SUMMARY
Borrelia burgdorferi, the spirochetal agent of Lyme disease is a zoonotic pathogen that is maintained in a natural cycle that typically involves mammalian reservoir hosts and a tick vector of the Ixodes species. During each stage of the enzootic cycle B. burgdorferi is exposed to environments that differ in temperature, pH, small molecules, and most importantly, nutrient sources. B. burgdorferi has a highly restricted metabolic capacity as it does not contain a tricarboxylic acid cycle, oxidative phosphorylation or any pathways for de novo biosynthesis of carbohydrates, amino acids or lipids. Thus, B. burgdorferi relies solely on glycolysis for ATP production and is completely dependent on the transport of nutrients and cofactors from extracellular sources. Herein, pathways for carbohydrate uptake and utilization in B. burgdorferi are described. Regulation of these pathways during the different phases of the enzootic cycle is discussed. In addition, a model for differential control of nutrient flux through the glycolytic pathway as the spirochete transits through the enzootic cycle is presented.
1. The Enzootic Cycle
Borrelia burgdorferi is the spirochetal agent of Lyme disease, the most commonly reported arthropod-borne disease in the United States (1-3). B. burgdorferi is a zoonotic pathogen that is maintained in a natural cycle involving mammalian reservoir hosts such as field mice, squirrels, and birds and an arthropod vector of the Ixodes species (4-6) (Figure 1). In the United States, the principal vector isIxodes scapularis, the common deer tick (5, 6). As there is no transovarial transmission of B. burgdorferi, newly hatched larvae acquire the spirochete during their first blood meal on an infected mammalian host reservoir (7, 8). The spirochete is maintained in the midgut of the tick during molting to the nymphal stage. At this point, the spirochete is in a non-motile state until the nymph begins to feed on the next mammalian host (9). The spirochete then begins rapidly replicating in the feeding nymphal midgut, leave the midgut and enter the hemolymph from which the bacteria migrate to the salivary glands and are transmitted to the next mammalian host (9-12) (Figure 1).
Figure 1.
Borrelia burgdorferi enzootic cycle. (1) Uninfected larva emerges from eggs. (2) Larval acquisition of B. burgdorferi during a blood meal on an infected reservoir host. (3) Infected fed larva molts to an unfed nymph. (4) Transmission of B. burgdorferi from a feeding nymph to an uninfected reservoir host during the nymphal blood meal. (5) Infected fed nymph molts to an adult. (6) Female and male adults mate on a large mammal (typically deer). The female adult feeds on the large mammal and lays eggs.
During each stage of the enzootic cycle B. burgdorferi is exposed to differing environments. Each milieu varies by temperature, pH, small molecules, and most importantly, nutrient sources. The drastic changes in environmental attributes require the spirochete to not only modulate the expression of colonization factors necessary for persistence in either the vector or the mammalian host, but to also adjust its metabolic state to adapt to the changing nutrient profile. While in the mammalian host, B. burgdorferi can be found in the skin at the tick bite site, in the circulatory system and at distal tissue sites, such as joints and heart (13). During larval acquisition, spirochetes enter the midgut concurrent with the blood meal and its associated nutrients and host factors. After molting to the nymphal stage, B. burgdorferi is confined to the midgut lumen of the unfed nymph. The lumen is a nutrient-poor environment consistent with the metabolically dormant state of the spirochetes at this stage (9, 14). When the nymph begins to feed, the incoming blood meal is surrounded by a peritrophic membrane that sequesters the blood meal and its accompanying nutrients away from the spirochetes within the midgut lumen (15). It is likely that the ability to utilize a variety of available carbohydrates during the enzootic cycle is essential for the survival of B. burgdorferi during the tick phases and pathogenesis in mammalian hosts, as has been observed for other pathogenic bacteria (16).
2. B. burgdorferi Genomics
B. burgdorferi has a complex genome. The segmented genome of the B31 type strain consists of one large linear chromosome (approximately 910 Kb) and twenty-one circular (cp) and linear plasmids (lp) (17, 18). A substantial portion of the predicted open reading frames (ORFs) is annotated as hypothetical or as having homology to other spirochete genes without any functional annotation (17, 18). ORFs located on the linear chromosome encode primarily housekeeping functions, including most genes associated with metabolism (17, 19). Plasmid content in individual B. burgdorferi isolates is variable, although lp54 and cp26 are uniformly present in most characterized isolates (20-22). Plasmids can be lost during in vitro cultivation, which typically has no effect on in vitro growth; however, several plasmids play an essential role in vivo (22-30). cp26 is absolutely required for survival of the bacterium (25, 31); it encodes several proteins involved in purine biosynthesis, transport, telomere resolution and outer surface protein C (OspC), an essential virulence factor (17, 25, 32-34). lp25 is required for mammalian infection (35). It has been shown that the critical gene on lp25 is pncA, which encodes a nicotinamidase is likely involved in NAD+ salvage (24, 32, 36). Additionally, lp28-1 is required for persistence during mammalian infection, likely as the result of antigenic variation in vlsE (23, 35, 37-39).
3. Regulation of Gene Expression during the Enzootic Cycle
A. RpoS
Bacteria typically regulate gene expression in response to environmental cues by processes that are often mediated by alternative sigma factors or two-component signaling systems (TCS) (40, 41). The B. burgdorferi genome encodes for only two alternative sigma factors and two TCS (17). Thus, to adapt to the different environments encountered during the enzootic cycle B. burgdorferi must rely on a limited repertoire of such components. Seminal studies by Norgard and co-workers demonstrated a link between one TCS, specifically Hk2-Rrp2, and the alternative sigma factors RpoN and RpoS (42, 43). The expression of several virulence genes is dependent on RpoS (42, 44). RpoS is also essential for repression of genes whose expression is required in the tick, but not in the mammalian host (45). As a result, RpoS is absolutely essential for mammalian infection, as well as migration through the tick during transmission (44, 46). Global transcriptome analyses of wild type and RpoS mutant strains under mammalian-like conditions defined the RpoS regulon and included both genes that are induced and repressed by RpoS. On this basis, RpoS has been referred to as a “gatekeeper” that controls the reciprocal expression of genes required for mammalian infection or maintenance in ticks (47).
In B. burgdorferi, RpoS expression is controlled by multiple layers of transcriptional and posttranscriptional regulation (48). The Rrp2-RpoN-RpoS pathway is required for transcription of rpoS (49-51). RpoN binds directly to a canonical −24/−12 sequence in the rpoS promoter to induce transcription (52). This interaction requires the activation of Rrp2, the encoded response regulator of the Hk2-Rrp2 two-component system (43, 50). It was assumed that Rrp2 would be phosphorylated and activated by Hk2, however, it was later demonstrated that Rrp2 could induce virulence gene expression independent of Hk2 (53). Xu et al. subsequently showed that acetyl phosphate can function as the phosphate group donor for Rrp2 (54). RpoS expression is also modulated by several other effectors including BosR (see below), DsrA (a small RNA), Hfq (an RNA chaperone), and CsrA (BB184) (55-58), although the role of CsrA has been recently challenged (59). It has also been suggested that BadR (BB693), a protein with sequence homology to the ROK family of proteins, represses the transcription of rpoS (60).
B. BosR
B. burgdorferi encodes a Fur/Per homolog, BosR (Borrelia oxidative stress regulator) (17, 61, 62). Its role in mediating the oxidative stress response is not clear, as B. burgdorferi bosR mutants are only slightly more sensitive to oxidative stress compared to wild type spirochetes (63, 64). Several studies have demonstrated that BosR is a transcriptional activator of rpoS (63, 65, 66). Ouyang et al. identified a “BosR box” to which the protein binds and demonstrated that the rpoS promoter contains three such binding sites; interestingly, bioinformatic analysis revealed the presence of 60 additional cis-acting “BosR boxes” in the B. burgdorferi genome suggesting that BosR likely regulates the expression of additional genes aside from its role as an activator of RpoS (67, 68). More recently, BosR was reported to be directly involved in repression of lipoprotein gene expression (69). The environmental signal(s) that controls bosR expression has not been fully elucidated. It has been suggested that transition metals may be involved, since bosR expression is Zn2+-dependent and is posttranscriptionally inhibited by Mn2+ (61, 70).
C. Hk1-Rrp1
The second TCS in B. burgdorferi has been designated as Hk1- Rrp1. The response regulator Rrp1 contains a GGDEF motif characteristic of diguanylate cyclases that convert two GTP molecules to a molecule of c-di-GMP and is the sole protein in the genome containing this motif (17, 71). Ryjenkov et al. have demonstrated that Rrp1 functions as a diguanylate cyclase and its activity is dependent on phosphorylation of its receiver domain (72). The secondary messenger c-di-GMP has gained attention as a global regulator in bacteria that is associated with virulence, motility and central metabolism (73, 74). Rrp1 receives its signal from the membrane-bound sensor histidine kinase Hk1 (75, 76). B. burgdorferi also encodes the other components of the c-di-GMP signaling system, including two phsophodiesterases (PdeA [BB363] and PdeB [BB374]) and a cyclic-di-GMP binding protein PlzA (BB733) (17, 77). The hk1-rrp1 operon appears to be constitutively expressed (76), although rrp1 and plzA expression may be elevated during tick feeding (71, 77). Both Rrp1 and Hk1 deletion mutants are infectious in mice, but are unable to survive in the tick vector (76, 78, 79); Rrp1 mutants also have defective motility (76, 79). Global transcriptome analyses of wild type and Rrp1 mutant spirochetes revealed that c-di-GMP regulates the expression of a substantial number of genes (71, 78). Taken together, the data suggest that c-di-GMP signaling plays a critical role during tick colonization (80).
4. Carbohydrate metabolism
B. burgdorferi has a very restricted metabolic capacity. Genes encoding functions related to carbohydrate transport and metabolism are listed in Table 1. The genome encodes enzymes of the glycolytic pathway, but not of the tricarboxylic acid cycle or oxidative phosphorylation (17). The spirochete does encode the oxidative branch of the pentose phosphate pathway (17), but as ribose cannot support in vitro growth when supplied as the principal carbon source (81), this pathway is not likely to play a role in energy production. Thus, B. burgdorferi relies solely on glycolysis for ATP generation. The bacterium does not encode any complete pathways for de novo biosynthesis of fatty acids, amino acids or nucleotides (17). For this reason, B. burgdorferi is completely dependent on the transport of nutrients and cofactors from extracellular sources and several salvage pathways (17, 25, 32, 33, 82-84). Consequently, B. burgdorferi has over 50 genes encoding transporters for carbohydrates, oligopeptides and amino acids (17, 32).
Table 1.
Borrelia burgdorferi genes encoding proteins involved in carbohydrate metabolism
| Transporters | |
|---|---|
| Gene Locus | Annotated Namea |
| bb448 | PTS; phophocarrier protein Hpr |
| bb557 | PTS; phophocarrier protein Hpr, ptsH |
| bb558 | PTS; phosphoenolpyruvate protein phosphocarrier EI, pstP |
| bb559 | PTS; glucose-specific EIIA, crr |
| bb645 | PTS; glucose-specific EIIBC, ptsG |
| bb116 | PTS; glucose-specific EIIABC, malX1 |
| bbb29 | PTS; glucose-specific EIIABC, malX2 |
| bb408 | PTS; fructose, mannose-specific EIIABC, fruA1 |
| bb629 | PTS; fructose, mannose-specific EIIABC, fruA2 |
| bbb04 | PTS; chitobiose-specific EIIC, chbC |
| bbb05 | PTS; chitobiose-specific EIIA, chbA |
| bbb06 | PTS; chitobiose-specific EIIB, chbB |
| bb240 | glycerol facilitator, glpF |
| bb677 | ABC transporter (glucose, ribose, galactose), ATP-binding protein, mglA |
| bb678 | ABC transporter (glucose, ribose, galactose), permease protein, mglC1 |
| bb679 | ABC transporter (glucose, ribose, galactose), permease protein, mglC2 |
| bb604 | lactate permease, lctP |
| Glycolysis | |
|---|---|
| Gene Locus | Annotated Name |
| bb730 | glucose-6-phosphate isomerase, pgi |
| bb727 | phosphofructokinase, pfk |
| bb020 | diphosphate-fructose-6-phosphate-1 -phosphotransferase, pfpB |
| bb445 | fructose-bisphosphate aldolase, class II, fbaA |
| bb055 | triose-phosphate isomerase, tpiA |
| bb056 | phosphoglycerate kinase, pgk |
| bb658 | phosphoglycerate mutase |
| bb337 | enolase, eno |
| bb348 | pyruvate kinase, pyk |
| bb087 | L-lactate dehydrogenase, ldh |
| Pentose Phosphate Pathway | |
|---|---|
| Gene Locus | Annotated Name |
| bb636 | glucose-6-phosphate-1-dehydrogenase, zwf |
| bb222 | 6-phosphoglyconolactonase, pgl |
| bb561 | 6-phophogluconate dehydrogenase, gnd |
| bb657 | ribose-5-isomerase, rpi |
| Other Carbohydrate Utilization Pathways | |
|---|---|
| Gene Locus | Annotated Name |
| Mannose: | |
| bb407 | mannose-6-phosphate isomerase, class I, manA |
| bb630 | 1 -phosphofructokinase, pfkB |
| bb835 | phosphomannomutase |
| bb644 | N-acetylmannosamine-6-phosphate epimerase |
| GlcNAc: | |
| bb004 | phosphoglucomutase |
| bb151 | N-acetylglucosamine-6-phosphate isomerase, nagA |
| bb152 | glucosamine-6-phosphate isomerase, nagB |
| Chitobiose: | |
| bb002 | chitobiase |
| bb620 | beta-glucosidase |
| bb831 | glucokinase |
| Maltose: | |
| bb116 | 4-alpha-glucanotransferase, malQ |
| Trehalose: | |
| bb381 | trehalase, treA |
| Glycerol: | |
| bb241 | glycerol kinase, glpK |
| bb243 | glycerol-3-phosphate dehydrogenase, glpD |
Annotations based on Fraser et al. (17) and NCBI Borrelia burgdoreri B31 genome (NC_001318.1 and NC_001903.1).
Typically, glycolysis yields a net of only two or three ATP molecules per glucose molecule; the reliance on only glycolysis for production of ATP could account for the spirochete's slow growth, even under optimal in vitro cultivation conditions. B. burgdorferi is not only restricted to the carbohydrate sources available in the distinct environments it encounters during the enzootic cycle, but also by the limited number of encoded carbohydrate uptake systems and catabolic pathways (17). von Lackum and Stevenson reported that B. burgdorferi can utilize six carbohydrates as the principal carbon source during in vitro growth in BSK medium—glucose, glycerol, maltose, mannose, N-acetylglucosamine (GlcNAc), and chitobiose (81). More recently, Hoon-Hanks et al. demonstrated that trehalose can also support B. burgdorferi in vitro growth (85).
A. Glycolysis
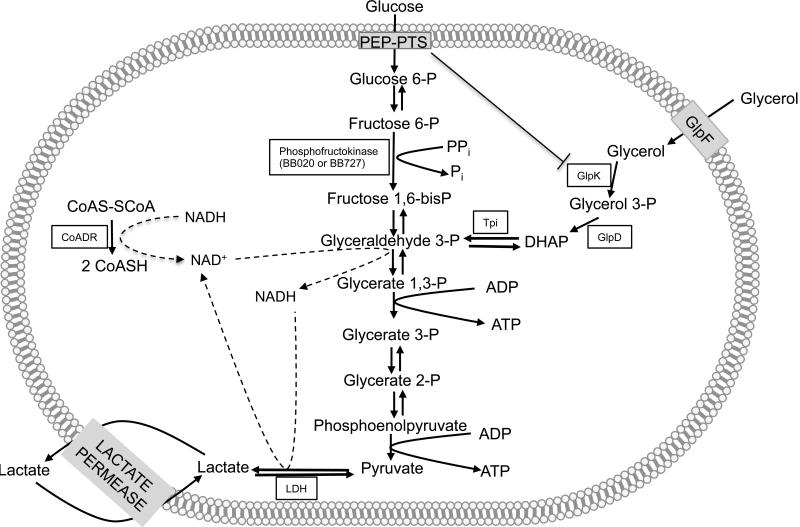
B. burgdorferi encodes all enzymes of the glycolytic pathway (17). Glucose enters as either glucose (via a putative ABC transporter [see below]) or as glucose 6-phosphate (via a phosphoenolpyruvate-phosphotransferase system [PEP-PTS]) (Figure 2). Acquired glucose would be converted to glucose 6-phosphate by the action of a putative glucokinase (BB831) and then to fructose 6-phosphate by glucose 6-phosphate isomerase (Pgi, BB730) (Figure 3). B. burgdorferi encodes two enzymes with putative phosphofructokinase activity (BB020, BB727). Both genes have been expressed and characterized in vitro; BB020 is an active pyrophosphate-dependent phosphofructokinase that functions as a dimer (86, 87), whereas BB727 exists as a multimer in solution with no measurable enzymatic activity in vitro (86). The fact that phosphofructokinase utilizes pyrophosphate as the phosphate donor rather than ATP is of particular interest because this would conserve intracellular ATP and increase the ATP yield per molecule of glucose. Cleavage of fructose 1,6-bisphosphate to dihydroxyacetone phosphate and glyceraldehyde-3-phosphate and their ultimate conversion to pyruvate proceeds by the action of the glycolytic pathway as expected. tpi, pgk, and gapdh comprise a single operon in B. burgdorferi (BB055-057) and are transcribed from a single promoter; this type of genetic organization has also been observed in other bacteria (88). B. burgdorferi does not encode for a pyruvate dehydrogenase or pyruvate oxidase (17); as a result the only disposition of pyruvate is conversion to lactate by lactate dehydrogenase. This results in regeneration of NAD+ that is required for continued glycolysis (Figure 3). The potential role of NADH/NAD+ ratio in regulating metabolite flux through the glycolytic pathway is discussed later in this chapter.
Figure 2.
Borrelia burgdorferi carbohydrate transporters. Schematic diagram indicates predicted or experimentally verified transport systems. BB numbers indicate gene locus in B. burgdorferi strain B31 (17). Based on von Lackum and Stevenson (81).
Figure 3.
The glycolytic pathway and control of glycolytic flux during the enzootic cycle.
B. Other Carbohydrate Utilization Pathways
Like most bacteria, glucose is the preferred carbohydrate for ATP generation. In addition to glycolysis, glucose 6-P can also enter the oxidative branch of the pentose phosphate pathway by conversion to 6-phosphogluconolactone through the action of glucose 6-phosphate dehydrogenase. As noted, several additional carbohydrates can support B. burgdorferi growth in vitro. Mannose can be taken up via a mannose-specific PTS and the resultant mannose-6-phosphate is converted to fructose-6-phosphate by the action of ManA (BB407) and can be directed into glycolysis (17) (Table 1).
B. burgdorferi requires GlcNAc to reach high density during in vitro cultivation, making its import and utilization an absolute requirement for growth (81, 89, 90). It is assumed that GlcNAc is taken up through a glucose-specific PTS. It is utilized for peptidoglycan biosynthesis, but it can also be converted to fructose 6-phosphate by the combined actions of NagA (BB151) and NagB (BB152) and thereby utilized as a substrate for glycolysis. Chitobiose, a dimer of GlcNAc, is a constituent of the tick cuticle and peritrophic membrane (15, 91). It has been demonstrated that chitobiose can substitute for the GlcNAc requirement during in vitro growth (90, 92). Studies have shown that a chitobiose-specific PTS is encoded on plasmid cp26 (bbb04, bbb05, bbb06) (Figure 2) and that ChbC mutants cannot utilize chitobiose to support in vitro growth (90). After uptake via the chitobiose-specific PTS, chitobiose 6-P is cleaved into GlcNAc and GlcNAc 6-P by chitobiase (BB002) and the monomers would enter the glycolytic pathway as fructose 6-P. The disaccharides maltose and trehalose can also be utilized after hydrolysis to glucose monomers by either amylomaltase (MalQ; BB166) or trehalase (TreA; BB381) (Figure 2).
In addition to mono- and di-saccharides, B. burgdorferi also contains a pathway for uptake and utilization of glycerol. The genes comprise an operon (bb240-bb243) which encodes an uptake facilitator (GlpF), glycerol kinase (GlpK) and glycerol 3-phosphate dehydrogenase (GlpD) (17). Once glycerol has entered into the cytosol, it is phosphorylated by GlpK to yield glycerol 3-phosphate; GlpD converts glycerol 3-phosphate to dihydroxyacetone phosphate which can enter the glycolytic pathway after conversion to glyceraldehyde 3-phosphate (81) (Figure 2).
C. Carbohydrate Transporters
Phosphotransferase systems (PTS or PEP-PTS) simultaneously import and phosphorylate a sugar substrate by coupling phosphorelay of a phosphoryl group from phosphoenolpyruvate (PEP) to carbohydrate transport (93). PTS are composed of a number of proteins referred to as enzyme I (EI), enzyme II (EII) and histidine phospocarrier protein (HPr). EII is composed of two cytoplasmic domains (EIIA, EIIB) and a transmembrane domain (EIIC). These domains can be located on a single polypeptide or separate protein molecules (94). PEP transfers a phosphoryl group to EI which in turn transfers it to HPr and ultimately, the phosphate is transferred to the sugar concomitant with its EIIC- mediated uptake (94, 95). Specificity of these systems is defined by the EII components downstream of the initial phosphorelay between PEP, EI and HPr (93, 94).
Based on genome sequence, B burgdorferi contains the complete PTS machinery (17) (Table 1). EI is encoded by (BB558) and, interestingly, there are two genes annotated as HPr (BB557, BB448). It is important to note, however, that very few of the PTS components have been definitively shown to function in their putative roles by direct biochemical or genetic studies. A schematic diagram showing the specific carbohydrate transporters is presented in Figure 2. Glucose import is mediated by the glucose-specific EII proteins (BB559, BB645, BB116, and BBB29); by homology, BB559 (EIIA) and BB645 (EIIBC) are annotated as glucose-specific, whereas BB116 and BBB29 (EIIABC) are annotated as maltose and glucose-specific (17). GlcNAc can also be transported into the cell via these EII components. It was thought that the disaccharides, maltose (α1-4-glucose-glucose) and trehalose (α1-1-glucose-glucose), are cleaved into two molecules of glucose by MalQ or TreA prior to transport into the cytosol (17, 96). However, it was recently demonstrated that malQ mutants are able to grow normally when maltose or trehalose were provided as the principal carbon source in vitro and these mutants successfully complete the experimental mouse-tick-mouse enzootic cycle (85). It is possible that a simultaneous disruption of both enzymes might prevent utilization of both disaccharides, but this has not been tested.
B. burgdorferi encodes two mannose-specific EIIABC components (BB408, BB629). These were initially annotated as fructose-specific (17), but fructose does not support in vitro growth (81). As a result, these are now presumed to be mannose-specific transporters (32, 81). In addition, the spirochete also possesses a dedicated chitobiose PTS (17). chbC (BBB04) is required for chitobiose transport and is induced during the tick phase of the enzootic cycle (12, 90, 92). Although the genes for the three EII components are adjacently located on cp26, the gene encoding the EIIC component, chbC, is divergently transcribed from those for chbA (BBB05) and chbB (BBB06); these latter genes are apparently not differentially expressed in ticks (92).
Glucose could potentially be transported into the cytosol by a putative ABC transporter MglAC1C2 (BB677-79) (17, 32, 97). This operon was initially annotated to encode a ribose/galactose transporter. It is unlikely that either sugar is utilized for ATP production since neither can support spirochetal growth in vitro (81). B. burgdorferi membranes contain mongalactosyl diacylglycerol (98) and its synthesis would require a source of galactose. If galactose were imported through the mglAC1C2 transporter, a uridylyltransferase would be required for synthesis of UDP-galactose; such an activity has not been identified in the B. burgdorferi genome. Alternatively, UDP-galactose could be produced from UDP-glucose by an epimerase (BB444) (32). If ribose were imported via this ABC transporter it could be utilized for nucleotide biosynthesis. The route from ribose to ribose 5-phosphate is unclear, but bb545 is annotated as a xylulokinase and it has been suggested that this enzyme could convert ribose to ribulose 5-phosphate and then ribose 5-phosphate by the action of ribose phosphate isomerase (BB657) (32). Ribose 5-phosphate could also be produced from glucose 6-phosphate via the oxidative branch of the pentose phosphate pathway. Taken together, it seems unlikely that the mglAC1C2 transporter is utilized for galactose or ribose uptake; rather it could serve as an additional route for glucose transport.
B. burgdorferi encodes one carbon-specific major facilitator super family protein, GlpF (17). GlpF is a member of a family of conserved aquaglyceroporins that mediate diffusion of glycerol into the cytosol (99, 100). Interestingly, expression of the glp operon (bb240-243) is significantly induced during the tick phase of the spirochete enzootic cycle and glycerol utilization is vital for maximal fitness of the spirochetes in the tick vector (12).
5. NAD+/NADH Balance
The balance between NAD+ and NADH is an indicator of the intracellular redox state for both eukaryotic and prokaryotic cells and NAD+ is a required co-factor for many cellular enzymes (101, 102). During glycolysis, NAD+ is reduced to NADH by GAPDH and the cell must regenerate NAD+ to maintain a balanced redox state and to allow glycolysis to continue (Figure 3). NAD+ pools can be replenished by biosynthesis or oxidative metabolism of pyruvate. Many bacteria encode biosynthetic pathways that utilize tryptophan or aspartic acid to generate NAD+ (101, 102). B. burgdorferi does not encode this biosynthetic capacity and cannot metabolize pyruvate oxidatively (17). Instead it must depend on alternative strategies to replenish NAD+. As noted, the only metabolic fate for pyruvate is its conversion to lactate by pyruvate dehydrogenase. During lactogenesis, NADH is oxidized producing NAD+ that can be recycled back into the glycolytic pathway (103) which directly couples NAD+ regeneration to glycolysis.
In an oxidative environment, organisms must deal with the presence of reactive oxygen species. Among the consequences is formation of the disulphide form of coenzyme A (CoA). To deal with this, B. burgdorferi produces a CoA disulphide reductase (CoADR) (encoded by bb728) that utilizes NADH exclusively as a cofactor producing reduced CoA and regenerating NAD+ (104, 105). A B. burgdorferi CoADR mutant was avirulent in mice and had reduced survival in feeding nymphs suggesting an important role in maintaining an optimal redox state in the spirochete (105).
Microbes contain pathways for nicotinamide salvage that can be employed for NAD+ production (102, 106). B. burgdorferi pncA (bbe22) encodes a functional nicotinamidase that can complement E. coli and S. typhimurium pncA deletion strains (17, 36). B. burgdorferi pncA is abosolutely required for both mammalian infection and persistence within the tick vector suggesting the importance of this pathway for maintenance of the intracellular NAD+ pool in B. burgdorferi (24, 36). Although a nicotinamide uptake system has not yet been identified in B. burgdorferi, enzymes that would mediate the step-wise conversion of nicotinamide to NAD+ (pncB [bb635], nadD [bb782], nadE [bb311]) are apparently encoded in the B. burgdorferi genome (17).
6. Regulation of Glycerol and Chitobiose and Utilization
Numerous global analyses of the wild type B. burgdorferi transcriptome during in vitro growth in a variety of environmental conditions (e.g., temperature, pH, redox state) or in a mammalian host-adapted state have been reported. Similar studies have been performed to identify members of the RpoS, Rrp1, Rrp2 and BosR regulons (reviewed in (48, 107)). A comprehensive survey of these studies revealed no definitive transcriptional control of carbohydrate uptake and utilization genes/pathways except for those involving glycerol and chitobiose (Figure 4). Perhaps this can best be understood with the reasonable assumption that glucose is the preferred carbohydrate throughout the enzootic cycle, and therefore constitutively utilized, except for certain tick stages during which glucose availability would be limited.
Figure 4.
Schematic diagram depicting reported regulatory circuits controlling glycerol and chitobiose utilization. Solid lines indicate interactions confirmed by in vivo studies; dashed lines indicate interactions observed in vitro only. Diagram is summary of data from references 43, 47, 50, 54, 60, 71, 72, 78, 111, and 112.
A. Glycerol Uptake and Utilization
Glycerol is a readily available carbohydrate in the tick vector and is produced by Ixodes spp. to serve as an anti-freeze during over-wintering (108, 109). The B. burgdorferi glp operon encodes the capacity for uptake of glycerol and its conversion to dihydroxyacetone phosphate (see section 3). Early global transcriptome studies showed that the glp operon is expressed at higher levels in cells grown at 23°C than at 35°C, suggesting a potential role in the vector (110). This was definitively confirmed by Pappas et al. who reported that glpF and glpD transcripts are substantially elevated during all tick stages as compared to mouse joints (12). The glp operon is subject to RpoS-mediated repression (47) and its expression is induced by Rrp1 (71, 78). The inability of Rrp1 mutants to survive in ticks was partially restored by complementation with the glp operon (78). Although a GlpD mutant could complete the experimental mouse-tick-mouse infection cycle, it had a fitness defect in ticks that was manifested in impaired growth and lower spirochete numbers in fed nymphs (12). Taken together, the findings demonstrate that the ability to utilize glycerol as a nutrient during the tick phase is critical and that modulation of glp operon expression is dependent, at least in part, on reciprocal regulation by RpoS and Rrp1.
B. Chitobiose Uptake and Utilization
Chitin, a polymer composed of chitobiose (glucosamine or GlcNAc dimer) units, is the primary component of the tick exoskeleton and the peritrophic membrane (15, 91). Chitobiose can substitute for GlcNAc, an essential requirement for in vitro cultivation, and can serve as the principal carbon source in vitro (81, 90). As described earlier (section 3B), chitobiose can be taken up via a dedicated PTS, ChbABC , cleaved to GlcNAc and GlcNAc 6-P and enter glycolysis following conversion to fructose 6-P. chbC is highly expressed during all tick stages and has little expression in mouse joints (12), an expected finding given that chitobiose is likely to be available as a nutrient only in the vector. However, the transcriptional regulators that are responsible for this differential expression are a matter of controversy. Rhodes et al. suggested an RpoS involvement in chbC expression (111), a finding at odds with the global transcriptome analysis of an RpoS mutant that found no effect on chbC expression (47). Recently, Sze et al. reported that chbC transcript levels were significantly repressed in an Rrp1 mutant (112), but in this case as well, previous global transcriptome analyses of Rrp1 mutants did not reveal any differences in chbC expression (71, 78). Curiously, Sze et al. suggested that chbC regulation by Rrp1 is mediated through BosR and RpoS, as expression of these transcriptional regulators was also repressed in their Rrp1 mutant. However, it must be noted that chbC expression is not altered in a BosR mutant and a BosR binding site has not been identified upstream of chbC (65, 67, 113). BadR has also been implicated as a repressor of chbC expression. Based on current understanding of the roles for RpoS and Rrp1 (the former being responsible for the mammalian phase regulon and the latter a tick phase regulon; see section 3) and the likelihood that chitobiose would only be available to the spirochete in the vector, it seems reasonable to conclude that chbC expression is regulated primarily by Rrp1 (and c-di-GMP) which functions during the vector phase of the enzootic cycle. Further studies to more clearly define the regulation of chitobiose uptake are warranted.
7. Control of Carbohydrate Uptake and Glycolytic Flux During the Enzootic Cycle
When multiple nutrient sources are available, bacteria will initially utilize the preferred carbon source, typically glucose and, in an effort to conserve energy, will also repress genes associated with the utilization of alternate nutrient sources; this process is referred to as carbon catabolite repression (95, 114). Unlike free-living bacteria, B. burgdorferi is restricted during its enzootic cycle to utilizing only those nutrients found in the tick vector or the mammalian host. Concentrations of glucose and glycerol in mouse plasma are approximately 150 and 2.8 mg/100 mL, respectively (115), and glycerol is abundantly present during all tick stages (109). It may be assumed that B. burgdorferi utilizes glucose while in the mammal and at early stages of tick feeding. At later feeding stages, or after cessation of vector feeding, glucose will be depleted due to its uptake by tick midgut epithelial cells. This triggers a switch to glycerol utilization that is mediated by elevated levels of glycerol pathway proteins. Pappas et al. proposed that this switch in carbohydrate utilization may represent the B. burgdorferi version of carbon catabolite repression, but did not provide a mechanism for this process (12).
B. burgdorferi carbohydrate metabolism has several unusual features (Figure 3). Among these are the sole reliance on glycolysis for ATP generation, a phosphofructokinase (PFK) that utilizes pyrophosphate as the donor for conversion of fructose 6-P to fructose 1,6-bisphosphate, the inability to utilize pyruvate as an energy source, and the presence of lactate permease (LctP; BB604). The rate-determining step of glycolysis in most eubacteria and eukaryotes is catalyzed by PFK, an allosteric enzyme whose activity is typically controlled by the ADP/ATP ratio (116). B. burgdorferi contains genes for two PFK enzymes (BB020, BB727). Interestingly, purified BB020 exists as a dimer in solution and, by comparison with other pyrophosphate-dependent PFKs (e.g., Treponema pallidum TP0542) is likely to be non-allosteric (86, 87, 117). If PFK does not sense the ADP/ATP ratio how is the flux of glycolytic intermediates through the pathway regulated in B. burgdorferi?
The end product of glycolysis is pyruvate and continued function of the pathway requires regeneration of NAD+. In B. burgdorferi this can only be accomplished by conversion of pyruvate to lactate and this reaction is the only metabolic fate for pyruvate as the spirochete lacks pyruvate dehydrogenase (and a TCA cycle), pyruvate-formate lyase or lactate oxidase (17). Extracellular lactate could be re-acquired through the action of lactate permease, likely functioning as a lactate:H+ symporter (118) (Figure 3); interestingly, lctP transcription is significantly induced in feeding ticks (Schwartz et al., unpublished observations). Typically, the acquired lactate would be utilized as an energy source by stepwise conversion to pyruvate (by lactate dehydrogenase [LDH]) and acetyl-CoA (via pyruvate dehydrogenase) and entry into the TCA cycle. However, the acquired lactate cannot be used in this manner in B. burgdorferi since pyruvate cannot be converted to any other metabolic intermediate. In addition, conversion of lactate to pyruvate by lactate dehydrogenase would generate NADH, altering the NAD+/NADH ratio in a manner unfavorable for continued glycolysis.
Taken together, these findings suggest a model for control of the glycolytic pathway in B. burgdorferi, particularly during the tick phases of the enzootic cycle, by the intracellular pyruvate/lactate ratio (Figure 3). When B. burgdorferi is growing in an environment with abundant glucose (e.g., a mammal), the sugar would enter the glycolytic pathway by uptake through its PTS and would ultimately be converted to lactate which is excreted. In an open system (mammalian tissue), excreted lactate would diffuse away and glycolysis would continue to function normally as long as sufficient glucose is available. During tick feeding glucose will also be available to B. burgdorferi and glycolysis would proceed initially as in the mammal. Further, this metabolic state enables a process referred to as inducer exclusion, whereby phosphorylated EIIAGlc interacts directly with GlpK and prevents conversion of available glycerol to glycerol 3-phosphate (119) (Figure 3). At later stages of feeding, glucose will become limiting (as a result of uptake by tick midgut epithelial cells). Additionally, excreted lactate will accumulate in the tick midgut because this is essentially a physically closed environment and LctP would be induced. As a result, B. burgdorferi will take up lactate which will be converted to pyruvate by LDH. Elevated pyruvate levels will alter the PEP/pyruvate ratio, a key factor in controlling glucose uptake through its cognate PTS. In E. coli, when the PEP/pyruvate ratio is low (i.e., when pyruvate is high), the EIIA component is dephosphorylated and the import of glucose as glucose 6-P by action of the PTS is blocked; indeed, the PEP/pyruvate ratio is directly correlated with the phosphorylation state of EIIAGlc (119-121). Furthermore, GlpK-mediated conversion of glycerol to glycerol 3-phosphate would resume. Under these circumstances, glycerol would become an important carbohydrate source for continued energy generation via glycolysis. The significant increase in glp operon expression during the tick feeding stages is consistent with this model. A second consequence of lactate uptake and conversion to pyruvate is the generation of an unfavorable NAD+/NADH ratio. This problem could be solved by the action of CoADR (see section 4), which would regenerate NAD+ and facilitate continued glycolysis (104, 105).
The precise details of the mechanism by which B. burgdorferi senses the levels of extracellular lactate, glucose and glycerol and transmits signals to the transcription machinery remains elusive. The Rrp2/RpoN/RpoS and Hk1/Rrp1 (c-di-GMP) circuits regulate transcription of genes during the mammalian and vector phases of the enzootic cycle. The involvement of these transcriptional regulators in modulating the expression of genes whose products facilitate transport and utilization of the alternative carbon sources glycerol and chitobiose argues that they play an important role in these processes. Elucidation of the interactions between these two regulatory systems would help to clarify how B. burgdorferi can alternate between different metabolic states in order to survive the drastically different environments encountered during its life cycle.
ACKNOWLEDGMENTS
We thank Justin Radolf for many helpful discussions. Studies conducted in the authors’ laboratory were supported by National Institutes of Health grants AR41511 and AI45801.
REFERENCES
- 1.Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease--United States, 1992-2006. MMWR Surveill Summ. 2008;57:1–9. [PubMed] [Google Scholar]
- 2.Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, Malawista SE. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 1983;308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 3.Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, Cameron DJ, Ziegler JL, Barbour AG, Burgdorfer W, Edelman R, Kaslow RA. Spirochetes isolated from the blood of two patients with Lyme disease. N. Engl. J. Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- 4.Levine JF, Wilson ML, Spielman A. Mice as reservoirs of the Lyme disease spirochete. Am. J. Trop. Med. Hyg. 1985;34:355–360. doi: 10.4269/ajtmh.1985.34.355. [DOI] [PubMed] [Google Scholar]
- 5.Tsao JI. Reviewing molecular adaptations of Lyme borreliosis spirochetes in the context of reproductive fitness in natural transmission cycles. Vet. Res. 2009;40:36. doi: 10.1051/vetres/2009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnarelli LA, Anderson JF, Fish D. Transovarial transmission of Borrelia burgdorferi in Ixodes dammini (Acari:Ixodidae). J. Infect. Dis. 1987;156:234–236. doi: 10.1093/infdis/156.1.234. [DOI] [PubMed] [Google Scholar]
- 8.Rollend L, Fish D, Childs JE. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick-borne Dis. 2013;4:46–51. doi: 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Dunham-Ems SM, Caimano MJ, Pal U, Wolgemuth CW, Eggers CH, Balic A, Radolf JD. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J. Clin. Invest. 2009;119:3652–3665. doi: 10.1172/JCI39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Silva AM, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am. J. Trop. Med. Hyg. 1995;53:397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- 11.Piesman J, Schneider BS, Zeidner NS. Use of quantitative PCR to measure density of Borrelia burgdorferi in the midgut and salivary glands of feeding tick vectors. J. Clin. Microbiol. 2001;39:4145–4148. doi: 10.1128/JCM.39.11.4145-4148.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappas CJ, Iyer R, Petzke MM, Caimano MJ, Radolf JD, Schwartz I. Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle. PLoS Pathog. 2011;7:e1002102. doi: 10.1371/journal.ppat.1002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J. Clin. Invest. 2004;113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pal U, Fikrig E. In: Tick Interactions. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Samuels DS, Radolf JD, editors. Caister Academic Press; Norfolk, UK.: 2010. pp. 279–298. [Google Scholar]
- 15.Shao L, Devenport M, Jacobs-Lorena M. The peritrophic matrix of hematophagous insects. Arch. Insect Biochem. Physiol. 2001;47:119–125. doi: 10.1002/arch.1042. [DOI] [PubMed] [Google Scholar]
- 16.Rohmer L, Hocquet D, Miller SI. Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends Microbiol. 2011;19:341–348. doi: 10.1016/j.tim.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van VR, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fuji C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 18.Casjens S, Palmer N, van VR, Huang WM, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson RJ, Haft D, Hickey E, Gwinn M, White O, Fraser CM. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 19.Brisson D, Drecktrah D, Eggers CH, Samuels DS. Genetics of Borrelia burgdorferi. Annu. Rev. Genet. 2012;46:515–536. doi: 10.1146/annurev-genet-011112-112140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iyer R, Kalu O, Purser J, Norris S, Stevenson B, Schwartz I. Linear and circular plasmid content in Borrelia burgdorferi clinical isolates. Infect. Immun. 2003;71:3699–3706. doi: 10.1128/IAI.71.7.3699-3706.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terekhova D, Iyer R, Wormser GP, Schwartz I. Comparative genome hybridization reveals substantial variation among clinical isolates of Borrelia burgdorferi sensu stricto with different pathogenic properties. J. Bacteriol. 2006;188:6124–6134. doi: 10.1128/JB.00459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casjens SR, Mongodin EF, Qiu WG, Luft BJ, Schutzer SE, Gilcrease EB, Huang WM, Vujadinovic M, Aron JK, Vargas LC, Freeman S, Radune D, Weidman JF, Dimitrov GI, Khouri HM, Sosa JE, Halpin RA, Dunn JJ, Fraser CM. Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS.One. 2012;7:e33280. doi: 10.1371/journal.pone.0033280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U.S.A. 2000;97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 2003;48:753–764. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- 25.Byram R, Stewart PE, Rosa P. The essential nature of the ubiquitous 26-kilobase circular replicon of Borrelia burgdorferi. J. Bacteriol. 2004;186:3561–3569. doi: 10.1128/JB.186.11.3561-3569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart PE, Byram R, Grimm D, Tilly K, Rosa PA. The plasmids of Borrelia burgdorferi: essential genetic elements of a pathogen. Plasmid. 2005;53:1–13. doi: 10.1016/j.plasmid.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Grimm D, Tilly K, Bueschel DM, Fisher MA, Policastro PF, Gherardini FC, Schwan TG, Rosa PA. Defining plasmids required by Borrelia burgdorferi for colonization of tick vector Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 2005;42:676–684. doi: 10.1093/jmedent/42.4.676. [DOI] [PubMed] [Google Scholar]
- 28.Chaconas G, Kobryn K. Structure, function, and evolution of linear replicons in Borrelia. Annu. Rev. Microbiol. 2010;64:185–202. doi: 10.1146/annurev.micro.112408.134037. [DOI] [PubMed] [Google Scholar]
- 29.Chaconas G, Norris SJ. Peaceful coexistence amongst Borrelia plasmids: getting by with a little help from their friends? Plasmid. 2013;70:161–167. doi: 10.1016/j.plasmid.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dulebohn DP, Bestor A, Rosa PA. Borrelia burgdorferi linear plasmid 28-3 confers a selective advantage in an experimental mouse-tick infection model. Infect. Immun. 2013;81:2986–2996. doi: 10.1128/IAI.00219-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jewett MW, Byram R, Bestor A, Tilly K, Lawrence K, Burtnick MN, Gherardini F, Rosa PA. Genetic basis for retention of a critical virulence plasmid of Borrelia burgdorferi. Mol. Microbiol. 2007;66:975–990. doi: 10.1111/j.1365-2958.2007.05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gherardini F, Boylan J, Lawrence K, Skare J. In: Metabolism and Physiology of Borrelia. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Samuels DS, Radolf JD, editors. Caister Academic Press; Norfolk, UK.: 2010. pp. 103–138. [Google Scholar]
- 33.Jewett MW, Lawrence KA, Bestor A, Byram R, Gherardini F, Rosa PA. GuaA and GuaB are essential for Borrelia burgdorferi survival in the tick-mouse infection cycle. J. Bacteriol. 2009;191:6231–6241. doi: 10.1128/JB.00450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, Rosa P. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 2006;74:3554–3564. doi: 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labandeira-Rey M, Skare JT. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 2001;69:446–455. doi: 10.1128/IAI.69.1.446-455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jewett MW, Jain S, Linowski AK, Sarkar A, Rosa PA. Molecular characterization of the Borrelia burgdorferi in vivo-essential protein PncA. Microbiology. 2011;157:2831–2840. doi: 10.1099/mic.0.051706-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang JR, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 38.Zhang JR, Norris SJ. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect. Immun. 1998;66:3698–3704. doi: 10.1128/iai.66.8.3698-3704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norris SJ. Antigenic variation with a twist--the Borrelia story. Mol. Microbiol. 2006;60:1319–1322. doi: 10.1111/j.1365-2958.2006.05204.x. [DOI] [PubMed] [Google Scholar]
- 40.Kazmierczak MJ, Wiedmann M, Boor KJ. Alternative sigma factors and their roles in bacterial virulence. Microbiol. Mol. Biol. Rev. 2005;69:527–543. doi: 10.1128/MMBR.69.4.527-543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beier D, Gross R. Regulation of bacterial virulence by two-component systems. Curr. Opin. Microbiol. 2006;9:143–152. doi: 10.1016/j.mib.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Hubner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12724–12729. doi: 10.1073/pnas.231442498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang XF, Alani SM, Norgard MV. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S .A. 2003;100:11001–11006. doi: 10.1073/pnas.1834315100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caimano MJ, Eggers CH, Hazlett KR, Radolf JD. RpoS is not central to the general stress response in Borrelia burgdorferi but does control expression of one or more essential virulence determinants. Infect. Immun. 2004;72:6433–6445. doi: 10.1128/IAI.72.11.6433-6445.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caimano MJ, Eggers CH, Gonzalez CA, Radolf JD. Alternate sigma factor RpoS is required for the in vivo-specific repression of Borrelia burgdorferi plasmid lp54-borne ospA and lp6.6 genes. J. Bacteriol. 2005;187:7845–7852. doi: 10.1128/JB.187.22.7845-7852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunham-Ems SM, Caimano MJ, Eggers CH, Radolf JD. Borrelia burgdorferi requires the alternative sigma factor RpoS for dissemination within the vector during tick-to-mammal transmission. PLoS Pathog. 2012;8:e1002532. doi: 10.1371/journal.ppat.1002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, Schwartz I, Radolf JD. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 2007;65:1193–1217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuels DS. Gene regulation in Borrelia burgdorferi. Annu. Rev. Microbiol. 2011;65:479–499. doi: 10.1146/annurev.micro.112408.134040. [DOI] [PubMed] [Google Scholar]
- 49.Ouyang Z, Blevins JS, Norgard MV. Transcriptional interplay among the regulators Rrp2, RpoN and RpoS in Borrelia burgdorferi. Microbiology. 2008;154:2641–2658. doi: 10.1099/mic.0.2008/019992-0. [DOI] [PubMed] [Google Scholar]
- 50.Blevins JS, Xu H, He M, Norgard MV, Reitzer L, Yang XF. Rrp2, a sigma54-dependent transcriptional activator of Borrelia burgdorferi, activates rpoS in an enhancer-independent manner. J. Bacteriol. 2009;191:2902–2905. doi: 10.1128/JB.01721-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouyang Z, Narasimhan S, Neelakanta G, Kumar M, Pal U, Fikrig E, Norgard MV. Activation of the RpoN-RpoS regulatory pathway during the enzootic life cycle of Borrelia burgdorferi. BMC. Microbiol. 2012;12:44. doi: 10.1186/1471-2180-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith AH, Blevins JS, Bachlani GN, Yang XF, Norgard MV. Evidence that RpoS (sigmaS) in Borrelia burgdorferi is controlled directly by RpoN (sigma54/sigmaN). J. Bacteriol. 2007;189:2139–2144. doi: 10.1128/JB.01653-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burtnick MN, Downey JS, Brett PJ, Boylan JA, Frye JG, Hoover TR, Gherardini FC. Insights into the complex regulation of rpoS in Borrelia burgdorferi. Mol. Microbiol. 2007;65:277–293. doi: 10.1111/j.1365-2958.2007.05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H, Caimano MJ, Lin T, He M, Radolf JD, Norris SJ, Gherardini F, Wolfe AJ, Yang XF. Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi. PLoS Pathog. 2010;6:e1001104. doi: 10.1371/journal.ppat.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lybecker MC, Samuels DS. Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol. Microbiol. 2007;64:1075–1089. doi: 10.1111/j.1365-2958.2007.05716.x. [DOI] [PubMed] [Google Scholar]
- 56.Lybecker MC, Abel CA, Feig AL, Samuels DS. Identification and function of the RNA chaperone Hfq in the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 2010;78:622–635. doi: 10.1111/j.1365-2958.2010.07374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karna SL, Sanjuan E, Esteve-Gassent MD, Miller CL, Maruskova M, Seshu J. CsrA modulates levels of lipoproteins and key regulators of gene expression critical for pathogenic mechanisms of Borrelia burgdorferi. Infect. Immun. 2011;79:732–744. doi: 10.1128/IAI.00882-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sze CW, Li C. Inactivation of bb0184, which encodes carbon storage regulator A, represses the infectivity of Borrelia burgdorferi. Infect. Immun. 2011;79:1270–1279. doi: 10.1128/IAI.00871-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ouyang Z, Zhou J, Norgard MV. CsrA (BB0184) is not involved in activation of the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. Infect. Immun. 2014;82:1511–1522. doi: 10.1128/IAI.01555-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller CL, Karna SL, Seshu J. Borrelia host adaptation Regulator (BadR) regulates rpoS to modulate host adaptation and virulence factors in Borrelia burgdorferi. Mol. Microbiol. 2013;88:105–124. doi: 10.1111/mmi.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boylan JA, Posey JE, Gherardini FC. Borrelia oxidative stress response regulator, BosR: a distinctive Zn-dependent transcriptional activator. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11684–11689. doi: 10.1073/pnas.2032956100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katona LI, Tokarz R, Kuhlow CJ, Benach J, Benach JL. The fur homologue in Borrelia burgdorferi. J. Bacteriol. 2004;186:6443–6456. doi: 10.1128/JB.186.19.6443-6456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hyde JA, Shaw DK, Smith R, III, Trzeciakowski JP, Skare JT. Characterization of a conditional bosR mutant in Borrelia burgdorferi. Infect. Immun. 2010;78:265–274. doi: 10.1128/IAI.01018-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Samuels DS, Radolf JD. Who is the BosR around here anyway? Mol. Microbiol. 2009;74:1295–1299. doi: 10.1111/j.1365-2958.2009.06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ouyang Z, Kumar M, Kariu T, Haq S, Goldberg M, Pal U, Norgard MV. BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol. Microbiol. 2009;74:1331–1343. doi: 10.1111/j.1365-2958.2009.06945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hyde JA, Shaw DK, Smith IR, Trzeciakowski JP, Skare JT. The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol. Microbiol. 2009;74:1344–1355. doi: 10.1111/j.1365-2958.2009.06951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ouyang Z, Deka RK, Norgard MV. BosR (BB0647) controls the RpoN-RpoS regulatory pathway and virulence expression in Borrelia burgdorferi by a novel DNA-binding mechanism. PLoS Pathog. 2011;7:e1001272. doi: 10.1371/journal.ppat.1001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ouyang Z, Zhou J, Brautigam CA, Deka R, Norgard MV. Identification of a core sequence for the binding of BosR to the rpoS promoter region in Borrelia burgdorferi. Microbiology. 2014;160:851–862. doi: 10.1099/mic.0.075655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang P, Dadhwal P, Cheng Z, Zianni MR, Rikihisa Y, Liang FT, Li X. Borrelia burgdorferi oxidative stress regulator BosR directly represses lipoproteins primarily expressed in the tick during mammalian infection. Mol. Microbiol. 2013;89:1140–1153. doi: 10.1111/mmi.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Troxell B, Ye M, Yang Y, Carrasco SE, Lou Y, Yang XF. Manganese and zinc regulate virulence determinants in Borrelia burgdorferi. Infect. Immun. 2013;81:2743–2752. doi: 10.1128/IAI.00507-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rogers EA, Terekhova D, Zhang HM, Hovis KM, Schwartz I, Marconi RT. Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol. Microbiol. 2009;71:1551–1573. doi: 10.1111/j.1365-2958.2009.06621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J. Bacteriol. 2005;187:1792–1798. doi: 10.1128/JB.187.5.1792-1798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hengge R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009;7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- 74.Sondermann H, Shikuma NJ, Yildiz FH. You've come a long way: c-di-GMP signaling. Curr. Opin. Microbiol. 2012;15:140–146. doi: 10.1016/j.mib.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Novak EA, Sultan SZ, Motaleb MA. The cyclic-di-GMP signaling pathway in the Lyme disease spirochete, Borrelia burgdorferi. Front. Cell.Infect. Microbiol. 2014;4:56. doi: 10.3389/fcimb.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caimano MJ, Kenedy MR, Kairu T, Desrosiers DC, Harman M, Dunham-Ems S, Akins DR, Pal U, Radolf JD. The hybrid histidine kinase Hk1 is part of a two-component system that is essential for survival of Borrelia burgdorferi in feeding Ixodes scapularis ticks. Infect. Immun. 2011;79:3117–3130. doi: 10.1128/IAI.05136-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Freedman JC, Rogers EA, Kostick JL, Zhang H, Iyer R, Schwartz I, Marconi RT. Identification and molecular characterization of a cyclic-di-GMP effector protein, PlzA (BB0733): additional evidence for the existence of a functional cyclic-di-GMP regulatory network in the Lyme disease spirochete, Borrelia burgdorferi. FEMS Immunol. Med. Microbiol. 2010;58:285–294. doi: 10.1111/j.1574-695X.2009.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He M, Ouyang Z, Troxell B, Xu H, Moh A, Piesman J, Norgard MV, Gomelsky M, Yang XF. Cyclic di-GMP is essential for the survival of the Lyme disease spirochete in ticks. PLoS Pathog. 2011;7:e1002133. doi: 10.1371/journal.ppat.1002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kostick JL, Szkotnicki LT, Rogers EA, Bocci P, Raffaelli N, Marconi RT. The diguanylate cyclase, Rrp1, regulates critical steps in the enzootic cycle of the Lyme disease spirochetes. Mol. Microbiol. 2011;81:219–231. doi: 10.1111/j.1365-2958.2011.07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Groshong AM, Blevins JS. Insights into the biology of Borrelia burgdorferi gained through the application of molecular genetics. Adv. Appl. Microbiol. 2014;86:41–143. doi: 10.1016/B978-0-12-800262-9.00002-0. [DOI] [PubMed] [Google Scholar]
- 81.von Lackum K, Stevenson B. Carbohydrate utilization by the Lyme borreliosis spirochete, Borrelia burgdorferi. FEMS Microbiol. Lett. 2005;243:173–179. doi: 10.1016/j.femsle.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 82.Das R, Hegyi H, Gerstein M. Genome analyses of spirochetes: a study of the protein structures, functions and metabolic pathways in Treponema pallidum and Borrelia burgdorferi. J. Mol. Microbiol. Biotechnol. 2000;2:387–392. [PubMed] [Google Scholar]
- 83.Jain S, Sutchu S, Rosa PA, Byram R, Jewett MW. Borrelia burgdorferi harbors a transport system essential for purine salvage and mammalian infection. Infect. Immun. 2012;80:3086–3093. doi: 10.1128/IAI.00514-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ouyang Z, He M, Oman T, Yang XF, Norgard MV. A manganese transporter, BB0219 (BmtA), is required for virulence by the Lyme disease spirochete, Borrelia burgdorferi. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3449–3454. doi: 10.1073/pnas.0812999106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoon-Hanks LL, Morton EA, Lybecker MC, Battisti JM, Samuels DS, Drecktrah D. Borrelia burgdorferi malQ mutants utilize disaccharides and traverse the enzootic cycle. FEMS Immunol. Med. Microbiol. 2012;66:157–165. doi: 10.1111/j.1574-695X.2012.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deng Z, Roberts D, Wang X, Kemp RG. Expression, characterization, and crystallization of the pyrophosphate-dependent phosphofructo-1-kinase of Borrelia burgdorferi. Arch. Biochem. Biophys. 1999;371:326–331. doi: 10.1006/abbi.1999.1446. [DOI] [PubMed] [Google Scholar]
- 87.Moore SA, Ronimus RS, Roberson RS, Morgan HW. The structure of a pyrophosphate-dependent phosphofructokinase from the Lyme disease spirochete Borrelia burgdorferi. Structure. 2002;10:659–671. doi: 10.1016/s0969-2126(02)00760-8. [DOI] [PubMed] [Google Scholar]
- 88.Gebbia JA, Backenson PB, Coleman JL, Anda P, Benach JL. Glycolytic enzyme operon of Borrelia burgdorferi: characterization and evolutionary implications. Gene. 1997;188:221–228. doi: 10.1016/s0378-1119(96)00811-6. [DOI] [PubMed] [Google Scholar]
- 89.Barbour AG. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 90.Tilly K, Elias AF, Errett J, Fischer E, Iyer R, Schwartz I, Bono JL, Rosa P. Genetics and regulation of chitobiose utilization in Borrelia burgdorferi. J. Bacteriol. 2001;183:5544–5553. doi: 10.1128/JB.183.19.5544-5553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hackman RH. Structure and function in tick cuticle. Annu. Rev. Entomol. 1982;27:75–95. doi: 10.1146/annurev.en.27.010182.000451. [DOI] [PubMed] [Google Scholar]
- 92.Rhodes RG, Atoyan JA, Nelson DR. The chitobiose transporter, chbC, is required for chitin utilization in Borrelia burgdorferi. BMC Microbiol. 2010;10:21. doi: 10.1186/1471-2180-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barabote RD, Saier MH., Jr. Comparative genomic analyses of the bacterial phosphotransferase system. Microbiol. Mol. Biol. Rev. 2005;69:608–634. doi: 10.1128/MMBR.69.4.608-634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clore GM, Venditti V. Structure, dynamics and biophysics of the cytoplasmic protein-protein complexes of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Trends Biochem. Sci. 2013;38:515–530. doi: 10.1016/j.tibs.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gorke B, Stulke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008;6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 96.Godany A, Vidova B, Janecek S. The unique glycoside hydrolase family 77 amylomaltase from Borrelia burgdorferi with only catalytic triad conserved. FEMS Microbiol. Lett. 2008;284:84–91. doi: 10.1111/j.1574-6968.2008.01191.x. [DOI] [PubMed] [Google Scholar]
- 97.Jahreis K, Pimentel-Schmitt EF, Bruckner R, Titgemeyer F. Ins and outs of glucose transport systems in eubacteria. FEMS Microbiol. Rev. 2008;32:891–907. doi: 10.1111/j.1574-6976.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- 98.Ostberg Y, Berg S, Comstedt P, Wieslander A, Bergstrom S. Functional analysis of a lipid galactosyltransferase synthesizing the major envelope lipid in the Lyme disease spirochete Borrelia burgdorferi. FEMS Microbiol. Lett. 2007;272:22–29. doi: 10.1111/j.1574-6968.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- 99.Jensen MO, Park S, Tajkhorshid E, Schulten K. Energetics of glycerol conduction through aquaglyceroporin GlpF. Proc. Natl. Acad. Sci. U. S. A. 2002;99:6731–6736. doi: 10.1073/pnas.102649299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen LY. Glycerol modulates water permeation through Escherichia coli aquaglyceroporin GlpF. Biochim. Biophys. Acta. 2013;1828:1786–1793. doi: 10.1016/j.bbamem.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Foster JW, Moat AG. Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems. Microbiol. Rev. 1980;44:83–105. doi: 10.1128/mr.44.1.83-105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gazzaniga F, Stebbins R, Chang SZ, McPeek MA, Brenner C. Microbial NAD metabolism: lessons from comparative genomics. Microbiol. Mol. Biol. Rev. 2009;73:529–541. doi: 10.1128/MMBR.00042-08. Table. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wolfe AJ. The acetate switch. Microbiol. Mol. Biol. Rev. 2005;69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boylan JA, Hummel CS, Benoit S, Garcia-Lara J, Treglown-Downey J, Crane EJ, III, Gherardini FC. Borrelia burgdorferi bb0728 encodes a coenzyme A disulphide reductase whose function suggests a role in intracellular redox and the oxidative stress response. Mol. Microbiol. 2006;59:475–486. doi: 10.1111/j.1365-2958.2005.04963.x. [DOI] [PubMed] [Google Scholar]
- 105.Eggers CH, Caimano MJ, Malizia RA, Kariu T, Cusack B, Desrosiers DC, Hazlett KR, Claiborne A, Pal U, Radolf JD. The coenzyme A disulphide reductase of Borrelia burgdorferi is important for rapid growth throughout the enzootic cycle and essential for infection of the mammalian host. Mol. Microbiol. 2011;82:679–697. doi: 10.1111/j.1365-2958.2011.07845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gazanion E, Garcia D, Silvestre R, Gerard C, Guichou JF, Labesse G, Seveno M, Cordeiro- Da-Silva A, Ouaissi A, Sereno D, Vergnes B. The Leishmania nicotinamidase is essential for NAD+ production and parasite proliferation. Mol. Microbiol. 2011;82:21–38. doi: 10.1111/j.1365-2958.2011.07799.x. [DOI] [PubMed] [Google Scholar]
- 107.Skare JT, Carroll JA, Yang XF, Samuels DS, Akins DR. Gene Regulation, Transcriptomics and Proteomics. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Caister Academic Press; Norfolk, UK.: 2010. pp. 67–101. [Google Scholar]
- 108.Lee RJ, Baust JG. Cold-hardiness in the Antartic tick, Ixodes uriae Physiol. Zool. 1987;60:499–506. [Google Scholar]
- 109.Vandyk JK, Bartholomew DM, Rowley WA, Platt KB. Survival of Ixodes scapularis (Acari: Ixodidae) exposed to cold. J. Med. Entomol. 1996;33:6–10. doi: 10.1093/jmedent/33.1.6. [DOI] [PubMed] [Google Scholar]
- 110.Ojaimi C, Brooks C, Casjens S, Rosa P, Elias A, Barbour A, Jasinskas A, Benach J, Katona L, Radolf J, Caimano M, Skare J, Swingle K, Akins D, Schwartz I. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 2003;71:1689–1705. doi: 10.1128/IAI.71.4.1689-1705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rhodes RG, Coy W, Nelson DR. Chitobiose utilization in Borrelia burgdorferi is dually regulated by RpoD and RpoS. BMC Microbiol. 2009;9:108. doi: 10.1186/1471-2180-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sze CW, Smith A, Choi YH, Yang X, Pal U, Yu A, Li C. Study of the response regulator Rrp1 reveals its regulatory role in chitobiose utilization and virulence of Borrelia burgdorferi. Infect. Immun. 2013;81:1775–1787. doi: 10.1128/IAI.00050-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hyde JA, Seshu J, Skare JT. Transcriptional profiling of Borrelia burgdorferi containing a unique bosR allele identifies a putative oxidative stress regulon. Microbiology. 2006;152:2599–2609. doi: 10.1099/mic.0.28996-0. [DOI] [PubMed] [Google Scholar]
- 114.Deutscher J. The mechanisms of carbon catabolite repression in bacteria. Curr. Opin. Microbiol. 2008;11:87–93. doi: 10.1016/j.mib.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 115.Maeda N, Funahashi T, Hibuse T, Nagasawa A, Kishida K, Kuriyama H, Nakamura T, Kihara S, Shimomura I, Matsuzawa Y. Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17801–17806. doi: 10.1073/pnas.0406230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moat AG, Foster JW, Spector MP. Microbial Physiology. 4 ed. Wiley-Liss; New York: 2002. [Google Scholar]
- 117.Roberson RS, Ronimus RS, Gephard S, Morgan HW. Biochemical characterization of an active pyrophosphate-dependent phosphofructokinase from Treponema pallidum. FEMS Microbiol. Lett. 2001;194:257–260. doi: 10.1111/j.1574-6968.2001.tb09479.x. [DOI] [PubMed] [Google Scholar]
- 118.Saier MH, Jr., Eng BH, Fard S, Garg J, Haggerty DA, Hutchinson WJ, Jack DL, Lai EC, Liu HJ, Nusinew DP, Omar AM, Pao SS, Paulsen IT, Quan JA, Sliwinski M, Tseng TT, Wachi S, Young GB. Phylogenetic characterization of novel transport protein families revealed by genome analyses. Biochim. Biophys. Acta. 1999;1422:1–56. doi: 10.1016/s0304-4157(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 119.Deutscher J, Ake FM, Derkaoui M, Zebre AC, Cao TN, Bouraoui H, Kentache T, Mokhtari A, Milohanic E, Joyet P. The bacterial phosphoenolpyruvate:carbohydrate phosphotransferase system: regulation by protein phosphorylation and phosphorylation-dependent protein-protein interactions. Microbiol. Mol. Biol. Rev. 2014;78:231–256. doi: 10.1128/MMBR.00001-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lengeler JW, Jahreis K. Bacterial PEP-dependent carbohydrate: phosphotransferase systems couple sensing and global control mechanisms. Contrib. Microbiol. 2009;16:65–87. doi: 10.1159/000219373. [DOI] [PubMed] [Google Scholar]
- 121.Hogema BM, Arents JC, Bader R, Eijkemans K, Yoshida H, Takahashi H, Aiba H, Postma PW. Inducer exclusion in Escherichia coli by non-PTS substrates: the role of the PEP to pyruvate ratio in determining the phosphorylation state of enzyme IIAGlc. Mol. Microbiol. 1998;30:487–498. doi: 10.1046/j.1365-2958.1998.01053.x. [DOI] [PubMed] [Google Scholar]