Abstract
Steatohepatitic hepatocellular carcinoma (SH-HCC) is a variant of hepatocellular carcinoma (HCC) with established association with non-alcoholic steatohepatitis (NASH), while its association with alcoholic steatohepatitis (ASH) is unclear. We studied two cohorts of patients who underwent resection for HCC in the setting of steatohepatitis. In our Mount Sinai (New York) cohort, we found SH-HCC in 17/24 (71%) patients with NASH and in 14/19 (74%) patients with ASH, while SH-HCC was the predominant tumor morphology in 12/24 (50%) in the NASH group and 9/19 (47%) in the ASH group. Upon review, 12/19 patients diagnosed with ASH also had diabetes and/or a BMI >30. When these patients were removed, we still found similar rates of SH-HCC [6/7(86%) showed SH-HCC while SH-HCC was predominant in 3/7 (43%)]. Interestingly, glycogenated hepatocyte nuclei were seen in non-tumor liver in 4/7 (57%) of these cases. In our Japan cohort we also found similar rates of SH-HCC in NASH and ASH patients with HCC, 15/66 (23%) and 16/46 (35%), respectively. We determined molecular subclassification of tumors from the Japan cohort and found no difference in distribution of S1, S2 and S3 subclasses among the ASH and NASH groups, though among cases of SH-HCC, there was a trend toward association of ASH with S1 (p=0.054) and NASH with S3 (p=0.052). Our study shows that SH-HCC is common in both ASH and NASH and that both underlying liver diseases produce tumors with similar molecular profiles, though different pathways may underlie the development of SH-HCC in ASH vs. NASH.
Keywords: Hepatocellular carcinoma, alcoholic steatohepatitis, non-alcoholic steatohepatitis
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer death worldwide,1 with the majority of cases arising in the setting of chronic liver disease such as chronic hepatitis B virus (HBV) infection, chronic hepatitis C virus (HCV) infection, alcoholic steatohepatitis (ASH), and nonalcoholic steatohepatitis (NASH).2 With the current obesity epidemic3,4 and the introduction of direct acting antivirals for the treatment of HCV5,6 steatohepatitis is growing as a cause of HCC. The heterogeneity of HCC even within tumor nodules is well-recognized and includes numerous histologic subtypes such as clear cell, pseudoglandular, macrotrabecular/solid, and lymphoepithelioma-like.7 In 2010, Salomao et al. first described steatohepatitic HCC (SH-HCC) as a histologic subtype with features that resemble non-tumor liver in steatohepatitis and showed its association with steatosis and/or steatohepatitis.8 This was further confirmed in a subsequent study showing that 15/42 (36%) of patients with steatohepatitis (alone or in combination with viral hepatitis) showed predominant SH-HCC pattern, while only 1/76 (1.3%) of patients without evidence of steatohepatitis showed predominant SH-HCC pattern.9 Though subgroups were small, they also found that 4 of 6 patients with NASH alone and 4 of 6 patients with ASH alone showed predominant SH-HCC subtype, suggesting that both alcoholic and non-alcoholic causes of steatohepatitis may be associated with this pattern of HCC. Of note, no patients with both ASH and NASH were described in this study. Jain et al. also studied SH-HCC finding that 5 of the 7 tumors with viable tissue identified in the NASH cirrhosis group showed at least focal (>5%) SH-HCC, while only 1 of 5 tumors identified in the ASH cirrhosis group showed at least focal SH-HCC. This led the authors to suggest that SH-HCC is related to NASH, but not ASH.10 Further, Shibahara et al. found that a higher proportion of patients with conventional HCC (43%) reported alcohol intake than those with SH-HCC (30%),11 suggesting that alcoholic liver disease was not associated with SH-HCC. These studies show that the link between NASH and SH-HCC is well established, while the link between ASH and SH-HCC is less clear. We also suspect that NASH may complicate cases of ASH, which may make account for the differing results.
Much recent work has focused on the molecular aspects of HCC. Meta-analysis of gene expression profiles from multiple data sets has uncovered three molecular subclasses: S1 –characterized by WNT pathway activation, S2 – characterized by proliferation and MYC and AKT activation, and S3 – characterized by hepatocytic differentiation.12 These molecular subclasses were subsequently shown to correlate with tumor morphology, with SH-HCC showing enrichment for S1 subclass.7
The aims of this study were twofold: first to better define the association between ASH and SH-HCC using two separate cohorts of patients, and second, to analyze molecular subclass differences in ASH- vs. NASH-associated HCC. As the obesity epidemic continues to grow, the likelihood of patients with ASH complicated by NASH is increasing. This study attempts to limit the effect of possible overlap of NASH in ASH patients by careful chart review.
Materials and Methods
Mount Sinai, New York Cohort
The Department of Pathology database at Mount Sinai Hospital was queried from Jan 2008 to May 2018 for HCC resection or explant specimens with a diagnosis of NASH or ASH without concurrent liver disease (i.e. no concomitant HBV, HCV, etc.). The following clinical data were collected from the medical record: age at surgery, sex, procedure, history of type 2 diabetes mellitus, hemoglobin A1c, body mass index (BMI), history of hypertension, history of hyperlipidemia, history of statin use, history of alcohol use, serum alpha-fetoprotein (AFP), and history of prior locoregional therapy. Obesity was defined as a BMI of >30. The clinical diagnoses of NASH and ASH was derived from assessment made by the treating hepatologist and/or liver surgeon within the medical record.
The number, size, location of tumors and presence or absence of microscopic and macroscopic vascular invasion was recorded from review of pathology reports. All available slides were retrieved from the archives and reviewed by two liver pathologists (JQ and SCW). At least 1 H+E slide with viable tumor and 1 H+E slide with non-tumor liver tissue was required for inclusion into the study. Slides from multiple tumor nodules from the same patient were evaluated when available and each tumor nodule was evaluated separately. Tumor grade (Edmondson Steiner13) and histologic subtype (steatohepatitic, steatotic, trabecular, clear cell, pseudoglandular, macrotrabecular/solid, lymphoepithelioma-like) and proportion of each histologic subtype were recorded for each tumor nodule as described previously.7 SH-HCC pattern (at least focal) was defined when at least 5% of a tumor nodule showed at least 3 of the following “steatohepatitis-like” features, that is steatosis, hepatocyte ballooning, Mallory-Denk bodies, inflammation, or pericellular fibrosis, as defined by Salomao et al.8 (Figure 1). A tumor nodule was considered to be predominantly SH-HCC if at least 50% of the nodule showed at least 3 of these features. A patient with multiple tumor nodules was considered positive for the SH-HCC pattern if at least one of the tumor nodules showed at least 3 of the “steatohepatitis-like” features in at least 5% of that nodule, while a patient with multiple tumor nodules was considered to have predominant SH-HCC pattern if at least one of the tumor nodules showed at least 3 of these features involving at least 50% of that tumor nodule. The tumors were staged according to the 8th Edition of American Joint Committee on Cancer.14 Steatohepatitis was graded according to Brunt classification15 and staged according to the NASH Clinical Research Network.16 The presence or absence of glycogenated hepatocyte nuclei in the non-tumor liver was also recorded.
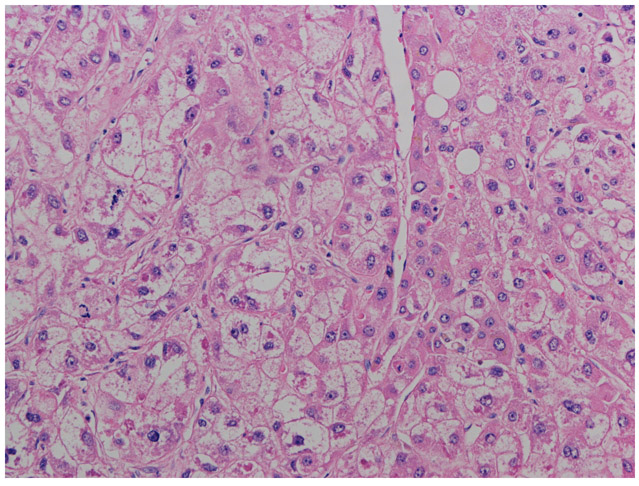
Figure 1.
Example of steatohepatitic hepatocellular carcinoma showing macrovesicular steatotosis, ballooning degeneration, pericellular fibrosis and Mallory-Denk hyalines (hematoxylin and eosin, 200X)
Japan Cohort
This cohort of cases consisted of retrospectively identified patients with a diagnosis of HCC in the setting of NASH or ASH without concurrent liver disease from Kumamoto University, Kumamoto, Japan (83 consecutive cases from 2003 to 2011) and Toranomon Hospital, Tokyo, Japan (20 cases with slides available 1988 to 2012). The patient’s age at surgery, sex, alcohol use, and serum AFP were recorded. The diagnosis of NASH was made according to practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology.17 Alcohol abuse was defined as lifetime alcohol consumption of greater than 500 kg and used to define ASH.
The number, size, and location of tumors, and presence or absence of microscopic and macroscopic vascular invasion was recorded from the medical record. One H+E slide with maximum tumor diameter was selected randomly for each case. Slides from Toranomon Hospital were reviewed by a liver pathologist (SCW) and liver surgeon (SN) with training in liver pathology and experience in evaluation of morphologic subtypes of HCC from prior collaboration.7 Slides from Kumamoto University were reviewed by SN with selected review by SCW. Nine tumors from Kumamoto University contained distinct morphologic areas within the slide, and these areas were evaluated for tumor grade and molecular profile separately. Tumor grade (Edmondson Steiner13) and presence or absence of steatohepatitic subtype as defined by Salamao et al.8 encompassing at least 30% of the tumor nodule on the reviewed slide were recorded. The tumors from the Japan cohort were classified as S1, S2 or S3 using a transcriptome based 30-gene panel composed of 10 signature genes for each of the 3 molecular subclassifications as described previously.7
Statistical analysis
Descriptive statistics (mean, standard deviation) were calculated. Two-tailed Student t test was used to compare means while two-tailed Fischer exact test was used for comparisons of categorical variables. A P value of < 0.05 was considered statistically significant.
Results
Mount Sinai Cohort
Sixty-two patients with HCC arising in the setting of steatohepatitis were identified by database search, 16 of which were excluded (4 - slides not available for review, 4 – no viable tumor on available slides, 3 - not ASH or NASH after further review, 2 – HCV infection status post treatment, 1 – cholangiocarcinoma, 1 - high grade dysplastic nodule, 1 – no further records available). Of the 46 patients included, 34 underwent liver transplantation and 12 underwent partial hepatectomy only (3 patients underwent partial hepatectomy prior to liver transplantation and 1 patient underwent 2 partial hepatectomy procedures). At the time of surgery, 19 patients were diagnosed as ASH clinically, while 24 patients were diagnosed as NASH clinically. In three cases, the distinction between ASH and NASH was not clear and these were classified as having overlapping features. When comparing the 19 patients with ASH to the 24 patients with NASH, the patients with ASH came to surgery at a younger age and showed a higher M:F ratio than the patients with NASH. There were no significant differences in procedure, history of prior locoregional therapy, fibrosis, tumor size, tumor number, tumor differentiation, presence of vascular invasion, or elevations in serum AFP levels. Importantly, patients with ASH and NASH showed similar incidence of tumors with at least focal (>5%) SH-HCC (74% vs 71%). When comparing patients with at least one tumor nodule showing predominantly SH-HCC pattern, there was also no difference between the ASH and NASH groups (47% vs 50%; see table 1).
Table 1.
Clinical and pathologic features of tumors arising in ASH and NASH (Mount Sinai cohort)
| ASH | NASH | ||
|---|---|---|---|
| Age, year, mean (SD) | 58 (7.2) | 69 (5.1) | p<0.0001 |
| M:F | 18:1 | 16:8 | p=0.055 |
| # tumors | 3.1 (SD 1.9) | 2.5 (SD 2.0) | p=0.36 |
| Tumor size, cm mean (SD) | 1.7 (SD 1.0) | 2.0 (SD 1.5) | p=0.13 |
| Tumor differentiation (per tumor nodule) | p=0.12 | ||
| Well | 12 (27%) | 8 (16%) | |
| Moderate | 31 (70%) | 35 (71%) | |
| Poor | 1 (3%) | 6 (12%) | |
| Microscopic vascular invasion | 10/19 (53%) | 16/24 (67%) | p=0.53 |
| Macroscopic vascular invasion | 1/19 (5%) | 1/24 (4%) | p=1 |
| AFP > 9 | 10/18 (56%) | 9/22 (41%) | p=0.53 |
| SH-HCC | 14/19 (74%) | 17/24 (71%) | p=1 |
| Predominant SH-HCC | 9/19 (47%) | 12/24 (50%) | p=1 |
| Glycogenated hepatocyte nuclei | 7/19 (37%) | 15/24 (63%) | p=0.13 |
Upon further chart review, many of the cases diagnosed as ASH at time of surgery also showed clinical features to suggest a possible component of NASH. Of the 19 patients diagnosed as having ASH, 9 (47%) had a BMI >30, 6 (32%) had type 2 diabetes, 7 (37%) had hypertension, and 7 (37%) had a lipid disorder, see table 2. Twelve patients that were originally diagnosed as ASH had a BMI >30 and/or at least 2 of the following (type 2 diabetes, hypertension, hyperlipidemia) and were re-classified as having overlapping features. Additionally, one patient initially classified as NASH had significant history of alcohol use and was also re-classified as overlapping features. After reclassification, this left 7 cases that were designated as revised ASH (rASH), 23 patients that were designated as revised NASH (rNASH) and 16 patients that showed overlapping features of ASH and NASH (overlap). Of the 7 patients classified as rASH, none had BMI>30 (no BMI was recorded for one patient, but this patient was not noted to be clinically obese), one patient had hypertension and one patient had hyperlipidemia; none of the patients had type 2 diabetes. The patient with hyperlipidemia was also the one without a recorded BMI. When comparing the 7 patients with rASH to the 23 patients with rNASH, the patients with rASH came to surgery at a younger age, but there was no significant difference in M:F ratio. There were no significant differences in procedure, history of prior locoregional therapy, fibrosis, tumor number, tumor differentiation, presence of vascular invasion, or elevation in serum AFP levels, though there was a trend toward smaller tumors in rASH patients (1.5cm vs 2.0 cm; p=0.07). Again, patients with rASH and rNASH showed similar incidence of at least focal SH-HCC (86% vs 70%). When comparing patients with at least one tumor nodule showing predominantly SH-HCC pattern, there was also no significant difference between rASH and rNASH patients (57% vs 65%), see table 3. Interestingly, glycogenated nuclei were identified in 4 of 7 patients with rASH, none of which had diabetes.
Table 2.
Clinical features of patients diagnosed as alcoholic steatohepatitis at time of surgery (Mount Sinai cohort)
| Case | BMI | Diabetes | Hypertension | Lipid disorder |
|---|---|---|---|---|
| 1 | 24.9 | |||
| 2 | 28.9 | |||
| 3 | 29.3 | |||
| 4 | 28.8 | |||
| 5 | 28.1 | |||
| 6 | 29.2 | |||
| 7 | ND | |||
| 8 | 33.6 | |||
| 9 | 30.4 | |||
| 10 | 37.7 | |||
| 11 | 31.5 | |||
| 12 | 29.7 | |||
| 13 | 22.5 | |||
| 14 | 23.1 | |||
| 15 | 37.2 | |||
| 16 | 32.9 | |||
| 17 | 30.1 | |||
| 18 | 32.7 | |||
| 19 | 36.2 | |||
| 9/18 (50%) | 6/19 (32%) | 7/19 (37%) | 7/19 (37%) | |
Note: BMI = body mass index, ND = no data. Shaded squares = BMI>30, filled squares = positive history of diabetes, hypertension and/or lipid disorder. Cases 1-7 cases were re-classified as revised alcoholic steatohepatitis while cases 8-19 were re-classified as having overlapping features of alcoholic steatohepatitis and nonalcoholic steatohepatitis.
Table 3.
Clinical and pathologic features of tumors arising in rASH, rNASH, and overlap groups (Mount Sinai cohort)
| rASH | rNASH | rASH v. rNASH |
overlap | |
|---|---|---|---|---|
| Age, y mean (SD) | 59 (9.0) | 69 (5.2) | p=0.0014 | 60 (8.2) |
| M:F ratio | 7:0 | 15:8 | p=0.14 | 15:1 |
| # tumorsmean (SD) | 3.9 ( 1.6) | 2.7 (2.0) | p=0.16 | 2.2 (1.8) |
| Tumor size, cmmean (SD) | 1.5 (0.75) | 2.0 (1.5) | p=0.073 | 2.0 (1.1) |
| Tumor differentiation(per tumor nodule) | p=0.24 | |||
| Well | 1 (4.5%) | 8 (17%) | 14 (52%) | |
| Moderate | 20 (91%) | 34 (71%) | 13 (48%) | |
| Poor | 1 (4.5%) | 6 (12%) | 0 (0%) | |
| Microscopic vascular invasion | 4/7 (57%) | 15/23 (65%) | p=1 | 8/16 (50%) |
| Macroscopic vascular invasion | 1/7 (14%) | 1/23 (4.3%) | p=0.418 | 0/16 (0%) |
| AFP > 9 | 4/6 (67%) | 9/21 (43%) | p=0.39 | 8/16 (50%) |
| Steatohepatic HCC | 6/7 (86%) | 16/23 (70%) | p=1 | 11/16 (69%) |
| Steatohepatic HCC predominant | 3/7 (43%) | 12/23 (52%) | p=1 | 7/16 (43%) |
| Glycogenated hepatocyte nuclei | 4/7 (57%) | 15/23 (65%) | p=1 | 6/16 (38%) |
Japan Cohort
Overall, 103 patients who underwent surgery for HCC arising in the setting of steatohepatitis were included, 45 with ASH and 58 with NASH. Histologic review revealed 9 cases that contained 2 distinct morphologic regions within one slide, with each component comprising at least 30% of the slide. These regions were evaluated separately for tumor grade and molecular subclassification leading to a total of 112 tumor regions assessed (46 from ASH patients and 66 from NASH patients). Of these 9 tumors showing heterogeneity, one from the NASH group contained a SH-HCC region and a non-SH-HCC region (this patient was counted as having SH-HCC), while the other 8 heterogeneous tumors (1 ASH patient and 7 NASH patients) contained 2 non-SH-HCC regions.
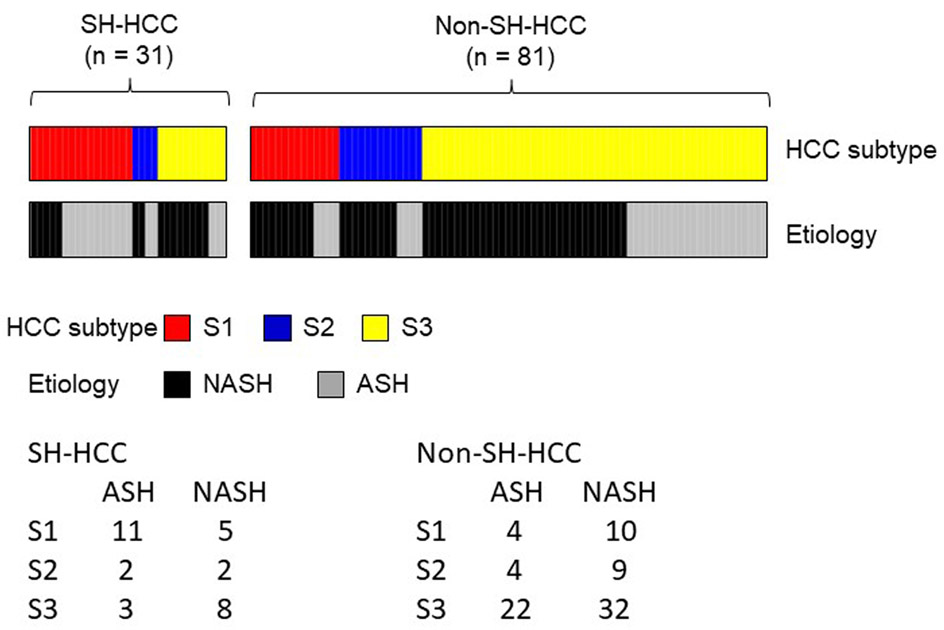
There was no significant difference in proportion of cases with SH-HCC (defined as comprising at least 30% of the tumor nodule on reviewed slide) between the ASH and NASH groups (16/45, 36% in the ASH group vs. 15/58, 26% in the NASH group, p = 0.39; see Table 4). Further, the diagnosis of SH-HCC did not distinguish between ASH and NASH (OR 0.55, 95%CI 0.24-1.27, p=0.163; see Table 5). There was no significant difference in distribution of molecular subclasses of the tumors between the ASH and NASH groups, p = 0.49, see Table 4 and Figure 2); however, there was an enrichment in S1 with associated reduction in S3 subclasses in SH-HCC cases compared with non-SH-HCC cases (P=0.0012; see Table 5). When broken down further, among cases of SH-HCC there was a trend toward association of ASH with S1 subclass and NASH with S3 subclass; however, this did not reach statistical significance (OR 0.23, 95%CI 0.05-1.03, p=0.054; and OR 4.95, 95%CI 0.97-20.0, p=0.052, respectively; see Figure 2).
Table 4.
Clinical, pathologic and molecular features of tumors arising in ASH and NASH (Japan cohort).
| ASH | NASH | ||
|---|---|---|---|
| N | 45 | 58 | |
| Age, y mean (SD) | 66.6 (7.7) | 70.2 (9.7) | p = 0.01 |
| M:F ratio | 45:0 | 45:13 | p < 0.001 |
| # tumors mean (SD) | 1.2 (0.7) | 1.3 (1.0) | p = 0.5 |
| Tumor size, cm Mean (SD) | 3.5 (2.1) | 5.2 (3.3) | p = 0.001 |
| Tumor differentiation (per tumor region) | p = 0.67 | ||
| Well | 4 (8.7%) | 6 (9.1%) | |
| Moderate | 38 (83%) | 57 (86%) | |
| Poor | 4 (8.7%) | 3 (4.5%) | |
| Microscopic vascular invasion | 8 (17.8%) | 5 (8.6%) | p = 0.14 |
| Macroscopic vascular invasion | 4 (8.9%) | 0 (0%) | p = 0.03 |
| AFP > 9 | 14 (31%) | 21 (36%) | p = 0.68 |
| Steatohepatitic HCC | 16 (36%) | 15 (26%) | p = 0.39 |
| Molecular subtype (per tumor region) | p = 0.49 | ||
| S1 | 15 (33%) | 15 (23%) | |
| S2 | 6 (13%) | 11 (17%) | |
| S3 | 25 (54%) | 40 (60%) |
Table 5.
Distribution of molecular subclassifications in SH-HCC and non-SH-HCC (Japan cohort)
| SH-HCC | Non-SH-HCC | P=0.0012 | |
|---|---|---|---|
| S1 | 16 (43%) | 14 (17%) | |
| S2 | 4 (13%) | 13 (16%) | |
| S3 | 11 (35%) | 54 (67%) |
Figure 2.
Distribution of molecular subclassifications in tumors arising in ASH vs NASH (Japan cohort). The presence of SH-HCC did not distinguish between ASH and NASH (OR 0.55, 95%CI 0.24-1.27, p=0.163). Among cases with SH-HCC, there was a trend toward association of ASH with S1 subclass significance (OR 0.23, 95%CI 0.05-1.03, p=0.054) and NASH with S3 subclass (OR 4.95, 95%CI 0.97-20.0, p=0.052).
Discussion
SH-HCC is a variant of HCC that shows histologic features resembling those seen in non-tumor liver with steatohepatitis and a strong association with NASH has been reported.8,9 Though prognosis of SH-HCC does not appear to differ significantly from conventional HCC,9,18,19 this variant can pose diagnostic difficulties, especially with biopsy material.20 Early work has shown that SH-HCC shows enrichment for the S1 molecular subclass,7 though molecular differences related to underlying liver disease in development of SH-HCC are not clear.
This paper reports the results of two studies assessing the steatohepatitic variant of HCC in the setting of alcoholic- and non-alcoholic steatohepatitis. The Mount Sinai cohort study was designed to evaluate the incidence of SH-HCC in ASH vs. NASH. All available slides were evaluated and medical records were extensively reviewed to minimize overlap of NASH with ASH in this group. The Japan cohort study was designed to assess for molecular differences between tumors arising in the setting of ASH and NASH and among morphologic subtypes, specifically SH-HCC here. This study had more limited clinical information and was based on evaluation of a single tumor slide per case. While the histologic features described by Salamao et al. for the definition of SH-HCC were used in both groups,8 different cut-off values were used. The Mount Sinai cohort used two previously reported cut-off values of at least 5%10 and at least 50%8 to determine whether these differences in cut-offs may have led to the different results reported in these studies. The Japan cohort used a cut-off of at least 30% that had been used in a prior collaboration correlating morphologic subtypes with molecular subclassification.7 Though these studies differ significantly in design, we show that SH-HCC occurs at similar rates in the setting of both alcoholic- and nonalcoholic steatohepatitis using different percent cut-offs and across both cohorts. In the Mount Sinai (New York) cohort we found that 74% of patients with underlying ASH showed at least focal SH-HCC, compared with 71% of patients with underlying NASH, while SH-HCC was the predominant morphology in 47% of patients with ASH compared with 50% of patients with NASH. In our cohort from Japan, we also found similar rates of SH-HCC in patients with underlying ASH (36%) and. NASH (26%).
The histologic features used in defining SH-HCC (steatosis, hepatocyte ballooning, Mallory-Denk bodies, inflammation, and pericellular fibrosis) established by Salamao et al.8 have been widely used, though different thresholds have been set to define SH-HCC, both in the literature and within our study. In our New York cohort, we required that tumors show at least three of the above five features and we examined two thresholds: at least focal SH-HCC (at least 5%) and predominant SH-HCC (at least 50%). In their study, Salamao et al. required three of the features to be present and involve at least 50% of the tumor on representative sections. They classified 4 of 6 (67%) cases of HCC arising in the setting of NASH and 4 of 6 (67%) cases arising in the setting of ASH as SH-HCC,9 comparable to the 47% and 50% rates we saw when using the same criteria. In their study, Jain et al. defined SH-HCC as “the unequivocal presence of steatosis, Mallory hyaline, hydropic change, inflammation, and variable fibrosis in some parts of the tumor… but always in more than 5% of its area.” They found in their cohort from India that 5 of 7 (71%) HCCs arising in the setting of NASH showed SH-HCC while only 1 of 5 (20%) HCCs arising in the setting of ASH showed HCC. Though their numbers were small, the authors suggested that NASH, but not ASH was associated with SH-HCC.10 In our New York cohort comprising 24 patients with NASH and 19 patients with ASH, we also analyzed the cases with the 5% cutoff used by Jain et al., but found no difference in the rate of SH-HCC in NASH vs. ASH (71% and 74%, respectively). In their study of a Japanese cohort showing that a higher proportion of patients with conventional HCC reported alcohol intake than those with SH-HCC, Shibahara et al. required that the tumor demonstrate 4 of these 5 criteria, either focally or predominantly.11 The Japanese cohort in our study used a threshold of at least 30% to define SH-HCC. While there was no significant difference in rates of SH-HCC between the ASH and NASH groups (p = 0.20), the rates appear to be lower in our Japan cohort than those seen in our New York cohort and that of Salamao et al (from Columbia University College of Physicians and Surgeons, in New York). It is unclear whether these differences in observed rates are due to differences in the patient population or study design.
One complicating factor in our analysis is the explosion of the obesity epidemic, especially within the United States. Singh et al. showed increasing rates of central obesity (50% to 66%; p = 0.002) and metabolic syndrome (26% to 37%; p = 0.044) in patients with alcoholic liver disease by comparing a historic cohort of patients with alcoholic liver disease from 1999-2001 to a contemporary cohort from 2009-2014.21 We now recognize that steatohepatitis can accelerate fibrosis progression in chronic liver diseases such as viral hepatitis22,23 and autoimmune hepatitis.24 In patients with alcoholic liver disease, obesity is associated with an increased risk of hepatocellular carcinoma,25 and type 2 diabetes is associated with increased risk of cirrhosis.26 Since the histology of the liver in ASH and NASH in most cases is indistinguishable and that the diagnosis of ASH largely relies on patient reported alcohol consumption, overlap of NASH and ASH can be difficult to establish. In our study, quantification of alcohol consumption was not well documented in the medical record in our Mount Sinai cohort, so we relied on the clinical impression of the hepatologist and/or liver surgeon for the initial diagnosis of ASH. In many published reports, the clinical finding of significant alcohol consumption is sufficient to exclude a patient from a NASH group and/or place them into an ASH group, though in some cases the patient may also have many of the risk factors for the development of NASH. We attempted to address this issue by diving deeper into the patients’ medical records in our Mount Sinai Cohort to define a group of patients with ASH with few or no risk factors for NASH (such as obesity, type 2 diabetes mellitus, hypertension, hyperlipidemia). After examining our original cohort of 19 patients diagnosed with ASH, we found that 12 had a BMI > 30 and/or a diagnosis of type 2 diabetes mellitus. When these patients were removed from the ASH cohort, we still found comparable rates of SH-HCC in the revised ASH and NASH cohorts (86% rASH vs. 70% rNASH with at least focal SH-HCC, 43% rASH vs 52% rNASH with predominant SH-HCC). These findings suggest that the rates of SH-HCC are truly similar between the ASH and NASH cohorts, and are not the result of an undiagnosed component of NASH.
Glycogenated hepatocyte nuclei can be seen in a number of diseases states, but their presence is commonly associated with diabetes mellitus.27,28,29 Interestingly, we found glycogenated hepatocyte nuclei in over a third of patients originally classified as having ASH in our Mount Sinai cohort. Further, when patients with type 2 diabetes and obesity were excluded from our ASH group, 4 of the 7 patients (57%) in this revised ASH group also showed glycogenated hepatocyte nuclei, similar to the 65% seen in the revised NASH group. This may indicate a subclinical glucose intolerance in these ASH patients, or that glycogenated hepatocyte nuclei may be less specific for diabetes/glucose intolerance in the setting of advanced ASH.
We examined the molecular subclasses of HCC in the Japan study cohort of 103 patients with either ASH or NASH using an FDA-approved platform.7 As shown previously, we found an enrichment in S1 subclass in SH-HCC compared with non-SH-HCC;7 however, we found no difference in distribution of S1, S2 and S3 subclasses among the ASH and NASH groups, suggesting that both disease processes can lead to tumors with heterogeneous molecular profiles. When limiting our examination to the 31 cases of SH-HCC, there was a trend toward association of ASH with S1 subclass (p=0.054) and NASH with S3 subclass (p=0.052). The implications of this are not clear, but if this finding is confirmed in larger studies it could indicate a more complex relationship between underlying liver disease, molecular pathways and tumor morphology.
In conclusion, we show that both ASH and NASH are associated with the steatohepatitic variant of HCC. This finding held true across cohorts in both the United States and Japan and using various diagnostic cutoffs previously described in the literature. Through careful chart review we attempted to minimize the possibility of NASH complicating ASH by excluding patients with obesity or type 2 diabetes from the ASH group. Using these revised cohorts, we still showed similar rates of SH-HCC among the two groups. These findings suggest that steatohepatitis itself, rather than the underlying liver disease, leads to SH-HCC. This was further supported by molecular studies which showed no difference in molecular subclass distribution in HCC developing in the setting of ASH or NASH. Future studies further evaluating relationships between molecular subtypes and underlying liver disease in specific morphologic variants of HCC are warranted.
Acknowledgments
Yujin Hoshida, MD, PhD funding sources
- NIH/NIDDK (DK099558)
- European Research Council (ERC-2014-AdG-671231)
- Cancer Prevention and Research Institute of Texas (RR180016)
- NIH/NCI (CA233794)
- NIH/NCI (CA226052)
References:
- 1.World Health Organization, News room, fact sheets, cancer. In: WHO; [Internet]. 1 February 2018. [cited 12 Sep 2018]. http://www.who.int/news-room/fact-sheets/detail/cancer. [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–30. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357 [DOI] [PubMed] [Google Scholar]
- 5.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma J Hepatol. 2018;68: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanwal F, Kramer J, Asch SM, et al. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents Gastroenterology. 2017;153: 996–1005. [DOI] [PubMed] [Google Scholar]
- 7.Tan PS, Nakagawa S, Goossens N, et al. Clinicopathological indices to predict hepatocellular carcinoma molecular classification. Liver Int. 2016;36:108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salomao M, Yu WM, Brown RS Jr, et al. Steatohepatitic hepatocellular carcinoma (SH-HCC): a distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am J Surg Pathol. 2010;34:1630–6. [DOI] [PubMed] [Google Scholar]
- 9.Salomao M, Remotti H, Vaughan R, et al. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum Pathol. 2012;43:737–46. [DOI] [PubMed] [Google Scholar]
- 10.Jain D, Nayak NC, Saigal S. Hepatocellular carcinoma in nonalcoholic fatty liver cirrhosis and alcoholic cirrhosis: risk factor analysis in liver transplant recipients. Eur J Gastroenterol Hepatol. 2012;24:840–8. [DOI] [PubMed] [Google Scholar]
- 11.Shibahara J, Ando S, Sakamoto Y, et al. Hepatocellular carcinoma with steatohepatitic features: a clinicopathological study of Japanese patients. Histopathology. 2014;64:951–62. [DOI] [PubMed] [Google Scholar]
- 12.Hoshida Y, Nijman SM, Kobayashi M, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. [DOI] [PubMed] [Google Scholar]
- 14.Amin MB, Edge SB, Greene FL, et al. , editors. AJCC cancer staging manual, 8th edn. New York, NY: Springer; 2017. [Google Scholar]
- 15.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, et al. , Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 17.Chalasani N, Younossi Z, Lavine JE, et al. ; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. [DOI] [PubMed] [Google Scholar]
- 18.Lee JS, Yoo JE, Kim H, et al. Tumor stroma with senescence-associated secretory phenotype in steatohepatitic hepatocellular carcinoma. PLoS One. 2017;12:e0171922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibahara J, Ando S, Sakamoto Y, et al. Hepatocellular carcinoma with steatohepatitic features: a clinicopathological study of Japanese patients. Histopathology. 2014;64(7):951–962. [DOI] [PubMed] [Google Scholar]
- 20.Olofson AM, Gonzalo DH, Chang M, et al. Steatohepatitic Variant of Hepatocellular Carcinoma: A Focused Review. Gastroenterology Res. 2018;11:391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh A, Amin H, Garg R, et al. Increased prevalence of obesity and metabolic syndrome in patients with alcoholic fatty liver disease. Dig Dis Sci. 2020. January 24. doi: 10.1007/s10620-020-06056-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Suliman I, Abdelgelil N, Kassamali F, et al. The effects of hepatic steatosis on the natural history of HBV infection. Clin Liver Dis. 2019;23:433–450. [DOI] [PubMed] [Google Scholar]
- 23.Adinolfi LE, Rinaldi L, Guerrera B, et al. NAFLD and NASH in HCV infection: Prevalence and significance in hepatic and extrahepatic manifestations. Int J Mol Sci. 2016. 25;17:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Luca-Johnson J, Wangensteen KJ, Hanson J, et al. Natural history of patients presenting with autoimmune hepatitis and coincident nonalcoholic fatty liver disease. Dig Dis Sci. 2016;61:2710–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair S, Mason A, Eason J, et al. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36:150–5. [DOI] [PubMed] [Google Scholar]
- 26.Raff EJ, Kakati D, Bloomer JR, et al. Diabetes mellitus predicts occurrence of cirrhosis and hepatocellular cancer in alcoholic liver and non-alcoholic fatty liver diseases. J Clin Transl Hepatol. 2015;3: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagore N, Scheuer PJ. The pathology of diabetic hepatitis. J Pathol. 1988;156:155–60. [DOI] [PubMed] [Google Scholar]
- 28.Silverman JF, O'Brien KF, Long S, et al. Liver pathology in morbidly obese patients with and without diabetes. Am J Gastroenterol. 1990;85:1349–55. [PubMed] [Google Scholar]
- 29.Abraham S, Furth EE. Receiver operating characteristic analysis of glycogenated nuclei in liver biopsy specimens: quantitative evaluation of their relationship with diabetes and obesity. Hum Pathol. 1994;25:1063–8. [DOI] [PubMed] [Google Scholar]