Abstract
Gastrointestinal stromal tumours (GISTs) are rare - and rectovaginal extragastrointestinal stromal tumours (RV-EGISTs) even rarer. We share a case of RV-EGIST, complemented by high-quality radiological and surgical images. A review of current literature pertaining to RV-EGIST is also included. Our case report highlights the diagnostic challenge presented by extragastrointestinal stromal tumours. Differentiated from overlapping pathologies only by targeted application of immunohistopathology and cytogenetics, the inclusion of RV-EGIST in the differential diagnosis of a rectovaginal tumour is essential to making this correct diagnosis. Primary surgery is the treatment of choice for RV-EGIST if complete cytoreduction can be achieved, combined with adjuvant tyrosine kinase inhibitor (TKI) therapy for those with high-risk features to further reduce rates of future recurrence.
Keywords: gynaecological cancer, pathology, surgical oncology
Background
Gastrointestinal stromal tumours (GISTs) are rare, affecting 0.001%–0.002% of the population - with an age-adjusted incidence of 1.5 per 100 000.1 2 GISTs account for only 1%–2% of all gastrointestinal (GI) malignancies, but represent the most common GI mesenchymal tumour.1 3 4
GISTs typically originate from within the wall of the GI tract - demonstrating intraluminal extension towards the mucosa and outward growth towards, or even beyond, the outer serosa.5–7 The most common site for GIST formation is the stomach, in up to 70% of cases, with 20%–30% affecting the small intestine and the remainder developing at a number of rarer locations along the GI tract - including the oesophagus, colon and rectum.8 9
GISTs originating from distant sites with no direct transmural communication with the GI lumen have also been described, but are rare - accounting only for at most 5%–7% of all GIST cases.9 Such lesions have been reported in the omentum, mesentery, retroperitoneal space, urinary bladder and female pelvis - and are referred to as extragastrointestinal stromal tumours or EGISTs.1 9–13
Rectovaginal EGISTs are especially rare, with only 22 cases reported in the literature up to 2019.13 Due to vague symptoms and significant overlap with the presentation and clinicohistological features of other pathologies, misdiagnosis of EGIST at this site is common.12 Clinical and pathological vigilance is therefore essential to include EGIST in the differential for any unusual vaginal swelling - and subsequently target immunohistochemistry appropriately to enable diagnostic distinction.13 While rectovaginal EGISTs are aggressive, the majority are amenable to primary debulking surgery and postoperative adjuvant treatment with tyrosine kinase inhibitors (TKI) can further reduce recurrence in high-risk cases - improving prognosis.14–21
Case presentation
A 55-year-old para 2 was referred from primary care to a cancer centre for further investigation of a suspicious vaginal mass found incidentally at routine smear-taking. The patient was asymptomatic - with no gynaecological, medical or surgical history; a non-smoker; and on no regular medications.
Speculum examination indeed revealed a large tumour - bulging into the vagina, but separate from the cervix and without breach of the overlying mucosa. The smooth, fixed mass was also palpable per rectum - raising suspicion for involvement of the rectovaginal septum.
Investigations
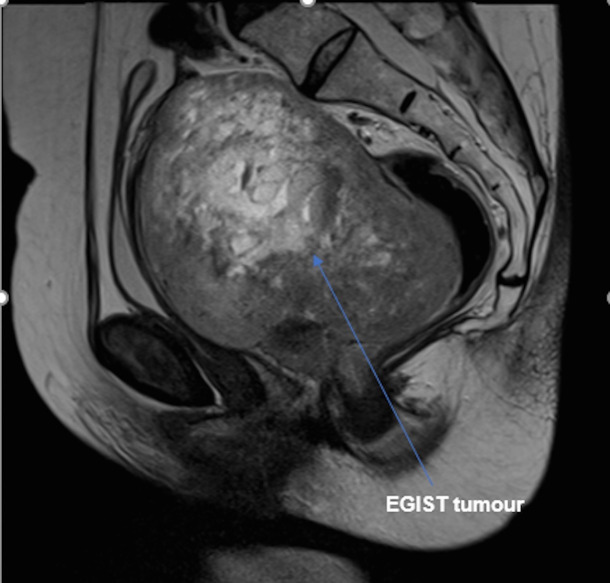
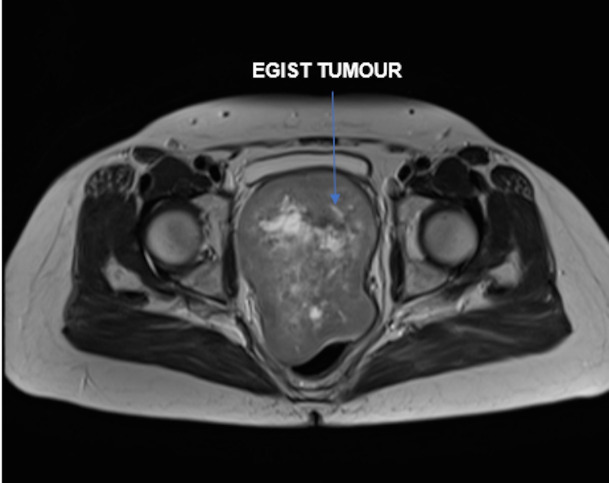
An MRI was undertaken and the imaging reviewed via the gynae-oncology multidisciplinary team (MDT) network. A 13×8×9.5 cm solid/cystic vaginal mass was identified - extending into the pelvis to displace the bladder anteriorly, the uterus superiorly and the rectum posteriorly; but without definite invasion into these surrounding structures (figures 1 and 2). A radiological differential diagnosis of leiomyoma, leiomyosarcoma or sarcomatoid carcinoma was suggested. Subsequent staging CT showed no evidence of lymphadenopathy nor distant metastases.
Figure 1.
MRI of the rectovaginal extragastrointestinal stromal tumour (EGIST) displacing the bladder anteriorly, the rectum posteriorly and the uterus superiorly.
Figure 2.
MRI of the rectovaginal extragastrointestinal stromal tumour (EGIST) displacing the bladder anteriorly and the rectum posteriorly.
Differential diagnosis
Given the diagnostic uncertainty and close proximity of tumour to the bladder and bowel, initial exploratory laparoscopy was recommended by the MDT - allowing consideration of biopsy under vision to aid diagnosis, as well as importantly facilitatingdirect visualisation of the mass for tumour-mapping and assessment of feasibility of primary surgery.
At laparoscopy, a large retroperitoneal mass was identified - invading the pelvic floor and adherent to the rectosigmoid, but with no other pelvic or upper abdominal disease. Primary debulking, with the aim of achieving R0 cytoreduction, was deemed possible. For this reason and the intention to proceed to en bloc resection, laparoscopic biopsy was omitted to avoid risk of iatrogenic seeding.
Treatment
Following further MDT discussion, the patient was counselled for primary surgical management - with input from the colorectal and stoma teams; and support from the cancer nurse specialist throughout.
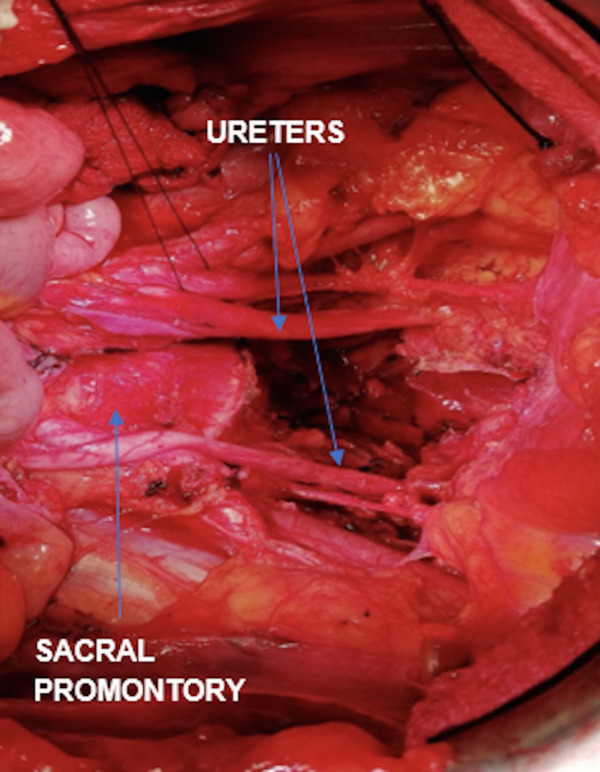
Infralevator posterior exenteration was performed - removing the uterus, fallopian tubes, ovaries, rectosigmoid, levator ani muscle and upper vagina en bloc (figure 3). Pelvic side wall dissection facilitated slinging of the anterior division of the internal iliac arteries, gaining early vascular control of the pelvis; and bilateral ureterolysis was performed. Lymph nodes were assessed and an enlarged paracaval node excised. On the intraoperative advice of plastic surgery, the pelvic floor was reconstructed using interrupted No1 monocryl sutures, without the need for flap or mesh insertion. An end-to-end colostomy was fashioned. The surgery took 7 hours to complete; the estimated blood loss was 1500 mL; and no intraoperative complications occurred. Postoperatively, the patient had a planned admission to high-dependency; was transfused one unit of packed red cells on day 1 for haemoglobin of 66 g/L; and was managed conservatively for paralytic ileus. She was discharged home well on day 9.
Figure 3.
Situs finale (view of the pelvis after complete removal of the tumour).
The final histopathology report described a large tumour in two fragments - measuring 3×2×1.5 cm and 20×15×4 cm - arising from thevagina, with extension into the rectum. The tumour had tan and haemorrhagic components, with gelatinous texture. Microscopic examination revealed a vaginal spindle cell tumour, invading the muscularis propria and submucosa of the rectum. Tumour margins were clear by at least 1 mm and no lymphovascular space invasion was identified. To further investigate the histopathological differential of this solid stromal tumour, a wide range of tailored immuno- and cytogenetic tests were applied. Immunohistochemistry was positive for CD117, DOG1 and CD34; while desmin and S100 stains were negative - enabling distinction of EGIST from overlapping pathologies of leiomyoma and leiomyosarcoma. Subsequent cytogenetics identified a c-KIT exon 11 mutation and mitotic rate of 1 mitosis per 50 high power field (HPF). With the bulk, and hence origin, of tumour agreed as vaginal - together with the above immunohistochemical profile - a final diagnosis of stage pT4N0 rectovaginal EGIST was confirmed.
The case was further discussed at the gynae-oncology and GI MDTs - with recommendation for adjuvant TKI therapy under the care of medical oncology. A baseline positron emission tomography CT (PET-CT) 6 weeks postoperatively showed no residual disease. The patient was commenced on matinib 200 mg once daily for 8 weeks after surgery, titrating up to 400 mg once daily after 1 week. After 10 days of treatment, however, due to a widespread skin rash, the imatinib was stopped. A rechallenge was attempted at a later date and at a lower starting dose of 100 mg once daily, but due to alopecia and derangement in liver function tests imatinib was once again discontinued.
Outcome and follow-up
The patient has subsequently remained under close surveillance - attending oncology outpatients and undergoing serial PET-CT imaging at 4 monthly intervals. She is currently 28 months postoperative, with no disease relapse to date and is awaiting surgical review to discuss colostomy reversal.
Discussion
Demographics
GISTs as a whole demonstrate male predominance and most typically affect adults between the fifth and seventh decades, with a mean age of 55–60 years at diagnosis reported.6 14 22 EGISTs of the female genital tract, however, may have an earlier age of onset - with one case series reporting 30% of rectovaginal EGISTs occurring <40 years of age.22 23
Clinical presentation
The presentation of GISTs is diverse and dependent on the tumour location.14 Patients with rectovaginal EGIST may present with a range of non-specific symptoms - including abnormal bleeding, pain, abdominal distension; or even with compression effects of urinary frequency, retention and constipation.4 6 14 Examination typically reveals a hard, well-circumscribed vaginal or rectal mass; with or without extension into the pelvis.4 A mean tumour size of 6.2 cm at presentation has been reported.4 EGISTs are less likely to metastasise than GISTs proper; with metastatic disease detected in 13% of cases at presentation, compared with in 28%–61% of GISTs truly originating from the GI tract.14 24 If metastasis of a rectovaginal EGIST does occur, however, it is more likely to spread to the lung, bone or adrenal gland - rather than the liver or peritoneal secondaries typical of GISTs at other sites.6 25 Such vague clinical symptoms and non-descript examination pose diagnostic difficulty, easily mistaking an EGIST for other more common gynaecological or colorectal pathologies.12 26 27
Pre-operative investigation
Serum tumour markers (CA125 and carcinoembryonic antigen) are of no diagnostic value in GIST of the female genital tract.4 14 Imaging is typically associated with an absence of any differentiating features - CT detects a non-specific, well-circumscribed soft tissue mass; while MRI depicts a tumour with uniform intermediate enhancement on T1-weighted images.4 28 Heterogeneous changes on either modality likely represent haemorrhage or necrosis within the tumour.4 28 In part due to this lack of specific radiological features, rates of correct pre-operative diagnosis of EGIST are low.4 29 Only 3 of 18 cases were correctly identified before definitive treatment in one case series, with the most common suspected radiological diagnosis for EGIST being either leiomyoma or leiomyosarcoma.4 12 23
Histopathology
Even once histopathology is obtained, the diagnosis of an EGIST can still be missed unless actively sought. GISTs are characterised by a broad morphological spectrum - including spindle, epithelioid and mixed morphology; and therefore also encompass many differential diagnoses - including leiomyoma, leiomyosarcoma, schwannoma, local extension of a primary retroperitoneal liposarcoma, benign and malignant vascular tumours, intra-abdominal fibromatosis, carcinoid with a spindle cell morphology, and metastatic disease (melanoma, spindle cell carcinoma).4 13 23 A high index of clinical and/or histopathological suspicion is thus required to seek out the diagnosis of EGIST, achieved only by employing appropriate immunohistochemistry and cytogenetics.13
The typical immunohistochemistry panel for a GIST will demonstrate positive staining for CD117 (95%), DOG1 (98%) and CD34 (70%).30 31 A combination of CD117 and DOG1 positivity is sufficient to confirm the diagnosis, with CD34 acting as a useful complementary test if either CD117 or DOG1 is negative.4 32 Demonstrating the absence of desmin and smooth muscle actin excludes leiomyoma and leiomyosarcoma.13 Negative pan-cytokeratin argues against an epithelial tumour type, while the absence of S100 rules out schwannoma.13 Cytogenetics also play a role in diagnosis. c-KIT mutations are found in 95% of GISTs.13 Mutations in the juxtamembrane domain (exon 11) are most common in GISTs of the GI tract proper, while mutations in the extracellular domain (exon 9) are more often observed in EGISTs.12
Treatment
Primary surgery
Primary surgical excision is the treatment of choice for GIST, offering the only curative option and most favourable prognosis.4 14 33 34 Due to their rarity, there is no standardised operative approach to rectovaginal EGIST.14 While any surgery undertaken should be individualised guided by detailed clinical evaluation and MDT involvement - the US National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) define R0 cytoreduction as the key objective.4 14 33 34 Conservative surgical approaches of enucleation and local excision should hence be avoided - reported to achieve R0 debulking in only 16% of such cases, compared with complete cytoreduction in 71% of patients undergoing radical surgery for GIST.14
Neoadjuvant therapy
While primary surgery is the gold standard treatment for operable GIST, both the NCCN and ESMO recommend neoadjuvant treatment with TKIs, such as Imatinib, in cases of advanced disease deemed inoperable or if primary debulking surgery is felt unlikely to achieve R0 status.33–39 Neoadjuvant TKIs downsize GISTs, with three large studies all reporting pre-operative regression by >60% with Imatinib.40–42 This reduction in bulk can reduce tumour burden to within operable limits, hence enabling subsequent interval debulking. R0 cytoreduction is achieved in 88%–96% of cases of advanced GIST undergoing surgery following completion of neoadjuvant TKI.40–42 TKIs may also reduce the radicality of surgery required, as well as potentially facilitating a less invasive transanal or transvaginal approach to resection - keeping the peritoneal cavity intact, reducing both surgical morbidity and risk of future peritoneal recurrence.43–45 One mechanism of action of neoadjuvant Imatinib is to devitalise the tumour bed, as demonstrated on post-TKI imaging, making tissues less vascular and less friable.46 Hence, patients receiving TKI in advance of surgery may benefit from reduced blood loss and lower rates of tumour rupture with intraoperative tissue handling.2 With recurrence rates of close to 100% in the context of such rupture, this is of important prognostic significance.47–50 The safety and efficacy of Imatinib have been demonstrated, with a generally favourable side effect profile: rash (9%), neutropaenia (8%) and less often nausea.40 41
Adjuvant therapy and prognosis
GISTs are associated with high rates of recurrence - with tumour diameter, mitotic activity, anatomical location and surgical margin status /rupture identified as significant prognostic factors.12 14 22 47 Tumours measuring >5 cm with mitotic rate of >5 per 50 HPF; or tumours >10 cm with any mitotic rate are deemed high risk for recurrence.22 51 52 Those with mutations in exon 9 exhibit better response rates to second-line targeted therapy.53 54
By comparison, EGISTs tend to be more aggressive in their clinical course with even higher rates of recurrence - in part attributed to greater difficulty in achieving clear surgical margins at these anatomical sites versus the more feasible complete resection of GISTs of the GI tract proper.6 14 23 A study of 14 patients with rectovaginal EGIST reported a 50% recurrence rate, occurring on average 11–82 months after primary surgery (mean: 48.4 months) and affecting the pelvic floor, rectal wall, vagina, and the presacral or pararectal space.14 Two patients within this cohort developed distant metastasis in the liver and adrenal gland at 6 and 14 months postoperatively; and 15% died by 3 years of follow-up.14 Of those with recurrence, four of seven patients with rectovaginal EGIST experienced multiple recurrences - five of whom had undergone local excision, two radical resection and in none had R0 cytoreduction been achieved.14 This correlation between surgical margin status and prognosis is reflected further in the results of another paper reporting a recurrence rate of 77% in patients undergoing local resection with close or positive margins, compared with relapse in 31% of patients undergoing radical resection achieving clear margins.55
Imatinib has been approved for use as an adjuvant therapy in GISTs identified as high risk based on a number of prognostic features as detailed above.56 Imatinib reduces relapse rates and improves disease-free survival in such cases - with one paper reporting a 4% versus 67% recurrence rate in high risk patients receiving adjuvant Imatinib compared with untreated matched controls.15–21
Learning points.
Rectovaginal extragastrointestinal stromal tumours (RV-EGISTs) are rare and difficult to diagnose.
Inclusion of RV-EGIST in the differential of a rectovaginal mass is essential to prompt the application of targeted immunohistochemistry, key to distinguishing EGISTs from other solid mesenchymal tumours and securing the correct diagnosis.
Primary debulking surgery is the treatment of choice for RV-EGIST if complete cytoreduction can be achieved.
‘High-risk’ tumour features identify those at greatest risk of recurrence and hence those patients known to benefit from adjuvant tyrosine kinase inhibitor (TKI) therapy.
In patients in whom primary resection is not feasible - there may be a role for neoadjuvant TKI to reduce tumour burden, followed by consideration of interval debulking surgery.
Acknowledgments
We would like to acknowledge the contributions of Dr Zoe Traill and Dr Negin Sadeghi in helping to produce this manuscript.
Footnotes
Contributors: SA: wrote the manuscript, performed the literature search. CJ: involvement in patient care, proof-reading and critique, provided radiological images and input. MA: involvement in patient care, proof-reading and critique. HSM: involvement in patient care, performed the surgery, cowrote the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Molina I, Seamon LG, Copeland LJ, et al. Reclassification of leiomyosarcoma as an extra-gastrointestinal stromal tumor of the gynecologic tract. Int J Gynecol Pathol 2009;28:458–63. 10.1097/PGP.0b013e31819c7fc1 [DOI] [PubMed] [Google Scholar]
- 2.Jakob J, Hohenberger P. Neoadjuvant therapy to Downstage the extent of resection of gastrointestinal stromal tumors. Visc Med 2018;34:359–65. 10.1159/000493405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ressing M, Wardelmann E, Hohenberger P, et al. Strengthening health data on a rare and heterogeneous disease: sarcoma incidence and histological subtypes in Germany. BMC Public Health 2018;18:235. 10.1186/s12889-018-5131-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng M, Liu C-H, Horng H-C, Chia-Hao L, Huann-Cheng H, et al. Gastrointestinal stromal tumor presenting as a rectovaginal septal mass. Medicine 2019;98:17. 10.1097/MD.0000000000015398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fletcher CDM, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 2002;33:459–65. 10.1053/hupa.2002.123545 [DOI] [PubMed] [Google Scholar]
- 6.Miettinen M, Furlong M, Sarlomo-Rikala M, et al. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol 2001;25:1121–33. 10.1097/00000478-200109000-00002 [DOI] [PubMed] [Google Scholar]
- 7.Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 2001;438:1–12. 10.1007/s004280000338 [DOI] [PubMed] [Google Scholar]
- 8.Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol 1999;30:1213–20. 10.1016/S0046-8177(99)90040-0 [DOI] [PubMed] [Google Scholar]
- 9.Reith JD, Goldblum JR, Lyles RH, et al. Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod Pathol 2000;13:577–85. 10.1038/modpathol.3880099 [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Monihan JM, Sarlomo-Rikala M, et al. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol 1999;23:1109–18. 10.1097/00000478-199909000-00015 [DOI] [PubMed] [Google Scholar]
- 11.Lasota J, Carlson JA, Miettinen M. Spindle cell tumor of urinary bladder serosa with phenotypic and genotypic features of gastrointestinal stromal tumor. Arch Pathol Lab Med 2000;124:894–7. 10.5858/2000-124-0894-SCTOUB [DOI] [PubMed] [Google Scholar]
- 12.Lam MM, Corless CL, Goldblum JR, et al. Extragastrointestinal stromal tumors presenting as vulvovaginal/rectovaginal septal masses: a diagnostic pitfall. Int J Gynecol Pathol 2006;25:288–92. 10.1097/01.pgp.0000215291.22867.18 [DOI] [PubMed] [Google Scholar]
- 13.Le BH, Nguyen J, Bossert A, et al. Surgically enucleated gastrointestinal tumor of the rectovaginal septum. Cureus 2019;11:e5019. 10.7759/cureus.5019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agaimy A, Vassos N, Märkl B, et al. Anorectal gastrointestinal stromal tumors: a retrospective multicenter analysis of 15 cases emphasizing their high local recurrence rate and the need for standardized therapeutic approach. Int J Colorectal Dis 2013;28:1057–64. 10.1007/s00384-013-1655-3 [DOI] [PubMed] [Google Scholar]
- 15.Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097–104. 10.1016/S0140-6736(09)60500-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh Y-Y, Yen C-C, Yeh C-N, et al. Effective salvage therapy of imatinib-resistant gastrointestinal stromal tumor with combination of imatinib and pegylated liposomal doxorubicin. J Chin Med Assoc 2011;74:272–4. 10.1016/j.jcma.2011.04.007 [DOI] [PubMed] [Google Scholar]
- 17.Chen Y-Y, Peng F-S, Lin H-H, et al. Gastrointestinal stromal tumor mimicking ovarian malignancy in a woman with type I neurofibromatosis. Taiwanese Journal of Obstetrics and Gynecology 2015;54:330–1. 10.1016/j.tjog.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 18.Tjhoi WEH, Li K, Shou C-H, et al. Long-Term adjuvant imatinib treatment for a patient who underwent complete resection of a localized recurrent gastrointestinal stromal tumor after preoperative imatinib treatment: a case report. Medicine 2019;98:e14477. 10.1097/MD.0000000000014477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J, Dai Y, Zheng H-L, et al. What is the appropriate duration of adjuvant imatinib mesylate treatment for primary gastrointestinal stromal tumors classified according to the strict definition of tumor rupture? Medicine 2019;98:e14177. 10.1097/MD.0000000000014177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nie Y, Sun W, Xiao Z, et al. Complete response to sunitinib for more than three years in a patient with a jejunum gastrointestinal stromal tumor. Medicine 2019;98:14060. 10.1097/MD.0000000000014060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsson B, Sjölund K, Kindblom L-G, et al. Adjuvant imatinib treatment improves recurrence-free survival in patients with high-risk gastrointestinal stromal tumours (GIST). Br J Cancer 2007;96:1656–8. 10.1038/sj.bjc.6603797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70–83. 10.1053/j.semdp.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 23.Pelz A-F, Agaimy A, Daniels M, et al. Gastrointestinal stromal tumor presenting as a rectovaginal mass. clinicopathologic and molecular-genetic characterization of a rare tumor with a literature review. Hum Pathol 2011;42:586–93. 10.1016/j.humpath.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 24.Rutkowski P, Nowecki ZI, Michej W, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol 2007;14:2018–27. 10.1245/s10434-007-9377-9 [DOI] [PubMed] [Google Scholar]
- 25.Barrière J, Thariat J, Vandenbos F, et al. Diplopia as the first symptom of an aggressive metastatic rectal stromal tumor. Onkologie 2009;32:345–7. 10.1159/000215712 [DOI] [PubMed] [Google Scholar]
- 26.Nagase S, Mikami Y, Moriya T, et al. Vaginal tumors with histologic and immunocytochemical feature of gastrointestinal stromal tumor: two cases and review of the literature. Int J Gynecol Cancer 2007;17:928–33. 10.1111/j.1525-1438.2007.00892.x [DOI] [PubMed] [Google Scholar]
- 27.Herawi M, Montgomery EA, Epstein JI. Gastrointestinal stromal tumors (GISTs) on prostate needle biopsy: a clinicopathologic study of 8 cases. Am J Surg Pathol 2006;30:1389–95. 10.1097/01.pas.0000209847.59670.c8 [DOI] [PubMed] [Google Scholar]
- 28.Vázquez J, Pérez-Peña M, González B, et al. Gastrointestinal stromal tumor arising in the rectovaginal septum. J Low Genit Tract Dis 2012;16:158–61. 10.1097/LGT.0b013e31823b52af [DOI] [PubMed] [Google Scholar]
- 29.Irving JA, Lerwill MF, Young RH. Gastrointestinal stromal tumors metastatic to the ovary: a report of five cases. Am J Surg Pathol 2005;29:920–6. 10.1097/01.pas.0000155161.55915.c3 [DOI] [PubMed] [Google Scholar]
- 30.Fülöp E, Marcu S, Milutin D, et al. Gastrointestinal stromal tumors: review on morphology, diagnosis and management. Rom J Morphol Embryol 2009;50:319–26. [PubMed] [Google Scholar]
- 31.West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of kit or PDGFRA mutation status. Am J Pathol 2004;165:107–13. 10.1016/S0002-9440(10)63279-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novelli M, Rossi S, Rodriguez-Justo M, et al. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology 2010;57:259–70. 10.1111/j.1365-2559.2010.03624.x [DOI] [PubMed] [Google Scholar]
- 33.Network NCCN . Soft tissue sarcoma version 2. NCCN clinical practice guidelines in oncology (NCCN guidelines), 2018. Available: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf
- 34.Casali PG, Blay J-Y, ESMO/CONTICANET/EUROBONET Consensus Panel of Experts . Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;5:98–1024. 10.1093/annonc/mdq208 [DOI] [PubMed] [Google Scholar]
- 35.Sicklick JK, Lopez NE. Optimizing surgical and imatinib therapy for the treatment of gastrointestinal stromal tumors. J Gastrointest Surg 2013;17:1997–2006. 10.1007/s11605-013-2243-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu H-B, Zhou Z-G, Feng X-Y, et al. Advanced gastrointestinal stromal tumor patients benefit from palliative surgery after tyrosine kinase inhibitors therapy. Medicine 2018;97:e9097. 10.1097/MD.0000000000009097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang J, Zhao R, Zheng X, et al. Using the recurrence risk score by Joensuu to assess patients with gastrointestinal stromal tumor treated with adjuvant imatinib: a retrospective cohort study. Medicine 2018;97:e11400. 10.1097/MD.0000000000011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu J, Chen S, Li X, et al. Gastrointestinal stromal tumors: fibrinogen levels are associated with prognosis of patients as blood-based biomarker. Medicine 2018;97:e0568. 10.1097/MD.0000000000010568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin X, Shen C, Yin Y, et al. Giant gastric stromal tumor mimicking as a posterior mediastinal mass: a case report and literature review. Medicine 2018;97:e12816. 10.1097/MD.0000000000012816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doyon C, Sidéris L, Leblanc G, et al. Prolonged therapy with imatinib mesylate before surgery for advanced gastrointestinal stromal tumor results of a phase II trial. Int J Surg Oncol 2012;2012:761576. 10.1155/2012/761576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurokawa Y, Yang H-K, Cho H, et al. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br J Cancer 2017;117:25–32. 10.1038/bjc.2017.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hohenberger P, Langer C, Wendtner CM, et al. Neoadjuvant treatment of locally advanced GIST: Results of APOLLON, a prospective, open label phase II study in KIT- or PDGFRA-positive tumors. JCO 2012;30:10031. 10.1200/jco.2012.30.15_suppl.10031 [DOI] [Google Scholar]
- 43.Wang J-P, Wang T, Huang M-J, et al. The role of neoadjuvant imatinib mesylate therapy in sphincter-preserving procedures for anorectal gastrointestinal stromal tumor. Am J Clin Oncol 2011;34:314–6. 10.1097/COC.0b013e3181dea970 [DOI] [PubMed] [Google Scholar]
- 44.Beham AW, Schaefer I-M, Schüler P, et al. Gastrointestinal stromal tumors. Int J Colorectal Dis 2012;27:689–700. 10.1007/s00384-011-1353-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jakob J, Mussi C, Ronellenfitsch U, et al. Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol 2013;20:586–92. 10.1245/s10434-012-2647-1 [DOI] [PubMed] [Google Scholar]
- 46.Dimitrakopoulou-Strauss A, Ronellenfitsch U, Cheng C, et al. Imaging therapy response of gastrointestinal stromal tumors (GIST) with FDG PET, CT and MRI: a systematic review. Clin Transl Imaging 2017;5:183–97. 10.1007/s40336-017-0229-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265–74. 10.1016/S1470-2045(11)70299-6 [DOI] [PubMed] [Google Scholar]
- 48.Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg 2010;97:1854–9. 10.1002/bjs.7222 [DOI] [PubMed] [Google Scholar]
- 49.Joensuu H, Eriksson M, Hall KS, et al. Risk factors for gastrointestinal stromal tumor recurrence in patients treated with adjuvant imatinib. Cancer 2014;120:2325–33. 10.1002/cncr.28669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hølmebakk T, Bjerkehagen B, Boye K, et al. Definition and clinical significance of tumour rupture in gastrointestinal stromal tumours of the small intestine. Br J Surg 2016;103:684–91. 10.1002/bjs.10104 [DOI] [PubMed] [Google Scholar]
- 51.Hornick JL, Fletcher CDM. The role of kit in the management of patients with gastrointestinal stromal tumors. Hum Pathol 2007;38:679–87. 10.1016/j.humpath.2007.03.001 [DOI] [PubMed] [Google Scholar]
- 52.Fletcher CDM, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 2002;33:459–65. 10.1053/hupa.2002.123545 [DOI] [PubMed] [Google Scholar]
- 53.Demetri GD. Targeting the molecular pathophysiology of gastrointestinal stromal tumors with imatinib. mechanisms, successes, and challenges to rational drug development. Hematol Oncol Clin North Am 2002;16:1115–24. 10.1016/s0889-8588(02)00052-7 [DOI] [PubMed] [Google Scholar]
- 54.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329–38. 10.1016/S0140-6736(06)69446-4 [DOI] [PubMed] [Google Scholar]
- 55.Changchien CR, Wu M-C, Tasi W-S, et al. Evaluation of prognosis for malignant rectal gastrointestinal stromal tumor by clinical parameters and immunohistochemical staining. Dis Colon Rectum 2004;47:1922–9. 10.1007/s10350-004-0687-8 [DOI] [PubMed] [Google Scholar]
- 56.Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265–72. 10.1001/jama.2012.347 [DOI] [PubMed] [Google Scholar]