Abstract
Background/Objectives:
Cognitive changes are commonly observed in older adults following surgical procedures. There are concerns that exposure to general anesthesia (GA) may contribute to an increased risk of Alzheimer’s disease. Our study examined the associations between exposure to GA compared to regional anesthesia (RA) administered for elective surgical procedures and the development of dementia.
Design:
Population-based, propensity matched retrospective cohort study.
Setting:
Linked administrative databases were accessed from ICES in Ontario, Canada.
Participants:
We included all community-dwelling individuals aged ≥ 66 years and older who underwent one of five elective surgical procedures in Ontario, Canada between April 1, 2007 and March 31, 2011. Individuals with evidence of dementia preceding cohort entry were excluded. Individuals who received GA were matched within surgical procedures to those who received RA on age, sex, cohort entry year and a propensity score to control for potential confounders.
Measurements:
The baseline characteristics of the study sample were compared before and after matching. Individuals were followed for up to 5 years following cohort entry for the occurrence of dementia using a validated algorithm. Cox-proportional hazards analysis were used to determine the hazard ratio (HR) and 95% CI for the association between anesthetic type and dementia. Subgroup and sensitivity analyses were undertaken
Results:
There were a total of 7,499 matched pairs included in the final analysis. Overall, there was no difference observed in risk of being diagnosed with dementia for individuals who received GA when compared to RA (HR=1.0, 95% CI: 0.8–1.2). There was also no association between anesthesia and dementia in most subgroup and sensitivity analyses.
Conclusion:
Elective surgery using GA was not associated with an overall elevated risk of dementia when compared to RA. Future studies are required to determine whether surgery is a risk factor for dementia irrespective of anesthetic technique.
Keywords: dementia, Alzheimer’s disease, surgery, anesthesia, cohort study
INTRODUCTION
In 2015, there were more than 47 million people worldwide living with Alzheimer’s disease (AD) and other forms of dementia, and this number is projected to increase in the future1. Many risk factors for AD are non-modifiable, including age, female sex, and genetic predisposition2. Currently, there are no disease-modifying treatments available for AD or other dementias, and there has been growing interest in prevention of dementia through identification of potentially modifiable risk factors3, 4.
There has been increasing interest in the possible associations between exposure to general anesthesia (GA) and the development of dementia. Cognitive changes are commonly observed following surgery and can manifest clinically in several different ways. Postoperative delirium is a typically reversible state which typically occurs within days surgery5, 6. Objective cognitive deficits accompanied by subjective cognitive complaints with 1–12 months after surgery are referred to as postoperative neurocognitive disorders (NCD)7, 8. NCD, by definition, does not extend beyond 1 year following surgery. Although controversial, there are concerns about potential persistent postoperative cognitive deficits and that perioperative neurocognitive disorders (an umbrella term for postoperative delirium and NCD) can be associated with long-term cognitive decline9. While often symptomatically reversible, there are reports of persistence of PND and some evidence to suggest that PND can be associated with development of cognitive impairment years later10.
While postoperative cognitive changes in older adults are common, the relationship between anesthesia and dementia is less certain. Some in-vitro11–13, animal11, 14, 15, and human studies16–18, have demonstrated increased production and aggregation of Aβ peptides and increased cerebrospinal fluid tau protein levels following exposure to different GAs. Studies have also observed increases in cerebrospinal fluid tau/Aβ ratios within 24–48 hours following surgery into the same range that are observed in AD patients17, 18. Previous observational studies have found either an increased risk of developing dementia following exposure to GA19–21, or no increase in risk22–24. Few studies have compared the effect of GA to regional anesthesia (RA) on the risk of developing dementia.
Few large-scale studies have explored the relationship between surgery, anesthesia, and development of dementia19, 20, 22, 23, 25–27. Limitations of these studies have included the use self-reported exposure data23, insufficient controlling for confounders20, 28, and used of outcome measures other than dementia22, 23. In addition, some studies included high risk procedures such as cardiac surgery in which RA cannot be utilized alone which limits generalizability to elective surgical procedures in which there are multiple anesthetic options20, 23, 26, 28. Therefore, we examined the association between exposure to GA versus RA among older adults undergoing elective surgical procedures and subsequent development of dementia.
METHODS
Study Design
We used a matched retrospective cohort study design to evaluate the diagnosis of dementia in a cohort of older individuals who received GA compared to RA for common elective surgical procedures.
Data sources
Several population-based, administrative databases were accessed from ICES in Ontario. Demographic information was available in the Registered Persons Database (RPDB). The Canadian Institute for Health Information - Discharge Abstract Database (CIHI-DAD) and Same Day Surgery (SDS) databases contain information on inpatient hospital surgical admissions, outpatient surgical procedures, and the type of anesthetic administered during surgery. The Ontario Health Insurance Plan (OHIP) database was used to identify physician services. The Ontario Drug Benefits (ODB) database contains information on prescription medications for all adults aged 65 and older in Ontario. Disease cohort databases (e.g., diabetes, heart failure) are also available at ICES and were used in this study. These datasets were linked using unique encoded identifiers and analyzed at ICES. The study was approved by the Queen’s University Health Sciences Research Ethics Board.
Study Sample
We included community-dwelling older adults who underwent one of five major elective surgeries in Ontario, Canada between April 1, 2007 and March 31, 2011. The study dates were selected to allow a minimum of 5 years of potential follow-up in order to identify dementia diagnoses. The study sample included individuals who underwent one of five elective surgical procedures that can be completed with either GA or RA: elective hip replacement, elective knee replacement, hernia repair, prostatectomy and hysterectomy. The index date for all analyses was the date of surgery. We included individuals who ≥ 66 years at the time of surgery, which allowed a 1-year lookback window for assessment of prescription medications within the ODB database.
We excluded individuals who had a diagnosis of dementia or who received any surgical procedure within the five years preceding cohort entry. We excluded nursing home residents as their baseline prevalence of dementia is high and their post-operative care processes in Ontario differ significantly from individuals residing in community settings29. We also excluded individuals who had relative contraindications to receiving spinal RA, including specific medical conditions that can preclude use of spinal RA use (e.g. aortic stenosis) as well as medications or medical conditions associated with an increased bleeding risk30. We excluded these individuals as individuals who had these contraindications to RA would likely only receive GA, and there would be no corresponding matches for these individuals among the group who had received RA.
Exposure definition
The exposure of interest was the type of anesthesia administered for surgery. Two categories of anesthesia were compared, GA and RA, which are recorded in the CIHI-DAD and SDS databases for all surgical procedures. GA includes both inhalational and intravenous anesthetic agents, although only a minority of surgical procedures utilize only intravenous medications. RA included both spinal and epidural anesthesia techniques without the use of GA. The accuracy of the coding for anesthesia type has been previously validated from CIHI-DAD and has an error rate of 6% after excluding individuals with missing data31. We excluded surgeries that involved a combination of GA and RA during the same procedure.
Outcome definition:
The primary outcome was incident physician-diagnosed dementia. The algorithm to determine newly diagnosed dementia used the following criteria: one hospitalization record with a dementia diagnosis during follow-up; the date of the first of three physician outpatient visits for dementia that were at least 30 days apart in a two-year period during follow-up; or one prescription drug reimbursement record for any cholinesterase inhibitor. This algorithm was previously validated against family physicians’ electronic medical records32.
Covariates
We identified potential covariates that may have acted as confounders for the association between anesthesia type and development of dementia33. Demographic information included age, sex, and calendar year of surgery. We also recorded measures of socioeconomic status. Measures of overall medical comorbidity were assessed using data from the three years prior to surgery, including the average number of Aggregated Diagnostic Groups (ADGs) using the Johns Hopkins Adjusted Clinical Grouping system34, the Charlson Comorbidity Index35, and the number of unique medications prescribed in the year prior to surgery36. We also recorded medical and psychiatric conditions potentially related to selection of anesthesia or dementia such as neurological conditions (e.g. stroke, Parkinsonism, head injury), cardiovascular disorders (e.g. hypertension, myocardial infarction) and respiratory conditions (e.g. asthma, chronic obstructive lung disease) and other medical conditions. Psychiatric conditions included history of anxiety disorders, mood disorders, and psychotic disorders. Medication exposure was recorded including the use of specific types of antidepressants, antipsychotics, benzodiazepines, opioids, statins and the Anticholinergic Risk Score37. The number of visits to a family physician and specialists involved in dementia evaluation including geriatricians, neurologists, and psychiatrists in the year prior to surgery were recorded. Health service utilization prior to surgery emergency department visits, medical and psychiatric hospitalizations in the year preceding surgery.
Consultations to anesthesiologists, cardiologists and respirologists within the 90 days preceding surgery were recorded as a marker of perioperative complexity. The hospital type in which the surgery occurred was categorized as academic (i.e. affiliated with a medical school) or as a small or large community hospital. Anesthesiologist billing codes based on the American Society of Anesthesiologists physical status score for each individual were recorded along with and postoperative complications.
Analysis
We first compared individuals who received GA to those who received RA in the unmatched sample. Differences in baseline covariates between those in the two exposure groups were assessed using the chi-squared test for categorical variables and the Mann-Whitney U test for continuous variables. Standardized differences were also used for this comparison, with standardized differences of 0.1 or greater used as a threshold to identify potentially meaningful imbalances in baseline variables between the two anesthesia exposure groups38. All variables that were imbalanced between the unmatched exposure groups were incorporated into a propensity score. The propensity score was estimated using a logistic regression model in which receipt of GA (vs. RA) was regressed on the imbalanced baseline covariates39. We then matched individuals who received GA to similar individuals who received RA on the logit of the propensity score40, and hard matching on the following variables: type of surgical procedure, age (within 1 year), sex, and calendar year of surgery. After matching, imbalances between anesthesia exposure groups were assessed again using standardized differences for all variables.
We use cumulative incidence function (CIF) curves and cause-specific proportional hazards models to assess the relationship between anesthesia and dementia. The following events were treated as competing risks: undergoing subsequent surgeries during follow-up, loss of health service coverage for greater than 1 year, and mortality prior to developing dementia. All individuals were followed from their date of surgery until a maximum of 5 years. All analyses were accounted for the matched nature of the data using a robust variance estimator41. We tested the difference in the CIF curves using Fine-Gray test of equality of strata42. Hazard ratios and 95% confidence intervals for the association between anesthesia and dementia were determined. All analyses were completed using SAS version 9.4. In our matched sample of 7,499 individuals who received GA and 7,499 individuals who received RA our study was powered to detect hazard ratios of <0.95 or >1.06 with a power of 0.8 and alpha = 0.05
Subgroup and Sensitivity Analysis
Subgroup analyses were completed according to age strata (categorized as: age 66–69, 70–74, 75–79, 80–84, and 85 years or older), sex, and by each surgical procedure. To account for the possibility that delirium may be misclassified as dementia postoperatively, we completed a sensitivity analysis that excluded new diagnoses of dementia within the first 90 days following surgery. We also completed a sensitivity analysis excluding individuals who died within 90 days of surgery to account for potential differences in early perioperative morbidity and mortality related to selection of anesthesia.
RESULTS
Description of Study Sample
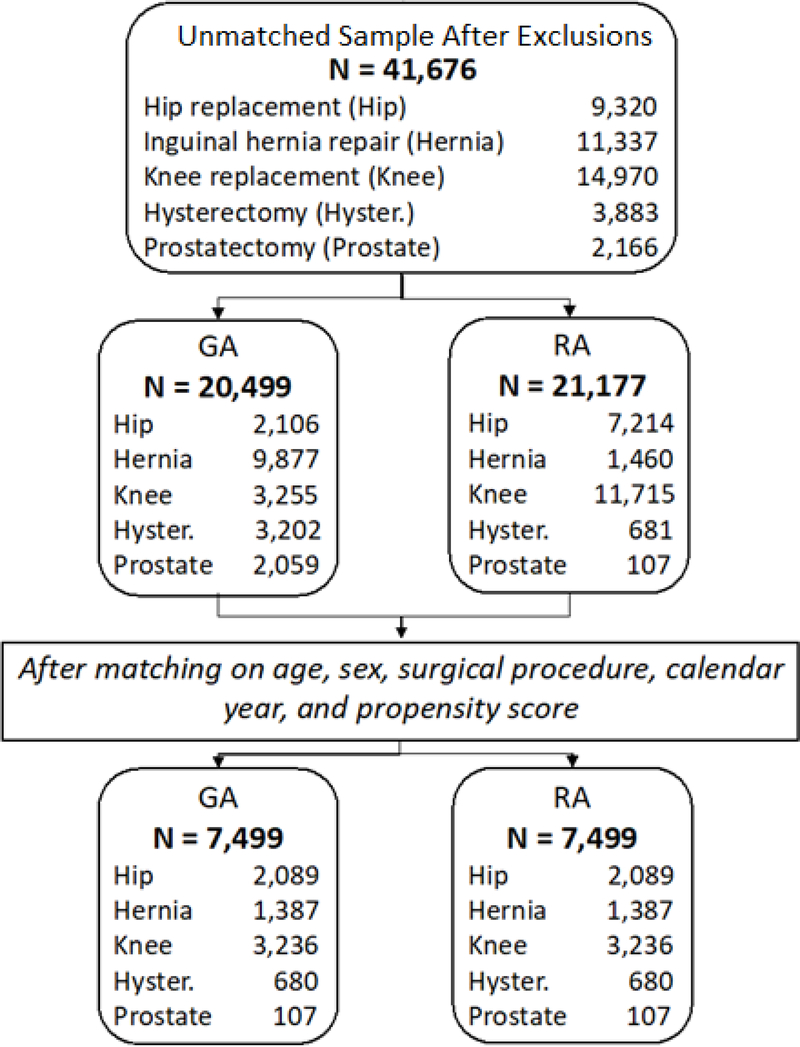
During the study time period, there were 41,676 individuals who were potentially eligible for inclusion in the final study sample, 20,499 (49.2%) who received GA and 21,177 (50.8%) who received RA (Figure 1). The average age of the entire unmatched sample was 73.6 years (SD=5.7 years), and 21,739 (52.2%) of the study sample were women (Table 1). The mean Charlson comorbidity scores and the mean number of unique medications prescribed differed significantly between groups. Additional covariate comparisons between the GA and RA unmatched groups are summarized in Supplementary Table S1 in supplementary material.
Figure 1:
Cohort Creation for Anesthesia and Dementia Study Population
Table 1.
Comparison of descriptive characteristics between unmatched GA and RA groups
| Variable | General Anesthesia (N = 20,499) | Regional Anesthesia (N = 21,177) | Test statistic | p-value | Std. Diff. |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, Mean (SD) | 72.8 (5.5) | 74.5 (5.9) | Z = −30.1 | <0.0001* | 0.3* |
| 66–69, N (%) | 7,191 (35.1) | 5,165 (24.4) | X2 = 570.7 (1) | <0.0001* | 0.2* |
| 70–74, N (%) | 6,491 (31.7) | 6,235 (29.4) | X2 = 24.3 (1) | <0.0001* | 0.05* |
| 75–79, N (%) | 4,062 (19.8) | 5,398 (25.5) | X2 = 191.1 (1) | <0.0001* | 0.1* |
| 80–84, N (%) | 2,042 (10.0) | 3,105 (14.7) | X2 = 212.6 (1) | <0.0001* | 0.1* |
| 85+, N (%) | 713 (3.5) | 1,274 (6.0) | X2 = 147.7 (1) | <0.0001* | 0.1* |
| Female sex, N (%) | 8,996 (43.9) | 12,743 (60.2) | X2 = 1107.5 (1) | <0.0001* | 0.3* |
| Medical comorbidity† | |||||
| ADG groups, Mean (SD) | 9.1 (3.3) | 8.9 (3.4) | Z = 5.7 | <0.0001* | 0.05 |
| Charlson comorbidity score, Mean (SD) | 0.6 (1.2) | 0.3 (0.7) | Z = 18.1 | <0.0001* | 0.3* |
| Number of unique medications, Mean (SD) | 6.9 (4.7) | 7.6 (4.7) | Z = −17.0 | <0.0001* | 0.2* |
| Medical conditions†, N (%) | |||||
| Head trauma | 419 (2.0) | 424 (2.0) | X2 = 0.1 (1) | 0.8 | 0 |
| Chronic renal disease | 1,105 (5.4) | 1,215 (5.7) | X2 = 2.4 (1) | 0.1 | 0.02 |
| Diabetes mellitus | 4,464 (21.8) | 5,018 (23.7) | X2 = 21.8 (1) | <0.0001* | 0.05 |
| Congestive heart failure | 585 (2.9) | 738 (3.5) | X2 = 13.5 (1) | 0.0002* | 0.04 |
| Hypertension | 13,633 (66.5) | 15,684 (74.1) | X2 = 285.1 (1) | <0.0001* | 0.17* |
| Ischemic heart disease | 536 (2.6) | 589 (2.8) | X2 = 1.1 (1) | 0.3 | 0.01 |
| Obesity | 220 (1.1) | 330 (1.6) | X2 = 18.8 (1) | <0.0001* | 0.04 |
| Stroke | 1,024 (5.0) | 1,056 (5.0) | X2 = 0.0 (1) | 1.0 | 0 |
| Anxiety disorder | 6,702 (32.7) | 6,738 (31.8) | X2 = 3.7 (1) | 0.1 | 0.02 |
| Depression | 6,848 (33.4) | 7,017 (33.1) | X2 = 0.4 (1) | 0.6 | 0.01 |
| Mood disorders | 1,056 (5.2) | 1,323 (6.2) | X2 = 23.2 (1) | <0.0001* | 0.05 |
| Parkinsonism | 215 (1.0) | 245 (1.2) | X2 = 1.1 (1) | 0.3 | 0.01 |
| Schizophrenia | 103 (0.5) | 108 (0.5) | X2 = 0.01 (1) | 0.9 | 0 |
| Asthma | 1,111 (5.4) | 1,273 (6.0) | X2 = 6.8 (1) | 0.009* | 0.03 |
| COPD | 3,502 (17.1) | 3,855 (18.2) | X2 = 9.0 (1) | 0.003* | 0.03 |
| Medication history† | |||||
| Any antidepressants, N (%) | 745 (3.6) | 1,050 (5.0) | X2 = 44.3 (1) | <0.0001* | 0.07 |
| Any antipsychotics, N (%) | 290 (1.4) | 218 (1.0) | X2 = 12.8 (1) | 0.0003* | 0.04 |
| Any benzodiazepines, N (%) | 2,398 (11.7) | 2,745 (13.0) | X2 = 15.4 (1) | <0.0001* | 0.04 |
| Any opioids, N (%) | 3,227 (15.7) | 5,170 (24.4) | X2 = 486.8 (1) | <0.0001* | 0.2* |
| Any statins, N (%) | 7,450 (36.3) | 8,075 (38.1) | X2 = 14.2 (1) | 0.0002* | 0.04 |
| Anticholinergic risk score, Mean (SD) | 0.3 (0.8) | 0.3 (0.9) | Z = −7.9 | <0.0001* | 0.07 |
| Health services utilization† | |||||
| Any ED visit, N (%) | 5,721 (27.9) | 5,332 (25.2) | X2 = 39.9 (1) | <0.0001* | 0.06 |
| Any inpatient admission, N (%) | 1,139 (5.6) | 907 (4.3) | X2 = 36.2 (1) | <0.0001* | 0.06 |
| Physician visits | |||||
| Family/General practitioner visits, Mean (SD) | 7.4 (5.3) | 7.7 (5.5) | Z = −6.0 | <0.0001* | 0.05 |
| Any geriatrician visit, N (%) | 725 (3.5) | 753 (3.6) | X2 = 0.01 (1) | 0.9 | 0 |
| Any neurologist visit, N (%) | 836 (4.1) | 984 (4.6) | X2 = 8.1 (1) | 0.005* | 0.03 |
| Any psychiatrist visit, N (%) | 327 (1.6) | 321 (1.5) | X2 = 0.4 (1) | 0.5 | 0.01 |
| Home care utilization | |||||
| Any home care service, N (%) | 2,267 (11.1) | 5,299 (25.0) | X2 = 1366.8 (1) | <0.0001* | 0.4* |
| Perioperative | |||||
| Index surgery, N (%) | |||||
| Hip replacement | 2,106 (10.3) | 7,214 (34.1) | X2 = 3395.9 (1) | <0.0001* | 0.6* |
| Hysterectomy | 3,202 (15.6) | 681 (3.2) | X2 = 1897.0 (1) | <0.0001* | 0.4* |
| Inguinal hernia repair | 9,877 (48.2) | 1,460 (6.9) | X2 = 8966.9 (1) | <0.0001* | 1.0* |
| Knee replacement | 3,255 (15.9) | 11,715 (55.3) | X2 = 7039.5 (1) | <0.0001* | 0.9* |
| Prostatectomy | 2,059 (10.0) | 107 (0.5) | X2 = 1923.7 (1) | <0.0001* | 0.4* |
| Hospital LOS days, Mean (SD) | 3.9 (2.6) | 4.4 (2.4) | Z = −28.1 | <0.0001 | 0.2* |
| Physician consultations†, N (%) | |||||
| Any anesthesiologist visit | 10,619 (51.8) | 15,369 (72.6) | X2 = 1914.6 (1) | <0.0001* | 0.4* |
| Any cardiologist visit | 11,286 (55.1) | 10,988 (51.9) | X2 = 42.1 (1) | <0.0001* | 0.06 |
| Any respirology visit | 1,415 (6.9) | 1,519 (7.2) | X2 = 1.2 (1) | 0.3 | 0.01 |
| Type of hospital, N (%) | |||||
| Missing | 495 (2.4) | 115 (0.5) | - | - | 0.2* |
| Community | 14,568 (71.1) | 15,029 (71.0) | X2 = 11.0 (1) | 0.0009* | 0 |
| Small | 350 (1.7) | 118 (0.6) | X2 = 128.8 (1) | <0.0001* | 0.1* |
| Teaching | 5,086 (24.8) | 5,915 (27.9) | X2 = 37.0 (1) | <0.0001* | 0.07 |
| ASA score, N (%) | |||||
| I/II – No Bill code | 11,097 (54.1) | 9,309 (44.0) | X2 = 431.7 (1) | <0.0001* | 0.2* |
| III | 8,726 (42.6) | 11,071 (52.3) | X2 = 393.9 (1) | <0.0001* | 0.2* |
| IV/V | 676 (3.3) | 797 (3.7) | X2 = 6.6 (1) | 0.01* | 0.03 |
| Any Complications, N (%) | 2,247 (11.0) | 4,055 (19.1) | X2 = 543.9 (1) | <0.0001* | 0.2* |
ADG = Aggregated diagnostic groups, ASA = American Society of Anesthesiologists, COPD = Chronic obstructive pulmonary disease, ED = Emergency department, Std. Diff. = Standardized difference
Statistically significant
Lookback periods: ADG groups, Charlson comorbidity = 3 years preceding index; Number of unique medications = 1 year preceding index; Medical conditions = 5 years preceding index; Medication history = 120 days preceding; Health services utilization = 1 year preceding index; Physician consultations = 90 days preceding index.
Baseline Comparisons of Matched GA and RA Groups
The proportion on individuals who received GA and RA varied between the different surgical procedures. Within each surgery, the maximum number of pairs that could be created corresponded to the number of individuals who received the least frequent type of anesthesia for that procedure. After matching, there were 14,998 individuals (7,499 pairs of individuals who received GA matched to those who received RA) representing a total of 98.6% of the potential eligible sample. After matching, most variables had standardized differences less than 0.05 when comparing the GA and RA groups (Table 2). The only variables that were not balanced between the anesthesia groups after matching were the number of geriatrician visits, cardiologist visits, and the average hospital length of stay. Additional variables in the matched sample are described in Supplementary Table S2 in supplementary material.
Table 2.
Descriptive characteristics for the GA and RA groups in the matched sample
| Variable | General Anesthesia (N = 7,499) | Regional Anesthesia (N = 7,499) | Test statistic | p-value | Std. Diff. |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, Mean (SD) | 74.3 (5.8) | 74.3 (5.8) | Z = −0.1 | 0.9 | 0 |
| 66–69, N (%) | 1,900 (25.3) | 1,892 (25.2) | X2 = 2.5 (1) | 0.1 | 0 |
| 70–74, N (%) | 2,255 (30.1) | 2,255 (30.1) | X2 = 0 (1) | 1.0 | 0 |
| 75–79, N (%) | 1,847 (24.6) | 1,842 (24.6) | X2 = 0.5 (1) | 0.5 | 0 |
| 80–84, N (%) | 1,075 (14.3) | 1,085 (14.5) | X2 = 2.6 (1) | 0.1 | 0 |
| 85+, N (%) | 422 (5.6) | 425 (5.7) | X2 = 1.3 (1) | 0.3 | 0 |
| Female sex, N (%) | 4,358 (58.1) | 4,358 (58.1) | X2 = 0 (1) | 1.0 | 0 |
| Medical comorbidity† | |||||
| ADG groups, Mean (SD) | 9.1 (3.3) | 9.0 (3.3) | Z = 2.7 | 0.007* | 0.05 |
| Charlson comorbidity score, Mean (SD) | 0.4 (0.9) | 0.3 (0.7) | Z = 3.3 | 0.0008* | 0.08 |
| Number of unique medications, Mean (SD) | 7.5 (4.8) | 7.4 (4.6) | Z = 1.1 | 0.3 | 0.03 |
| Medical conditions†, N (%) | |||||
| Head trauma | 179 (2.4) | 157 (2.1) | X2 = 1.5 (1) | 0.2 | 0.02 |
| Chronic renal disease | 411 (5.5) | 411 (5.5) | X2 = 0 (1) | 1.0 | 0 |
| Diabetes mellitus | 1,763 (23.5) | 1,634 (21.8) | X2 = 7.1 (1) | 0.008* | 0.04 |
| Congestive heart failure | 248 (3.3) | 269 (3.6) | X2 = 0.9 (1) | 0.3 | 0.02 |
| Hypertension | 5,464 (72.9) | 5,464 (72.9) | X2 = 0 (1) | 1.0 | 0 |
| Ischemic heart disease | 190 (2.5) | 245 (3.3) | X2 = 7.5 (1) | 0.006* | 0.04 |
| Obesity | 114 (1.5) | 99 (1.3) | X2 = 1.1 (1) | 0.3 | 0.02 |
| Stroke | 407 (5.4) | 384 (5.1) | X2 = 0.7 (1) | 0.4 | 0.01 |
| Anxiety disorder | 2,521 (33.6) | 2,425 (32.3) | X2 = 2.8 (1) | 0.1 | 0.03 |
| Depression | 2,577 (34.4) | 2,533 (33.8) | X2 = 0.6 (1) | 0.5 | 0.01 |
| Mood disorders | 436 (5.8) | 467 (6.2) | X2 = 1.1 (1) | 0.3 | 0.02 |
| Parkinsonism | 72 (1.0) | 89 (1.2) | X2 = 1.8 (1) | 0.2 | 0.02 |
| Schizophrenia | 44 (0.6) | 27 (0.4) | X2 = 4.1 (1) | 0.04* | 0.03 |
| Asthma | 412 (5.5) | 473 (6.3) | X2 = 4.4 (1) | 0.04* | 0.03 |
| COPD | 1,315 (17.5) | 1,466 (19.5) | X2 = 10.2 (1) | 0.001* | 0.05 |
| Medication history† | |||||
| Any antidepressants, N (%) | 330 (4.4) | 361 (4.8) | X2 = 1.5 (1) | 0.2 | 0.02 |
| Any antipsychotics, N (%) | 101 (1.3) | 79 (1.1) | X2 = 2.8 (1) | 0.1 | 0.03 |
| Any benzodiazepines, N (%) | 967 (12.9) | 970 (12.9) | X2 = 0.01 (1) | 0.9 | 0 |
| Any opioids, N (%) | 1,804 (24.1) | 1,673 (22.3) | X2 = 11.2 (1) | 0.001* | 0.04 |
| Any statins, N (%) | 2,810 (37.5) | 2,807 (37.4) | X2 = 0.0 (1) | 1.0 | 0 |
| Anticholinergic risk score, Mean (SD) | 0.3 (0.8) | 0.3 (0.8) | Z = −7. | 0.5 | 0 |
| Health services utilization† | |||||
| Any ED visit, N (%) | 1,929 (25.7) | 2,006 (26.8) | X2 = 2.1 (1) | 0.1 | 0.02 |
| Any inpatient admission, N (%) | 347 (4.6) | 318 (4.2) | X2 = 1.4 (1) | 0.3 | 0.02 |
| Physician visits | |||||
| Family/General practitioner visits, Mean (SD) | 7.9 (5.5) | 7.5 (5.6) | Z = 5.2 | <0.0001* | 0.07 |
| Any geriatrician visit, N (%) | 493 (6.6) | 218 (2.9) | X2 = 115.5 (1) | <0.0001* | 0.2* |
| Any neurologist visit, N (%) | 336 (4.5) | 329 (4.4) | X2 = 0.1 (1) | 0.8 | 0 |
| Any psychiatrist visit, N (%) | 122 (1.6) | 114 (1.5) | X2 = 0.3 (1) | 0.6 | 0.01 |
| Home care utilization | |||||
| Any home care service, N (%) | 1,653 (22.0) | 1,629 (21.7) | X2 = 0.3 (1) | 0.6 | 0.01 |
| Perioperative | |||||
| Index surgery, N (%) | |||||
| Hip replacement | 2,089 (27.9) | 2,089 (27.9) | X2 = 0 (1) | 1.0 | 0 |
| Hysterectomy | 680 (9.1) | 680 (9.1) | X2 = 0 (1) | 1.0 | 0 |
| Inguinal hernia repair | 1,387 (18.5) | 1,387 (18.5) | X2 = 0 (1) | 1.0 | 0 |
| Knee replacement | 3,236 (43.2) | 3,236 (43.2) | X2 = 0 (1) | 1.0 | 0 |
| Prostatectomy | 107 (1.4) | 107 (1.4) | X2 = 0 (1) | 1.0 | 0 |
| Hospital LOS days, Mean (SD) | 4.5 (2.6) | 4.2 (2.4) | Z=9.0 | <0.0001 | 0.1 |
| Physician consultations†, N (%) | |||||
| Any anesthesiologist visit | 4,860 (64.8) | 4,909 (65.5) | X2 = 1.1 (1) | 0.3 | 0.01 |
| Any cardiologist visit | 4,251 (56.7) | 3,770 (50.3) | X2 = 64.5 (1) | <0.0001* | 0.1* |
| Any respirology visit | 594 (7.9) | 551 (7.3) | X2 = 1.8 (1) | 0.2 | 0.02 |
| Type of hospital, N (%) | |||||
| Community | 5,591 (74.6) | 5,619 (74.9) | X2 = 0.4 (1) | 0.6 | 0.01 |
| Small | 61 (0.8) | 73 (1.0) | X2 = 1.5 (1) | 0.2 | 0.02 |
| Teaching | 1,847 (24.6) | 1,807 (24.1) | X2 = 0.8 (1) | 0.4 | 0.01 |
| ASA score, N (%) | |||||
| I/II – No Bill code | 3,533 (47.1) | 3,456 (46.1) | X2 = 2.4 (1) | 0.1 | 0.02 |
| III | 3,674 (49.0) | 3,759 (50.1) | X2 = 2.8 (1) | 0.1 | 0.02 |
| IV/V | 292 (3.9) | 284 (3.8) | X2 = 0.2 (1) | 0.7 | 0.01 |
| Any Complications, N (%) | 1,233 (16.4) | 1,239 (16.5) | X2 = 0.02 (1) | 0.9 | 0 |
ADG = Aggregated diagnostic groups, ASA = American Society of Anesthesiologists, COPD = Chronic obstructive pulmonary disease, ED = Emergency department, Std. Diff. = Standardized difference
Statistically significant
Lookback periods: ADG groups, Charlson comorbidity = 3 years preceding index; Number of unique medications = 1 year preceding index; Medical conditions = 5 years preceding index; Medication history = 120 days preceding; Health services utilization = 1 year preceding index; Physician consultations = 90 days preceding index.
Association between Anesthesia and Time to Diagnosis of Dementia
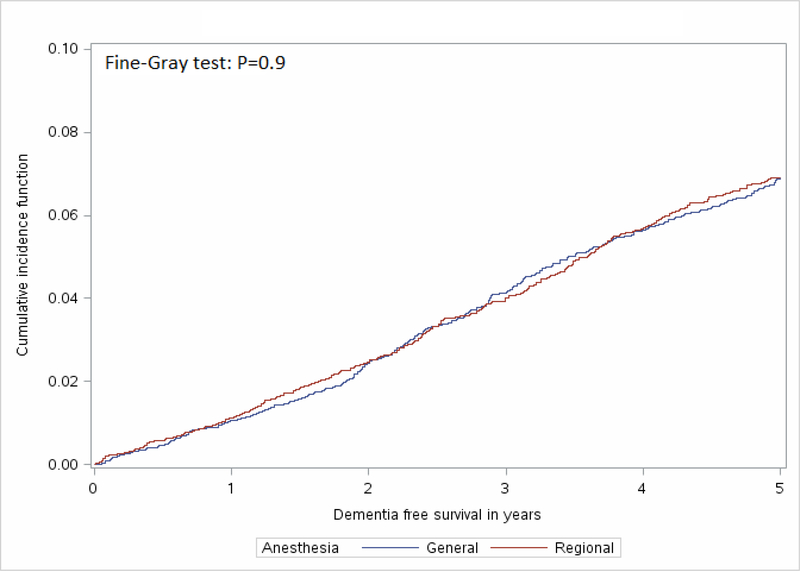
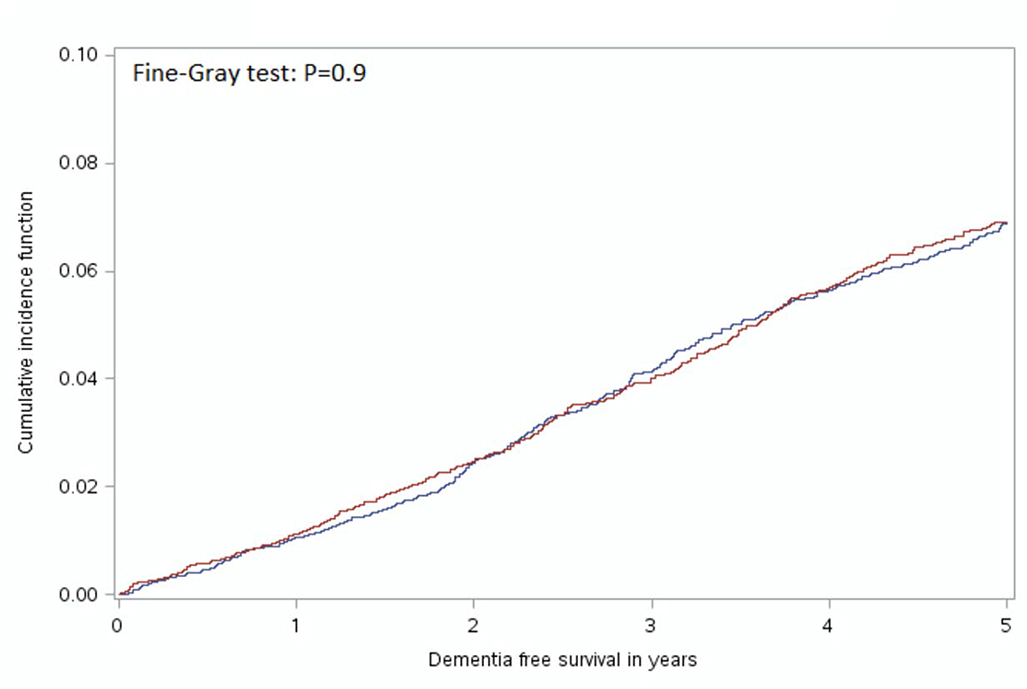
In the unmatched sample, GA was associated with a reduced risk of developing dementia (HR: 0.82, 95% CI: 0.75 – 0.90). The Fine-Gray test for equality of strata from the cumulative incidence function curves (Figure 2) indicated no significant difference between the GA and RA group curves (χ2<0.01, P=0.9). The median duration of follow-up for the GA group was 1,580 days (4.3 years) and 1,578 days (4.3 years) in the RA group. Overall, 363 (4.8%) people administered GA and 362 (4.8%) receiving RA developed dementia within five years following surgery. Amongst people receiving GA for surgery in the matched sample, there were 371 (4.9%) who died without developing dementia and 368 (4.9%) who died in the RA group (4.9%). In the cause-specific models incorporating death as a competing risk and the robust variance estimator there was no difference in the risk of dementia for GA compared to RA (HR=1.0, 95% CI: 0.9–1.1, P=0.8).
Figure 2:
Cumulative Incidence Function Curve Comparing Time to Diagnosis of Dementia for Individuals who received General Anesthesia when compared to Regional Anesthesia
Subgroup and Sensitivity Analyses
There were no statistically significant associations between type of anesthesia in different age groups (Table 3). There was also no difference in the dementia risk noted for men or women in the overall study sample. Subgroup analyses based on type of surgery did not find any difference dementia risk for individuals who underwent hip replacement, knee replacement or prostatectomy. There was an increased risk of dementia in association with GA noted in the hysterectomy subgroup (HR: 1.6, 95% CI: 1.2 – 2.3, P<0.01) and slightly decreased risk of dementia with GA noted in the inguinal hernia subgroup group (HR: 0.8, 95% CI: 0.7 – 0.9, P<0.01).
Table 3.
Cox proportional hazards models using RA as the reference group and sensitivity analyses comparing GA to RA for the matched sample.
| Group | Events | Total N | Hazard Ratio | 95% CI | Wald Chi-Square (D.F.) | P value |
|---|---|---|---|---|---|---|
| Entire Cohort | 725 | 14,998 | 1.0 | 0.8–1.2 | 0.0 (1) | 0.9 |
| Subgroup Analyses | ||||||
| Age | ||||||
| 66–69 | 54 | 3,766 | 1.1 | 0.8 – 1.6 | 0.8 (1) | 0.4 |
| 70–74 | 129 | 4,460 | 1.0 | 0.8 – 1.3 | 0.1 (1) | 0.7 |
| 75–79 | 220 | 3,634 | 0.9 | 0.7 – 1.1 | 1.1 (1) | 0.3 |
| 80–84 | 199 | 2,122 | 1.0 | 0.6 – 1.2 | 0.0 (1) | 0.8 |
| 85+ | 108 | 840 | 1.1 | 0.8 – 1.4 | 0.3 (1) | 0.5 |
| Females | 395 | 8,716 | 1.0 | 0.9 – 1.2 | 0.0 (1) | 0.8 |
| Males | 330 | 6,282 | 1.0 | 0.9 – 1.2 | 0.0 (1) | 0.9 |
| Index surgery | ||||||
| Hip replacement | 184 | 4,178 | 1.1 | 0.9 – 1.3 | 0.0 (1) | 0.4 |
| Knee replacement | 266 | 6,472 | 1.0 | 0.9 – 1.2 | 0.1 (1) | 0.8 |
| Inguinal hernia repair | 210 | 2,774 | 0.8 | 0.7 – 0.9 | 6.6 (1) | 0.01 |
| Hysterectomy | 60 – 65 | 1,360 | 1.6 | 1.2 – 2.3 | 8.2 (1) | 0.004 |
| Prostatectomy | ≤5 | 214 | 1.1 | 0.2 – 5.3 | 0.0 (1) | 0.9 |
| Sensitivity Analyses | ||||||
| Excluding developed dementia within 90 days | 680 | 14,956 | 1.0 | 0.9 – 1.2 | 0.0 | 1.0 |
| Excluding death within 90 days following surgery | 723 | 14,928 | 1.0 | 0.9 – 1.2 | 0.0 | 1.0 |
CI = confidence interval, D.F. = degrees of freedom, N = numbers
In the sensitivity analysis excluding anyone diagnosed with dementia within 90 days of surgery there was no difference in dementia diagnosis between anesthesia exposure groups (HR=1.0, 95% CI: 0.8–1.2, P=1.0). Similarly, exclusion of individuals who died within 90 days of surgery also found no difference in risk for dementia diagnosis between anesthesia groups (HR=1.0, 95% CI: 0.9–1.2, P=1.0).
DISCUSSION:
In this study of older adults without dementia undergoing common elective surgical procedures with a median of over 4 years of follow-up, we found no difference in the risk of developing dementia for individuals who received GA versus RA. This result remained consistent in most subgroups; we observed no significant differences in the association between type of anesthesia and dementia risk in different age strata, in the overall sex-specific analyses for women and men, and in the three largest subgroups based on different surgical procedures. These findings were also robust to different analytic assumptions and in a series of sensitivity analyses.
There have been relatively few studies which have evaluated dementia risk comparing GA versus RA. A systematic review and meta-analysis of case-control studies published in 2011 did not find any significant difference in the risk of dementia for individuals exposed to GA when compared to RA43. Since that time, several additional studies have been published with inconsistent results. A retrospective cohort study comparing GA and RA found that GA was associated with lower risk of dementia compared to RA for individuals undergoing either hernia repair (adjusted HR=0.7, 95% CI: 0.5–0.9), or prostatectomy (adjusted HR=0.7, 95% CI: 0.5–0.8). However this study controlled for a limited number of confounders which may have biased these results27. Another second retrospective cohort study evaluating a broad range of surgical procedures identified that undergoing surgery with RA was associated with an increased risk of dementia when compared to GA (HR: 1.4, 95% CI: 1.2 – 1.6) although this study did not directly compare individuals undergoing the same surgical procedures and controlled for a limited number of confounders20. Our study provides additional evidence that there does not appear to be an increased risk of dementia associated with undergoing surgical procedures with GA when compared to RA exposure.
While there have been few studies comparing GA and RA, there is a wider literature examining whether undergoing surgical procedures under any type of anesthesia is associated with an increased risk of dementia. There have been two previous systematic reviews examining GA and subsequent AD risk, neither of which found any clear associations between AD and anesthesia43, 44. The first review included a meta-analysis that found no association between dementia and prior history of surgery with anesthesia in 15 case-control studies (OR: 1.1 95% CI: 0.9 – 1.2, P=0.4)43. The second review examined the relationship between type of anesthesia and a range of cognitive disorders, ranging from POCD to AD which concluded that there was also no consistent evidence for any associations between surgery and dementia44. A more recent case-control study also reported no increase in dementia in association with any exposure to surgery involving GA (OR:0.9, 95% CI: 0.7 – 1.1, P = 0.3) or with the total number of procedures involving GA24. Another large retrospective cohort study comparing outcomes in patients undergoing surgical procedures requiring GA as compared to non-surgical controls found an association between surgery with GA and subsequent development of dementia (HR: 1.29, 95% CI: 1.26–1.38)25. Two recent studies have found that hospitalizations for both nonsurgical conditions were associated with an increased risk of cognitive decline45 and dementia46 when compared to surgical admissions highlighting the roles that illness and physiological stress may play in cognitive impairment and development of dementia. The incidence of dementia in our study population was 4.8% over 4 years of follow-up. This is higher than the 5-year incidence rates of 2 – 3% observed in similar aged populations from other epidemiological studies47. The higher incidence of dementia observed in our study may reflect differences in the baseline risk of our population which was not randomly selected from the general population. Therefore, while our data showed that anesthesia type administered for surgery did not influence the dementia risk overall, further research is required to determine if undergoing surgery, irrespective of anesthesia, is a risk factor for dementia.
There were several strengths to our study. Our study evaluated the real-world outcomes of older adults and included a large sample size which allowed us to examine several surgical procedures and subgroups of patients; these, result should generalize to a broad range of settings. By limiting our analysis to adults ≥ 66 and years we also examined individuals who at a relatively high risk for developing dementia. We also controlled for several variables in our analysis to minimize the chance that any observed associations were due to confounding. This study also employed multiple analytic strategies and sensitivity analyses which demonstrated that our main results of the study were robust to different analytic approaches and underlying assumptions. Our study also evaluated whether there may be specific subgroups who are at increased risk of developing dementia following exposure to GA or RA and did not find any associations in the majority of the subgroups we evaluated.
There are potential limitations to our study. This was a retrospective study and thus there is the potential that unmeasured confounders may not have been accounted for. While our primary analysis and most subgroup analyses did not find an association between anesthesia type and dementia, in two of the smaller surgical subgroups statistically significant associations, both positive and negative, were observed so there may be differences in the risk of dementia associated with anesthesia type in certain surgical procedures. We do not have detailed information on the cognitive functioning of individuals prior to surgery and although our analysis excluded individuals with pre-existing diagnosed dementia, it is possible that there may have been baseline differences between exposure groups in cognition or preclinical AD pathology that are not captured in our databases. Additionally, we restricted our analyses to a single anesthesia exposure and as such we cannot conclude that there is no association between cumulative exposure to multiple surgeries under a single anesthetic type and dementia risk. Our databases also do not contain detailed information related to anesthesia such as the specific medications administered or quantities of medication which may also influence postoperative cognitive outcomes. Our study outcome was physician-diagnosed dementia, so more subtle forms of cognitive impairment may have remained undiagnosed48. For example, individuals with mild cognitive impairment may have been preferentially given RA given concerns about the cognitive effects of GA in this population which might have attenuated potential associations between GA and the development of dementia. Whether individuals with mild cognitive impairment or early biomarker evidence of AD pathology (i.e. preclinical AD) may have an increased risk of developing dementia following surgery with GA versus AD cannot be answered with this data.
This large observational study with over 4 years of follow-up found no difference between GA and RA and the development of dementia in our primary analysis. These results were consistent across sensitivity analyses and in the majority of subgroups. Our findings provide some reassurance that there does not appear to be any increased risk of being diagnosed with dementia in the years following exposure to GA versus RA for some common surgical procedures. These findings may contribute to decision-making about the comparative risks of anaesthesia type for various common elective surgeries. Future studies may want to examine whether repeated exposures to surgery and anesthesia, exposure to anesthesia in in other surgical populations, or the duration and dosage of specific anesthetic medications may be associated with dementia risk.
Supplementary Material
IMPACT STATEMENT:
This work is novel and expands upon previous clinical research related to the relationship between anesthesia and dementia. A systematic review in 2011 found no significant increase in risk of dementia following surgery with GA (pooled OR: 1.05; 95% CI: 0.93–1.19)(1). More recent observational studies have been limited by small sample sizes(2), insufficient control of potential confounders(3, 4), or the inclusion of surgeries that could only be completed under GA(3–6). There have been relatively few small randomized controlled trials addressing this topic(7). We conducted a large-scale retrospective cohort study to evaluate the association between exposure to either GA or RA administered for elective surgical procedures and the subsequent risk of developing dementia among older adults. Overall, our results did not find that there was an increased risk of dementia in association with GA when compared to RA.
REFERENCE LIST FOR IMPACT STATEMENT
- 1.Seitz DP, Shah PS, Herrmann N, et al. : Exposure to general anesthesia and risk of Alzheimer’s disease: a systematic review and meta-analysis. BMC Geriatr 2011; 11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sprung J, Jankowski CJ, Roberts RO, et al. : Anesthesia and incident dementia: a population-based, nested, case-control study. Mayo Clin Proc 2013; 88:552–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen PL, Yang CW, Tseng YK, et al. : Risk of dementia after anaesthesia and surgery. Br J Psychiatry 2014; 204:188–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim CT, Myung W, Lewis M, et al. : Exposure to general anesthesia and risk of dementia: a nationwide population-based cohort study. Journal of Alzheimer’s Disease 2018; 63:395–405 [DOI] [PubMed] [Google Scholar]
- 5.Lee TA, Wolozin B, Weiss KB, et al. : Assessment of the emergence of Alzheimer’s disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. J Alzheimers Dis 2005; 7:319–324 [DOI] [PubMed] [Google Scholar]
- 6.Aiello Bowles EJ, Larson EB, Pong RP, et al. : Anesthesia Exposure and Risk of Dementia and Alzheimer’s Disease: A Prospective Study. J Am Geriatr Soc 2016; 64:602–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Pan N, Ma Y, et al. : Inhaled sevoflurane may promote progression of amnestic mild cognitive impairment: a prospective, randomized parallel-group study. Am J Med Sci 2013; 345:355–360 [DOI] [PubMed] [Google Scholar]
Acknowledgements
Clive Velkers was supported by an interdisciplinary fellowship award from the Canadian Frailty Network. This work was completed as part of Mr. Velkers’ requirements for the MSc in Epidemiology at Queen’s University. Funds for this project were provided from the Southeastern Ontario Academic Medical Organization Academic Funding Plan Innovation Fund. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Dr. Austin was supported in part by a Mid-Career Investigator award from the Heart and Stroke Foundation. Dr. Berger acknowledges support from National Institutes of Health K76 AG057022. Dr. Rochon holds the RTO/ERO Chair in Geriatric Medicine from the University of Toronto.
REFERENCES
- [1].Prince M, Wimo A, Guerchet M, Ali G, Wu Y, Prina M. World Alzheimer Report 2015: The global impact of dementia: An analysis of prevalence, incidence, cost and trends. London, UK: Alzheimer’s Disease International, 2015. [Google Scholar]
- [2].Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16: 391–460. [Google Scholar]
- [3].Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurology. 2011;10: 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396: 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dyer CB, Ashton CM, Teasdale TA. Postoperative delirium. A review of 80 primary data-collection studies. Arch Intern Med. 1995;155: 461–465. [DOI] [PubMed] [Google Scholar]
- [6].Vijayakumar B, Elango P, Ganessan R. Post-operative delirium in elderly patients. Indian J Anaesth. 2014;58: 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery—2018. J Alzheimers Dis. 2018;66: 1–10. [DOI] [PubMed] [Google Scholar]
- [8].Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351: 857–861. [DOI] [PubMed] [Google Scholar]
- [9].Avidan MS, Evers AS. The fallacy of persistent postoperative cognitive decline. Anesthesiology. 2016;124: 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344: 395–402. [DOI] [PubMed] [Google Scholar]
- [11].Dong Y, Zhang G, Zhang B, et al. The common inhalational anesthetic sevoflurane induces apoptosis and increases beta-amyloid protein levels. Arch Neurol. 2009;66: 620–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xie Z, Dong Y, Maeda U, et al. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104: 988–994. [DOI] [PubMed] [Google Scholar]
- [13].Eckenhoff RG, Johansson JS, Wei H, et al. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101: 703–709. [DOI] [PubMed] [Google Scholar]
- [14].Perucho J, Rubio I, Casarejos MJ, et al. Anesthesia with isoflurane increases amyloid pathology in mice models of Alzheimer’s disease. J Alzheimers Dis. 2010;19: 1245–1257. [DOI] [PubMed] [Google Scholar]
- [15].Whittington RA, Virag L, Marcouiller F, et al. Propofol directly increases tau phosphorylation. PLoS One. 2011;6: e16648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Palotás A, Reis HJ, Bogáts G, et al. Coronary artery bypass surgery provokes Alzheimer’s disease-like changes in the cerebrospinal fluid. J Alzheimers Dis. 2010;21: 1153–1164. [DOI] [PubMed] [Google Scholar]
- [17].Berger M, Nadler JW, Friedman A, et al. The effect of propofol versus isoflurane anesthesia on human cerebrospinal fluid markers of Alzheimer’s disease: results of a randomized trial. J Alzheimers Dis. 2016;52: 1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tang JX, Baranov D, Hammond M, Shaw LM, Eckenhoff MF, Eckenhoff RG. Human Alzheimer and inflammation biomarkers after anesthesia and surgery. Anesthesiology. 2011;115: 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen CW, Lin CC, Chen KB, Kuo YC, Li CY, Chung CJ. Increased risk of dementia in people with previous exposure to general anesthesia: a nationwide population-based case-control study. Alzheimers Dement. 2014;10: 196–204. [DOI] [PubMed] [Google Scholar]
- [20].Chen PL, Yang CW, Tseng YK, et al. Risk of dementia after anaesthesia and surgery. Br J Psychiatry. 2014;204: 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu Y, Pan N, Ma Y, et al. Inhaled sevoflurane may promote progression of amnestic mild cognitive impairment: a prospective, randomized parallel-group study. Am J Med Sci. 2013;345: 355–360. [DOI] [PubMed] [Google Scholar]
- [22].Sprung J, Roberts RO, Knopman DS, et al. Association of Mild Cognitive Impairment With Exposure to General Anesthesia for Surgical and Nonsurgical Procedures: A Population-Based Study. Mayo Clinic Proc. 2016;91: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aiello Bowles EJ, Larson EB, Pong RP, et al. Anesthesia Exposure and Risk of Dementia and Alzheimer’s Disease: A Prospective Study. J Am Geriatr Soc. 2016;64: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sprung J, Jankowski CJ, Roberts RO, et al. Anesthesia and incident dementia: a population-based, nested, case-control study. Mayo Clin Proc. 2013;88: 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim CT, Myung W, Lewis M, et al. Exposure to General Anesthesia and Risk of Dementia: A Nationwide Population-Based Cohort Study. J Alzheimers Dis. 1–11. [DOI] [PubMed] [Google Scholar]
- [26].Lee TA, Wolozin B, Weiss KB, Bednar MM. Assessment of the emergence of Alzheimer’s disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. J Alzheimers Dis. 2005;7: 319–324. [DOI] [PubMed] [Google Scholar]
- [27].Vanderweyde T, Bednar MM, Forman SA, Wolozin B. Iatrogenic risk factors for Alzheimer’s disease: surgery and anesthesia. J Alzheimers Dis. 2010;22 Suppl 3: 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim CT, Myung W, Lewis M, et al. Exposure to general anesthesia and risk of dementia: a nationwide population-based cohort study. J Alzheimers Dis. 2018;63: 395–405. [DOI] [PubMed] [Google Scholar]
- [29].Seitz DP, Gill SS, Gruneir A, et al. Effects of dementia on postoperative outcomes of older adults with hip fractures: a population-based study. J Am Med Direc Assoc. 2014;15: 334–341. [DOI] [PubMed] [Google Scholar]
- [30].Seitz DP, Gill SS, Bell CM, et al. Postoperative medical complications associated with anesthesia in older adults with dementia. J Am Geriatr Soc. 2014;62: 2102–2109. [DOI] [PubMed] [Google Scholar]
- [31].Richards J, Brown A, Homan C. The data quality study of the Canadian discharge abstract database. Proceedings of Statistics Canada Symposium Ottawa: Statistics Canada, 2001. https://www150.statcan.gc.ca/n1/en/pub/11-522-x/2001001/session16/6282-eng.pdf?st=nAGgqNXk [Google Scholar]
- [32].Jaakkimainen RL, Bronskill SE, Tierney MC, et al. Identification of physician-diagnosed Alzheimer’s disease and related dementias in population-based administrative data: a validation study using family physicians’ electronic medical records. J Alzheimers Dis. 2016;54: 337–349. [DOI] [PubMed] [Google Scholar]
- [33].Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11: 718–726. [DOI] [PubMed] [Google Scholar]
- [34].Austin PC, van Walraven C, Wodchis WP, Newman A, Anderson GM. Using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49: 932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173: 676–682. [DOI] [PubMed] [Google Scholar]
- [36].Schneeweiss S, Seeger JD, Maclure M, Wang PS, Avorn J, Glynn RJ. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154: 854–864. [DOI] [PubMed] [Google Scholar]
- [37].Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168: 508–513. [DOI] [PubMed] [Google Scholar]
- [38].Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stats Med. 2009;28: 3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Austin PC. Some methods of propensity‐score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biometrical J. 2009;51: 171–184. [DOI] [PubMed] [Google Scholar]
- [41].Lin DY, Wei LJ. The Robust Inference for the Cox Proportional Hazards Model. J Am Stat Assoc. 1989;84: 1074–1078. [Google Scholar]
- [42].Szychowski JM, Roth DL, Clay OJ, Mittelman MS. Patient death as a censoring event or competing risk event in models of nursing home placement. Stat Med. 2010;29: 371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Seitz DP, Shah PS, Herrmann N, Beyene J, Siddiqui N. Exposure to general anesthesia and risk of Alzheimer’s disease: a systematic review and meta-analysis. BMC Geriatr. 2011;11: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bilotta F, Qeva E, Matot I. Anesthesia and cognitive disorders: a systematic review of the clinical evidence. Expert Rev Neurother. 2016;16: 1311–1320. [DOI] [PubMed] [Google Scholar]
- [45].Krause BM, Sabia S, Manning HJ, Singh-Manoux A, Sanders RD. Association between major surgical admissions and the cognitive trajectory: 19 year follow-up of Whitehall II cohort study. BMJ. 2019;366: l4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Eriksson LI, Lundholm C, Narasimhalu K, et al. Hospitalization, surgery, and incident dementia. Alzheimers Dement. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. New Eng J Med. 2016;374: 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Berger M, Burke J, Eckenhoff R, Mathew J. Alzheimer’s disease, anesthesia, and surgery: a clinically focused review. J Cardiothorac Vasc Anesth. 2014;28: 1609–1623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.