Abstract
In persistent high-risk HPV infection, viral gene expression can trigger some important early changes to immune capabilities which act to protect the lesion from immune attack and subsequently promote its growth and ability for sustained immune escape. This includes immune checkpoint-inhibitor ligand expression (e.g. PD-L1) by tumour or associated immune cells that can block any anti-tumour T-cell effectors. While there are encouraging signs of efficacy for cancer immunotherapies including with immune checkpoint inhibitors, therapeutic vaccines and adoptive cell therapies, overall response and survival rates remain relatively low. HPV oncogene vaccination has shown some useful efficacy in treatment of patients with high-grade lesions but was unable to control later stage cancers. To maximally exploit anti-tumour immune responses, the suppressive factors associated with HPV carcinogenesis must be countered. Importantly, a combination of chemotherapy, reducing immunosuppressive myeloid cells, with therapeutic HPV vaccination significantly improves impact on cancer treatment. Many clinical trials are investigating checkpoint inhibitor treatments in HPV associated cancers but response rates are limited; combination with vaccination is being tested. Further investigation of how chemo- and/or radio-therapy can influence the recovery of effective anti-tumour immunity is warranted. Understanding how to optimally deploy and sequence conventional and immunotherapies is the challenge.
Keywords: HPV therapeutic Vaccines, Immune checkpoint inhibitors, Myeloid derived suppressor cells, Exhausted T cells, HPV oncogenes, Adoptive cell therapy
1. Introduction
For most virus infections the natural immune response provides for initial control, then elimination plus protection against future attack through immune memory [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10]]. There is now widespread recognition of the importance of immune factors in the control [11,12] and/or the promotion of tumour development and growth [13,14]. This has been exemplified by the successful impact of immune checkpoint inhibitors in the treatment of some types of cancer [15]. However, the plethora of immune-suppressive cells that can accumulate in the tumour microenvironment (TME), including both specific- and nonspecific-induced Tregs, M2 macrophages, myeloid-derived suppressor cells (MDSC) not withstanding tumour, and their associated fibroblasts, negative contributions through checkpoint expression and/or other inhibitory mechanisms, present significant barriers to effective immune control and the elimination of neoplasia [16]. This article will review the role of immune responses in natural HPV infection control and how the oncogenic virus types can sometimes deviate immunity promoting carcinogenesis. Progress and hurdles in meeting the challenges of delivering efficacious immune interventions in HPV associated cancer are discussed.
2. Immune mediated clearance of an HPV infection
For those high-risk HPV infections associated with cervical cancer, the critical target cell is in the basal layer of the epithelium of the transformation zone [17]. Infection of the basal cell occurs following minor damage to the epithelium thereby exposing the basement membrane components which act to alter conformation of the virions to facilitate virus uptake [18]. The completion of the life cycle of the virus is subsequently intimately linked to the differentiation of the epithelium as HPV reprogrammes the cells to support viral replication and production [19]. Phased viral gene expression subverts and hijacks the cellular machinery of the differentiating cells so completed virions are delivered in the terminally differentiated cells as they slough off and are replaced in the natural turnover of the epithelium. This whole process can occur without any significant disruption to the tissue and with no virus associated cell death or viremia. This type of infection can sometimes fly under the radar of the first line of defence, innate immunity. Such failure to properly invoke immune control can facilitate the persistence of an oncogenic HPV infection and it is this that drives the risk of developing cervical intraepithelial neoplasia [20]. Time provides opportunity for integration of the virus with the host genome, increasing viral oncogene expression thereby altering epithelial differentiation and undermining virion production while interfering with host genome repair processes [21]. The latter provides a driver for cumulative genetic errors which can be selected to yield a metastatic cancer avoiding cellular and immune controls.
In most cases of productive high-risk HPV infection of different tissue targets, it is likely that resident antigen presenting cells (APCs) like dendritic or Langerhans cells, sense damage and/or HPV associated molecular patterns using pattern recognition receptors like Toll-like receptors (TLRs). This causes activation of local non-specific effectors as well as secretion of interferons to help control the infection [22]. Further, there is production of proinflammatory cytokines and chemokines which support the activation of the APCs, viral antigen processing and their migration to the local lymph nodes. In the secondary lymphoid tissue, the APC are able to selectively activate and cause to expand relevant HPV specific T cells. Importantly, it is the initial signals received at the infection site by the APCs that flavour the spectrum of T cell subtype differentiation. In an optimal balance of response, CD4 T helper cells would act to activate specific B cells that subsequently produce neutralising antibodies against L1 capsid proteins as well as support the differentiation of cytotoxic CD8 T cells targeting viral targets like the E6 and E7 oncogenes (Fig. 1: Immune Control). Indeed, HPV induced premalignant lesions with evidence of infiltration by CD4 and CD8 T cells rather than T regulatory cells or macrophages are more likely to show regression [23,24]. Long lived plasma cells can provide sufficient levels of antibodies for protection against a subsequent infection while T cells specific for early antigens can clear virus infected cells and also contribute to protection through immune memory. This is supported by evidence of HPV oncogene-specific CTLs being associated with the clearance of cervical intra-epithelial neoplasia (CIN) plus the lack of such effectors in progressing high-grade lesions or cancers [23,25] and the increased susceptibility to HPV-associated lesions in immune-suppressed patients [26].
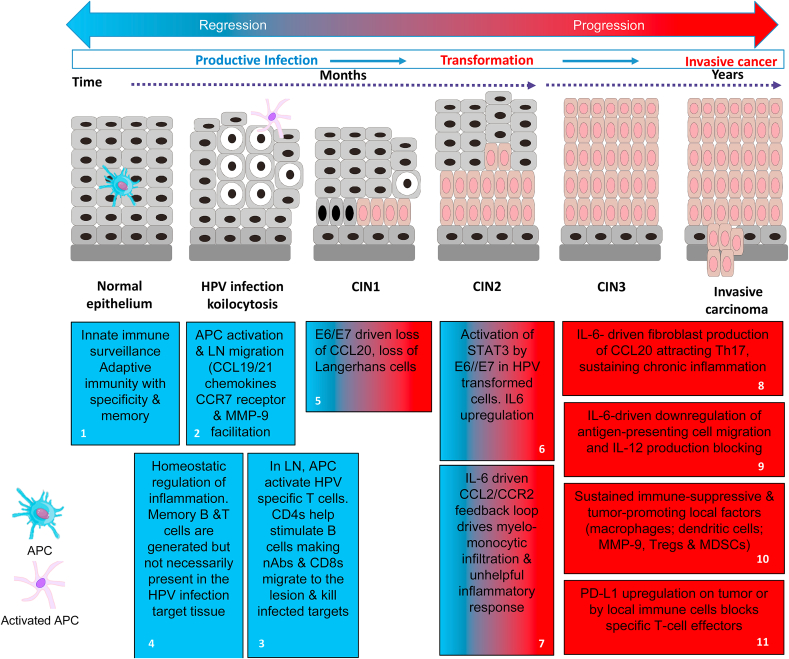
Fig. 1.
Immune control or immune deviation in HPV infection and neoplasiaImmune Control: (blue boxes). Blue box 1: APC detect tissue damage or the presence of HPV through sensor systems (e.g. TLRs) and act as the pivotal messengers to elicit adaptive immunity. Blue box 2: Induction of adaptive immunity requires migration of activated APCs to the local lymph nodes (LN) [1]. This occurs in response to gradients of the chemokines CCL19/CCL21 through CCR7 receptors on the activated APCs [2], plus production of MMP-9 facilitating passage through the extracellular matrix [3]. Blue box 3: APCs process and present viral antigens to activate specific T cells requiring CD80/CD86 and CD28 co-stimulation plus secretion of enabling cytokines like IL-12; without such co-stimulation immune tolerance and T-cell anergy can result [4,5]. Optimally activated T cells multiply and differentiate across a spectrum of associated cytokine production and effector properties to engage the viral threat. For example, helper CD4 T cells facilitate specific B cell stimulation leading to secretion of highly virus specific neutralising antibodies while also supporting the differentiation of CD8 cytotoxic T cells (CTL) able to kill virus infected cells directly or through the secretion of cytokines like IFN and tumour necrosis factor (TNF). Other types of T cell such as regulatory cells (Tregs) and/or the balance of cytokines produced can act to limit local responses to prevent tissue damage [6]. Blue box 4: This is a dynamic process, as when the viral threat is reduced, inhibitory signals (immune checkpoints) between T cells and APCs mediated by CTLA-4/CD28 and PD-1/PD-L1-type interactions act to induce a contraction of the specific effector T cell populations [7,8]. Earlier in the infection, subsets of memory B and T cells are produced so that the immune system can respond faster and more effectively upon the specific virus reinfection [9,10].
Immune Deviation: (blue & red or red boxes) reflect decreasing potential for immune control and increasing immune suppression respectively). Blue/red box 5: Some cases of HPV infection, E6/E7 expression can lead to loss of local APC (Langerhans cells) through down-regulation of the chemokine CCL20 [[33], [34], [35]]. This event inhibits innate immune detection of the viral infection and promotes viral persistence. Blue/red boxes 6 &7: E6/E7 upregulation of STAT-3 in HPV associated cells induces IL-6 can drive a myelo-monocytic cell lesion infiltration through the CCL2/CCR2 axis which is self-reinforcing [[42], [43], [44]]. This type of inflammation is able to provide a significantly immunosuppressive environment further facilitating viral persistence. With time, viral integration can occur with transformation of the cells breaking the link with the epithelial differentiation required for completion of the virus lifecycle. Viral inhibition of DNA repair allows for variants with additional means for immune escape. Red boxes 8 & 9: The emerging tumour microenvironment evolves through multiple IL-6 mediated influences including tumour fibroblast production of CCL20 attracting Th17 cells and the downregulation of antigen-presenting cell migration and IL-12 production blocking immune activation and chronic inflammation [46]. Red boxes 10 & 11: The TME accumulates high levels of immune-suppressive and tumour-promoting local factors including macrophages and dysfunctional dendritic cells: local MMP-9 production, Tregs and myeloid-derived suppressor cells (MDSCs) [31,57]. This milieu of immune players and factors can up-regulate PD-L1 by tumour or the associated immune cells thereby blocking anti-tumour specific T-cell effectors [47].
Interestingly, significant levels of HPV neutralising antibodies are not always detected in individuals who have apparently resolved an oncogenic HPV infection [27]. The precise extent of cellular and/or humoral HPV specific immunity that provides for protection to any subsequent HPV infection is really not known. However, many individuals are clearly exposed to high-risk HPV infection without suffering any persistent infections so natural immunity is the likely agent of resolution and protection. The development of HPV virus-like particle (VLP) based prophylactic vaccines able to induce supra-normal levels of neutralising antibodies against the most important and prevalent oncogenic HPV types can now provide for long lived protection in vaccinees but have no therapeutic value [28]. Limitations in vaccine coverage and/or secondary screening programmes particularly in the developing world plus the existing burden of HPV associated precancers and cancers provides a continuing challenging agenda for therapeutic interventions [29,30]. Where secondary screening is available, high grade intraepithelial lesions of the cervix can be detected and treated surgically. HPV associated cancers developing at other sites do not have such organized screening options and/or the best success of surgical intervention is usually linked to early diagnosis.
3. Immune deviation in HPV associated carcinogenesis
For unknown reasons in some normal healthy people high-risk HPV infections are not cleared and such persistent infection provides for a significant risk of developing neoplasia. While high-risk HPV infection alone is not sufficient to cause tumour development, viral gene expression does influence many of the key pathways that under pin cancer control including mechanisms that modulate immune factors [28]. Indeed, both viral gene expression and the chronic infection per se contribute to the neoplastic progression [31]. Importantly oncogene driven interference with cell cycle control allows for double-stranded DNA breaks, genomic instability and mutations that if of advantage will be selected. Table 1 summarizes the viral gene influences which contribute to transformation process and interference in immune control in HPV associated neoplasia [28]. Significantly, over an extended period of time, a diverse array of interactive events can establish a state of immune deviation which can act as key drivers in the evolution of a metastasizing tumour [32].
Table 1.
HPV gene expression influencing neoplastic transformation and immune control [28]
| Epithelial cell behaviour | HPV gene: Targets |
|---|---|
| Sustained proliferation & signalling | E7: ↓RB/HDAC; E7: ↑KDM6A/B; E5: ↑EGFR |
| Enabling replicative immortality | E6: ↑Telomerase |
| Resisting cell death | E6: ↓p53/BAK; E6: ↑BCL-2 |
| Deregulating cellular energetics | E6: ↑ mTORC1/MYC |
| Genome instability & mutation | E7: ↑dsDNA breaks |
| Inducing angiogenesis | E7: ↑ HIF1α; E6/E7: ↑VEGF & IL8 |
| Activating invasion & metastasis | E6: ↓PDZ proteins |
| Evading growth suppression | E6: ↓p53; E7: ↑RB |
| Impacts on immune control | |
| Avoiding immune destruction | E5: ↓MHCI/II/TAP; E7: ↓CXCL14; IFNk E7: ↓IRF1 & cGAS-STING; E6: ↓IRF3 |
| Tumour promoting inflammation | E6/E7: ↓CCL20/& local APC E6/E7: ↑IL6 & monocyte CCL2 & MMP9 |
Abbreviations: RB, RetinoBlastoma tumour suppressor protein; HDAC, Histone DeACetylase; KDMA/B, Lysine DeMethylases; EGFR, Epithelial Growth Factor Receptor; p53, DNA-binding transcription factor; Bcl-2, apoptosis suppressor protein; BAK, Bcl-2 homologous antagonist/killer protein; mTORC1, Mammalian Target of Rapamycin Complex-1; MYC, proto-oncogene; ds, double stranded; HIF1α, Hypoxia-Inducible Factor-1-alpha; VEGF, Vascular Endothelial Growth Factor; IL8, InterLeukin 8 cytokine; PDZ domain binding proteins; MHC, Major Histocompatibility Complex I/II; TAP, Transport associated with Antigen Processing; IFN, Interferon; IRF, Interferon Regulatory Factor; cGAS, cyclic GMP-AMP and STING, cyclic GMP-AMP receptor of interferon genes; CXCL14, Chemokine (C-X-C motif)-14; CCL20, Chemokine (C–C motif)-20; APC, Antigen Presenting Cell; CCL2, Chemokine (C–C motif)-2 or Monocyte Chemoattractant Protein-1 (MCP-1); MM9, Matrix Metallopeptidase-9.
Cells have evolved strategies to detect and combat virus infection but HPVs have counter mechanisms to undermine such pathways in addition to the means to hijack the cellular machinery as required to complete the virus life cycle (Table 1). For example, the cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS) detects intracellular DNA and signals through the adapter protein STING to initiate the antiviral response to DNA viruses. Sensing of the viral DNA by the cGAS-STING machinery results in a type I interferon (IFN)-mediated anti-viral response. HPV E7 is able to specifically inhibit this cGAS-STING pathway antagonizing DNA sensing with the inhibition dependent on the highly conserved LXCXE motif which is also essential for blockade of the Retinoblastoma (RB) tumour suppressor protein [33,34] Further, transcriptome analyses have shown that oncogenic HPV gene expression is associated with the downregulation of multiple cellular targets including the antiviral genes (IFIT1 and MX1), genes involved in IFN signalling (STAT1), proapoptotic genes (TRAIL and XAF1), and pathogen recognition receptors (TLR3, RIG-I, and MDA5). An important theme is the impact on the constitutive expression of interferon (IFN)-stimulated genes (ISGs). For example, reduced expression of pathogen receptors leads to significantly lower induction of IFN-β and IFN-λs. IFN-κ is usually constitutively expressed by keratinocytes but in HPV infected cells it is significantly repressed by mainly viral E6 but also E7. It appears that IFN-κ expression is modulated by DNA methylation [35]. Reducing the susceptibility of the infected cells to interferon also impacts immune recognition and activation processes.
In the context of a persistent high HPV infection, viral gene expression can trigger some important early changes to immune capabilities which act to protect the lesion from immune attack and subsequently promote its growth and ability for sustained immune escape (Fig. 1: Immune Deviation). An example is the reduced numbers of APCs and the low levels of inflammatory chemokines which are found in HPV- infected compared to normal tissues [36]. Normal keratinocytes produce CCL20, a chemoattractant for epidermal APCs (Langerhans cells) that express the CCR6 receptor with these cells critical to innate immune activation in the skin or mucosa. Mucosal HPV 16 E6 and E7 expression have been shown to suppress the NF-κB transcription-factor-dependent CCL20 induction [37]. For cutaneous HPV 8 infections, E7 controls differentiation-dependent CCL20 induction through binding the transcription factor C/EBPβ, preventing binding to the promoter of CCL20, thus repressing CCL20 transcription [38]. Thus, two important signalling pathways that regulate CCL20 levels by keratinocytes have been shown to be inhibited by the action of HPV oncogenes and this could account for a lack of recruitment of APCs to the HPV-infected epithelium, undermining innate immune activation and promoting virus infection.
Viral gene expression influences a wide range of defensive mechanisms of innate immunity through pleotropic effects on the detection, activation and migration of immune cells. Thus, viral E5, E6 and E7 expression can inhibit several pathways which compromise antigen processing and HLA presentation of viral peptides at the infected cell surface thereby promoting immune escape [39]. Another example of influence is through E7 mediated downregulation of CXCL14 via promoter DNA hyper-methylation. This leads to important changes in the capacity for migration of Langerhans cells and effector lymphocytes from and to the HPV associated lesions [40,41]. Such virus-mediated immune interference combines to promote the persistence of the infection and thereby promote the risk of cancer [see 28,31].
The development of a cancer from a premalignant lesion can take many years and during this time there is an infiltration by myelo-monocytic cells. HPV-E6/E7 induced STAT-3 in the transformed keratinocytes drives IL-6 production and acts on lesion-associated myeloid and inflammatory cells in a paracrine manner This causes activation of STAT3 in monocytes which induces CCL2, a potent monocyte-attracting chemokine and an autocrine CCL2/CCR2 loop sustains and further skews the inflammatory microenvironment favouring immune suppression and carcinogenesis [42,43]. For example, low CCL2 in myeloid cells is linked with better outcomes in cervical cancer while downstream CCL2 signalling induces high matrix metallopeptidase (MMP)-9 expression associated with cancer progression [43,44]. Chronic IL-6 produced during cervical carcinogenesis can also downregulate mature activated APC CCR7 expression inhibiting the response to chemokines that direct migration to the local lymph nodes (LNs) while stimulating their MMP-9 expression [45]. Thus, APC and other myeloid cells accumulate in the tumour stroma and their MMP-9 expression can promote tumour growth and spread through angiogenesis. In definitive tumours, constitutive IL-6 production attracts CD4/IL-17/CCR6-positive T cells which are tumour promoting [46]. In addition, CCL20 transcription is regulated by paracrine production of IL-6, activating the C/EBPβ pathway in fibroblasts associated with the tumour. This illustrates the dynamic nature of the changes that occur over the natural history of the HPV driven carcinogenesis. At the start CCL20 is suppressed by HPV oncoproteins in the neoplastic epithelial cells while in advanced stages it is expressed in the stroma promoting a chronic inflammatory response.
The integrated effect of these factors promotes lesion infiltration and differentiation of immune cells including macrophages, migration inhibited APCs with local MMP-9 production, Tregs and myeloid-derived suppressor cells (MDSCs) establishing an immune-suppressive and tumour-promoting environment [31]. Importantly, the combination of local immune factors also upregulates the expression of the checkpoint-inhibitor ligands like PD-L1, by tumour or associated immune cells and this acts to block any anti-tumour-specific T-cell effectors [47].
4. The effect of chronic viral infections on CD8 T cell function
It is apparent that the TME is a battleground where effective immune tumour control depends on access for tumour specific T cell effectors that are able to deliver their therapeutic payloads overcoming any competing negative influences. Unfortunately, HPV associated neoplasia favours many events that lead to a tumour promoting immune environment and this can result in a failure of specific T cell effectors to access the tumour [28,48] and/or their local suppression by other infiltrating immune cells [48,49]. and/or loss of function with induction of anergy or an exhausted state [50].
The exhaustion of T cells derivative from a chronic viral infection is well illustrated by lymphocytic choriomeningitis virus (LCMV) mouse models. In acute viral infection activated APC migrate to the local LN and present processed viral peptides to stimulate naïve antigen specific CD8 T cells [51]. These rapidly proliferate and differentiate to CD8 effectors that migrate to the infection site and act to clear the virus. With the reduction in viral load, homeostatic mechanisms downregulate the CD8 effector numbers but recirculating or lymphoid tissue resident memory cell populations are delineated. The consequences of a chronic LCMV infection are rather different and can lead to the functional exhaustion of CD8 T cells. These cells are characterised by overexpression of several inhibitory receptors (e.g. PD-1), major changes in T cell receptor and cytokine signalling pathways, altered expression of genes controlling chemotaxis, adhesion, and migration, expression of a distinct transcriptional signature plus display significant metabolic and bioenergetic deficiencies [52,53]. The continuous stimulation of naïve T cells in the LN eventually skews the population CD8 effectors at the infection sites where the functional T cell exhaustion is progressive but distinct from anergy [51]. It appears to be the consequence of both active suppression and passive defects in signalling and metabolism. In these circumstances, a backstop mechanism to protect the repertoire of virus specific T cells generates a novel stem cell-like CD8 non-recirculating population which can be found in the T cell zones of lymphoid tissues along with the naïve T cells [54]. These cells are quiescent but retain proliferative potential and act as the emergency resource to maintain a supply of activated specific T cells in a chronic virus infection. T cell factor-1 (Tcf-1) is a transcription factor essential for commitment to both the T cell and the innate lymphoid cell lineages in mammals. Tcf-1 is critical for the generation of this PD-1+ Tcf-1+ CXCR5+ CD8+ T cell subset and they are capable of self-renewal and differentiation into more terminally differentiated cells that downregulate Tcf-1 and with a transitory population of CD101-Tim3+ converting to CD101+ Tim3+ cells. CD101 is a heavily glycosylated transmembrane molecule which exhibits negative-costimulatory functions and promotes Treg function. TIM3 is part of a module that contains multiple co-inhibitory receptors (checkpoint receptors), which are co-expressed and co-regulated on dysfunctional or ‘exhausted’ T cells in chronic viral infections and cancer. It is the recently generated CD101-Tim3+ cells that proliferate in vivo, contribute to viral control, and have an effector-like transcriptional signature including expression of CX3CR1, pro-inflammatory cytokines, and granzyme B. Importantly PD-1 pathway blockade increased the numbers of CD101-Tim3+ CD8+ T cells, suggesting that these newly generated transitional cells play a critical role in PD-1-based immunotherapy [53]. Both the stemlike CD8 T cells and their terminally differentiated progeny showed minimal migration during chronic infection. The lack of recirculation of the PD-1+ Tcf-1+CXCR5+ stemlike CD8 T cells resident in lymphoid tissues suggests the need for a specialized niche as essential for maintaining their quiescence and stemlike program under conditions of a chronic viral infection [53].
There is increasing evidence that such cells are also generated in the context of chronic stimulation of tumour specific CD8 T cells. Thus, distinct populations of terminally differentiated and stem-like CD8 T cells can be detected in human tumours with the proliferation of the stem-like cells producing terminally differentiated, effector-molecule-expressing progeny [55,56]. The stem-like T cells reside in dense antigen-presenting-cell niches within the tumour, and in the absence of such structures there is a lack of T cell infiltration which correlates with more progressive disease. It seems that in the battle with a virus and/or a tumour there is a requirement to generate tertiary lymphoid type structures to provide a suitable local niche to protect these novel CD8 T stem cell populations.
5. Optimising immunotherapeutic strategies for cancer treatment
As accounted above there are a cornucopia of tumour extrinsic and intrinsic mechanisms which can provide extensive primary resistances to immune control but these can, together with tumour genomic instability, also drive secondary immune escape events. Individual cancers can be extremely heterogenous in respect of the genetics of the immune selected cells as well as in the composition of their tumour microenvironment. Unsurprisingly, while there are encouraging signs of efficacy in several different immunotherapeutic approaches for cancer treatment including with immune checkpoint inhibitors, therapeutic vaccines and adoptive cell therapies, overall response and survival rates remain relatively low [13,14]. It is obvious that to maximally exploit any potential anti-tumour immune response, the plethora of immunosuppressive factors associated with and/or driving the carcinogenic process must be addressed. Myeloid cells are key players in the orchestration of the immunosuppression blocking effective natural immunity and immunotherapy development [57]. Likewise, the impact of the current standard of care treatments (radiation and/or chemotherapy), either positive or negative, on natural or therapy induced tumour immunity must also be considered [58]. It is clearly immensely challenging to document the precise combination of resistance mechanisms for every individual tumour in order to configure the most appropriate immune treatment options. Animal models can help to identify and investigate the means to target particular resistance mechanisms while human tumour samples and clinical trials can be used to validate the developed therapeutic strategies [14]. Unfortunately, the complexity of immune local and systemic interacting factors in cancer aetiology and progression challenges clinical trial designs which are mostly not well co-ordinated.
Our knowledge of the natural history of HPV associated tumours may provide some greater consistency for designing intervention opportunities not the least through the obligate requirement for HPV oncogene driving tumorgenicity and as exogenous tumour specific targets. Table 2 and Fig. 2 summarize the several phases from initial immune awareness to the potential killing of cancer cells and where and which therapeutic interventions might be able to provide more effective cancer control.
Table 2.
Immune barriers and interventions in HPV associated cancer.
| Immune event | Negative immune factors | Therapeutic Options |
|---|---|---|
| Antigen Release | Inherently stealthy pre-cancer stage of persistent infection provides for facilitating immune deviation including through reduced APC numbers | Selective chemo-, radio- and/or targeted therapies might alter the balance of local factors to boost natural immunity and could help early lesion resolution. |
| Antigen Presentation | Defective activity of APC & macrophages; accumulation of Tregs, MDSC & establishment of immune deviation in TME. | Any effective therapeutic vaccine against E6/E7 viral oncogenes aims to generate specific killer T cell activity either by boosting natural and/or stimulating new populations. Combination treatments which counter the negative local TME affects by promoting APC activity and the activation of fully functional T cell effectors include provision cytokines like IL-12 cytokines and/or TLR agonists may be useful. |
| Immune Activation/Priming | Deficiency in macrophages & APC function; sub-optimal stimulation of T cells; unhelpful cytokine skew; inhibitory immune checkpoint expression. | Chronic virus infection positively reinforces the immunosuppressive intensity of the TME. This can ultimately deplete the repertoire although stem-like CD8 cells can preserve this capacity. By this point, checkpoint inhibitors may provide a means to alter the balance of immune factors in favour of regaining control but where additional treatments such as low dose IL-2, or agonists like anti-CD40 might give synergy. |
| Immune Trafficking | Dysregulation of chemokine and chemokine expression & IFN signalling pathways | Advanced cancer TME may be particularly resilient to immune attacks based on stimulating existing or de novo T cell responses particularly as the latter may not get the guidance signals for tumour homing. ACT of expanded TILS may circumvent some such barriers quantitatively and for homing. Such TIL expanded effectors may also target wider tumour immunogenic genetic changes. CAR T cells might provide sufficiency in effector numbers but target selection is more problematic. |
| Immune Infiltration | TME driven changes influencing cancer angiogenesis, adhesion, and extravasation plus immune cell apoptosis and activation of the stroma to reduce immune cell infiltration. | The complexity and breadth of TME effects on immune control could be influenced by anti-angiogenesis treatments. Local treatment with immune response modulators (cytokines, TLR agonists) could be helpful but does not address metastatic cancers. |
| Immune recognition & killing | Evasion of T cell (NK) killing; Upregulation of inhibitory signals like PD-1, PD-L1, LAG-3, TIM etc; consolidation of actively immunosuppressive TME through combination of cellular (Tregs, M2 macrophages, MDSC) and soluble factors (TGFB, IDO, IL-10, etc) | In end stage cancers, the maximal number and extent of TME immunosuppressive influences may require extensive use of combinations of treatments influencing several immune checkpoints, cellular, secreted and metabolic inhibitory factors. The key challenge is to deploy these options including in context of SOC in the best array but also with the optimal sequencing. |
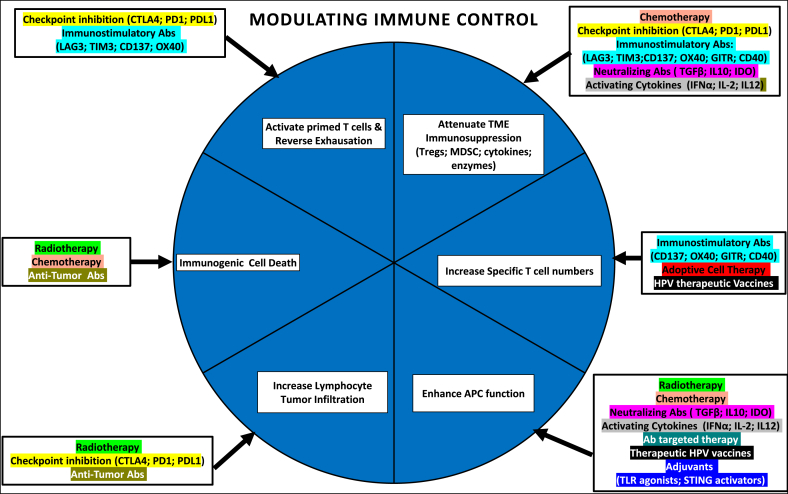
Fig. 2.
Potential synergies in combined immunotherapies [after 107]
Citations for examples of antibodies: for immune-stimulation [108]; direct anti-tumour therapy [109] neutralising cytokines [110,111]; activating cytokines [112]. Effector and targets: Abs, antibodies; LAG3 negatively regulates cellular proliferation, activation/homeostasis of T cells; CD137 crosslinking enhances T cell proliferation, IL-2 secretion, survival and cytolytic activity; OX40, a tumour necrosis factor receptor superfamily member acts as a secondary co-stimulatory immune checkpoint molecule, expressed after 24 h after activation; GITR, (glucocorticoid-induced tumour necrosis factor receptor) is a surface receptor molecule involved in inhibiting the suppressive activity of T-regulatory cells and extending T-effector cell survival; CD40 is a costimulatory protein found on APC and required for their activation so agonistic Abs activate an anti-tumour T cell response via activation of dendritic cells; TGFβ, transforming growth factor beta is a pleiotropic cytokine that can exhibit both tumour-suppressive and oncogenic functions; IDO, indoleamine 2, 3-dioxygenase 1 is a rate-limiting enzyme that metabolizes the essential amino acid, tryptophan, to kynurenine which leads to inhibition of immune cell effector functions and/or facilitates T cell death.
5.1. Immune checkpoint inhibitors
In cancer, immune checkpoint homeostatic mechanisms can be hijacked via upregulation of the appropriate ligands in the tumour microenvironment (TME) allowing evasion of anti-tumour immunity; the blocking of these pathways with specific antibodies can recover useful immune responses in some patients [15,59]. The first checkpoint inhibitor approved for treatment of metastatic melanoma patients was ipilimumab (anti-CTLA-4 monoclonal antibody) in 2011. The dramatic impact on previously treatment unresponsive tumours like metastatic melanoma acted to remove the “blinkers” of many oncologists and scientists to the relevance and potential of immunity in cancer. Subsequently, the PD-1 inhibitors nivolumab, pembrolizumab, cemiplimab and PD-L1 inhibitors atezolizumab, avelumab, and durvalumab joined the list of approved agents for treatment of several types of advanced cancers [60]. Unfortunately, the impact of these revolutionary approaches is limited by the relatively low proportion of patients who respond to blockade of immune checkpoints, the occurrence of significant, albeit generally manageable, toxicities in some patients and the high cost of the treatment schedules. One issue that could be extremely useful in targeting the therapies to those most likely to respond is the concept that the level of inhibitory molecule tumour expression can predict patient responses. While such tests have been adopted in relation to some treatment options there is a lack of consistency in the methodologies being used (different antibodies, variable cell types defined in the assessment, different density measurements, automated versus visual scoring; scoring by global or spatial interaction patterns) and it is unclear whether their use is yet of significant added value [61,62].
In respect of HPV associated cancers there are many ongoing clinical trials investigating checkpoint inhibition therapy in cervical cancer [63] and oral pharyngeal squamous cell carcinoma (OPSCC), a subset of head and neck cancers (HNSCC) [64]. Various different investigations of PD-L1 expression by squamous and adenocarcinomas of the cervix have reported inconsistent associations with survival [65]. This undoubtedly reflects the complex and interactive contributions of different cell types to clinical impact, both in time and through the architecture of the tumours. Thus, the report of an association of PD-L1 macrophages with poorer outcomes might be explained by the knowledge that IL-6 can drive monocyte differentiation to PD-L1 positive CD163+ and CD14+ macrophages (M2) thereby inducing Tregs, effecting lower IL-12 but higher IL-10 levels which then can modulate T-cell activation [66]. In another study, suppressive PD-L1+/CD14+ M2 macrophages, MDSCs, PD-1 or CTLA-4 positive T cells and Tregs were detected in tumour positive but not negative lymph nodes. The Tregs and other immunosuppressive cells were found surrounding the PD-L1 positive metastatic tumour cells in the LNs [67]. This can be interpreted as a means by which the early immune deviation events in HPV associated carcinogenesis can subsequently enable metastatic spread [68]. At this time, only pembrolizumab is licenced for treatment of recurrent or metastatic cervical cancer progressing after chemotherapy and in PD-L1 tumour positive patients. This approval was given via an accelerated process based on the evidence from cohort E of the Keynote-158 trial showing overall response rate of 14% [60].
HPV positive (compared to negative) OPSCC patients have better clinical outcome to standard of care (SOC) chemo-radiation treatment, most likely as a result of increased radio-sensitivity (residual p53 activity compared to HPV negative tumours where p53 is most often mutated) [69]. A functional role for CD8 T cell activity in HPV positive versus negative OPSCC is supported by a higher density of CD8 T cells in the stroma being associated with improved clinical outcome [70]. Other investigations have scored the different types of PD-L1 positive cells in tumours and their location in attempts to identify a useful prognostic and/or treatment deployment measure [[71], [72], [73], [74]]. It is complicated, as illustrated by the results from a study which used a multiplex scoring of CD8, PD-L1 and CD68 marker expression in an OPSCC patient cohort with long follow up [75]. As might be expected, the best prognosis patients within the HPV positive OPSCC group were those with high-density CD8 T cells in the stroma and low tumour levels of PD-L1. However, an improved prognosis for HPV negative tumour patients was linked to high CD68 PD-L1 positive macrophage infiltration. A more recent study tested a new automatic approach to select tissue and quantify the frequencies of cell-cell spatial interactions occurring in the PD1/PD-L1 pathway, hypothesised to reflect immune escape in the same OPSCC cohort. In this analysis, a high frequency of proximal CD8 or PD-1 marked T cells with PD-L1 positive cells was found to be prognostic for poor overall survival in patients only in the HPV negative OPSCC [76]. These types of results reveal the limitations of the PD-L1 biomarker as a means to decide how to allocate checkpoint inhibitor treatment in OPSCC. It is likely that any useful prognostic will have to utilize multiple marker and spatial information. At this point, nivolumab or pembrolizumab are licenced in recurrent or metastatic HNSCC that has progressed on or after platinum-based therapy. In addition, pembrolizumab is available as a first-line therapy for patients with metastatic or unresectable, recurrent HNSCC either as monotherapy in patients whose tumour expresses PD-L1 (combined positive score ≥ 1%) or in combination with platinum and fluorouracil [60]. It is important to note that HNSCC can result from exposure to environmental carcinogens or oncogenic HPV transformation [77]. Interestingly, immune cells within HPV positive and negative tumours display a spectrum of transcriptional signatures, with helper CD4+ T cells and B cells being relatively divergent and CD8+ T cells and CD4+ regulatory T cells being relatively similar [78]. These transcription patterns were contextualized using multispectral immunofluorescence and putative cell-cell interaction based on spatial proximity evaluated. This yielded a gene expression signature associated with CD4+ T follicular helper cells linked to longer progression-free survival in HNSCC patients. This type of study emphasises the heterogeneity of immune control mechanisms that may operate in a “single” tumour type with variable aetiology and the difficulties in defining simple useful prognostic markers. A recent study has shown that HNSCC tumours expressing HPV E5 are resistant to anti–PD-L1 immunotherapy in orthotopic models. In addition, patients with high expression of HPV16 E5 have a worse survival. The antiviral E5 inhibitor rimantadine demonstrated significant single-agent antitumor activity and this offers the prospect for improved outcomes for head and neck cancer patients [79].
Tumour-specific CD8 T cells in solid tumours are frequently dysfunctional, allowing tumours to progress. Under such chronic stimulation, a recovery pathway where lymphoid tissue resident stem-like CD8 T cells are induced is deployed. Importantly, these cells can also be detected in dense antigen-presenting-cell niches within some tumours but patients with progressive disease lack these immune niches and show low levels of T cell infiltration [55,56]. With checkpoint blockade, Tcf1+PD-1+ cells are detected in tumour-reactive CD8+ T cells in the blood and tumour infiltrating lymphocytes (TILs) of primary human melanoma patients [56]. In has now been shown that checkpoint blockade depends not on reversal of T cell exhaustion programs, but on the proliferation of the stem-like population [56,80,81]. These cells are released from their quiescence and are able to engage with antigen presentation, mobilize, expand and differentiate to deliver effector functions. The checkpoint blockade may also influence effector function at the target sites with increased killing and cytokine release. A greater understanding of how to best mobilize this unique stem-like population in a chronic viral driven tumour will be important to maximizing the impact of therapies which seek to harness the immune response. Any therapeutic value will still need to overcome the sum total of all the immunosuppressive factors that may be present in the TME. The dynamic process involving stem, transitory, differentiated effector cells may be maximised for therapeutic potential by the use of drug combinations which not only influence CD8 prevalence and function but also override other TME limitations synergistically.
5.2. Therapeutic vaccines
The obligate viral oncogene expression in HPV associated cancers provides the seemingly ideal tumour specific targets for therapeutic vaccines. A recent study used proteome-wide profiling of HPV-16–specific T cell responses comparing HPV-associated OPC patients and healthy individuals investigated the potential wider breadth for HPV immune targeting. HPV-specific T cell responses from OPC patients were not restricted to E6 and E7 antigens, E1, E2, E4, E5, and L1 proteins were also recognized by virus-specific CD8+ and CD4+ T cells [82]. However, it is not clear whether all the components of this multi-targeted overall human T cell response to HPV are important in tumour control or simply derivative from the natural history of HPV infections in these OPC patients. In the majority of HPV associated cancers, viral integration with the host genome disrupts the viral E2 expression that negatively controls E6/E7 oncogene expression and with the latter upregulated, epithelial differentiation is altered thereby blocking virus production while promoting transformation. Since the other viral early genes are not required for carcinogenesis and/or are not expressed by the transformed cells they are not attractive targets for therapeutic cancer vaccines; the late gene encoded capsid proteins are only made in terminally differentiating cells. However, it is highly likely that specific T cell mediated immunity to several of the early gene viral components is relevant in the clearance of natural infection [83]. It is possibly that in some HPV associated tumours, non-integrated HPV expression may be required for oncogenesis and so cellular immunity to a broader spectrum of HPV early gene proteins could be relevant although this has not been proven [82].
The great majority of therapeutic HPV vaccines have targeted HPV 16 and/or 18 oncogenes (E6 and/or E7) with the first one tested in clinical trials more than 3 decades ago. The best principles of vaccine design and application of insights from the contemporary understanding of immune activation and control have been employed during this time for a multiplicity of approaches. The unifying goal has been to deliver vaccines with the ability to induce strong CD8 T-cell responses [84]. Many different types of vaccines have been tested for efficacy in patients with HPV associated cancers or high-grade neoplasia but even when immunogenic, demonstrating significant clinical benefit as been elusive [[85], [86], [87], [88], [89]]. It seems likely that important common limitations are the failure to overcome the impact of immune suppressive factors including those associated with chronic viral infection in the natural history of the neoplasia and the generally underpowered clinical trial designs. Added to this the vaccine trial patients were often tested after SOC treatment where the chemotherapy and radiation could have significantly reduced their immune response potential. To be provocative, the impacts of SOC for many cancers should really be reappraised in the light of the recognition of the central role of the immune response in limiting tumour emergence. Getting back the potential of cancer immune control should be considered as a more primary component in future treatment strategies especially where more (higher, longer dosing SOC) may indeed provide less [58]. Many of the various HPV oncogene vaccine candidates that are currently being evaluated need to build on these insights through different treatment scheduling and clinical trial design. Data from clinical trials of three different HPV vaccine types, protein (TA-CIN, HPV 16 L2E6E7 fusion), synthetic long overlapping peptides (ISA101, SLPs of HPV 16 E6 and E7) and a DNA plasmid (VGX-3100 encoding HPV16/18 E6 and E7) have reported evidence of immunogenicity linked to encouraging clinical responses. These results serve to illustrate some aspects of design and combination treatment approaches that can help to provide a useful platform for future studies able to deliver efficacious treatment strategies.
Clinical trials in HPV 16 -associated high grade vulvar intraepithelial neoplasia (VIN) patients, although much less prevalent than those with CIN-3, can provide some key logistical advantages. For the latter, surgery is an extremely effective treatment so vaccine investigational studies will be limited by the requirement for timely curative treatment. By contrast, for high-grade VIN patients, surgery is not always an option and/or the other limited treatments available are not curative. This provides a much longer window for investigation of vaccine and associated treatment immunological effects both locally and systemically.
Imiquimod is an innate immune response TLR7/8 agonist that can negate local immunosuppressive factors, so lesion pretreatment might act to alter the TME and facilitate the impact of vaccine stimulation of pre-existing or de novo HPV vaccine generated anti-oncogene T cells [90]. Treatment of high-grade VIN lesions with Imiquimod followed by unadjuvanted TA-CIN vaccination of the patients delivered 63% complete regression at one year [91]. Immunohistochemistry showed that CD8 and CD4 T cell lesion infiltration was significantly increased in the clinical responder patients compared to those with unresponsive VIN which showed a significantly increased density of Tregs. Following vaccination, only the clinical responders showed significantly increased lympho-proliferation of peripheral blood lymphocytes to the HPV vaccine antigens; these were also those patients with pre-existing responses. These results suggest that in the refractory VIN patients both local and systemic factors cannot be overcome by this immune response modifier and vaccination combination treatment. Future studies will need to explore the specific mechanisms from the complex immunosuppressive armoury whereby chronic viral stimulation establishes the T cell dysfunctional state in some VIN patients. Ongoing work will use a phase I study to investigate TA-CIN vaccine as therapy in previously treated HPV16 positive cervical cancer patients with stable disease with analysis of pre- and post-vaccine responses (NCT02405221). Future studies should explore formulation of the fusion protein with a suitable vaccine adjuvant and/or in combination with a checkpoint inhibitor strategy.
The testing of the ISA101 vaccine (13 peptides of 25–35 amino acids covering overlapping sequences of the HPV 16 E6 and E7 proteins, adjuvanted with montanide) in high grade VIN patients demonstrated T cell immunogenicity and significant clinical responses [92]. Clinical efficacy of ISA101 vaccination was related to the strength of vaccine-induced HPV16-specific T-cell immunity [93]. However, when this vaccination therapy was tested in patients with advanced or recurrent gynaecological carcinoma there was no measurable clinical impact [94]. A preclinical investigation of treatment of HPV tumour-bearing mice with standard carboplatin and paclitaxel chemotherapy plus vaccination significantly improved survival indicating the potential for combination therapies [95]. This chemotherapy was shown to reduce the immunosuppressive myeloid cell population in the blood and the tumour but did not alter tumour-specific T-cell responses. A clinical trial of carboplatin-paclitaxel in advanced cervical cancer patients confirmed a reduction in the numbers of circulating myeloid cells while boosting patient T-cell responses. The minimum level of circulating myeloid cells was detected at two weeks following the second chemotherapy cycle [96]. This information was used for the testing of the ISA101 immunization timing which was shown to elicit strong and durable HPV16-specific T-cell responses to a single dose of the vaccine. A clinical trial assessing the safety, tolerability and the HPV-specific immune responses of different doses of the ISA101 long peptide HPV16 vaccine with or without pegylated IFN-α as combination therapy with carboplatin and paclitaxel has now reported [97]. The underpinning reasoning was that the chemotherapy would enhance the tumour-specific immunity and synergize with cancer immunotherapy with the addition of pegylated IFN-α aimed at further improving the immune response. 77 patients with Stage IIIb/IVa or metastatic or recurrent Stage IVb HPV 16 positive cervical cancer received the vaccine plus IFN after the 2nd, 3rd, and 4th of six chemotherapy cycles. Overall, the treatment was safe, well tolerated and not different from the chemotherapy given alone. The reduction in myeloid cell numbers was confirmed and strong specific T cell responses were detected to all vaccine doses. A lymphocyte depleting affect was only associated with a low frequency of HPV specific T cells in about one third of the patients. Tumour regressions were observed in 43% of 72 evaluable patients. The patients with higher median vaccine induced response lived significantly longer as compared to those with a lower than median response and this difference did not reflect immune competence or any pre-existing HPV specific immunity. These are very encouraging results but the study did not investigate effects on tumour infiltration measured pre- and post-treatment nor was there any chemotherapy alone arm or randomization in the trial design. The overall group median survival in this study is similar to those reported for first line SOC (chemotherapy and bevacizumab) but show a flattened tail in the survival plots offering a platform for further improvement. However, this particular combination treatment is unlikely to override other barriers to vaccine efficacy including through Tregs effects, tumour associated checkpoint inhibition or HLA I downregulation which can all contribute to tumour cell escape in the face of effective T cell immunity.
There are now various clinical trials that are exploring the use of ISA101 SLP vaccine in combination with other treatments of HPV related disease. For example, a phase II trial of nivolumab (anti-PD-1) and HPV-16 vaccination in patients with HPV 16 positive incurable solid tumours. The goal is to see if nivolumab combined with the ISA101 SLP vaccine is safe and can help to control cancer that has spread (NCT02426892). Another clinical study is testing utomilumab (humanized monoclonal antibody (mAb) versus 4-1BB), recognizing the CD-137 co-stimulation receptor expressed by CD4, and CD8 T cells plus natural killer (NK) cells, given intravenously alone or in combination with the ISA101 vaccine for ability to reduce or slow the growth of tumours in patients with incurable HPV 16 positive OPSCC. The rationale is that the anti-CD137 will stimulate and increase the number of immune cells and therefore enhance anti-tumour function (NCT03258008).
The DNA plasmid VGX-3100 vaccine, delivered by electroporation intramuscularly, has been evaluated for safety, efficacy and immunogenicity in a randomized, double blind, placebo-controlled Phase 2b study in patients with CIN2/3 lesions and demonstrated significant lesion regression and viral clearance [98]. The trial design was able to show an impact on the composition, magnitude, and quality of immune responses in the target lesions by this systemic immunization. Importantly these analyses allowed the investigation of the type of immunologic responses associated with successful resolution of HPV-induced premalignancy, identifying the effector upregulation of perforin as a key component [48]. A prospective, randomized, double-blind, placebo-controlled Phase 3 study to determine the efficacy, safety, and tolerability of VGX-3100 administered intramuscularly by electroporation in adult women with histologically confirmed cervical high grade squamous intraepithelial lesion (HSIL) (CIN grade 2 or 3) associated with HPV16 and/or HPV 18 is in progress. A clinical trial of treatment of patients with HPV 16 and/or HPV 18 high grade VIN with a combination of VGX-3100 vaccination and Imiquimod is nearing completion (NCT03180684). VGX-3100 vaccination is also being tested with more advanced disease including a prospective study in patients with HPV associated head and neck squamous cell carcinoma (NCT02163057) and in patients with either inoperable invasive cervical carcinoma associated after standard chemoradiation therapy or with persistent/recurrent cervical cancer following salvage therapy (NCT02172911). A trial combining VGX-3100 vaccination with Durvalumab in HPV positive OPSCC is recruiting (NCT03162224).
The above examples serve to show a range of vaccine approaches that are being investigated but where the momentum was maintained in the early clinical studies by trial designs incorporating investigation of effects on the TME as well as systemic immune influences relating to clinical responses. Many other HPV therapeutic vaccine designs should follow this experience in order to maximise their potential to deliver.
5.3. Adoptive cell therapies (ACT)
Adoptive transfer of ex vivo expanded tumour infiltrating lymphocytes (TIL) can be efficacious with response rates of about 30% in patients with treatment refractory metastatic melanoma [99]. This contrasts with a general history of failure to improve patient responses by first enriching for putative tumour specific antigen T cell populations. In choosing a particular target antigen it must be assumed that any ex vivo expanded immunity is going to be relevant whereas the reality is that tumour immune control is likely to be directed against multiple tumour antigens reflecting the natural history of cancer and its’ genetic heterogeneity. It appears that such multi-target specific effectors, previously inactive due to various immunosuppressive factors in the tumour, when expanded ex vivo and transplanted into patients following a conditioning regime to favour their proliferation, can sometimes deliver efficacious functionality.
For HPV associated cancers the necessary viral oncogene expression provides some rational for attempting to expand TILs with this specificity although other genetic changes may also be targets for useful and potentially recoverable tumour immunity. In one study, advanced cervical cancer patients (after chemotherapy or chemo-radiotherapy) were treated with a single infusion of tumour-infiltrating T cells (stimulated when possible for HPV E6 and E7 reactivity) with the cell infusion preceded by lymphocyte-depleting chemotherapy followed by IL-2. Objective tumour responses were seen in 3/9 patients with the two complete responses sustained for 15–22 months. Importantly, an association of HPV reactivity of the adoptive cells and the observed clinical responses was reported (NCT01585428). There are many factors that might impact efficacy of TIL treatment, any anti-tumour activity needs to overcome the negative influences both in the isolated TIL (by preferentially expansion) and in the local TME. While impressive clinical responses can be seen, this is not predictable, and this type of approach is only available where individual tumours can be harvested and the ACT capability available [100].
The engineering of T-cell receptors (TCR) or chimeric antigen receptors (CAR) and their expression by effector lymphocytes can enable a more generic approach to ACT in some cancers [101,102]. Using an engineered HPV oncogene specific TCR will have a particular HLA restriction [103] but the targeting of a single epitope in a particular MHC context can easily escape via HLA down-regulation, a frequent event in cervical and other cancers [104]. CAR-T cells require a cell surface tumour specific target [105], e. g CD19 in treatment of B cell leukaemia and lymphoma [106], and thus there is no potential for targeting HPV antigens.
6. Conclusions
HPV associated intraepithelial anogenital neoplastic lesions, identified by screening (cervical vulval, vaginal, anal, penile) represent an early stage when the accumulation of immunosuppressive factors, the size and heterogeneity are relatively limited. Nether the less it is apparent that some lesions remain refractory to vaccine or vaccine combination immunotherapies. To approach the near complete cure rates for surgical intervention in treating CIN, further investigation of the factors that prevent some lesion resolution is required. The goal must be to find acceptable and safe combinations of treatments that can reset the immune system so that it can fully utilize its adaptive immune repertoire to eliminate all elements of any residual HPV oncogenic threat with sufficient efficacy. Any treatment incorporating a therapeutic vaccine approach would also need to provide immunity against at least the most prevalent high-risk HPVs oncogenes. Unfortunately, many HPV driven cancers remain undetected including to the point where curative surgery is not an option. In spite of individual tumour genetic heterogeneity plus the magnitude of local and systemic immune suppressive influences, immune interventions can deliver efficacious outcomes in some cases. In later stage disease, the threshold for the deployment of more complex immune intervention strategies is lower but the dividends may be greater, especially if integrated where appropriate with SOC. Evidence of chemo- and radio-therapeutic components of SOC able to contribute to the recovery of effective anti-tumour immunity should provide momentum for further investigation [[113], [114], [115]].
In this context, platforms which can integrate multiplexed expression data (RNA, protein markers) from both individual cells and their context in the tissue architecture can provide a more dynamic perspective and understanding of immune related variations in health and disease [116,117]. In particular, T cell migration across vascular endothelium mediated by specific tissue-homing receptors is essential for any anti-tumour response [118] while the development of tertiary lymphoid structures following high endothelial venule neogenesis is thought to facilitate the generation of tissue-destroying lymphocytes inside chronically inflamed tissues and cancers [119]. How such trafficking is regulated during inflammation is poorly understood but being able to manipulate this aspect can allow the development of better immune therapies. Indeed, understanding the pathways involved in regulating the recruitment of all immune players in cancer including γδ T cells [120] or neutrophils [121] which are frequently overlooked is required. It is now clear that innate immunity can also be trained [122] and indeed be manipulated to deliver useful myeloid responses in some circumstances [123]. Evaluating how immunosuppressive “imprinting” may be reversed is at the heart of progress. Understanding how and which immune and SOC type interventions to deploy and sequence is the challenge. A first step should be to define some key systemic and tumour associated immune factors to be assessed in all treatment trials. In summary, the prospects are good for harnessing immunity to HPV associated cancers to deliver more effective treatments than the current regimens.
Declaration of competing interest
The author declares that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tvr.2021.200212.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Garcon N., Stern P., Cunningham T., Stanberry L. Elsevier; 2011. Understanding Modern Vaccines.http://www.sciencedirect.com/science/journal/22107622 [Google Scholar]
- 2.Johnson L.A., Jackson D.G. Control of dendritic cell trafficking in lymphatics by chemokines. Angiogenesis. 2014 Apr;17(2):335–345. doi: 10.1007/s10456-013-9407-0. PMID: 24232855. [DOI] [PubMed] [Google Scholar]
- 3.Brown G.T., Murray G.I. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2015;237(3):273–281. doi: 10.1002/path.4586. [DOI] [PubMed] [Google Scholar]
- 4.Alegre M.L., Frauwirth K.A., Thompson C.B. T-cell regulation by CD28 and CTLA-4. Nat. Rev. Immunol. 2001;1(3):220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 5.Aakanksha J., Chandrashekhar P. Innate control of adaptive immunity: beyond the three-signal paradigm. J. Immunol. 2017 May 15;198(10):3791–3800. doi: 10.4049/jimmunol.1602000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker L.S.K. Treg and CTLA-4: two intertwining pathways to immune tolerance. J. Autoimmun. 2013;45(100):49–57. doi: 10.1016/j.jaut.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fife B.T., Pauken K.E. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann. N. Y. Acad. Sci. 2011;1217:45–59. doi: 10.1111/j.1749-6632.2010.05919.x. [DOI] [PubMed] [Google Scholar]
- 8.Fife B.T., Bluestone J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008 Aug;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi Y., Kelsoe G. Role of germinal centers for the induction of broadly-reactive memory B cells. Curr. Opin. Immunol. 2017 Apr;45:119–125. doi: 10.1016/j.coi.2017.03.002. Epub 2017 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wingren A.G., Parra E., Varga M. T cell activation pathways: B7, LFA-3, and ICAM-1 shape unique T cell profiles. Crit. Rev. Immunol. 2017;37(2–6):463–481. doi: 10.1615/CritRevImmunol.v37.i2-6.130. [DOI] [PubMed] [Google Scholar]
- 11.Galon J., Mlecnik B., Bindea G. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J. Pathol. 2014;232(2):199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mlecnik B., Bindea G., Kirilovsky A. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci. Transl. Med. 2016;8(327) doi: 10.1126/scitranslmed.aad6352. [DOI] [PubMed] [Google Scholar]
- 13.Pan C., Liu H., Robins E. Next-generation immuno-oncology agents: current momentum shifts in cancer immunotherapy. J. Hematol. Oncol. 2020;13(1):29. doi: 10.1186/s13045-020-00862-w. Published 2020 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Elsas M.J., van Hall T., van der Burg S.H. Future challenges in cancer resistance to immunotherapy. Cancers. 2020;12(4):935. doi: 10.3390/cancers12040935. Published 2020 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359(6382):1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalathil S.G., Thanavala Y. High immunosuppressive burden in cancer patients: a major hurdle for cancer immunotherapy. Cancer Immunol. Immunother. 2016;65(7):813–819. doi: 10.1007/s00262-016-1810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirkovic J., Howitt B.E., Roncarati P. Carcinogenic HPV infection in the cervical squamo-columnar junction. J. Pathol. 2015;236(3):265–271. doi: 10.1002/path.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day P.M., Kines R.C., Thompson C.D. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8(3):260–270. doi: 10.1016/j.chom.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doorbar J., Quint W., Banks L. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30(Suppl 5):F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 20.Kjær S.K., Frederiksen K., Munk C., Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J. Natl. Cancer Inst. 2010;102(19):1478–1488. doi: 10.1093/jnci/djq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Canc. 2002;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 22.Ye L., Schnepf D., Staeheli P. Interferon-λ orchestrates innate and adaptive mucosal immune responses. Nat. Rev. Immunol. 2019;19(10):614–625. doi: 10.1038/s41577-019-0182-z. [DOI] [PubMed] [Google Scholar]
- 23.van der Burg S.H., Arens R., Ossendorp F., van Hall T., Melief C.J. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat. Rev. Canc. 2016;16(4):219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 24.de Jong A., van Poelgeest M.I., van der Hulst J.M. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Canc. Res. 2004;64(15):5449–5455. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 25.Trimble C.L., Clark R.A., Thoburn C. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J. Immunol. 2010;185(11):7107–7114. doi: 10.4049/jimmunol.1002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denny L.A., Franceschi S., de Sanjosé S., Heard I., Moscicki A.B., Palefsky J. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine. 2012;30(Suppl 5):F168–F174. doi: 10.1016/j.vaccine.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 27.Mollers M., Vossen J.M., Scherpenisse M., van der Klis F.R., Meijer C.J., de Melker H.E. Review: current knowledge on the role of HPV antibodies after natural infection and vaccination: implications for monitoring an HPV vaccination programme. J. Med. Virol. 2013;85(8):1379–1385. doi: 10.1002/jmv.23616. [DOI] [PubMed] [Google Scholar]
- 28.Roden R.B.S., Stern P.L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Canc. 2018;18(4):240–254. doi: 10.1038/nrc.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brisson M., Kim J.J., Canfell K. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395(10224):575–590. doi: 10.1016/S0140-6736(20)30068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arbyn M., Weiderpass E., Bruni L. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smola S., Trimble C., Stern P.L. Human papillomavirus-driven immune deviation: challenge and novel opportunity for immunotherapy. Ther Adv Vaccines. 2017;5(3):69–82. doi: 10.1177/2051013617717914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Litwin T.R., Clarke M.A., Dean M., Wentzensen N. Somatic host cell alterations in HPV carcinogenesis. Viruses. 2017;9(8):206. doi: 10.3390/v9080206. Published 2017 Aug 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau L., Gray E.E., Brunette R.L., Stetson D.B. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science. 2015;350(6260):568–571. doi: 10.1126/science.aab3291. [DOI] [PubMed] [Google Scholar]
- 34.Shaikh M.H., Bortnik V., McMillan N.A., Idris A. cGAS-STING responses are dampened in high-risk HPV type 16 positive head and neck squamous cell carcinoma cells. Microb. Pathog. 2019;132:162–165. doi: 10.1016/j.micpath.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Reiser J., Hurst J., Voges M., Krauss P., Münch P., Iftner T., Stubenrauch F. High-risk human papillomaviruses repress constitutive kappa interferon transcription via E6 to prevent pathogen recognition receptor and antiviral-gene expression. J. Virol. 2011;85(21):11372–11380. doi: 10.1128/JVI.05279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guess J.C., McCance D.J. Decreased migration of Langerhans precursor-like cells in response to human keratinocytes expressing human papillomavirus type 16 E6/E7 is related to reduced macrophage inflammatory protein-3alpha production. J. Virol. 2005;79(23):14852–14862. doi: 10.1128/JVI.79.23.14852-14862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karim R., Meyers C., Backendorf C. Human papillomavirus deregulates the response of a cellular network comprising of chemotactic and proinflammatory genes. PloS One. 2011;6(3) doi: 10.1371/journal.pone.0017848. Published 2011 Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sperling T., Ołdak M., Walch-Rückheim B. Human papillomavirus type 8 interferes with a novel C/EBPβ-mediated mechanism of keratinocyte CCL20 chemokine expression and Langerhans cell migration. PLoS Pathog. 2012;8(7) doi: 10.1371/journal.ppat.1002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moerman-Herzog A., Nakagawa M. Early defensive mechanisms against human papillomavirus infection. Clin. Vaccine Immunol. 2015;22(8):850–857. doi: 10.1128/CVI.00223-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cicchini L., Westrich J.A., Xu T., Vermeer D.W. Suppression of antitumor immune responses by human papillomavirus through epigenetic downregulation of CXCL14. mBio. 2016;7(3) doi: 10.1128/mBio.00270-16. e00270-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasegawa T., Feng Z., Yan Z., Ngo K.H., Hosoi J., Demehri S. Reduction in human epidermal Langerhans cells with age is associated with decline in CXCL14-mediated recruitment of CD14+ monocytes. J. Invest. Dermatol. 2020;140(7):1327–1334. doi: 10.1016/j.jid.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schröer N., Pahne J., Walch B., Wickenhauser C., Smola S. Molecular pathobiology of human cervical high-grade lesions: paracrine STAT3 activation in tumor-instructed myeloid cells drives local MMP-9 expression. Canc. Res. 2011;71(1):87–97. doi: 10.1158/0008-5472.CAN-10-2193. [DOI] [PubMed] [Google Scholar]
- 43.Walch-Rückheim B., Pahne-Zeppenfeld J., Fischbach J. STAT3/IRF1 pathway activation sensitizes cervical cancer cells to chemotherapeutic drugs. Canc. Res. 2016;76(13):3872–3883. doi: 10.1158/0008-5472.CAN-14-1306. [DOI] [PubMed] [Google Scholar]
- 44.Zijlmans H.J., Fleuren G.J., Baelde H.J., Eilers P.H., Kenter G.G., Gorter A. The absence of CCL2 expression in cervical carcinoma is associated with increased survival and loss of heterozygosity at 17q11.2. J. Pathol. 2006;208(4):507–517. doi: 10.1002/path.1918. [DOI] [PubMed] [Google Scholar]
- 45.Pahne-Zeppenfeld J., Schröer N., Walch-Rückheim B. Cervical cancer cell-derived interleukin-6 impairs CCR7-dependent migration of MMP-9-expressing dendritic cells. Int. J. Canc. 2014;134(9):2061–2073. doi: 10.1002/ijc.28549. [DOI] [PubMed] [Google Scholar]
- 46.Walch-Rückheim B., Mavrova R., Henning M. Stromal fibroblasts induce CCL20 through IL6/C/EBPβ to support the recruitment of Th17 cells during cervical cancer progression. Canc. Res. 2015;75(24):5248–5259. doi: 10.1158/0008-5472.CAN-15-0732. [DOI] [PubMed] [Google Scholar]
- 47.Yang W., Song Y., Lu Y.L., Sun J.Z., Wang H.W. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology. 2013;139(4):513–522. doi: 10.1111/imm.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrow M.P., Kraynyak K.A., Sylvester A.J. Clinical and immunologic biomarkers for histologic regression of high-grade cervical dysplasia and clearance of HPV16 and HPV18 after immunotherapy. Clin. Canc. Res. 2018;24(2):276–294. doi: 10.1158/1078-0432.CCR-17-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frey A.B. Suppression of T cell responses in the tumor microenvironment. Vaccine. 2015 Dec 16;33(51):7393–7400. doi: 10.1016/j.vaccine.2015.08.096. [DOI] [PubMed] [Google Scholar]
- 50.Crespo J., Sun H., Welling T.H., Tian Z., Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr. Opin. Immunol. 2013;25(2):214–221. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wherry E.J., Ha S.J., Kaech S.M. Molecular signature of CD8+ T cell exhaustion during chronic viral infection [published correction appears in Immunity. 2007;27(5):824. doi: 10.1016/j.immuni.2007.09.006. Immunity. 2007;27(4):670-684. [DOI] [PubMed] [Google Scholar]
- 52.Im S.J., Hashimoto M., Gerner M.Y. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hudson W.H., Gensheimer J., Hashimoto M. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1+ stem-like CD8+ T cells during chronic infection. Immunity. 2019;51(6):1043–1058. doi: 10.1016/j.immuni.2019.11.002. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Im S.J., Konieczny B.T., Hudson W.H., Masopust D., Ahmed R. PD-1+ stemlike CD8 T cells are resident in lymphoid tissues during persistent LCMV infection. Proc. Natl. Acad. Sci. U. S. A. 2020;117(8):4292–4299. doi: 10.1073/pnas.1917298117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jansen C.S., Prokhnevska N., Master V.A. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019;576(7787):465–470. doi: 10.1038/s41586-019-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siddiqui I., Schaeuble K., Chennupati V. Intratumoral Tcf1+PD-1+cd8+ T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 2019;50(1):195–211. doi: 10.1016/j.immuni.2018.12.021. e10. [DOI] [PubMed] [Google Scholar]
- 57.Galliverti G., Wullschleger S., Tichet M. Myeloid cells orchestrate systemic immunosuppression, impairing the efficacy of immunotherapy against HPV+cancers. Cancer Immunol Res. 2020;8(1):131–145. doi: 10.1158/2326-6066.CIR-19-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dalgleish A.G., Stern P.L. The failure of radical treatments to cure cancer: can less deliver more? Ther Adv Vaccines Immunother. 2018;6(5–6):69–76. doi: 10.1177/2515135518815393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azoury S.C., Straughan D.M., Shukla V. Immune checkpoint inhibitors for cancer therapy: clinical efficacy and safety. Curr. Cancer Drug Targets. 2015;15(6):452–462. doi: 10.2174/156800961506150805145120. [DOI] [PubMed] [Google Scholar]
- 60.Vaddepally R.K., Kharel P., Pandey R., Garje R., Chandra A.B. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020;12(3):738. doi: 10.3390/cancers12030738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shen X., Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. 2018;362:k3529. doi: 10.1136/bmj.k3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen E.E.W., Bell R.B., Bifulco C.B. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC) J Immunother Cancer. 2019;7(1):184. doi: 10.1186/s40425-019-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grywalska E., Sobstyl M., Putowski L., Roliński J. Current possibilities of gynecologic cancer treatment with the use of immune checkpoint inhibitors. Int. J. Mol. Sci. 2019;20(19):4705. doi: 10.3390/ijms20194705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saada-Bouzid E., Peyrade F., Guigay J. Immunotherapy in recurrent and or metastatic squamous cell carcinoma of the head and neck. Curr. Opin. Oncol. 2019;31(3):146–151. doi: 10.1097/CCO.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 65.Heeren A.M., Punt S., Bleeker M.C. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod. Pathol. 2016;29(7):753–763. doi: 10.1038/modpathol.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Esch E.M., van Poelgeest M.I., Trimbos J.B., Fleuren G.J., Jordanova E.S., van der Burg S.H. Intraepithelial macrophage infiltration is related to a high number of regulatory T cells and promotes a progressive course of HPV-induced vulvar neoplasia. Int. J. Canc. 2015;136(4):E85–E94. doi: 10.1002/ijc.29173. [DOI] [PubMed] [Google Scholar]
- 67.Heeren A.M., de Boer E., Bleeker M.C. Nodal metastasis in cervical cancer occurs in clearly delineated fields of immune suppression in the pelvic lymph catchment area. Oncotarget. 2015;6(32):32484–32493. doi: 10.18632/oncotarget.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heeren A.M., Koster B.D., Samuels S. High and interrelated rates of PD-L1+CD14+ antigen-presenting cells and regulatory T cells mark the microenvironment of metastatic lymph nodes from patients with cervical cancer. Cancer Immunol Res. 2015;3(1):48–58. doi: 10.1158/2326-6066.CIR-14-0149. [DOI] [PubMed] [Google Scholar]
- 69.Ang K.K., Harris J., Wheeler R. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oguejiofor K., Hall J., Slater C. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br. J. Canc. 2015;113(6):886–893. doi: 10.1038/bjc.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lyford-Pike S., Peng S., Young G.D., Taube J.M., Westra W.H., Akpeng B., Bruno T.C., Richmon J.D., Wang H., Bishop J.A., Chen L., Drake C.G., Topalian S.L. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Canc. Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ukpo O.C., Thorstad W.L., Lewis J.S., Jr. B7-H1 expression model for immune evasion in human papillomavirus-related oropharyngeal squamous cell carcinoma. Head Neck Pathol. 2013;7:113–121. doi: 10.1007/s12105-012-0406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Badoual C., Hans S., Merillon N. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Canc. Res. 2013;73(1):128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 74.Gooden M.J., de Bock G.H., Leffers N., Daemen T., Nijman H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br. J. Canc. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oguejiofor K., Galletta-Williams H., Dovedi S.J., Roberts D.L., Stern P.L., West C.M. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV- oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget. 2017;8(9):14416–14427. doi: 10.18632/oncotarget.14796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsakiroglou A.M., Fergie M., Oguejiofor K. Spatial proximity between T and PD-L1 expressing cells as a prognostic biomarker for oropharyngeal squamous cell carcinoma. Br. J. Canc. 2020;122(4):539–544. doi: 10.1038/s41416-019-0634-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cillo A.R., Kürten C.H.L., Tabib T. Immune landscape of viral- and carcinogen-driven head and neck cancer. Immunity. 2020;52(1):183–199. doi: 10.1016/j.immuni.2019.11.014. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miyauchi S., Sanders P.D., Guram K. HPV16 E5 mediates resistance to PD-L1 blockade and can Be targeted with rimantadine in head and neck cancer. Canc. Res. 2020;80(4):732–746. doi: 10.1158/0008-5472.CAN-19-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kamphorst A.O., Pillai R.N., Yang S. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc. Natl. Acad. Sci. U. S. A. 2017;114(19):4993–4998. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kamphorst A.O., Wieland A., Nasti T. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355(6332):1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhatt K.H., Neller M.A., Srihari S. Profiling HPV-16-specific T cell responses reveals broad antigen reactivities in oropharyngeal cancer patients. J. Exp. Med. 2020;217(10) doi: 10.1084/jem.20200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Jong A., van Poelgeest M.I., van der Hulst J.M. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Canc. Res. 2004;64(15):5449–5455. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 84.van der Burg S.H., Arens R., Ossendorp F., van Hall T., Melief C.J. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat. Rev. Canc. 2016;16(4):219–233. doi: 10.1038/nrc.2016.16. [DOI] [PubMed] [Google Scholar]
- 85.Clark K.T., Trimble C.L. Current status of therapeutic HPV vaccines. Gynecol. Oncol. 2020;156(2):503–510. doi: 10.1016/j.ygyno.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 86.van der Burg S.H., Melief C.J. Therapeutic vaccination against human papilloma virus induced malignancies. Curr. Opin. Immunol. 2011;23(2):252–257. doi: 10.1016/j.coi.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 87.Yang A., Farmer E., Lin J., Wu T.C., Hung C.F. The current state of therapeutic and T cell-based vaccines against human papillomaviruses. Virus Res. 2017;231:148–165. doi: 10.1016/j.virusres.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chabeda A., Yanez R.J.R., Lamprecht R., Meyers A.E., Rybicki E.P., Hitzeroth Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res. 2018;5:46–58. doi: 10.1016/j.pvr.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barra F., Della Corte L., Noberasco G. Advances in therapeutic vaccines for treating human papillomavirus-related cervical intraepithelial neoplasia. J. Obstet. Gynaecol. Res. 2020;46(7):989–1006. doi: 10.1111/jog.14276. [DOI] [PubMed] [Google Scholar]
- 90.Kobold S., Wiedemann G., Rothenfußer S., Endres S. Modes of action of TLR7 agonists in cancer therapy. Immunotherapy. 2014;6(10):1085–1095. doi: 10.2217/imt.14.75. [DOI] [PubMed] [Google Scholar]
- 91.Daayana S., Elkord E., Winters U. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br. J. Canc. 2010;102(7):1129–1136. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kenter G.G., Welters M.J., Valentijn A.R. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med. 2009;361(19):1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 93.van Poelgeest M.I., Welters M.J., Vermeij R. Vaccination against oncoproteins of HPV16 for noninvasive vulvar/vaginal lesions: lesion clearance is related to the strength of the T-cell response. Clin. Canc. Res. 2016;22(10):2342–2350. doi: 10.1158/1078-0432.CCR-15-2594. [DOI] [PubMed] [Google Scholar]
- 94.Poelgeest M.I., Welters M.J., van Esch E.M. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J. Transl. Med. 2013;11:88. doi: 10.1186/1479-5876-11-88. Published 2013 Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Welters M.J., van der Sluis T.C., van Meir H. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci. Transl. Med. 2016;8(334) doi: 10.1126/scitranslmed.aad8307. 334ra52. [DOI] [PubMed] [Google Scholar]
- 96.van Meir H., Nout R.A., Welters M.J. Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. OncoImmunology. 2016;6(2) doi: 10.1080/2162402X.2016.1267095. Published 2016 Dec 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Melief C.J.M., Welters M.J.P., Vergote I. Strong vaccine responses during chemotherapy are associated with prolonged cancer survival. Sci. Transl. Med. 2020;12(535) doi: 10.1126/scitranslmed.aaz8235. eaaz8235. [DOI] [PubMed] [Google Scholar]
- 98.Trimble C.L., Morrow M.P., Kraynyak K.A. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386(10008):2078–2088. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baruch E.N., Berg A.L., Besser M.J., Schachter J., Markel G. Adoptive T cell therapy: an overview of obstacles and opportunities. Cancer. 2017;123(S11):2154–2162. doi: 10.1002/cncr.30491. [DOI] [PubMed] [Google Scholar]
- 100.Pillai M., Davies M.M., Thistlethwaite F.C. Delivery of adoptive cell therapy in the context of the health-care system in the UK: challenges for clinical sites. Ther Adv Vaccines Immunother. 2020;8 doi: 10.1177/2515135520944355. PMID: 33015538; PMCID: PMC7509731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harris D.T., Kranz D.M. Adoptive T cell therapies: a comparison of T cell receptors and chimeric antigen receptors. Trends Pharmacol. Sci. 2016;37(3):220–230. doi: 10.1016/j.tips.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mo Z., Du P., Wang G., Wang Y. The multi-purpose tool of tumor immunotherapy: gene-engineered T cells. J. Canc. 2017;8(9):1690–1703. doi: 10.7150/jca.18681. Published 2017 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Draper L.M., Kwong M.L., Gros A. Targeting of HPV-16+ epithelial cancer cells by TCR gene engineered T cells directed against E6. Clin. Canc. Res. 2015;21(19):4431–4439. doi: 10.1158/1078-0432.CCR-14-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garrido F. HLA class-I expression and cancer immunotherapy. Adv. Exp. Med. Biol. 2019;1151:79–90. doi: 10.1007/978-3-030-17864-2_3. [DOI] [PubMed] [Google Scholar]
- 105.Rafiq S., Hackett C.S., Brentjens R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020;17(3):147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Halim L., Maher J. Therapeutic Advances in Vaccines and Immunotherapy. January 2020. CAR T-cell immunotherapy of B-cell malignancy: the story so far. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Melero I., Berman D.M., Aznar M.A. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat. Rev. Canc. 2015;15(8):457–472. doi: 10.1038/nrc3973. [DOI] [PubMed] [Google Scholar]
- 108.Cabo M., Offringa R., Zitvogel L., Kroemer G., Muntasell A., Galluzzi L. Trial Watch: immunostimulatory monoclonal antibodies for oncological indications. OncoImmunology. 2017;6(12) doi: 10.1080/2162402X.2017.1371896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abdollahpour-Alitappeh M., Lotfinia M., Gharibi T. Antibody-drug conjugates (ADCs) for cancer therapy: strategies, challenges, and successes. J. Cell. Physiol. 2019;234(5):5628–5642. doi: 10.1002/jcp.27419. [DOI] [PubMed] [Google Scholar]
- 110.Ciardiello D., Elez E., Tabernero J., Seoane J. Clinical development of therapies targeting TGFβ: current knowledge and future perspectives. Ann. Oncol. 2020 Oct;31(10):1336–1349. doi: 10.1016/j.annonc.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 111.Lippitz B.E. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14(6):e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 112.Conlon K.C., Miljkovic M.D., Waldmann T.A. Cytokines in the treatment of cancer. J. Interferon Cytokine Res. 2019;39(1):6–21. doi: 10.1089/jir.2018.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.van Meir H., Nout R.A., Welters M.J., Loof N.M., de Kam M.L., van Ham J.J. Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. OncoImmunology. 2017;6(2) doi: 10.1080/2162402X.2016.1267095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schuler P.J., Harasymczuk M., Schilling B., Saze Z., Strauss L., Lang S. Effects of adjuvant chemoradiotherapy on the frequency and function of regulatory T cells in patients with head and neck cancer. Clin. Canc. Res. 2013;19(23):6585–6596. doi: 10.1158/1078-0432.CCR-13-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zheng X., Guo Y., Wang L., Zhang H., Wang S., Wang L. Recovery profiles of T-cell subsets following low-dose total body irradiation and improvement with cinnamon. Int. J. Radiat. Oncol. Biol. Phys. 2015;93(5):1118–1126. doi: 10.1016/j.ijrobp.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 116.Spitzer M.H., Gherardini P.F., Fragiadakis G.K., Bhattacharya N., Yuan R.T., Hotson A.N. IMMUNOLOGY. An interactive reference framework for modeling a dynamic immune system. Science. 2015 Jul 10;349(6244):1259425. doi: 10.1126/science.1259425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abeler-Dörner L., Laing A.G., Lorenc A., Ushakov D.S., Clare S., Speak A.O. High-throughput phenotyping reveals expansive genetic and structural underpinnings of immune variation. Nat. Immunol. 2020 Jan;21(1):86–100. doi: 10.1038/s41590-019-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chimen M., Apta B.H., Mcgettrick H.M. Introduction: T cell trafficking in inflammation and immunity. Methods Mol. Biol. 2017;1591:73–84. doi: 10.1007/978-1-4939-6931-9_6. [DOI] [PubMed] [Google Scholar]
- 119.Jones E., Gallimore A., Ager A. Defining high endothelial venules and tertiary lymphoid structures in cancer. Methods Mol. Biol. 2018;1845:99–118. doi: 10.1007/978-1-4939-8709-2_7. [DOI] [PubMed] [Google Scholar]
- 120.Hayday A.C. Γδ T cell update: adaptate orchestrators of immune surveillance. J. Immunol. 2019 Jul15;203(2):311–320. doi: 10.4049/jimmunol.1800934. [DOI] [PubMed] [Google Scholar]
- 121.Coffelt S.B., Wellenstein M.D., de Visser K.E. Neutrophils in cancer: neutral no more. Nat. Rev. Canc. 2016 Jul;16(7):431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 122.Mitroulis I., Ruppova K., Wang B., Chen L.S., Grzybek M., Grinenko T. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell. 2018 Jan 11;172(1–2):147–161. doi: 10.1016/j.cell.2017.11.034. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020 Jun;20(6):375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.