Abstract
Background
Sickle cell disease (SCD) causes significant morbidity, pre-mature mortality and high disease burden, resulting in frequent healthcare use. Co-management may improve utilization and patient adherence with treatments such as Hydroxyurea. The purpose of this study was to describe acute care utilization in Medicaid enrolled patients with SCD, patient factors associated with co-management and adherence to Hydroxyurea.
Methods
Data from 2,790 patients diagnosed with SCD, age 1 to 65+, enrolled at least 1 month in North Carolina Medicaid between March 2016 and February 2017 were analyzed. Out-patient visits were categorized as: primary care, hematologist and non-hematologist specialist. Nurse practitioners or physician assistants with unidentified specialty type or family practice were categorized separately. Co-management was defined as a minimum of one primary care and one hematologist visit/patient during the study period.
Results
There were notable age related differences in utilization of health care services. Only 34.82% of the sample was co-managed. Co-management was higher in the 1-9 (44.88%) and 10-17 year-old groups (39.22%) versus the 31-45 (30.26%) and 65+ (18.75%) age groups. Age had the greatest influence (AUC=0.599) on whether or not a patient was co-managed. Only a third of the sample (32.24%) had at least one Hydroxyurea prescription. Age was the most predictive factor of good Hydroxyurea adherence (AUC 0.6503). Prediction by co-management was minimal with an AUC=0.5615.
Conclusion
Co-management was a factor in predicting Hydroxyurea adherence, but further studies are needed to identify the frequency and components of co-management needed to increase adherence and reduce acute care utilization.
Keywords: Sickle Cell Disease, co-management, Medicaid, emergency department, hospitalization, hydroxyurea
Introduction
Sickle cell disease (SCD) is a genetic disorder and the most common, rare blood disease in the United States (U.S.).1 Patients suffer from complications, including but not limited to stroke, acute chest syndrome, and severe painful crisis that contribute to morbidity and pre-mature mortality. Individuals also suffer from significant disease burden, often resulting in a high number of hospitalizations, re-admissions, emergency department (ED) visits, and other associated costs to the healthcare system. In 2006 people with SCD had an estimated 232,382 ED visits, 68,410 hospitalizations from the ED, accounting for an estimated $2.4 billion dollars.2 In 2010, SCD was associated with the highest 30 day re-admission rate (31.9%) among all diagnoses excluding cancer and cancer-related conditions.3 Other national data representing about 1/3 of the SCD population found a similar 30 day re-admission rate of 33.4% and 14 day re-admission rate of 22.1%.4 Extremely high ED use has also been well described.5,6 In a prospective cohort study of SCD, healthcare utilization was examined in three EDs over two years and found 342 unique patients had a total of 2,934 ED visits.6 Understanding there is significant healthcare utilization due to disease severity, there has been an increasing effort to leverage out-patient primary care providers using a care model in which hematology specialists and primary care providers (PCPs) partner to co-manage SCD. Primary care and outpatient management using evidence-based guidelines can improve disease burden, improve patient outcomes, and prevent the need for ED visits and in-patient hospitalizations.7–10
The National Heart Lung and Blood Institute (NHLBI) published the Evidence-Based Management of SCD report, in 2014. This comprehensive guide consists of health maintenance and prevention information, including the use of hydroxyurea (HU). HU is an oral agent found to reduce the median number of SCD pain episodes per year by 44%, but is historically underutilized.11 Only 32% of general internists and 35% of pediatricians report feeling comfortable caring for SCD patients.12 In a study of 129 PCP’s with greater than ten years (median) since graduating from medical school, 80% reported relying on knowledge gained in residency when deciding how to manage SCD patients and 68% did not regularly communicate with other providers regarding their SCD patients.12 In a survey of 53 primary care physicians in North Carolina, 65% indicated they are already comfortable co-managing hydroxyurea prescriptions with a SCD specialist.9 More specific to family physicians, in a large survey of over 1000 family physicians, comfort level for managing patients with SCD was low (20%), however, 80% and 68% of respondents indicated they would be willing to co-manage pediatric and adults patients with a SCD specialist.13 Thus a gap exists in the implementation of current evidence based practices in the management of SCD in the ambulatory setting.
As adults with SCD age, they are at risk for developing co-morbidities unrelated to SCD. Thus, individuals with SCD are recommended to have both a PCP and a SCD specialist co-manage their care. Co-management is defined as collaborative and coordinated care that is conceptualized, planned, delivered, and evaluated by two or more health care providers. Nationally, there is often a lack of providers and co-management for individuals with SCD is not common practice.11 It is possible that co-management of patients with SCD, in combination with dissemination of evidence based guidelines, could lead to an increased comfort level of family physicians, interests and pediatricians. Therefore we designed a dissemination and implementation project to promote co-management between SCD specialists and PCPs. Our team partnered with Community Care North Carolina (CCNC), a statewide primary case management program for North Carolina (NC) Medicaid enrollees.14 CCNC spans 14 community-based primary care networks throughout the state and works collaboratively with physicians and other healthcare professionals to promote quality improvement and care management initiatives.14 For the purposes of this project, we defined co-management as a minimum of one PCP and one hematologist visit/patient during the study period. We adapted the NHLBI recommendations into user-friendly tools (health maintenance charts and treatment algorithms) designed specifically for and with the input of PCP’s and ED providers, which are easy to access and use. These tools are available on two websites (sickleemergency.duke.edu and www.communitycarenc.org) as well as via a mobile application (www.scdtoolbox.com).
This paper reports baseline Medicaid data from NC prior to dissemination efforts, including: 1) ED encounters and re-encounters within 7, 14 and 30 days, hospitalization and re-hospitalization within 7, 14 and 30 days; 2) PCP, hematologist, non-hematology specialist visits and co-management (at least 1 PCP and hematologist visit per patient during a one year period); and 3) HU prescription fills and adherence. In addition, this paper will identify predictors of HU adherence and co-management for patients insured under Medicaid in NC.
METHODS
Dataset - Medicaid North Carolina
In fiscal year 2017 almost 2 million people (approximately one-fifth of NC’s population) were covered by Medicaid; making it the third largest Medicaid population in states that did not expand the program.15 In fiscal year 2016 there were 68,583 NC Medicaid providers. 15 In partnership with CCNC, we obtained a dataset of Medicaid administrative claims of enrollees with a diagnosis of SCD including HbSS, HbSC, and HbS-thalassemia, excluding sickle cell trait (International Classification of Diseases, [ICD] 10 CM codes: D57.0x, D57.1, D57.2x, D57.8x).
Sample
Data is reported for a cohort of 2,045 patients with a diagnosis of SCD, age 1 to 65+, enrolled at least 12 months in NC Medicaid between March 1, 2016 and February 28, 2017. Table 1 further describes the sample. IRB approval and waiver of consent were obtained.
Table 1:
Demographic Characteristics and Enrollment in CCNC Programs (Sample = 2045)
| Characteristics | Statistic |
|---|---|
| Sex, n (%) | |
| Female | 1162 (56.82) |
| Male | 883 (43.18) |
| Age, mean (sd) | 22.87 (16.41) |
| N (%) | |
| 1-9 years old | 499 (24.40) |
| 10-17 years old | 436 (21.32) |
| 18-30 years old | 537 (26.26) |
| 31-45 years old | 347 (16.97) |
| 46-64 years old | 194 (9.49) |
| ≥65 years old | 32 (1.56) |
| CCNC Program Months Enrolled*, mean (sd) | 10.67 (3.43) |
| Dual Eligible Medicare and Medicaid, n (%) | |
| Yes | 417 (20.39) |
| No | 1628 (79.61) |
| Residence**, n (%) | |
| Metro | 1558 (76.19) |
| Non-Metro adjacent to Metro | 440 (21.52) |
| Non-Metro un-adjacent to Metro | 47 (2.30) |
| CCNC Network, n (%) | |
| Access East | 390 (19.07) |
| Access Care | 180 (8.80) |
| Carolina Collaborative Community Care | 125 (6.11) |
| Carolina Community Health Partnership | 30 (1.47) |
| Community Care Partners of Greater Mecklenburg | 320 (15.65) |
| Community Care of Southern Piedmont | 66 (3.23) |
| Community Care of Wake/Johnston Counties | 217 (10.61) |
| Community Care of Western North Carolina | 20 (0.98) |
| Community Care of the Lower Cape Fear | 112 (5.48) |
| Community Care of the Sandhills | 102 (4.99) |
| Community Health Partners | 37 (1.81) |
| Northern Piedmont Community Care | 133 (6.5) |
| Northwest Community Care Network | 134 (6.55) |
| Partnership for Community Care | 179 (8.75) |
CCNC program enrollment is defined as having active full Medicaid coverage and being linked to a medical home.
Residence categories were determined using the United States Department of Agriculture 2013 Rural-Urban Continuum Codes
Measures
Emergency Department Encounters and Hospitalization:
ED encounters were identified using a CCNC developed logic model that used revenue codes (450-452, 456 and 459) and excluding Medicare part A crossover-inpatient, inpatient, management fee, drug and capitation claim type codes. ED re-encounters and re-hospitalizations within 7, 14 and 30 days were identified using the time between the date of service listed on the ED or hospital claim and the next date of service in the subsequent claim. Individual ED Reliance (EDR) score was calculated as the total number of ED encounters divided by the total ambulatory visits (outpatient + ED encounters) per enrollee.16,17 An EDR of >0.33 is a high score.17 Inpatient claims were identified using category of service code (0015, 0017, 0018, 0040, 0041, 0051 and 0058) and with either a Medicare Part A crossover-inpatient or inpatient claim type.
Outpatient Visits and Co-Management:
Outpatient visits were identified by the Current Procedural Terminology (CPT) code listed on the claim. Outpatient visit types (PCP, hematologists and non-hematology specialists) were identified using the descriptions from the Medicare Provider/Supplier to Healthcare Provider Taxonomy that matched the billing provider code and the rendering provider code listed on the claim.18 Visits were classified into the following categories: 1) PCP (including pediatrician); 2) hematologist (including pediatric hematologists); 3) non-hematology specialist (see Table 2 for the list of non-hematology specialists); and 4) Nurse Practitioner (NP) or Physician Assistant (PA) visits. NP and PA visits in which a PCP, hematology or non-hematology specialty was indicated on the claim were included into the corresponding category. When we were unable to determine if an NP or PA visit provided primary care, hematology, or other specialty care, they were included in the NP or PA unidentified specialty type or family practice. The “Other Specialty” category includes outpatient visits not historically linked to SCD care or with a frequency of < 1% of the total number of non-hematology specialty visits. If the billing provider or rendering provider code was either missing or did not link to a specific provider type, then those visits were placed in the “NULL” category to signify they were unidentifiable. “Acute care visits” signified medical care occurred outside the ED, and were not in-patient stays but occurred in an acute care location. Co-management is defined as a minimum of one PCP and one hematologist visit/patient during the study period. Our team discussed this definition at length and agreed this is a bare minimum and co-management should include more than one visit by both the PCP and SCD specialist. However, because co-management for patients with SCD is so rare, we agreed to examine a bare minimum of one visit for each.
Table 2:
List of Non-Hematology Specialist by type and visit frequency for age 1− 65+ Sample N=2045
| Specialty Type | Frequency |
|---|---|
| Primary Care Visits | 6251 |
| Hematology Specialty Visits | 2792 |
| Non-Hematology Specialty Visits | 8827 |
| Acute Care* | 2743 |
| Physician Assistant (Unidentified Specialty or Family Practice) | 1477 |
| Nurse Practitioner (Unidentified Specialty or Family Practice) | 1144 |
| Unidentifiable (Null)** | 458 |
| Obstetrician/Gynecologist | 383 |
| Other Specialty Visits*** | 377 |
| Orthopedic Medicine | 295 |
| Ophthalmology/Optometry | 282 |
| Surgery | 235 |
| Cardiology | 191 |
| Neurology | 178 |
| Pulmonary | 174 |
| Oncology | 161 |
| Nephrology | 122 |
| Otolaryngology | 116 |
| Anesthesiology | 103 |
| Gastroenterology | 96 |
| Physical and Rehabilitation Medicine | 91 |
| Foot & Ankle Surgery/Podiatric Medicine | 87 |
| Urology | 82 |
| Psychology | 32 |
Acute care visit- a visit that occurred in an out-patient acute setting
Unidentifiable (Null) category includes office visits with a billing provider code for “multi-specialty” or “single specialty” with no rendering provider information.
Other specialty visits- include outpatient visits not historically linked to SCD care or a frequency of visits within the specialty category ≤ 1% of the total number of specialty visits. Excludes: SCD and general NP or PA visits; Includes: addiction medicine, allergy and immunology, anatomic pathology, critical care medicine, dermatology, development behavior, diagnostic radiology, endocrinology, geriatric medicine, infectious disease, special hospital, neonatal-perinatal medicine, neuro-development, rheumatology, sleep medicine, sports medicine, vascular and interventional.
Hydroxyurea Prescription Fills and Adherence:
HU claims were identified using the drug name. Only those enrolled in Medicaid for 12 months were included in this analysis. The number of HU prescriptions filled per enrollee by age group was determined by summing the number of filled HU prescriptions over the study period for each eligible enrollee. The number of HU days supplied is the sum of the days of supply on the prescription (e.g. 30 day supply) in a 12-month period per person. The duration of HU treatment days was measured as the number of days between the date of the first HU prescription filled and the last day of the study period. The number of days between breaks in treatment is the sum of days with no HU supplied, divided by the number of gaps (missing next HU prescription fill) per person.
HU adherence was categorized into one of the followings: 1) Good- if number of days supplied is ≥80% of duration of HU treatment; 2) Fair or Moderate- if number of days supplied is 60-79% of duration of HU treatment; 3) Poor- if number of days supplied is < 60% of duration of HU treatment. 19,20
Predictor Measures
Age related differences in SCD health service utilization have been found in several studies.16,21–24 Young adults that are transition-aged (16 – 25 years) have particularly high rates of acute (emergency and in-patient) care utilization.23 However, 18 to 30-year-olds with SCD have the highest reported rates of acute care encounters per patient per year, before decreasing throughout middle and older ages.25 Differences in EDR depending on age have also been previously noted in SCD patients, with highest EDR noted in those transitioning to adulthood. 16 For this study, patients were categorized into age categories similar to previously published groups. 26,27The following age groups were utilized: 1-9, 10-17, 18-30, 31-45, 46-64 and >65 years.26 Gender differences in frequency and intensity of pain have previously been reported. Several studies have found an increased sensitivity and lower tolerance to pain in women and have shown related increased health service utilization.28–30 However, prior studies in reporting SCD pain have reported no difference in pain experiences between men and women, but higher healthcare utilization by men.31,32 For this study enrollees were categorized as either male or female. Number of months enrolled in the CCNC network (1-12 months) was obtained in order to account for variations in co-management and HU adherence that could be affected by the length of time in the network. Differences in the geographic location of people with SCD have been linked to differences in health service utilization.33 Prior studies indicate that the further away clients with SCD are from clinics, the lower the rate of health service utilization. 33,34In this study patient zip codes were used to categorize the sample by county using the US Department of Agriculture Economic Research Service (ERS) 2013 Rural-Urban Continuum Codes classification to distinguish metropolitan (metro) counties by population size and nonmetropolitan (non-metro) counties by their degree of urbanization and adjacency to metro areas.35 This method allows for consideration of regional factors such as proximity to an urban area that may influence utilization of healthcare services.
Analysis
Descriptive statistics, means and standard deviations for interval variables and frequencies and percentages for categorical variables, were computed to summarize demographic characteristics of the sample. Inpatient and ambulatory healthcare and HU utilization were summarized by frequencies, medians and interquartile ranges (IQR), due to skewed distributions. Utilization summaries were presented for the overall sample and by 6 age categories. Only those participants age 1 to 65+, who were enrolled in Medicaid for all 12 months and had at least one Medicaid claim for HU were included in the calculation of the HU utilization summary statistics. There were no HU Medicaid claims in the 65+ age group, thus they were excluded from the analysis. Logistic regression models were used to evaluate participant factors’ influence on co-management and HU treatment adherence (good versus fair or poor). Both models were conditional on age, gender, rural residence and months enrolled in the CCNC network. A regression analysis including primary care and hematology visits as main effects with their interaction (co-management) were added as predictors in the HU adherence model. For each outcome (co-management and HU adherence), an area under the curve-receiver operating characteristic curve (AUC-ROC) was created. AUC-ROC provides an estimate of how capable the model is of distinguishing between the outcomes. The higher the AUC the better the model is at predicting good adherence vs fair or poor adherence. When AUC=0.5, the model does not distinguish between levels of adherence.
RESULTS
The participants in the sample were majority female (56.82%), lived in metropolitan areas (76.19%) and had a mean age of 22.87 years old (SD=16.41), see Table 1. Table 3 displays the summary of utilization of acute care and outpatient services of the sample.
Table 3:
Emergency Department Encounters, In-Patient Stays, Out-Patient Visits, Emergency Department Reliance and Co-Management for North Carolina Medicaid Enrolled 12 months and aged 1− 65+ (Sample=2045)
| Total ED Encounters | Within 7-day Re-Encounters | Within 14-day Re-Encounters | Within 30-day Re-Encounters | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Participants n (%) | Encounters n (mean) | Median (IQR) | Participants n (%) | Encounters n (mean) | Median (IQR) | Participants n (%) | Encounters n (mean) | Median (IQR) | Participants n (%) | Encounters n (mean) | Median (IQR) |
| 1-9 (n=499) | 348 (69.74) | 930 (1.86) | 1 (0, 3) | 75 (15.03) | 102 (0.20) | 0 (0, 0) | 96 (19.24) | 146 (0.29) | 0 (0, 0) | 114 (22.85) | 220 (0.44) | 0 (0, 0) |
| 10-17 (n=436) | 268 (61.47) | 643 (1.47) | 1 (0, 2) | 47 (10.78) | 61 (0.14) | 0 (0, 0) | 60 (13.76) | 84 (0.19) | 0 (0, 0) | 74 (16.97) | 128 (0.29) | 0 (0, 0) |
| 18-30 (n=537) | 423 (78.77) | 2801 (5.22) | 2 (1, 6) | 166 (30.91) | 934 (1.74) | 0 (0, 1) | 189 (35.20) | 1253 (2.33) | 0 (0, 1) | 229 (42.64) | 1663 (3.10) | 0 (0, 2) |
| 31-45 (n=347) | 258 (74.35) | 1804 (5.20) | 2 (0, 4) | 84 (20.49) | 703 (2.03) | 0 (0, 0) | 105 (30.36) | 914 (2.63) | 0 (0, 1) | 125 (36.02) | 1176 (3.39) | 0 (0, 2) |
| 46-64 (n=194) | 132 (68.04) | 607 (3.13) | 1 (0, 4) | 37 (19.07) | 148 (0.76) | 0 (0, 0) | 44 (22.68) | 209 (1.08) | 0 (0, 0) | 55 (28.35) | 297 (1.53) | 0 (0, 1) |
| 65+ (n=32) | 22 (68.75) | 49 (1.53) | 1 (0, 2) | 1 (3.13) | 1 (0.03) | 0 (0, 0) | 2 (6.25) | 2 (0.06) | 0 (0, 0) | 6 (18.75) | 9 (0.28) | 0 (0, 0) |
| All (n=2045) | 1451 (70.95) | 6834 (3.34) | 1 (0, 4) | 410 (20.05) | 1949 (0.94) | 0 (0, 0) | 496 (24.25) | 2608 (1.28) | 0 (0, 0) | 603 (29.49) | 3493 (1.71) | 0 (0, 1) |
| Total IP Hospitalizations | Within 7-day Re-hospitalization | Within 14-day Re-hospitalization | Within 30-day Re-hospitalization | |||||||||
| Age | Participants n (%) | Stays n (mean) | Median (IQR) | Participants n (%) | Stays n (mean) | Median (IQR) | Participants n (%) | Stays n (mean) | Median (IQR) | Participants n (%) | Stays n (mean) | Median (IQR) |
| 1-9 (n=499) | 176 (35.27) | 312 (0.63) | 0 (0, 1) | 13 (2.61) | 13 (0.03) | 0 (0, 0) | 16 (3.21) | 20 (0.04) | 0 (0, 0) | 26 (5.21) | 43 (0.09) | 0 (0, 0) |
| 10-17 (n=436) | 133 (30.50) | 324 (0.74) | 0 (0, 1) | 10 (2.29) | 10 (0.02) | 0 (0, 0) | 20 (4.59) | 25 (0.06) | 0 (0, 0) | 30 (6.88) | 56 (0.13) | 0 (0, 0) |
| 18-30 (n=537) | 318 (59.22) | 1211 (2.26) | 1 (0, 2) | 45 (8.38) | 84 (0.16) | 0 (0, 0) | 73 (13.59) | 234 (0.44) | 0 (0, 0) | 100 (18.62) | 479 (0.89) | 0 (0, 0) |
| 31-45 (n=347) | 184 (53.03) | 618 (1.78) | 1 (0, 2) | 22 (6.34) | 34 (0.10) | 0 (0, 0) | 32 (9.22) | 81 (0.23) | 0 (0, 0) | 46 (13.26) | 208 (0.60) | 0 (0, 0) |
| 46-64 (n=194) | 88 (45.36) | 225 (1.16) | 0 (0, 1) | 4 (2.06) | 7 (0.04) | 0 (0, 0) | 9 (4.64) | 22 (0.11) | 0 (0, 0) | 17 (8.76) | 55 (0.28) | 0 (0, 0) |
| 65+ (n=32) | 17 (53.13) | 19 (0.59) | 1 (0, 1) | 0 (0) | 0 (0) | 0 (0, 0) | 0 (0) | 0 (0) | 0 (0, 0) | 0 (0) | 0 (0) | 0 (0, 0) |
| All (n=2045) | 916 (44.79) | 2709 (1.32) | 0 (0, 1) | 94 (4.60) | 148 (0.07) | 0 (0, 0) | 150 (7.33) | 382 (0.19) | 0 (0, 0) | 219 (10.71) | 841 (0.41) | 0 (0, 0) |
| Total Outpatient Visits | PCP Visits | Hematology Specialty Visits | Non-Hematology Specialty Visits | |||||||||
| Age | Participants n (%) | Visits n (mean) | Median (IQR) | Participants n (%) | Visits n (mean) | Median (IQR) | Participants, n (%) | Visits n (mean) | Median (IQR) | Participants n (%) | Visits n (mean) | Median (IQR) |
| 1-9 (n=499) | 486 (97.39) | 4044 (8.10) | 6 (4, 11) | 437 (87.58) | 1853 (3.71) | 3 (1, 5) | 257 (51.50) | 761 (1.53) | 1 (0, 2) | 345 (69.14) | 1430 (2.87) | 2 (0, 4) |
| 10-17 (n=436) | 410 (94.04) | 3299 (7.57) | 5 (3, 9) | 344 (78.90) | 1101 (2.53) | 2 (1, 3) | 209 (47.94) | 667 (1.53) | 0 (0, 2) | 329 (75.46) | 1531 (3.51) | 2 (1, 4) |
| 18-30 (n=537) | 482 (89.76) | 4174 (7.77) | 6 (2, 11) | 332 (61.82) | 1226 (2.28) | 1 (0, 3) | 214 (39.85) | 668 (1.24) | 0 (0, 2) | 406 (75.61) | 2280 (4.25) | 2 (1, 6) |
| 31-45 (n=347) | 319 (91.93) | 3708 (10.69) | 8 (4, 16) | 254 (73.20) | 1214 (3.50) | 2 (0, 5) | 134 (38.62) | 459 (1.32) | 0 (0, 1) | 271 (78.10) | 2035 (5.86) | 3 (1, 8) |
| 46-64 (n=194) | 180 (92.78) | 2286 (11.78) | 9 (4, 17) | 140 (72.16) | 716 (3.69) | 2 (0, 5) | 72 (37.11) | 222 (1.14) | 0 (0, 1) | 162 (83.51) | 1348 (6.95) | 4 (2, 10) |
| 65+ (n=32) | 30 (93.75) | 359 (11.22) | 9 (4.5,14) | 26 (81.25) | 141 (4.41) | 4 (1.5, 6) | 6 (18.75) | 15 (0.47) | 0 (0, 0) | 27 (84.38) | 203 (6.34) | 4.5 (1, 8) |
| All (n=2045) | 1907 (93.25) | 17870 (8.74) | 7 (3, 12) | 1533 (74.96) | 6251 (3.06) | 2 (0, 4) | 892 (43.62) | 2792 (1.37) | 0 (0, 2) | 1540 (75.31) | 8827 (4.32) | 2 (1, 6) |
| ED Reliance Score | Co-management | |||||||||||
| Age | % of sample with EDR ≥ 0.33 | Mean (sd) | n (Row %) | |||||||||
| 1-9 (n=499) | 21.84 | 0.19 (0.21) | 224 (44.88) | |||||||||
| 10-17 (n=436) | 23.17 | 0.19 (0.24) | 171 (39.22) | |||||||||
| 18-30 (n=537) | 44.69 | 0.35 (0.31) | 153 (28.49) | |||||||||
| 31-45 (n=347) | 34.01 | 0.27 (0.28) | 105 (30.26) | |||||||||
| 46-64 (n=194) | 24.23 | 0.20 (0.25) | 53 (27.32) | |||||||||
| 65+ (n=32) | 12.50 | 0.14 (0.20) | 6 (18.75) | |||||||||
| All (n=2045) | 30.27 | 0.25 (0.27) | 712 (34.82) | |||||||||
PCP= Primary Care Physcian, ED= Emergency Department, EDR= Emergency Department Reliance
ED and hospital utilization
Of the 6,834 total ED encounters, 70.95% of the total sample had an ED encounter during the 12-month study period. There was a mean of 3.34 (SD=7.51) and median of 1 (IQR = 0 - 4) ED encounters per patient for the sample. Those who were 18-30 years old had the highest mean and median ED encounters per patient (5.22, SD= 9.38 and 2, IQR 1 to 6). The 31-45 year old group had the second most, with 5.20 (SD= 12.15) total ED encounters. The percentage of the sample with an ED re-encounter within 7, 14, and 30 days was also highest among the 18-30 year old group (30.91%, 35.20% and 42.64%) followed by those 31-45 years old (20.49%, 30.36%, and 36.02%), respectively. The mean EDR was highest among 18-30 year old patients (0.35) and 44.69% of this age group had an EDR of 0.33 or greater. In the 31-45 year-old age group, the mean EDR was 0.27 and 34.01% had an EDR of 0.33 or greater. The overall sample had a mean of 1.32 (SD= 2.78) hospitalizations/patient. The 18-30 year old age group also had the highest mean total hospitalizations (2.26, SD= 3.91) and mean re-hospitalizations within 7 (0.16; SD=0.78), 14 (0.44; SD=1.78), and 30 (0.89; SD=2.97) days. The 31-45 age group had the second most hospitalizations/patient and re-hospitalizations (Table 3).
Outpatient visits and co-management
The 46-64 years old and the oldest (65+) of the sample had the highest median number of total outpatient visits (9 each), as well as non-hematology specialty visits (4 and 4.5), respectively. The youngest (1-9 years old) and the oldest (65+) had the highest median number of PCP visits (3 and 4, respectively). Participants that were age 1-9 years had the highest median number of hematology visits (1). Overall, there were a large total number of non-hematology specialist visits 8,827 (Table 2). Outpatient acute care visits, PA and NP visits accounted for 60% of the total number of non-hematology specialist visits. Only 34.82%, however, of the study sample met our definition of being co-managed. Co-management was higher in the 1-9 (44.88%) and 10-17 year-old groups (39.22%) versus the 31-45 (30.26%) and 65+ (18.75%) age groups, see table 3. Age had the greatest influence (AUC=0.599) on whether or not a patient was co-managed, whereas, gender, months enrolled in CCNC and residence had very little predictive influence on co-management (figure 1).
Figure 1:
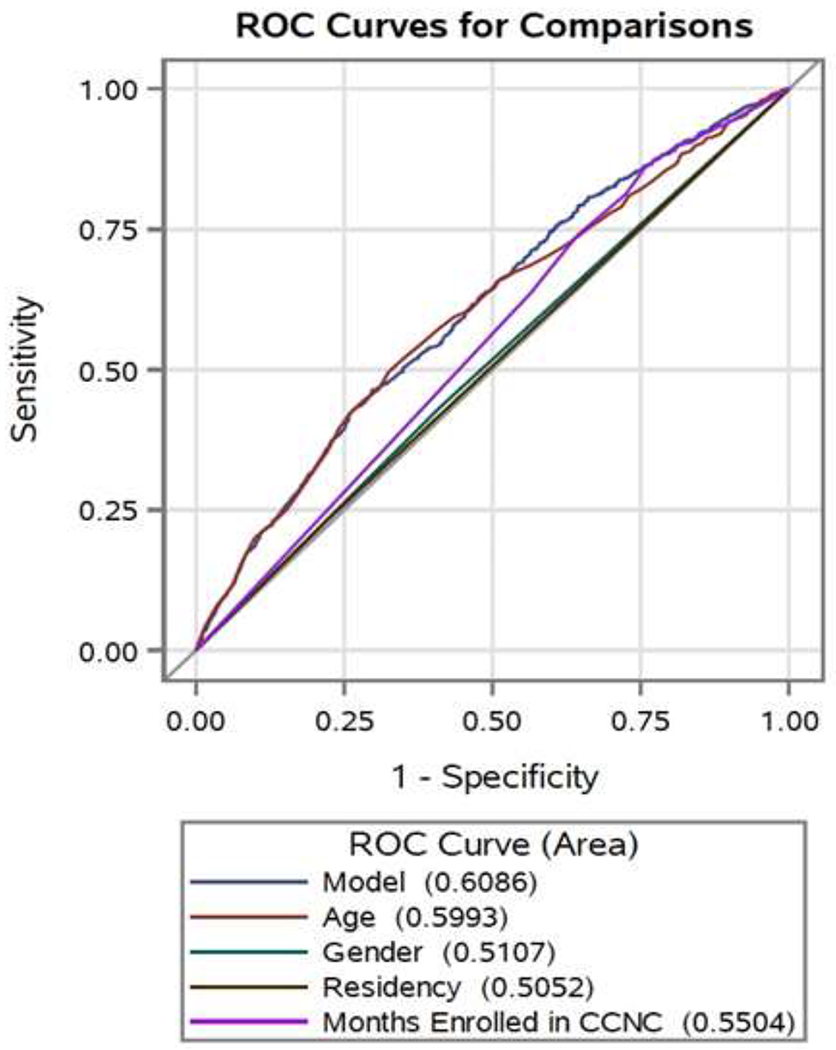
Area Under the Curve Receiver Operating Characteristics (AUC-ROC) curve for co-management
Performance of predictor measures and a combined model including age, gender, residency and months enrolled in Community Care of North Carolina (CCNC) for co-management with area under the curve values for each.
Hydroxyurea utilization
A third of the sample (32.24%) had at least 1 HU prescription during the study period (Table 4). Those who were 1-9 years old had the highest median number of days supplied (221; range 21-750), the least median days between breaks in HU treatment (14.21; range 0-318), and the longest duration of HU treatment days (median 340; range 0-364). Those who were 18-30 years old had the lowest number of median days supplied (110; range 4-366) and the most median days between treatment (49.3; range 0-337). The 1-9 year olds also had the highest number of patients classified as good HU adherence (47.50%) and conversely the lowest classified as poor HU adherence (37.50%). In contrast, the 18-30 year old age group had the lowest good HU adherence (18.03%) and the highest poor HU adherence (69.40%) in the sample. The 31-45 year old age groups had the next lowest good HU adherence (20.93%) and next highest poor HU adherence (60.47%). Age was the strongest predictive factor of good HU adherence (AUC 0.6503). Prediction by co-management was minimal with an AUC=0.5615, but it was greater than the prediction of primary care only (AUC=0.5481) or hematology care only (AUC=0.5481) (Figure 2). Gender, residency and number of months enrolled in CCNC had little influence on HU adherence.
Table 4 -.
Hydroxyurea (HU) Prescription (Rx) Fills and Adherence for North Carolina Medicaid Enrolled at least 12-months Age 1-64 (N=2013)*
| Medicaid Enrolled for 12- months by Age Group | ||||||
|---|---|---|---|---|---|---|
| 1-9 (n=499) | 10-17 (n=436) | 18-30 (n=537) | 31-45 (n=347) | 46-64 (n=194) | All (n=2013) | |
| Participants with a HU Rx Filled | ||||||
| N (% of eligible sample) | 200 (40.08) | 201 (46.10) | 183 (34.08) | 43 (12.39) | 22 (11.34) | 649 (32.24) |
| Description of usage for participants with at least one HU Rx Enrolled in Medicaid for 12-months | ||||||
| 1-9 (n=200) | 10-17 (n=201) | 18-30 (n=183) | 31-45 (n=43) | 46-64 (n=22) | All (n=649) | |
| Number of HU Rx Filled | ||||||
| Median (IQR) | 7 (4, 10) | 6 (4, 8) | 4 (1, 7) | 4 (2, 7) | 5.5 (2, 10) | 5 (2, 8) |
| Number of Days Supplied** | ||||||
| Median (IQR) | 221 (104.5, 319) | 180 (105, 270) | 110 (30, 210) | 120 (58, 210) | 165 (90, 300) | 159 (85, 270) |
| Duration of HU Treatment (days)*** | ||||||
| Median (IQR) | 340 (301, 349) | 334 (285, 350) | 322 (266, 345) | 336 (305, 347) | 334 (287, 357) | 334 (284, 349) |
| Number of Days between breaks in treatment+ | ||||||
| Median (IQR) | 14.21 (0, 50.75) | 22.8 (7.75, 51.50) | 49.33 (19.67, 139) | 40.40 (21.60, 103.50) | 35.45 (0, 89.67) | 29.6 (7, 72.5) |
| HU Adherence++, n (%) | ||||||
| Good | 95 (47.50) | 60 (29.85) | 33 (18.03) | 9 (20.93) | 8 (36.36) | 205 (31.59) |
| Fair | 30 (15.00) | 48 (23.88) | 23 (12.57) | 8 (18.60) | 2 (9.09) | 111 (17.10) |
| Poor | 75 (37.50) | 93 (46.27) | 127 (69.40) | 26 (60.47) | 12 (54.55) | 333 (51.31) |
There were zero participants on HU in the 65+ age group so they were excluded from this analysis.
Number of days supplied is the sum of the days of supply on the Rx (e.g. 30 day supply) in a 12-month period per person
Duration of HU treatment days is the number of days between the first HU Rx filled and February 28, 2017
Number of days between breaks in treatment is the sum of days of no HU coverage divided by the number of gaps (missing next HU Rx fill) per person
HU adherence is considered Good- if number of days supplied is ≥80% of duration of HU treatment ; Fair or Moderate- if number of days supplied is 60-79% of duration of HU treatment; Poor- if number of days supplied is > 60% of duration of HU treatment
Figure 2:
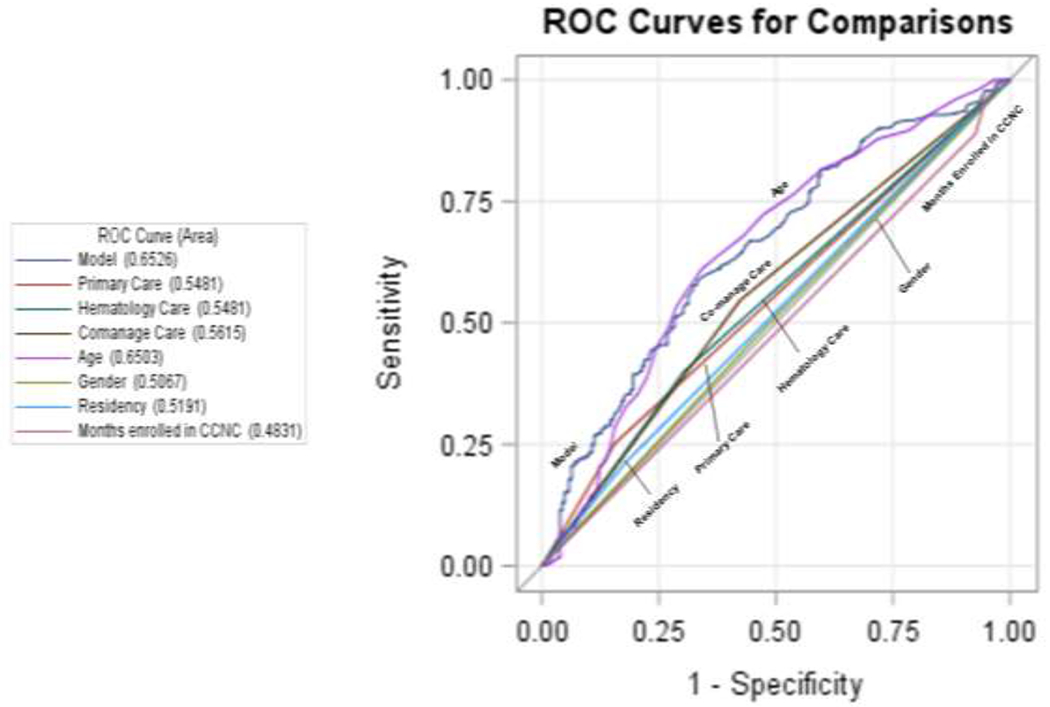
Area Under the Curve Receiver Operating Characteristics curve for Hydroxyurea adherence (Good vs Fair or Poor).
Performance of predictor measures and a combined model including Primary Care visit alone, Hematology visit alone, Co-management, age, gender, residency and months enrolled in Community Care of North Carolina (CCNC) for good versus fair or poor hydroxyurea adherence with area under the curve values for each.
DISCUSSION
Our study revealed high health service utilization and low rates of HU adherence, which are consistent with prior studies of SCD healthcare utilization.16,25,36–39 We also found, however, a strikingly low rate of co-management between the PCP and SCD specialist, simply defined as one visit to both a PCP and hematologist. Furthermore, we noted high utilization of additional services by non-hematologist specialist and significant variations in HU adherence by age group.
Emergency Department Encounters and Hospitalization
Prior national assessments of ED encounters in one year were estimated to be 232,381 and primarily by adult patients (81% by adults, 19% by those <18).40 Similarly, our sample revealed 18-30 and 31-45 year-old age groups had the highest rate of ED encounters, with close to three quarters of those age groups having at least one ED encounter. This rate was lower than prior rates of up to 90% reported by Brousseau et al. 25 In the <18 year-old group, our sample had a lower ED encounter rate (65.88%) compared to the national estimate of 67%. 40 Additionally, previous studies have highlighted the highest EDR among the patients transitioning from pediatrics to adult medical care with an associated high healthcare cost. 41 Our sample also highlighted poor transition to adult medical care with a significant percentage of 18-30 year old patients (44.69% of the sample) having greater than 0.33 EDR, which indicates a high reliance on the ED. 17
Patients with SCD are frequently admitted to the hospital for further pain control after initially being seen in the ED, supported by our findings that almost half of our sample were hospitalized during the study period. Furthermore, SCD has a higher re-hospitalization rate than other chronic diseases such as heart failure and diabetes and re-hospitalization is a measure of quality of care related to hospitalization 42. A significant contributor to health care costs in the chronically ill is 30-day re-hospitalization rates, which may reflect the lack of access and quality of ambulatory care for patients with SCD.25,38,43,44 We found 7, 14 and 30 day re-hospitalization rates were lower than prior findings (5% to 16%). 25,42 These lower rates may be due to state based efforts by CCNC to reduce hospital admissions and re-admissions, variations in access to care and insurance benefits by state and previous studies performing analysis on data over a decade ago. Risk factors for re-hospitalizations such as age, (particularly the age at transition from pediatric to adult care) and having public health insurance have been previously identified. 45 Our study supports prior findings indicating a spike in utilization that coincides with the age of transition from pediatric to adult SCD care and high utilization in a sample of NC Medicaid enrollees. Moreover, it indicates a continued need to develop interventions for SCD patients and providers that will improve care throughout the care transition period in this population. Finally, a lack of follow-up care with the PCP or hematologist has also been consistently reported as an important factor associated with re-hospitalizations and return to the ED within 30 days of discharge.36,38,42,45,46
Outpatient Visits
In addition to evaluating PCP visits and hematology visits, our study included data on the number of outpatient visits with various specialists. We found a large number of non-hematology specialty visits, many by PAs and NPs, and age related differences in the types of outpatient services utilized. To the best of our knowledge, we are the first group to report this level of complex outpatient care and the use of multiple specialty services by SCD patients. This important finding has implications for care coordination. Use of patient navigators may be one intervention to assist patients with the high number of specialists and associated appointments required to manage SCD.47 Two projects funded by PCORI are currently evaluating the use of community health workers and peer mentoring to improve the transition period for emergency adults with SCD.48,49 These projects hope to generate evidence that will support the routine use of some type of support systems necessary to navigate the complex healthcare needs for individuals with SCD.
There was little difference between the percentages of enrollees that had a PCP visit and a non-hematology specialist, although the total and mean number non-hematology specialist visits exceeded the total and mean number of PCP visits. This demonstrates the wide variety and high frequency of specialty care requirements for this population. The 1- 17 year old sample age groups had similar total out-patient visit rates to the 18 > age groups. Prior studies have reported a higher number of mean out-patient visits (12.6 vs. 7.9 in our sample) for Medicaid enrolled children.50 However, there was a downward trend in the rate of hematology specialists visits in older SCD age groups. This trend was reversed in the non-hematology specialist visits, which were lowest in the youngest age group and highest in the oldest age group. Studies of SCD patients have previously shown that the absence of a PCP and missing or forgetting outpatient visits were associated with both hospitalizations and re-hospitalizations of SCD patients. 36,38 The effect of non-hematologist specialty visits on ED or inpatient health service utilization, in contrast, has not been reported. Information on the type of services provided by non-hematology specialists and the mechanisms (e.g. referral processes) used by patients to access these services are needed. Service delivery may be influenced by restrictions in advanced practice provider practice, which vary by state. Patterns of outpatient specialist utilization are needed. This includes non-hematology specialists visits, prior to ED and hospitalizations for common complaints such as pain, as this is the most common reason for SCD patient hospitalization, is complex and difficult to manage.36,37,51,52 These findings support the need for alternative care models. However, models such as the patient- centered medical home (PCMH) care model have been difficult to implement and for patients to access.7,53 A recent national survey of 1060 family practice physicians, found that only 20% reported being comfortable with overall management of SCD.13 However, 80% of respondents indicated they would be willing to co-manage pediatric patients, and 68% reported they would be willing to co-manage adult patients.13
Co-Management
It has been previously identified that there is a need to improve co-management between specialty and PCPs for chronic diseases. In a large survey of 702 primary care practices, only 27% of primary care patients with chronic diseases were co-managed, despite evidence that co-management can improve care of chronically ill patients.54 For example, co-management by nephrologists and PCPs has been found to improve the PCP’s ability to identify chronic kidney disease, referral to nephrologists, execution of co-management plans and improved monitoring of anemia and metabolic bone disease.55,56 Co-management between rheumatology and primary care has also been suggested to improve management of patients with rheumatoid arthritis and cardiovascular disease.57 Our sample rate of co-management was 34.82%, but varied by age group. Co-management was lower in the 18 and older age groups and age also contributed the most to our co-management prediction model. This finding, along with the high number of non-hematology specialist visits in adults suggest a need to promote co-management of SCD patients to a wider group of providers including PAs, NPs and other specialists such as obstetrician/gynecologists that have not been considered part of usual care for SCD patients. Similar to other chronic diseases, a co-management model using a combination of a PCP and a hematology specialist to manage the care of SCD patients could potentially improve SCD quality of care by reducing disease complications and high acute care utilization. For example, co-management models of care could specifically focus on increasing the rate of prescription and use of HU, which is often under prescribed and the mainstay of therapy for SCD.
Hydroxyurea Prescription Fills and Adherence
HU has been shown to reduce the frequency of pain episodes, acute chest syndrome, need for red blood cell transfusions, hospitalizations and mortality.10,58 Reported HU adherence rates vary widely depending on the assessment method used and the sample size.59–62 Although there is no perfect measurement of adherence, HU pharmacy refills have been used to identify SCD patients at risk for poor response due to non-adherence. 62 Using Medicaid HU prescription claims, we found only a third of patients were classified as having good HU adherence, which was similar to the rate reported in a prior study (35%) of NC Medicaid enrollees, using prescription claims data to obtain medication possession ratio as a measure of adherence. 20 Factors leading to poor adherence include patient negative perceptions about benefits of HU, adverse symptoms and poor emotional response to SCD. 58,63,64 Age has been shown to have a significant effect on HU adherence, with older age being associated with poorer adherence. 59 We found a similar trend, with increasing age and decreasing number of participants with a filled HU prescription, the number of HU prescriptions filled, number of days supplied and adherence. HU adherence in the 65+ age group was excluded from our analysis because they had no HU prescription fills. Age was more highly associated with good versus fair or poor HU adherence than the proposed statistical model. However, co-management was associated with HU adherence, despite low overall rates of co-management in our sample. This finding warrants further analysis of the relationship of co-management to HU adherence, which may in turn influence ED and inpatient utilization.
Study Limitations
There were several limitations of our study. Our study only included Medicaid enrollees with SCD, a group that has been found to have higher rates of health service utilization in several other studies.25,37,40,65–67 Only Medicaid claims from 2016 to 2017, after the initiation of the Patient Protection and Affordable Care Act (ACA) were used in our study. Although NC did not expand Medicaid coverage, studies on ED and outpatient health service utilization after the ACA went into effect have varied greatly between states. 68–70 Variations in utilization may also result from between state differences in the number of new Medicaid enrollees, coverage limits and the number of years since expansion occurred. Future comparisons of health service utilization that considers these payer differences (Medicaid expanded and not expanded) by state would provide a more robust understanding of utilization in the SCD population.
Differences in the number of SCD related ED visits have been noted depending on the volume and proximity of the ED to metropolitan areas.40 Our data did not include the location of the ED where the encounter occurred, so this level of analysis was excluded from our study. Additionally, the study observation period was shorter, (12 months) in our study compared to other studies that have included more than two years of utilization data.25,40,65,71 The episodic nature of SCD, including intermittent periods of very high service utilization followed by periods of lower service utilization may have led to over or under representation of data during our 12-month study period. We were also unable to classify claims with no rendering provider information. These claims were categorized into an unidentifiable (null) category, but likely caused an underrepresentation of another non-hematology specialist category. We were also unable to determine the practice type of PA’s and NP’s, specifically whether they were SCD specialists or primary care. Additionally, we were unable to review medical records to describe the type of co-management that occurred between the specialist and PCP, the type of care provided by the PCP’s, or “who” prescribed HU. This paper reports our baseline data, prior to dissemination of the NHLBI evidence based guidelines and we cannot yet comment on how they will affect practice.
CONCLUSION
In our study, age was the most important factor in predicting co-management and HU adherence and there were notable age related differences in healthcare utilization. As in prior studies, the age at which ED and inpatient use increases, coincides with the period of transition from pediatric to adult care.16,27,72 Increased utilization from acute care services has been described in several studies of pediatric to adult care transition.11,22 Our study further supports the need for increased focus on acute care utilization in the 18-45 year-old age group and considerations for improved care transition interventions. 73 Interventions that improve HU adherence are of particular importance in reducing acute care utilization. Importantly, we found co-management is associated with HU adherence. Further studies of the influence of co-management on known drivers of high SCD acute care utilization such as acute pain episodes are needed, as well as studies that identify the frequency and components of co-management needed to increase adherence and reduce acute care utilization. While we were able to report preliminary data on outpatient utilization including utilization of non-hematology specialist, we did not report specifics such as procedures or the chief complaint associated with these visit (e.g. pain related) or include non-hematology specialist utilization in our co-management or HU adherence models. Future research that considers non-hematology specialist in understanding of SCD health service utilization is also needed.
Acknowledgments
Funding Statement: Supported by National Heart, Lung and Blood Institute (R18 RHS024501A)
Footnotes
Conflicting and Competing Interests
The authors do not have any conflicting or competing interests to report
Contributor Information
Nancy Crego, Duke University School of Nursing.
Christian Douglas, Duke University School of Nursing.
Emily Bonnabeau, Duke University School of Nursing.
Marian Earls, Community Care of North Carolina.
Kern Eason, Community Care of North Carolina.
Elizabeth Merwin, University of Texas College of Nursing.
Gary Rains, Duke University School of Medicine.
Paula Tanabe, Duke University School of Nursing.
Nirmish Shah, Duke University Medical Center.
References
- 1.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4 Suppl):S512–521. [DOI] [PubMed] [Google Scholar]
- 2.Lanzkron S, Carroll CP, Haywood C Jr. The burden of emergency department use for sickle-cell disease: an analysis of the national emergency department sample database. Am J Hematol. 2010;85(10):797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elixhauser A, Steiner CA. Readmissions to U.S. Hospitals by Diagnosis, 2010. Agency for Healthcare Research and Quality; August 2013 2013. Statistical Brief #153. [PubMed] [Google Scholar]
- 4.Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303(13):1288–1294. [DOI] [PubMed] [Google Scholar]
- 5.Tanabe P, Artz N, Mark Courtney D, et al. Adult emergency department patients with sickle cell pain crisis: a learning collaborative model to improve analgesic management. Acad Emerg Med. 2010;17(4):399–407. [DOI] [PubMed] [Google Scholar]
- 6.Tanabe P, Hafner JW, Martinovich Z, Artz N. Adult emergency department patients with sickle cell pain crisis: results from a quality improvement learning collaborative model to improve analgesic management. Acad Emerg Med. 2012;19(4):430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raphael JL, Oyeku SO. Sickle cell disease pain management and the medical home. Hematology American Society of Hematology Education Program. 2013;2013:433–438. [DOI] [PubMed] [Google Scholar]
- 8.Aljuburi G, Phekoo KJ, Okoye NO, et al. Patients’ views on improving sickle cell disease management in primary care: focus group discussion. JRSM Short Rep. 2012;3(12):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lunyera J, Jonassaint C, Jonassaint J, Shah N. Attitudes of Primary Care Physicians Toward Sickle Cell Disease Care, Guidelines, and Comanaging Hydroxyurea With a Specialist. J Prim Care Community Health. 2017;8(1):37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. Jama. 2014;312(10):1033–1048. [DOI] [PubMed] [Google Scholar]
- 11.Adams-Graves P, Bronte-Jordan L. Recent treatment guidelines for managing adult patients with sickle cell disease: challenges in access to care, social issues, and adherence. Expert Rev Hematol. 2016;9(6):541–552. [DOI] [PubMed] [Google Scholar]
- 12.Whiteman LN, Haywood C Jr., Lanzkron S, Strouse JJ, Feldman L, Stewart RW. Primary Care Providers’ Comfort Levels in Caring for Patients with Sickle Cell Disease. South Med J. 2015;108(9):531–536. [DOI] [PubMed] [Google Scholar]
- 13.Mainous AG III, Tanner RJ, Harle CA, Baker R, Shokar NK, Hulihan MM. Attitudes toward management of sickle cell disease and its complications: a national survey of academic family physicians. Anemia. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tilson EC. Dissemination and Adoption of Guidelines: The Experience of Community Care of North Carolina. North Carolina Medical Journal. 2015;76(4):251–255. [DOI] [PubMed] [Google Scholar]
- 15.North Carolina Institute of Medicine. Understanding Medicaid in North Carolina Primer. 2018; http://nciom.org/wp-content/uploads/2018/01/NC_Medicaid_CHIP_Primer.pdf. Accessed September 27, 2018.
- 16.Blinder MA, Duh MS, Sasane M, Trahey A, Paley C, Vekeman F. Age-Related Emergency Department Reliance in Patients with Sickle Cell Disease. J Emerg Med. 2015;49(4):513–522 e511. [DOI] [PubMed] [Google Scholar]
- 17.Kroner EL, Hoffmann RG, Brousseau DC. Emergency department reliance: a discriminatory measure of frequent emergency department users. Pediatrics. 2010;125(1):133–138. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Medicare and Medicaid Services. Crosswalk Medicare Provider/Supplier to Healthcare Provider Taxonomy. 2018; https://data.cms.gov/Medicare-Enrollment/CROSSWALK-MEDICARE-PROVIDER-SUPPLIER-to-HEALTHCARE/j75i-rw8y, 2018 .
- 19.Walsh KE, Cutrona SL, Kavanagh PL, et al. Medication adherence among pediatric patients with sickle cell disease: a systematic review. Pediatrics. 2014;134(6):1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Candrilli SD, O’Brien SH, Ware RE, Nahata MC, Seiber EE, Balkrishnan R. Hydroxyurea adherence and associated outcomes among Medicaid enrollees with sickle cell disease. American Journal of Hematology. 2011;86(3):273–277. [DOI] [PubMed] [Google Scholar]
- 21.Andemariam B, Owarish-Gross J, Grady J, Boruchov D, Thrall RS, Hagstrom JN. Identification of risk factors for an unsuccessful transition from pediatric to adult sickle cell disease care. Pediatr Blood Cancer. 2014;61(4):697–701. [DOI] [PubMed] [Google Scholar]
- 22.Kayle M, Docherty SL, Sloane R, et al. Transition to adult care in sickle cell disease: A longitudinal study of clinical characteristics and disease severity. Pediatr Blood Cancer. 2019;66(1):e27463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClish DK, Smith WR, Levenson JL, et al. Comorbidity, Pain, Utilization, and Psychosocial Outcomes in Older versus Younger Sickle Cell Adults: The PiSCES Project. Biomed Res Int. 2017;2017:4070547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115(17):3447–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute Care Utilization and Rehospitalizations for Sickle Cell Disease. Journal of the American Medical Association. 2010;303(13):1288–1294. [DOI] [PubMed] [Google Scholar]
- 26.Brousseau DC, Panepinto JA, Nimmer M, Hoffmann RG. The number of people with sickle cell disease in the United States: National and state estimates. American Journal of Hematology. 2009;85(1):77–78. [DOI] [PubMed] [Google Scholar]
- 27.Steiner CA, Miller JL. Sickle Cell Disease Patients in U.S. Hospitals, 2004. HCUP Statistical Brief #21 2006; http://www.hcup-us.ahrq.gov/reports/statbriefs/sb21.pdf. Accessed December, 2018. [PubMed]
- 28.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL, 3rd. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartley EJ, Fillingim RB. Sex Differences in Pain: a brief review of clinical and experimental findings. British Journal of Anaeshesia. 2013;111(1):52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorge RE, Strath LJ. Sex Differences in Pain Responses. Current Opinion in Physiology. 2018;6:75–81. [Google Scholar]
- 31.McClish DK, Levenson JL, Penberthy LT, et al. Gender Differences in Pain and Healthcare Utilization for Adult Sckle Cell Patients: The PiSCES Project. Journal of Women’s Health. 2006;15(2):146–154. [DOI] [PubMed] [Google Scholar]
- 32.Udezue E, Girshab AM. Differences between males and females in adult sickle cell pain crisis in eastern Saudi Arabia. Ann Saudi Med. 2004;24(3):179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haque A, Telfair J. Socioeconomic Distress and Health Status: The Urban-rural Dichotomy of Services Utilization for People with Sickle Cell Disorder in North Carolina. Journal of Rurual Health. 2000;16(1):43–55. [DOI] [PubMed] [Google Scholar]
- 34.Asnani MR, Knight Madden J, Reid M, Greene LG, Lyew-Ayee P. Socio-environmental exposures and health outcomes among persons with sickle cell disease. PLoS One. 2017;12(4):e0175260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.United States Department of Agriculture. Metropolitan Statistical Areas Metropolitan Divisions Micropolitan Statistical Areas and Combined Statistical Areas and Guidance on Uses of the Delineations of These Areas. 2013; https://obamawhitehouse.archives.gov/sites/default/files/omb/bulletins/2013/b-13-01.pdf. Accessed September 26, 2018.
- 36.Cronin RM, Hankins JS, Byrd J, et al. Risk factors for hospitalizations and readmissions among individuals with sickle cell disease: results of a U.S. survey study. Hematology. 2019;24(1):189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benenson I, Jadotte Y, Echevarria M. Factors influencing utilization of hospital services by adult sickle cell disease patients: a systematic review. JBI Database System Rev Implement Rep. 2017;15(3):765–808. [DOI] [PubMed] [Google Scholar]
- 38.Brodsky MA, Rodeghier M, Sanger M, et al. Risk Factors for 30-Day Readmission in Adults with Sickle Cell Disease. Am J Med. 2017;130(5):601 e609–601 e615. [DOI] [PubMed] [Google Scholar]
- 39.Lanzkron S, Little J, Field J, et al. Increased acute care utilization in a prospective cohort of adults with sickle cell disease. Blood Advances. 2018;2:2412–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanzkron S, Carroll CP, Haywood C, Jr. The burden of emergency department use for sickle-cell disease: an analysis of the national emergency department sample database. Am J Hematol. 2010;85(10):797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blinder MA, Vekeman F, Sasane M, et al. Age-Related Emergency Department Reliance and Healthcare Resource Utilization in Patients with Sickle Cell Disease. Blood. 2012;120(475). [Google Scholar]
- 42.Leschke J, Panepinto JA, Nimmer M, Hoffmann RG, Yan K, Brousseau DC. Outpatient follow-up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58(3):406–409. [DOI] [PubMed] [Google Scholar]
- 43.Hemker BG, Brousseau DC, Yan K, Hoffman RG, Panepinto JA. When Children with Sickle-Cell Disease become adults: Lack of outpatient care Leads to Increased use of the Emergency Department. American Journal of Hematology. 2011;86(10):863–865. [DOI] [PubMed] [Google Scholar]
- 44.Frei-Jones MJ, Field JJ, DeBaun MR. Risk factors for hospital readmission within 30 days: a new quality measure for children with sickle cell disease. Pediatr Blood Cancer. 2009;52(4):481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol. 2005;79(1):17–25. [DOI] [PubMed] [Google Scholar]
- 46.Field TS, Ogarek J, Garber L, Reed G, Gurwitz JH. Association of early post-discharge follow-up by a primary care physician and 30-day rehospitalization among older adults. J Gen Intern Med. 2015;30(5):565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rollins M, Milone F, Suleman S, Vojvoda D, Sgro M, Barozzino T. Patient Navigators: Mapping the route toward accessibility in health care. Paediatrics & Child Health. 2019;24(1):19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rubin DM. Community Health Worker and Mobile Health Programs to Help Young Adults with Sickle Cell Disease Transition to Using Adult Healthcare Services: The COMETS STudy. 2017; https://www.pcori.org/research-results/2017/community-health-worker-and-mobile-health-programs-help-young-adults-sickle. Accessed March 23, 2019.
- 49.Osunkwo I Comparitive Effectiveness of Peer Mentoring versus Structured Education-Based Transition Programming for the Management of Care Transitions in Emerging Adults with Sickle Cell Disease. 2017; https://www.pcori.org/research-results/2017/comparative-effectiveness-peer-mentoring-versus-structured-education-based. Accessed March 23, 2019.
- 50.Mvundura M, Amendah D, Kavanagh PL, Sprinz PG, Grosse SD. Health care utilization and expenditures for privately and publicly insured children with sickle cell disease in the United States. Pediatr Blood Cancer. 2009;53(4):642–646. [DOI] [PubMed] [Google Scholar]
- 51.Cronin RM, Hankins JS, Byrd J, et al. Modifying factors of the health belief model associated with missed clinic appointments among individuals with sickle cell disease. Hematology. 2018;23(9):683–691. [DOI] [PubMed] [Google Scholar]
- 52.Carroll CP, Cichowitz C, Yu T, et al. Predictors of acute care utilization and acute pain treatment outcomes in adults with sickle cell disease: The role of non-hematologic characteristics and baseline chronic opioid dose. Am J Hematol. 2018;93(9):1127–1135. [DOI] [PubMed] [Google Scholar]
- 53.Raphael JL, Rattler TL, Kowalkowski MA, Mueller BU, Giordano TP. The medical home experience among children with sickle cell disease. Pediatr Blood Cancer. 2013;60(2):275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larochelle JL, Feldman DE, Levesque JF. The primary-specialty care interface in chronic diseases: patient and practice characteristics associated with co-management. Healthcare policy = Politiques de sante. 2014;10(2):52–63. [PMC free article] [PubMed] [Google Scholar]
- 55.Samal L, Wright A, Waikar SS, Linder JA. Nephrology co-management versus primary care solo management for early chronic kidney disease: a retrospective cross-sectional analysis. BMC Nephrol. 2015;16:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haley WE, Beckrich AL, Sayre J, et al. Improving care coordination between nephrology and primary care: a quality improvement initiative using the renal physicians association toolkit. Am J Kidney Dis. 2015;65(1):67–79. [DOI] [PubMed] [Google Scholar]
- 57.Bartels CM, Roberts TJ, Hansen KE, et al. Rheumatologist and Primary Care Management of Cardiovascular Disease Risk in Rheumatoid Arthritis: Patient and Provider Perspectives. Arthritis Care Res (Hoboken). 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3(3):255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loiselle K, Lee JL, Szulczewski L, Drake S, Crosby LE, Pai AL. Systematic and Meta-Analytic Review: Medication Adherence Among Pediatric Patients With Sickle Cell Disease. J Pediatr Psychol. 2016;41(4):406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patel NG, Lindsey T, Strunk RC, DeBaun MR. Prevalence of daily medication adherence among children with sickle cell disease: a 1-year retrospective cohort analysis. Pediatr Blood Cancer. 2010;55(3):554–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Hematol Oncol Clin North Am. 2010;24(1):199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thornburg CD, Calatroni A, Telen M, Kemper AR. Adherence to Hydroxyurea Therapy in Children with Sickle Cell Anemia. The Journal of Pediatrics. 2010;156:415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Badawy SM, Thompson AA, Lai JS, Penedo FJ, Rychlik K, Liem RI. Adherence to hydroxyurea, health-related quality of life domains, and patients’ perceptions of sickle cell disease and hydroxyurea: a cross-sectional study in adolescents and young adults. Health Qual Life Outcomes. 2017;15(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haywood C, Beach MC, Bediako S, et al. Examining the characteristics and beliefs of hydroxyurea users and nonusers among adults with sickle cell disease. American Journal of Hematology. 2011;86(1):85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carroll CP, Haywood C, Jr., Fagan P, Lanzkron S. The course and correlates of high hospital utilization in sickle cell disease: Evidence from a large, urban Medicaid managed care organization. Am J Hematol. 2009;84(10):666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mayer ML, Konrad TR, Dvorak CC. Hospital Resource Utilization Among Patients with Sickle Cell Disease. Journal of Health Care for the Poor and Underserved. 2003;14(1):122–135. [PubMed] [Google Scholar]
- 67.Yusuf HR, Atrash HK, Grosse SD, Parker CS, Grant AM. Emergency department visits made by patients with sickle cell disease: a descriptive study, 1999–2007. Am J Prev Med. 2010;38(4 Suppl):S536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gingold DB, Pierre-Mathieu R, Cole B, Miller AC, Khaldun JS. Impact of the Affordable Care Act Medicaid expansion on emergency department high utilizers with ambulatory care sensitive conditions: A cross-sectional study. Am J Emerg Med. 2017;35(5):737–742. [DOI] [PubMed] [Google Scholar]
- 69.Nikpay S, Freedman S, Levy H, Buchmueller T. Effect of the Affordable Care Act Medicaid Expansion on Emergency Department Visits: Evidence From State-Level Emergency Department Databases. Ann Emerg Med. 2017;70(2):215–225 e216. [DOI] [PubMed] [Google Scholar]
- 70.Sommers BD, Blendon RJ, Orav EJ, Epstein AM. Changes in Utilization and Health Among Low-Income Adults After Medicaid Expansion or Expanded Private Insurance. JAMA Intern Med. 2016;176(10):1501–1509. [DOI] [PubMed] [Google Scholar]
- 71.Ter-Minassian M, Lanzkron S, Derus A, Brown E, Horberg MA. Quality Metrics and Health Care Utilization for Adult Patients with Sickle Cell Disease. J Natl Med Assoc. 2018. [DOI] [PubMed] [Google Scholar]
- 72.Jordan L, Swerdlow P, Coates TD. Systematic Review of Transition from Adolescent to Adult Care in Patients with Sickle Cell Disease. Journal of Pediatric Hematology and Oncology. 2013;35(3):165–169. [DOI] [PubMed] [Google Scholar]
- 73.Minniti CP, Vichinsky E. Lifespan care in SCD: Whom to transition, the patients or the health care system? Am J Hematol. 2017;92(6):487–489. [DOI] [PubMed] [Google Scholar]


