Abstract
Objective:
COVID-19 is associated with derangement in biomarkers of coagulation and endothelial function and has been likened to the coagulopathy of sepsis. However, clinical laboratory metrics suggest key differences in these pathologies. We sought to determine if plasma coagulation and fibrinolytic potential in patients with COVID-19 differ compared to healthy donors and critically-ill patients with sepsis.
Approach and Results:
We performed comparative studies on plasmas from a single-center, cross-sectional observational study of 99 hospitalized patients (46 with COVID-19, 53 with sepsis) and 18 healthy donors. We measured biomarkers of endogenous coagulation and fibrinolytic activity by immunoassays, thrombin and plasmin generation potential by fluorescence, and fibrin formation and lysis by turbidity. Compared to healthy donors, patients with COVID-19 or sepsis both had elevated fibrinogen, D-dimer, soluble thrombomodulin, and plasmin-antiplasmin complexes. Patients with COVID-19 had increased thrombin generation potential despite prophylactic anticoagulation, whereas patient with sepsis did not. Plasma from patients with COVID-19 also had increased endogenous plasmin potential, whereas patients with sepsis showed delayed plasmin generation. The collective perturbations in plasma thrombin and plasmin generation permitted enhanced fibrin formation in both COVID-19 and sepsis. Unexpectedly, the lag times to thrombin, plasmin, and fibrin formation were prolonged with increased disease severity in COVID-19, suggesting a loss of coagulation-initiating mechanisms accompanies severe COVID-19.
Conclusion:
Both COVID-19 and sepsis are associated with endogenous activation of coagulation and fibrinolysis, but these diseases differently impact plasma procoagulant and fibrinolytic potential. Dysregulation of procoagulant and fibrinolytic pathways may uniquely contribute to the pathophysiology of COVID-19 and sepsis.
Keywords: COVID-19, sepsis, thrombin, plasmin, fibrinogen
INTRODUCTION
The SARS-CoV-2 coronavirus emerged in Wuhan, China in December, 2019 and has infected close to 37 million people worldwide.1 The associated disease, COVID-19, has claimed over one million lives as of October, 2020.1 Severe COVID-19 cases present with hypoxia, acute respiratory distress syndrome (ARDS), and multi-organ involvement.2 Thrombotic complications have been observed in patients with COVID-19, including macrothrombi in arteries and veins, and small vessel thrombosis in multiple organs.3–14 Post-mortem analyses of lungs from patients with COVID-19 reveal fibrin in the pulmonary microvasculature and alveolar space3, 6, 11, 15, suggesting fibrin deposition contributes to morbidity and mortality. Treatment with low molecular weight heparin (LMWH) has become standard of care at many hospitals. However, elevated prothrombotic biomarkers and thrombosis have been reported even in COVID-19 patients on thromboprophylaxis, suggesting hypercoagulability persists in spite of anticoagulation.2, 4–7, 9, 10, 12, 14, 16–24 Accordingly, clinical trials are underway to determine if higher-intensity anticoagulation is warranted for patients with COVID-19.
Growing evidence documents elevated pro-inflammatory cytokines, (e.g., interleukin-6) in patients with COVID-19, which is thought to lead to derangement in vascular function and blood composition.20 Accordingly, increased circulating von Willebrand factor (VWF), thrombomodulin, and factor VIII suggests COVID-19 induces endothelial activation17, 18, and elevated C-reactive protein and fibrinogen indicates the infection activates the acute phase response. Patients with COVID-19 also have elevated circulating D-dimer, a biomarker of fibrin formation and degradation, and D-dimer correlates with COVID-19 disease severity and is predictive of outcomes.25, 26 Although the presence of D-dimer suggests fibrinolytic pathways are intact and actively dissolving (lysing) fibrin, the discovery of fibrin deposits in lungs and other organs suggests dysregulation of the balance in fibrin-forming (i.e., thrombin generation [TG]) and fibrin-dissolving (i.e., plasmin generation [PG]) pathways is a major aspect of COVID-19 pathogenesis. Importantly, thrombin, fibrin, and plasmin have central roles not only in thrombosis, but also as effectors in infection and host responses27, 28, suggesting dysregulation of these pathways may contribute in multiple ways to the pathophysiology of SARS-CoV-2 infection.
Similar to COVID-19, critically-ill patients with sepsis exhibit exaggerated inflammatory responses, including elevated C-reactive protein and markers of endothelial dysfunction (e.g., VWF, thrombomodulin) and also have increased risk of thrombotic complications.29 Furthermore, elevated D-dimer predicts mortality in critically-ill patients with sepsis. Accordingly, COVID-19 has been compared conceptually to sepsis. However, unlike COVID-19, up to 35% of patients with sepsis present with disseminated intravascular coagulation (DIC), with consumption of coagulation factors marked by prolonged prothrombin times (PT) and activated partial thromboplastin times (aPTT).30 Consequently, the observation that PT and aPTT remain relatively preserved in patients with COVID-19 until end stages of the disease course suggest fundamental differences in these diseases. Thus far, changes in procoagulant and fibrinolytic activity in patients with COVID-19 have not been directly compared to a critically-ill cohort of patients with sepsis. Filling this knowledge gap is necessary to understand pathophysiological changes in COVID-19, determine how these changes are similar or dissimilar to sepsis, and enable the selection of appropriate interventions, some of which may have already been tested in sepsis or other diseases where disordered coagulation and thrombosis occur.
Here, we employed assays of circulating biomarkers and plasma procoagulant and fibrinolytic potential to define the functional coagulation status of hospitalized patients with COVID-19 or sepsis. Comparison of these data revealed both similarities and key differences in the pathophysiology of these diseases.
METHODS
Sources of materials and additional methods are provided in the Supplemental Materials.
Study participants.
COVID-19 participants were a subset of subjects enrolled in previous studies of platelet gene expression21 and neutrophil extracellular traps22 in COVID-19. Briefly, hospitalized patients (N=46 total, 20 intensive care unit [ICU] and 26 non-ICU) with acute SARS-CoV-2 infections confirmed by RT-PCR were recruited from the University of Utah Health Sciences Center in Salt Lake City between March 17 and August 1, 2020. Enrollment criteria included positive SARS-CoV-2 testing, respiratory symptoms (cough, shortness of breath) or fever, hospital admission, age >18, and informed consent. The primary triage decision to admit COVID-19 patients to the ICU was dictated by the need for advanced oxygen therapies such as high nasal flow cannula, noninvasive ventilation (continuous positive airway pressure or bilevel positive airway pressure) and mechanical ventilation due severity of lung injury. Therefore, the majority of ICU patients were critically-ill due to acute respiratory distress syndrome. Critically-ill patients with sepsis (N=53) were recruited from the Medical Intensive Care Unit at the University of Utah Health Sciences Center in Salt Lake City between July, 2017 and March, 2020. Enrollment criteria included SEPSIS-3 criteria, MICU admission, age >18, and informed consent. All patients underwent clinically-directed investigations to identify the pathogen causing infection, including bacterial, respiratory, and urinary cultures, and antigen testing, as directed by the treating clinical team. As is common in sepsis patients, only 30% of patients had a pathogen specifically identified. Pathogens that were identified include Escherichia coli (N=4/53), Klebsiella pneumoniae (N=4/53), Streptococcus (N=3/53), Staphylococcus (N=3/53), and Influenza A (N=1/53) (Supplemental Table I). In addition to recording the pathogen, when identified, we also tracked the clinically-identified site or organ of the primary infection. These included pneumonia (N=19/53, 36%), skin and soft tissue infection (N=11/53, 20%), urosepsis (N=10/53, 19%), intra-abdominal infection (N=6/52, 15%), blood (N=2/53, 4%), pharyngitis (N=1/53, 2%), or was unknown (N=2/53, 4%) (Supplemental Table II). All patients were enrolled within 72 hours of ICU admission and were recruited under protocols approved by the Institutional Review Board of the University of Utah (IRB#: 00102638, 00093575). Healthy age- and sex-matched donors (N=18) were enrolled under a separate protocol (IRB#: 0051506). Healthy donors had no known bleeding disorder, liver or kidney disease, cancer, or history of surgery or thrombotic event within the past 3 months, and were not on antiplatelet or anticoagulant therapy. Each study participant or their Legal Authorized Representative gave written informed consent for study enrollment in accordance with the Declaration of Helsinki. Clinical laboratory values (platelet counts, white blood cell counts, anti-factor Xa levels, PT, and aPTT) were performed by ARUP Laboratories in Salt Lake City. Clinical laboratory test values at time enrollment were included in our analysis if available. Reference ranges as of August, 2020 were provided by ARUP Laboratories.
Statistical methods.
Biomarkers and TG in the presence and absence of tissue factor (TF), fibrin formation, fibrinolysis, and PG parameters were compared by Kruskal-Wallis tests using Dunn’s multiple comparisons comparing healthy donors and patients with COVID-19 or sepsis. Comparisons between non-ICU and ICU patients, patients on LMWH, patients with COVID-19 or sepsis in the ICU, and ventilation status were analyzed with Mann-Whitney ranked sum tests. TG assays in the presence and absence of anti-TF antibody were compared by Wilcoxon matched-pairs signed rank test. Associations between clinical, laboratory, and kinetic parameters were analyzed using Spearman’s rank correlations. All analyses were performed using Prism 8.4.3. P<0.05 was considered statistically significant.
RESULTS
Patients with COVID-19 and critically-ill patients with sepsis have altered coagulation biomarkers.
Clinical characteristics of the healthy donors and patients with COVID-19 or sepsis are summarized in Supplemental Table III; values were consistent with characteristics reported previously for this cohort21, 22. Subjects were well-matched by age and sex. Patients were more frequently of Hispanic or Latino descent, and compared to patients with sepsis, significantly more patients with COVID-19 were of Hispanic or Latino descent, consistent with reports suggesting these populations have increased risk of severe SARS-CoV-2 infection.31 Co-morbidities in both disease cohorts included obesity, diabetes, hypertension, and chronic lung disease; patients with COVID-19 were more likely to have diabetes. Compared to patients with sepsis, patients with COVID-19 had similar BMI, chronic lung disease, and mortality, but lower Sequential Organ Failure Assessment (SOFA) scores, as patients with sepsis commonly have multiple organ dysfunction syndrome (Supplemental Table III). Patients with COVID-19 also had lower white blood cell count, but higher platelet counts. Compared to patients with sepsis, patients with COVID-19 had similar aPTTs; however, PTs were slightly prolonged in sepsis and within the normal range in COVID-19. Using these findings to approximate a score greater than “5” in the International Society of Thrombosis and Haemostasis criteria32, four patients with sepsis, but none with COVID-19, had overt disseminated intravascular coagulation. COVID-19 patients in the ICU had similar demographic characteristics and co-morbidities as non-ICU patients (Supplemental Table IV).
At the time of blood draw, 90% of patients with COVID-19 or sepsis were receiving anticoagulants to prevent thrombosis, as ordered by the patient’s primary team in accordance with institutional guidelines. Most patients with COVID-19 were on LMWH (Lovenox), although some were on unfractionated heparin or factor Xa inhibitors (rivaroxaban, apixaban) (Supplemental Table III). Sepsis patients were more frequently on unfractionated heparin than COVID-19 patients (53% vs. 4.3%, P=0.0006). Most patients on anticoagulants were on weight-based prophylactic dosing per institutional guidelines, except three septic patients who were on therapeutic heparin. Four non-ICU COVID-19 patients and five patients with sepsis were not on anticoagulants due to increased bleeding risk. In patients in which anti-factor Xa levels were measured, levels were similar in COVID-19 and sepsis. The standard of care at the University of Utah Hospital does not include screening ultrasound, and clinically-diagnosed thrombotic events were rare; only two patients with sepsis had thrombotic events (one ischemic stroke and one venous thrombosis).
We first assessed circulating biomarkers of coagulation and fibrinolysis. Consistent with prior reports, compared to healthy donors, patients with COVID-19 or sepsis had elevated fibrinogen and D-dimer (Fig 1A–B). Patients with sepsis had higher D-dimer than patients with COVID-19 (Fig 1B). Compared to healthy donors, patients with COVID-19 or sepsis had similarly elevated circulating soluble thrombomodulin (Fig 1C). Compared to healthy donors, circulating thrombin-antithrombin (TAT) complexes were not different in patients with COVID-19 or sepsis (Fig 1D), but plasmin-antiplasmin (PAP) complexes were elevated in both disease groups (Fig 1E), suggesting both groups have endogenous activation of fibrinolysis. COVID-19 patients in the ICU and on the ward had similar biomarkers, except ICU patients had higher D-dimer (P=0.048). When COVID-19 patients were stratified by the need for mechanical ventilation, D-dimer and soluble thrombomodulin were higher in ventilated patients (P=0.0164).
Figure 1. Increased plasma biomarkers of coagulation and fibrinolysis activation in patients with COVID-19 or sepsis.
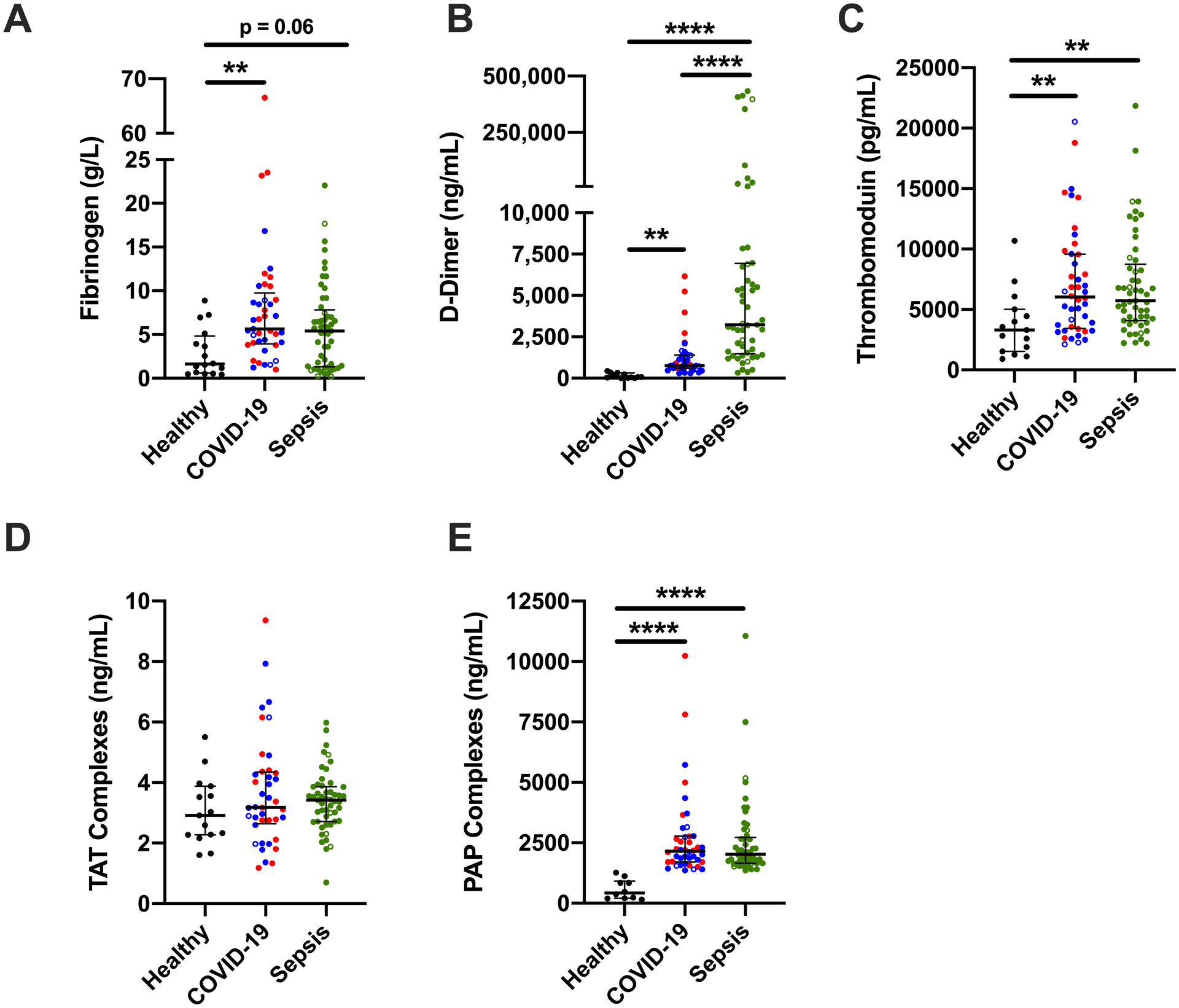
(A) Fibrinogen, (B) D-dimer, (C) thrombomodulin, (D) thrombin-anti-thrombin (TAT) complexes, and (E) plasmin-antiplasmin (PAP) complexes were measured by ELISA in healthy donors and hospitalized patients with COVID-19 or sepsis. Symbols: COVID-19 non-ICU (blue) and ICU (red) patients; patients who did (closed circles) or did not (open circles) receive anticoagulation. Lines show medians and interquartile range; symbols indicate individual donors; **P<0.01, ***P<0.001, ****P<0.0001
Patients with COVID-19 or sepsis have increased plasma TG potential.
To compare effects of COVID-19 and sepsis on plasma coagulation potential, we first measured TG in reactions initiated with TF. Representative curves are shown in Fig 2A. TG parameters were not different between COVID-19 patients stratified by ICU status or the need for mechanical ventilation (Supplemental Fig I and data not shown) and were combined. Compared to healthy donors, patients with COVID-19 had similar lag time (LT) and time to peak (TTP), whereas LT and TTP were prolonged in sepsis (Fig 2B, C). Interestingly, even though patients were receiving guideline-recommended anticoagulation, the mean TG velocity and peak were higher in COVID-19 patients than either healthy donors or patients with sepsis (Fig 2D, E), although there was a wide range of values within each group. Endogenous thrombin potential (ETP) was not different between healthy donors and COVID-19 patients, but was higher in COVID-19 than in sepsis (Fig 2F). This pattern persisted in subanalyses comparing only COVID-19 and sepsis patients in the ICU (Supplemental Fig II) or on LMWH (Supplemental Fig III).
Figure 2. Increased thrombin generation (TG) in plasma from COVID-19 patients.
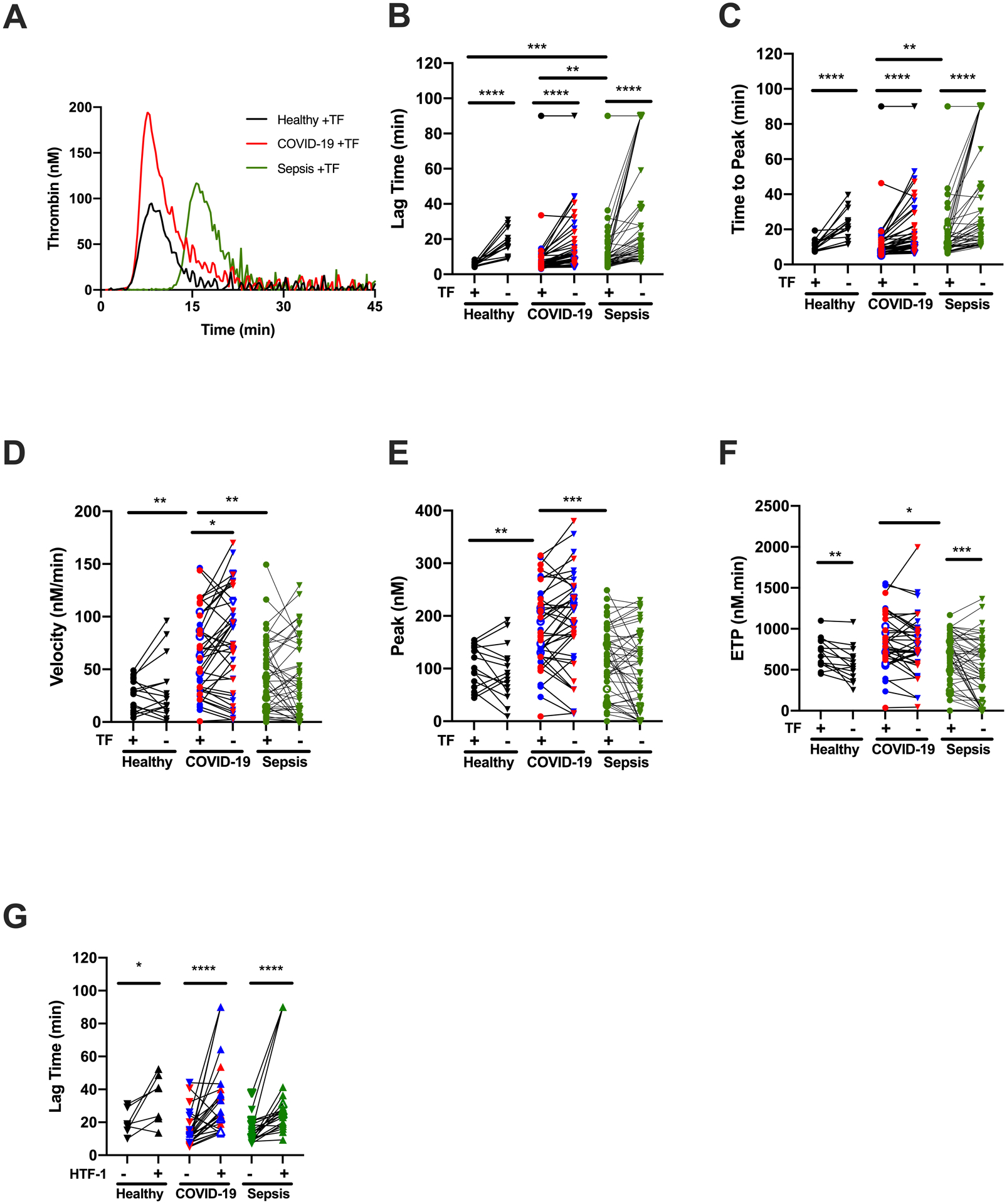
TG was initiated with the addition of CaCl2 and phospholipids, with (circles) or without (downward triangles) tissue factor (TF), as indicated. (A) Representative TG curves; each curve shows the mean of duplicate reactions in one individual’s plasma. The quantitative parameters of (B) lag time, (C) time to peak, (D) velocity, (E) peak thrombin, and (F) endogenous thrombin potential (ETP) were derived from the TG curves. (G) TG lag time in plasma from COVID-19 patients treated with (triangles) or without (downward triangles) anti-TF antibody (10 μg/mL HTF-1). Symbols: COVID-19 non-ICU (blue) and ICU (red) patients; patients who did (closed symbols) or did not (open symbols) receive anticoagulation. Lines connect individual plasmas; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001
Since it has been speculated that both COVID-19 and sepsis are associated with increased circulating initiators of coagulation, we also measured TG in reactions recalcified in the absence of added TF. As expected, these reactions showed prolonged LT and TTP compared to reactions initiated by exogenous TF (Fig 2B, C). Addition of inhibitory anti-TF antibody to TG reactions further prolonged the LT in plasmas from all three groups (Fig 2G), implicating circulating TF as at least one potential endogenous initiator of coagulation in both diseases.
Patients with COVID-19 or sepsis have enhanced plasma fibrin formation potential.
Fibrin formation is influenced by the local concentrations of both fibrinogen and thrombin.33 To determine the net impact of elevated fibrinogen (Fig 1A) and enhanced TG (Fig 2) on fibrin-forming potential, we measured fibrin formation kinetics by turbidity in reactions initiated with TF. Representative curves are shown in Fig 3A. Fibrin formation parameters were similar in non-ICU and ICU COVID-19 patients (data not shown) and were combined. Compared to healthy donors, the LT was not altered in patients with COVID-19; however, the LT was prolonged in patients with sepsis compared to either healthy donors or patients with COVID-19 (Fig 3B). The TTP was not different between groups (Fig 3C); however, both fibrin formation rate (Vmax) and maximum turbidity change were increased in patients with COVID-19 or sepsis (Fig 3D, E). These effects persisted in subanalyses comparing only patients in the ICU or on LMWH (data not shown).
Figure 3. Increased fibrin formation in plasma from patients with COVID-19 or sepsis.
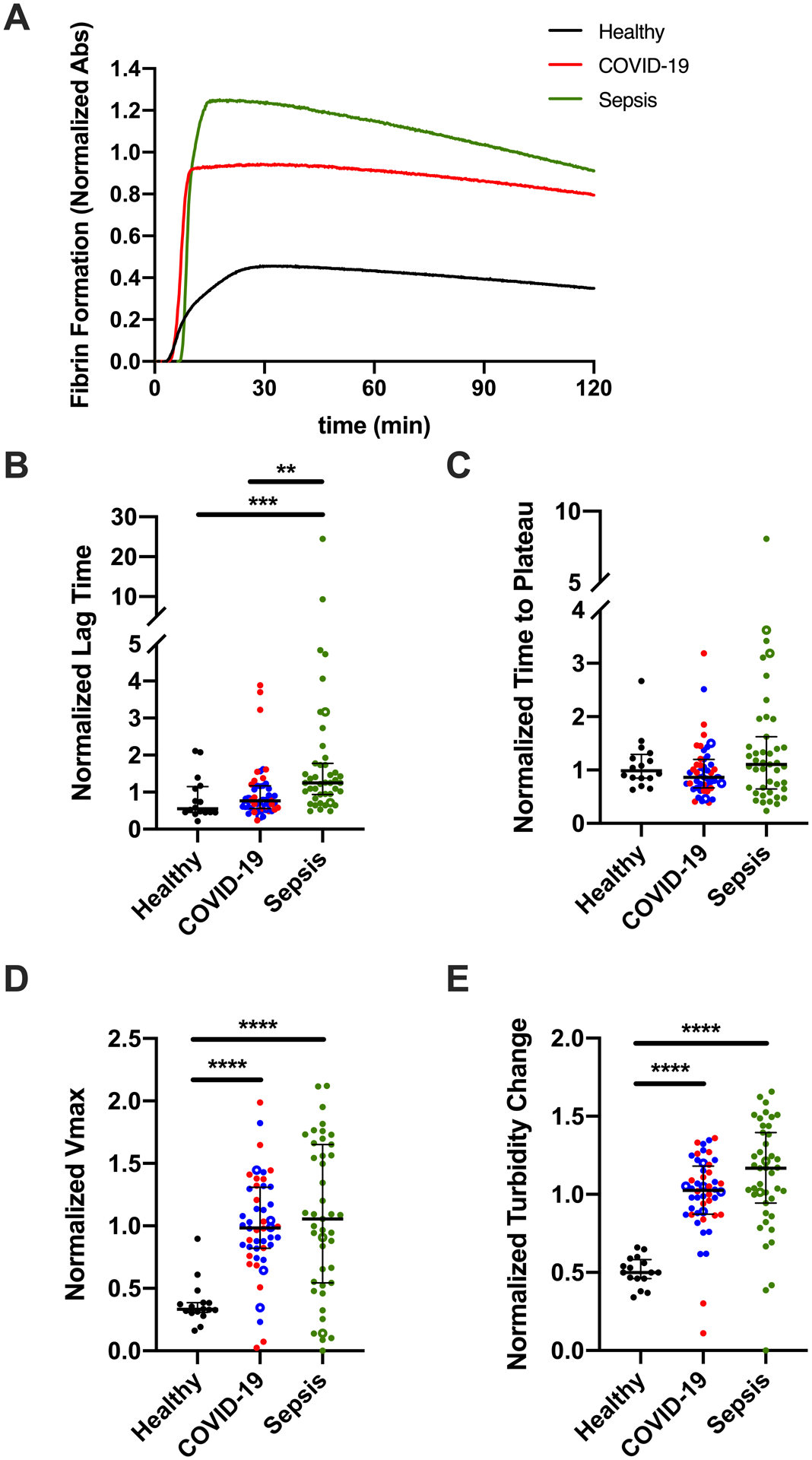
Fibrin formation was initiated with TF, CaCl2, and phospholipids, and measured by absorbance at 405 nm. Turbidity was normalized to COVID-19 samples run on each plate. (A) Representative fibrin formation curves; each curve shows the mean of duplicate reactions in one individual’s plasma. The quantitative parameters of (B) lag time, (C) time to plateau, (D) maximum velocity (Vmax), and (E) turbidity change were derived from the fibrin formation curves. Symbols: COVID-19 non-ICU (blue) and ICU (red) patients; patients who did (closed circles) or did not (open circles) receive anticoagulation. Lines show medians and interquartile range; symbols indicate individual donors; **P<0.01, ***P<0.001, ****P<0.0001
Patients with sepsis have delayed plasma PG, whereas patients with COVID-19 have increased PG potential.
Fibrin is a cofactor for tPA-mediated generation of plasmin.34 Given increased fibrin formation potential in both COVID-19 and sepsis, we then compared plasmin-generating potential in these settings using a calibrated assay35. Representative curves are shown in Fig 4A. Within COVID-19 patients, PG parameters were similar except for a slight prolongation in the LT and TTP in ICU versus non-ICU patients or when stratifying by the need for mechanical ventilation (Supplemental Fig IV and data not shown) and were combined. Compared to healthy donors, patients with COVID-19 had a similar LT but prolonged TTP, whereas patients with sepsis had a prolonged LT and TTP (Fig 4B, C). There was no difference in PG velocity between groups (Fig 4D). Interestingly, compared to healthy donors or patients with sepsis, patients with COVID-19 had an increased plasmin peak and endogenous plasmin potential (EPP) (Fig 4E, F), and this pattern was also seen in subanalyses of patients in the ICU (Supplemental Fig V) or on LMWH (Supplemental Fig VI). Together, these data suggest plasma from patients with COVID-19 has enhanced capacity to generate plasmin in response to addition of rtPA.
Figure 4. Delayed plasmin generation (PG) in plasma from patients with sepsis, but enhanced PG potential in patients with COVID-19.
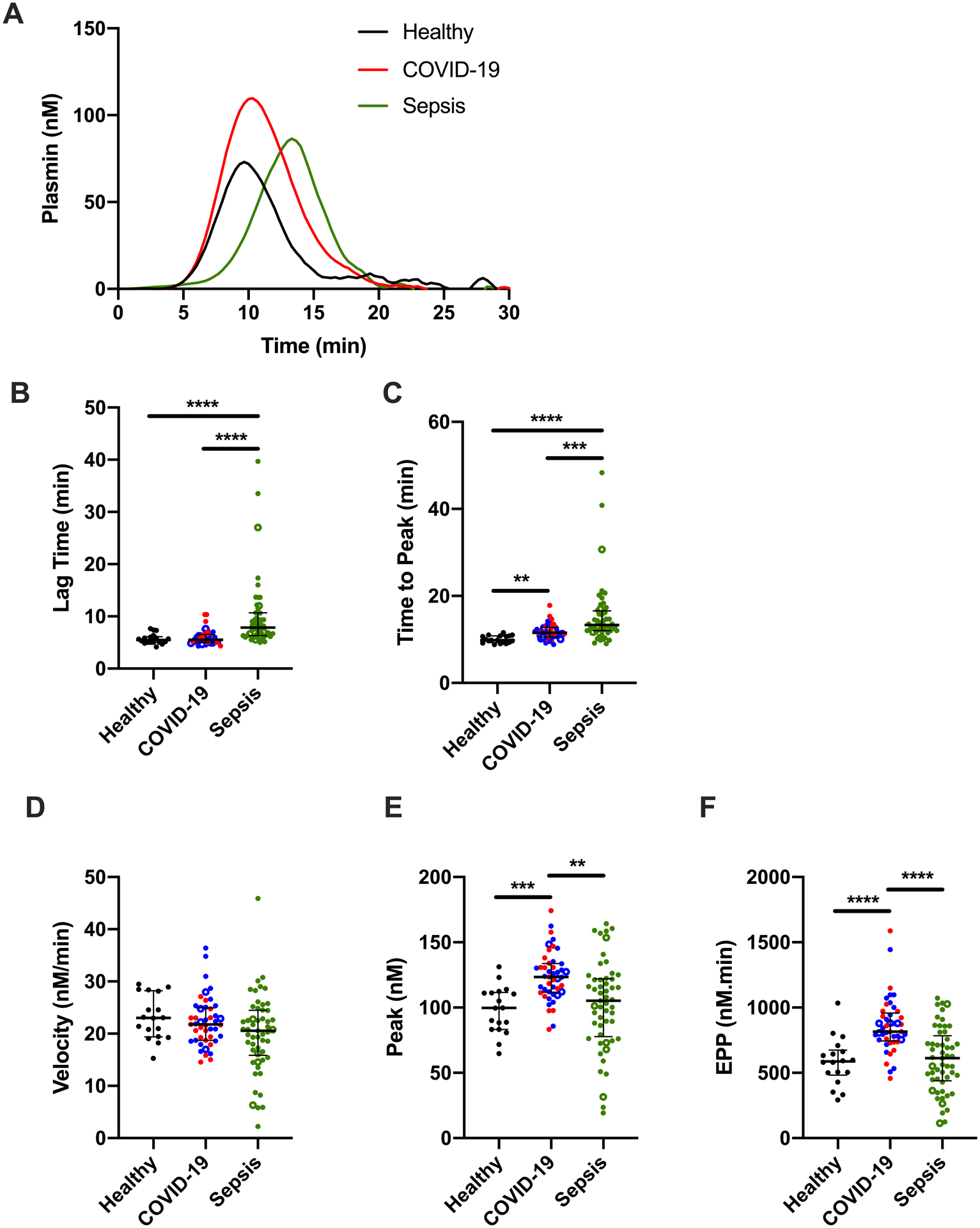
PG was initiated with TF, CaCl2, and phospholipids, in the presence of recombinant tissue plasminogen activator (rtPA). (A) Representative PG curves; each curve shows the mean of duplicate reactions in one individual’s plasma (spline fit). The quantitative parameters of (B) lag time, (C) time to peak, (D) velocity, (E) peak plasmin, and (F) endogenous plasmin potential (EPP) were derived from the PG curves. Symbols: COVID-19 non-ICU (blue) and ICU (red) patients; patients who did (closed circles) or did not (open circles) receive anticoagulation. Lines show medians and interquartile range; symbols indicate individual donors; **P<0.01, ***P<0.001, ****P<0.0001
Plasma clots from patients with sepsis have increased resistance to fibrinolysis.
To characterize the net impact of changes in TG, fibrin formation, and PG potential on clot stability, we then clotted plasma from healthy donors and patients with COVID-19 or sepsis in the presence of rtPA and followed clot formation and dissolution by turbidity. Representative curves are shown in Fig 5A. Fibrinolysis parameters were similar in non-ICU and ICU patients (data not shown) and were combined. Compared to healthy donors, the LT and TTP were not different in patients with COVID-19, but were significantly prolonged in patients with sepsis (Fig 5B, C). As seen in fibrin formation assays, plasma from patients with COVID-19 or sepsis both had increased fibrin formation rates and higher turbidity change signifying increased fibrin-forming potential (Fig 5D, E). In spite of increased PG potential in plasma from patients with COVID-19, compared to healthy donors, the time to return to baseline turbidity trended longer in plasma from patients with COVID-19 and was significantly delayed in plasma from patients with sepsis (Fig 5F).
Figure 5. Enhanced plasma clot stability in patients with COVID-19 or sepsis.
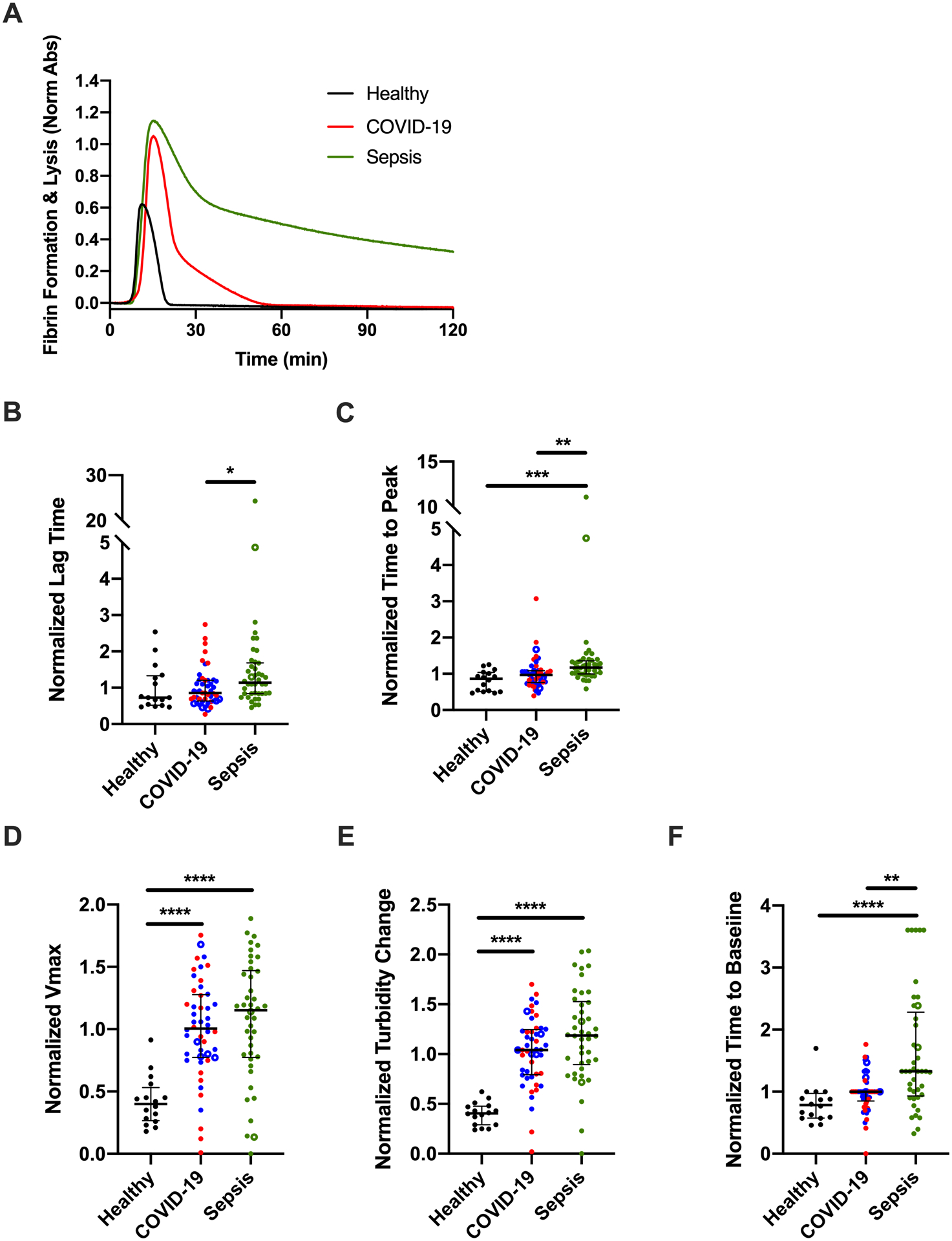
Clot formation and fibrinolysis were initiated with TF, calcium, and phospholipids, in the presence of rtPA, and measured by turbidity at 405 nm. (A) Representative fibrin formation curves; each curve shows the mean of duplicate reactions in one individual’s plasma. The quantitative parameters of (B) lag time, (C) time to plateau, (D) maximum velocity (Vmax), and (E) peak turbidity change, and (F) time to baseline were derived from the fibrinolysis curves. Symbols: COVID-19 non-ICU (blue) and ICU (red) patients; patients who did (closed circles) or did not (open circles) receive anticoagulation. Lines show medians and interquartile range; symbols indicate individual donors; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001
Imbalanced TG and PG in COVID-19 promotes a fibrin-permissive environment.
To quantify the imbalance between TG and PG in patients with COVID-19 and sepsis, we then calculated the TG/PG ratio for each parameter (Fig 6A–E). In patients with sepsis, changes in TG were generally rebalanced by parallel changes in PG, yielding overall TG/PG ratios that were similar to those seen in healthy donors. In contrast, in patients with COVID-19, increased TG was not sufficiently offset by changes in PG. The TTP ratio in COVID-19 trended faster than in healthy donors, the velocity ratio was higher in COVID-19 patients than in healthy donors or septic patients, and the peak ratio was significantly higher in patients with COVID-19 versus sepsis. Collectively, these data expose and quantify a net procoagulant (fibrin-permissive) environment in plasmas from patients with COVID-19. Particularly in the context of elevated fibrinogen (Fig 1A), these changes could enable increased deposition of stable fibrin in both extravascular and intravascular (thrombotic) settings.
Figure 6. The metric of TG/PG ratio differentiates net functional activity in patients with COVID-19 versus sepsis.
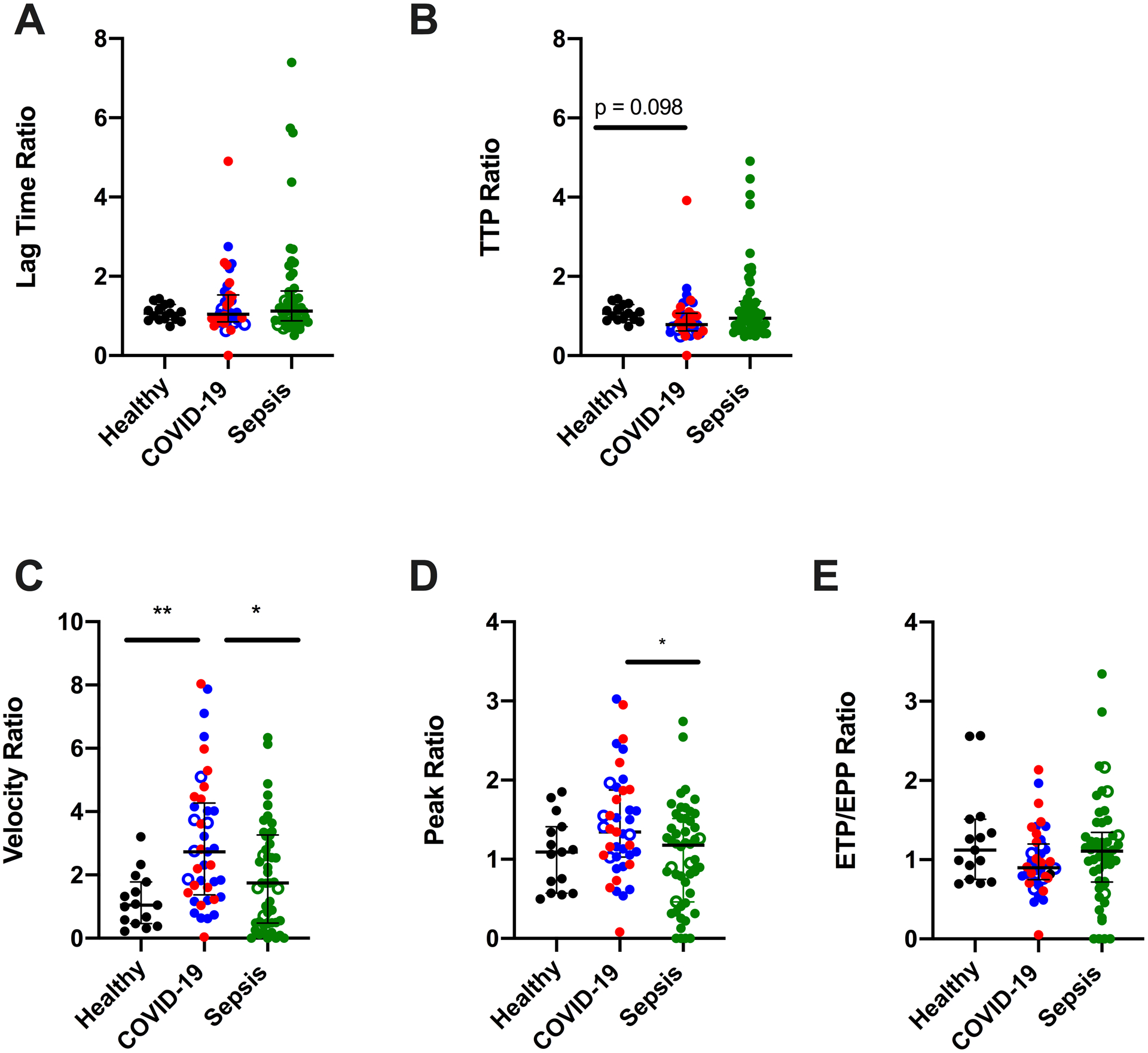
TG/PG ratios were calculated by dividing each TG parameter by the corresponding PG parameter. Panels show TG/PG ratios for (A) lag time, (B) TTP, (C) velocity, (D) peak, and (E) ETP/EPP. Symbols: COVID-19 non-ICU (blue) and ICU (red) patients; patients who did (closed circles) or did not (open circles) receive anticoagulation Lines show medians and interquartile range; symbols indicate individual donors; *P<0.05, **P<0.01. (F-H)
Increased COVID-19 disease severity is associated with delayed onset of plasma coagulation and fibrinolytic mechanisms.
To further explore relationships between plasma coagulation and fibrinolysis potential, we performed Spearman correlation analyses. Several expected associations were present in both healthy donors and patients. All subjects showed generally consistent patterns within TG parameters; LT correlated positively with TTP but negatively with velocity, peak, and ETP in both the presence (Fig 7A–C, Supplemental Tables V–VII) and absence (data not shown) of exogenous TF. Likewise, patterns within fibrin formation and fibrinolysis parameters were generally maintained in all three groups, wherein LT correlated positively with TTP, but negatively with the rate and peak of each parameter (Fig 7A–C). The PG LT and TTP correlated positively with velocity, peak, and EPP in healthy donors but not in patients, suggesting mechanisms regulating PG potential are altered in both COVID-19 and sepsis. In spite of similar group sizes, correlations were less robust in COVID-19 versus sepsis (Fig 6G, H, Supplemental Tables V–VII), implying increased heterogeneity in COVID-19.
Figure 7. Relationships between procoagulant and fibrinolytic biomarkers and parameters differ between patients with COVID-19 versus sepsis.
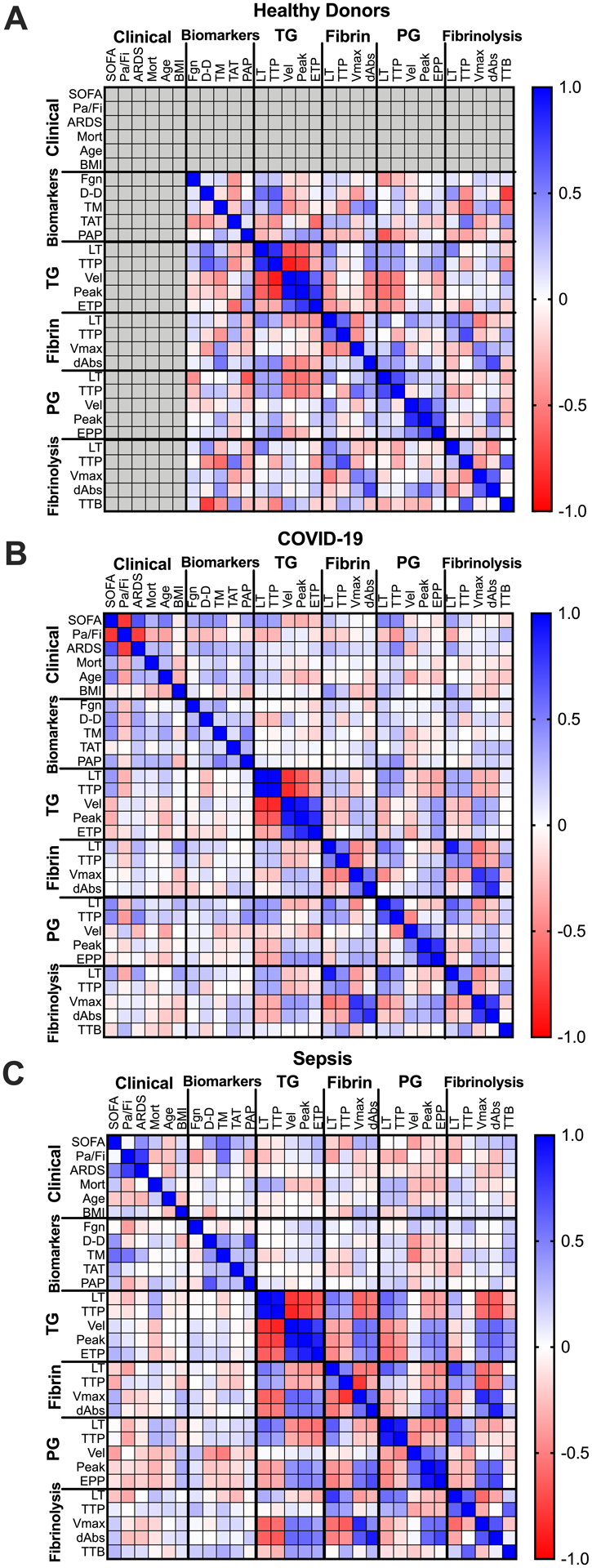
Spearman’s rank correlations were performed for clinical metrics, plasma biomarkers, and coagulation and fibrinolytic parameters measured in (A) healthy donors, (B) patients with COVID-19, and (C) patients with sepsis. Positive correlations are indicated in blue and negative correlations are indicated in red, as defined in the legend. Abbreviations are: SOFA, Sequential Organ Failure Assessment; Pa/Fi, ratio of arterial oxygen partial pressure to fractional inspired oxygen; ARDS, acute respiratory distress syndrome; Mort, mortality; BMI, body mass index; Fgn, fibrinogen; D-D, D-dimer; TM, thrombomodulin; TAT, thrombin-antithrombin complexes; PAP, plasmin-antiplasmin complexes; LT, lag time; TTP, time to peak; Vel, velocity; ETP, endogenous thrombin potential; EPP, endogenous plasmin potential; TTB, time to baseline.
Finally, to identify parameters that differentiate clinical status in patients with COVID-19 and sepsis and discern additional clues to the underlying pathophysiology, we correlated biomarker and assay readouts with SOFA scores, a clinical measure of disease severity (Fig 7B, C, Supplemental Tables V–VII, Supplemental Figs VII–VIII). Both soluble thrombomodulin and D-dimer correlated positively and significantly with SOFA scores in patients with COVID-19, suggesting endothelial dysfunction and fibrin degradation are aspects of both diseases. However, markers of in vivo activation of TG or PG differed in COVID-19 and sepsis; TAT complexes correlated with SOFA scores in patients with sepsis but not COVID-19, whereas PAP complexes correlated with SOFA scores in COVID-19 but not sepsis. Interestingly, the LT in TG, PG, and fibrinolysis assays correlated positively with SOFA scores in COVID-19 but not sepsis. These data indicate a loss of procoagulant activity accompanies development of severe COVID-19 and suggest COVID-19 patients acquire a consumptive coagulopathy in severe (later) stages of disease. Collectively, these data suggest pathophysiologic changes that occur in COVID-19 and sepsis differently impact plasma procoagulant and fibrinolytic potential. These differences may have implications for identifying patients with severe disease or developing approaches to alter procoagulant and fibrinolytic activity in these two disease settings.
DISCUSSION
COVID-19 is associated with morbidity and mortality, but pathophysiologic mechanisms are unclear. Studies of coagulation biomarkers and post-mortem findings have associated both extravascular fibrin deposition and thrombosis with disease progression, organ failure, and poor outcomes. Two major gaps limit our current understanding of the implications of these findings. First, few studies have characterized TG, and none have measured PG – key pathways that mediate fibrin formation and stability. Second, clinical context for this disease has not been established. COVID-19 has been compared to sepsis and DIC; however, loss of coagulation factors and acquisition of a DIC-like phenotype is not typically seen in COVID-19 until later stages of severe disease. In contrast, in sepsis DIC often occurs earlier in the disease course. Thus, the extent to which disordered coagulation overlaps in these two diseases is currently unknown. This information is important for understanding, preventing, and treating COVID-19 complications. Our study helps fill these knowledge gaps by comprehensively quantifying coagulation and fibrinolysis pathways in a cross-sectional cohort of patients with COVID-19 and comparing these to patients with sepsis. Our findings reveal similarities as well as differences in these diseases.
Abnormalities in circulating biomarkers in patients with COVID-19 or sepsis were similar, indicating endogenous activation of coagulation and enhanced fibrin formation occur in both disease settings. Moreover, analysis of plasma TG potential showed evidence of circulating TF activity in both COVID-19 and sepsis. These data are consistent with our previous findings in critically-ill patients with influenza A1/H1N136 and the hypothesis that procoagulant initiators such as TF-positive microvesicles contribute to the pathophysiology of vascular disease.37–39 Since clinical blood samples are not typically collected into contact pathway inhibitors (e.g., corn trypsin inhibitor), we were unable to determine whether patients with COVID-19 or sepsis also have increased contact pathway activation. Future studies in which the contact pathway is inhibited are necessary to elucidate the relative roles of TF-positive microvesicles, contact pathway activation, and/or other procoagulant proteases in these disorders.
Our analysis also revealed differences in plasma TG kinetics between COVID-19 and sepsis. Whereas plasma from patients with sepsis showed delayed initiation of TG, plasma from patients with COVID-19 showed increased TG velocity and peak. In other viral diseases, enhanced coagulation can help limit infectivity by stimulating protease-activated receptors and toll-like receptors on macrophages, epithelial cells, and endothelial cells.40 Whether increased TG alters the risk of SARS-CoV-2 infection and/or contributes to COVID-19 are unknown. Elevated TG in plasmas from COVID-19 patients is also noteworthy because most patients were receiving prophylactic anticoagulation. Few studies have examined the effect of LMWH on TG potential in critically-ill patients. Studies of sepsis have shown therapeutic anticoagulation (warfarin or heparin) significantly suppresses all TG parameters, but prophylactic anticoagulation with heparin only slightly prolongs the LT and decreases the TG peak.41, 42 Since we were unable to assess TG in sufficient numbers of patients not on heparinoids, the extent to which LMWH may have reduced TG in this cohort remains unknown. However, our findings are consistent with reports showing persistently elevated TG in plasmas from anticoagulated COVID-19 patients, even in the absence of overt thrombosis.23, 43 Together with reports documenting VTE in COVID-19 patients receiving anticoagulation, our results imply that current prophylactic dosing regimens of LMWH, even if weight-based, may not sufficiently reduce plasma coagulation potential in these patients. Given observations of enhanced platelet activation and increased platelet-neutrophil immunothrombi in COVID-19 patients21, additional antithrombotic measures, including platelet antagonists, may be needed to reduce thrombotic risk is associated with COVID-19. These types of strategies are now being tested in clinical trials, and our findings add support to the rationale and need for these studies.
Both patient groups showed evidence of an operational fibrinolytic pathway, including increased circulating PAP complexes and D-dimer, and measurable plasmin-generating potential in vitro. Unexpectedly, the plasma PG peak and EPP were elevated in COVID-19 compared to healthy donors or patients with sepsis. Although our PG assay is not sensitive to plasma levels of plasminogen activator inhibitor-135, enhanced PG potential may arise from increased fibrinolytic proteins (e.g., plasminogen) or decreased fibrinolysis inhibitors (e.g., α2-antiplasmin); further work is needed to define these molecular mechanisms. Intriguingly, in spite of enhance PG potential, we and others detected hypofibrinolysis of plasma clots from patients with COVID-19.16, 23 In light of post-mortem observations of fibrin in lungs, spleen, and other organs, the data suggest endogenous fibrinolytic mechanisms are insufficient to compensate for enhanced procoagulant activity in patients with COVID-19. Differences in the balance between TG and PG suggest one potential mechanism in which enhanced TG is not sufficiently offset by enhanced PG potential. Ultimately, elevated fibrinogen may drive fibrin deposition in both COVID-19 and sepsis. Elevated fibrinogen is associated with increased risk of thrombosis, as well as inflammatory disease. In vitro studies show clots with increased fibrin(ogen) have increased network density and enhanced resistance to fibrinolysis.44 Thus, it appears likely that the combination of increased TG and elevated fibrinogen promotes fibrin formation, leading to extravascular fibrin deposition as well as thrombosis. Changes in other plasma proteins may also enhance fibrin formation and stability. For example, resistance of clots to fibrinolysis in critically-ill septic patients has been attributed to cell-free DNA that impairs plasmin-mediated clot lysis.45 Since patients with COVID-19 have increased circulating cell-free DNA from neutrophil extracellular trap formation22, cell-free DNA may further enhance fibrin stability in COVID-19 patients.
No patients with COVID-19 and only two patients with sepsis enrolled in our study had clinically-evident thrombosis, and the standard of care at the University of Utah Hospital does not include screening ultrasound. Therefore, we were unable to directly correlate clinical aspects (e.g., ventilation, ICU status) or abnormal coagulation and fibrinolytic potential with thrombosis. The lack of overt thrombosis may also explain the normal level of TAT complexes seen in these patient cohorts. However, our data revealed relationships between coagulation and fibrinolytic biomarkers and clinical disease severity (SOFA score) that provide clues to the underlying pathophysiology of COVID-19 and sepsis. We and others have observed increased soluble thrombomodulin in patients with COVID-19 or sepsis, and soluble thrombomodulin correlated with disease severity in both settings.18, 46 Elevated soluble thrombomodulin is a biomarker of endothelial dysfunction, although it remains unclear whether this arises from disease-induced effects on the endothelium and/or is a predisposing risk factor for either pathology. We recently detected a thrombomodulin-dependent delay in PG in a mouse model of diet-induced obesity35, and obesity is a risk factor for COVID-19. Interestingly, here soluble thrombomodulin did not correlate with BMI in either patient group. Additional studies are needed to define the roles of obesity, soluble thrombomodulin, and PG in illness severity.
Among the functional assays, LT was the primary kinetic parameter to correlate significantly with SOFA score in patients with COVID-19. Surprisingly, this relationship, which differed from patients with sepsis, was positive across several assays (TG, PG, and fibrinolysis), indicating patients with the most severe COVID-19 disease have delayed coagulation and fibrinolytic activity. While initially counterintuitive, these data appear consistent with observations that later stages of severe COVID-19 are associated with a shift to a consumptive coagulopathy and a decrease in plasma procoagulants that initiate coagulation.47
Our study has several limitations. First, our population was relatively small and it was not possible to synchronize patient groups with respect to disease onset. Consequently, heterogeneity in coagulation and fibrinolytic parameters in both COVID-19 and sepsis groups may have limited statistical power to detect further differences. However, the cohort size enabled the application of multiple assays with moderate throughput but high clinical relevance to characterize biomarkers and plasma coagulation and fibrinolytic potential in these cohorts. Our findings expose unexpected patterns and define heterogeneity in these groups which is essential for understanding these changes and designing future trials. Second, since most patients were receiving guideline-recommended anticoagulation, changes in procoagulant and fibrinolytic potential specifically induced by the diseases are difficult to parse. Methods to quantify extent of anticoagulation as well as strategies to neutralize anticoagulants are challenging to standardize and may introduce artifacts when assessing coagulation pathways.48 However, direct comparisons between COVID-19 and sepsis patients receiving weight-based LMWH dosing revealed significant differences between groups that warrant further evaluation. Third, it is difficult to extrapolate from measurements of plasma function in vitro to mechanisms occurring in vivo. For example, increased PG potential in vitro could reflect either enhanced PG in vivo or suppression of endogenous PG that preserves levels of PG system components. Thus, although our findings document changes in procoagulant and fibrinolytic potential in both COVID-19 and sepsis, the mechanisms leading to these changes will require more study using both targeted and agnostic methods. Some abnormalities (e.g., elevated fibrinogen) have already been identified. However, other molecular changes that alter coagulation and fibrinolysis will require broader screens.
In conclusion, our results suggest COVID-19 is associated with dysregulation of procoagulant and fibrinolytic pathways. Given central roles of thrombin, plasmin, and fibrin not only in thrombosis, but also in infection and host responses27, 28, our findings advance understanding of the pathophysiology of COVID-19 as well as common and divergent aspects of these pathways during COVID-19 and sepsis.
Supplementary Material
Highlights.
COVID-19 patients had increased plasma thrombin and plasmin potential compared to healthy donors.
Sepsis patients had no change in thrombin generation and delayed plasmin generation compared to healthy donors.
The lag times to thrombin, plasmin, and fibrin formation correlated positively with disease severity in COVID-19 but not sepsis patients.
Changes in the balance of procoagulant and fibrinolytic activity may contribute to COVID-19 pathophysiology.
ACKNOWLEDGEMENTS
The authors thank Dr. Matthew J. Flick for helpful conversations and reading the manuscript.
FUNDING
This work was supported by grants from the NIH (U24NS107228 to F.D., K01AG059892 to R.A.C., R01HD093826 to C.C.Y., R01HL142804, R01AG048022, R56AG059877, and R01HL130541 to M.T.R, and U01HL143403 and HL126974 to A.S.W.), the University of Utah Triple I Program (E.A.M.), and Fonds voor Wetenschappelijk Onderzoek Vlaanderen FWO 12U7818N (F.D.). This work was also supported in part by Merit Review Award Number I01 CX001696 to MTR from the United States (U.S.) Department of Veterans Affairs Clinical Sciences R&D (CSRD). This material is the result of work supported with resources and the use of facilities at the George E. Wahlen VA Medical Center, Salt Lake City, Utah. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
ABBREVIATIONS
- aPTT
activated partial thromboplastin time
- BMI
Body mass index
- EPP
Endogenous plasmin potential
- ETP
Endogenous thrombin potential
- IRB
Institutional Review Board
- LT
Lag time
- LMWH
Low molecular weight heparin
- ICU
Intensive Care Unit
- PAP
Plasmin anti-plasmin
- PG
Plasmin generation
- PT
Prothrombin time
- rtPA
Recombinant tissue-type plasminogen activator
- SOFA
Sequential Organ Failure Assessment
- TAT
Thrombin anti-thrombin
- TF
Tissue factor
- TG
Thrombin generation
- TM
Thrombomodulin
- TTP
Time to peak
- VTE
Venous thromboembolism
- VWF
von Willebrand factor
Footnotes
CONFLICT OF INTEREST DISCLOSURE
C.C.Y. has received grant funding from PEEL Therapeutics, Inc. during the conduct of this study. J.D.S. is employed by and shareholder of PEEL Therapeutics, Inc. BdL is employed by Synapse Research Institute, a not-for-profit member of the STAGO Diagnostic group that produces calibrated automated thrombography for thrombin generation measurements in plasma. Synapse Research Institute holds the patent on calibrated thrombin generation and plasmin generation analysis. M.T.R. is a Member of the Scientific Advisory Board for Acticor Biotech SAS.
REFERENCES
- 1.Johns hopkins coronavirus resource center. 2020;2020
- 2.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quere I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH, Global Covid-19 Thrombosis Collaborative Group EbtINE, the Iua SbtESCWGoPC, Right Ventricular F. Covid-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: Jacc state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, Galli M, Catena E, Tosoni A, Gianatti A, Nebuloni M. Pulmonary post-mortem findings in a series of covid-19 cases from northern italy: A two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with covid-19 in a new york city health system. JAMA. 2020;324:799–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanif A, Khan S, Mantri N, Hanif S, Saleh M, Alla Y, Chinta S, Shrestha N, Ji W, Attwood K, Adrish M, Jain KR. Thrombotic complications and anticoagulation in covid-19 pneumonia: A new york city hospital experience. Ann Hematol. 2020;99:2323–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary arterial thrombosis in covid-19 with fatal outcome : Results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173:350–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M, Merouani K. High incidence of venous thromboembolic events in anticoagulated severe covid-19 patients. J Thromb Haemost. 2020;18:1743–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackman N, Antoniak S, Wolberg AS, Kasthuri R, Key NS. Coagulation abnormalities and thrombosis in patients infected with sars-cov-2 and other pandemic viruses. Arterioscler Thromb Vasc Biol. 2020;40:2033–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Muller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N. Incidence of venous thromboembolism in hospitalized patients with covid-19. J Thromb Haemost. 2020;18:1995–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S, Lille ICUHC-G. Pulmonary embolism in patients with covid-19: Awareness of an increased prevalence. Circulation. 2020;142:184–186 [DOI] [PubMed] [Google Scholar]
- 11.Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, Hochman JS, Reynolds HR. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in covid-19: A case series. EClinical Medicine. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoliquido A, Porfidia A, Nesci A, De Matteis G, Marrone G, Porceddu E, Camma G, Giarretta I, Fantoni M, Landi F, Gasbarrini A, Pola R, Group GAC-, D’Alfonso ME, Lo Monaco MR. Incidence of deep vein thrombosis among non-icu patients hospitalized for covid-19 despite pharmacological thromboprophylaxis. J Thromb Haemost. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early-phase 2019 novel coronavirus (covid-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP. Covid-19 and coagulation: Bleeding and thrombotic manifestations of sars-cov-2 infection. Blood. 2020;136:489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe covid-19 infection: A report of five cases. Transl Res. 2020;220:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blasi A, von Meijenfeldt FA, Adelmeijer J, Calvo A, Ibanez C, Perdomo J, Reverter JC, Lisman T. In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in covid-19 patients on anticoagulation. J Thromb Haemost. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escher R, Breakey N, Lammle B. Severe covid-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, Dela Cruz CS, Dumont A, Halene S, Hwa J, Koff J, Menninger H, Neparidze N, Price C, Siner JM, Tormey C, Rinder HM, Chun HJ, Lee AI. Endotheliopathy in covid-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Angles-Cano E, Sattler L, Mertes PM, Meziani F, Group CT. High risk of thrombosis in patients with severe sars-cov-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, Klein M, Weinberger T. Elevated levels of il-6 and crp predict the need for mechanical ventilation in covid-19. J Allergy Clin Immunol. 2020;146:128–136 e124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C, Petrey AC, Tolley ND, Guo L, Cody M, Weyrich AS, Yost CC, Rondina MT, Campbell RA. Platelet gene expression and function in patients with covid-19. Blood. 2020;136:1317–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, Mostyka M, Baxter-Stoltzfus A, Borczuk AC, Loda M, Cody MJ, Manne BK, Portier I, Harris ES, Petrey AC, Beswick EJ, Caulin AF, Iovino A, Abegglen LM, Weyrich AS, Rondina MT, Egeblad M, Schiffman JD, Yost CC. Neutrophil extracellular traps contribute to immunothrombosis in covid-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nougier C, Benoit R, Simon M, Desmurs-Clavel H, Marcotte G, Argaud L, David JS, Bonnet A, Negrier C, Dargaud Y. Hypofibrinolytic state and high thrombin generation may play a major role in sars-cov2 associated thrombosis. J Thromb Haemost. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of covid-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippi G, Favaloro EJ. D-dimer is associated with severity of coronavirus disease 2019: A pooled analysis. Thromb Haemost. 2020;120:876–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun H, Ringdahl U, Homeister JW, Fay WP, Engleberg NC, Yang AY, Rozek LS, Wang X, Sjobring U, Ginsburg D. Plasminogen is a critical host pathogenicity factor for group a streptococcal infection. Science. 2004;305:1283–1286 [DOI] [PubMed] [Google Scholar]
- 28.Sun H, Wang X, Degen JL, Ginsburg D. Reduced thrombin generation increases host susceptibility to group a streptococcal infection. Blood. 2009;113:1358–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan D, Casper TC, Elliott CG, Men S, Pendleton RC, Kraiss LW, Weyrich AS, Grissom CK, Zimmerman GA, Rondina MT. Vte incidence and risk factors in patients with severe sepsis and septic shock. Chest. 2015;148:1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, International Sepsis Definitions C. 2001 sccm/esicm/accp/ats/sis international sepsis definitions conference. Intensive Care Med. 2003;29:530–538 [DOI] [PubMed] [Google Scholar]
- 31.Vahidy FS, Nicolas JC, Meeks JR, Khan O, Pan A, Jones SL, Masud F, Sostman HD, Phillips R, Andrieni JD, Kash BA, Nasir K. Racial and ethnic disparities in sars-cov-2 pandemic: Analysis of a covid-19 observational registry for a diverse us metropolitan population. BMJ Open. 2020;10:e039849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor FB Jr., Toh CH, Hoots WK, Wada H, Levi M, Scientific Subcommittee on Disseminated Intravascular Coagulation of the International Society on T, Haemostasis. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–1330 [PubMed] [Google Scholar]
- 33.Wolberg AS. Thrombin generation and fibrin clot structure. Blood Rev. 2007;21:131–142 [DOI] [PubMed] [Google Scholar]
- 34.Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982;257:2912–2919 [PubMed] [Google Scholar]
- 35.Miszta A, Kopec AK, Pant A, Holle LA, Byrnes JR, Lawrence DA, Hansen KC, Flick MJ, Luyendyk JP, de Laat B, Wolberg AS. A high-fat diet delays plasmin generation in a thrombomodulin-dependent manner in mice. Blood. 2020;135:1704–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rondina MT, Tatsumi K, Bastarache JA, Mackman N. Microvesicle tissue factor activity and interleukin-8 levels are associated with mortality in patients with influenza a/h1n1 infection. Crit Care Med. 2016;44:e574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aras O, Shet A, Bach RR, Hysjulien JL, Slungaard A, Hebbel RP, Escolar G, Jilma B, Key NS. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103:4545–4553 [DOI] [PubMed] [Google Scholar]
- 38.Baker JV, Huppler Hullsiek K, Bradford RL, Prosser R, Tracy RP, Key NS. Circulating levels of tissue factor microparticle procoagulant activity are reduced with antiretroviral therapy and are associated with persistent inflammation and coagulation activation among hiv-positive patients. J Acquir Immune Defic Syndr. 2013;63:367–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogura H, Kawasaki T, Tanaka H, Koh T, Tanaka R, Ozeki Y, Hosotsubo H, Kuwagata Y, Shimazu T, Sugimoto H. Activated platelets enhance microparticle formation and platelet-leukocyte interaction in severe trauma and sepsis. J Trauma. 2001;50:801–809 [DOI] [PubMed] [Google Scholar]
- 40.Antoniak S, Mackman N. Multiple roles of the coagulation protease cascade during virus infection. Blood. 2014;123:2605–2613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlier L, Hunault G, Lerolle N, Macchi L. Ex vivo thrombin generation patterns in septic patients with and without disseminated intravascular coagulation. Thromb Res. 2015;135:192–197 [DOI] [PubMed] [Google Scholar]
- 42.Schmitt FCF, Manolov V, Morgenstern J, Fleming T, Heitmeier S, Uhle F, Al-Saeedi M, Hackert T, Bruckner T, Schochl H, Weigand MA, Hofer S, Brenner T. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: Results of an observational pilot study. Ann Intensive Care. 2019;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zermatten MG, Gomez FJ, Pantet O, Papadimitriou-Olivgeris M. Hugli O, Bart P-A, Aliotta A, Bertaggia Calderara D, Mean M, Mazzolai, Alberio L. Thrombin generation in severe covid-19 patients hospitalized in the icu. Annual Meeting of the International Society on Thrombosis and Haemostasis. 2020 [Google Scholar]
- 44.Machlus KR, Cardenas JC, Church FC, Wolberg AS. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood. 2011;117:4953–4963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gould TJ, Vu TT, Stafford AR, Dwivedi DJ, Kim PY, Fox-Robichaud AE, Weitz JI, Liaw PC. Cell-free DNA modulates clot structure and impairs fibrinolysis in sepsis. Arterioscler Thromb Vasc Biol. 2015;35:2544–2553 [DOI] [PubMed] [Google Scholar]
- 46.Guo X, Liu Y, Li D, Li Y. Plasma thrombomodulin levels are associated with endothelial injury in patients with bacterial infections. Clin Lab. 2019;65. [DOI] [PubMed] [Google Scholar]
- 47.Harenberg J, Favaloro E. Covid-19: Progression of disease and intravascular coagulation - present status and future perspectives. Clin Chem Lab Med. 2020;58:1029–1036 [DOI] [PubMed] [Google Scholar]
- 48.Hardy M, Douxfils J, Bareille M, Lessire S, Gouin-Thibault I, Fontana P, Lecompte T, Mullier F. Studies on hemostasis in covid-19 deserve careful reporting of the laboratory methods, their significance and their limitations. J Thromb Haemost. 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


