Abstract
Background
Vaccine-associated anaphylaxis is a rare event (1.34 events/million doses; 0.00017% occurrence over 26 years). Several reports of allergic reactions concerning for anaphylaxis have been reported early into the Pfizer-BioNTech and Moderna coronavirus disease 2019 (COVID-19) vaccine campaign in the United States, Canada, and the United Kingdom.
Objective
To perform a cost-effectiveness analysis characterizing the risks of COVID-19 versus vaccine anaphylaxis, comparing universal COVID-19 vaccination versus risk-stratified vaccination approaches.
Methods
Cohort analysis models were created to evaluate the cost-effectiveness of universal vaccination versus risk-stratified vaccination (eg, contraindicated in persons with a history of any previous episode of anaphylaxis) with a threshold for cost-effective care at $10,000,000 per death prevented. In the base case, risk of anaphylaxis was estimated at 0.1%, with case-fatality estimated at 0.3%.
Results
On a population level (n = 300,000,000 simulated persons), universal vaccination was associated with a cost-savings of $503,596,316 and saved 7,607 lives, but the cost-savings was sensitive to increasing rates of vaccine-associated anaphylaxis. The universal strategy dominated at higher rates of COVID-19 infection and low rates of vaccine-associated anaphylaxis in both the health care and societal perspectives. When the risk of vaccine-associated anaphylaxis exceeded 0.8%, the risk-stratified approach to vaccination was the most cost-effective strategy. There was also an interaction between anaphylaxis risk and anaphylaxis fatality, with a risk-stratified approach becoming cost-effective as each risk increased concurrently. Stratified observation time by anaphylaxis history (15 minutes vs 30 minutes) was not cost-effective until a 1% anaphylaxis case-fatality was assumed and risk of vaccine anaphylaxis exceeded 6%.
Conclusions
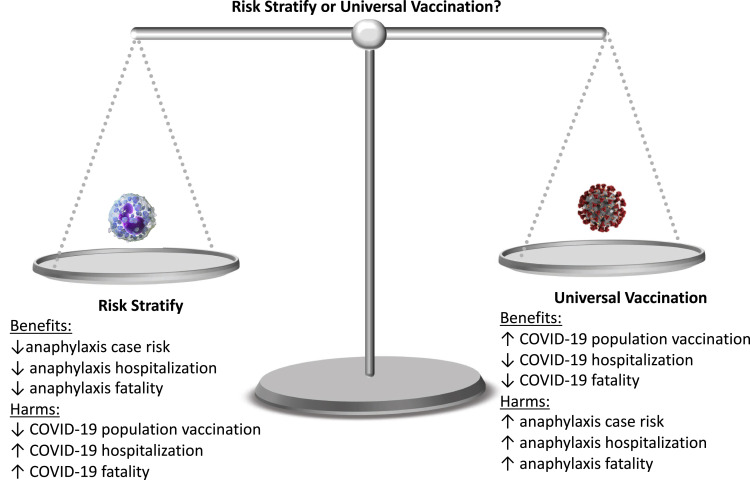
This study demonstrates that unless vaccine anaphylaxis rates exceed 0.8%, a universal vaccination approach dominates a risk-stratified approach where persons with any history of anaphylaxis would be contraindicated from vaccination, with lower cost and superior health outcomes.
Key words: COVID-19, Vaccination, Anaphylaxis, Risk-stratification, Vaccine Adverse Events Registry System, Adverse events, Cost-effectiveness, CDC
Abbreviations used: CDC, Centers for Disease Control and Prevention; COVID-19, Coronavirus disease 2019; EUA, Emergency use authorization; ICER, Incremental cost-effectiveness ratio; PEG, Polyethylene glycol
What is already known about this topic? Despite robust safety data in the randomized controlled trial, allergic reactions suspicious for anaphylaxis have been reported with early Pfizer-BioNTech and Moderna coronavirus disease 2019 (COVID-19) vaccine administration in the United Kingdom, Canada, and the United States.
What does this article add to our knowledge? In a cost-effectiveness model comparing universal versus risk-stratified vaccination, universal vaccination is cost saving and provides superior health outcomes. With low COVID-19 risk and vaccine-associated anaphylaxis rates higher than 0.8%, a risk-stratified approach can be cost-effective.
How does this study impact current management guidelines? Approaches to COVID-19 vaccination will be influenced by vaccine-associated anaphylaxis risk and COVID-19 infection risk. A universal vaccination model provides superior health outcomes to a risk-stratified approach, unless vaccine anaphylaxis risk surpasses 0.8%.
Introduction
As of December 13, 2020, the novel coronavirus disease 2019 (COVID-19), caused by the pathogen severe acute respiratory syndrome coronavirus 2, had infected more than 72 million people internationally, with more than 1.6 million global deaths.1 On December 11, 2020, the US Food and Drug Administration issued its first emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to be distributed in the United States for persons 16 years and older.2 The US Food and Drug Administration subsequently issued an EUA for the Moderna COVID-19 vaccine.3 Efficacy of the Pfizer-BioNTech COVID-19 vaccine is estimated to be 95% (95% CI, 90.3%-97.6%) after the second dose, with the 2 doses administered intramuscularly 3 weeks apart.4 For the Moderna vaccine, efficacy is estimated to be 94.1% (95% CI, 89.3%-96.8%), administered intramuscularly 1 month apart.3 Not surprisingly, COVID-19 vaccines are expected to be a cost-effective intervention to help overcome the ongoing pandemic.5
The Pfizer-BioNTech COVID-19 vaccine EUA was based on safety data of 37,586 participants in an ongoing multinational randomized placebo controlled trial including the United States, who were followed for a median of 2 months after the second dose of vaccine administration.6 The most commonly reported side effects, such as headache, fatigue, and injection-site pain, were mild and short-lasting.6 There was no statistically significant difference in hypersensitivity-related adverse events in the vaccine group (0.63%) compared with the placebo group (0.51%) in the randomized placebo controlled trial.7 , 8 The Moderna vaccine EUA was based on data from 30,420 participants followed for 14 days after second vaccination, which noted a 0.4% excess rate of hypersensitivity of any severity, and no attributable increase in anaphylaxis.3 , 9 However, on the first day of Pfizer-BioNTech vaccine administration in the United Kingdom, there were 2 reports of reactions suspicious for anaphylaxis among 2 recipients, both with a previous history of a severe allergic reaction (1 to food, 1 to a drug) and both of whom carried epinephrine autoinjectors.7 Both these health care workers recovered with standard treatment for a presumed allergic reaction. There have since been reports of reactions concerning for potential anaphylaxis among 5 vaccine recipients in the United States within the first 2 weeks both the Pfizer-BioNTech and Moderna vaccines were available, including events occurring in persons both with and without a history of previous allergic disease.10 , 11 Given the vaccine distribution patterns to date, the overwhelming majority of cases have occurred in health care workers, with vaccine administration in a health care setting. In January 2021, the Centers for Disease Control and Prevention (CDC) COVID-19 Response Team reported 21 cases of anaphylaxis adjudicated by the Brighton Collaboration case definition out of 1,893,360 first doses of the Pfizer-BioNTech COVID-19 vaccine, and 10 cases occurring within the first 4,041,396 doses of the Moderna vaccine.12 , 13 If these cases are truly anaphylaxis, this would represent a rate of 11.1 per million doses administered for the Pfizer-BioNTech vaccine and 2.5 per million doses administered for the Moderna vaccine12 , 13; however, many of these cases did not clearly fulfill anaphylaxis definitions proposed by the National Institute of Allergy and Infectious Disease and the Food Allergy and Anaphylaxis Network or the 2020 World Allergy Organization criteria.14, 15, 16
In response to these events, the UK Medicines and Healthcare Products Regulatory Agency placed a contraindication for individuals with a history of a severe allergic reaction to any vaccine, medication, or food to receive the Pfizer-BioNTech (no contraindication was placed for Moderna because this was not planned to be administered in the United Kingdom).17 The CDC has recommended similar but slightly less broad restriction, that a severe allergic reaction to a vaccine or injectable medication is a precaution to COVID-19 vaccination with the Pfizer-BioNTech and Moderna vaccines.18 In addition, the CDC recommends that patients with such a history necessitating this precaution should be observed for 30 minutes after vaccination, versus the standard observation time of 15 minutes.18 In Canada, similar contraindication against receiving the Pfizer-BioNTech product for persons with allergies to any of the ingredients in the vaccine was issued.19 Within days of issuing these precautions, the CDC further specified that individuals having severe allergic reactions (defined by use of epinephrine or requiring treatment in the hospital) with their initial COVID-19 vaccine dose should not receive the second dose of either the Pfizer-BioNTech or Moderna vaccine.18 Notably, an approach of vaccine deferral in patients with history of non–COVID-19 vaccine anaphylaxis was quickly revised in the United Kingdom, and in the United States a history of anaphylaxis (unrelated to a COVID-19 vaccine component) was never a contraindication to vaccination.18 , 20 Still, the precautionary approaches implemented in patients with histories of anaphylaxis agents not related to COVID-19 vaccines may lead to a higher rate of vaccine deferral as a result of anxiety and inconvenience.21 , 22
The approval of the Pfizer-BioTech and Moderna COVID-19 vaccines represents the first true opportunity to mitigate COVID's devastating impact. There is a need to contextualize the risk of vaccine-associated anaphylaxis to the real and ongoing threat that COVID-19 poses to national health and to the economy (both directly as related to detection and management of the infection, and indirectly as a result of measures taken to reduce community spread).23 As a result, a cost-effectiveness analysis was undertaken to characterize the risks of COVID-19 versus vaccine anaphylaxis, comparing universal COVID-19 vaccination versus risk-stratified vaccination where persons with a certain past allergic history would be excluded from vaccination secondary to the risk of a vaccine-associated reaction.
Methods
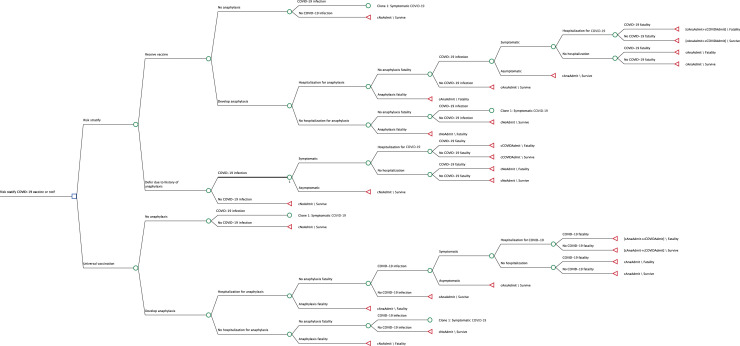
TreeAge Pro (Williamstown, Mass) was used to construct a decision tree comparing risks of COVID-19 infection and COVID-19 vaccine anaphylaxis (Figure 1 ). Strategies compared were (1) universal COVID-19 vaccination versus (2) risk-stratified vaccination based on history of anaphylaxis attributable to any trigger, where vaccination is deferred in patients with a history of self-reported anaphylaxis (see Figure E1 in this article's Online Repository at www.jaci-inpractice.org, closely approximating the previous recommendation of the Medicines and Healthcare Products Regulatory Agency, which was broader than that recommended by the CDC). In the universal model, rates of vaccine deferral were assumed to be equivalent between patients with and without anaphylaxis, and in each model vaccine efficacy in recipients was equivalent regardless of anaphylaxis history. The primary outcomes of total hospitalizations and total fatalities from anaphylaxis or COVID-19 infection were evaluated from a health care perspective. To create a parsimonious model, downstream consequences and costs of COVID-19 infection (such as post–COVID-19 symptoms) or nonanaphylactic sequela of vaccination were not included in the model. As well, productivity losses as a result of COVID-19 or anaphylaxis fatalities were not included. Cohort analysis was performed to evaluate strategy cost, episodes of anaphylaxis, hospitalization from anaphylaxis and COVID-19 infection, and fatality from anaphylaxis and COVID-19 infection. All costs were expressed in 2020 dollars and, because the time horizon was 1 year, discounting and all-cause age-adjusted mortality was not applied. The threshold for cost-effective care was set at $10,000,000 per death prevented.24 Cost-effectiveness was indicated as the incremental cost-effectiveness ratio (ICER), reported as cost per death prevented, where a negative ICER represents a dominated (higher cost, lower effectiveness) scenario characterized by increased spending on both hospitalization and fatality. The analysis adhered to the Consolidated Health Economic Evaluation Reporting Standards statement.25 Evaluation from a societal perspective was included with additional emergency department costs and indirect costs as recommended by the Second Panel on Cost-Effectiveness in Health and Medicine.26 Outcomes were evaluated at individual and population (n = 300,000,000 persons) levels. This simulation did not involve research in human subjects and was exempt from review by the Colorado Multiple Institutional Review Board.
Figure 1.
Approaches to COVID-19 vaccine evaluated included universal vaccination vs a risk-stratified approach, with primary outcomes of total hospitalizations and fatalities evaluated from a health care perspective.
Figure E1.
Decision tree. Decision tree depicting health states and transitions in a risk-stratification vs a universal vaccination strategy. cAnaAdmit, Cost of anaphylaxis hospitalization; cCOVIDAdmit, cost of COVD-19 hospitalization.
Risk of COVID infection was estimated from 16,067,965 cases reported as of December 13, 2020, and the US population of 330,703,412 individuals and modeled at 5%.1 , 27 Based on estimates from the CDC, 60% of individuals contracting COVID-19 were assumed to be symptomatic.28 COVID hospitalization rate of symptomatic individuals was estimated at 279 per 100,000 population.29 COVID-19 fatality was estimated from 297,843 deaths per 16,067,965 cases to reach a 2% symptomatic case-fatality rate.1 It was estimated that in the risk-stratified approach, 5% of the population would have vaccination deferred because of history of self-reported anaphylaxis.30 Risk of anaphylaxis associated with COVID-19 vaccination was estimated at 0.1%,2 with anaphylaxis hospitalization estimated at 22%31 and case-fatality assumed at 0.3%.32 Risk stratification was assumed to be 95% protective against anaphylaxis risk for the purpose of this model. Anaphylaxis hospitalization cost was estimated at $21,897 per hospitalization,33 , 34 with COVID hospitalization cost estimated at $73,300.35 The vaccine was assumed to be 95% effective in the base case.2 Model assumptions are presented in Table I .
Table I.
Simulation model inputs
| Probabilities | Rate/Cost | Sensitivity | Source |
|---|---|---|---|
| Contract COVID-19 | 5% | 2%-40% | Johns Hopkins University Coronavirus Resource Center,1 United States Census Bureau27 |
| Symptomatic COVID | 60% | 30%-90% | Centers for Disease Control and Prevention28 |
| COVID hospitalization if symptomatic | 6% | 5%-30% | Centers for Disease Control and Prevention29 |
| COVID-19 fatality | 2% | 1%-10% | Johns Hopkins University Coronavirus Resource Center1 |
| Risk-stratification vaccine deferral | 5% | 1%-20% | Wood et al30 |
| COVID vaccine anaphylaxis | 0.1% | 0.0%-1% | 2 |
| Anaphylaxis hospitalization | 22% | 10%-30% | Clark et al31 |
| Anaphylaxis case-fatality | 0.33% | 0.1%-0.5% | Ma et al32 |
| Vaccine protection from COVID | 95% | 90%-99% | 2 |
| Anaphylaxis hospitalization | $21,897 | $5,000-$25,000 | Candrilli and Kurosky,33 US Department of Labor, Bureau of Labor Statistics34 |
| COVID Hospitalization | $73,300 | $25,000-$100,000 | 35 |
| Risk-stratification anaphylaxis protection | 95% | 90%-99% | Assumption |
Sensitivity analyses
Deterministic sensitivity analyses were performed for higher COVID-19/lower anaphylaxis risk, lower COVID-19/higher anaphylaxis risk, lower vaccine effectiveness (floor rate of 50%), and for each variable across specified ranges (Table I). Given uncertainty in model variables and potential contemporaneous variable instability, probabilistic sensitivity analyses were performed across triangular distributions bounded by upper and lower sensitivity ranges, with median values set at the base-case variable assumption. Alternative probability beta distributions and cost gamma distributions were used for validation, with SDs set to half the mean value for gamma distributions and beta distributions, setting alpha equal to the observed number of events (r) and the at-risk population not experiencing the event representing the beta. Alternative random number seeding was also used to evaluate distributions.
Evaluations from a societal perspective included incorporation of ambulance and emergency department costs for anaphylaxis and emergency department costs for symptomatic COVID-19 infection, as well as job-related opportunity costs assumed for anaphylaxis and symptomatic COVID-19 infection. Ambulance and emergency department visit costs were estimated at $854 and $1554, respectively.31 Hourly wage for opportunity costs was estimated at $29, assuming loss of 7 days for anaphylaxis and 21 days for COVID-19 infection, with opportunity costs applied to individuals requiring hospitalization.34
Risk stratification by time
A supplemental model evaluated risk stratification by time (a universal observation of 15 minutes vs 30 minutes for patients at risk for anaphylaxis). In this analysis, an extended observation period provided a 95% reduction in anaphylaxis hospitalization and anaphylaxis fatality; however, it was associated with a 5% rate of vaccine deferral in the population with a history of previous anaphylaxis.
Results
Health care perspective
From a health care perspective, universal vaccination was more effective decreasing individual fatality risk (3.3 × 10−5 universal vs 5.9 ×10−5 with risk stratification) and had a lower individual cost ($11 universal approach vs $13 risk-stratified approach). When considered on a population level (n = 300,000,000 persons), universal vaccination was associated with a cost savings of $503,596,316 and saved 7,607 lives; however, this approach was associated with an additional 37,215 hospitalizations. The individual net monetary benefit of universal vaccination ($9,999,656) was greater when compared with risk stratification ($9,999,400), with risk stratification dominated (eg, lower cost, higher benefit) by a universal approach (ICER, −$66,201 per death prevented; please refer to the methods for an interpretation of the negative ICER) (Table II ).
Table II.
Simulation results
| Analysis | Cost (US $) | Anaphylaxis events | Anaphylaxis hospitalization | Anaphylaxis fatality | COVID-19 hospitalization | COVID-19 fatality | Total hospitalizations | Total fatalities | NMB | ICER (per death prevented) | Population estimate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Base case (per patient) | 300,000,000 | ||||||||||
| Universal vaccination | $11 | 0.000998056 | 0.00022 | 0.0000033 | 8.99997 × 10−05 | 2.99999× 10−05 | 0.00031 | 3.32999× 10−05 | $9,999,656 | — | |
| Risk stratified | $13 | 4.74077× 10−05 | 0.00001045 | 1.5675 × 10−07 | 0.0001755 | 5.85 × 10−05 | 0.00018595 | 5.86567× 10−05 | $9,999,400 | −$66,201 | |
| Universal vs risk stratified | |||||||||||
| Per patient | −$2 | 0.000950649 | 0.00020955 | 3.14325× 10−06 | −8.55003× 10−05 | −2.85 × 10−05 | 0.00012405 | −2.53568 × 10−05 | $255 | ||
| Per 300 million persons | −$503,596,316 | 285,195 | 62,865 | 943 | −25,650 | −8,550 | 37,215 | −7,607 | $76,574,129,208 | ||
| Higher COVID risk; Lower anaphylaxis risk (per patient) | 300,000,000 | ||||||||||
| Universal vaccination | $540 | 0.000982018 | 0.0001 | 0.000001 | 0.005399995 | 0.001799998 | 0.005499995 | 0.001800998 | $9,981,450 | — | |
| Risk stratified | $1,053 | 4.66459× 10−05 | 0.00000475 | 4.75 × 10−08 | 0.01053 | 0.00351 | 0.01053475 | 0.003510047 | $9,963,847 | −$299,889 | |
| Universal vs risk stratified | |||||||||||
| Per patient | −$513 | 0.000935372 | 0.00009525 | 9.525 × 10−07 | −0.005130005 | −0.00171 | −0.005034755 | −0.001709049 | $17,603 | ||
| Per 300 million persons | −$153,757,279,305 | 280,612 | 28,575 | 286 | −1,539,002 | −513,001 | −1,510,427 | −512,715 | $5,280,904,922,805 | ||
| Lower COVID risk; Higher anaphylaxis risk (per patient) | 300,000,000 | ||||||||||
| Universal vaccination | $225 | 0.029979105 | 0.009 | 0.00015 | 1.49978 × 10−05 | 2.99955× 10−06 | 0.009014998 | 0.000153 | $9,998,245 | −$1,527,990 | |
| Risk stratify | $11 | 0.001424007 | 0.0004275 | 0.000007125 | 2.92499× 10−05 | 5.84998× 10−06 | 0.00045675 | 1.2975 × 10−05 | $9,999,859 | — | |
| Universal vs risk stratify | |||||||||||
| Per patient | $214 | 0.028555098 | 0.0085725 | 0.000142875 | −1.42521× 10−05 | −2.8504× 10−06 | 0.008558248 | 0.000140025 | −$1,614 | ||
| Per 300 million persons | $64,186,858,927 | 8,566,529 | 2,571,750 | 42,863 | −4,276 | −855 | 2,567,474 | 42,007 | −$484,260,573,051 | ||
| Second-order Monte-Carlo simulations (n = 10,000 simulations) | 300,000,000 | ||||||||||
| Universal vaccination | $59 | 0.003661082 | 0.000761315 | 1.14038× 10−05 | 0.000687309 | 0.000218603 | 0.001448623 | 0.000230007 | $9,997,641 | — | |
| SD | $42 | 0.00221648 | 0.000493234 | 7.6776 × 10−06 | 0.000594858 | 0.000202776 | — | — | $2,054 | — | |
| Risk stratified | $116 | 0.000178587 | 3.71331× 10−05 | 5.565 × 10−07 | 0.001746735 | 0.000553022 | 0.001783868 | 0.000553578 | $9,994,348 | −$178,125 | |
| SD | $104 | 0.000130277 | 2.85387× 10−05 | 4.415 × 10−07 | 0.001497153 | 0.000499743 | — | — | $5,060 | — | |
| Universal vs risk stratified | |||||||||||
| Per patient | −$57 | 0.003482495 | 0.000724182 | 1.08473× 10−05 | −0.001059426 | −0.00033442 | −0.000335244 | −0.000323571 | $3,293 | ||
| Per 300 million persons | −$17,100,000,000 | 1,044,748 | 217,254 | 3,254 | −317,828 | −100,326 | −100,573 | −97,071 | $987,900,000,000 |
NMB, Net monetary benefit.
Base-case assumptions: 5% COVID-19 infection rate; 6% symptomatic COVID-19 hospitalization rate; 2% COVID-19 fatality rate; anaphylaxis hospitalization 10%; hospital costs $10,000 for anaphylaxis and $73,300 for COVID-19. Higher COVID-19 risk/Lower anaphylaxis risk: 40% COVID-19 infection rate; 90% symptomatic COVID-19; 30% symptomatic COVID-19 hospitalization rate; 10% COVID-19 fatality rate; 0.1% COVID-19 vaccine anaphylaxis rate;10% anaphylaxis hospitalization rate; 0.1% anaphylaxis fatality rate; hospital costs $5,000 for anaphylaxis and $100,000 for COVID-19. Lower COVID-19 risk/Higher anaphylaxis risk: 2% COVID-19 infection rate; 30% symptomatic COVID-19; 5% symptomatic COVID-19 hospitalization rate; 1% COVID-19 fatality rate; 3% COVID-19 vaccine anaphylaxis rate; 30% anaphylaxis hospitalization rate; 0.5% anaphylaxis fatality rate; hospital costs $25,000 for anaphylaxis and $25,000 for COVID-19.
Sensitivity analyses
From a health care perspective, when higher COVID-19 infection risk was assumed along with a lower vaccine-associated anaphylaxis risk, a risk-stratified approach was even more dominated (ICER, −$299,899 per death prevented) with larger differences in net monetary benefit between strategies (individual NMB $9,981,450 for the universal strategy vs $9,963,847 for the risk-stratified strategy), with the universal approach resulting in 1,510,427 fewer hospitalizations on a population level (Table II). Conversely, under assumptions of lower COVID-19 infection risk combined with a higher vaccine-associated anaphylaxis risk, a universal approach became dominated by the risk-stratified approach (ICER, −$1,527,990 per death prevented).
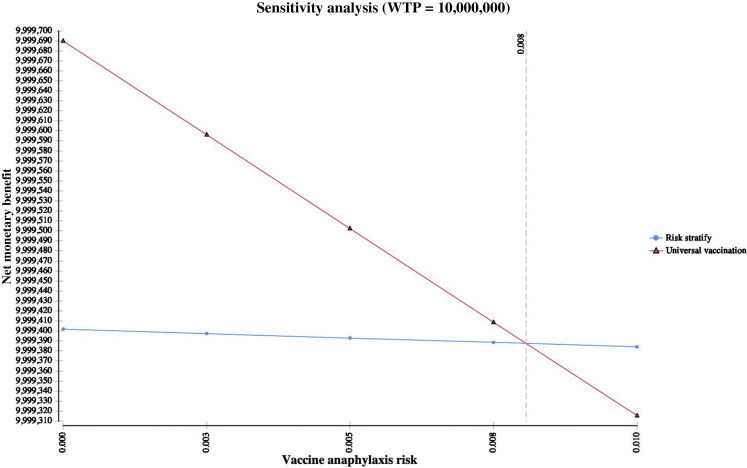
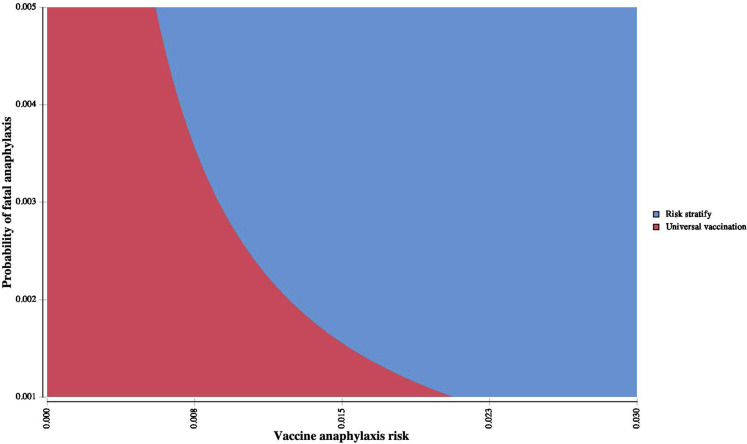
The universal vaccination cost-effectiveness exceeded $10,000,000 per death prevented with increasing rates of vaccine-associated anaphylaxis. When risk of vaccine-associated anaphylaxis exceeded 0.8%, the risk-stratified approach was the most cost-effective strategy (Figure 2 ). An interaction was noted between anaphylaxis risk and anaphylaxis fatality, with a risk-stratified approach becoming cost-effective as each risk increased concurrently (Figure 3 ). In addition, as risk of COVID-19 increased, cost-effectiveness of a risk-stratified approach required vaccine deferral at higher anaphylaxis risk thresholds (see Figure E2 in this article's Online Repository at www.jaci-inpractice.org). Additional deterministic sensitivity analyses did not reveal any univariate levers for a cost-effective risk-stratified approach (Figure 4 ). Assuming only 50% vaccine effectiveness, the universal approach still remained the most cost-effective approach (ICER $108,774 per death prevented). Evaluation of probabilistic sensitivity analysis using second-order Monte-Carlo simulations (n = 10,000 simulations) demonstrated that a universal vaccination approach was most likely to be cost-effective across a range of simultaneously randomized variable assumptions. Universal vaccination produced a cost-saving of more than $17 billion in second-order Monte-Carlo simulations and resulted in 97,071 fewer fatalities (Table II). Probabilistic sensitivity analysis demonstrated that a universal approach was the most cost-effective strategy in 99.58% of simulations (Figure 5 ). Using both alternative seeding and alternative distributions, simulations continued to demonstrate a universal approach to be most cost-effective (99.53% of simulations with alternative seeding and 90.08% of simulations with alternative distributions).
Figure 2.
One-way sensitivity analysis of anaphylaxis risk. When risk of vaccine anaphylaxis exceeds 0.8%, risk stratification was the most cost-effective strategy (WTP = 10,000,000 per death prevented). Typical rates of vaccine anaphylaxis are estimated at 1.3 per million, with COVID-19 anaphylaxis rates estimated at 11.1 per million.12,36WTP, Willingness to pay.
Figure 3.
Two-way sensitivity analysis of anaphylaxis risk and anaphylaxis fatality. An interaction was noted between anaphylaxis risk and anaphylaxis fatality, with a risk-stratification approach becoming cost-effective as each risk increased concurrently. At an anaphylaxis case-fatality rate of 0.33%, when risk of vaccine anaphylaxis exceeds 0.8%, risk stratification was the most cost-effective strategy (WTP = 10,000,000 per death prevented). WTP, Willingness to pay.
Figure E2.
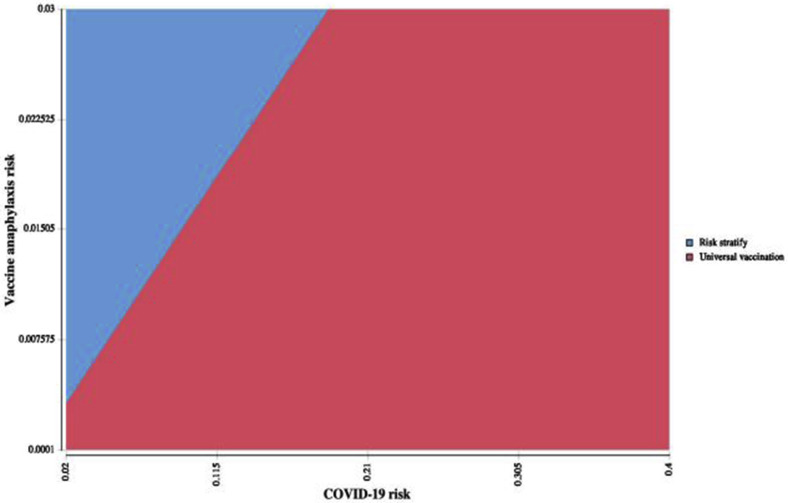
Two-way sensitivity analysis of anaphylaxis risk and COVID-19 risk. As risk of COVID-19 increased, cost-effectiveness of a risk-stratified approach required vaccine deferral at higher anaphylaxis risk thresholds.
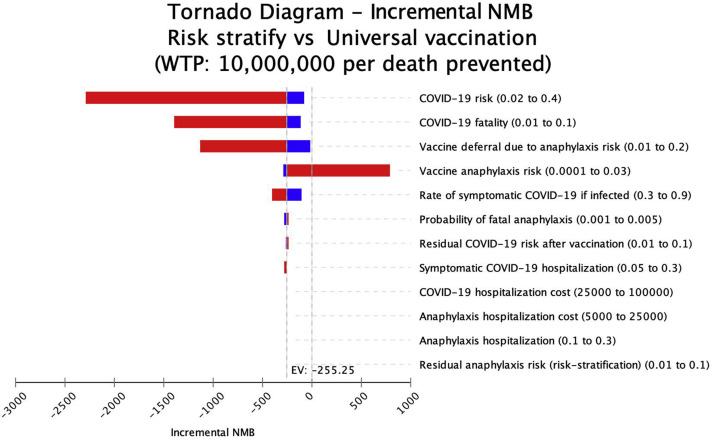
Figure 4.
Tornado plot of deterministic sensitivity analyses. As vaccine anaphylaxis risk exceeded 0.8%, a risk-stratification approach was cost-effective (WTP = 10,000,000 per death prevented). Incremental net monetary benefit of a risk-stratification approach vs universal vaccination is shown. Blue bars represent assumptions below the base case, and red bars depict assumptions above the base case. Positive incremental NMB represents cost-effectiveness of a risk-stratification approach, and negative NMB reflects cost-effectiveness of the universal strategy. An incremental net monetary benefit with a negative EV indicates risk stratification is not cost-effective. EV, Expected value; NMB, net monetary benefit; WTP, willingness to pay.
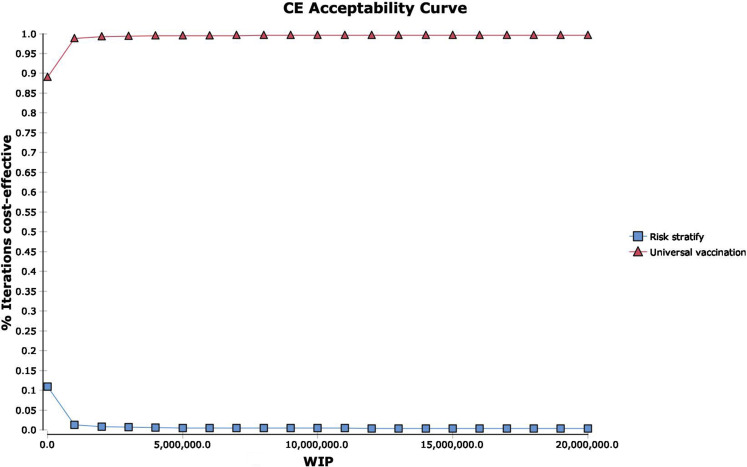
Figure 5.
Probabilistic sensitivity analysis. Probabilistic sensitivity analysis demonstrated a universal vaccination strategy to be the most cost-effective strategy in 99.58% of simulations. (A) Cost-effectiveness acceptability curve. (B) Acceptability at WTP. (C) Incremental cost-effectiveness scatter plot, risk stratification vs universal vaccination. WTP = 10,000,000 per death prevented. WTP, Willingness to pay.
Societal perspective
In evaluations from a societal perspective inclusive of ambulance transport and emergency department evaluation for anaphylaxis, emergency department evaluation of symptomatic COVID infection, and indirect costs, the findings were similar to the analysis from the health care perspective. From the societal perspective, universal vaccination demonstrated savings in cost, lower total fatalities, and overall incremental population net monetary benefit of $76,568,934,350 compared with a risk-stratified approach (see Table E1 in this article's Online Repository at www.jaci-inpractice.org). From the societal perspective, universal vaccination was the most cost-effective approach unless risk of vaccine anaphylaxis exceeded 0.76%.
Risk stratification by time
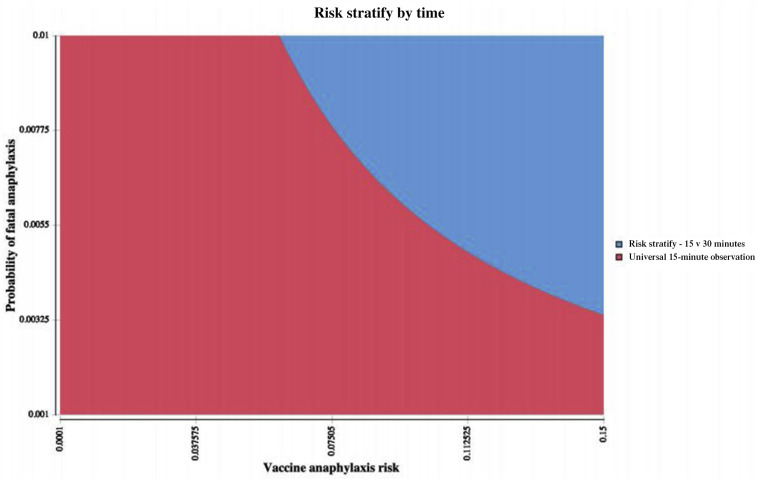
Evaluating risk stratification of anaphylaxis risk by observation time (health care perspective), a universal approach of 15-minute observation was preferred until risk of anaphylaxis and anaphylaxis case-fatality rates rose significantly. However, if a 1% anaphylaxis case-fatality was assumed, a risk-stratified approach of 30-minute (vs 15-minute) observation became cost-effective when vaccine anaphylaxis exceeded 6.1% (see Figure E3 in this article's Online Repository at www.jaci-inpractice.org). Findings were similar when evaluated from a societal perspective.
Figure E3.
Two-way sensitivity analysis of risk stratification by time. Evaluating risk stratification of anaphylaxis risk by observation time, a universal approach of 15-minute observation was preferred until risk of anaphylaxis and anaphylaxis case-fatality rates rose significantly. However, if a 1% anaphylaxis case-fatality was assumed, a risk-stratified approach of 30-minute (vs 15-minute) observation became cost-effective when vaccine anaphylaxis exceeded 6% (WTP = 10,000,000 per death prevented). WTP, Willingness to pay.
Discussion
Two cases of apparent anaphylaxis occurring in the first 500 doses in the United Kingdom prompted health authorities to issue a precaution for vaccination in those with a history of a severe drug or vaccine reaction (and extended to also include a history of a severe food allergy in the United Kingdom) early in the vaccine effort, and similar but less restrictive precautions and contraindications were issued in the United States and Canada. Despite a lack of high-certainty evidence linking a history of allergies unrelated to the COVID-19 vaccine to a risk for adverse vaccine reaction, precautions remain in place for individuals with a history of all-cause anaphylaxis, as well as those with a history of any immediate reaction to any vaccination or injectable therapy.18 It is unclear whether such broad restrictions on COVID-19 vaccination are necessary, or cost-effective—and the degree to which these precautions create a barrier to broader population vaccine acceptance is uncertain. This analysis demonstrates that a universal vaccination approach is cost-saving and provides superior health outcomes compared with a risk-stratified approach that, in our analysis, broadly excluded anyone with a history of self-reported anaphylaxis from being vaccinated. However, in situations characterized by low COVID-19 infection risk together with significant rates of vaccine-associated anaphylaxis, a risk-stratified approach (assuming risk stratification can prevent 95% of vaccine-related anaphylaxis) becomes cost-effective (ie, as risk of anaphylaxis from the vaccine increased above 0.8%). Although stratified observation time by anaphylaxis history (15 minutes vs 30 minutes) was not cost-effective in the base case, at a 1% anaphylaxis case-fatality rate, more prolonged observation could be cost-effective when risk of vaccine anaphylaxis exceeded 6%.
Vaccine-related anaphylaxis is a significant but treatable medical event for trained, experienced clinicians. A 2019 review of the Vaccine Adverse Events Registry System noted that among 467,960 total reports from 1990 to 2016, there were only 828 cases of anaphylaxis, a cumulative incidence rate of 0.0017%. Although it is possible not every case may have been reported to the Vaccine Adverse Events Registry System in that time interval, such events are exceedingly rare, statistically. In the United States, vaccinations are recommended to be administered only by personnel trained and equipped to manage severe reactions such as anaphylaxis. Allergic reactions to vaccines may result from an excipient, such as gelatin (Measles Mumps Rubella vaccine), ovalbumin (eg, egg, influenza, and yellow fever), latex (in the stopper on vials), and yeast (hepatitis B, human papillomavirus). Historically, although the CDC Advisory Committee on Immunization Practices has issued contraindications against vaccinating individuals allergic to the vaccine or the excipient, allergists have devised desensitization or graded challenge protocols allowing safe vaccination or shown that the risk of reaction was rare enough that observation in a specialist's office is sufficient (or occurred no more than in the general population).36, 37, 38 It is unusual for an allergy to completely prevent receiving a vaccination in some fashion, presuming there is a favorable risk-benefit standpoint, following evaluation by a board-certified allergist. In the case of the Pfizer-BioNTech and Moderna vaccines, the provoking vaccine culprit has not been identified, but has been suspected to be either (1) naked mRNA particles themselves through direct mast cell degranulation via Toll-like receptor stimulation or (2) polyethylene glycol (PEG).39, 40, 41, 42 PEG is an agent that has been linked to a small quantity of allergic reactions, felt to potentially be IgE mediated though detection of anti-PEG IgE has been somewhat elusive.39 , 40 Desensitization to PEG and PEGylated products has been described in cases in which re-administration of the PEG-containing product is desired or necessary.43 A recent article by an author group from Mass General Brigham and Vanderbilt Health systems that described a series of ad hoc consensus recommendations has advocated for and outlined an approach to testing to PEG and restricting mRNA vaccination to persons with positive PEG or polysorbate skin testing result (polysorbate is potentially cross-reactive with PEG).21 However, recently published American College of Allergy, Asthma & Immunology COVID-19 Task Force recommendations do not recommend PEG testing, instead describing (but not endorsing) an option of graded vaccine challenge as a potential avenue for subsequent vaccination in those with a previous severe allergic reaction to a COVID-19 vaccine or any of its components.44
Hypersensitivity-related adverse events in the randomized controlled Pfizer-BioNTech (0.12% numerical difference) and the Moderna vaccine trials (0.4% excess hypersensitivity occurrence with no attributable anaphylaxis) were low.7, 8, 9 , 45 However, even if the mRNA vaccines were associated with an excess 0.1% anaphylaxis rate (eg, equivalent to a rate of 1000 cases per million vaccine doses), this would be much higher than typical rates of vaccine anaphylaxis (given the aforementioned historical rate of 1.3 cases of anaphylaxis per million vaccine doses). For perspective, our threshold for when the strategies flip in this analysis to support restricting the opportunity to offer vaccination to someone with a history of previous anaphylaxis occurs when the rate reaches 7600 to 8000 cases per million doses (and this assumes risk stratification can effectively mitigate this rate, which is unproven). It should be understood how even further disproportionately high this threshold rate of vaccine-associated anaphylaxis would be compared with historical estimates, to reinforce how exaggerated the margins of the analysis are to the reader, given numeracy with risk reporting can be difficult to accurately translate. Readers should also keep in mind that over a 26-year period in which several new vaccines were brought to market (1990-2016, including vaccines for hepatitis A and B, rotavirus, Streptococcus pneumonia, Neisseria meningitis, human papillomavirus, varicella/zoster, tetanus, and influenza), there were only 828 anaphylaxis events total reported to the Vaccine Adverse Events Registry System. Thus, although such a threshold rate of anaphylaxis exists, it should be understood how highly unlikely that is to be achieved. To reach a point where the optimal strategy would no longer be to universally vaccinate anyone irrespective of their allergic history, either the cumulative incidence of reports in the past 26 years would need to grow 10-fold or the rate of anaphylaxis occurring in real life would have to be 8 times the rate of all attributable hypersensitivity-related adverse events reported in the clinical trial that served as the basis for the EUA. Therefore, although we show the levers of how and where strategies may shift, the feasibility of achieving these levers must be kept in proper perspective.
This study has limitations. Foremost, it is a cohort simulation with very limited inputs. This is a novel vaccine with limited reported vaccination outcomes for a new and still evolving illness, and the initial precautions were issued based on 2 persons among 500 individuals vaccinated (occurring outside the clinical trial, after the EUA) having a reaction concerning for anaphylaxis. Importantly, few cases of allergic reactions were noted in the clinical trials for either mRNA vaccine, with no significant difference seen between trials arms in terms of observed rates of this event.46 It is unclear what has provoked these reactions reported since the vaccine approval, and to what degree having a previous severe reaction to another vaccine, parenterally administered medication, or any past history of anaphylaxis predisposes someone to a risk of anaphylaxis from this vaccine. It is unclear whether additional reactions will be noted upon the second doses, though the CDC has quickly moved to contraindicate additional doses in anyone with an initial severe reaction.18 Our definition of self-reported anaphylaxis prompting vaccine deferral in the base-case assumption was deliberately broad and overinclusive, incorporating reported adult rates of any source of anaphylaxis from a recent large nationally representative survey, and simulated up to a very high rate (20%).30 Although initial guidance in the United Kingdom restricting vaccination in those with a history of anaphylaxis has been revised (and recommendations in the United States and Canada were never as restrictive), there is still concern that a patient reporting a history of a previous episode of anaphylaxis (from any source) may be perceived at higher risk, and this perception could act as a deterrent to vaccination efforts through either prolonged wait times or fear.18 , 20 The only standing contraindication at the time of each vaccine EUA release was a history of previous reaction to this vaccine's components, which is considerably narrower than what we are simulating. However, there is distinct advantage for being broadly overinclusive for understanding where the margins of the cost-effectiveness lie, particularly because it is likely that vaccine hesitancy would extend the margins of vaccine deferral in those concerned about anaphylaxis risk.47 , 48 Costs for events and event rates were taken from the published literature, but may vary, though broad ranges were used to ensure maximal sensitivity of the assumptions. Although international vaccination approaches may continue to evolve, we have broadly modeled the health and economic outcomes of strategies aimed at mitigating anaphylaxis risk, with findings demonstrating the larger public health risk is likely to be the COVID-19 pandemic. Lastly, it is unclear whether other vaccines that are either currently available or are close to an EUA will be associated with any postmarketing events, given similar experience that few allergic events have been reported in their clinical trials.
Conclusions
Although health authorities in the United Kingdom, Canada, and the United States have issued precautions against vaccinating individuals with certain underlying allergic risk factors against mRNA COVID-19 vaccines, when exploring the value of such actions, the decision to limit routine contraindications (pending evaluation by an allergist-immunologist) to individuals with previous anaphylaxis to a known vaccine component seems a cost-effective approach, and there is limited value present only under very particular contexts for wider exclusions.
Footnotes
This study was supported by Dartmouth-Hitchcock Medical Center Department of Medicine.
Conflicts of interest: M. Shaker is a member of the Joint Taskforce on Allergy Practice Parameters; has a family member who is CEO of Altrix Medical; and serves on the Editorial Board of the Journal of Food Allergy and the Annals of Allergy, Asthma, and Immunology. E. M. Abrams is on the National Advisory Board for Food Allergy Canada, is on the National Food Allergy Action Plan Action Steering Team for Food Allergy Canada, and has received moderator fees from Novartis and AstraZeneca. M. Greenhawt has served as a consultant for the Canadian Transportation Agency, Thermo Fisher, Intrommune, and Aimmune Therapeutics; has served as a member of physician/medical advisory boards for Aimmune Therapeutics, DBV Technologies, Sanofi/Genzyme, Genentech, Nutricia, Kaleo Pharmaceutical, Nestle, Acquestive, Allergy Therapeutics, Pfizer, US World Meds, Allergenis, Aravax, and Monsanto; is a member of the scientific advisory council for the National Peanut Board; has received honorarium for lectures from Thermo Fisher, Aimmune, DBV, Before Brands, multiple state allergy societies, the American College of Allergy Asthma and Immunology, the Eurpean Academy of Allergy and Clinical Immunology; is an associate editor for the Annals of Allergy, Asthma, and Immunology; and is a member of the Joint Taskforce on Allergy Practice Parameters.
Online Repository.
Table E1.
Simulation results, societal perspective
| Analysis | Cost (US $) | Anaphylaxis events | Anaphylaxis hospitalization | Anaphylaxis fatality | COVID-19 hospitalization | COVID-19 fatality | Total hospitalizations | Total fatalities | NMB | C/E | ICER | Population estimate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base case (per patient) | 300,000,000 | |||||||||||
| Universal vaccination | $17 | 0.000998056 | 0.00022 | 0.0000033 | 9.00 × 10−05 | 3.00 × 10−05 | 0.00031 | 3.33 × 10−05 | $9,999,650 | $17 | — | |
| Risk stratify | $19 | 4.74 × 10−05 | 0.00001045 | 1.57 × 10−07 | 0.0001755 | 5.85 × 10−05 | 0.00018595 | 5.87 × 10−05 | $9,999,395 | $19 | −$65,518 | |
| Universal vs risk stratify | ||||||||||||
| Per patient | −$2 | 0.000950649 | 0.00020955 | 3.14 × 10−06 | −8.55 × 10−05 | −2.85× 10−05 | 0.00012405 | −2.54× 10−05 | $255 | |||
| Per 300 million persons | −$498,401,457 | 285,195 | 62,865 | 943 | −25,650 | −8,550 | 37,215 | −7,607 | $76,568,934,350 | |||
C/E, Cost-effectiveness.
References
- 1.Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu Available from: Accessed December 13, 2020.
- 2.Pfizer BioNTech. Vaccines and Related Biological Products Advisory Committee Meeting in December 10, 2020. FDA. 2020. https://www.fda.gov/media/144245/download Available from:
- 3.ModernaTX Vaccines and Related Biological Products Advisory Committee Meeting in December 17, 2020. FDA. 2020. https://www.fda.gov/media/144673/download Available from:
- 4.Centers for Disease Control and Prevention COVID-19 vaccine planning. https://www.cdc.gov/vaccines/covid-19/planning/index.html Available from: Accessed February 10, 2021. [PubMed]
- 5.Appleby J. Will covid-19 vaccines be cost effective—and does it matter? BMJ. 2020;371:m4491. doi: 10.1136/bmj.m4491. [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration FDA takes key action in fight against COVID-19 by issuing emergency use authorization for first COVID-19 vaccine. https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19 Available from: Accessed December 20, 2020.
- 7.Mahase E. Covid-19: people with history of significant allergic reactions should not receive Pfizer vaccine, says regulator. BMJ. 2020;371:m4780. doi: 10.1136/bmj.m4780. [DOI] [PubMed] [Google Scholar]
- 8.US Food and Drug Administration Vaccines and related biological products advisory committee meeting. December 10. 2020. https://www.fda.gov/media/144245/download Available from: Accessed December 20, 2020.
- 9.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiland N., LaFraniere S., Baker M., Thomas K. 2 Alaska health workers got emergency treatment after receiving Pfizer’s vaccine. The New York Times. Updated December 22, 2020. https://www.nytimes.com/2020/12/16/health/covid-pfizer-vaccine-allergic-reaction.html Available from: Accessed December 20, 2020.
- 11.Reuters Staff CDC issues guidelines on COVID-19 vaccination after allergic reactions. Reuters. December 19, 2020. https://www.reuters.com/article/healthcoronavirus-usa-vaccine-cdc/cdc-issues-guidelines-on-covid-19-vaccination-after-allergic-reactions-idINL4N2IZ08G Available from: Accessed February 10, 2021.
- 12.CDC COVID-19 Response Team Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine – United States, December 14-23. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC COVID-19 Response Team Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine – United States, December 21, 2020–January 10,2021. MMWR Morb Mortal Wkly Rep. 2021;70:125–129. doi: 10.15585/mmwr.mm7004e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner P.J., Worm M., Ansotegui I.J., El-Gamal Y., Rivas M.F., Fineman S., et al. Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ J. 2019;12:100066. doi: 10.1016/j.waojou.2019.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaker M.S., Wallace D.V., Golden D.B.K., Oppenheimer J., Bernstein J.A., Campbell R.L., et al. Anaphylaxis—a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145:1082–1123. doi: 10.1016/j.jaci.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Cardona V., Ansotegui I.J., Ebisawa M., El-Gamal Y., Fernandez Rivas M., Fineman S., et al. World Allergy Organization anaphylaxis guidance 2020. World Allergy Organ J. 2020;13:100472. doi: 10.1016/j.waojou.2020.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gov.UK Confirmation of guidance to vaccination centres on managing allergic reactions following COVID-19 vaccination with the Pfizer/BioNTech vaccine. https://www.gov.uk/government/news/confirmation-of-guidance-to-vaccination-centres-on-managing-allergic-reactions-following-covid-19-vaccination-with-the-pfizer-biontech-vaccine Available from: Accessed February 10, 2021.
- 18.Centers for Disease Control and Prevention Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fpfizer%2Fclinical-considerations.html Available from:
- 19.Government of Canada Pfizer-BioNTech COVID-19 vaccine: Health Canada recommendations for people with serious allergies. https://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2020/74543a-eng.php Available from:
- 20.BSACI. Update on MHRA decision re: Pfizer COVID-19 vaccination 30.12.20. https://www.bsaci.org/update-on-mhra-decision-re-pfizer-covid-19-vaccination/ Available from: Accessed February 10, 2021.
- 21.Banerji A., Wickner P.G., Saff R., Stone C.A., Jr., Robinson L.B., Long A.A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenhawt M., Abrams E.M., Oppenheimer J., Vander Leek T.K., Mack D., Singer A.G., et al. The COVID-19 pandemic in 2021: avoiding overdiagnosis of anaphylaxis risk while safely vaccinating the world. J Allergy Clin Immunol Pract. 2021;9:1438–1441. doi: 10.1016/j.jaip.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Global Preparedness Monitoring Board A world in disorder. https://apps.who.int/gpmb/assets/annual_report/GPMB_AR_2020_EN.pdf Available from:
- 24.US Department of Transportation Economic values used in analysis. https://www.transportation.gov/regulations/economic-values-used-in-analysis Available from: Accessed December 20, 2020.
- 25.Husereau D., Drummond M., Petrou S., Carswell C., Moher D., Greenberg D., et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049. doi: 10.1136/bmj.f1049. [DOI] [PubMed] [Google Scholar]
- 26.Sanders G.D., Neumann P.J., Basu A., Brock D.W., Feeny D., Krahn M., et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316:1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 27.US Census Bureau U.S. and world population clock. https://www.census.gov/popclock/world Available from: Accessed December 20, 2020.
- 28.Centers for Disease Control and Prevention COVID-19 pandemic planning scenerios. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html Available from: Accessed February 10, 2021.
- 29.Centers for Disease Control and Prevention COVIDView. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html Available from: Accessed December 20, 2020.
- 30.Wood R.A., Camargo C.A., Jr., Lieberman P., Sampson H.A., Schwartz L.B., Zitt M., et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133:461–467. doi: 10.1016/j.jaci.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Clark S., Wei W., Rudders S.A., Camargo C.A., Jr. Risk factors for severe anaphylaxis in patients receiving anaphylaxis treatment in US emergency departments and hospitals. J Allergy Clin Immunol. 2014;134:1125–1130. doi: 10.1016/j.jaci.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Ma L., Danoff T.M., Borish L. Case fatality and population mortality associated with anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133:1075–1083. doi: 10.1016/j.jaci.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Candrilli S., Kurosky S. Recent trends in anaphylaxis-related hospitalization in the United States. Value Health. 2012;18:PA503. [Google Scholar]
- 34.US Bureau of Labor Statistics CPI inflation calculator. October 22, 2020. https://www.bls.gov/data/inflation_calculator.htm Available from: Accessed December 20, 2020.
- 35.FAIR Health Inc. Costs for a hospital stay for COVID-19. https://www.fairhealth.org/article/costs-for-a-hospital-stay-for-covid-19 Available from: Accessed December 20, 2020.
- 36.Kelso J.M., Greenhawt M.J., Li J.T., Nicklas R.A., Bernstein D.I., Blessing-Moore J., et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130:25–43. doi: 10.1016/j.jaci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Greenhawt M.J., Li J.T., Bernstein D.I., Blessing-Moore J., Cox L., Khan D., et al. Administering influenza vaccine to egg allergic recipients: a focused practice parameter update. Ann Allergy Asthma Immunol. 2011;106:11–16. doi: 10.1016/j.anai.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Greenhawt M., Turner P.J., Kelso J.M. Administration of influenza vaccines to egg allergic recipients: a practice parameter update 2017. Ann Allergy Asthma Immunol. 2018;120:49–52. doi: 10.1016/j.anai.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Stone C.A., Jr., Liu Y., Relling M.V., Krantz M.S., Pratt A.L., Abreo A., et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7:1533–1540.e8. doi: 10.1016/j.jaip.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wenande E., Garvey L.H. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46:907–922. doi: 10.1111/cea.12760. [DOI] [PubMed] [Google Scholar]
- 41.Kulka M., Alexopoulou L., Flavell R.A., Metcalfe D.D. Activation of mast cells by double-stranded RNA: evidence for activation through Toll-like receptor 3. J Allergy Clin Immunol. 2004;114:174–182. doi: 10.1016/j.jaci.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 42.Lund J.M., Alexopoulou L., Sato A., Karow M., Adams N.C., Gale N.W., et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turgay Yagmur I., Guzelkucuk Z., Yarali N., Ozyoruk D., Toyran M., Civelek E., et al. Evaluation of hypersensitivity reactions to cancer chemotherapeutic agents in pediatric patients. Ann Allergy Asthma Immunol. 2020;124:350–356. doi: 10.1016/j.anai.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Murphy K.R., Patel N.C., Ein D., Hudelson M., Kodoth S., Marshall G.D., Jr., et al. Insights from American College of Allergy, Asthma, and Immunology COVID-19 Vaccine Task Force: allergic reactions to mRNA SARS-CoV-2 vaccines. Ann Allergy Asthma Immunol. 2021;126:319–320. doi: 10.1016/j.anai.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.US Food and Drug Administration Emergency Use Authorization (EUA) for an unapproved product review memorandum. December 17, 2020. https://www.fda.gov/media/144673/download Available from:
- 46.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherman S.M., Smith L.E., Sim J., Amlôt R., Cutts M., Dasch H., et al. COVID-19 vaccination intention in the UK: results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey. Hum Vaccin Immunother. 2020:1–10. doi: 10.1080/21645515.2020.1846397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldman R.D., Yan T.D., Seiler M., Parra Cotanda C., Brown J.C., Klein E.J., et al. Caregiver willingness to vaccinate their children against COVID-19: cross sectional survey. Vaccine. 2020;38:7668–7673. doi: 10.1016/j.vaccine.2020.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]