [Google Scholar]
- [221].Machado LG, Savi MA, Braz. J. Med. Biol. Res 2003, 36, 683. [DOI] [PubMed] [Google Scholar]
- [222].Biscarini A, Mazzolai G, Tuissi A, Recent Pat. Biomed. Eng 2008, 1, 180. [Google Scholar]
- [223].Hoare D, Bussooa A, Neale S, Mirzai N, Mercer J, Advanced Science 2019, 6, 1900856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Bussooa A, Hoare D, Kirimi MT, Mitra S, Mirzai N, Neale SL, Mercer JR, Advanced Science, DOI: 10.1002/advs.2019029991902999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


