Abstract
Background
Numerous studies have investigated age‐based declines in semen traits, but the impact of paternal age on semen parameter values remains inconclusive.
Objectives
The aim of this study was to detect an impact of age on semen quality was studied in healthy nonsmoking men exposed to traffic air pollution.
Methods
Semen samples from 150 Prague City policemen aged 23 to 63 years were examined for standard semen parameters, sperm DNA fragmentation and high DNA stainability.
Results
A significant positive correlation was found between age and %DFI (r = .359, P < .001), and negative correlations were found between age and sperm vitality (r = −.247, P < .001), the % acrosome‐intact sperm (r = −.202, P = .013) and the % normal sperm heads (r = −.204, P = .012). A weak but significant negative correlation was found for high DNA stainability (% HDS) vs age (r = −.161, P = .050). No significant correlation was detected between male age and the other investigated semen quality parameters. At ages of 23 to 30, 31 to 40, 41 to 50, and 51 to 63 years, the mean %DFI values were 12.7 ± 7.18, 14.7 ± 7.42, 19.6 ± 11.25, and 34.2 ± 15.08, respectively.
Conclusion
Our study shows a strong relationship (P < .001) between the age of men and sperm DNA fragmentation in an occupational cohort at risk of exposure to heavy traffic‐related air pollution in a large city center.
Keywords: aging effect, DNA fragmentation, high DNA stainability, semen analysis
1. INTRODUCTION
In recent years, the average age of men at the time of conception of their first child has significantly increased, which has enhanced interest in the effects of aging on male reproductive functions. It was shown that not only levels of reproductive hormones, general testicular functions and conventional semen parameters but also sperm DNA integrity, de novo mutation rates, telomere lengths, chromosomal structure, and epigenetic modifications of sperm chromatin have been negatively affected by the increasing paternal age. 1 , 2 Despite a number of published studies focused on age‐related decline in reproductive functions and semen quality, the impact of paternal age on semen parameters still remains inconclusive. 3 The testing of the quality of sperm by standard semen analysis is often insufficient in predicting the outcome of assisted reproductive technology, and the detection of sperm chromatin integrity is therefore increasingly being performed. A positive correlation between increasing male age and DNA damage has been reported in many studies, and a high percentage of DNA fragmentation was found associated with low fertility. Sperm DNA damage has been studied recently because it is associated with reduced fertilization and pregnancy rates, limited embryo quality, and increased risk of spontaneous miscarriage. 4 , 5 , 6 , 7 , 8 , 9 The main possible mechanisms inducing sperm DNA breaks are apoptosis, impaired sperm chromatin maturation, and oxidative stress. These effects can be induced by lifestyle factors, various diseases, exposure to pollutants, and aging. 10 , 11
Sperm DNA fragmentation can be assessed by various methods, among which the single‐cell gel electrophoresis (Comet assay), the terminal deoxyuridine nick‐end labeling (TUNEL) assay, the sperm chromatin structure assay (SCSA), and the sperm chromatin dispersion (SCD) test are most common. Significant correlations have been found between these assays, although these methods probably detect different types of DNA damage. 12 , 13 In our study, we used SCSA, which enabled us to determine the percentage of sperm with fragmented DNA (DNA fragmentation index, DFI), the degree of DNA damage, and the level of uncondensed chromatin (HDS) simultaneously, and had an exact fixed standardized protocol. 14 The aim of this study was to detect the age‐related changes in conventional semen parameters and sperm DNA damage in healthy nonsmoking Prague City policemen.
2. MATERIALS AND METHODS
2.1. Study design and population
The study group consisted of 150 nonsmoking city policemen living and working in Prague, Czech Republic. The average age of the policemen was 37.8 ± 9.1 years (range 23‐63 years). Data on each participant's reproductive and general health and on factors that might impact his semen quality were collected by questionnaire. Policemen with chronic or andrological diseases and long‐term treatment were excluded from the study. Special attention was paid to diabetes, varicocele, accessory gland infection, and chlamydial infection. Alcohol or drug abuse was also monitored. In addition, reproductive history, the number of children and possible fertility problems were recorded. All participants signed an informed consent form and could cancel their participation at any time during the study, in accord with the Helsinki II declaration. The study was approved by the ethical committee of the Institute of Experimental Medicine AS CR in Prague, approval number: 2018/09.
2.2. Inhalation exposure
The Czech Hydrometeorological Institute, Prague, performed stationary monitoring. Average daily air‐pollutant concentrations recorded by stationary monitoring for 90 days preceding the collection of the semen samples were evaluated for different city districts and the whole territory of Prague. The concentrations of major traffic‐associated pollutants (PM 2.5, NO2, benzene, and benzo[a]pyrene) are shown in Table 1.
TABLE 1.
Air pollution levels for 90 days preceding the collection of semen samples
| PM 2.5 (𝜇g/m3) | 11.7 a | (11.3‐12.6) b |
| NO2 (𝜇g/m3) | 21.5 | (20.2‐32.4) |
| Benzene (𝜇g/m3) | 1.2 | (1.1‐1.3) |
| Benzo[a]pyrene (ng/m3) | 1.7 | (1.4‐1.9) |
Abbreviation: PM, particulate matter.
Average concentrations related to the whole territory of Prague.
Ranges of average concentrations in 10 districts of Prague.
2.3. Semen collection and analysis
Semen samples were collected on site by masturbation into clean glass containers at the end of the winter season. Abstinence interval of 2 to 7 days was requested. After liquefaction at room temperature, standard semen parameters were assessed according to the guidelines of the World Health Organization. 15 The parameters included the semen volume, sperm concentration, sperm morphology (head shape, midpiece, and tail defects), sperm motility, acrosomal reaction, and sperm plasma membrane integrity. Sperm counts were determined using a Neubauer chamber. Sperm motility was evaluated under a light microscope at ×200 magnification. Acrosome‐intact sperm rates were analyzed by the Pisum sativum (PSA) lectin staining of fixed semen smears. 16 Sperm vitality was estimated by assessing the percentage of sperm with plasma membrane damage detected by staining with eosin‐nigrosin. 15 The percentage of morphologically normal sperm was determined by examining 200 sperm per sample stained with a Diff‐Quik rapid staining kit at ×1000 magnification under oil immersion and classifying them according to strict criteria as described by the World Health Organization. 15
Sperm DNA damage was analyzed after acridine orange staining by the SCSA using a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, California) as previously described. 17 Semen samples were exposed to 488 nm monochromatic laser light, and red (ssDNA) and green (dsDNA) fluorescence values were recorded in 5000 spermatozoa per sample. SCSA‐Soft software (SCSA Diagnostics, Inc, Brookings, South Dakota) was used to assess the rates of sperm with fragmented DNA (DNA fragmentation index, DFI) and high‐density staining (representing mainly immature sperm, HDS).
2.4. Cotinine assay
The level of tobacco smoke exposure reported in the lifestyle questionnaires was verified by detection of the urinary levels of cotinine as the major nicotine metabolite by radioimmunoassay. 18 Subjects with cotinine levels higher than 500 ng/mg of creatinine were considered active smokers and were excluded. In our cohort, the median cotinine concentration was 5.2 ng/mg of creatinine with a minimum of 1.5 and a maximum of 177 ng/mg of creatinine.
2.5. Statistical analysis
Statistical analysis was performed by nonparametric exact tests using the SPSS software package, version 18 for Windows (SPSS, Inc. Chicago, Illinois). Because not all variables are normally distributed (Kolmogorov‐Smirnov test) nonparametric analyses were applied. The Mann‐Whitney test was used to compare means. Regression and correlation analyses with continuous variables were performed using Spearman's rank correlation coefficient.
3. RESULTS
Descriptive statistics for the semen analysis are presented in Table 2. Our results showed that the study group consisted largely of normospermic individuals 15 according to the semen volume and sperm concentration, morphology, vitality, and motility.
TABLE 2.
Results of the semen analysis in the study group
| Parameter | Mean ± SD |
|---|---|
| Sperm volume (mL) | 3.2 ± 1.7 |
| Sperm concentration (x106/mL) | 96.1 ± 79.43 |
| Total motility (%) | 57.0 ± 11.06 |
| Vitality (%) | 72.4 ± 8.66 |
| Sperm morphology, normal form (%) | 17.5 ± 8.9 |
| Sperm head morphology, normal form (%) | 24.1 ± 12.76 |
| Acrosome‐intact sperm (%) | 82.5 ± 6.93 |
| DFI (%) | 17.0 ± 10.64 |
| HDS (%) | 11.9 ± 5.61 |
Abbreviations: DFI, DNA fragmentation index; HDS, high DNA stainability.
A significant positive correlation was found between age and %DFI (r = .359, P < .001), and negative correlations were found between age and sperm vitality (r = −.247, P < .001), the % acrosome‐intact sperm (r = −.202, P = .013), and the % normal sperm heads (r = −.204, P = .012). A weak but significant negative correlation was found for high DNA stainability (% HDS) vs age (r = −.161, P = .050). The individual data points and the regression line demonstrated a positive correlation between age and the proportion of DNA fragmentation (Figure 1). No significant correlation was revealed between male age and the other investigated semen quality parameters (sperm volume, concentration, and total motility). At the ages of 23 to 30, 31 to 40, 41 to 50, and 51 to 63, the means for selected semen parameters are shown in Table 3. Tables 3 and 4 include only parameters for which a significant correlation with age was found. The proportions of men with a DFI ≥25% were 8.6%, 10.8%, 25.6%, and 72.7% in age groups 23 to 30, 31 to 40, 41 to 50, and 51 to 63, respectively. The differences between the groups were significant except between the first and second groups. When we divided the cohort under study into two groups of 23 to 40 years and 41 to 63 years (Table 4), the first group showed no significant correlation of the semen parameters with age, while in the second group, the correlation was relatively high (r = .460, P < .001). The proportions of men with a DFI ≥25% in the two groups were 10% and 36%, respectively (P < .001). In the cohort under study, 20% of men exhibited HDS >15%, with values of 18% in the 23 to 40 age group and 24% in the 41 to 63 age group. However, the difference between the groups was not significant. In terms of the relationship between %DFI and various WHO semen parameters in our cohort, a significant (P < .001) negative correlation was found between %DFI, total motility (r = −.414), vitality (r = −.471), and acrosome‐intact sperm (r = −.301). A weak but significant correlation was observed between DFI and the sperm concentration (r = −.165, P = .043).
FIGURE 1.
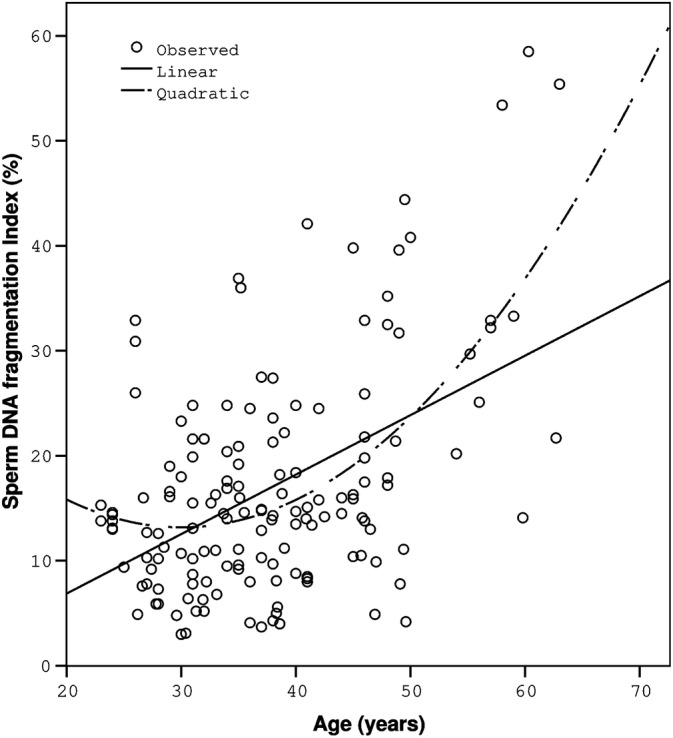
Correlation between age and the proportion of DNA fragmentation. Individual data points and the corresponding regression lines are shown. Spearman's rank correlation coefficient = 0.359; P < .001
TABLE 3.
Relationship of DFI and selected semen parameters to age
| Variable | 23–30 y (n = 35)a, a | 31–40 y (n = 65)b | 41–50 y (n = 39)c | 51–63 y (n = 11)d |
|---|---|---|---|---|
| Age (y) | 27.2 ± 2.25 | 35.4 ± 2.86 | 45.5 ± 2 .93 | 58.4 ± 2.92 |
| 27.4 (23, 30) | 35.1 (31, 40) | 45.0 (41, 50) | 58.0 (54, 63) | |
| DFI (%) | 12.7 ± 7.18 | 14.7 ± 7.42 | 19.6 ± 11.25 | 34.2 ± 15.08 |
| 12.6 (3.0, 32.9) | 14.5 (3.7, 36.9) | 15.9 (4.2, 44.4) | 32.2 (14.1, 58.5) | |
| HDS (%) | 12.2 ± 4.96 | 14.7 ± 7.42 | 11.7 ± 6.27 | 10.3 ± 6.63 |
| 12.3 (4.0, 28.5) | 10.8 (4.2, 27.5) | 8.9 (4.3, 27.0) | 7.6 (4.5, 27.2) | |
| Vitality (%) | 74.4 ± 7.70 | 73.2 ± 8.44 | 71.4 ± 9.28 | 65.2 ± 7.26 |
| 73.5 (63, 90) | 73.5 (44, 87) | 72.5 (46, 89) | 68.5 (53, 73) | |
| Intact acrosome (%) | 83.9 ± 5.24 | 83.1 ± 6.96 | 81.1 ± 7.93 | 79.9 ± 6.95 |
| 83.0 (74.5, 95.0) | 84.0 (55.5, 95.0) | 83.0 (58.5, 94.5) | 81.5 (70.5, 92.0) |
Note: Data presented as mean ± SD and median (min,max),
Age: P < .001 for all subgroups (mutually), DFI: P < .001 for subgroups a,b,c vs d and a vs c.
Vitality: P < .05 for subgroups a,b vs d.
Abbreviations: DFI, DNA fragmentation index; HDS, high DNA stainability.
Age subgroups.
TABLE 4.
Comparison of DFI and selected semen parameters in the two age subgroups
| Variable | 23–40 y (n = 100) | 41‐63 y (n = 50) | p value |
|---|---|---|---|
| Age (y) | 32.5 ± 4.76 | 48.4 ± 6.10 | <.001 |
| 32.8 (23,40) | 46.7 (41,63) | ||
| DFI (%) | 14.0 ± 7.36 | 22.8 ± 13.49 | <.001 |
| 13.6 (3.0,36.9) | 17.7 (4.2,58.5) | ||
| HDS (%) | 12.1 ± 5.25 | 11.4 ± 6.31 | .087 |
| 11.4 (4.0,28.5) | 8.7 (4.3,27.2) | ||
| Vitality (%) | 73.6 ± 8.17 | 70.0 ± 9.18 | .035 |
| 73.5 (45,90) | 70.8 (46,89) | ||
| Intact acrosome (%) | 83.5 ± 6.23 | 79.4 ± 8.21 | .011 |
| 83.8 (55.5,95.0) | 82.8 (58.5,94.5) |
Note: Data presented as mean ± SD and median (min,max).
Abbreviations: DFI, DNA fragmentation index; HDS, high DNA stainability.
4. DISCUSSION
The impact of age on semen quality in men has been described in a number of studies, but the men involved were mostly fertility clinic patients. In our study, all samples were obtained from men from a non‐clinical setting. None of the participants reported fertility problems, all were living in the same locality and had the same occupation, and non‐smoking was confirmed by the measurement of cotinine levels in the urine. All examinations were performed in a single laboratory.
4.1. Age and chromatin analysis
A significant positive correlation was found between age and %DFI. The correlation coefficients published so far exhibit a relatively wide range, most often between .10 and .55 depending on the method used and the population studied. 19 , 20 , 21 , 22 , 23 , 24 , 25 They are usually slightly lower than those observed in this study. This may be due to the fact that city policemen in the performance of their professional duties are exposed to exhaust gases from road transport for extended periods. In our previous study, we observed that a concentration of 1 ng benzo[a]pyrene/m3 increases DNA fragmentation in mature spermatozoa. 17 In the present study, the concentrations of B[a]P in the monitoring period ranged between 1.4 and 1.9 ng/m3 in different Prague districts (Table 1).
In some studies, the examined men have been divided into groups according to their ages. For men under 35 and over 40 years of age, the mean %DFI values are 13 to 15 and 17 to 22, respectively. 20 , 22 , 25 , 26 Our results correspond to the lower limit for men under 35 years and the upper limit for men over 40 years. Evenson et al 26 estimated that the change point in the scope of age vs %DFI is an age of 41.6 years with a 95% confidence interval (40.4, 42.8), which is consistent with our results.
It has been assessed that men with DFI higher than 25% to 27% are at infertility risk for both natural and IUI fertilization. 27 , 28 In our group, the percentage of men with a DFI ≥25% was 16.7%, in the subgroup of 41 to 63‐year‐old men it was 36%.
Evenson et al 26 reported the mean DFI to be 40% for men aged 60 to 65 years, with a minimum DFI of 7% and a maximum of 82%. In our cohort, men over 60 years of age exhibited a mean DFI of 37.4% with a minimum of 14.1 and a maximum of 58.5%. Two men aged 60 and 63 years presented low mean DFI values of 15.5% and 20.9%. This suggests that the %DFI can be low in some individuals even at this relatively advanced age. Our previous study shows that endogenous DNA fragmentation in human sperm can be modulated by a polymorphism in metabolic and DNA repair genes. 17 , 29 The results obtained by Darbandi et al 30 demonstrated that fertility decline is associated with sperm DNA fragmentation but not with the age of men. The authors found that in men with normal sperm DFIs and antioxidant capacity levels, there were no significant changes in fertilization outcomes with increasing age.
A weak but significant negative correlation was found for high DNA stainability (% HDS) vs age. This is consistent with studies published previously. 26 , 31 , 32 HDS population of sperm have immature sperm chromatin, which is reflected in increased DNA staining using acridine orange. Jerre et al. (2019) reported a small but increased risk of miscarriage after ICSI in men with HDS >15%. However, the increased risk was not observed in men undergoing IVF in general. The authors inferred that increased %HDS can predict an elevated risk of miscarriage following ICSI treatment. This might be associated with high sperm aneuploidy rates in such men; however, the miscarriages may also be due to the abnormal chromatin structure that prevents normal timely gene products causing the embryo to die. 26
4.2. Age and conventional sperm parameters
Regarding the impact of age on conventional sperm parameters, the published results are not unambiguous. Using data from 90 studies, Johnson et al 3 quantified the effect of paternal age on semen volume, the sperm concentration, the total sperm count, sperm morphology, total motility, and progressive motility. The analyses suggested that male age is associated with a decrease in all of these semen parameters except for the sperm concentration. Based on conventional sperm parameters, our study found significant negative correlations between age and vitality, the % acrosome‐intact sperm and the % normal sperm heads. However, no significant correlation was found between male age and the other investigated semen quality parameters (semen volume, sperm concentration, total sperm count, sperm morphology, and total motility). Sperm morphology showed a negative but nonsignificant correlation with age. A significant, although relatively low, correlation was found in the percentage of normal sperm heads, which is likely to be more age related than the condition of other sperm parts. Vinnakota et al 33 examined a large group of subfertile patients and sperm donors and found that men with a high DFI were older and exhibited a lower percentage of motile sperm than men with a normal DFI. On the strength of their results, they recommended the testing of chromatin integrity in patients over age 45 with sperm motility <40%. It follows from our results that the association of motility with age is contradictory. In our study, there was no significant correlation of total motility with age, while there was a highly significant negative correlation with %DFI.
We found a significant negative correlation between age and sperm vitality. Unfortunately, sperm vitality analysis has been included in only a few studies. Moskovtsev et al 34 described a significant decline in sperm vitality in a group of men aged over 45 years. Kaarouch et al 35 did not find any significant difference in vitality or other conventional sperm parameters between groups of men under and over 40 years of age. In the present study, we found a correlation between age and the proportion of acrosome‐intact sperm. There is still a lack of information on the impact of age on the percentage of acrosome‐intact sperm, even though this parameter has a significant effect on fertility. Only spermatozoa with an intact acrosome and capable of the acrosome reaction can penetrate the zona pellucida. 36 The percentage of acrosome‐intact sperm found in our study is in accordance with published data. 37
4.3. DFI and conventional sperm parameters
In terms of the relationship between chromatin fragmentation and various WHO semen parameters in our cohort, a significant negative correlation was found between %DFI and total motility, vitality, and acrosome‐intact sperm. A weak but significant correlation was observed between DFI and the sperm concentration. Some studies have shown significant negative correlations between %DFI and vitality 38 as well as with the sperm concentration and sperm motility 25 (Mettler et al., 2019).
4.4. Conclusion
Our study shows a strong relationship between the age of men and semen quality in a cohort of healthy normospermic subjects who were all nonsmokers with the same occupation, living in the same location and working outdoors in the same city center. To the best of our knowledge, this is the first study of an occupational cohort at risk of exposure to air pollution from heavy traffic in a city.
CONFLICT OF INTEREST
The authors declare there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Jiri Rubes, Radim J Sram
Formal Analysis: Jiri Rubes, Petra Musilova
Funding Acquisition: Jiri Rubes
Investigation: Vera Kopecka, Anna Pastorkova, Jaroslav Sipek
Methodology: Miluse Vozdova, Petra Prinosilova
Project Administration: Jiri Rubes, Radim J Sram
Resources: Jan Topinka, Vlasta Svecova
Supervision: Radim J Sram
Visualization: Jiri Rubes
Writing—Original Draft: Jiri Rubes
Writing—Review & Editing: Jiri Rubes, Petra Musilova, Miluse Vozdová
All authors have read and approved the final version of the manuscript.
Jiri Rubes had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
TRANSPARENCY STATEMENT
The lead author affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Rubes J, Sipek J, Kopecka V, et al. The effects of age on DNA fragmentation, the condensation of chromatin and conventional semen parameters in healthy nonsmoking men exposed to traffic air pollution. Health Sci Rep. 2021;4:e260. 10.1002/hsr2.260
Funding information “Healthy Aging in Industrial Environment HAIE”, Grant/Award Number: CZ.02.1.01/0.0/0.0/16_019/0000798
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bergh C, Pinborg A, Wennerholm UB. Parental age and child outcomes. Fertil Steril. 2019;111(6):1036‐1046. 10.1016/j.fertnstert.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 2. Sharma R, Agarwal A, Rohra VK, Assidi M, Abu‐Elmagd M, Turki RF. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol Endocrinol. 2015;13:35. 10.1186/s12958-015-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age‐dependent declines in human semen quality: a systematic review and meta‐analysis. Ageing Res Rev. 2015;19:22‐33. 10.1016/j.arr.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 4. Chen Q, Zhao JY, Xue X, Zhu GX. The association between sperm DNA fragmentation and reproductive outcomes following intrauterine insemination, a meta analysis. Reprod Toxicol. 2019;86:50‐55. 10.1016/j.reprotox.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 5. Deng C, Li T, Xie Y, et al. Sperm DNA fragmentation index influences assisted reproductive technology outcome: a systematic review and meta‐analysis combined with a retrospective cohort study. Andrologia. 2019;51(6):e13263. 10.1111/and.13263. [DOI] [PubMed] [Google Scholar]
- 6. Esteves SC, Santi D, Simoni M. An update on clinical and surgical interventions to reduce sperm DNA fragmentation in infertile men. Andrology. 2020;8(1):53‐81. 10.1111/andr.12724. [DOI] [PubMed] [Google Scholar]
- 7. Humm KC, Sakkas D. Role of increased male age in IVF and egg donation: is sperm DNA fragmentation responsible. Fertil Steril. 2013;99(1):30‐36. 10.1016/j.fertnstert.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 8. McQueen DB, Zhang J, Robins JC. Sperm DNA fragmentation and recurrent pregnancy loss: a systematic review and meta‐analysis. Fertil Steril. 2019;112(1):54‐60.e3. 10.1016/j.fertnstert.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 9. Tan J, Taskin O, Albert A, Bedaiwy MA. Association between sperm DNA fragmentation and idiopathic recurrent pregnancy loss: a systematic review and meta‐analysis. Reprod Biomed Online. 2019;38(6):951‐960. 10.1016/j.rbmo.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 10. Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol. 2017;14(8):470‐485. 10.1038/nrurol.2017.69. [DOI] [PubMed] [Google Scholar]
- 11. Muratori M, Baldi E. Effects of FSH on sperm DNA fragmentation: review of clinical studies and possible mechanisms of action. Front Endocrinol (Lausanne). 2018;9:734. 10.3389/fendo.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agarwal A, Majzoub A, Esteves SC, Ko E, Ramasamy R, Zini A. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol. 2016;5(6):935‐950. 10.21037/tau.2016.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Javed A, Talkad MS, Ramaiah MK. Evaluation of sperm DNA fragmentation using multiple methods: a comparison of their predictive power for male infertility. Clin Exp Reprod Med. 2019;46(1):14‐21. 10.5653/cerm.2019.46.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. Andrology Lab Corner J Androl. 2002;23(1):25‐43. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization . Laboratory Manual for the Examination and Processing of Human Semen. Fith ed. Geneva, Switzerland: WHO Press; 2010. [Google Scholar]
- 16. Mortimer D. Sperm fertilizing ability testing. In: Mortimer D, ed. Practical Laboratory Andrology. New York: Oxford University Press; 1994:209‐212. [Google Scholar]
- 17. Rubes J, Rybar R, Prinosilova P, et al. Genetic polymorphisms influence the susceptibility of men to sperm DNA damage associated with exposure to air pollution. Mutat Res. 2010;683(1–2):9‐15. 10.1016/j.mrfmmm.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 18. Langone JJ, Van Vunakis H. Radioimmunoassay of nicotine, cotinine, and gamma‐(3‐pyridyl)‐gamma‐oxo‐N‐methylbutyramide. Methods Enzymol. 1982;84:628‐640. 10.1016/0076-6879(82)84050-0. [DOI] [PubMed] [Google Scholar]
- 19. Belloc S, Benkhalifa M, Cohen‐Bacrie M, Dalleac A, Amar E, Zini A. Sperm deoxyribonucleic acid damage in normozoospermic men is related to age and sperm progressive motility. Fertil Steril. 2014;101(6):1588‐1593. 10.1016/j.fertnstert.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 20. Das M, Al‐Hathal N, San‐Gabriel M, et al. High prevalence of isolated sperm DNA damage in infertile men with advanced paternal age. J Assist Reprod Genet. 2013;30(6):843‐848. 10.1007/s10815-013-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mettler AD, Govindarajan M, Srinivas S, Mithraprabhu S, Evenson D, Mahendran T. Male age is associated with sperm DNA/chromatin integrity. Aging Male. 2019;1‐8. 10.1080/13685538.2019.1600496. [DOI] [PubMed] [Google Scholar]
- 22. Petersen CG, Mauri AL, Vagnini LD, et al. The effects of male age on sperm DNA damage: an evaluation of 2,178 semen samples. JBRA Assist Reprod. 2018;22(4):323‐330. 10.5935/1518-0557.20180047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Plastira K, Msaouel P, Angelopoulou R, et al. The effects of age on DNA fragmentation, chromatin packaging and conventional semen parameters in spermatozoa of oligoasthenoteratozoospermic patients. J Assist Reprod Genet. 2007;24(10):437‐443. 10.1007/s10815-007-9162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim MK, Park JK, Jeon Y, Seok SH, Chang EM, Lee WS. Effects of paternal age on human embryo development in in vitro fertilization with preimplantation genetic screening. Clin Exp Reprod Med. 2019;46(1):22‐29. 10.5653/cerm.2019.46.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang H, Li G, Jin H, Guo Y, Sun Y. The effect of sperm DNA fragmentation index on assisted reproductive technology outcomes and its relationship with semen parameters and lifestyle. Transl Androl Urol. 2019;8(4):356‐365. 10.21037/tau.2019.06.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Evenson DP, Djira G, Kasperson K, Christianson J. Relationships between the age of 25,445 men attending infertility clinics and sperm chromatin structure assay (SCSA®) defined sperm DNA and chromatin integrity. Fertil Steril. 2020;114(2):311‐320. 10.1016/j.fertnstert.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 27. Bungum M, Humaidan P, Axmon A, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22(1):174‐179. 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 28. Virro MR, Larson‐Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004;81(5):1289‐1295. 10.1016/j.fertnstert.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 29. Rubes J, Selevan SG, Sram RJ, Evenson DP, Perreault SD. GSTM1 genotype influences the susceptibility of men to sperm DNA damage associated with exposure to air pollution. Mutat Res. 2007;625:20‐28. 10.1016/j.mrfmmm.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 30. Darbandi S, Darbandi M, Khorshid HRK, et al. The effect of paternal age on semen quality and fertilization outcome in men with normal sperm DNA compaction, reactive oxygen species, and total antioxidant capacity levels. Turk J Urol. 2019;45(3):164‐170. 10.5152/tud.2019.74944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Evenson DP, Brannian J, Hansen K, Kasperson K, Christianson J. Relationships between sperm DNA fragmentation, age of donors and patients and children with psychic disorders. Fertil Steril. 2014;102(suppl 3):e97. 10.1016/j.fertnstert.2014.07.330. [DOI] [Google Scholar]
- 32. Jerre E, Bungum M, Evenson D, Giwercman A. Sperm chromatin structure assay high DNA stainability sperm as a marker of early miscarriage after intracytoplasmic sperm injection. Fertility and Sterility. 2019;112(1):46‐53.e2. 10.1016/j.fertnstert.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 33. Vinnakota C, Cree L, Peek J, Morbeck DE. Incidence of high sperm DNA fragmentation in a targeted population of subfertile men. Syst Biol Reprod Med. 2019;65(6):451‐457. 10.1080/19396368.2019.1668077. [DOI] [PubMed] [Google Scholar]
- 34. Moskovtsev SI, Willis J, Mullen JB. Age‐related decline in sperm deoxyribonucleic acid integrity in patients evaluated for male infertility. Fertil Steril. 2006;85(2):496‐499. 10.1016/j.fertnstert.2005.05.075. [DOI] [PubMed] [Google Scholar]
- 35. Kaarouch I, Bouamoud N, Madkour A, et al. Paternal age: negative impact on sperm genome decays and IVF outcomes after 40 years. Mol Reprod Dev. 2018;85(3):271‐280. 10.1002/mrd.22963. [DOI] [PubMed] [Google Scholar]
- 36. Egeberg Palme DL, Rehfeld A, Bang AK, et al. Viable acrosome‐intact human spermatozoa in the ejaculate as a marker of semen quality and fertility status. Hum Reprod. 2018;33(3):361‐371. 10.1093/humrep/dex380. [DOI] [PubMed] [Google Scholar]
- 37. Rahiminia T, Hosseini A, Anvari M, Ghasemi‐Esmailabad S, Talebi AR. Modern human sperm freezing: effect on DNA, chromatin and acrosome integrity. Taiwan J Obstet Gynecol. 2017;56(4):472‐476. 10.1016/j.tjog.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 38. Yuan M, Huang L, Leung WT, et al. Sperm DNA fragmentation valued by SCSA and its correlation with conventional sperm parameters in male partner of recurrent spontaneous abortion couple. Biosci Trends. 2019;13(2):152‐159. 10.5582/bst.2018.01292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


