Abstract
Background/Purpose:
Choledochal cysts are congenital dilations of the bile ducts, and are associated with an increased risk of malignant transformation. The purpose of this study is to report the outcomes of a large series of patients with choledochal cysts and to highlight our analysis of one patient who developed malignancy after cyst resection.
Methods:
We conducted a retrospective review of patients <18 years of age with a choledochal cyst who underwent surgical resection between 1995 and 2018. Molecular testing of resected choledochal cyst specimens using the UCSF500 gene panel was performed on three patients including a 3-month-old boy and a 7-year-old girl who have remained cancer-free, and a 16-year-old girl who subsequently developed cholangiocarcinoma less than two years after resection.
Results:
One patient of the 48 included in our study developed cholangiocarcinoma after choledochal cyst resection. We observed de novo somatic mutations in TP53 and RBM10, and KRAS amplification in this patient’s tumor.
Conclusions:
In our series, the rate of malignancy after choledochal cyst resection was low. One patient developed de novo mutations in the remnant bile ducts after cyst resection. While it is a rare occurrence, the risk of malignancy following cyst resection supports the need for lifelong surveillance.
Keywords: Choledochal cysts, Cholangiocarcinoma, Molecular genetics, KRAS
Choledochal cysts (CCs) are a rare congenital anomaly characterized by dilation of the biliary tree. The most common type of CC is a fusiform dilation of the extrahepatic bile ducts for which surgical resection and creation of a biliary-enteric anastomosis are performed. Compared to the 0.0001–0.008% incidence of cholangiocarcinoma in the general population, [1] the rate of malignant transformation in patients with CC prior to resection is ~10%, [2] resulting in a 1000-fold increase in the incidence of cancer in these patients. The prevailing theory of oncogenesis in patients with CC postulates that abnormal mixing of biliary and pancreatic fluid contributes to chronic inflammation and malignant transformation [3]. This abnormal mixing of fluids also occurs in patients with an anomalous pancreaticobiliary junction (APBJ) which occurs concurrently with CC in more than 70% of patients [4] and is independently associated with an increased risk for bile duct cancer [4]. Despite the association between abnormal mixing of biliary and pancreatic fluids in CC and the development of malignancy, this association alone does not account for the ~90% of patients who do not develop cancer despite chronic exposure to these fluids. We reviewed our institutional experience of surgical resections of CC in pediatric patients and identified one patient who developed malignancy after CC resection.
1. Materials and methods
1.1. Study design and patient cohort
Pediatric patients (18 years of age or less) who had undergone resection of a choledochal cyst between 1995 and 2018 at the University of California San Francisco (UCSF) were retrospectively reviewed. A total of 12 fellowship-trained, board-certified pediatric surgeons performed operations for choledochal cyst in the patient cohort. Four surgeons performed 7–8 operations each, constituting more than 60% of the cases. Two surgeons did 4 cases, one surgeon did 3 cases, and one surgeon performed 2 cases. Four surgeons performed a single case each in this series. Each surgeon had at least 2 years of experience in performing these operations. Demographic information, details of their diagnosis (imaging, reason for imaging, labs), and details of their surgery (timing, type of procedure, post-operative complications) were collected from the patients’ charts. Follow-up timing and imaging, if applicable, were included.
1.2. UCSF500 choledochal cyst analysis
Molecular testing was performed on samples from three patients: one patient with a confirmed malignancy that developed after CC resection (the initial resected cyst specimen and the tumor were included), one patient deemed at low risk for malignancy, and one patient deemed at high risk for malignancy. The determination of high risk and low risk for malignancy was based on the prevailing theory that abnormal mixing of pancreatic and biliary fluid promotes malignant transformation. Therefore, we identified one patient as being low risk based on the patient’s young age and the absence of APBJ. Conversely, one patient was identified as high risk based on the patient being older and the presence of an APBJ.
The UCSF500 test is a molecular test of ~500 different genes, making it one of the most comprehensive cancer tests currently available. This assay is a DNA sequencing assay targeting the coding regions of 479 cancer-related genes and introns from 42 genes that are often rearranged or altered in cancer for structural variants detection, and intergenic regions along each chromosome for copy number assessment [5]. The analysis performed by UCSF500 used the human reference sequence UCSC build hg19 (NCBI build 37). Single nucleotide variants (SNVs) and small indels were detected using a Bayesian genetic variant detector FreeBayes [6] and a breakpoints detection toolbox Pindel [7], followed by annotations against databases including Exac, 1000Genome, dbSNP to filter out germline mutations. Copy number variations were inferred and visualized in a python library and command-line software toolkit CNVkit [8], and structural variants were detected with the integrated structural variant detector Delly [9]. Integrated Genome Viewer [10] was used to visualize and validate the SNVs.
1.3. Study approval
The UCSF Institutional Review Board (IRB) committee approved the collection of these patient data.
2. Results
2.1. Patient series
Between 1995 and 2018, 48 patients underwent resection of a CC at our institution (Table 1). The mean age at resection was four years, with 33.3% (n = 16) diagnosed prenatally. Sixty five percent (n = 31) were female. The patient population was composed of a variety of ethnicities: 31% Hispanic/Latino (n = 15), 23% Caucasian (n = 11), 17% Asian (n = 8), 10% Native Hawaiian or Pacific Islander (n = 5), 4% African American (n = 2), and 15% of unknown ethnicity (n = 7). More than half (56%, n = 27) were symptomatic. The average time between diagnosis and resection was 7.3 months. Three of the patients in this series were included in a previous publication from our institution in 2004, detailing the laparoscopic excision of three type I choledochal cysts [11]. Four patients were noted on preoperative imaging to have an anomalous pancreaticobiliary junction. Most patients in the current series had a type I CC (56.3%, n = 27) and underwent CC excision with a hepaticoduodenostomy (43%, n = 21).
Table 1.
Demographic and surgical data.
| n (%) | |
|---|---|
| Age at resection | |
| 0–3 months | 8 (17%) |
| 3–12 months | 9 (19%) |
| 1–4 years | 14 (29%) |
| 4–10 years | 12 (25%) |
| >10 years | 5 (10%) |
| Gender | |
| F | 31 (65%) |
| M | 17 (35%) |
| Ethnicity | |
| Hispanic/Latino | 15 (31%) |
| Caucasian | 11 (23%) |
| Asian | 8 (17%) |
| Native Hawaiian or Pacific Islander | 5 (10%) |
| African American | 2 (4%) |
| Unknown | 7 (15%) |
| Type of CC | |
| Type I | 28 (58%) |
| Type II | 1 (2%) |
| Type III | 1 (2%) |
| Type IV | 7 (15%) |
| Type V | 0 (0%) |
| Unknown | 11 (23%) |
| Symptomatic | |
| Yes | 27 (56%) |
| No | 21 (44%) |
| Time between diagnosis and resection | |
| <1 week | 12 (25%) |
| 1 week-1 month | 6 (12%) |
| 1 month-1 year | 20 (42%) |
| >1 year | 6 (12%) |
| Not recorded | 4 (9%) |
| Type of biliary-enteric reconstruction | |
| Hepaticoduodenostomy | 21 (43%) |
| Roux-en-Y Hepaticojejunostomy | 19 (40%) |
| Other type | 8 (17%) |
2.2. Surgical outcomes
Seventeen (35%) patients developed a postoperative complication. Sixteen percent (n = 8) developed an early postoperative complication (defined as within 30 days of surgery.) This included four patients who developed an infection (one patient developed a urinary tract infection, the second patient developed both a urinary tract infection and pneumonia, the third developed cholangitis requiring only antibiotic therapy, and the fourth patient developed enterococcal bacteremia of unclear source). Two patients developed a biliary leak requiring surgical revision. Two patients who underwent a Roux-en-Y hepaticojejunostomy required reoperation for bowel ischemia.
Nineteen percent (n = 9) of patients developed late complications (defined as greater than 30 days following surgery.) This included seven patients who developed chronic abdominal pain, one patient who died secondary to volvulus, and one patient who died following the development of cholangiocarcinoma (discussed further below).
2.3. Malignancy
Seventy-nine percent of patients (n = 38) were followed at our institution. The median follow-up time was 15 months (range 0–14 years). One patient in our series developed cholangiocarcinoma after CC resection (2.0%, n = 1). This patient was a 16-year-old girl who presented with several months of abdominal pain and emesis. An MRCP revealed a large fusiform dilation of the common bile duct (Fig. 1A). Within 5 days, she underwent a laparoscopic excision of a Type I CC with hepaticoduodenostomy. A postoperative MRCP revealed interval resolution of extrahepatic biliary dilation and mild intrahepatic dilation. Sixteen months later, she presented with abdominal pain and elevation of her CA19–9 to 73 U/mL. An MRCP performed at that time was unremarkable, demonstrating unchanged mild intrahepatic biliary dilation. Almost two years after surgery, she presented with worsening abdominal pain, a CA19–9 of 1859 U/mL, and an MRCP showing an infiltrative mass in the porta hepatis and new severe intra- and extrahepatic ductal dilation (Fig. 1B). Biopsies obtained via endoscopy confirmed cholangiocarcinoma and the patient was subsequently started on chemotherapy (Gemcitabine/Cisplatin). Restaging after 4 cycles revealed >50% reduction in the primary tumor and nodal size. However, in the ensuing months her tumor progressed and she died three years after her initial CC resection.
Fig. 1.
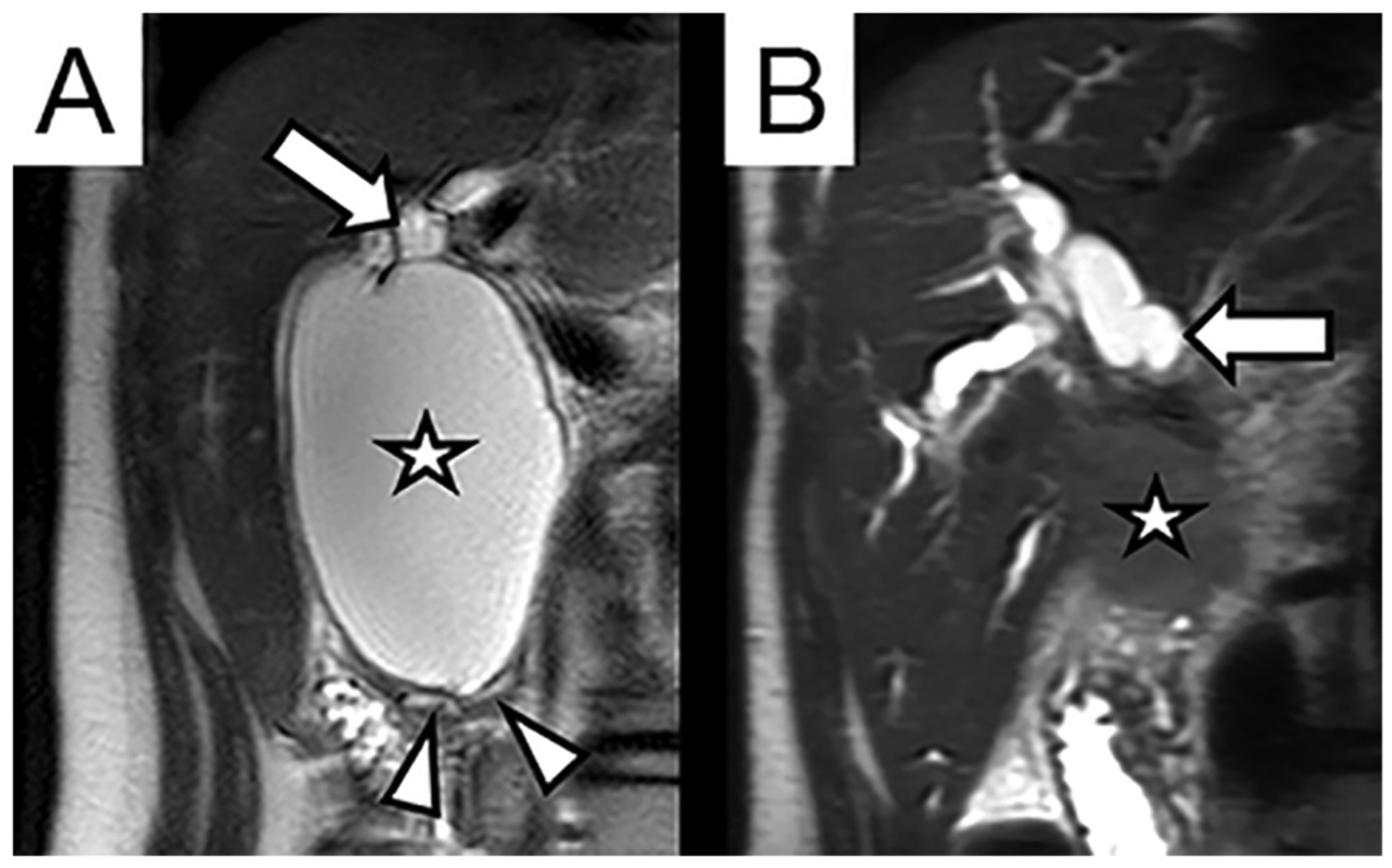
Coronal T2-weighted MRI images of the right upper abdomen. (A) 16-year-old female with two months of intermittent severe abdominal pain, found to have focal cystic dilation of the common bile duct (Type I choledochal cyst, annotated with star). The lumen of the cyst communicates with the dilated common hepatic duct (arrow). The cyst involves the distal intrapancreatic bile duct, resulting in splaying and displacement of the pancreatic parenchyma and pancreatic duct (arrow heads). (B) Same patient two years after cyst resection, presenting with new progressive pain, jaundice, and elevated serum bilirubin and CA19–9. MRI demonstrates new ill-defined infiltrative mass in porta hepatis (star) with severe intrahepatic biliary dilation (arrow).
2.4. UCSF500 assay analysis
Resected CC specimens from three patients were analyzed for genetic alterations using the UCSF500 assay (Table 2) [5]. The resected CC specimen and tumor of the patient who developed cholangiocarcinoma were sequenced, as well as the CC specimens from two younger control patients. Patient 1 was the previously described 16-year-old girl. Patient 2 was a 7-year-old girl who underwent cyst excision with hepaticoduodenostomy, and Patient 3 was a 3-month-old boy who underwent cyst excision with hepaticoduodenostomy. After filtering against multiple databases to remove any documented germline mutations and possible sequencing and alignment artifacts, two somatic mutations were identified: TP53 p.C135Y and RBM10 p.R836fs in the cholangiocarcinoma. These two mutations were considered somatic mutations owing to the fact that their mutation allelic frequencies were 27% and 13%, respectively, and were therefore unlikely a technical artifact, and because these two mutations were not detected in the matched and unmatched CC specimens. In the copy number analysis, setting a copy number log2 ratio of 2 as a threshold, we identified a high-level KRAS amplification in the tumor specimen (copy number log2 ratio of ~3.5) that was absent in the matched CC specimen and the other two CC specimens (Fig. 2). No other outstanding copy number amplifications or deletions (log2 ratio >2 or <−2) were found.
Table 2.
Description of the three patients whose specimens underwent UCSF500 molecular analysis.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age | 16 years | 7 years | 3 months |
| Gender | F | F | M |
| Surgery | Laparoscopic excision of cyst with hepaticoduodenostomy | Laparoscopic excision of cyst with hepaticoduodenostomy | Laparoscopic excision of cyst with hepaticoduodenostomy |
| Time between diagnosis and resection | 5 days | 3 years | 1 week |
| APBJ channel | Normal common channel | Long common channel, anomalous insertion of normal pancreatic duct | Normal common channel |
Fig. 2.
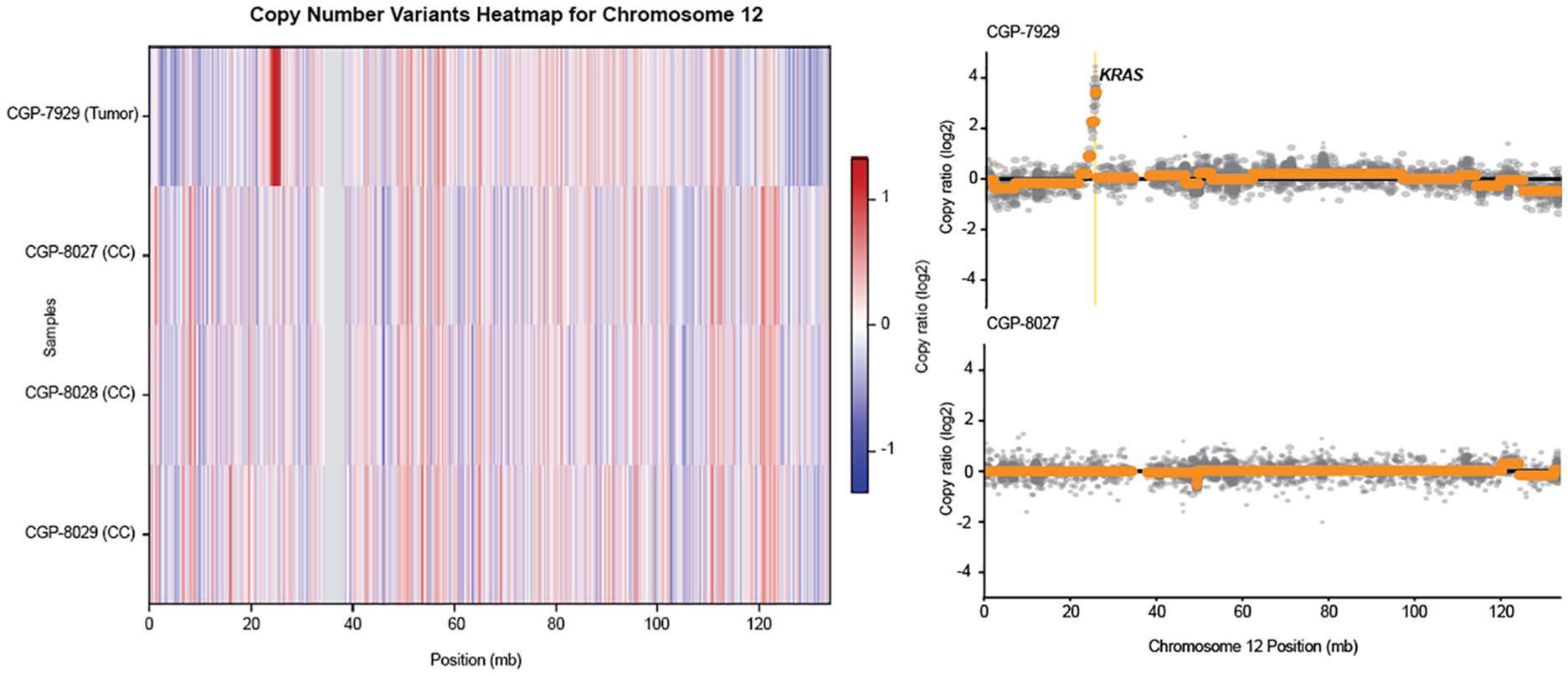
Unique copy number alterations in the tumor specimen of patient 1, not seen in the other choledochal cyst (CC) specimens of patients 1, 2 or 3.
3. Discussion
In this study, we report our experience with surgery for CC and describe the presence of somatic mutations in TP53 and RBM10, and amplification of KRAS in one patient who developed cholangiocarcinoma after CC resection. Notably, none of these mutations appeared in control CC specimens, indicating that mutations occurred de novo in the patient who developed malignant transformation after CC resection.
The risk of malignant transformation in CC is well documented. Eight percent to 15% of untreated patients with CC develop carcinoma, most commonly cholangiocarcinoma [12] although squamous cell carcinoma, gallbladder adenocarcinoma, and intraductal biliary papillomatosis have also been described [13]. Xia et al. found that of the 33 patients who developed malignancy (out of a total 196 patients with a history of CC), all had experienced cholangitis [14]. Types I and IV carry the highest risk of malignancy [15]. A SEER-Medicare population-based study revealed an odds ratio between CC and intrahepatic cholangiocarcinoma of 36.9 and 47.1 for extrahepatic cholangiocarcinoma [16]. In the largest case series from a Western institution, Edil et al. described five of their 92 patients having malignant disease associated with their CC at the time of resection (3 cholangiocarcinoma, 1 gallbladder cancer, and 1 embryonal rhabdomyosarcoma) of which only 2 patients had positive margins at resection. They also found an additional three patients who developed malignancy after their resection (2 cholangiocarcinoma, 1 pancreatic adenocarcinoma), and died 8, 9, and 21 years following their initial resection [17].
While CCs are typically thought of as a pediatric disease, today 70% of patients diagnosed with CC are adults [18]. In the largest case series of CC, Lee et al. examined the risk of malignancy in 808 patients with a history of CC diagnosed in adulthood in Korea. Almost 10% of patients had a biliary malignancy found at the time of CC resection. Furthermore, the risk of a biliary malignant tumor was age-related: 1.3% in the 21–30 year old group versus 23.5% in the 71–80 year old patient group [18]. Their univariate analysis revealed age > 40, preoperative serum carcinoembryonic antigen >0.05 ng/ml, preoperative serum cancer antigen 19–9 (CA 19–9) >37 U/L and the presence of APBJ to be associated with biliary malignancy in patients with a history of CC. On multivariate analysis, only CA 19–9 remained a significant factor [18].
Hepaticojejunostomy and hepaticoduodenostomy are the two most common types of biliary-enteric reconstitution after CC resection. A meta-analysis of six retrospective studies, encompassing a total of 679 patients, evaluated the outcomes after these two techniques and revealed hepaticoduodenostomy to have a higher postoperative rate of bile reflux gastritis [19]. However, rates of other complications, including cholangitis and reoperation, did not differ significantly between the two patient groups. No patient developed cancer in the included articles; thus, malignancy rate was not addressed. There are limited case reports describing cholangiocarcinoma after CC resection, after both hepaticoduodenostomy and hepaticojejunostomy [20,21], thus making it difficult to draw conclusions as to which reconstruction technique best mitigates long-term cancer risk. The 2017 Japanese clinical guidelines state that while a hepaticojejunostomy is most often adopted, owing to its ability to prevent reflux gastritis, there is no uniform view as to which technique is better (level B) [22]. The potential need to access the biliary-enteric anastomosis via endoscopic retrograde cholangiopancreatography has made hepaticoduodenostomy the preferred reconstructive option for our practice.
The specific molecular pathways leading to malignancy in patients with CC are poorly understood [23]. Malignancy most commonly develops at the hepatic duct at or near the biliary-enteric anastomosis, followed by the intrahepatic duct, and distal choledochus [18]. Recently, Weinberg et al. showed that intrahepatic and extrahepatic cholangiocarcinomas have different tumor biology, with extrahepatic tumors having a higher rate of KRAS, CDKN2A, and BRCA1 mutations [3,24]. In our patient, an extrahepatic cholangiocarcinoma was found to have KRAS amplification, along with somatic mutations in TP53 and RBM10. Mutations in TP53 and RBM10 have been previously observed in larger cohorts of cholangiocarcinoma at a frequency of 8.9% and 1.1% respectively [25,26]. As in our patient, the survival rates after treatment of cholangiocarcinoma in patients who had a CC resected in childhood are poor, with 50% at 2 years and 25% at 3 years [27]. With the known unfavorable outcome of cholangiocarcinoma even with aggressive treatment, close post-resection surveillance is essential.
The sequence of events that leads to malignant transformation in CC is unknown. The prevailing theory is that malignancy develops in patients with a history of choledochal cyst resection secondary to chronic inflammation of the remaining bile ducts, suggesting that mutations that lead to oncogenic transformation accumulate over time. However, our case of a patient who developed de novo KRAS amplification and mutations in TP53 and RBM10 shortly after her cyst resection suggests that the malignant transformation seen in this patient was sporadic. Broad conclusions regarding the molecular pathway leading to malignancy after CC cannot be drawn based on the single patient in our series who developed malignancy. Moreover, the UCSF500 panel is a molecular test that does not account for all potential pathways leading to malignant transformation.
There are no standard guidelines on the recommended frequency of follow-up for patients after resection of a CC. A review of the literature on malignancy in patients with a history of choledochal cyst led Madadi-Sanjani et al. to establish the following guidelines: a yearly abdominal ultrasound and “laboratory controls of the parameters of cholestasis”, to which CA19–9 is added once the patient reaches adulthood [3]. MRCP and US are the most commonly used imaging techniques for postop surveillance [28]. For adults with a history of choledochal excision in childhood, the University of British Columbia advocates for biochemical analysis (aspartate aminotransferase, alkaline phosphatase) every 4 months for 2 years then every 6 months for 5 years, as well as a yearly ultrasound for 5 years [29]. We routinely follow our patients with an annual CA19–9 and US every 5 years. Elevations in CA19–9 warrants further investigation by MRCP and/or ERCP with brushings. Regular follow-up is imperative given the persistent risk of developing cholangiocarcinoma even after CC resection.
4. Conclusions
In this study, we report a large series of patients with CC who underwent close postoperative follow-up. One patient in our series developed cholangiocarcinoma after CC resection and was found to have de novo somatic mutations and KRAS amplification. Further work is needed to determine the precise sequence of molecular events that lead to these mutations and the progression to cancer in patients with CC. We recommend close lifetime follow-up for these patients.
Acknowledgments
Thank you to Dr. Jessica Van Ziffle and her team who performed the UCSF500 molecular analyses. We are grateful to Barbara Bratton for her meticulous upkeeping of the patient database.
Funding: This work was supported in part by funds awarded to the UCSF Liver Center (NIH P30 DK026743; Dr. Mattis, Pathology Core).
Abbreviations:
- CC
choledochal cyst
- APBJ
anomalous pancreaticobiliary junction
References
- [1].Banales JM, Cardinale V, Carpino G, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13(5):261–80. 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- [2].Malik MN, Saleem T, Aslam S, et al. Cholangiocarcinoma in a resected biliary cyst: importance of follow-up. Cureus 2019;11(4):e4532. 10.7759/cureus.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Madadi-Sanjani O, Wirth TC, Kuebler JF, et al. Choledochal cyst and malignancy: a plea for lifelong follow-up. Eur J Pediatr Surg 2019;29(2):143–9. 10.1055/s-0037-1615275. [DOI] [PubMed] [Google Scholar]
- [4].Ragot E, Mabrut JY, Ouaissi M, et al. Pancreaticobiliary maljunctions in European patients with bile duct cysts: results of the multicenter study of the French Surgical Association (AFC). World J Surg 2017;41(2):538–45. 10.1007/s00268-016-3684-x. [DOI] [PubMed] [Google Scholar]
- [5].Kline CN, Joseph NM, Grenert JP, et al. Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro Oncol 2017;19(5):699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Garrison E, Marth G. Haplotype-based variant detection from short-read sequencing. arXiv preprint arXiv:12073907. 2012. [Google Scholar]
- [7].Ye K, Schulz MH, Long Q, et al. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics 2009;25(21):2865–71. 10.1093/bioinformatics/btp394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Talevich E, Shain AH, Botton T, et al. CNVkit: genome-wide copy number detection and visualization from targeted DNA sequencing. PLoS Comput Biol 2016;12(4): e1004873. 10.1371/journal.pcbi.1004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rausch T, Zichner T, Schlattl A, et al. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics 2012;28(18):i333–9. 10.1093/bioinformatics/bts378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Robinson JT, Thorvaldsdottir H, Winckler W, et al. Integrative genomics viewer. Nat Biotechnol 2011;29(1):24–6. 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee H, Hirose S, Bratton B, et al. Initial experience with complex laparoscopic biliary surgery in children: biliary atresia and choledochal cyst. J Pediatr Surg 2004;39(6): 804–7 [discussion 804-807]. [DOI] [PubMed] [Google Scholar]
- [12].Xia HT, Dong JH, Yang T, et al. Selection of the surgical approach for reoperation of adult choledochal cysts. J Gastrointest Surg 2015;19(2):290–7. 10.1007/s11605-014-2684-0. [DOI] [PubMed] [Google Scholar]
- [13].Baison GN, Rekman JF. Is cholangiocarcinoma in western adult patients with choledochal cyst (CC) disease and anomalous pancreaticobiliary junction (APBJ)? Hepatobil Surg Nutr 2019;8(Suppl. 1). [Google Scholar]
- [14].Xia HT, Wang J, Yang T, et al. Sphincter of Oddi dysfunction and the formation of adult choledochal cyst following cholecystectomy: a retrospective cohort study. Medicine (Baltimore) 2015;94(47):e2088. 10.1097/MD.0000000000002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54(1): 173–84. 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Welzel TM, Graubard BI, El-Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case–control study. Clin Gastroenterol Hepatol 2007;5(10):1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Edil BH, Cameron JL, Reddy S, et al. Choledochal cyst disease in children and adults: a 30-year single-institution experience. J Am Coll Surg 2008;206(5):1000–5 discussion 1005–1008 10.1016/j.jamcollsurg.2007.12.045. [DOI] [PubMed] [Google Scholar]
- [18].Lee SE, Jang JY, Lee YJ, et al. Choledochal cyst and associated malignant tumors in adults: a multicenter survey in South Korea. Arch Surg 2011;146(10):1178–84. 10.1001/archsurg.2011.243. [DOI] [PubMed] [Google Scholar]
- [19].Narayanan SK, Chen Y, Narasimhan KL, et al. Hepaticoduodenostomy versus hepaticojejunostomy after resection of choledochal cyst: a systematic review and meta-analysis. J Pediatr Surg 2013;48(11):2336–42. 10.1016/j.jpedsurg.2013.07.020. [DOI] [PubMed] [Google Scholar]
- [20].Santore MT, Behar BJ, Blinman TA, et al. Hepaticoduodenostomy vs hepaticojejunostomy for reconstruction after resection of choledochal cyst. J Pediatr Surg 2011;46(1):209–13. 10.1016/j.jpedsurg.2010.09.092. [DOI] [PubMed] [Google Scholar]
- [21].Goto N, Yasuda I, Uematsu T, et al. Intrahepatic cholangiocarcinoma arising 10 years after the excision of congenital extrahepatic biliary dilation. J Gastroenterol 2001;36 (12). 10.1007/s005350170010. [DOI] [PubMed] [Google Scholar]
- [22].Ishibashi H, Shimada M, Kamisawa T, et al. Japanese clinical practice guidelines for congenital biliary dilatation. J Hepatobiliary Pancreat Sci 2017;24(1):1–6. 10.1002/jhbp.415.10.1002/jhbp.415. [DOI] [PubMed] [Google Scholar]
- [23].Kim BH, Cho NY, Shin SH, et al. CpG island hypermethylation and repetitive DNA hypomethylation in premalignant lesion of extrahepatic cholangiocarcinoma. Virchows Arch 2009;455(4):343–51. 10.1007/s00428-009-0829-4. [DOI] [PubMed] [Google Scholar]
- [24].Weinberg BA, Xiu J, Lindberg MR, et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J Gastrointest Oncol 2019;10(4):652–62. 10.21037/jgo.2018.08.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Farshidfar F, Zheng S, Gingras MC, et al. Integrative genomic analysis of cholangiocarcinoma identifies distinct IDH-mutant molecular profiles. Cell Rep 2017;19(13): 2878–80. 10.1016/j.celrep.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zou S, Li J, Zhou H, et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat Commun 2014;5:5696. 10.1038/ncomms6696. [DOI] [PubMed] [Google Scholar]
- [27].Ohashi T, Wakai T, Kubota M, et al. Risk of subsequent biliary malignancy in patients undergoing cyst excision for congenital choledochal cysts. J Gastroenterol Hepatol 2013;28(2):243–7. 10.1111/j.1440-1746.2012.07260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hukkinen M, Koivusalo A, Lindahl H, et al. Increasing occurrence of choledochal malformations in children: a single-center 37-year experience from Finland. Scand J Gastroenterol 2014;49(10):1255–60. 10.3109/00365521.2014.946084. [DOI] [PubMed] [Google Scholar]
- [29].Wiseman K, Buczkowski AK, Chung SW, et al. Epidemiology, presentation, diagnosis, and outcomes of choledochal cysts in adults in an urban environment. Am J Surg 2005;189(5):527–31 [discussion 531]. [DOI] [PubMed] [Google Scholar]


