Abstract
A 35-year-old nurse, who was 27 weeks pregnant at the time, was admitted to hospital with a short history of cough, fever and worsening shortness of breath. Oral and nasopharyngeal swabs were positive for SARS-CoV-2 on real-time viral PCR. During her admission, her breathing further deteriorated and she developed type 1 respiratory failure. A decision was made to trial treatment with continuous positive airway pressure (CPAP) as a means of avoiding intubation. The patient tolerated this well and made rapid improvements on this therapy. She was quickly weaned off and fully recovered before being discharged home. This case highlights the potential for CPAP to be used as a means of avoiding mechanical ventilation and iatrogenic preterm birth in COVID-19 pneumonia in pregnancy. Furthermore, it highlights the need for robust evidence to support this treatment.
Keywords: CPAP, infectious diseases, respiratory medicine, obstetrics and gynaecology, pregnancy
Background
The COVID-19 is a novel viral illness that originated in Wuhan, China and has been responsible for an ongoing global pandemic, with more than 40 million confirmed cases and over 1 million deaths across 189 countries as of October 2020.1 It is thought to have similar effects on pregnant patients and the general population, where those with pre-existing comorbidities are more prone to severe outcomes.2 Recent evidence suggests that concurrent COVID-19 infection during pregnancy may also increase the risk of preterm birth.3 While the use of continuous positive airway pressure (CPAP) in acute respiratory distress syndrome (ARDS) has been documented to reduce the rate of endotracheal intubation in certain cases,4 minimal information is available on the use of CPAP in the deteriorating pregnant patients in the context of COVID-19. This is partially due to a very small proportion of patients in this cohort developing severe illness, with an even smaller proportion with critical illness requiring mechanical ventilation.5 Consequently, the potential for the use of this treatment is yet to be evaluated. We present an unusual and important case of a pregnant patient who was successfully trialled on and treated with CPAP therapy for COVID-19 pneumonia.
Case presentation
A 35-year-old Filipino nurse who was 27 weeks’ pregnant (G4 P1+2) presented to the emergency department in April 2020 with a 9-day history of fever, dry cough, sore throat and breathlessness. She has a medical history of latent tuberculosis which had been treated with rifampicin, isoniazid and pyridoxine in 2011 and no other comorbidities. She has never smoked and has no known passive smoking exposure. She is a nurse caring for patients with COVID-19 on the acute medical wards and had been self-isolating at home with her family following the development of her symptoms. She had not taken any regular medication and she reported no recent travel history.
On examination, her respiratory rate was 21 breaths per minute with oxygen saturations (SpO2) of 95% on 2 L/min of oxygen via nasal cannulae. She was tachycardic with a heart rate of 111 bpm, her blood pressure was 103/70 mm Hg and she was apyrexial with a temperature of 35.9°C. There were bilateral basal crepitations on chest auscultation. Systemic examination revealed a gravid uterus but was otherwise normal.
Investigations
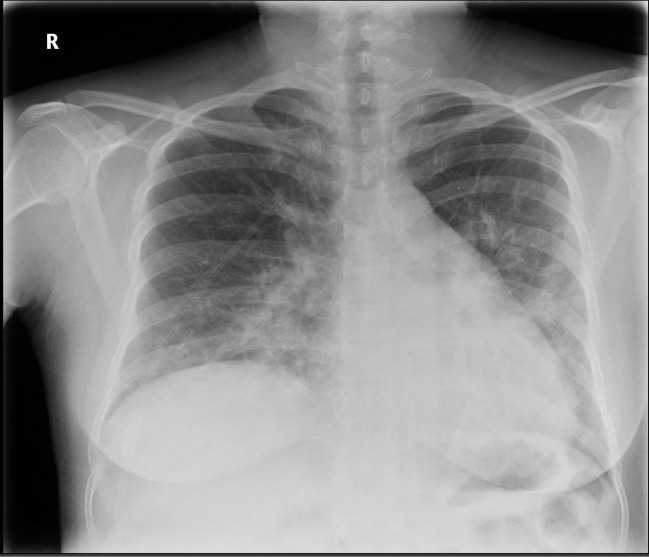
Routine blood tests revealed a mild lymphopaenia of 0.93×109/L (normal range >1) and an elevated C reactive protein of 117 mg/L (normal range <5) with no other significant abnormalities (table 1). An anterioposterior chest radiograph on admission demonstrated bilateral mid and lower zone consolidation in keeping with COVID-19 infection (figure 1). Based on her history, blood test results and chest radiograph findings, she was managed for likely COVID-19 infection and was admitted under general internal medicine. The results of oral and nasopharyngeal swabs taken on admission for viral PCR returned later with a positive result for COVID-19, confirming this diagnosis.
Table 1.
Blood test results taken from the patient on admission and upon respiratory deterioration
| Investigation | 03/04/2020 On admission | 05/04/2020 On deterioration |
| White cell count (×109/L) | 10.3 | 10.3 |
| Differential | ||
| Neutrophils (×109/L) | 8.76 | 9.06 |
| Lymphocytes (×109/L) | 1.04 | 0.93 |
| Platelet count (×109/L) | 268 | 321 |
| Haemoglobin (g/L) | 114 | 109 |
| C reactive protein (mg/L) | 114 | 109 |
| Creatinine (µmol/L) | 37 | 46 |
| eGFR (mL/min) | >90 | >90 |
eGFR, Estimated glomerular filtration rate.
Figure 1.
Chest radiograph taken on admission to hospital showing bilateral mid and lower zone consolidation.
Treatment
The patient had required a small amount of supplemental oxygen in the first 2 days of her admission. She was treated with a course of intravenous co-amoxiclav to give adequate antimicrobial coverage in the case of possible bacterial pneumonia. Appropriate venous thromboembolism prophylaxis was given using 5000 units of subcutaneous dalteparin once a day. On day 3 of admission, her work of breathing had increased, with a recorded respiratory rate of 32–34 breaths per min. At that time, her SpO2 was 93% on 4 L/min of oxygen via nasal cannulae, which was within the target saturations of 92%–96%. However, her SpO2 began to fall below her target saturations prompting the need of slow-up titration of her oxygen therapy, starting at 40% FiO2 and eventually reaching 60% FiO2 via a humidified circuit. Consequently, several multidisciplinary discussions took place between the medical, obstetric and intensive care teams. It was decided that two doses of 12 mg of dexamethasone would be given intramuscularly on day 2 of admission in order to promote fetal lung maturation in the case of early delivery. Furthermore, a magnesium sulphate infusion was administered on day 3 to protect against the risks of neurological compromise in preterm delivery.6
An arterial blood gas (ABG) revealed type 1 respiratory failure (table 2), with a PaO2 of 10.3 and an elevated alveolar-arterial gradient of 41.2 kPa. The PaO2/FiO2 ratio was 17 mm Hg. A posterioanterior (PA) chest radiograph was performed on the same day, showing worsening of the consolidation in keeping with progressive COVID-19 pneumonia (figure 2). The decision was made to trial her on CPAP in the respiratory support unit (a high dependency ward) with a view that if this was successful, it would avoid the need for intubation and therefore a possible preterm caesarean section. She was started on CPAP at 8 cm H2O and 60% oxygen and tolerated this well.
Table 2.
Arterial blood gas results taken from the patient on admission and upon respiratory deterioration
| ABG | 03/04/2020 On admission | 05/04/2020 Prior to continuous positive airway pressure |
| pH | 7.46 | 7.453 |
| pCO2 | 4.06 | 4.26 |
| pO2 | 9.01 | 10.30 |
| HCO3 | 23.1 | 23.5 |
| Est O2 sat | 94.6% | 96% |
| Lactate | 0.68 | 1.56 |
| FiO2 | 28.0% | 60% |
ABG, Arterial blood gas.
Figure 2.
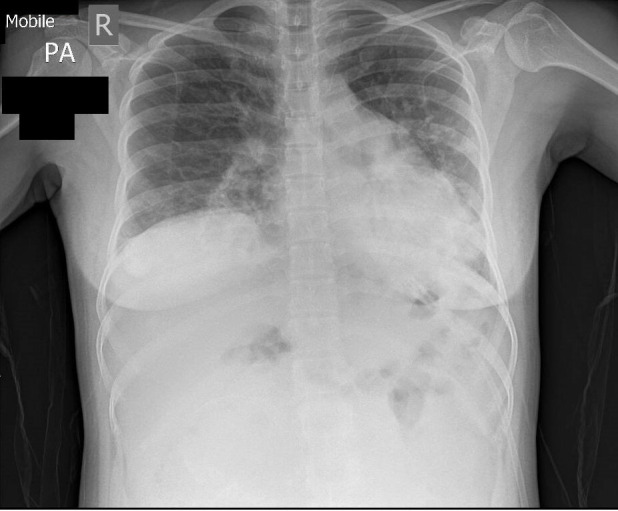
Repeat of chest radiograph on deterioration of patient showing worsening of consolidation.
Outcome and follow-up
CPAP was continued for a total of 12 hours overnight after which the patient made a marked improvement. She was successfully weaned down to oxygen delivered at 4 L/min via nasal cannulae within a few hours of discontinuing CPAP. The patient was eventually weaned off oxygen completely approximately 48 hours after the removal of CPAP. Unfortunately, further arterial blood gases were not taken as she had shown a vast clinical improvement. She had a total inpatient stay of 5 days, 12 hours of which were on CPAP. Ultimately, she was discharged home once she remained well off oxygen and showed no signs of respiratory distress. A repeat PA chest radiograph 6 weeks following her admission radiograph demonstrates significant resolution of the patchy changes noted on her initial X-ray (figure 3). In September 2020, the patient gave birth following an induction of labour for a post-term pregnancy (41+3) and reduced fetal movements. She had a rotational forceps delivery and has given birth to a healthy baby boy.
Figure 3.
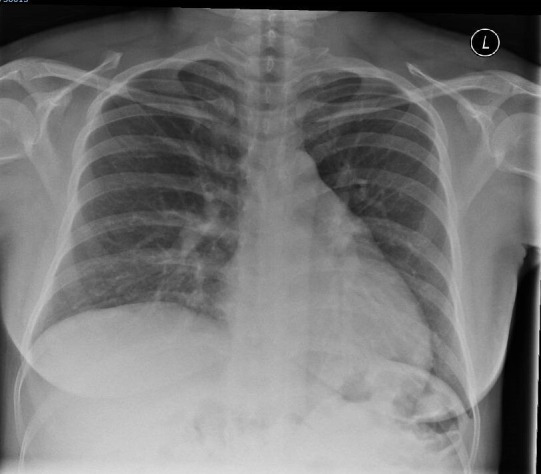
Repeat chest radiograph after 6 weeks showing marked resolution of consolidation.
Discussion
This case report highlights some of the complexities of caring for the pregnant patient in the context of COVID-19 infection due to the risk of rapid respiratory deterioration and fatality. Ideally, to address the health, risks and outcomes for both mother and fetus, the role of the multidisciplinary team plays a crucial part in helping to create escalation plans in the event of deterioration. Obstetric airway management offers its own unique challenges, with failure reported to carry an incidence of 0.4% globally.7 Moreover, preterm delivery may lead to significant complications such as neonatal ARDS, visual and hearing problems, poorer neurodevelopmental outcomes and learning difficulties in childhood.8 In the context of our case, the team-based approach proved to be particularly helpful in aiming to limit adverse outcomes to our patient and the fetus should respiratory deterioration have occurred.
Physiologically, pregnancy has multiple concurrent effects on the respiratory system from endocrine and mechanical changes. Most significantly, the upward pressure of the distending gravid uterus results in earlier closure of the small airways and consequent reduction of functional residual capacity.9 Positive-end expiratory pressure in the form of CPAP has been well documented to improve arterial oxygenation and reduce the work of breathing, by recruiting collapsed alveoli and increasing functional residual capacity, potentially reversing hypoxaemia.10 11
Pneumonia can complicate pregnancy and can result in significant morbidity. Previous studies have demonstrated that a high number of pregnant patients with SARS required hospital admission, with one-third of cases needing mechanical ventilation.12 More recent studies favour a better outcome in pregnant patients diagnosed with COVID-19,13 and it has been suggested that outcomes in this patient cohort are comparable to those of the general population.14 Nevertheless, there is not enough data currently to establish the true impact of COVID-19 infection on morbidity and mortality in this cohort.
In the early stages of the pandemic, early intubation and mechanical ventilation posed the mainstay of treatment; however, evolving evidence has increasingly supported the early use of CPAP as a treatment for respiratory compromise in COVID-19 pneumonia.15 CPAP is advocated in patients with severe COVID-19 infection as a means of either avoiding or delaying the need for intubation, or as a bridging therapy until mechanical ventilation becomes available.16 While these benefits are established among the general population, little is known about its benefits in pregnant women positive for COVID-19. According to the Royal College of Obstetricians and Gynaecologists, pregnant women are not deemed to be more at risk of acquiring COVID-19 nor to follow a severe disease course should they become infected.2 Furthermore, it is estimated that only 8% of pregnant patients develop severe disease, and only 1% become critically unwell requiring mechanical ventilation.17 Collectively, these factors may contribute to the paucity of evidence regarding the role of CPAP in this patient cohort.
As of October 2020, we have noted three case reports or case series which detail the use of either CPAP or non-invasive ventilation (NIV) as a treatment for respiratory deterioration in pregnant patients with COVID-19.18–20 These results involving data collected from China, France and Italy are summarised in table 3.
Table 3.
Summary table of case reports and case series detailing the use of CPAP or NIV in the treatment of pregnant patients with COVID-19
| Paper | Yan et al18 | Kayem et al19 | Savasi et al20 |
| Country of origin | China | France | Italy |
| Cases (n) | 65 | 617 | 77 |
| Number of patients treated with NIV (n (%)) | 6 (9.2) | 10 (1.6) | 2 (2.6) |
| CPAP or BiPAP | Not documented | Not documented | CPAP |
| Escalation to mechanical ventilation (n (%))* | 1 (0.2) | Not documented | Not documented |
| Escalation to ECMO (n (%))* | 1 (0.2) | Not documented | Not documented |
| Maternal deaths (n (%))* | 0 (0) | 1 (0.01) | 0 (0) |
| Maternal deaths in patients treated with CPAP (n (%))* | 0 (0) | Not documented | 0 (0) |
| Neonatal deaths (n (%))* | 1 (0.2) | 1 (0.01) | 0 (0) |
*Recorded as a percentage of patients treated with NIV.
BiPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; ECMO, extracorporeal membrane oxygenation; NIV, non-invasive ventilation.
There has been a total of 18 reported cases where NIV has been used as a treatment in pregnant patients with COVID-19. Only one patient required escalation to higher level care (mechanical ventilation, then extracorporeal membrane oxygenation).18 While the use of CPAP as a bridging measure for ventilatory support has been recommended by a number of studies,21 22 the evidence to support this is still sparse. Intubation during pregnancy is recognised to have a higher rate of failure, due to a range of factors including upper airway oedema, as well as a higher risk of aspiration due to the loss of oesophageal tone and delayed gastric emptying.23 It has been recommended that intubation should be avoided if clinically possible21 and CPAP presents a means of doing so if its use is successful.
As far as the limited evidence allows, this case report details the only case of a pregnant patient at this gestation (27 weeks) who was successfully treated for COVID-19 pneumonia with CPAP. While a comparable patient aged 32 years and at 28 weeks gestation was treated for COVID pneumonia with NIV,18 they subsequently had a caesarean section with pneumonia as the indication at 28+1 weeks. Our case presents a scenario where this outcome was avoided. This case report also reflects emerging evidence that early intervention with CPAP (treatment within 7 days or less) leads to significantly improved outcomes in patients with COVID-19.15 It also reflects the notion that patients admitted under general internal medicine for COVID-19 tend to have better outcomes than those admitted under intensive care.24
We must acknowledge, however, that this patient was treated in the very early stages of the pandemic, before there was sufficient evidence to support any drug treatments for COVID-19 pneumonia. As such, no antivirals or immunomodulatory agents were administered. Admittedly, this patient did receive two doses of dexamethasone 12 mg, although for the promotion fetal lung maturity rather than as a treatment for COVID-19. This drug would later be proven to reduce the incidence of death in patients requiring oxygen, or ventilated with COVID-19.25 Our patient did recover quickly following a short period of treatment with CPAP, and it is likely that there was a cumulative effect of both CPAP and steroid treatment resulting in this. While this case is encouraging, we would caution its use in isolation in informing clinical decisions. There is a need for more robust data and evidence to support the use of CPAP for the treatment of pregnant patients with COVID-19.
Currently, pregnant patients have been excluded from some studies concerning COVID-19. For example, in the UK, the RECOVERY-RS clinical trial which aims to study NIV treatment modalities in COVID-19 explicitly excludes pregnant women. Emerging evidence suggests a higher rate of iatrogenic preterm birth as a result of emergency caesarean delivery in pregnant patients with COVID-19 pneumonia. This is largely as a result of respiratory deterioration requiring intubation.26 In light of this, we argue that pregnant women encompass a patient population who may stand to gain a great deal from an evidence-based recommendation for the use of NIV in COVID-19. This treatment has great potential for benefit in this patient cohort, especially in pregnancies which are not yet at full term. With a greater evidence base to inform our guidelines and clinical reasoning, it is possible that a greater proportion of pregnant patients may eventually be able to avoid mechanical ventilation and iatrogenic preterm delivery when being treated for COVID-19.
Patients perspective.
I was contacted by the respiratory team immediately after my discharge and I also had follow up over thephone over the course of 7 days. I thankfully remained well, and I am grateful for such good follow up. Asa nurse, I am particularly happy that I benefited from CPAP - I felt my breathing improved significantlywhilst I was on the machine. I am also happy as this avoided early delivery of my baby and possibleintubation too.
Learning points.
This case report provides an account of the successful use of continuous positive airway pressure (CPAP) as a treatment modality in COVID-19 in pregnancy.
CPAP could be a useful treatment in the context of COVID-19 infection in pregnancy, especially as a means to avoid intubation and iatrogenic preterm deliveries.
Multidisciplinary input is essential in order to make these complex clinical decisions regarding both maternal and fetal health.
There is a need for robust evidence regarding the safe and efficacious use of CPAP in the pregnant cohort with COVID-19.
Footnotes
Contributors: MR took the lead in writing the case report and completed part of the supporting literature review. JN assisted with writing and editing the case report and the literature review. TI conceived the idea for this case report and assisted with writing and editing the case report, as well as the literature review.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Coronavirus Resource Centre . John Hopkins University of medicine. Available: https://coronavirus.jhu.edu/map.html [Accessed 19 Oct 2020].
- 2.Royal College of Obstetricians and Gynaecologists . Information for healthcare professionals coronavirus (COVID-19) infection in pregnancy 2020.
- 3.Al-Lami RA, Alrammahi AM, Algburi AMA. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol 2021;223:1 https://pubmed.ncbi.nlm.nih.gov/33476599/ 10.1016/j.ajog.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu X-P, Zhang X-C, SL H. Noninvasive ventilation in acute hypoxemic nonhypercapnic respiratory failure: a systematic review and meta-analysis. Crit Care Med 2017;45:e727. 10.1097/CCM.0000000000002361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020;395:809–15. 10.1016/S0140-6736(20)30360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle LW, Crowther CA, Middleton P. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the foetus. Cochrane database of systematic reviews. John Wiley and Sons Ltd 2009. [DOI] [PubMed] [Google Scholar]
- 7.Yao WY, Li SY, Yuan YJ, et al. Comparison of Supreme laryngeal mask airway versus endotracheal intubation for airway management during general anesthesia for cesarean section: a randomized controlled trial. BMC Anesthesiol 2019;19:123. 10.1186/s12871-019-0792-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel JP, Chawanpaiboon S, Moller AB. The global epidemiology of preterm birth, best practice and research: clinical obstetrics and gynaecology. Bailliere Tindall Ltd 2018;52:3–12. [DOI] [PubMed] [Google Scholar]
- 9.LoMauro A, Aliverti A. Respiratory physiology of pregnancy. Breathe 2015;11:297–301. 10.1183/20734735.008615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayalesi P, Maggiore S. Positve end-expiratory pressure. : Tobin MJ, . Principles and practice of mechanical ventilation. 3rd edn. New York, NY: McGraw-Hill Medical, 2013: 253–302. [Google Scholar]
- 11.Wild M, Alagesan K. Peep and CPAP. Vol. 3. British Journal of Anaesthesia CEPD Reviews 2001. [Google Scholar]
- 12.Wong SF, Chow KM, Leung TN, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol 2004;191:292–7. 10.1016/j.ajog.2003.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu D, Li L, Wu X, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol 2020;215:127–32. 10.2214/AJR.20.23072 [DOI] [PubMed] [Google Scholar]
- 14.Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ 2020;369:m2107 https://www.npeu.ox.ac 10.1136/bmj.m2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashish A, Unsworth A, Martindale J, et al. Cpap management of COVID-19 respiratory failure: a first quantitative analysis from an inpatient service evaluation. BMJ Open Respir Res 2020;7:e000692. 10.1136/bmjresp-2020-000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.England NHS, Improvement NHS . Specialty guides for patient management during the coronavirus pandemic guidance for the role and use of non-invasive respiratory support in adult patients with coronavirus (confirmed or suspected), 2020. Available: https://www.nice.org.uk/guidance/ng159 [Accessed 17 May 2020].
- 17.Bruce Aylward (WHO); Wannian Liang (PRC) . Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19). Vol. 1, the WHO-China joint mission on coronavirus disease 2019, 2020. Available: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf [Accessed 17 May 2020].
- 18.Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol 2020;223:111.e1 10.1016/j.ajog.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kayem G, Lecarpentier E, Deruelle P, et al. A snapshot of the Covid-19 pandemic among pregnant women in France. J Gynecol Obstet Hum Reprod 2020;49:101826. 10.1016/j.jogoh.2020.101826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savasi VM, Parisi F, Patanè L, et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19). Obstet Gynecol 2020;136:252–8. 10.1097/AOG.0000000000003979 [DOI] [PubMed] [Google Scholar]
- 21.Vega M, Hughes F. Bernstein PSet al. from the trenches: inpatient management of COVID-19 in pregnancy. Am J Obstet Gynecol MFM 2020;2:100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashokka B, Loh M-H, Tan CH, et al. Care of the pregnant woman with coronavirus disease 2019 in labor and delivery: anesthesia, emergency cesarean delivery, differential diagnosis in the acutely ill parturient, care of the newborn, and protection of the healthcare personnel. Am J Obstet Gynecol 2020;223:66–74. 10.1016/j.ajog.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapinsky SE. Management of acute respiratory failure in pregnancy. Semin Respir Crit Care Med 2017;38:201–7. 10.1055/s-0037-1600909 [DOI] [PubMed] [Google Scholar]
- 24.Pezzuto A, Tammaro A, Tonini G, et al. Copd influences survival in patients affected by COVID-19, comparison between subjects admitted to an internal medicine unit, and subjects admitted to an intensive care unit: an Italian experience. J Med Virol 2021;93:1239–41 https://onlinelibrary.wiley.com/doi/ 10.1002/jmv.26585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turan O, Hakim A, Dashraath P, et al. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS‐CoV‐2 infection among hospitalized pregnant women: a systematic review. Int J Gynecol Obstet 2020;151:7–16. 10.1002/ijgo.13329 [DOI] [PMC free article] [PubMed] [Google Scholar]