Abstract
Urinary tract infections (UTIs) are common, recurrent infections that can be mild to life-threatening. The continued emergence of antibiotic resistance, together with our increasing understanding of the detrimental effects conferred by broad-spectrum antibiotic use on the health of the beneficial microbiota of the host, has underscored the weaknesses in our current treatment paradigm for UTIs. In this Review, we discuss how recent microbiological, structural, genetic and immunological studies have expanded our understanding of host-pathogen interactions during urinary tract pathogenesis. These basic scientific findings have the potential to shift the strategy for UTI treatment away from broad-spectrum antibiotics targeting conserved aspects of bacterial replication towards pathogen-specific antibiotic-sparing therapeutics that target core determinants of bacterial virulence at the host-pathogen interface.
Table of content:
In this Review, Klein and Hultgren discuss recent advances in our understanding of the interplay between pathogens and host during urinary tract infections, and how the insights into host-pathogen interactions and pathogenesis are guiding the development of antibiotic-sparing therapeutics.
Introduction
Urinary tract infections (UTIs) are common infections caused predominantly by uropathogenic Escherichia coli (UPEC), which cause approximately 80% of UTIs. The annual incidence of physician-diagnosed UTIs in the United States is greater than 10% for females and 3% for males, and more than 60% of females will be diagnosed with a UTI in their lifetime1,2. Individual risk for infection depends on various factors, including age, sexual activity, family history, medical comorbidities and an individual history of UTI (Box 1) 3. Recurrent UTIs (rUTIs; defined as three or more UTIs during a 12 month period 4) contribute greatly to the incidence and morbidity rates of UTI, as more than 30% of women will suffer from a subsequent infection within 12 months of resolution of initial symptoms despite appropriate antibiotic therapy 5. UTIs are becoming increasingly difficulty to treat owing to the rapid spread of drug resistance among Gram-negative organisms, including UPEC 6.
Box 1: Understanding host susceptibility.
Many established risk factors, such as female sex and sexual activity, are consistent with the hypothesis that mechanical transfer of resident pathogens from the perianal and perineal areas to urinary meatus leads to inoculation of the urinary tract 3,18. However, the molecular basis for other reported associations has only recently begun to be appreciated. Although it has long been known that a history of urinary tract infections (UTIs) is the greatest independent risk factor for the development of a UTI, the recent establishment of a mouse model of recurrent cystitis has provided fresh insight into the molecular mechanism for susceptibility (Supplementary figure S1). Transurethral infection of C3H/HeN mice with uropathogenic Escherichia coli (UPEC) isolate UTI89 resulted in two distinct outcomes; development of persistent, chronic cystitis or spontaneous resolution of infection within 14 days 173. These findings are consistent with the range of infection outcomes observed in untreated human UTIs 15. Outcome of the initial infection was strongly correlated with the response of the mouse to a second bacterial challenge following antibiotic treatment and a period of convalescence. Mice that spontaneously resolved the first infection were resistant to subsequent UTI, whereas mice that had previously progressed to chronic cystitis were more susceptible to severe recurrent UTI, even if the challenge strain differed from the initial sensitizing strain 173. Examination of the bladder epithelium revealed that the superficial umbrella cells of sensitized mice were smaller than those from resolved mice or age-matched adult naive controls and lacked markers of terminal differentiation 173. Furthermore, proteomic and transcriptomic analyses revealed that infection can leave a molecular imprint on the bladder, even after clearance of the infection with antibiotic therapy, which can predispose to recurrent UTI (rUTI). Susceptibility to recurrent UTI was strongly correlated with the expression of cyclooxygenase-2 (COX-2) following challenge infection and could be reduced by treatment with a selective COX-2 inhibitor. This is consistent with previous observations showing that activation of a host-pathogen checkpoint influences the outcome of infection 40,174.
Recent studies have also revealed a molecular basis for the association between vaginal dysbiosis and increased UTI risk 175. Transient colonization with Gardnerella vaginalis, an organism that is overgrown in patients with bacterial vaginosis, triggered activation of latent UPEC reservoirs in a mouse model of infection 176. G. vaginalis colonization leads to the exfoliation of superficial umbrella cells by inducing apoptosis in the bladder epithelium. These effects were not seen with transient colonization of Lactobacillus crispatus, a normal component of a healthy vaginal microbiota with proven efficacy in reducing the risk of rUTI 177. Additionally, G. vaginalis causes kidney inflammation in an interleukin-1α (IL-1α)-dependent manner, predisposing colonized animals to UPEC-dependent pyelonephritis 176. Together, these findings provide mechanistic support for previous observations noting the overlap between bacterial vaginosis and pyelonephritis risk factors 178,179.
Most UTIs are initiated when UPEC enter the urinary tract through the urinary meatus before ascending up the urethra and into the bladder lumen. Isolated infections of the bladder and lower urinary tract without signs or symptoms of upper tract or systemic infection are referred to as ‘uncomplicated’ cystitis or ‘simple’ cystitis (Table 1). Acute cystitis in non-pregnant, premenopausal patients without functional urinary tract abnormalities is classified as uncomplicated7. Cystitis is classified as ‘complicated’ cystitis in pregnant or immunocompromised patients and patients with functional urinary tract abnormalities, an indwelling catheter or a history of renal transplantation 8. In approximately 0.34% of cases, pathogens causing cystitis ascend further through the ureters into the kidney, where they cause an infection of the renal pelvis, calices and cortex that leads to the clinical signs and symptoms of pyelonephritis 9. If left untreated, pathogens may spread from the kidney into the bloodstream (bacteremia) and, if a concurrent systemic inflammatory response is present this may lead to septicemia 10. In patients presenting to the emergency department with sepsis, 27% of all cases can be attributed to a previous urinary isolate, and are thus termed urosepsis 11. In hospitalized patients, the proportion of sepsis cases attributed to UTI increases to 42% 12. There is an urgent need to develop antibiotic-sparing therapeutics that can break the vicious cycle of rUTI, antibiotic resistance and the interconnection to sepsis.
Table 1:
Classification of urinary tract infections, symptoms, host risk factors and treatment. [Au: would it work to set the table up as indicated?]
| Infection type | Cystitis (bladder infection) | Pyelonephritis (kidney infection) |
|---|---|---|
| Classification | Uncomplicated UTI Complicated UTI |
Complicated UTI |
| Symptoms | - Painful urination (dysuria) - Urinary frequency - Urinary urgency - Suprapubic pain - Bloody urine (hematuria) |
- Fever - Flank pain - Costovertebral angle tenderness |
| Host risk factors | - Positive individual UTI history - Positive family history - Sexual activity - New sexual partner - Postmenopausal age - Vaginal dysbiosis - Recent antibiotic use |
- Positive individual UTI history - Positive family history - Sexual activity - New sexual partner - Postmenopausal age - Vaginal dysbiosis - Recent antibiotic use - Anatomic urogenital abnormalities - Vesicoureteral reflux - Diabetes mellitus - Pregnancy - Catheterization - Urolithiasis - Immunosuppression - History of pyelonephritis |
| Diagnosis | - Positive urine culture - Pyuria - Hematuria* |
- Positive urine culture - Pyuria - Hematuria* |
| Treatment (low MDR risk**) | Empiric treatment with nitrofurantoin or fosfomycin | Treatment with ciprofloxacin or levofloxacin |
| Treatment (high MDR risk**) | Treatment with oral β-lactams and fluoroquinolones until culture sensitivity results are acquired | Treatment with ceftriaxone or ertapenem and trimethoprim-sulfamethoxazole, Augmentin or a 3rd-generation cephalosporin |
MDR, multi-drug resistant; UTI, urinary tract infection.
Diagnosis and treatment of acute simple cystitis is often alone, although symptoms are not considered diagnostic.
MDR risk factors include an extended stay at a hospital facility, recent travel to India, Israel, Spain and Mexico, and/or recent antibiotic use.
The risk of UTI progression to pyelonephritis and urosepsis is dependent, in part, on the setting in which the UTI was acquired. For example, the pathogen population that causes healthcare-associated UTIs due to indwelling urethral catheters (catheter-associated UTIs (CAUTIs)) comprises a larger group of bacteria from those responsible for community-acquired UTIs. Insertion of a urinary catheter into the urethra and bladder results in the release of host factors such as fibrinogen, which coat the catheter. These factors enable diverse pathogens, including members of the genera Enterococcus and Staphylococcus, to bind and assemble biofilms on the catheter surface 13. The genomes of these pathogens encode fibrinogen-binding adhesins that are crucial to biofilm formation on the catheter, and confer increased mortality if the infections progress to pyelonephritis and urosepsis 14,15.
Traditionally, broad-spectrum antibiotics have been the drug of choice to combat both community- and hospital-associated UTIs. However, the continued emergence of antibiotic resistance, coupled with our increasing appreciation for the role of commensal members of the host microbiota, have underscored the urgent need for antibiotic-sparing therapeutics that can selectively treat UTIs without altering the structure of the gut and vaginal microbiota. In the gastrointestinal tract, antibiotic consumption increases inflammation, undercuts the host immune environment and promotes the proliferation of E. coli by increasing nitrate availability 16. As the gastrointestinal tract is frequently the ultimate reservoir for UPEC, expansion of UPEC within the gastrointestinal habitat is also associated with increased risk of subsequent UTI recurrence 17,18. Antibiotic treatment can also disrupt the protective vaginal microbiota by diminishing colonization with peroxide-producing Lactobacillus species that suppress vaginal UPEC colonization and subsequent bacterial ascension 19,20. Thus, paradoxically, the antibiotics used to treat UTIs are also risk factors for rUTIs, probably because of their effect on the gut and vaginal microbiotas 16, 21, 22.
In this Review, we discuss recent advances in our understanding of the dynamic and diverse interplay between pathogens and the host during infection, and how these molecular insights into host-pathogen interactions are guiding the design and development of antibiotic-sparing therapeutics. We first outline the pathogenic cascade of uncomplicated acute UTI and critical virulence factors involved in disease progression, followed by a discussion of CAUTI pathogenesis and virulence factors involved in establishing colonization and infection. We discuss how new insights into host-pathogen interactions are leading to the development of promising antibiotic-sparing therapeutics including small-molecular inhibitors of adhesion, inhibitors of nutrient uptake, immunomodulatory therapy that alters the host response to infection and vaccination against microbial targets. Such promising antibiotic-sparing therapeutics have the potential to curb recurrence and circumvent existing antimicrobial resistance.
UPEC pathogenesis during UTI
Recent metagenomic and metatranscriptomic studies on a panel of clinical isolates, combined with in vitro, in vivo, animal and human studies have provided tremendous molecular insights into the wide range of virulence factors used by UPEC to overcome the innate and adaptive immune response of the host to facilitate bladder colonization during UTI 23. These studies have also extended to virulence factors promoting the colonization of extraurinary tissues, including the vaginal, peri-urethral and gastrointestinal tracts en route to ascending UTI. In this section, we provide an overview of host-pathogen interactions critical in uncomplicated UTI. A discussion of novel insights into the interplay between virulence factor carriage and bacterial virulence is found in Box 2.
Box 2: Understanding uropathogenic Escherichia coli urovirulence based on host and pathogen factors.
Despite our growing appreciation for the function of different virulence factors during uropathogenic Escherichia coli (UPEC) urinary tract infection (UTI) pathogenesis, the identification of genomic signatures that can reliably predict urovirulence has proven challenging. Analysis of 43 E. coli strains and collected from women suffering from recurrent UTIs and 46 reference strains spanning multiple clades revealed a set of ‘core’ genes that comprise 60%-75% of the total genome, whereas the remaining 25%-40% varied between strains 23. Pathogenic E. coli strains are traditionally categorized into one of several pathotypes, each of which contains a combination of virulence factors that facilitates a specific pathogenic cascade within a defined host habitat 180. Although E. coli strains within a given pathotype are often clustered into the same phylogenetic groups based on population genetic studies, this is not the case for UPEC 181. For example, over 50% of E. coli strains isolated from patients with UTIs in the United States and Europe belong to clade B2, whereas 25%-50% belong to clades A, B1 and D 182,183. Additionally, this proportion is subject to a high degree of geographic variation; 42% of urinary isolates from East Asia belong to group D, whereas fewer than 30% belong to B2 184. Although studies have shown that B2 strains typically encode a greater number of putative urovirulence factors than strains from other groups, analysis of virulence factor carriage alone does not elucidate a clear genomic signature of urovirulence 23,166,182,185. However, analysis of E. coli metabolic activity during colonization, coupled with the expression pattern of certain genes participating in core bacterial functions during growth in liquid culture in vitro, may effectively predict uropathogenic potential 23,186. A systematic, interdisciplinary study of diverse clinical urine-associated E. coli isolates revealed a new conceptual model of UTI in which UTI risk and outcome are determined by a combination of dynamic host susceptibility determinants and diverse bacterial urovirulence phenotypes that are driven not only by variations in gene content but by differential expression of conserved functions 23.
Putative virulence factor carriage and expression also correlate with antibiotic resistance 75,78. A study examining the relationship between virulence factor carriage and resistance to fluoroquinolones in UPEC revealed that resistance is negatively associated with the carriage of specific virulence factors, including P pili and α-hemolysin 182. Specific associations, such as that between ciprofloxacin susceptibility and the carriage of papG-II, hylA and kpsMTK1 (a capsule gene), have also been described 187. Based on these data, it seems that the ability of individual E. coli strains to colonize the urinary tract is dependent on the interaction between bacterial determinants of virulence and individual host susceptibility factors. Further studies suggest that recurrent UTI and persistent bladder colonization by diverse pathogens may also be associated with poor treatment response in patients with overactive bladder 188. These studies illustrate the layers of complexity that underlie urovirulence, and underscore the need for continued interrogation of the E. coli genome and transcriptome in UTI isolates.
The UTI cycle and host immune response.
Over the past 20 years, various mouse models have been developed to better dissect the molecular mechanisms of UTI pathogenesis (Supplementary Figure S1). Because biopsy is not typically performed in patients with UTI, urine cytology and metabolic analysis are often necessary to correlate findings in mouse models to human disease. This has recently been accomplished by metatranscriptomic analyses of UPEC isolated directly from the urine of patients with UTIs 24. Knowledge gained from numerous mouse models of UTI pathogenesis have proven to be translatable to humans. After reaching the mouse bladder lumen, UPEC adhere to the surface of superficial umbrella cells using thin adhesive filaments called type 1 pili (Figure 1). Following attachment, bacteria are internalized into epithelial cells, where they multiply within this protected intracellular niche to form large biofilm-like intracellular bacterial communities (IBCs). Here, UPEC can evade Toll-Like receptor 4 (TLR-4)-mediated expulsion and replicate in the urothelial cell cytoplasm before fluxing out back into the bladder lumen and adhering to nearby cells 25,26. Formation of IBCs is the result of clonal expansion of a single bacterium, enabling the infection to sidestep efficient host clearance of extracellular bacteria while decreasing of the overall genetic diversity of the population 27. Although the kinetics of attachment, internalization, and IBC formation in humans may differ from their mouse counterparts, the presence of IBCs has been verified in several human adult and pediatric UTI studies 28–31.
Figure 1: Overview of urinary tract infection pathogenesis by UPEC.
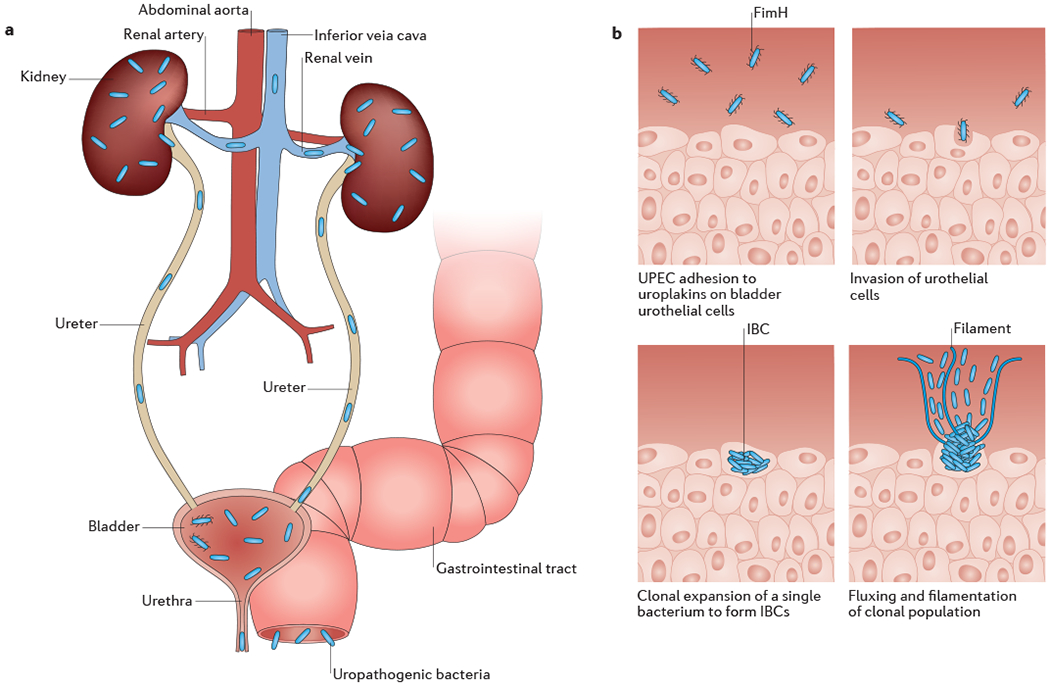
A) Uropathogenic Escherichia coli (UPEC) colonizing the gastrointestinal tract, perineum or vagina inoculate the urethra and ascend into the bladder. Isolated infections of the bladder are termed cystitis, and result in the classic urinary tract infection (UTI) symptoms, such as urinary frequency, urinary urgency, dysuria, and suprapubic tenderness. Bacteria can also ascend the ureters to the kidneys, where they cause a kidney infection termed pyelonephritis that can result in fever, chills and flank pain. Finally, bacteria can invade the bloodstream, causing bacteremia that can eventually lead to septic shock. B) In the bladder, individual bacteria adhere to and invade superficial urothelial umbrella cells in a FimH-dependent manner. There, they undergo clonal expansion to form biofilm-like intracellular bacterial communities (IBCs), where they can evade mechanical and immunological clearance mechanisms by the host. Bacteria then form filaments that flux out of the umbrella cells into the bladder lumen, where they can subsequently bind to neighboring cells and begin a new infection cycle.
In response to infection of naïve C3H/HeN mice with human UTI isolates, host secretion of interleukin-6 (IL6), IL-17, tumour necrosis factor (TNF), C-X-C motif chemokine 1 (CXCL1), CXCL2 and CXCL5 results in extensive neutrophil recruitment to the urothelium 32–35. This response is followed by the recruitment of monocytes and the induction of programmed cell death and exfoliation, which results in an initial decrease in bacterial burden in the bladder but also exposes the underlying transitional epithelium 36,37. Histologically, these processes are accompanied by lymphonodular hyperplasia and loss of terminal markers of differentiation on mouse superficial facet cells, both of which have also been observed in humans with rUTI 38. In mouse models, this immune-mediated exfoliation can lead to bacterial attachment and invasion of the transitional epithelium, which results in the formation of small quiescent intracellular reservoirs (QIRs) that persist even after resolution of bacteriuria that are capable of seeding rUTI 39. Although the presence of QIRs has yet to be observed in humans, these models suggest that exfoliation can be a double-edged sword wherein the initial bacterial UPEC burden is reduced at the expense of long-term persistence and recurrence.
Upregulation of cyclooxygenase 2 (COX2) expression in urothelial cells and neutrophils in response to UTI is associated with neutrophil transmigration across the bladder epithelium, and is crucial for the associated mucosal damage that ensues 40. Following this transmigration in mouse models of infection, persistent high-titer bacteriuria accompanied by severe immunopathology and ablation of the terminally differentiated superficial umbrella cells can develop39,41. Chronic cystitis is a prolonged infection observed in mice that is characterized by persistent high-titer bacteriuria with high bladder bacterial burdens. Histologically, this is manifested as chronic inflammation with lymphonodular hyperplasia of the submucosa and urothelial hyperplasia with loss of terminal differentiation 41. Placebo studies have shown that 50% of infections in humans do not resolve in the absence of effective antibiotic treatment, and histological features of chronic cystitis observed in mice have also been observed in humans 38,42. Studies of serum biomarkers in premenopausal women with acute cystitis caused by UPEC reveal that chronic and recurrent cystitis can be predicted 24 hours post-infection by increased levels of the serum biomarkers IL-5, IL-6, CXCL1 and granulocyte colony-stimulating factor (G-CSF) 40,41. Similarly, in the sera of young women with acute cystitis, increased levels of soluble biomarkers involved in myeloid cell development and chemotaxis were predictive of future UTI recurrence 41.
An episode of acute cystitis can result in either a protective response or render the host more susceptible to recurrences. In a mouse model of rUTI, the dynamics of TNF signalling activation differed when the initial infection was chronic (sensitized) compared with self-limiting (resolved). Inoculation of mice who had experienced a prior bladder infection with UPEC resulted in an earlier onset of TNF-mediated inflammation compared with age-matched naïve mice. This early-phase TNF signaling was shown to decrease colonization by facilitating rapid recruitment of neutrophils and exfoliation of infected bladder cells. By contrast, mice who suffered from chronic UTI following initial infection experienced a prolonged TNF signaling period, which exacerbated inflammation and worsened infection. Thus, the regulation of TNF dynamics and COX-2 expression (Box 1) could explain how a prior infection can modulate susceptibility to future infections 43.
Chaperone-usher pathway pili.
Chaperone-usher pathway (CUP) pili are proteinaceous extracellular appendages found on the outer membrane of Gram-negative bacteria. The E. coli pangenome encodes 38 distinct CUP pili, and individual E. coli genomes encode an average of 12 CUP operons, each of which likely mediates binding to a specific molecular receptor on biotic or abiotic surfaces 44. The binding specificity of each pilus type is typically conferred by a two-domain adhesin located at the distal tip of a fiber comprising hundreds of major structural subunits (Fig 2a). P pili and type 1 pili are two CUP pili that have well-established roles in UTI pathogenesis. The PapG adhesin at the tip of P pili binds to a Galα1-4Gal core on globoside glycolipids in the kidney and contributes to pyelonephritis, whereas the FimH adhesin at the tip of the type 1 pilus binds mannosylated glycoproteins on superficial epithelial cells with stereochemical specificity (Fig 2a) 36,45–47. This type-1-pilus-mediated adhesion mediates colonization and invasion of the bladder epithelium and facilitates the formation of the biofilm-like IBCs (discussed above).
Figure 2: Bacterial urovirulence factors.
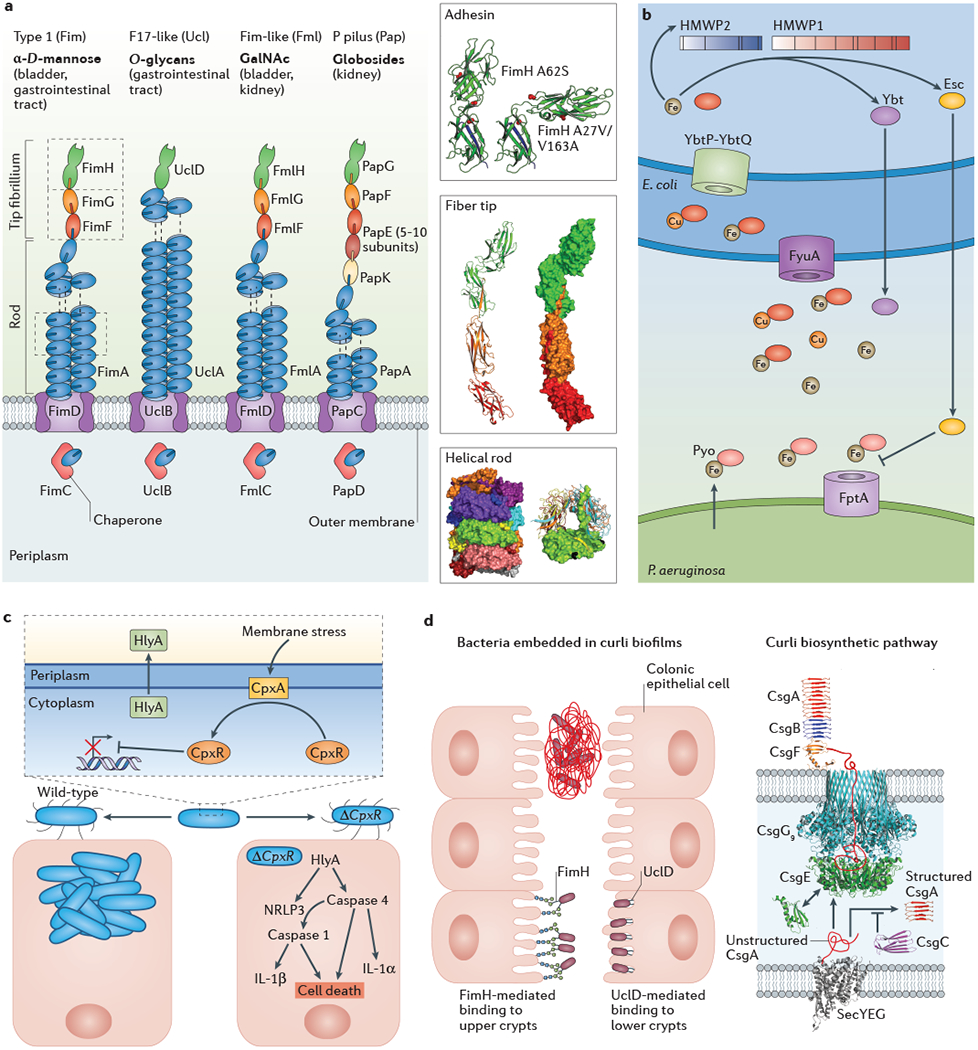
A) Four chaperone-usher pathway pili, including type 1 pili (also known as Fim pili), F17-like pili (also known as Ucl pili), Fim-like pili (also known as Fml pili) and P pili (also known as Pap pili), are involved in urinary tract infections (UTIs) through various mechanisms (left panel). Type 1 pili bind mannosylated uroplakins in the bladder and mannosylated mucous components in the gastrointestinal tract using the two-domain FimH adhesin. F17-like pili bind O-glycans in the gastrointestinal tract, helping to establish a reservoir for recurrent UTIs. Fim-like pili bind GalNAc moieties found in the inflamed bladder and kidneys, which promotes chronic UTIs. P pili bind globosides in the kidney and are essential for progression to pyelonephritis. Biophysical studies performed on type 1 pili revealed the importance of conformational dynamics in the adhesin and pilus rod during infection (right panel). More specifically, positive selection at positions 27, 62, and 163 influencesconformational changes in the FimH adhesion that modulate the affinity of the binding pocket for mannose residues on the bladder epithelium. The FimHA62S variant preferentially adopts a ‘tense’ conformation with a low affinity for mannose, whereas the FimHA27V/V163A variant preferentially adopts a ‘relaxed’ conformation with high affinity for mannose. In the wild type protein, it is thought that an equilibrium between these species is necessary for maximum virulence. These adhesin subunits are noncovalently linked to the pilus tip and rod via a process known as donor strand exchange, wherein a donor strand from the preceding pilus completes the beta strand of the immunoglobulin-like fold. Furthermore, interactions between neighboring subunits within the Fim pilus rod when it adopts its native helical conformation modulate the force-length characteristics of the type 1 pilus in response to external force. Alterations to these interactions lead to an attenuation of virulence in mouse models of cystitis.
B) Uropathogenic Escherichia coli (UPEC) preferentially express a subset of siderophores, including yersiniabactin (Ybt), during infection of the bladder. Ybt binds extracellular iron and copper ions and delivers them to the FyuA outer membrane receptor for internalization. The YbtP-YbtQ complex then transports the copper-Ybt complexes to the cytoplasm. The presence of depressed cytoplasmic metal levels upregulates transcription of the HWMP1 and HWMP2, which subsequently synthesize Ybt. A metabolic byproduct of the HMWP1/2-mediated Ybt synthesis pathway, escherichelin (Esc), engages in interbacterial competition by disrupting prochelin (Pyo)-mediated iron uptake through the FptA outer membrane transporter in Pseudomonas aeruginosa. C) Periplasmic CpxA (the kinase of the CpxA-CpxR two component system) can sense protein misfolding (periplasmic stress) in E. coli during UTI, leading to the induction of genes responsible for the production of the pore-forming toxin α-hemolysin (HlyA) by the CpxR response regulator. Approximately 40-50% of UPEC strains encode HlyA, which contributes to activation of the NRLP3 inflammasome, caspase-1 and caspase-4, interleukin-1β (IL-1β) and IL-1α, leading to urothelial cell death in the host. Deletion of the CpxR in UPEC results in overexpression of HlyA and loss of bacterial fitness in mouse models of acute and chronic cystitis by triggering the caspase-mediated inflammatory cell death pathway and early vigorous urothelial exfoliation. Such vigorous exfoliation overcomes the ability of wild type UPEC to subvert the exfoliation response and invade underlying epithelium. D) Mechanisms of gastrointestinal colonization and curli biofilm formation. In the gastrointestinal environment, E. coli can embed themselves in a complex extracellular matrix containing curli fibers, cellulose and DNA fragments to promote persistence and prevent clearance. Curli comprise repeating amyloidogenic CsgA subunits that are assembled into extracellular fibers. The periplasmic chaperone CsgE interacts with unfolded CsgA to facilitate the transport of CsgA to and through the nonameric CsgG outer membrane pore. CsgE and the chaperone-like protein CsgC prevent premature CsgA polymerization in the periplasm. Following transport to the CsgG pore, CsgA is secreted across the outer membrane where curli fiber formation is facilitated by CsgB, which is held to the outer membrane by CsgF. CsgB nucleates the folding of CsgA resulting fiber polymerization. The FimH adhesin at the tip of type 1 pili and UclD adhesin at the tip of F17-like pili also mediate binding to the intestinal crypts, helping to establish a stable gastrointestinal reservoir.
In the past few years, a wealth of new information has emerged about the role of other CUP pilus types in UTI pathogenesis 48,49. For example, expression of the Fim-like (Fml) pilus, which is enriched in the genomes of clinical UPEC isolates, was shown to occur in the urine of women with UTI, and in mice, the presence of the Fml pilus confers a fitness advantage to UPEC in the inflamed bladder 50–52. The Fml pilus adhesin FmlH recognizes GalNAc-containing receptors expressed on inflamed bladder tissue, Gal(β1-3)GalNAc in the core 1 Thomsen-Friedenreich antigen, and core 2 O-glycans found on glycoproteins excreted by the kidney epithelium 52.
CUP pili also facilitate the colonization of numerous other habitats, such as the gastrointestinal tract. The observation that UPEC strains isolated from the urine of women with UTI often concurrently dominate the rectal florae of those patients suggests that CUP adhesins with tropism for the gastrointestinal tract may influence the urovirulence of a strain 18. Genomic analyses of clinical UTI isolates causing recurrence revealed a strong enrichment for the carriage of the F17-like pilus, also known as the Ucl pilus, relative to the general E. coli pangenome 53. In a mouse model of gastrointestinal colonization, deletion of the ucl operon was associated with a stark competitive defect, validating its role in colonization of this habitat 53. The F17-like adhesin, UclD, uses a canonical β-sandwich topology to bind O-glycans on epithelial cells in the lower crypts of the colon 53. Phylogenetic analyses indicate that UPEC acquired the ucl operon from Proteus mirabilis, and that strains encoding this operon were subsequently better adapted for gastrointestinal persistence and seeding of the urinary tract 53. Additionally, FimH also has a role in gastrointestinal colonization by binding the upper crypts of the intestine in a mannose-dependent manner 53.
Structural and kinetic analyses revealed that UPEC can engage in a low affinity FimH-mediated multivalent bonding with mannosylated N-glycans on host cells within the bladder under static conditions, whereas the shear forces induced by rapid urine flow induce a high affinity catch-bond conformation that prevents bacterial expulsion in the urine stream 54. Recent biophysical analysis has also revealed that soluble FimH adhesin exists in two distinct conformational states that engage with mannose via unique binding modes with markedly different affinities 55. In addition to the catch-bond mechanism, positively-selected residues identified by prior genetic studies were also found to modulate the allosteric interconversion between these low- and high-affinity states 55–57. In the high-affinity state, the FimH adhesin is able to transition between a wide range of conformational states, enabling the tethering of bacteria to the luminal surface of the bladder. Allelic variants favoring the high-affinity conformation bind bladder cells with high affinity and invade transitional epithelial cells in vitro, but are attenuated in in vivo mouse models of cystitis 55. Thus, it seems that evolutionary selection has fine-tuned conformational states of the FimH adhesin in solution to modulate molecular tethering to receptors that are crucial for virulence in UTIs.
The role of type 1 rod dynamics has also been the subject of recent investigation. The major subunits of many CUP pili are assembled into a helical rod that is capable of unwinding into a linear fiber upon the application of external force to dampen the force at the adhesin-receptor interface, providing a ‘shock absorber’ effect which prevents bacterial dissociation from host tissues 58,59 (Fig 2a). Because interactions formed between neighboring pilin subunits within the helical rod dictates the strength of this unwinding force, the biomechanical properties of individual pili seem to be evolutionarily selected to optimize adhesion within different habitats 60. For example, P pili have adapted to the constant urine flow in the kidney by requiring a greater force to unwind, whereas the CS20 fimbriae that mediate intestinal attachment in enterotoxigenic E. coli (ETEC) strains are more easily unwound to accommodate the short, intense forces bacteria experience in the intestine 60. Structural and biophysical studies of the type 1 pilus rod revealed that residues responsible for the interaction between adjacent pilus subunits involved in winding and unwinding are subject to purifying selection. Mutation of these residues leads to substantial attenuation during in vivo infection in a mouse model 61,62. Thus, the unwinding dynamics of the pilus rod has a crucial function in host-pathogen interactions. Conversely, the high rate of polymorphism among outward-facing residues of the rod reflects high rates of positive, diversifying selection, which are likely to aid in immune evasion 61.
Finally, the role of the outer-membrane usher has also been subject to extensive biochemical and structural investigation. Recent high-resolution structures of the P pilus and type 1 pilus ushers in complex with their respective chaperone-adhesin complexes have revealed a putative mechanism by which the chaperone-adhesin complex is transferred from the amino-terminal to the carboxy-terminal periplasmic domain of the usher where the donor strand exchange reaction is subsequently catalyzed 63,64. For P pili, transfer requires formation of a tripartite interface between the incoming PapD chaperone and the N-terminal and C-terminal domains of PapC usher. The PapC-PapD-PapG structure revealed a potential mechanism for how bicyclic 2-pyridone compounds are able to inhibit pilus biogenesis by blocking interactions necessary for the formation of the tripartite complex 65. Such compounds hold promise in the treatment of infectious diseases.
Metal sequestration.
Sequestration of metals and other trace minerals by the host is perhaps the best-studied example of nutritional immunity 66,67. For example, the host response to infection includes increased expression of several iron-binding proteins, including lactoferrin and lipocalins, to sequester iron away from invading pathogens 68–70. To combat this, bacterial pathogens have evolved various genes that encode siderophores [G] 71(Fig 2b). Metabolomic characterization of E. coli during colonization has elucidated the interspecies diversity in metal acquisition systems between E. coli isolated from various habitats 72. Clinical UPEC isolates preferentially express two siderophores, yersiniabactin and salmochelin, at higher levels than gastrointestinal E. coli isolates, which suggests that optimization of iron uptake in the bladder environment is important for UPEC pathogenesis 73. Yersiniabactin binds extracellular iron and copper ions and delivers them to the FyuA outer membrane receptor (Fig 2b), which is associated with UPEC virulence in the bladder and is required for the establishment of septicemia 71,74–76. UPEC strains lacking the fyuA gene experience a bladder and kidney colonization defect relative to a wild-type control 77. Expression of an essential peptide for yersiniabactin synthesis , high-molecular weight protein 2 (HMWP2) encoded by the irp2 gene, is regulated by environmental iron levels and is associated with increased UPEC virulence and antibiotic resistance74,78. HMWP2 associates with HMWP1 to catalyze the formation of yersiniabactin via a series of biosynthetic precursors in the presence of low iron levels. Further metabolomic and biochemical characterization of the siderophore biosynthetic pathways has revealed metabolic precursors that may have secondary functions, such as microbial antagonism 79. For example, the production of escherichelin, a product of the yersiniabactin biosynthetic pathway, interferes with pyochelin iron uptake in Pseudomonas aeruginosa79
Two-component systems.
α-hemolysin (HlyA) is a proteinaceous pore-forming toxin that assembles into a water-filled channel that perforates the outer membrane of host cells, leading to lysis and cell death that increases nutrient availability to pathogens 80. HlyA is found in approximately 50% of recurrent UTI clinical isolates from women aged 18-39 versus 37% and 31% of isolates from first and second UTIs, respectively. This represents an enrichment over isolates found in the peri-urethral and fecal environments from women in those same age groups, where prevalence is 26% and 15%, respectively 81. HlyA is known to cause damage to urothelial and renal tubular cells, promoting exfoliation and inflammatory changes 82–84. Expression of hlyA in E. coli is regulated, in part, by the CpxR-CpxA two-component signal transduction system 84 (Fig 2c). Dysregulation of the CpxR-CpxA system alters the expression profiles of several virulence factors, including hlyA, as deletion of the response regulator CpxR in UPEC results in overexpression of hemolysin and loss of bacterial fitness in mouse models of acute and chronic cystitis 84. Mechanistic studies have revealed that HlyA contributes to activation of the host inflammasome by activating the caspase-1 and caspase-4. The CpxR-CpxA system itself is regulated by the interaction of CpxP with unfolded pilus subunits, further highlighting the complex interplay between regulators of bacterial virulence 85,86. Additional two-component systems known to affect UPEC virulence include the YdpA-YdpB and BtsS-BtsR systems, which together upregulate transporter genes in acute and chronic mouse models of UTI and in response to the presence of environmental biomolecules such as pyruvate 87,88. The QseB-QseC system, which is composed of the QseB response regulator and QseC kinase, is known to regulate UPEC virulence factors in response to high levels ferric iron 89–91. Finally, recent work has shown that crosstalk between the QseB-QseC and the PmrA-PmrB two-component systems has a role in antimicrobial resistance 92,93.
Biofilms.
Formation of biofilms by UPEC isolates is associated with increased bladder fitness and antimicrobial resistance. In a prospective study of 105 patients with E. coli UTIs, biofilm production was noted in 62% of UPEC isolates versus only 1% of control isolates, and was associated with higher rates of antimicrobial resistance 94. Recent studies have revealed that the reduced oxygen tension found in the bladder, coupled with the presence of terminal electron receptors within urine, facilitates the preferential expression of E. coli biofilms 95. The expression of other factors, such as the quinol oxidase cytochrome bd, facilitates biofilm complexity and resistance to extracellular stressors by altering the abundance of extracellular matrix components 96.
Curli fibers are a functional amyloid that forms the major proteinaceous component of many Gram-negative bacterial biofilms (Fig 2d) 97,98,99,100. The presence of curli fibers within these biofilms provide a competitive advantage in mouse models of UTI by promoting adhesion to bladder epithelial cells 101,102. This adhesion is further augmented by the presence of phosphoethanolamine cellulose concurrently produced by UPEC 102. Structural, biochemical and biophysical analyses have elucidated the molecular mechanisms of curli biogenesis by E. coli and other pathogens. Curli comprise repeating amyloidogenic CsgA subunits that are assembled into sodium dodecyl sulphate-resistant and protease-resistant extracellular fibers by several periplasmic and extracellular assembly factors. Specifically, CsgE is a periplasmic chaperone that interacts with unfolded CsgA via a series of charge-charge interactions to facilitate the transport of CsgA to and through the nonameric CsgG outer membrane pore 103(Fig 2d). The concerted efforts of CsgE and the chaperone-like protein CsgC prevent premature CsgA polymerization in the periplasm 104 (Fig 2d). Following transport to the CsgG pore, CsgA is secreted across the outer membrane where curli fiber formation is facilitated by CsgB, which is held to the outer membrane by CsgF. CsgB nucleates the folding of CsgA into its fibrillar β-solenoid conformation, resulting in a nucleation-elongation reaction that promotes fiber polymerization 105. Following a one-step nucleation process, elongation of individual curli fibers proceeds through stop-and-go dynamics and can be inhibited by CsgC 106. Further, forward-genetic screening examining curli production has revealed a number of genes essential for curli biosynthesis in a human pyelonephritis isolate that had not been previously characterized 107. Four genes with newly confirmed roles in curli biosynthesis (purF, purD, purM and purK) belong to the purine biosynthesis operon, and one gene (yrfF) controls the Rcs phosphorelay system important for colonic acid biosynthesis 108,109.
Catheter-associated UTI
Although UPEC are the most common causative agents in both community-acquired and CAUTI, the proportion of infections attributed to Gram-positive bacteria and fungi are enriched in CAUTI 14,110. Although reported prevalence rates vary depending on geographical location, sampling technique and diagnosis cutoffs, a recent multistate study in the United States revealed that Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species (ESKAPE) are the most common causative healthcare-associated UTI pathogens after E. coli 110, and many of these species are commonly associated with nosocomial infections and antibiotic resistance 111,112. As with UPEC, the proliferation of antibiotic-resistant bacterial strains in the healthcare environment has increased the cost and complexity of treating these infections 113. Efforts to reduce the incidence of CAUTI by limiting the frequency and duration of catheter use have been successful to some extent114,115. However, strict adherence to clinical guidelines to limit the screening and treatment of asymptomatic bacteriuria in the absence of symptomatic indications during catheterization, along with new treatment approaches, are needed to limit the total disease burden and proliferation of antimicrobial resistance among CAUTI strains 116. Recent studies examining the interplay of host response to indwelling urinary catheters and bacterial virulence factors have revealed hitherto unappreciated mechanisms of catheter colonization as described below.
Catheterization alters bladder ecology.
Implantation of a urinary catheter in the bladder is accompanied by various immunological and histological changes, including edema, epithelial exfoliation, metaplasia and inflammation 117,118. in a mouse model of urinary catheterization, edema occurs within three hours of bladder implantation, and continues for up to 24 hours post-implantation (as indicated by the thicker bladder outline (Fig 3))119. An inflammatory response mediated by interleukins IL-1β, IL-6, IL12 p40 and IL-17 is also observed and can be partially inhibited by treatment with glucocorticoids (Fig 3). The physiologic response to catheterization also includes the release of fibrinogen into the bladder lumen, which coats the catheter 120. A study conducted in a swine model of sterile catheterization revealed a Toll-like receptor 9 (TLR9)-nuclear factor-κB (NF-κB)-mediated increase in neutrophil migration into bladder tissues driven by increased levels of circulating mitochondrial DNA (which is associated with tissue damage and organ dysfunction)121. This results in bladder congestion, edema and hemorrhage that can be partially mitigated by coating catheters with chloroquine and N-acetylcysteine to decrease the levels of circulating cytokines121. In sum, catheterization alters the bladder ecology and creates an environment that is more amenable to colonization by common CAUTI pathogens, including Enterococcus and Staphylococcus species.
Figure 3:
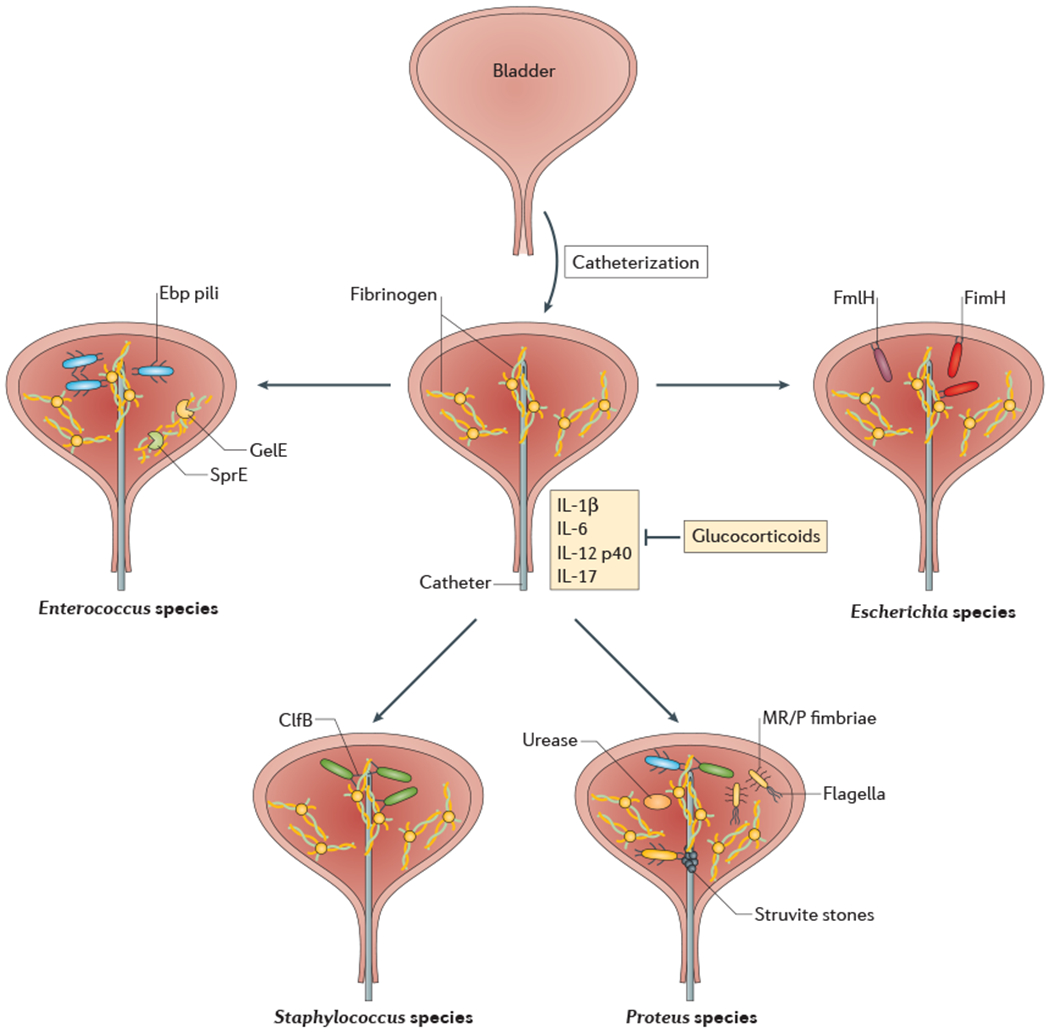
Pathogenesis of catheter-associated urinary tract infections. Introduction of a urinary catheter into the bladder environment results in inflammation and the generation of fibrinogen, which is deposited on the urinary catheter. Urinary pathogens exploit this fibrinogen deposition in various ways. Enterococci utilize Ebp pili to bind directly to the fibrinogen, and the GelE and SprE proteases to promote fibrinogen cleavage and urinary growth using fibrinogen as a food source. Staphylococci use clumping factor B (ClfB) to adhere to the fibrinogen-coated catheter and potentiates bladder inflammation to promote persistence. Proteus species use various virulence factors, including mannose-resistant Proteus-like (MR/P) fimbriae, flagella and urease to promote the formation of bladder stones and crystalline biofilms that permit bacterial persistence in the catheterized bladder. Finally, Escherichia coli use the FimH adhesin to bind fibrinogen coating the catheters.
Enterococcus.
Enteroccocus faecalis and E. faecium are Gram-positive bacteria responsible for up to 17% of nosocomial CAUTIs 110,113. Over 85% of E. faecium and 7% of E. faecalis clinical CAUTI isolates tested were found to be resistant to vancomycin 113. Using a mouse foreign-body implantation model of UTI (Supplementary figure S1), it was found that Enterococci utilize endocarditis- and biofilm-associated pili (Ebp) to form biofilms on catheters and overcome the neutrophil-mediated immune response 119 (Fig 3). Ebp pili are tipped with a fibrinogen-binding adhesin known as EbpA, which binds to the fibrinogen coating the catheter. Mutation of the N-terminal metal-ion-dependent fibrinogen-binding domain of the EbpA adhesin, or blockade with antibodies raised against the N-terminal domain of EbpA, prevents bacterial adhesion to fibrinogen-coated catheters in vitro and in mouse models of infection 120. Enterococci also use fibrinogen as a nutrient source, enabling their growth in urine 120. Enterococcal virulence is dependent on two secreted proteases, GelE and SprE, which are upregulated during infection to cleave the α-, β- and γ-chains of fibrinogen to potentiate biofilm formation (Fig 3) 122. This provides further evidence that Enterococcus species utilize fibrinogen as both a binding platform and food source during biofilm formation and growth. Activation of SprE by host proteases was further shown to promote bacterial dissemination in the kidneys, whereas inhibition of either host or bacterial proteases was able to curb bacterial dissemination 120. Ebp pili are also important for cell-cell aggregation and interspecies gene transfer, facilitating the spread of genetic elements that encode antibiotic resistance and other virulence factors 123.
Staphylococcus aureus.
Although S. aureus accounts for fewer than 3% of all positive urine cultures, S. aureus bacteriuria is more likely to progress to bacteremia than bacteriuria caused by other pathogens 14,113,124,125 Additionally, S. aureus is a major cause of asymptomatic bacteriuria and UTI during pregnancy in many developing countries 126. The frequency of complications, combined with methicillin-resistance rates of up to 86%, has prompted researchers to investigate the pathogenesis of S. aureus UTIs 113,125. Urinary catheterization is the most important risk factor for complicated methicillin-resistant S. aureus (MRSA) UTI 125,127. As with Enteroccocus CAUTI, interaction with fibrinogen is key for MRSA pathogenesis in various habitats 128. However, MRSA strains do not encode pili, but instead use microbial surface components recognizing adhesive matrix molecules (MSCRAMMS) to mediate binding to fibrinogen-coated catheters 129 (Fig 3). Specifically, clumping factor B (ClfB) has been shown to have an active role in MRSA CAUTI pathogenesis, while ClfA is important for systemic infection 130,131. Further, MRSA potentiates catheter-mediated inflammation by up-regulating the expression of IL-1α, IL-1β, IL-6, and TNF to promote fibrinogen release and bacterial persistence in the bladder in a mouse model of CAUTI 130(Fig 3).
Proteus mirabilis.
Although short-term community- and catheter-associated UTIs are mostly caused by monomicrobial infections, most CAUTIs in patients catheterized for long periods (>30 days) are polymicrobial in nature 132,133. p. mirabilis, a Gram-negative bacterium that is nearly ubiquitous in soil and water, is a common constituent of CAUTI polymicrobial communities in chronically catheterized patients 133,134. p. mirabilis uses various canonical virulence factors to establish infection in the bladder, including flagella and adhesive mannose-resistant Proteus-like MR/P fimbriae 135 (Fig 3). In addition, P. mirabilis expresses high levels of urease, an enzyme that can hydrolyze urea to ammonia and raise the pH of urine to facilitate the formation of kidney stones 135. Although carriage of this enzyme is not uncommon in uropathogens, P. mirabilis urease activity generates a substantially higher urinary pH than any of its uropathogenic counterparts, enabling the formation of crystalline biofilms that provide a protective niche during antibiotic treatment, promoting recurrence 136. Recent work has revealed that, like UPEC, P. mirabilis invades the superficial umbrella cells of the bladder and forms IBCs in the urothelium 137.
However, these IBCs are smaller and more scarce than their UPEC counterparts. Instead, most P. mirabilis bacteria form large extracellular clusters in the lumen of the bladder that function as the nidus for the formation of bladder stones 137. These clusters are dependent on both MR/P fimbriae and urease (Fig 3). Additionally, coinfection with Escherichia, Enterococcus, Klebsiella or Pseudomonas species in a mouse model of CAUTI enhances the urease activity of P mirabilis, resulting in increased disease severity and dissemination to other organ systems 138.
Emerging therapeutics
The mechanistic insights provided from the interdisciplinary studies outlined above have informed the development of novel antibiotic-sparing therapeutic approaches for the treatment and prevention of UTIs. These approaches, which target both bacterial and host processes, are outlined briefly below and summarized in Table 2. Given that 9% of all antibiotic prescriptions in the ambulatory setting in the United States are written for the treatment of UTI, UTIs are at the forefront of the antibiotic resistance problem 139. Thus, there is an urgent need to develop antibiotic-sparing therapeutics for the treatment of UTI.
Table 2:
Emerging therapeutics for the treatment of urinary tract infections.
| Therapeutic | Bacterial target | Disease target | Evidence |
|---|---|---|---|
| Anti-adhesives | |||
| Mannosides | UPEC; type 1 pili (FimH) | Acute cystitis | Reduced bacterial burdens in a mouse bladder following treatment and as a prophylactic agent in mouse models53,143,144 |
| Galactosides | UPEC; Fim-like (Fml) pili | Chronic cystitis and/or pyelonephritis | Reduced bacterial burden in the bladder and kidney in mouse models of chronic UTIs146,172 |
| Vaccine | |||
| FimCH | UPEC expressing type 1 pili | Acute and chronic cystitis | Phase 1 clinical trial showing no safety concerns and a reduction in total UTI recurrence in treatment cohort; approved for compassionate use as an investigational intervention150 |
| EbpANTD | Enterococcus species | CAUTI | Reduces bacterial titers in the bladder and catheter adhesion in in vitro mouse models153 |
| Four-component (Two capsular polysaccharides, mutated α-toxin, clumping factor A) | Staphylococcus species | Staphylococcal UTI | Shown efficacy in mouse models, has completed phase I clinical trials with no safety concerns154 |
| Other | |||
| NSAIDs | UPEC | Chronic and recurrent cystitis | In vivo mouse models demonstrate reduced bladder remodeling, and human clinical studies demonstrate effective resolution of symptoms with a reduction in overall antibiotic used when used in place of antibiotics40,156–158 |
| HIF-1α inhibition (AKB-4924) | UPEC | Decreased UPEC adherence and invasion of cultured human uroepithelial cells, decreased inflammation and bacterial load in mouse models of infection160 | |
| Probiotics | UPEC | rUTI | Reduced frequency of infection in a variety of patient populations with rUTI161–163 |
| Lactoferrin | UPEC | Cystitis | Reduction in UPEC adherence to human bladder epithelial cell lines and reduced mouse bladder bacterial burdens following treatment with exogenous lactoferrin70 |
CAUTI, catheter-associated urinary tract infection; MRSA, methicillin-resistant Staphylococcus aureus; NSAIDs, nonsteroidal anti-inflammatories; NTD, amino-terminal domain; UPEC, uropathogenic Escherichia coli; UTI, urinary tract infection.
Glycomimetic anti-adhesives.
Many common uropathogens have developed various strategies to adhere to host tissues to colonize specific habitats and cause infection. Pilus-mediating binding of UPEC and other Gram-negative bacteria to sugar moieties on glycoproteins can be inhibited by presence of glycomimetic small molecules that competitively outcompete interactions with the receptor by occupying the binding pocket of the fimbrial adhesin. Mannosides are high-affinity mannose analogues that bind the FimH adhesin at the tip of type 1 pili, preventing adhesion to mannosylated glycoproteins in the bladder and colon 53,140–142. Elucidation of the structure-activity relationship has enabled the development of mannosides that inhibit FimH function with a 106-fold higher potency than α-D-mannose and have considerably improved the bioavailability and half-life of these compounds 143,144. Studies examining the role of conformational dynamics in FimH-glycan interactions have revealed new classes of mannose analogues, the flexibility of which exploits known conformational changes within binding loops of the adhesive domain to further increase affinity 145. Mannosides are orally bioavailable and treatment reduces bladder bacterial burdens by up to 4-logs in mouse models of UTI after just 6 hours post infection 144.
The gastrointestinal tract is the ultimate reservoir for UPEC, and FimH and has been shown to have a critical role in maintaining colonization of this habitat in mice. Depletion of this reservoir within a given host would be expected to reduce the rate of recurrent UTIs. In mice, oral administration of mannosides selectively extirpates UPEC from the urinary and gastrointestinal habitats simultaneously. Unlike antibiotics that reduce the complexity of the gut microbiota, mannosides do not alter the structure of the native microbiota in mouse models of acute cystitis and gastrointestinal colonization53. Interestingly, those same experiments revealed that, while UPEC was selectively depleted, the abundance of intestinal Enterobacteriaceae in the healthy microbiota was not affected by mannoside treatment, which led the authors to speculate that not all E. coli strains express type 1 pili in the gastrointestinal environment53.
Recent studies have also focused on the use of small-molecule receptor analogues for the inhibition of Fml pilus-mediated adhesion to GalNAc-containing receptors expressed in the epithelia of kidneys and chronically infected bladders52. These high-affinity ‘galactosides’ have a 2000-fold higher affinity for FmlH than GalNAc and can reduce bacterial bladder and kidney in infected mice by approximately 1.5-logs 146. When given to mice in combination with mannosides, galactosides can reduce kidney burdens below the limit of detection and reduce bladder burdens to a greater degree than monotherapy with galactosides or mannosides146. These data suggest that glycomimetic compounds hold promise as antibiotic-sparing therapeutics for the prevention and treatment of acute, chronic and recurrent UPEC UTI. However, the efficacy of small molecular inhibitors of adhesion need to be fully assessed in humans; a candidate mannoside has been selected for clinical development in humans 147.
Immunization.
Active vaccination has proven highly efficacious in reducing the incidence and severity of UTI in several animal models. Vaccination with the type 1 pilus adhesin, FimH, has been shown to greatly decrease the incidence of UTI in mouse models of infection and in nonhuman primates 148. Vaccination with the N-terminal adhesive domain of FmlH decreases the bacterial burden 2-3 days post-infection in a mouse model of chronic cystitis 52. Boosting the humoral immune response using the truncated flagellin (FliC) protein from enteroaggregative E. coli (EAEC) as an adjuvant has also shown promise in a mouse model of immunization 149.
A phase 1a/1b clinical trial was recently completed to evaluate the safety and IgG response to immunization with the FimCH chaperone-adhesin complex in both healthy women and women experiencing at least five UTIs in the 24 months prior to enrollment 150. Co-administration with a synthetic adjuvant, phosphorylated hexaacyl disaccharide (PHAD), led to a 152 to 330-fold increase in antibody titers at 7 months and a 50-fold sustained increase in antibody titers at month 12 with no severe systemic reactions. In the 8 months following FimCH immunization, women in the rUTI cohorts experienced a 72% decrease in total UTI recurrence relative to the 8 months prior to the immunization. Based on these results, and the lack of serious complications arising from vaccination, the administration of the FimCH vaccine has been allowed by the FDA for compassionate use for individuals suffering from rUTI who no longer respond to standard of care, including last-line carbapenem agents 150.
Outer-membrane iron-acquisition proteins, including siderophores and heme receptors, also represent promising vaccine candidates against E. coli UTIs 151. These proteinaceous outer-membrane targets are required for urinary tract colonization by UPEC, expressed during infection and immunogenic during experimental infection 51,152. A recent study focusing on four antigens, including the yersiniabactin receptor FyuA, the heme receptor Hma, a catecholate receptor IreA and aerobactin receptor IutA, revealed the presence IgG titers against these antigens in females with a history of UTI and in males with no history of UTI 151. These data suggest that exposure to these antigens at other body sites create subprotective baseline immunologic responses that could be augmented by the administration of multivalent vaccines.
Vaccination also holds promise against Gram-positive bacterial pathogens during CAUTI. Immunization of mice with the EbpA N-terminal domain (EbpANTD) can reduce bladder and catheter bacterial burdens and protect against CAUTI caused by diverse enterococcal strains, including vancomycin-resistance isolates 120,153. Antibodies generated by EbpANTD-vaccinated mice can prevent bacterial adhesion to fibrinogen-coated catheters in vitro, and passive antibody transfer can reduce bladder and catheter bacterial burdens in mice with preexisting infections and naïve mice following subsequent bacterial challenge 153. It was also found that catheter-induced inflammation of the bladder increases the levels of serum antibody present in the bladder lumen, even in the absence of infection. Exposure of mice to E. faecalis results in an antibody response ~1000-fold lower than EbpANTD vaccination and is not protective against subsequent challenge infection 153. These results suggest active and passive immunity may hold promise as both a therapeutic and prophylactic option for the treatment of CAUTI. Vaccines against MRSA that have been promising in mouse models have not proven efficacious in clinical trials, although a newer four-component vaccine has successfully completed phase I clinical trials with no safety concerns 154. Thus, immunization against proteinaceous mediators of bacterial adhesion is a promising approach against various Gram-positive and Gram-negative uropathogens.
Immunomodulatory therapies.
Mouse studies have implicated inflammation-induced remodeling of the bladder epithelium as a mediator of increased UTI susceptibility (Box 1). In humans with persistent bacteriuria and rUTI, biopsies revealed B-cell and plasma cell migration with development of lymphoid nodules in the submucosa 38. Cyclooxygenase-1 (COX-1) and COX-2 are responsible for the synthesis of proinflammatory prostaglandins, which are the target of the nonsteroidal anti-inflammatory (NSAID) drug class, including naproxen, ibuprofen, aspirin and indomethacin 155. COX-2 activity predominates at sites of inflammation, is increased in neutrophils and urothelial cells in the human and mouse bladders during acute cystitis, and is strongly predictive of progression to chronic cystitis 40 (Box 1). COX-2 can be selectively inhibited using celecoxib to avoid gastrointestinal ulceration associated with nonspecific inhibition of COX-1. Inhibition of COX-2, but not COX-1, reduces the severity of acute cystitis and decreases the incidence of chronic infection in mouse models of cystitis by preventing mucosal damage and enabling the regeneration of the bladder epithelium 40. These data are supported by human trials showing some efficacy of oral NSAID use for symptomatic resolution of UTIs in human patients156. Although women treated with NSAIDs alone generally report a longer duration of symptoms and some require subsequent antibiotic use, NSAIDs seem to be a possible treatment option for symptomatic treatment in women who wish to avoid antibiotic use 157,158. Unfortunately, a side effect profile that includes chronic interstitial nephritis and gastrointestinal ulceration may limit the use of nonselective NSAID therapy in women suffering from chronic rUTIs.
Host factors that facilitate the inflammatory response of the bladder to UPEC colonization have also been examined as putative therapeutic targets. One such target, hypoxia-inducible factor 1α (HIF-1α, is a master transcriptional activator that has been shown to have a major immunomodulatory role under hypoxic conditions in mouse models of bacterial infection 159. Stabilization of HIF-1α by the novel drug candidate AKB-4924 (initially developed for inflammatory bowel disease) in in vivo mouse models of infection decreases the host inflammatory response to infection by reducing the levels of IL-1 β, IL-6, IL-8 (CXCL8) and CXCL1 160. This, in turn, reduced the level of epithelial hyperplasia in the bladder and the subsequent bacterial burden. If these findings are translatable to humans it would provide insight into the possibility of repurposing AKB-4924 for the treatment of UTI.
Probiotics.
Although probiotic use is most commonly associated with the gastrointestinal tract, the clinical use of antibacterial competition and interference has also been implicated in the urinary tract. The efficacy of asymptomatic urinary tract colonization with E. coli isolate 83972 as an antibiotic-sparing alternative for the prevention of rUTI has been shown in various patient populations and was endorsed by the European Urology Guidelines [https://uroweb.org/wp-content/uploads/EAU-Extended-Guidelines-2015-Edn..pdf] in 2015 161–163. The ability of E83972 to maintain stable colonization of the urinary tract for prolonged periods without the development of symptomatic UTI has been attributed to robust biofilm formation and rapid growth in urine, poor expression of flagella and lack of functional P pili or type 1 pili. Three alternative asymptomatic bacteriuria isolates that can outcompete E83972 in vivo in the absence of urovirulence have recently been identified based on these criteria 164. These strains belong to different phylogroups and display different sets of urovirulence factors, and their increased fitness in urine is thought to be due primarily to the expression of nutrient uptake and metabolite synthesis pathways 165,166. Although the competitiveness of these strains against various uropathogenic bacterial isolates remains under study, the use of asymptomatic colonization of the urinary tract with avirulent strains to exploit bacterial interference holds promise in the prevention of UTI. Additional studies aimed at engineering E. coli strains to contain CsgA fusion proteins have shown that fusion of bioactive molecules to the CsgA curli subunit can enhance bacterial interactions with intestinal mucins and promote persistence 167. This work suggests that directed bioengineering, together with microbiological characterization of existing members of the beneficial host microbiota, may lead to the development of therapeutic strains with enhanced probiotic activity for the prevention of UTI. Additional potential for probiotic use extends into the vaginal microbiome. A recent literature review suggests that probiotics containing Lactobacillus species, in conjunction with vaginal estrogen creams, may have clinical use in the prevention of UTI 168.
Other approaches.
The targeting of other virulence factors known to participate in the host-pathogen interaction of uropathogens has also been proposed. Because CpxA and CpxR are highly conserved among many Gram-negative bacterial species and are known to regulate various virulence factors, CpxA inhibitors have potential use in the treatment of UTIs 169. The differential expression of siderophores, including yersiniabactin and salmochelin, in UPEC isolates suggests that targeting metal sequestration systems may also be effective in selectively attenuating virulence in the bladder, especially given the high degree of cross-talk between siderophore systems 72. Additionally, treatment of human bladder epithelial cells with exogenous human lactoferrin reduces UPEC adherence and increases neutrophil-mediated bacterial killing 70. The therapeutic promise of this approach is further supported by murine studies showing increased clearance of UPEC in vivo with a single extravesicular treatment of human lactoferrin. However, the precise mechanism by which these effects occur requires further study.
The unique pathogenesis of CAUTI also presents several enticing antivirulence targets. The role of both MR/P fimbriae and urease in mono- and polymicrobial CAUTI suggests that targeting these central virulence factors may represent a viable antibiotic-sparing therapeutic option. Furthermore, our increasing understanding of the inflammatory changes that follow catheterization and subsequent bladder infection have suggested that an immunomodulatory approach may be efficacious for the treatment of some CAUTIs to decrease the morbidity and mortality associated with MRSA bacteriuria.
Outlook
Community- and healthcare associated UTIs affect millions of women worldwide every year. In the United States, hospitalizations for a primary diagnosis of UTI result in a total cost of $2.8 billion USD annually, in addition to 1 million visits to the emergency department and 7 million office visits 170. Development of UTI in hospitalized patients, especially those with an indwelling urinary catheter, extends the duration of patient stay and increases the complexity of patient treatment. Additionally, urosepsis comprises 25% of all adult sepsis cases, and is associated with an overall mortality rate of 20-40% 171. Patients suffering from a symptomatic UTI are commonly treated with broad-spectrum antibiotics; however the increase in antibiotic resistance and the impact of antibiotics on the gut microbiota raises the need for alternative antibiotic-sparing therapeutics that can selectively treat UTIs. A detailed mechanistic understanding of UTI pathogenesis is crucial for our ability to develop novel antimicrobial agents to effectively treat UTIs.
Although UTIs are caused by diverse pathogens harboring various virulence factors, we are gaining a greater appreciation for the complexity of the host-pathogen interface during colonization and infection. Through metabolomic and gene expression studies, we are beginning to understand the dynamic regulation of bacterial virulence throughout the infectious cycle, which enables us to pinpoint determinants of bacterial virulence and target them appropriately. Such treatments include small-molecular inhibitors of adhesion, inhibitors of nutrient update and immunization against bacterial antigens. Similarly, studies of animal models of infection and human immunology have identified components of the human immune response that influence infection outcome, which suggests a possible role for immunomodulatory therapy in the treatment of UTIs.
Although there is still much work that needs to be done to translate our structural and functional understanding of urovirulence factors to effective antibiotic-sparing therapeutics for humans, new breakthroughs hold promise to improve the lives and the health of millions of women suffering from this disease. Additionally, the strategies that have been developed to translate an understanding of UTI pathogenesis into therapeutic leads may be applicable to the treatment of other infections, enabling us to expand our therapeutic arsenal by targeting bacteria in the precise habitat in which they cause infection or reside asymptomatically as a reservoir.
Display Items
Supplementary Material
Acknowledgements
The authors thank K. W. Dodson and T. J. Hannan for their helpful suggestions and comments on the manuscript. Work in the author’s laboratory was supported by grants AI099099, AI095542, AI029549 and AI048689 from the US National Institution of Allergy and Infectious Diseases (NIAID), DK051406 and DK 108840 from the US National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and Medical Scientist Training Program Grant T32GM07200 from the US National Institute of General Medical Sciences. The authors apologize to researchers whose work was not included in this review due to space constraints.
Glossary
- Urinary meatus
The opening of the urethra through which urine exits in males and females, sometimes referred to as the external urethral orifice.
- Uncomplicated cystitis
an isolated infection of the bladder and/or lower urinary tract without signs or symptoms of upper tract or systemic infection in a patient without significant comorbid conditions, such as pregnancy or structural urinary tract abnormalities.
- Complicated cystitis
An infection of the upper urinary tract leading to upper tract signs or systemic symptoms, or any urinary tract infection in pregnant women, immunocompromised patients, or patients with functional urinary tract abnormalities.
- Pyelonephritis
An infection of the renal pelvic, calices and/or cortex.
- catheter-associated UTIs (CAUTIs)
Catheter-associated urinary tract infection.
- Fibrinogen
A glycoprotein released into the bladder lumen in response to inflammation and infection, and can coat urinary catheters and serve as a nidus for bacterial binding.
- Umbrella cells
Also known as facet cells, umbrella cells are large, polarized superficial cells that line bladder lumen.
- C3H/HeN mice
An inbred mouse strain commonly used for the study of a variety of disease processes, including UTI.
- Lymphonodular hyperplasia
Enlargement of mucosal lymphoid nodules seen via histology.
- Bacteriuria
The presence of bacterial in a urine system not attributable to contamination. Can be symptomatic or asymptomatic.
- Nutritional Immunity
Sequestration of nutrients by a host organism to prevent colonization and proliferation by pathogens.
- Siderophores
Low-molecular-weight compounds secreted by the host systems to bind metal ions and transport them across cellular membranes.
- Biofilms
Large collections of microbial organisms embedded within a complex extracellular matrix comprising polysaccharides, proteinaceous fibers, and extracellular DNA
Footnotes
Competing interests:
S.J.H. has an ownership interest in Fimbrion Therapeutics, and may benefit if the company is successful in marketing mannosides. S.J.H. is also the chief scientific officer of QureTech Bio. R.D.K. declares no competing interests.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41579-020-0324-0
Related links:
European Urology Guidelines: https://uroweb.org/wp-content/uploads/EAU-Extended-Guidelines-2015-Edn..pdf
References
- 1.Foxman B, Barlow R, D’Arcy H, Gillespie B & Sobel JD Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol 10, 509–515 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Foxman B & Brown P Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am 17, 227–241 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Scholes D et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis 182, 1177–1182, doi: 10.1086/315827 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Epp A, Larochelle A, Urogynaecology C & Family Physicians Advisory C Recurrent urinary tract infection. J Obstet Gynaecol Can 32, 1082–1090, doi: 10.1016/S1701-2163(16)34717-X (2010). [DOI] [PubMed] [Google Scholar]
- 5.Foxman B Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am 28, 1–13, doi: 10.1016/j.idc.2013.09.003 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Echols RM, Tosiello RL, Haverstock DC & Tice AD Demographic, clinical, and treatment parameters influencing the outcome of acute cystitis. Clin Infect Dis 29, 113–119, doi: 10.1086/520138 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Hooton TM Gupta K Acute simple cystitis in women. UpToDate. (ed Calderwood SB, Bloom A ) (2018). [Google Scholar]
- 8.Hooton TM Clinical practice. Uncomplicated urinary tract infection. N Engl J Med 366, 1028–1037, doi: 10.1056/NEJMcp1104429 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Katchman EA et al. Three-day vs longer duration of antibiotic treatment for cystitis in women: systematic review and meta-analysis. Am J Med 118, 1196–1207, doi: 10.1016/j.amjmed.2005.02.005 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Hsu DD & Melzer M Strategy to reduce E. coli bacteraemia based on cohort data from a London teaching hospital. Postgrad Med J 94, 212–215, doi: 10.1136/postgradmedj-2017-135454 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Seymour CW et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med 376, 2235–2244, doi: 10.1056/NEJMoa1703058 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatfield KM et al. Assessing Variability in Hospital-Level Mortality Among U.S. Medicare Beneficiaries With Hospitalizations for Severe Sepsis and Septic Shock. Crit Care Med 46, 1753–1760, doi: 10.1097/CCM.0000000000003324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flores-Mireles AL et al. Fibrinogen Release and Deposition on Urinary Catheters Placed during Urological Procedures. J Urol 196, 416–421, doi: 10.1016/j.juro.2016.01.100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study Illustrates the molecular mechanism by which fibrinogen deposition on urinary catheters facilitates bladder colonization.
- 14.Flores-Mireles AL, Walker JN, Caparon M & Hultgren SJ Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 13, 269–284, doi: 10.1038/nrmicro3432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foxman B The epidemiology of urinary tract infection. Nat Rev Urol 7, 653–660, doi: 10.1038/nrurol.2010.190 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Spees AM et al. Streptomycin-induced inflammation enhances Escherichia coli gut colonization through nitrate respiration. MBio 4, doi: 10.1128/mBio.00430-13 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koves B et al. Benefits and Harms of Treatment of Asymptomatic Bacteriuria: A Systematic Review and Meta-analysis by the European Association of Urology Urological Infection Guidelines Panel. Eur Urol 72, 865–868, doi: 10.1016/j.eururo.2017.07.014 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto S et al. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J Urol 157, 1127–1129 (1997). [PubMed] [Google Scholar]
- 19.Mayer BT et al. Rapid and Profound Shifts in the Vaginal Microbiota Following Antibiotic Treatment for Bacterial Vaginosis. J Infect Dis 212, 793–802, doi: 10.1093/infdis/jiv079 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macklaim JM, Clemente JC, Knight R, Gloor GB & Reid G Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb Ecol Health Dis 26, 27799, doi: 10.3402/mehd.v26.27799 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooton TM et al. Amoxicillin-clavulanate vs ciprofloxacin for the treatment of uncomplicated cystitis in women: a randomized trial. JAMA 293, 949–955, doi: 10.1001/jama.293.8.949 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Hooton TM, Roberts PL & Stapleton AE Cefpodoxime vs ciprofloxacin for short-course treatment of acute uncomplicated cystitis: a randomized trial. JAMA 307, 583–589, doi: 10.1001/jama.2012.80 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schreiber H. L. t. et al. Bacterial virulence phenotypes of Escherichia coli and host susceptibility determine risk for urinary tract infections. Sci Transl Med 9, doi: 10.1126/scitranslmed.aaf1283 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subashchandrabose S & Mobley HLT Virulence and Fitness Determinants of Uropathogenic Escherichia coli. Microbiol Spectr 3, doi: 10.1128/microbiolspec.UTI-0015-2012 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson GG et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301, 105–107, doi: 10.1126/science.1084550 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Song J et al. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc Natl Acad Sci U S A 106, 14966–14971, doi: 10.1073/pnas.0900527106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz DJ, Chen SL, Hultgren SJ & Seed PC Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect Immun 79, 4250–4259, doi: 10.1128/IAI.05339-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen DA, Hooton TM, Stamm WE, Humphrey PA & Hultgren SJ Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med 4, e329, doi: 10.1371/journal.pmed.0040329 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Nisco NJ et al. Direct Detection of Tissue-Resident Bacteria and Chronic Inflammation in the Bladder Wall of Postmenopausal Women with Recurrent Urinary Tract Infection. J Mol Biol, doi: 10.1016/j.jmb.2019.04.008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robino L et al. Detection of intracellular bacterial communities in a child with Escherichia coli recurrent urinary tract infections. Pathog Dis 68, 78–81, doi: 10.1111/2049-632X.12047 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Robino L et al. Intracellular bacteria in the pathogenesis of Escherichia coli urinary tract infection in children. Clin Infect Dis 59, e158–164, doi: 10.1093/cid/ciu634 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duell BL et al. Innate transcriptional networks activated in bladder in response to uropathogenic Escherichia coli drive diverse biological pathways and rapid synthesis of IL-10 for defense against bacterial urinary tract infection. J Immunol 188, 781–792, doi: 10.4049/jimmunol.1101231 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Ingersoll MA, Kline KA, Nielsen HV & Hultgren SJ G-CSF induction early in uropathogenic Escherichia coli infection of the urinary tract modulates host immunity. Cell Microbiol 10, 2568–2578, doi: 10.1111/j.1462-5822.2008.01230.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sivick KE, Schaller MA, Smith SN & Mobley HL The innate immune response to uropathogenic Escherichia coli involves IL-17A in a murine model of urinary tract infection. J Immunol 184, 2065–2075, doi: 10.4049/jimmunol.0902386 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiwon M et al. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 156, 456–468, doi: 10.1016/j.cell.2014.01.006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulvey MA et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282, 1494–1497 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Schaale K et al. Strain- and host species-specific inflammasome activation, IL-1beta release, and cell death in macrophages infected with uropathogenic Escherichia coli. Mucosal Immunol 9, 124–136, doi: 10.1038/mi.2015.44 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Schlager TA, LeGallo R, Innes D, Hendley JO & Peters CAB cell infiltration and lymphonodular hyperplasia in bladder submucosa of patients with persistent bacteriuria and recurrent urinary tract infections. J Urol 186, 2359–2364, doi: 10.1016/j.juro.2011.07.114 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Mysorekar IU & Hultgren SJ Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A 103, 14170–14175, doi: 10.1073/pnas.0602136103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hannan TJ et al. Inhibition of Cyclooxygenase-2 Prevents Chronic and Recurrent Cystitis. EBioMedicine 1, 46–57, doi: 10.1016/j.ebiom.2014.10.011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML & Hultgren SJ Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog 6, e1001042, doi: 10.1371/journal.ppat.1001042 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferry SA, Holm SE, Stenlund H, Lundholm R & Monsen TJ The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand J Infect Dis 36, 296–301, doi: 10.1080/00365540410019642 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Yu L et al. Mucosal infection rewires TNFα signaling dynamics to skew susceptibility to recurrence. eLife, 46677 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wurpel DJ, Beatson SA, Totsika M, Petty NK & Schembri MA Chaperone-usher fimbriae of Escherichia coli. PLoS One 8, e52835, doi: 10.1371/journal.pone.0052835 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides an excellent overview of the phenotypic relationships of chaperone-usher pathway pili throughout all E. coli species.
- 45.Stapleton AE, Stroud MR, Hakomori SI & Stamm WE The globoseries glycosphingolipid sialosyl galactosyl globoside is found in urinary tract tissues and is a preferred binding receptor In vitro for uropathogenic Escherichia coli expressing pap-encoded adhesins. Infect Immun 66, 3856–3861 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dodson KW et al. Structural basis of the interaction of the pyelonephritic E. coli adhesin to its human kidney receptor. Cell 105, 733–743 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Hung CS et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol Microbiol 44, 903–915 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Backhed F et al. Identification of target tissue glycosphingolipid receptors for uropathogenic, F1C-fimbriated Escherichia coli and its role in mucosal inflammation. J Biol Chem 277, 18198–18205, doi: 10.1074/jbc.M111640200 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Luterbach CL & Mobley HLT Cross Talk between MarR-Like Transcription Factors Coordinates the Regulation of Motility in Uropathogenic Escherichia coli. Infect Immun 86, doi: 10.1128/IAI.00338-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wurpel DJ et al. F9 fimbriae of uropathogenic Escherichia coli are expressed at low temperature and recognise Galbeta1-3GlcNAc-containing glycans. PLoS One 9, e93177, doi: 10.1371/journal.pone.0093177 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Subashchandrabose S et al. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc Natl Acad Sci U S A 111, 18327–18332, doi: 10.1073/pnas.1415959112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conover MS et al. Inflammation-Induced Adhesin-Receptor Interaction Provides a Fitness Advantage to Uropathogenic E. coli during Chronic Infection. Cell Host Microbe 20, 482–492, doi: 10.1016/j.chom.2016.08.013 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study elucidates the role of the F9 pilus adhesin, FmlH, in colonization of chronically-infected bladders via interaction with galactose moieties exposed by inflammation.
- 53.Spaulding CN et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature 546, 528–532, doi: 10.1038/nature22972 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the efficacy of mannose analogues in the selective and simultaneous extrirpation of UPEC from the bladder and gastrointestinal niches without a concamitant disruption of the beneficial microbiota.
- 54.Sauer MM et al. Binding of the bacterial adhesin FimH to its natural, multivalent high-mannose type glycan targets. J Am Chem Soc, doi: 10.1021/jacs.8b10736 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Kalas V et al. Evolutionary fine-tuning of conformational ensembles in FimH during host-pathogen interactions. Sci Adv 3, e1601944, doi: 10.1126/sciadv.1601944 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen SL et al. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc Natl Acad Sci U S A 106, 22439–22444, doi: 10.1073/pnas.0902179106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartz DJ et al. Positively selected FimH residues enhance virulence during urinary tract infection by altering FimH conformation. Proc Natl Acad Sci U S A 110, 15530–15537, doi: 10.1073/pnas.1315203110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abraham SN et al. Glycerol-induced unraveling of the tight helical conformation of Escherichia coli type 1 fimbriae. J Bacteriol 174, 5145–5148 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aprikian P et al. The bacterial fimbrial tip acts as a mechanical force sensor. PLoS Biol 9, e1000617, doi: 10.1371/journal.pbio.1000617 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mortezaei N et al. Structure and function of enterotoxigenic Escherichia coli fimbriae from differing assembly pathways. Mol Microbiol 95, 116–126, doi: 10.1111/mmi.12847 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spaulding CN et al. Functional role of the type 1 pilus rod structure in mediating host-pathogen interactions. Elife 7, doi: 10.7554/eLife.31662 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hospenthal MK et al. The Cryoelectron Microscopy Structure of the Type 1 Chaperone-Usher Pilus Rod. Structure 25, 1829–1838 e1824, doi: 10.1016/j.str.2017.10.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du M et al. Handover mechanism of the growing pilus by the bacterial outer-membrane usher FimD. Nature 562, 444–447, doi: 10.1038/s41586-018-0587-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Omattage NS et al. Structural basis for usher activation and intramolecular subunit transfer in P pilus biogenesis in Escherichia coli. Nat Microbiol 3, 1362–1368, doi: 10.1038/s41564-018-0255-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pinkner JS et al. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc Natl Acad Sci U S A 103, 17897–17902, doi: 10.1073/pnas.0606795103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miethke M & Marahiel MA Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71, 413–451, doi: 10.1128/MMBR.00012-07 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopez CA & Skaar EP The Impact of Dietary Transition Metals on Host-Bacterial Interactions. Cell Host Microbe 23, 737–748, doi: 10.1016/j.chom.2018.05.008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weichhart T, Haidinger M, Horl WH & Saemann MD Current concepts of molecular defence mechanisms operative during urinary tract infection. Eur J Clin Invest 38 Suppl 2, 29–38, doi: 10.1111/j.1365-2362.2008.02006.x (2008). [DOI] [PubMed] [Google Scholar]
- 69.Reigstad CS, Hultgren SJ & Gordon JI Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J Biol Chem 282, 21259–21267, doi: 10.1074/jbc.M611502200 (2007). [DOI] [PubMed] [Google Scholar]
- 70.Patras KA et al. Augmentation of Urinary Lactoferrin Enhances Host Innate Immune Clearance of Uropathogenic Escherichia coli. J Innate Immun, 1–15, doi: 10.1159/000499342 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaturvedi KS, Hung CS, Crowley JR, Stapleton AE & Henderson JP The siderophore yersiniabactin binds copper to protect pathogens during infection. Nat Chem Biol 8, 731–736, doi: 10.1038/nchembio.1020 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robinson AE, Heffernan JR & Henderson JP The iron hand of uropathogenic Escherichia coli: the role of transition metal control in virulence. Future Microbiol 13, 745–756, doi: 10.2217/fmb-2017-0295 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Henderson JP et al. Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog 5, e1000305, doi: 10.1371/journal.ppat.1000305 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson JR et al. Contribution of yersiniabactin to the virulence of an Escherichia coli sequence type 69 (“clonal group A”) cystitis isolate in murine models of urinary tract infection and sepsis. Microb Pathog 120, 128–131, doi: 10.1016/j.micpath.2018.04.048 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Parker KS, Wilson JD, Marschall J, Mucha PJ & Henderson JP Network Analysis Reveals Sex- and Antibiotic Resistance-Associated Antivirulence Targets in Clinical Uropathogens. ACS Infect Dis 1, 523–532, doi: 10.1021/acsinfecdis.5b00022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koh EI, Robinson AE, Bandara N, Rogers BE & Henderson JP Copper import in Escherichia coli by the yersiniabactin metallophore system. Nat Chem Biol 13, 1016–1021, doi: 10.1038/nchembio.2441 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brumbaugh AR et al. Blocking yersiniabactin import attenuates extraintestinal pathogenic Escherichia coli in cystitis and pyelonephritis and represents a novel target to prevent urinary tract infection. Infect Immun 83, 1443–1450, doi: 10.1128/IAI.02904-14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paniagua-Contreras GL et al. Comprehensive expression analysis of pathogenicity genes in uropathogenic Escherichia coli strains. Microb Pathog 103, 1–7, doi: 10.1016/j.micpath.2016.12.008 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Ohlemacher SI et al. Enterobacteria secrete an inhibitor of Pseudomonas virulence during clinical bacteriuria. J Clin Invest 127, 4018–4030, doi: 10.1172/JCI92464 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the importance of metal acquisition in UPEC virulence and identifies a siderophore metabolic byproduct that can inhibit iron update by competing bacteria.
- 80.Welch RA Uropathogenic Escherichia coli-Associated Exotoxins. Microbiol Spectr 4, doi: 10.1128/microbiolspec.UTI-0011-2012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marrs CF et al. Variations in 10 putative uropathogen virulence genes among urinary, faecal and peri-urethral Escherichia coli. J Med Microbiol 51, 138–142, doi: 10.1099/0022-1317-51-2-138 (2002). [DOI] [PubMed] [Google Scholar]
- 82.Hannan TJ et al. LeuX tRNA-dependent and -independent mechanisms of Escherichia coli pathogenesis in acute cystitis. Mol Microbiol 67, 116–128, doi: 10.1111/j.1365-2958.2007.06025.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mobley HL et al. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun 58, 1281–1289 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nagamatsu K et al. Dysregulation of Escherichia coli alpha-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc Natl Acad Sci U S A 112, E871–880, doi: 10.1073/pnas.1500374112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Otto K & Silhavy TJ Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc Natl Acad Sci U S A 99, 2287–2292, doi: 10.1073/pnas.042521699 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tschauner K, Hornschemeyer P, Muller VS & Hunke S Dynamic interaction between the CpxA sensor kinase and the periplasmic accessory protein CpxP mediates signal recognition in E. coli. PLoS One 9, e107383, doi: 10.1371/journal.pone.0107383 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Behr S, Fried L & Jung K Identification of a novel nutrient-sensing histidine kinase/response regulator network in Escherichia coli. J Bacteriol 196, 2023–2029, doi: 10.1128/JB.01554-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steiner BD et al. Evidence of Cross-Regulation in Two Closely Related Pyruvate-Sensing Systems in Uropathogenic Escherichia coli. J Membr Biol 251, 65–74, doi: 10.1007/s00232-018-0014-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clarke MB & Sperandio V Transcriptional autoregulation by quorum sensing Escherichia coli regulators B and C (QseBC) in enterohaemorrhagic E. coli (EHEC). Mol Microbiol 58, 441–455, doi: 10.1111/j.1365-2958.2005.04819.x (2005). [DOI] [PubMed] [Google Scholar]
- 90.Breland EJ, Zhang EW, Bermudez T, Martinez CR 3rd & Hadjifrangiskou M The Histidine Residue of QseC Is Required for Canonical Signaling between QseB and PmrB in Uropathogenic Escherichia coli. J Bacteriol 199, doi: 10.1128/JB.00060-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kostakioti M, Hadjifrangiskou M, Pinkner JS & Hultgren SJ QseC-mediated dephosphorylation of QseB is required for expression of genes associated with virulence in uropathogenic Escherichia coli. Mol Microbiol 73, 1020–1031, doi: 10.1111/j.1365-2958.2009.06826.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guckes KR et al. Strong cross-system interactions drive the activation of the QseB response regulator in the absence of its cognate sensor. Proc Natl Acad Sci U S A 110, 16592–16597, doi: 10.1073/pnas.1315320110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guckes KR et al. Signaling by two-component system noncognate partners promotes intrinsic tolerance to polymyxin B in uropathogenic Escherichia coli. Sci Signal 10, doi: 10.1126/scisignal.aag1775 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shah C, Baral R, Bartaula B & Shrestha LB Virulence factors of uropathogenic Escherichia coli (UPEC) and correlation with antimicrobial resistance. BMC Microbiol 19, 204, doi: 10.1186/s12866-019-1587-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eberly AR et al. Biofilm Formation by Uropathogenic Escherichia coli Is Favored under Oxygen Conditions That Mimic the Bladder Environment. Int J Mol Sci 18, doi: 10.3390/ijms18102077 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals the mechanisms by which UPEC biofilm formation is triggered within the bladder in response to an oxygen-poor environment.
- 96.Beebout CJ et al. Respiratory Heterogeneity Shapes Biofilm Formation and Host Colonization in Uropathogenic Escherichia coli. MBio 10, doi: 10.1128/mBio.02400-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reichhardt C & Cegelski L Solid-state NMR for bacterial biofilms. Molecular Physics 112, 887–894, doi: 10.1080/00268976.2013.837983 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chapman MR et al. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295, 851–855, doi: 10.1126/science.1067484 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Gerven N, Klein RD, Hultgren SJ & Remaut H Bacterial amyloid formation: structural insights into curli biogensis. Trends Microbiol 23, 693–706, doi: 10.1016/j.tim.2015.07.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Biesecker SG, Nicastro LK, Wilson RP & Tukel C The Functional Amyloid Curli Protects Escherichia coli against Complement-Mediated Bactericidal Activity. Biomolecules 8, doi: 10.3390/biom8010005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cegelski L et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat Chem Biol 5, 913–919, doi: 10.1038/nchembio.242 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hollenbeck EC et al. Phosphoethanolamine cellulose enhances curli-mediated adhesion of uropathogenic Escherichia coli to bladder epithelial cells. Proc Natl Acad Sci U S A 115, 10106–10111, doi: 10.1073/pnas.1801564115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Klein RD et al. Structure-Function Analysis of the Curli Accessory Protein CsgE Defines Surfaces Essential for Coordinating Amyloid Fiber Formation. MBio 9, doi: 10.1128/mBio.01349-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Evans ML et al. The Bacterial Curli System Possesses a Potent and Selective Inhibitor of Amyloid Formation. Mol Cell, doi: 10.1016/j.molcel.2014.12.025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schubeis T et al. Structural and functional characterization of the Curli adaptor protein CsgF. FEBS Lett, doi: 10.1002/1873-3468.13002 (2018). [DOI] [PubMed] [Google Scholar]
- 106.Sleutel M et al. Nucleation and growth of a bacterial functional amyloid at single-fiber resolution. Nat Chem Biol, doi: 10.1038/nchembio.2413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nhu NTK et al. Discovery of New Genes Involved in Curli Production by a Uropathogenic Escherichia coli Strain from the Highly Virulent O45:K1:H7 Lineage. MBio 9, doi: 10.1128/mBio.01462-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith DR et al. The Production of Curli Amyloid Fibers Is Deeply Integrated into the Biology of Escherichia coli. Biomolecules 7, doi: 10.3390/biom7040075 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Majdalani N, Heck M, Stout V & Gottesman S Role of RcsF in signaling to the Rcs phosphorelay pathway in Escherichia coli. J Bacteriol 187, 6770–6778, doi: 10.1128/JB.187.19.6770-6778.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Magill SS et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370, 1198–1208, doi: 10.1056/NEJMoa1306801 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rice LB Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 197, 1079–1081, doi: 10.1086/533452 (2008). [DOI] [PubMed] [Google Scholar]
- 112.Santajit S & Indrawattana N Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed Res Int 2016, 2475067, doi: 10.1155/2016/2475067 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weiner LM et al. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 37, 1288–1301, doi: 10.1017/ice.2016.174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prevention, C. f. D. C. a. (2016). [Google Scholar]
- 115.Daniels KR, Lee GC & Frei CR Trends in catheter-associated urinary tract infections among a national cohort of hospitalized adults, 2001-2010. Am J Infect Control 42, 17–22, doi: 10.1016/j.ajic.2013.06.026 (2014). [DOI] [PubMed] [Google Scholar]
- 116.Meddings J, Rogers MA, Macy M & Saint S Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis 51, 550–560, doi: 10.1086/655133 (2010). [DOI] [PubMed] [Google Scholar]
- 117.Delnay KM, Stonehill WH, Goldman H, Jukkola AF & Dmochowski RR Bladder histological changes associated with chronic indwelling urinary catheter. J Urol 161, 1106–1108; discussion 1108–1109 (1999). [PubMed] [Google Scholar]
- 118.Peychl L & Zalud R [Changes in the urinary bladder caused by short-term permanent catheter insertion]. Cas Lek Cesk 147, 325–329 (2008). [PubMed] [Google Scholar]
- 119.Guiton PS, Hannan TJ, Ford B, Caparon MG & Hultgren SJ Enterococcus faecalis overcomes foreign body-mediated inflammation to establish urinary tract infections. Infect Immun 81, 329–339, doi: 10.1128/IAI.00856-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Flores-Mireles AL, Pinkner JS, Caparon MG & Hultgren SJ EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci Transl Med 6, 254ra127, doi: 10.1126/scitranslmed.3009384 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Puyo CA et al. Mitochondrial DNA induces Foley catheter related bladder inflammation via Toll-like receptor 9 activation. Sci Rep 8, 6377, doi: 10.1038/s41598-018-24818-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu W et al. Host and bacterial proteases influence biofilm formation and virulence in a murine model of enterococcal catheter-associated urinary tract infection. NPJ Biofilms Microbiomes 3, 28, doi: 10.1038/s41522-017-0036-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.La Rosa SL, Montealegre MC, Singh KV & Murray BE Enterococcus faecalis Ebp pili are important for cell-cell aggregation and intraspecies gene transfer. Microbiology 162, 798–802, doi: 10.1099/mic.0.000276 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhanel GG et al. Antibiotic resistance in outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents 26, 380–388, doi: 10.1016/j.ijantimicag.2005.08.003 (2005). [DOI] [PubMed] [Google Scholar]
- 125.Al Mohajer M, Musher DM, Minard CG & Darouiche RO Clinical significance of Staphylococcus aureus bacteriuria at a tertiary care hospital. Scand J Infect Dis 45, 688–695, doi: 10.3109/00365548.2013.803291 (2013). [DOI] [PubMed] [Google Scholar]
- 126.Gilbert NM et al. Urinary tract infection as a preventable cause of pregnancy complications: opportunities, challenges, and a global call to action. Glob Adv Health Med 2, 59–69, doi: 10.7453/gahmj.2013.061 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Routh JC, Alt AL, Ashley RA, Kramer SA & Boyce TG Increasing prevalence and associated risk factors for methicillin resistant Staphylococcus aureus bacteriuria. J Urol 181, 1694–1698, doi: 10.1016/j.juro.2008.11.108 (2009). [DOI] [PubMed] [Google Scholar]
- 128.Cheng AG et al. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J 23, 3393–3404, doi: 10.1096/fj.09-135467 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Walker JN et al. The Staphylococcus aureus ArlRS two-component system is a novel regulator of agglutination and pathogenesis. PLoS Pathog 9, e1003819, doi: 10.1371/journal.ppat.1003819 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Walker JN et al. Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract. Proc Natl Acad Sci U S A 114, E8721–E8730, doi: 10.1073/pnas.1707572114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McAdow M et al. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog 7, e1002307, doi: 10.1371/journal.ppat.1002307 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Warren JW, Tenney JH, Hoopes JM, Muncie HL & Anthony WC A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis 146, 719–723 (1982). [DOI] [PubMed] [Google Scholar]
- 133.Armbruster CE, Prenovost K, Mobley HL & Mody L How Often Do Clinically Diagnosed Catheter-Associated Urinary Tract Infections in Nursing Homes Meet Standardized Criteria? J Am Geriatr Soc 65, 395–401, doi: 10.1111/jgs.14533 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors demonstrate the molecular basis for synergy among members of polymicrobial communities during colonization of urinary catheters in the healthcare setting.
- 134.Jacobsen SM, Stickler DJ, Mobley HL & Shirtliff ME Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin Microbiol Rev 21, 26–59, doi: 10.1128/CMR.00019-07 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Coker C, Poore CA, Li X & Mobley HL Pathogenesis of Proteus mirabilis urinary tract infection. Microbes Infect 2, 1497–1505 (2000). [DOI] [PubMed] [Google Scholar]
- 136.Broomfield RJ, Morgan SD, Khan A & Stickler DJ Crystalline bacterial biofilm formation on urinary catheters by urease-producing urinary tract pathogens: a simple method of control. J Med Microbiol 58, 1367–1375, doi: 10.1099/jmm.0.012419-0 (2009). [DOI] [PubMed] [Google Scholar]
- 137.Schaffer JN, Norsworthy AN, Sun TT & Pearson MM Proteus mirabilis fimbriae- and urease-dependent clusters assemble in an extracellular niche to initiate bladder stone formation. Proc Natl Acad Sci U S A 113, 4494–4499, doi: 10.1073/pnas.1601720113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Armbruster CE et al. The Pathogenic Potential of Proteus mirabilis Is Enhanced by Other Uropathogens during Polymicrobial Urinary Tract Infection. Infect Immun 85, doi: 10.1128/IAI.00808-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shapiro DJ, Hicks LA, Pavia AT & Hersh AL Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother 69, 234–240, doi: 10.1093/jac/dkt301 (2014). [DOI] [PubMed] [Google Scholar]
- 140.Klein T et al. FimH antagonists for the oral treatment of urinary tract infections: from design and synthesis to in vitro and in vivo evaluation. J Med Chem 53, 8627–8641, doi: 10.1021/jm101011y (2010). [DOI] [PubMed] [Google Scholar]
- 141.Cusumano CK et al. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci Transl Med 3, 109ra115, doi: 10.1126/scitranslmed.3003021 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schonemann W et al. Improvement of Aglycone pi-Stacking Yields Nanomolar to Sub-nanomolar FimH Antagonists. ChemMedChem, doi: 10.1002/cmdc.201900051 (2019). [DOI] [PubMed] [Google Scholar]
- 143.Jarvis C et al. Antivirulence Isoquinolone Mannosides: Optimization of the Biaryl Aglycone for FimH Lectin Binding Affinity and Efficacy in the Treatment of Chronic UTI. ChemMedChem 11, 367–373, doi: 10.1002/cmdc.201600006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mydock-McGrane L et al. Antivirulence C-Mannosides as Antibiotic-Sparing, Oral Therapeutics for Urinary Tract Infections. J Med Chem 59, 9390–9408, doi: 10.1021/acs.jmedchem.6b00948 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Touaibia M et al. Sites for Dynamic Protein-Carbohydrate Interactions of O- and C-Linked Mannosides on the E. coli FimH Adhesin. Molecules 22, doi: 10.3390/molecules22071101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kalas V et al. Structure-based discovery of glycomimetic FmlH ligands as inhibitors of bacterial adhesion during urinary tract infection. Proc Natl Acad Sci U S A, doi: 10.1073/pnas.1720140115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Therapeutics F The Opportunity: Mannosides as Therapeutics <https://www.fimbrion.com/pipeline> (2019). [Google Scholar]
- 148.Langermann S et al. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis 181, 774–778, doi: 10.1086/315258 (2000). [DOI] [PubMed] [Google Scholar]
- 149.Savar NS et al. In silico and in vivo studies of truncated forms of flagellin (FliC) of enteroaggregative Escherichia coli fused to FimH from uropathogenic Escherichia coli as a vaccine candidate against urinary tract infections. J Biotechnol 175, 31–37, doi: 10.1016/j.jbiotec.2014.01.037 (2014). [DOI] [PubMed] [Google Scholar]
- 150.Eldridge G Personal Communication. (Scott Hultgren) (2018). [Google Scholar]
- 151.Sarkissian CA, Alteri CJ & Mobley HLT UTI patients have pre-existing antigen-specific antibody titers against UTI vaccine antigens. Vaccine, doi: 10.1016/j.vaccine.2019.07.031 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mobley HL & Alteri CJ Development of a Vaccine against Escherichia coli Urinary Tract Infections. Pathogens 5, doi: 10.3390/pathogens5010001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Flores-Mireles AL et al. Antibody-Based Therapy for Enterococcal Catheter-Associated Urinary Tract Infections. MBio 7, doi: 10.1128/mBio.01653-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kane TL, Carothers KE & Lee SW Virulence Factor Targeting of the Bacterial Pathogen Staphylococcus aureus for Vaccine and Therapeutics. Curr Drug Targets 19, 111–127, doi: 10.2174/1389450117666161128123536 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hawkey CJ COX-1 and COX-2 inhibitors. Best Pract Res Clin Gastroenterol 15, 801–820, doi: 10.1053/bega.2001.0236 (2001). [DOI] [PubMed] [Google Scholar]
- 156.Bleidorn J, Gagyor I, Kochen MM, Wegscheider K & Hummers-Pradier E Symptomatic treatment (ibuprofen) or antibiotics (ciprofloxacin) for uncomplicated urinary tract infection?--results of a randomized controlled pilot trial. BMC Med 8, 30, doi: 10.1186/1741-7015-8-30 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gagyor I et al. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ 351, h6544, doi: 10.1136/bmj.h6544 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kronenberg A et al. Symptomatic treatment of uncomplicated lower urinary tract infections in the ambulatory setting: randomised, double blind trial. BMJ 359, j4784, doi: 10.1136/bmj.j4784 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zinkernagel AS, Johnson RS & Nizet V Hypoxia inducible factor (HIF) function in innate immunity and infection. J Mol Med (Berl) 85, 1339–1346, doi: 10.1007/s00109-007-0282-2 (2007). [DOI] [PubMed] [Google Scholar]
- 160.Lin AE et al. Role of Hypoxia Inducible Factor-1alpha (HIF-1alpha) in Innate Defense against Uropathogenic Escherichia coli Infection. PLoS Pathog 11, e1004818, doi: 10.1371/journal.ppat.1004818 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sunden F, Hakansson L, Ljunggren E & Wullt B Escherichia coli 83972 bacteriuria protects against recurrent lower urinary tract infections in patients with incomplete bladder emptying. J Urol 184, 179–185, doi: 10.1016/j.juro.2010.03.024 (2010). [DOI] [PubMed] [Google Scholar]
- 162.Darouiche RO et al. Multicenter randomized controlled trial of bacterial interference for prevention of urinary tract infection in patients with neurogenic bladder. Urology 78, 341–346, doi: 10.1016/j.urology.2011.03.062 (2011). [DOI] [PubMed] [Google Scholar]
- 163.Koves B et al. Rare emergence of symptoms during long-term asymptomatic Escherichia coli 83972 carriage without an altered virulence factor repertoire. J Urol 191, 519–528, doi: 10.1016/j.juro.2013.07.060 (2014). [DOI] [PubMed] [Google Scholar]
- 164.Stork C et al. Characterization of Asymptomatic Bacteriuria Escherichia coli Isolates in Search of Alternative Strains for Efficient Bacterial Interference against Uropathogens. Front Microbiol 9, 214, doi: 10.3389/fmicb.2018.00214 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hagan EC, Lloyd AL, Rasko DA, Faerber GJ & Mobley HL Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog 6, e1001187, doi: 10.1371/journal.ppat.1001187 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen SL et al. Genomic diversity and fitness of E. coli strains recovered from the intestinal and urinary tracts of women with recurrent urinary tract infection. Sci Transl Med 5, 184ra160, doi: 10.1126/scitranslmed.3005497 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Duraj-Thatte AM, Praveschotinunt P, Nash TR, Ward FR & Joshi NS Modulating bacterial and gut mucosal interactions with engineered biofilm matrix proteins. Sci Rep 8, 3475, doi: 10.1038/s41598-018-21834-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Smith AL et al. Treatment and Prevention of Recurrent Lower Urinary Tract Infections in Women: A Rapid Review with Practice Recommendations. J Urol 200, 1174–1191, doi: 10.1016/j.juro.2018.04.088 (2018). [DOI] [PubMed] [Google Scholar]
- 169.Dbeibo L et al. Evaluation of CpxRA as a Therapeutic Target for Uropathogenic Escherichia coli Infections. Infect Immun 86, doi: 10.1128/IAI.00798-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Simmering JE, Tang F, Cavanaugh JE, Polgreen LA & Polgreen PM The Increase in Hospitalizations for Urinary Tract Infections and the Associated Costs in the United States, 1998-2011. Open Forum Infect Dis 4, ofw281, doi: 10.1093/ofid/ofw281 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wagenlehner FM et al. Diagnosis and management for urosepsis. Int J Urol 20, 963–970, doi: 10.1111/iju.12200 (2013). [DOI] [PubMed] [Google Scholar]
- 172.Maddirala A et al. Biphenyl Gal and GalNAc FmlH Lectin Antagonists of Uropathogenic E. coli (UPEC): Optimization through iterative rational drug design. J Med Chem, doi: 10.1021/acs.jmedchem.8b01561 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides immunological insight into the long history of epidemilogic data suggesting that a history of UTI is the greatest risk factor for the development of subsequent UTI by identifying long-term damage caused by immune-mediated urothelial exfoliation.
- 173.O’Brien VP et al. A mucosal imprint left by prior Escherichia coli bladder infection sensitizes to recurrent disease. Nat Microbiol 2, 16196, doi: 10.1038/nmicrobiol.2016.196 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hannan TJ et al. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev 36, 616–648, doi: 10.1111/j.1574-6976.2012.00339.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sumati AH & Saritha NK Association of urinary tract infection in women with bacterial vaginosis. J Glob Infect Dis 1, 151–152, doi: 10.4103/0974-777X.56254 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gilbert NM, O’Brien VP & Lewis AL Transient microbiota exposures activate dormant Escherichia coli infection in the bladder and drive severe outcomes of recurrent disease. PLoS Pathog 13, e1006238, doi: 10.1371/journal.ppat.1006238 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stapleton AE et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis 52, 1212–1217, doi: 10.1093/cid/cir183 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Scholes D et al. Risk factors associated with acute pyelonephritis in healthy women. Ann Intern Med 142, 20–27 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bautista CT et al. Bacterial vaginosis: a synthesis of the literature on etiology, prevalence, risk factors, and relationship with chlamydia and gonorrhea infections. Mil Med Res 3, 4, doi: 10.1186/s40779-016-0074-5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kaper JB, Nataro JP & Mobley HL Pathogenic Escherichia coli. Nat Rev Microbiol 2, 123–140, doi: 10.1038/nrmicro818 (2004). [DOI] [PubMed] [Google Scholar]
- 181.Touchon M et al. Organised genome dynamics in the Escherichia coli species results in highly diverse adaptive paths. PLoS Genet 5, e1000344, doi: 10.1371/journal.pgen.1000344 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Piatti G, Mannini A, Balistreri M & Schito AM Virulence factors in urinary Escherichia coli strains: phylogenetic background and quinolone and fluoroquinolone resistance. J Clin Microbiol 46, 480–487, doi: 10.1128/JCM.01488-07 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ejrnaes K et al. Characteristics of Escherichia coli causing persistence or relapse of urinary tract infections: phylogenetic groups, virulence factors and biofilm formation. Virulence 2, 528–537, doi: 10.4161/viru.2.6.18189 (2011). [DOI] [PubMed] [Google Scholar]
- 184.Wang Y et al. Drug resistance and virulence of uropathogenic Escherichia coli from Shanghai, China. J Antibiot (Tokyo) 67, 799–805, doi: 10.1038/ja.2014.72 (2014). [DOI] [PubMed] [Google Scholar]
- 185.Nielsen KL et al. Whole-genome comparison of urinary pathogenic Escherichia coli and faecal isolates of UTI patients and healthy controls. Int J Med Microbiol 307, 497–507, doi: 10.1016/j.ijmm.2017.09.007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mann R, Mediati DG, Duggin IG, Harry EJ & Bottomley AL Metabolic Adaptations of Uropathogenic E. coli in the Urinary Tract. Front Cell Infect Microbiol 7, 241, doi: 10.3389/fcimb.2017.00241 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lavigne JP et al. Resistance and virulence potential of uropathogenic Escherichia coli strains isolated from patients hospitalized in urology departments: a French prospective multicentre study. J Med Microbiol 65, 530–537, doi: 10.1099/jmm.0.000247 (2016). [DOI] [PubMed] [Google Scholar]
- 188.Chen Z et al. The urinary microbiome in patients with refractory urge incontinence and recurrent urinary tract infection. Int Urogynecol J 29, 1775–1782, doi: 10.1007/s00192-018-36792 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


