Abstract
Background:
The use of living donor liver transplantation (LDLT) for primary liver transplant (LT) may quell concerns about allocating deceased donor organs if the need for re-transplantation (re-LT) arises because the primary LT did not draw from the limited organ pool. However, outcomes of re-LT after LDLT are poorly studied. The purpose of this study was to analyze the Adult to Adult Living Donor Liver Transplantation Study (A2ALL) data to report outcomes of re-LT after LDLT, with a focus on long-term survival after re-LT.
Methods:
A retrospective review of A2ALL data collected between 1998–2014 was performed. Patients were excluded if they received a deceased donor liver transplant. Demographic data, post-operative outcomes and complications, graft and patient survival, and predictors of re-LT and patient survival were assessed.
Results:
Of the 1065 patients who underwent LDLT during the study time period, 110 recipients (10.3%) required re-LT. In multivariable analyses, HCV, longer LOS at LDLT, HAT, biliary stricture, infection, and disease recurrence were associated with an increased risk of re-LT. Patient survival among re-LT patients was significantly inferior to those who underwent primary transplant only at 1 (86 vs. 92%), 5 (64 vs. 82%), and 10 years (44 vs. 68%).
Conclusions:
Approximately 10% of A2ALL patients who underwent primary LDLT required re-LT. Compared with patients who underwent primary LT, survival among re-LT recipients was worse at 1, 5, and 10 years after LT, and re-LT was associated with a significantly increased risk of death in MV modeling (HR 2.29, p<0.001).
INTRODUCTION
Survival after liver transplantation (LT) remains excellent, with one and five-year survival rates of approximately 90% and 70%, respectively. However, 10–22% of LT recipients will ultimately require re-transplantation (re-LT),1,2 and survival after re-LT is known to be inferior to survival after primary LT.1 The causes of graft failure leading to re-LT vary according to the amount of time elapsed since LT. Common early indications for re-LT (defined in the literature as occurring anywhere between 0–7 months after LT) include primary graft failure and vascular complications, while late indications are generally due to recurrent disease or chronic rejection.3,4 And following re-LT, graft and patient survival are known to be impacted by recipient age, preoperative ventilator dependence, donor age, donor quality, and etiology of liver disease.1,3,4 While it is widely recognized that re-LT is essential for patients who experience graft failure after primary LT, concerns about re-LT include higher costs compared with primary LT and the ethical issue of allocating limited deceased donor organs to patients who have already received an allograft.5
In the United States (US), approximately 6% of all LT performed each year are living donor liver transplants (LDLT). However there is very little data on re-LT after LDLT in North America, and the one study that investigated this question addressed only one-year mortality.6 In that study, which compared re-LT following LDLT versus deceased donor liver transplant (DDLT) using data from the Organ Procurement and Transplantation Network (OPTN)/United Network for Organ Sharing (UNOS), LDLT recipients were more likely to require early re-LT for vascular thrombosis and were more often critically ill prior to re-LT, but there was ultimately no difference in one-year mortality between the groups.6 Understanding risk factors for, and outcomes following, re-LT after LDLT is important, because utilizing a deceased donor organ in these patients is ethically more palatable, as they have yet to draw from the deceased donor organ pool. Given the overall paucity of data on this subject, the purpose of this study was to perform secondary analyses of the large, multi-center data collected as part of the Adult to Adult Living Donor Liver Transplantation Study (A2ALL) to report outcomes of re-LT after LDLT, particularly long-term survival outcomes after re-LT.
MATERIALS & METHODS
Institutional review board approval was obtained prior to the initiation of this study.
Study Population
The A2ALL study collected data from nine North American liver transplant centers. Data was obtained retrospectively on adult LDLT recipients between 1998–2004, and prospectively from 2004–2009, with follow-up data available through 2014. The A2ALL data now resides in the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) repository and is available for use following a successful application process. Both the retrospective and prospective A2ALL data were obtained from the NIDDK for secondary analyses as outlined below. This study included all participants receiving a LDLT who had corresponding donor data and relevant dates for transplant and follow-up.
Statistical analyses
The descriptive analyses performed by our group have been previously described,7 but in brief included recipient demographics, outcomes and complications following LDLT, and donor characteristics, which were described with medians (interquartile ranges (IQR)) and frequencies (percentages). Cohort characteristics were stratified by re-LT versus primary LDLT alone. Differences were analyzed using Wilcoxon rank sum, Chi square, and Fisher’s exact tests, as appropriate.
Primary outcomes of interest included re-LT (defined as a subsequent transplant (DDLT or LDLT) after primary LDLT) and patient survival (defined as a patient death). To assess the outcome of re-LT, patients were followed from the date of LDLT to the date of subsequent transplant, death, or last follow-up. Patients dying without re-LT or remaining alive were censored. To assess the outcome of patient survival, patients were followed from the date of LDLT to death or last follow-up at which time patients were censored. Patients were not censored at the time of re-LT but rather followed beyond re-LT to death (event) or last follow-up (censored).
The Kaplan-Meier method was used to estimate patient survival after LDLT and compare survival by re-LT using the log-rank test. These comparisons utilized two approaches (1) comparing survival from primary LDLT stratified by re-LT and (2) comparing survival from LDLT for those without re-LT to survival after re-LT for those with re-LT (essentially comparing survival after most recent LT).
Cox proportional hazards regression estimated hazard ratios (HR) and 95% confidence intervals (CI) were compared for characteristics associated with (1) re-LT and (2) death. Post-LT complications were modeled as time-varying covariates such that a patient was classified as not having the complication until the time the complication occurred. Characteristics with a univariate p value <0.1 were evaluated in the multivariable model. The final multivariable model was built using backward selection (p>0.05 for elimination).
The impact of timing of graft failure was also analyzed, using cutoffs of both 14 and 30 days after LDLT as “early” graft failure.
Statistical significance was set at p<0.05. Statistical analyses were completed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
The final cohort included 1065 donor/recipient pairs who underwent LDLT in the A2ALL study between 1998 and 2014. Of this cohort, 110 recipients (10.3%) required re-LT following initial LDLT and the remaining 955 (89.6%) underwent a single LDLT only during the study follow-up period. Basic demographics are presented in Table 1. Re-LT patients were 65.5% male with a median age of 49 years (IQR 41–57) which was significantly younger than patients who underwent LDLT alone (median age 53 years (IQR 46–59), p<0.001). There were otherwise no significant demographic differences among re-LT and LDLT alone. Table 2 compares major post-transplant complications between re-LT and LDLT alone. As expected, re-LT patients had a significantly greater incidence of hepatic artery thrombosis (HAT, 38.1% vs. 3.2%, p<0.001), bile leak (36.2% vs. 26.9%, p=0.046), infection (39% vs. 23.6%, p<0.001), and recurrent disease (9.5% vs. 2.6%, p<0.001).
Table 1:
Basic demographic data comparing patients who underwent re-LT versus those who had a single transplant (LDLT only).
| Variable | Re-LT (n=110) | LDLT only (n=955) | P-value |
|---|---|---|---|
| Male, N (%) | 72 (65.5%) | 548 (57.4%) | 0.13 |
| Caucasian | 104 (94.5%) | 849 (89.8%) | 1.00 |
| Age at LDLT, median (IQR) | 49 (41–57) | 53 (46–59) | <0.001 |
| BMI, median (IQR) | 25 (23–28) | 26 (23–30) | 0.11 |
| MELD, median (IQR) | 16 (12–20) | 15 (11–19) | 0.11 |
| ALD, N (%) | 17 (15.5%) | 151 (15.8%) | 1.00 |
| HCV, N (%) | 50 (45.5%) | 353 (37.0%) | 0.10 |
| NASH or cryptogenic cirrhosis, N (%) | 10 (9.1%) | 96 (10.0%) | 0.75 |
| Cholestatic disease, N (%)* | 23 (20.9%) | 219 (22.9%) | 0.63 |
| HCC, N (%) | 12 (10.9%) | 157 (16.4%) | 0.17 |
| Left lobe, N (%) | 68 (7.1%) | 7 (6.4%) | 1.00 |
| Related donor, N (%) | 65 (59.1%) | 633 (66.4%) | 0.14 |
Cholestatic disease included primary biliary cholangitis and primary sclerosing cholangitis.
Table 2:
Complications after initial LDLT stratified by re-LT versus LDLT only.
| Variable | Re-LT | LDLT only | P-value |
|---|---|---|---|
| Hepatic artery thrombosis, N (%) | 40 (38.1%) | 26 (3.2%) | <0.001 |
| Bile leak, N (%) | 38 (36.2%) | 219 (26.9%) | 0.046 |
| Biliary stricture, N (%) | 30 (28.6%) | 275 (33.8%) | 0.29 |
| Rejection, N (%) | 10 (9.5%) | 88 (10.8%) | 0.69 |
| Infection, N (%) | 41 (39.0%) | 192 (23.6%) | <0.001 |
| Recurrent disease, N (%) | 10 (9.5%) | 21 (2.6%) | <0.001 |
Non-HCV recurrent disease included acute liver failure, alcoholic liver disease, cryptogenic cirrhosis, PSC, autoimmune hepatitis, HBV, other chronic liver disease, and HCC.
Factors Associated with Re-LT
Table 3 shows the results of univariate (UV) and multivariable (MV) analyses evaluating variables associated with re-LT. In MV analysis, a diagnosis of HCV, longer length of stay (LOS) at LDLT, HAT, biliary stricture, infection, and disease recurrence were all associated with increased risk of re-LT, while older age at LDLT was associated with reduced risk of re-LT.
Table 3:
Factors associated with risk of Re-LT
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Recipient age (LDLT) | 0.98 | 0.96–0.99 | 0.003 | 0.97 | 0.95–0.98 | <0.001 |
| Bilirubin (LDLT) | 1.03 | 1.01–1.05 | 0.01 | -- | ||
| Female sex | 0.70 | 0.47–1.03 | 0.07 | --- | ||
| HCV | 1.41 | 0.97–2.05 | 0.08 | 1.83 | 1.21–2.76 | 0.004 |
| HCC | 0.73 | 0.40–1.32 | 0.29 | --- | ||
| Donor age | 1.03 | 1.01–1.05 | <0.001 | --- | ||
| Ethnicity | 0.88 | 0.50–1.55 | 0.66 | --- | ||
| Left lobe | 1.10 | 0.51–2.38 | 0.80 | --- | ||
| LOS (at LDLT) | 1.02 | 1.01–1.02 | <0.001 | 1.02 | 1.01–1.02 | <0.001 |
| Bile leak | 2.16 | 1.43–3.25 | <0.001 | -- | ||
| HAT | 24.98 | 16.71–37.36 | <0.001 | 26.3 | 17.3–40.0 | <0.001 |
| GI Infection* | 2.70 | 1.30–5.62 | 0.008 | 3.55 | 1.67–7.57 | 0.001 |
| Disease recurrence | 5.47 | 2.48–12.06 | <0.001 | 4.07 | 1.80–9.21 | <0.001 |
GI infection included c. difficile colitis or a GI tract infection of bacterial, fungal, or viral origin.
Timing of Re-LT
The literature defines “early” graft failure as need for re-LT anywhere from 7 days to 7 months following initial transplant. We compared 1, 5, and 10-year survival among patients who underwent early versus late re-LT, defining early as within 14 days (Figure 1) and within 30 days (Figure 2). There was no significant difference in survival between the groups when defining early graft failure as 14 days (p=0.11) or when using 30 days as the cutoff (p=0.47). We also evaluated time from original LDLT on a continuum in attempt to identify a point at which the risk of death after re-LT changed significantly, however there was no significant association between time from primary LDLT to re-LT and risk of death after re-LT, nor was there an inflection point where this risk changed significantly.
Figure 1:
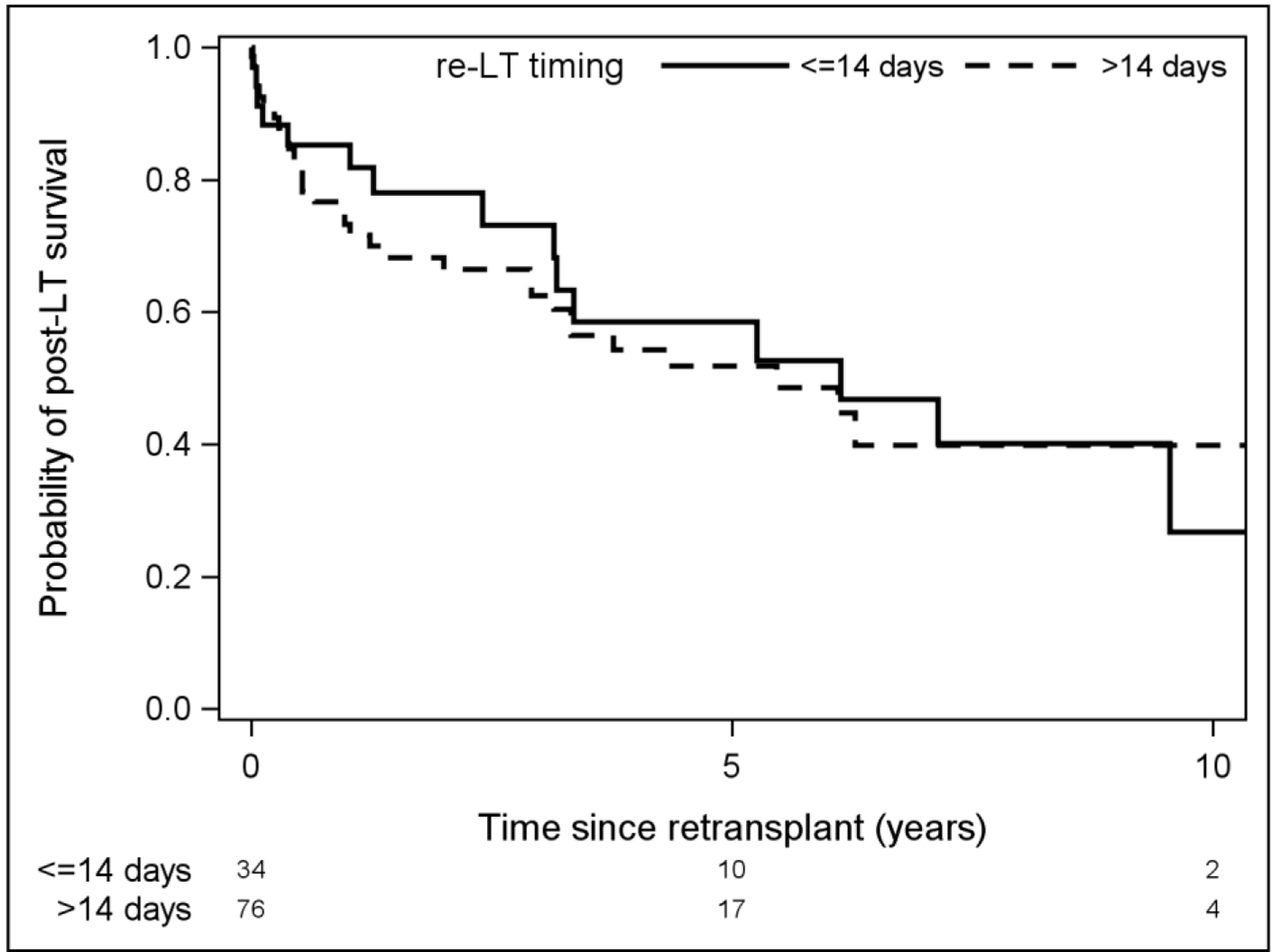
Comparison of survival among patients who required re-LT before or after 14 days post-LDLT.
Figure 2:
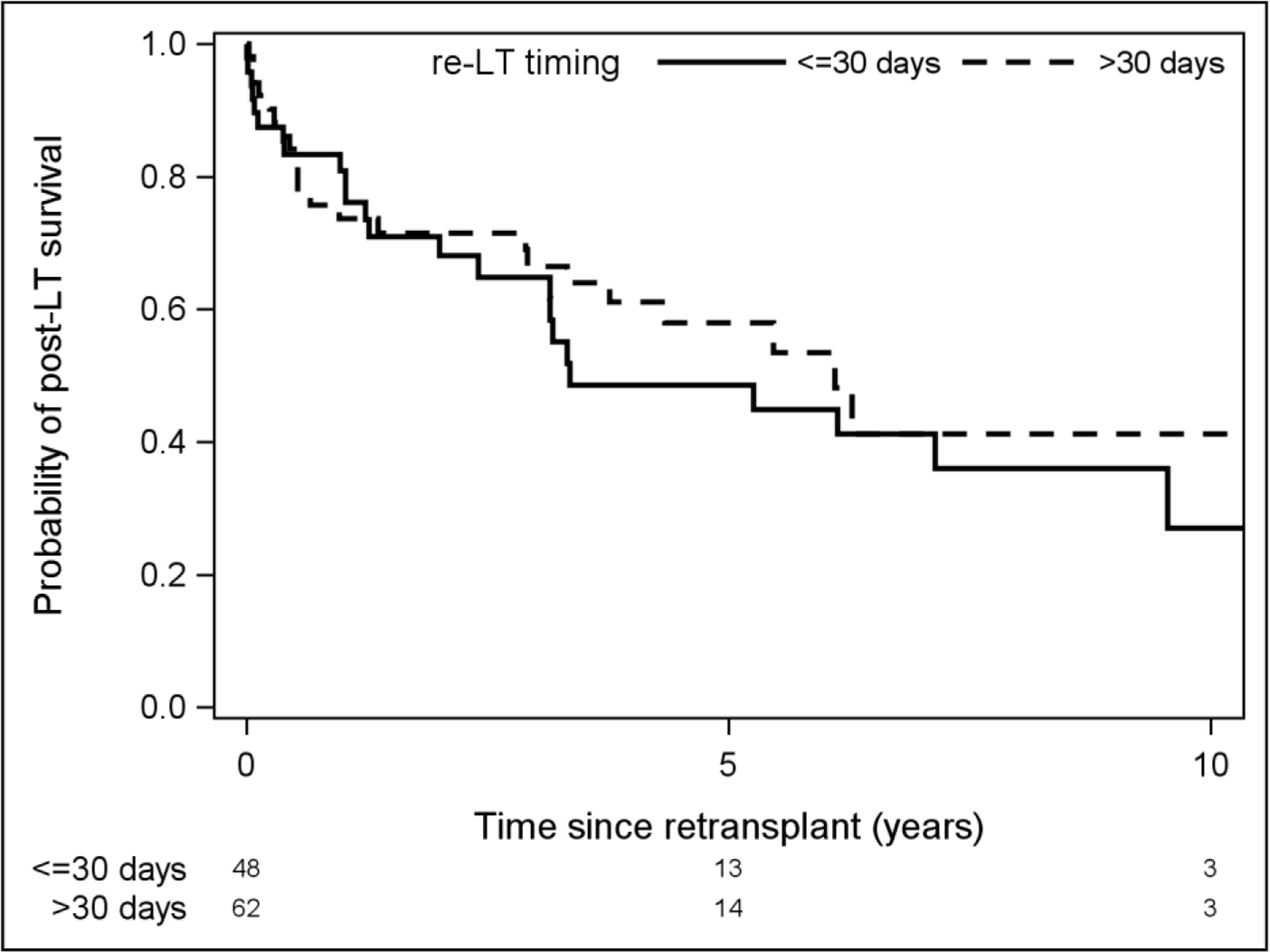
Comparison of survival among patients who required re-LT before or after 30 days post-LDLT.
Patient Survival
Overall patient survival from the time of original LDLT was significantly lower among patients who required re-LT (Figure 3). At one year from original LDLT, survival in the re-LT cohort was 86% (CI 78–92%) compared with 92% (CI 90–93%) for the LDLT only cohort. Similarly, at 5 and 10 years following LDLT, survival in the re-LT group remained significantly worse (64% (53–75%) vs. 82% (79–85%) and 44% (31–56%) vs. 68% (64–72%), respectively, p<0.001). We also compared overall survival from the time of re-LT in patients who underwent re-LT and LDLT in patients who did not require re-LT (Figure 4). Again, survival was inferior in the re-LT group vs. LDLT alone at 1 (77% (67–84%) vs. 92% (90–93%)), 5 (54% (43–64%) vs. 82% (79–85%)), and 10 years post-transplant (34% (20–49%) vs. 68% (64–72%), p<0.001).
Figure 3:

Survival comparison between patients requiring re-LT and those who did not from the time of original transplant.
Figure 4:
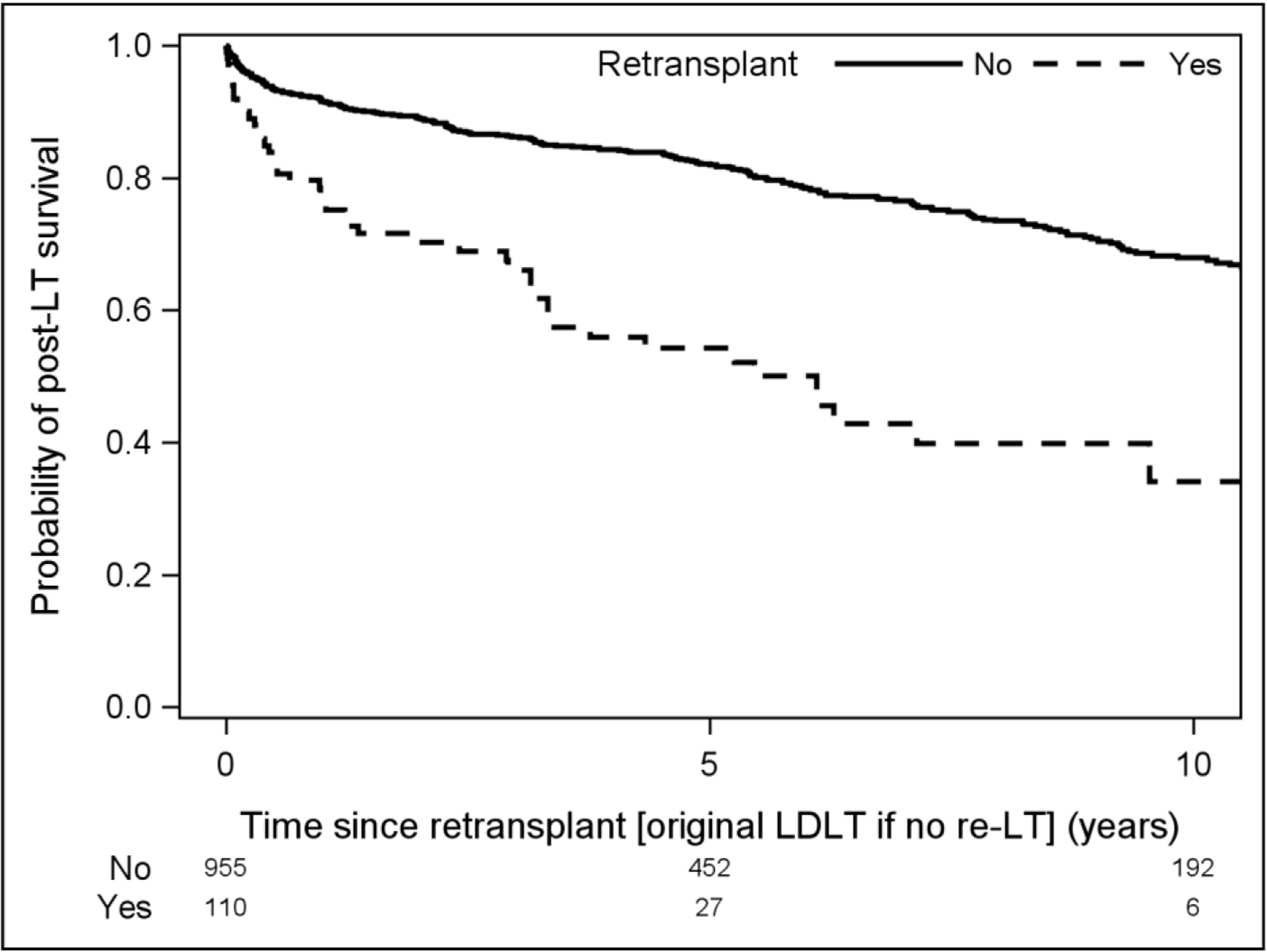
Survival comparison between patients requiring re-LT and those who did not from time of re-LT (or time of initial transplant for those who only underwent primary LT).
Factors Associated with Risk of Death
Table 4 illustrates UV and MV models that address factors associated with risk of death. In the MV model, recipient age, creatinine at LDLT, HCV, HCC, LOS, re-LT, HAT, and pulmonary complications were associated with an increased risk of death.
Table 4:
Factors associated with risk of death among the entire cohort.
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Recipient age (LDLT) | 1.03 | 1.01–1.04 | <0.001 | 1.02 | 1.01–1.03 | <0.001 |
| MELD (LDLT) | 1.02 | 1.00–1.04 | 0.025 | --- | ||
| Creatinine (LDLT) | 1.36 | 1.19–1.16 | <0.001 | 1.37 | 1.20–1.56 | <0.001 |
| Female sex | 0.77 | 0.60–0.99 | 0.04 | --- | ||
| HCV | 1.60 | 1.26–2.03 | <0.001 | 1.32 | 1.03–1.70 | 0.03 |
| HCC | 1.73 | 1.29–2.33 | <0.001 | 1.40 | 1.02–1.91 | 0.04 |
| Ethnicity | 0.99 | 0.70–1.38 | 0.94 | --- | ||
| Left lobe | 1.12 | 0.64–1.96 | 0.69 | |||
| LOS | 1.02 | 1.01–1.02 | <0.001 | 1.01 | 1.01–1.02 | <0.001 |
| Bile leak | 1.21 | 0.93–1.58 | 0.16 | |||
| Biliary stricture | 1.12 | 0.84–1.50 | 0.45 | |||
| HAT | 2.63 | 1.75–3.96 | <0.001 | 1.69 | 1.05–2.72 | 0.03 |
| Disease recurrence | 1.70 | 0.95–3.06 | 0.075 | |||
| Pulmonary Infection | 2.28 | 1.56–3.34 | <0.01 | 1.68 | 1.13–2.5 | 0.01 |
| Re-LT | 3.56 | 2.60–4.89 | <0.001 | 2.29 | 1.55–3.39 | <0.001 |
DISCUSSION
The purpose of this study was to compare long-term outcomes among patients who underwent LDLT alone versus those who underwent LDLT and required subsequent re-LT. The significant findings of this work were two-fold. First, a diagnosis of HCV, longer LOS at LDLT, HAT, biliary stricture, infection, and disease recurrence were associated with an increased risk of re-LT. Second, among patients who undergo primary LDLT, patient survival at 1, 5, and 10 years after transplantation is significantly worse for patients who require re-LT.
The current literature on re-LT after LDLT is derived primarily from Asian centers. In 2005, Tanaka et al. published data from a single center in Japan indicating the rate of re-LT after LDLT was approximately 3%.8 Because of the scarcity of deceased donors in this country, the majority of re-LT were performed with a second LDLT. In 2013, Hwang et al. presented an overview of re-LT after LDLT, which included results from Asan Medical Center, and found that 1-year survival after re-LT following LDLT was 60%, compared with their survival rates of 91% in patients undergoing first time LT.9 More recently in 2017, the Hong Kong group reviewed their single center data from 2000–2016 to evaluate outcomes after re-LT following initial LDLT or DDLT. The cohort in this study included 1114 patients who underwent primary LT (478 DDLT, 636 LDLT) and identified 44 patients who underwent a total of 48 re-LT. There was no significant difference in the percentage of patients with initial DDLT or LDLT who required re-LT and, when stratified by re-DDLT or re-LDLT, there was no difference in graft or patient survival at 5 years following transplantation.10 Most recently in 2017, Bitterman et al. analyzed OPTN/UNOS data and compared one-year mortality among patients who underwent primary DDLT versus LDLT and required re-LT. At one-year following re-LT, there was no significant difference in survival for patients who initially underwent DDLT (73.6%) compared with initial LDLT (76.1%).6 In sum, these studies demonstrate early survival rates of 60–80% in patients who undergo LDLT and require re-LT. The A2ALL data analyzed in the present study revealed a one-year survival rate of 86% after re-LT, which is slightly higher than previously published data. Unlike others, however, this analysis reports long-term outcomes after re-LT and shows that at 5 and 10 years after initial LDLT, patients who require re-LT continue to have inferior survival rates.
A variety of manuscripts have commented on risk factors associated with re-LT and outcomes following re-LT, and the majority of this data is derived from the DDLT literature. Well established causes of early graft failure after primary LT include primary non-function and vascular complications, while late graft failure is caused by chronic rejection, recurrent disease, and late biliary or vascular complications.3,4 Predictors of outcome after re-LT have most recently included recipient age, MELD at re-LT, creatinine at re-LT, cause of graft failure, timing of re-LT, status at re-LT, location at re-LT, and DRI.6 In the present study, we identified a diagnosis of HCV, LOS at initial LDLT, HAT, biliary stricture, and disease recurrence as predictors of graft failure after initial LDLT. Similar to prior studies, the present analysis also identified recipient age, creatinine at LDLT, HCV, HCC, LOS, re-LT, HAT, and pulmonary complications as having an association with increased risk of death.
The controversy surrounding re-LT presents a palimpsest, with many layers of ethical, financial, and medical questions that must be addressed in an era where deceased donor organs remain a scarce resource. All patients in this analysis of A2ALL data underwent primary LDLT, and approximately 10% required re-LT. LDLT is known to be associated with a higher risk of biliary and vascular complications due to the unique challenges of smaller, and partial, grafts,11–13 so we should accept a slightly higher rate of need for re-LT in this population, particularly as the common causes for early re-LT include vascular complications. While it is well established that the hospital costs associated with re-LT will be greater than initial LT, one would hope that because these patients initially utilized live donor grafts and assumed a larger risk of vascular complications without drawing from the deceased donor pool, they would be viewed more favorably from the ethical quandaries associated with re-LT.
As with any study, there are several limitations to the present investigation. First, these patients underwent LDLT prior to the introduction of direct antiviral agents and nearly 50% of the cohort was transplanted for HCV. HCV was associated with an increased risk of both re-LT and of death, so we would anticipate that a similar cohort transplanted now might have fewer instances of disease recurrence resulting in either re-LT or death. Second, the A2ALL study includes data on patients who undergo re-LT, however we do not have granular details regarding the transplants performed after initial LDLT and linking of the A2ALL data to UNOS/OPTN/SRTR is prohibited. Despite these two limitations, we believe the data presented here identifies important factors associated with outcomes following re-LT after primary LDLT and reports long term survival in these patients for the first time.
CONCLUSION
Approximately 10% of A2ALL patients who underwent primary LDLT required re-LT. Factors associated with re-LT included a diagnosis of HCV, longer LOS at LDLT, HAT, biliary stricture, and disease recurrence. Among patients who underwent re-LT, there was no difference in survival when timing of re-LT was stratified into early versus late. Compared with patients who underwent primary LDLT alone, patients who underwent re-LT had worse survival at one, five, and ten years after transplantation.
Acknowledgments
The A2ALL study was conducted by the A2ALL Study Investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The data from the A2ALL study reported here were supplied by the NIDDK Central Repositories. This manuscript was not prepared in collaboration with Investigators of the A2ALL study and does not necessarily reflect the opinions or views of the A2ALL study, the NIDDK Central Repositories, or the NIDDK.
Funding: This project was supported by the National Institutes of Health Grant Number T32AI125222. Partial support for statistical analyses was provided by the UCSF Liver Center (P30 DK026743). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- A2ALL
Adult to Adult Living Donor Liver Transplantation Study
- CI
confidence intervals
- DDLT
deceased donor liver transplantation
- HAT
hepatic artery thrombosis
- HCC
hepatocellular carcinoma
- HCV
Hepatitis C virus
- HR
hazard ratios
- IQR
interquartile ranges
- LDLT
living donor liver transplantation
- LOS
length of stay
- LT
liver transplantation
- MELD
Model for End Stage Liver Disease
- MV
multivariable
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- OPTN
Organ Procurement and Transplantation Network
- Re-LT
re-transplantation
- UNOS
United Network for Organ Sharing
- US
United States
- UV
univariate
Footnotes
Disclosures: the authors declare no conflicts of interest
REFERENCES
- 1.Azoulay D, Linhares MM, Huguet E, et al. Decision for retransplantation of the liver: an experience- and cost-based analysis. Annals of surgery. 2002;236(6):713–721; discussion 721. doi: 10.1097/01.SLA.0000036264.66247.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markmann JF, Markowitz JS, Yersiz H, et al. Long-term survival after retransplantation of the liver. Annals of surgery. 1997;226(4):408–418; discussion 418–420. doi: 10.1097/00000658-199710000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo PS, Umman V, Rodriguez-Davalos MI, Emre SH. Retransplantation of the liver: review of current literature for decision making and technical considerations. Transplant Proc. 2013;45(3):854–859. doi: 10.1016/j.transproceed.2013.02.063 [DOI] [PubMed] [Google Scholar]
- 4.Zarrinpar A, Hong JC. What is the prognosis after retransplantation of the liver? Adv Surg. 2012;46:87–100. doi: 10.1016/j.yasu.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 5.Rosen HR, Madden JP, Martin P. A model to predict survival following liver retransplantation. Hepatology. 1999;29(2):365–370. doi: 10.1002/hep.510290221 [DOI] [PubMed] [Google Scholar]
- 6.Bittermann T, Shaked A, Goldberg DS. When Living Donor Liver Allografts Fail: Exploring the Outcomes of Retransplantation Using Deceased Donors. Am J Transplant. 2017;17(4):1097–1102. doi: 10.1111/ajt.14037 [DOI] [PubMed] [Google Scholar]
- 7.Braun HJ, Dodge JL, Grab JD, et al. Living Donor Liver Transplant for Alcoholic Liver Disease: Data from the Adult to Adult Living Donor Liver Transplantation Study (A2ALL). Transplantation. 2020. 104(2):285–292. doi: 10.1097/TP.0000000000002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka K, Yamada T. Living donor liver transplantation in Japan and Kyoto University: what can we learn? J Hepatol. 2005;42(1):25–28. doi: 10.1016/j.jhep.2004.11.004 [DOI] [PubMed] [Google Scholar]
- 9.Hwang S, Ahn CS, Kim KH, et al. Liver retransplantation for adult recipients. Korean journal of hepato-biliary-pancreatic surgery. 2013;17(1):1–7. doi: 10.14701/kjhbps.2013.17.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chok KSH, Chan ACY, Fung JYY, et al. Comparable short- and long-term outcomes in deceased-donor and living-donor liver retransplantation. Hepatol Int. 2017;11(6):517–522. doi: 10.1007/s12072-017-9821-2 [DOI] [PubMed] [Google Scholar]
- 11.Freise CE, Gillespie BW, Koffron AJ, et al. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am J Transplant. 2008;8(12):2569–2579. doi: 10.1111/j.1600-6143.2008.02440.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samstein B, Smith AR, Freise CE, et al. Complications and Their Resolution in Recipients of Deceased and Living Donor Liver Transplants: Findings From the A2ALL Cohort Study. Am J Transplant. 2016;16(2):594–602. doi: 10.1111/ajt.13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmerman MA, Baker T, Goodrich NP, et al. Development, management, and resolution of biliary complications after living and deceased donor liver transplantation: a report from the adult-to-adult living donor liver transplantation cohort study consortium. Liver Transpl. 2013;19(3):259–267. doi: 10.1002/lt.23595 [DOI] [PMC free article] [PubMed] [Google Scholar]


