Abstract
Eupalinilide E was assessed for ex-vivo expansion activity on hematopoietic stem cells (HSCs) from human cord blood (CB) CD34+ cells in serum-free, SCF, TPO and FL stimulated 7 day cultures. Eupalinilide E ex-vivo enhanced phenotyped (p) HSCs and glycolysis of CD34+ cells isolated 7 days after culture as measured by extracellular acidification rate, but did not alone show enhanced NSG engrafting capability of HSCs as determined by chimerism and numbers of SCID Repopulating cells, a quantitative measure of functional human HSCs. This is another example of pHSCs not necessarily recapitulating functional activity of these cells. Lack of effect on engrafting HSCs may be due to a number of possibilities, including down regulation of CXCR4 or of the homing capacity of these treated cells. However, Eupalinilide did act in an additive to synergistic fashion with UM171 to enhance ex vivo expansion of both pHSCs, and functionally engrafting HSCs. While reasons for the disconnect between pHSC and function of HSCs with Eupalinilide E alone cultured CB CD34+ cells is yet to be determined, the data suggest possible future use of Eupalinilide and UM171 together to enhance ex vivo production of CB HSCs for clinical hematopoietic cell transplantation.
Keywords: Hematopoietic Stem Cells, Ex Vivo Expansion, Eupalinilide E, UM171, Glycolysis
Introduction
Hematopoietic stem (HSCs) and progenitor (HPCs) cells and the process of hematopoiesis, in which mature blood cells are produced from HSCs and HPCs, are regulated by biologically active molecules such as cytokines/chemokines, and microenvironmental and accessory cells and their products [1–3]. Many of these biological molecules manifest their positive, negative and additive/synergistic effects through receptor-mediated intracellular signaling events [1]. Some of the biologically active molecules and the cells that they act on have been used to good advantage to enhance treatment of malignant and non-malignant disorders. However, there are other natural and synthesized agents that have been used to good advantage in combination with recombinant cytokines/chemokines to enhance in vitro, ex-vivo, and in vivo cellular functions, especially in context of ex vivo expansion of HSCs [4–18].
An active area of hematological studies is the enhancement of ex-vivo expansion of HSCs/HPCs for both pre-clinical [4–18] and clinical use [9, 19–21]. There are currently three sources of cells that have been used for clinical transplantation. This includes bone marrow (BM), cytokine and/or other reagent induced mobilized peripheral blood (mPB), and umbilical cord blood (CB) obtained at the birth of a baby. CB, which has pros and cons for its use, and which has been used to treat over 35,000 patients with malignant and non-malignant disorders, has a limitation in numbers of HSCs and HPCs collected in a single CB unit [4,5]. This may in part be responsible for the delayed time to neutrophil, platelet and immune cell recovery compared to that of BM and mPB. Hence, there have been a number of preclinical studies to expand numbers of CB HSC and HPC ex vivo, some such as SR1, UM171, and nicotinamide which are currently in clinical trials [19–21]. None of the ex vivo procedures work well, if at all, without addition of cytokines such as stem cell factor (SCF), thrombopoietin (TPO), and Flt3-ligand (FL) during the ex vivo culture period. Hence, the need to add the reagent of choice with SCF, TPO, and FL during the ex vivo culture period. Use of serum free cultures and a short time of cell culture has benefits for potential use of the derived cells for clinical applicability.
In a collaborative effort, we assessed the effectiveness of Eupalinilide E to enhance the 7 day ex vivo expansion of human CB HSCs using serum-free culture medium in the presence of SCF, TPO and FL. Eupalinilide E was originally isolated from Eupatorium lindleyanum, a plant which was shown to have use as an antibacterial and antihistamine, with selective antiproliferative activity against an established lung cancer cell line with a KRAS mutation [22]. Eupalinilide E was synthetically produced [22] and promoted an increase in cytokine stimulated phenotypically-defined human CB CD34+ cells and HPCs (assessed through in vitro colony assays) [22–25]. However, none of these studies demonstrated whether or not HSCs were actually expanded. We used very rigorously-defined phenotypes of HSCs and multi-potent progenitor (MPP cells), and engraftment studies of input HSCs and 7 day ex-vivo output cultures in sublethally-irradiated NSG immune deficient mice to assess human cell chimerism, and limiting dilution analysis of input and 7 day ex vivo cultured output to calculate SCID Repopulating Cells (SRC, a quantitative measure of numbers of functionally active human HSCs). Our results show that Eupalinalide at appropriate concentrations enhanced cytokine stimulated- ex vivo expansion of phenotyped HSCs induced by Eupalinalide E, with increased glycolysis noted in CD34+ cells isolated after 7 days. While showing no enhanced engrafting capacity after ex vivo culture, Eupalinilide E acted in an additive to synergistic fashion when combined with UM171 for numbers of phenotyped and functionally engrafting HSCs in NSG mice.
Materials and Methods
Mice.
6–8 weeks old NSG (NOD.Cg-PrkdcscidIL2rgtm1Wjl/SzJ) mice were supplied by the In Vivo Therapeutics Core of the Indiana University School of Medicine (IUSM; supported in part by DK U54 106846; NIDDK Cooperative Centers of Excellence in Hematology), and maintained in the Laboratory Animal Resource Center (LARC) at IUSM. All experimental protocols were approved by The Institutional Animal Care and Use Committee of IUSM.
Eupalinilide E was synthesized starting from (R)-carvone as previously described [22].
Isolation of human CB CD34+ cells and in vitro cell culture
Normal human cord blood samples were obtained from Cord:Use Cord Blood Bank (Orlando, FL, USA). Mononuclear cells were isolated by density-gradient centrifugation over Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ, USA). CD34+ cells were enriched with immunomagnetic selection kit (Miltenyi Biotec, Auburn, CA, USA) following manufacturer’s instructions. Freshly isolated CD34+ cells were seeded at the density of 50,000 cells/mL into 24‐well plates with 1 mL serum‐free medium (StemSpan™ SFEM II, STEMCELL Technologies Inc, Vancouver, BC, Canada), which was supplemented with 100 ng/mL SCF (#7466-SC-010/CF, R&D Systems, Minneapolis, MN, USA), 100 ng/mL TPO (#288-TP-200/CF, R&D Systems), 100 ng/mL FL (#710802, BioLegend, San Diego, CA, USA) with a vehicle control, Eupalinilide E, UM171 (35nM) (#72914, STEMCELL Technologies Inc) alone, or in combination for 7 days. Eupalinilide E doses of 0.6, 1, 1.2, and 2.4 μM were tested. UM171 was used for possible additive/synergistic effects. Dimethyl sulfoxide (DMSO) (#D2650, Sigma-Aldrich, St. Louis, MO, USA) was used as vehicle control. Cells were cultured at 37°C in a humidified atmosphere containing lowered (5%) O2 and 5% CO2.
Immunostaining and Flow cytometry.
Immunostaining and flow cytometry analysis were performed as previously described. Cells were counted on day 7 and collected by centrifugation at 300 g for 10 min, washed twice with cold PBS, and resuspended in 200 μL of PBS. Cells were stained at 4°C for 30 minutes with the following fluorescence conjugated antibodies: anti-Lineage cocktail (Lin)-FITC, anti-CD34-APC (581), anti-CD38-PE (HIT2), anti-CD45RA-PE-CF594 (HI100), anti-CD90-PE-Cy7 (5E10), anti-CD49f-PerCP-Cy5.5 (GoH3), anti-CD45-APC (HI30), anti-CD3-FITC (UCHT1), anti-CD19-PE (HIB19) and anti-CD33-PE-Cy7 (WM53). All the antibodies were purchased from BD Bioscience (San Jose, CA, USA). Cells were washed with cold PBS and cell pellets were fixed with 1% formaldehyde. Samples were analyzed on an LSR4 flow cytometer (BD Biosciences). By flow analysis, we can determine the frequencies and numbers of CD34+ cells, CD34+CD38− cells, pHSCs (Lin−CD34+CD38−CD45RA−CD49f+CD90+) and pMPPs (Lin−CD34+CD38−CD45RA−CD49f−CD90−).
In vivo transplantation.
The progeny cells of 50,000 CD34+ CB cells before ex-vivo culture and after being treated with vehicle, Eupalinilide E, UM171 or Eupalinilide E plus UM171 for 7 days were intravenously transplanted into sublethally irradiated NSG recipient mice (350 cGy; 137Cs source, single dose). Four months after transplantation, recipient mice were sacrificed and the percentage of human CD45+, CD33+, CD19+ and CD3+ cells in the bone marrow was assessed by immunostaining and flow cytometry studies.
Limiting dilution analysis.
The frequency of human SCID repopulating cells (SRCs) was determined by limiting dilution analysis (LDA) as previously reported [17,26–30]. Increasing doses of uncultured, vehicle, Eupalinilide E, UM171 or Eupalinilide E plus UM171 treated CD34+ cells (500, 2,500 or 10,000 cells) were intravenously injected into sublethally irradiated NSG recipient mice (350 cGy; 137Cs source, single dose). The percentage of human CD45+ cell chimerism was analyzed by immuno-staining and flow cytometry 4 months after transplantation. Based on our immuno-staining and flow cytometry analysis, we found that negative control samples sometimes show 0.1~0.3 % human CD45+ cells. To exclude non-specific staining, mice were considered positive when at least 1% CD45+ human cells were detected among the mouse BM cells, unless otherwise indicated. SRCs was calculated using L-Calc software (Stem Cell Technologies Inc, Vancouver, BC, Canada) and plotted using ELDA software (bioinf.wehi.edu.au/software/elda/).
Seahorse extracellular flux assay.
The extracellular acidification rate (ECAR) or oxygen consumption rate (OCR) of human CB CD34+ cells was determined using a Seahorse XF Extracellular Flux Analyzer (Agilent Technologies) [30]. XF Calibrant buffer 200 μL (#100840–000, Seahorse Bioscience) was added into the wells of a Seahorse Bioscience 96-well utility plate, and then the plate was incubated at 37℃ overnight. The cell culture plate (#101085–004, Seahorse Bioscience) was coated with Cell-Tak cell and tissue adhesive solution (#354241, CORNING, NY, USA) at room temperature for one hour. Ex vivo expanded CB CD34+ cells were purified using human CD34 MicroBead Kit (Miltenyi Biotec, Auburn, CA, USA), cells were resuspended with assay medium, and cell number was determined using a hemacytometer. 100,000 purified CD34+ cells per well were plated into cell culture plates. The plates were centrifuged at 1,000 g for 10 min. Glucose (#G8644, Sigma-Aldrich), oligomycin (#75351, Sigma-Aldrich), 2-DG (#D8375, Sigma-Aldrich) medium or oligomycin, FCCP (#C2920, Sigma-Aldrich), rotenone (#R8875, Sigma-Aldrich) medium was sequentially added into the wells of a utility plate for ECAR or OCR analysis. The utility plate with the loaded sensor cartridge was placed on the instrument tray for calibration. When prompted, replace the calibration plate with the cell culture microplate then click “Start” to evaluate ECAR or OCR of CB CD34+ cells.
Statistical analysis.
Statistical comparisons were performed using the Student t-tests or one‐way analysis of variance (ANOVA). Two-tailed Students t-tests were performed for statistical analysis between two groups. One-way analysis of variance (ANOVA) was used to compare differences in means between more than two groups where indicated. Results are expressed as mean values ± standard deviation (SD). A P value less than 0.05 was considered as statistically significant (e.g., *p<0.05, **p<0.01, #p<0.05, ##p<0.01).
Results and Discussion
While there are a number of ex vivo HSC/HPC expansion studies reported in a preclinical situation [4–18] and a few now reported for clinical transplantation for CB HCT [9, 19–21], it is not at all clear which, if any of these procedures will be of wide-use nationally and internationally in context of CB HCT, or for BM HCT in cases where there are limited numbers of BM cells collected. We were intrigued by a few studies that reported that Eupalinalide E, originally isolated from the plan Euratorium lindleyanum, and then synthesized might have a capacity for some ex vivo expansion of CB HPCs [22–25]. While these reports mentioned effects on CB HSCs, this was based on an imprecise phenotypic description of HSCs, mainly using CD34 as a marker, when HSCs are a rare population within CD34+ cell populations. Moreover, no functional activity of the in vivo engrafting capacity of these human cells were undertaken in immune deficient mice. Thus, in a collaborative effort between groups, we assessed the capability of Eupalinalide E to ex vivo expand rigorously defined phenotypic and NSG mouse engrafting capability of human CB HSCs in a serum free cytokine (SCF, TPO, FL) stimulated culture system by comparing absolute numbers of input (Day 0) vs. output (Day 7 cultured) CB cells.
Effects on phenotyped HSC and HPC cells.
The experimental procedure was diagrammed in Fig. 1A for input vs. output of CD34+ (Fig. 1B), CD34+CD38− (more enriched populations for HSCs; Fig. 1C), rigorously phenotyped (p) HSCs (Fig. 1D) and multipotential progenitor (pMPPs) cells (Fig. 1E). The combination of SCT, TPO, and FL significantly enhanced ex vivo expansion output compared to Day 0 input cells, and this increase was significantly enhanced by 0.6uM-1uM Eupalinilide E, for CD34+ and CD34+CD38− cells, by 0.6uM-2.4uM for pHSCs and by 0.6uM-1.2uM for pMPPs. UM171 is a potent ex vivo HSC expansion reagent [14] being tested in clinical trials [21], so we assessed the effects of Eupalinilide E and UM171, each alone and in combination on the ex-vivo expansion (diagrammatically represented in Fig. 2A) of CD34+ CB cells for effects on CD34+ cells (Fig. 2B), CD34+CD38− cells (Fig. 2C), pHSCs (Fig. 2D), and pMPPs (Fig. 2E). Eupalinilide E and UM1717, each alone significantly enhanced these phenotyped immature cell populations (Fig. 2B–E). Of interest, the combination of these two reagents had an additive effect on expanding the pHSC population (Fig. 2D).
Figure 1. Dose-response effect of Eupalinilide E on ex vivo expansion of cytokine stimulated human phenotyped CB HSCs and HPCs.
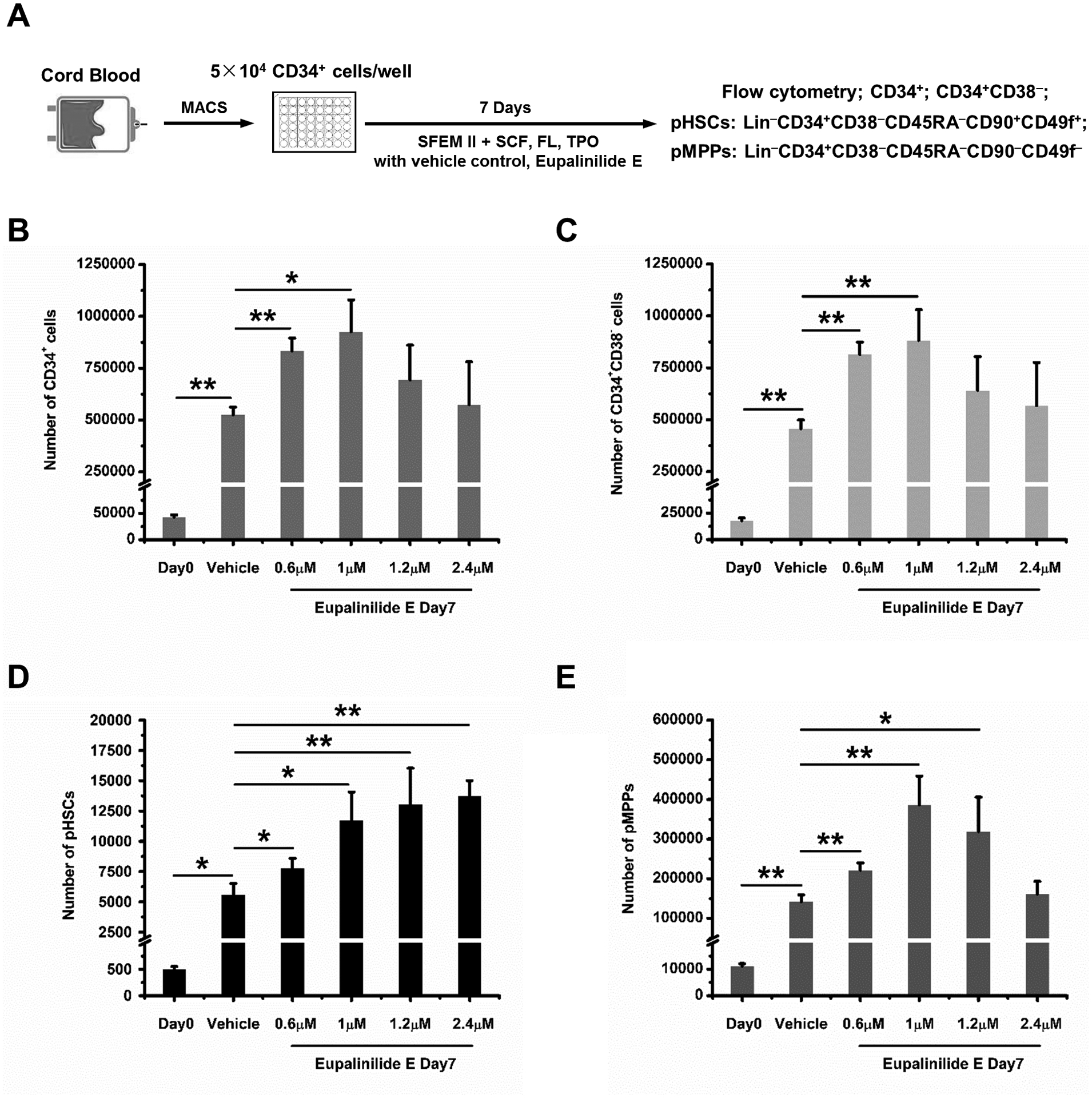
(A) Experimental strategy for evaluating the dose-response effect of Eupalinilide E on human CB HSCs and HPCs ex-vivo expansion. Frequencies of CD34+, CD34+CD38−, phenotypic (p) HSCs (Lin−CD34+CD38−CD45RA−CD49f+CD90+), and MPPs (Lin−CD34+CD38−CD45RA−CD49f−CD90−) were determined. (B-E) 50,000 CB CD34+ cells per well, were put into suspension culture at day 0, and input (Day 0) and output (Day 7) numbers calculated by total cell number counting, immunostaining and flow cytometry analysis. Data were pooled from 4 independent experiments and shown as mean ± standard deviation (SD) (n=triplicates per experiment). One-way analysis of variance (ANOVA). Significance, * p<0.05, ** p<0.01.
Figure 2. Cytokine stimulated ex-vivo expansion of human CB CD34+ cells cultured with Eupalinilide E, UM171, each alone or in combination.
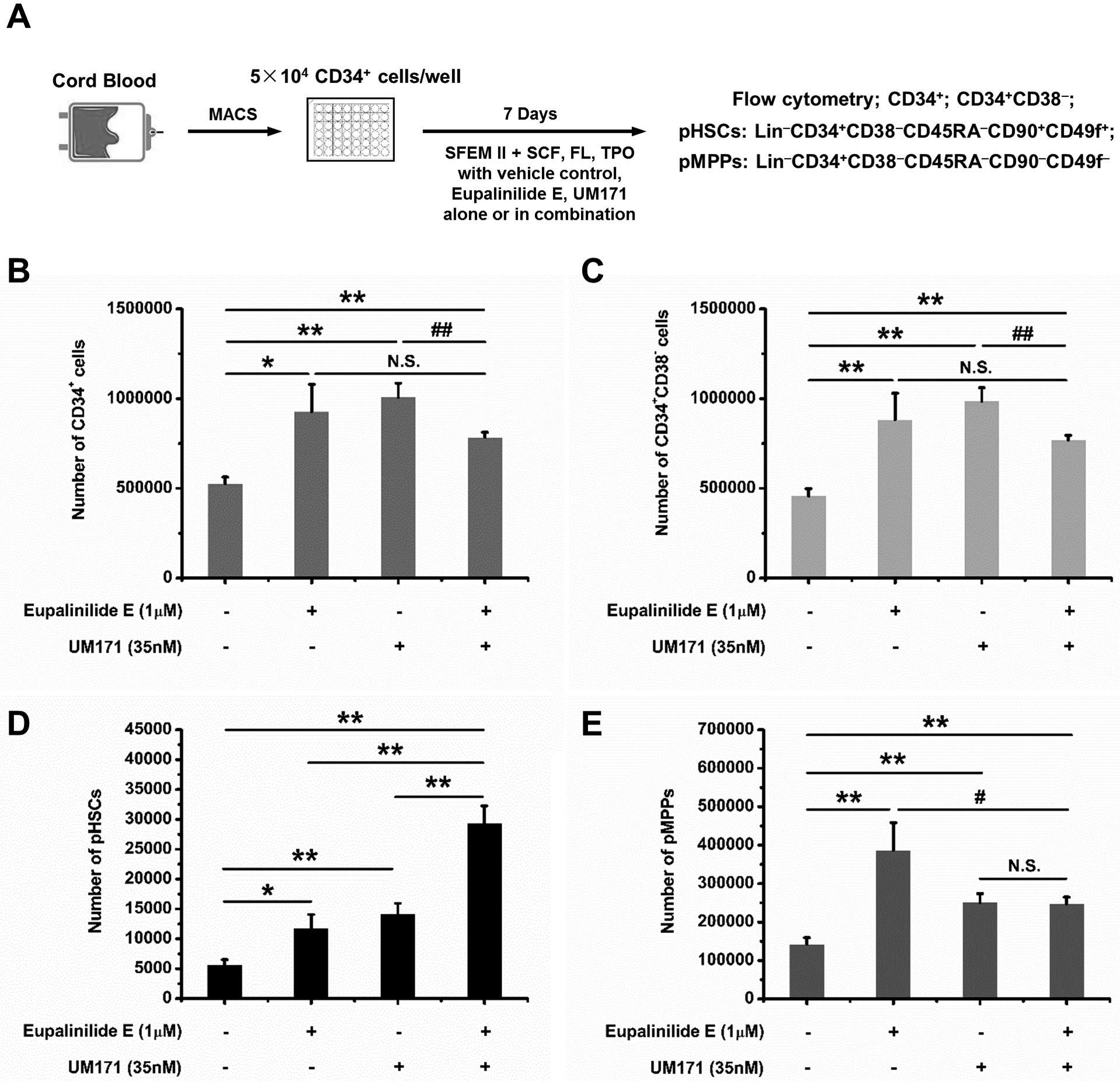
(A) Schematic illustration of human CB CD34+ cell ex vivo expansion. (B-E) Quantification data of ex-vivo expansion of CB CD34+ cells, CD34+CD38− cells, pHSCs, and pMPPs in presence of vehicle, Eupalinilide E, UM171, alone or in combination for 7 days. Data were pooled from three independent experiments, shown as mean ± SD (triplicates per experiment). One-way ANOVA. N.S., not significant; Significance: * p<0.05, ** p<0.01; Hash mark indicates statistically significant decrease, # p<0.05, ## p<0.01.
Effects on NSG engrafting human CB CD34+ cells.
HSC phenotype does not always recapitulate their functional engrafting capability [29,31], so as diagrammed in Fig. 3A, we assessed the NSG engrafting capability of cells from SCF, TPO, and FL ex vivo cultures set up in the presence of Eupalinilide E and UM171 alone and in combination. A sample flow analysis of human CD45 engraftment is shown in Fig. 3B. The combination of the two had synergistic effects on engraftment in BM of primary sublethally-irradiated NSG mice at month 4 of engraftment (Fig. 3C; by using a two-way analysis of variance (ANOVA) in SAS version 9.4, the combined Eupalinilide E and UM171 effects on percentage of human CD45 chimerism produced a statistically significant positive interaction effect, which suggested a synergistic rather than an additive effect) and on the % of myeloid cells (Fig. 3D), T-cells (Fig. 3E) and B-cells (Fig. 3F) 4 months after in vivo engraftment, but with no apparent effects on the resultant myeloid/lymphoid ratio (Fig. 3G). Limiting dilution analysis of donor cell populations allows one to calculate out numbers of SRCs (a quantitative measure of engrafted human HSCs) [17,27–30]. The numbers of SRCs were significantly enhanced by UM171, and the combination of UM171 plus Eupalinilide E resulted in additive to synergistic increases in ex vivo expansion of SRCs.
Figure 3. Engraftment of cytokine stimulated human CB CD34+ cells cultured with Eupalinilide E, UM171, alone or in combination, in sublethally irradiated NSG mice.
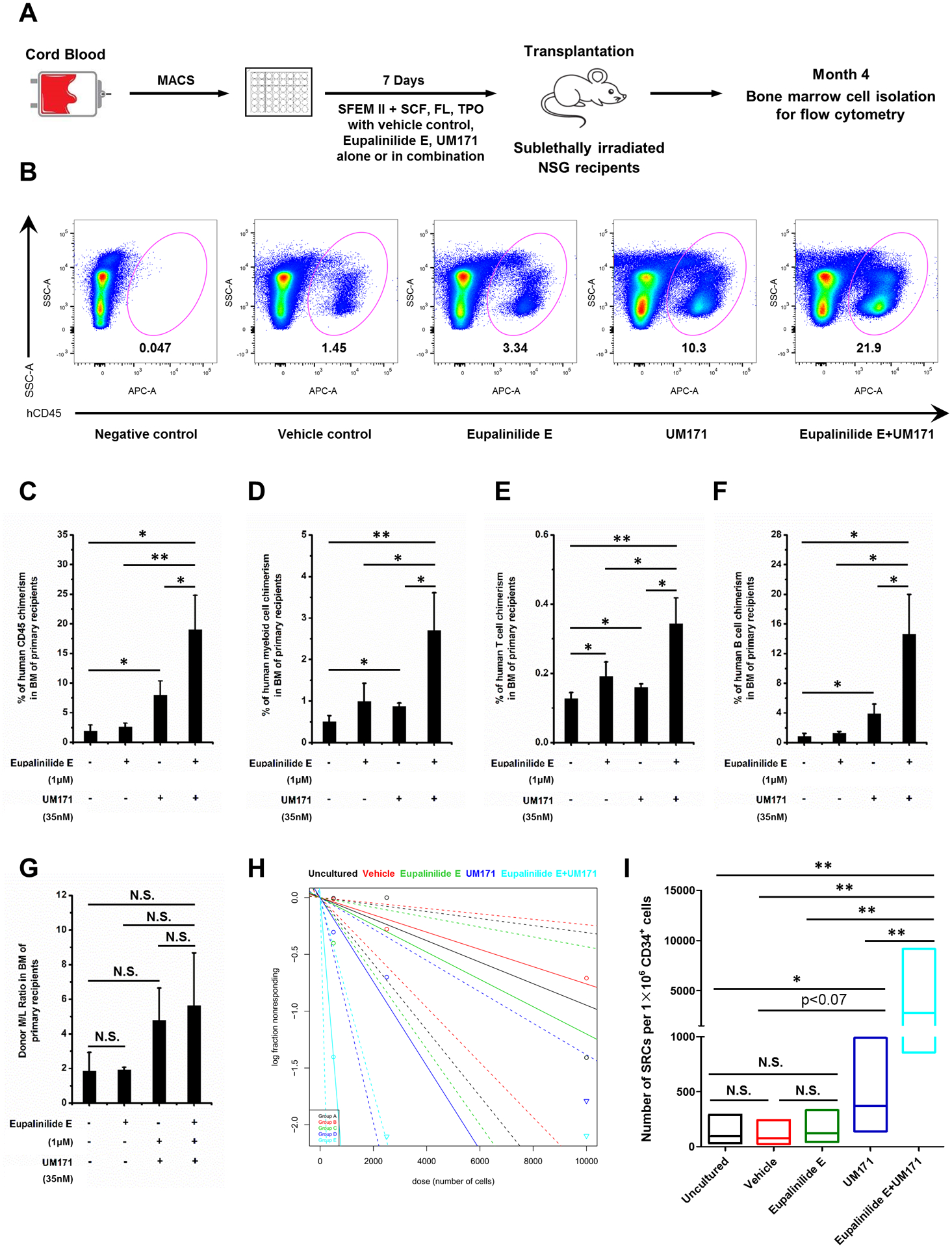
Schematic representation of NSG mice transplanted with progeny of 50,000 vehicle-, Eupalinilide E-, UM171- or Eupalinilide E plus UM171-treated human CB CD34+ cells. Human engraftment in BM of NSG mice was analyzed 4 months after transplantation. (B) Representative FACS plots (from n=4 independent experiments) showing percentage engraftment of vehicle-, Eupalinilide E-, UM171- or Eupalinilide E plus UM171-treated human CB CD34+ cells in the BM of recipient NSG mice. BM cells from mice that did not undergo transplantation were used as the negative control. (C) The percentage of human CD45+ cells in BM at 4 months after transplantation of NSG mice with the progeny of 50,000 CB CD34+ cells that were treated with vehicle, Eupalinilide E, UM171, alone or in combination for 7 days (n= 4 mice per group). One-way ANOVA. * p<0.05, ** p<0.01. (D) Myeloid cell (CD33+), (E) T cell (CD3+) and (F) B cell (CD19+) chimerism in NSG mice bone marrow 4 months after transplantation (n=4 mice per group). One-way ANOVA. * p<0.05, ** p<0.01. (G) Donor Myeloid/Lymphoid (M/L) ratio in BM of NSG recipient mice 4 months after transplantation with progeny of CB CD34+ cells cultured with vehicle, Eupalinilide E, UM171, alone or in combination for 7 days (n= 4 mice per group). One-way ANOVA. N.S., not significant. Plot (H) and quantification (I) of frequency of human SRCs in uncultured CB CD34+ cells (black line; group A) or in progeny of an equivalent number of CD34+ cells treated with vehicle (red line; group B), Eupalinilide E (green line; group C), UM171 (dark blue line; group D) or Eupalinilide E plus UM171 (light blue line; group E) for 7 days. Graded doses of uncultured or of vehicle-, Eupalinilide E-, UM171- or Eupalinilide E plus UM171-treated CB CD34+ cells were transplanted into irradiated NSG mice, and the percentage of human CD45+ cells in BM was analyzed at 4 months after transplantation (n=4 mice per group). Poisson statistical analysis of data is shown in Tables 1 and Table 2. The percentage of mice that show negative engraftment for each dose of cells is plotted. Solid lines represent the best-fit linear model for each group, dotted lines indicate 95% confidence intervals. (I) HSC frequencies (line in the box) and 95% confidence intervals (box) presented as numbers of SRCs in 1×106 CD34+ cells. N.S., not significant; * p<0.05, ** p<0.01; by Poisson distribution analysis.
However, Eupalinilide by itself, while having significant enhancing effects on pHSCs (Fig. 1D and 2D), did not show expansion of functional engrafting HSCs (Fig. 3C, H, and I). This thus provides another example to that of phenotyped HSCs not recapitulating their functional engrafting capabilities [29,31]. The reason for this phenotype/functional assessment disconnect is not at present known, although it could possibly reflect changes in the homing capability of the ex vivo cultured cells exposed to Eupalinilide E alone, effects that will have to be determined in future experiments.
Metabolic analysis of Eupalinilide E effects on ex vivo cultured human CB CD34+ cells.
To gain mechanistic insight into how Eupalinilide E promotes ex vivo expansion of phenotyped HSCs, we assessed the metabolic effects of Eupalinilide E, UM171, and the combination of Eupalinilide and UM171 on cultured cells, using a Seahorse extracellular flux assay to measure extracellular acidification rate (ECAR; Fig. 4A and C) and oxygen consumption rate (OCR; Fig. 4B). Eupalinilide E demonstrated increased ECAR (Fig. 4A and C), with no effect on OCR (Fig. 4B) on isolated CB CD34+ cells cultured after 7 days compared to vehicle control cultured cells (all cultures containing SCF, TPO, and FL). While further mechanistic insight into the actions of Eupalinilide E are needed, this suggests that Eupalinilide E enhances glycolysis without compromising mitochondrial metabolism. UM171 alone had no effect on ECAR and the combination of Eupalinilide E plus UM171 was similar to that of Eupalinilide E alone.
Figure 4. Culture of human CB CD34+ cells with Eupalinilide E for 7 days switches on glycolysis.
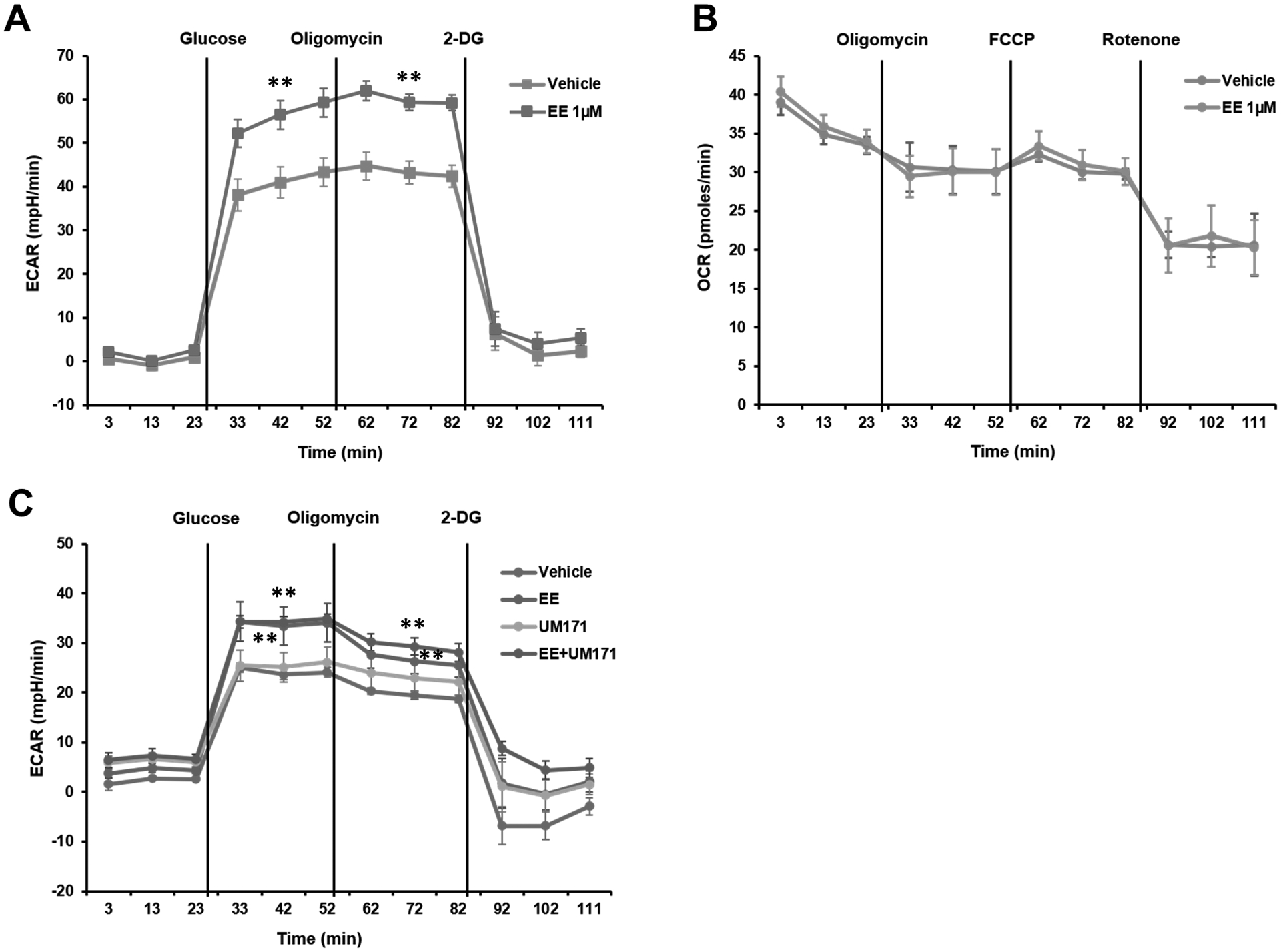
(A) ECAR measurements in purified CB CD34+ cells following a 7 day culture with vehicle or Eupalinilide E (1 μM). Data pooled from three independent experiments are shown as mean ± SD. Two-tailed Student’s t-test. ** indicates p<0.01. Oligomycin is an inhibitor of ATP synthase. 2-DG, 2-deoxyglucose. (B) OCR measurements in purified CB CD34+ cells following a 7 day culture with vehicle or Eupalinilide E. Carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) is a potent mitochondrial oxidative phosphorylation uncoupler; rotenone is an inhibitor of mitochondrial respiratory chain complex I. Representative data from three independent experiments are shown as mean ± SD. (C) ECAR measurements in purified CB CD34+ cells following a 7-d culture with vehicle, Eupalinilide E, UM171 (35 nM), alone or in combination. Data pooled from three independent experiments are shown as mean ± SD. One-way ANOVA. ** indicates p<0.01 versus vehicle group.
Table 1A.
Determination of SRC frequency determined by limiting dilution assay in NSG mice.
| Culture conditions | Cell transplanted | Number of mice with >1% human cell chimerism/total number of mice | |
|---|---|---|---|
| Day 0 Uncultured | 500 | 0/3 | |
| 2500 | 0/3 | ||
| 10000 | ¾ | ||
| Number of CD34+ cells at starting | Number of CD34+ cells at transplanted | ||
| Vehicle control | 500 | 1.9×104±1.8×103 | 0/4 |
| 2500 | 8.9×104±9.1×103 | ¼ | |
| 10000 | 3.6×105±3.6×104 | 2/4 | |
| Eupalinilide E | 500 | 1.5×104±2.8×103 | 1/3 |
| 2500 | 7.7×104±1.4×104 | 0/4 | |
| 10000 | 3.1×105±5.6×104 | ¾ | |
| UM171 | 500 | 1.3×104±1.1×103 | ¼ |
| 2500 | 6.3×104±5.4×103 | 2/4 | |
| 10000 | 2.5×105±2.2×104 | 3/3 | |
| Eupalinilide E +UM171 | 500 | 9.0×103±0.3×103 | ¾ |
| 2500 | 4.5×104±1.7×103 | 4/4 | |
| 10000 | 1.8×105±6.9×103 | 4/4 | |
Calculations (Table 1A) were from input (Day 0) CB CD34+ cells, and from output (Day 7) of cytokine stimulated cultures in presence of vehicle control, Eupalinilide E, UM171, and Eupalinilide E plus UM171.
Table 1B.
SRC frequency for input (Day 0) vs. output (7 day cultured) cells.
| Culture conditions | SRC frequency | 95% confidence interval | Number of SRC in 1×106 CD34+ starting cells | SRC frequency in total number of cells transplanted | 95% confidence interval of cells transplanted |
|---|---|---|---|---|---|
| Day 0 Uncultured | 1/10555 | 1/32471–1/3431 | 95 | 1/10555 | 1/32471–1/3431 |
| Vehicle control | 1/13152 | 1/41826–1/4135 | 76 | 1/499776 | 1/1493188–1/147620 |
| Eupalinilide E | 1/8329 | 1/23114–1/3001 | 120 | 1/249870 | 1/709190–1/91958 |
| UM171 | 1/2701 | 1/7229–1/1009 | 370 | 1/70226 | 1/182171–1/25427 |
| Eupalinilide E +UM171 | 1/357 | 1/1166–1/109 | 2801 | 1/6426 | 1/21044–1/1967 |
Calculations (Table 1B) were from input (Day 0) CB CD34+ cells, and from output (Day 7) of cytokine stimulated cultures in presence of vehicle control, Eupalinilide E, UM171, and Eupalinilide E plus UM171. SRC frequency was calculated by Poission statistics from the data provided in Table 1A using L-Calc and ELDA software.
Highlights.
Ex vivo expansion of HSCs has potential clinical applicability
Eupalinilide E enhances ex vivo expansion of phenotypic cord blood (CB) HSCs.
Eupalinilide E increases CB CD34+ cells.
Eupalinilide E and UM171 additively/synergistically enhance HSC expansion.
Acknowledgments
These studies were supported by US Public Health Service Grants to HEB: R35 HL139599, R01 DK 109188, and U54 DK 106846. MLC was supported by T32 DK 007519 (to HEB) for some of these studies.
Footnotes
Competing Interest Statement
Neither: JZ, XH, BG, SC, MLC, TCJ, DRS, or HEB have any conflicts of interest.
References
- 1.Shaheen M, Broxmeyer HE. Cytokine/Receptor Families and Signal Transduction. In: Hematology: Basic Principles and Practice. 7th Edition (Hoffman R, Benz E, Silberstein L, Heslop H, Weitz JI, and Anastasi J, Salama ME, and Abutalib SA, Editors). 2018;Chapter 16. Pages 163–175. [Google Scholar]
- 2.Shaheen M, Broxmeyer HE. Hematopoietic Cytokines and Growth Factors. In: Cord Blood Biology, Transplantation, Banking, and Regulation. (Editor: Broxmeyer HE). AABB Press, Bethesda, MD. 2011;pp. 35–74. [Google Scholar]
- 3.Wei Q, Frenette PS. Niches for Hematopoietic Stem Cells and Their Progeny. Immunity. 2018;48(4):632‐648. doi: 10.1016/j.immuni.2018.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broxmeyer HE, Milano F. 2020. Cord Blood Transplantation: State of the Science. Cord Blood Association (CBA). In Press. Available at http://www.cb-association.org/state-of-the-science. On-line March 2020. [Google Scholar]
- 5.Mayani H, Wagner JE, Broxmeyer HE. Cord blood research, banking, and transplantation: achievements, challenges, and perspectives. Bone Marrow Transplant. 2020;55(1):48‐61. doi: 10.1038/s41409-019-0546-9 [DOI] [PubMed] [Google Scholar]
- 6.Broxmeyer HE, Hoggatt J, O’Leary HA, et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med. 2012;18(12):1786‐1796. doi: 10.1038/nm.2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo B, Huang X, Lee MR, Lee SA, Broxmeyer HE. Antagonism of PPAR-γ signaling expands human hematopoietic stem and progenitor cells by enhancing glycolysis. Nat Med. 2018;24(3):360‐367. doi: 10.1038/nm.4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang X, Guo B, Capitano M, Broxmeyer HE. Past, present, and future efforts to enhance the efficacy of cord blood hematopoietic cell transplantation. F1000Res. 2019;8:F1000 Faculty Rev-1833. Published 2019 Oct 31. doi: 10.12688/f1000research.20002.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cutler C, Multani P, Robbins D, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122(17):3074‐3081. doi: 10.1182/blood-2013-05-503177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(2):232‐236. doi: 10.1038/nm.2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam P, Horwitz ME. Small-molecule nicotinamide for ex vivo expansion of umbilical cord blood. Exp Hematol. 2019;80:11‐15. doi: 10.1016/j.exphem.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 12.Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells [published correction appears in Science. 2011 May 6;332(6030):664]. Science. 2010;329(5997):1345‐1348. doi: 10.1126/science.1191536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stiff PJ, Montesinos P, Peled T, et al. Cohort-Controlled Comparison of Umbilical Cord Blood Transplantation Using Carlecortemcel-L, a Single Progenitor-Enriched Cord Blood, to Double Cord Blood Unit Transplantation. Biol Blood Marrow Transplant. 2018;24(7):1463‐1470. doi: 10.1016/j.bbmt.2018.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fares I, Chagraoui J, Gareau Y, et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science. 2014;345(6203):1509‐1512. doi: 10.1126/science.1256337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo B, Huang X, Lee MR, Lee SA, Broxmeyer HE. Antagonism of PPAR-γ signaling expands human hematopoietic stem and progenitor cells by enhancing glycolysis. Nat Med. 2018;24(3):360‐367. doi: 10.1038/nm.4477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bari S, Zhong Q, Fan X, et al. Ex Vivo Expansion of CD34+ CD90+ CD49f+ Hematopoietic Stem and Progenitor Cells from Non-Enriched Umbilical Cord Blood with Azole Compounds. Stem Cells Transl Med. 2018;7(5):376‐393. doi: 10.1002/sctm.17-0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capitano ML, Mor-Vaknin N, Saha AK, et al. Secreted nuclear protein DEK regulates hematopoiesis through CXCR2 signaling. J Clin Invest. 2019;129(6):2555‐2570. Published 2019 May 20. doi: 10.1172/JCI127460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Qian P, Shao W, et al. Suppression of m6A reader Ythdf2 promotes hematopoietic stem cell expansion [published correction appears in Cell Res. 2018 Aug 27;:]. Cell Res. 2018;28(9):904‐917. doi: 10.1038/s41422-018-0072-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner JE Jr, Brunstein CG, Boitano AE, et al. Phase I/II Trial of StemRegenin-1 Expanded Umbilical Cord Blood Hematopoietic Stem Cells Supports Testing as a Stand-Alone Graft. Cell Stem Cell. 2016;18(1):144‐155. doi: 10.1016/j.stem.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horwitz ME, Chao NJ, Rizzieri DA, et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest. 2014;124(7):3121‐3128. doi: 10.1172/JCI74556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen S, Roy J, Lachance S, et al. Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1–2 safety and feasibility study. Lancet Haematol. 2020;7(2):e134‐e145. doi: 10.1016/S2352-3026(19)30202-9 [DOI] [PubMed] [Google Scholar]
- 22.Johnson TC, Chin MR, Siegel D. Synthetic Route Development for the Laboratory Preparation of Eupalinilide E. J Org Chem. 2017;82(9):4640‐4653. doi: 10.1021/acs.joc.7b00266 [DOI] [PubMed] [Google Scholar]
- 23.Johnson TC, Siegel D. Directing Stem Cell Fate: The Synthetic Natural Product Connection. Chem Rev. 2017;117(18):12052‐12086. doi: 10.1021/acs.chemrev.7b00015 [DOI] [PubMed] [Google Scholar]
- 24.Johnson TC, Chin MR, Han T, Shen JP, Rana T, Siegel D. Synthesis of Eupalinilide E, a Promoter of Human Hematopoietic Stem and Progenitor Cell Expansion. J Am Chem Soc. 2016;138(18):6068‐6073. doi: 10.1021/jacs.6b03055 [DOI] [PubMed] [Google Scholar]
- 25.de Lichtervelde L, Boitano AE, Wang Y, et al. Eupalinilide E inhibits erythropoiesis and promotes the expansion of hematopoietic progenitor cells. ACS Chem Biol. 2013;8(5):866‐870. doi: 10.1021/cb4000234 [DOI] [PubMed] [Google Scholar]
- 26.Cooper SH, Broxmeyer HE, Capitano ML. Experimental Mouse Models of Mouse and Human Hematopoietic Stem Cell Transplantation. In, Methods in Molecular Biology: Hematopoietic Stem Cells (Eds: Pelus LM and Hoggatt J). Springer Nature, New York, 2020;In Press. [DOI] [PubMed] [Google Scholar]
- 27.Guo B, Huang X, Cooper S, Broxmeyer HE. Glucocorticoid hormone-induced chromatin remodeling enhances human hematopoietic stem cell homing and engraftment. Nat Med. 2017;23(4):424‐428. doi: 10.1038/nm.4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang X, Guo B, Liu S, Wan J, Broxmeyer HE. Neutralizing negative epigenetic regulation by HDAC5 enhances human haematopoietic stem cell homing and engraftment. Nat Commun. 2018;9(1):2741. Published 2018 Jul 16. doi: 10.1038/s41467-018-05178-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y, Yao C, Teng Y, et al. Phorbol ester induced ex vivo expansion of rigorously-defined phenotypic but not functional human cord blood hematopoietic stem cells: a cautionary tale demonstrating that phenotype does not always recapitulate stem cell function. Leukemia. 2019;33(12):2962‐2966. doi: 10.1038/s41375-019-0528-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantel C, Messina-Graham S, Moh A, et al. Mouse hematopoietic cell-targeted STAT3 deletion: stem/progenitor cell defects, mitochondrial dysfunction, ROS overproduction, and a rapid aging-like phenotype. Blood. 2012;120(13):2589‐2599. doi: 10.1182/blood-2012-01-404004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capitano ML, Griesenauer B, Guo B, Cooper S, Paczesny S, Broxmeyer HE. The IL-33 Receptor/ST2 Acts as a Positive Regulator of Functional Mouse Bone Marrow Hematopoietic Stem and Progenitor Cells. Blood Cells Molecules and Diseases. 2020;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]


