Abstract
Cells of the oligodendrocyte (OLG) lineage engage in highly motile behaviors that are crucial for effective central nervous system (CNS) myelination. These behaviors include the guided migration of OLG progenitor cells (OPCs), the surveying of local environments by cellular processes extending from differentiating and pre-myelinating OLGs, and during the process of active myelin wrapping, the forward movement of the leading edge of the myelin sheath’s inner tongue along the axon. Almost all of these motile behaviors are driven by actin cytoskeletal dynamics initiated within a lamellipodial structure that is located at the tip of cellular OLG/OPC processes and is structurally as well as functionally similar to the neuronal growth cone. Accordingly, coordinated stoichiometries of actin filament (F-actin) assembly and disassembly at these OLG/OPC growth cones have been implicated in driving process outgrowth and guidance, and the initiation of myelination. Nonetheless, the functional importance of the OLG/OPC growth cone still remains to be fully understood, and, as a unique aspect of actin cytoskeletal dynamics, F-actin depolymerization and disassembly start to predominate at the transition from myelination initiation to myelin wrapping. This review provides an overview of the current knowledge about OLG/OPC growth cones, and it proposes a model in which actin cytoskeletal dynamics in OLG/OPC growth cones are a main driver for morphological transformations and motile behaviors. Moreover, these activities, at least at the later stages of OLG maturation, may be regulated independently from the transcriptional gene expression changes typically associated with CNS myelination.
Keywords: Oligodendrocyte, growth cone, actin cytoskeleton, migration, myelination
GRAPHICAL ABSTRACT
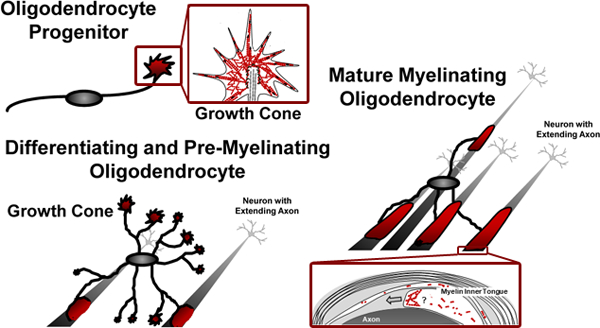
1 -. INTRODUCTION
Oligodendrocytes (OLGs) are specialized cells of the central nervous system (CNS) that generate the axon enwrapping myelin sheath, which enables rapid and efficient saltatory conduction and provides metabolic axonal support (Stadelmann et al., 2019). During development, cells of the OLG lineage originate as bipolar and migratory OLG progenitor cells (OPCs) within distinct regions of the CNS (Miller, 2005; Naruse et al., 2017; Ono et al., 2018; Richardson et al., 2006). From their points of origin, OPCs migrate throughout the CNS along pathways that are guided by various extracellular cues including the endothelial surface of blood vessels (Jarjour & Kennedy, 2004; Miller et al., 1997; Small et al., 1987; Tsai et al., 2016). Once OPCs reach their final destination they differentiate and ultimately myelinate axonal segments. This process of OLG differentiation occurs in a stepwise progression that is characterized by gene expression patterns, which are controlled by both intrinsic and extrinsic signals, and ultimately include typical myelin genes such as proteolipid protein (Plp1) and myelin basic protein (Mbp) (Elbaz & Popko, 2019; Emery & Lu, 2015; Gregath & Lu, 2018; Liu et al., 2016; Pol et al., 2017; Wheeler & Fuss, 2016). In addition, OLG differentiation features extensive changes in morphology as OPCs mature first into differentiating, pre-myelinating OLGs, which extend a complex and expanded process network, and then into mature OLGs generating a fully functional myelin sheath (Bauer et al., 2009; Michalski & Kothary, 2015; Pfeiffer et al., 1993).
Within the past few years, it has been recognized that myelination likely follows a basic intrinsic program that functions in the absence of extrinsic molecular instruction. This intrinsic program is thought to be modulated by a so-called ‘adaptive program’ initiated by extrinsic molecular signals and axonal electrical activity (Bechler et al., 2015; Bechler et al., 2018; de Faria et al., 2018; Foster et al., 2019; Gibson et al., 2018). Thus, the regulation of myelination via the action of extracellular factors, here referred to as ‘extrinsically modulated myelination’, could be considered a form of ‘adaptive’ myelination. Remarkably, such extrinsically modulated myelination has been reported to operate on a neuron/axon individual basis (Hines et al., 2015; Koudelka et al., 2016; Lundgaard et al., 2013; Mitew et al., 2018), hence requiring each of the multiple processes of differentiating OLGs to respond to a signal in an independent fashion. This idea is further supported by histological studies describing the presence of ‘transitional’ OLGs, which retain most of their radial processes but have few single processes leading each to a newly forming myelin sheath (Butt et al., 1997; Hardy & Friedrich, 1996). Such individualized responses require specialized sensing features at the tips of outgrowing processes. In this regard, the tips of the leading processes of migratory OPCs have been shown to feature structural and functional similarities to neuronal growth cones (Jarjour & Kennedy, 2004; Schmidt et al., 1997; Simpson & Armstrong, 1999), and similar observations have been made for the tips of processes extending from differentiating OLGs (Asou et al., 1995; Fox et al., 2006; Hardy & Friedrich, 1996; Kachar et al., 1986; Michalski et al., 2016; Rumsby et al., 2003; Song et al., 2001; Zuchero et al., 2015). Thus, OLG/OPC growth cones emerge as a characteristic feature of OLG lineage cells.
In neurons, growth cone behavior has been well-established to be driven to a large extent by the dynamic features of the actin cytoskeleton (Dent et al., 2011; Gomez & Letourneau, 2014; Vitriol & Zheng, 2012). Likewise, OLG/OPC growth cones and their actin cytoskeletal dynamics are thought to play a key role in guiding the morphological changes associated with each of the stages of the OLG lineage. This idea is consistent with the critical functions ascribed to the actin cytoskeleton in controlling overall OLG morphology (Brown & Macklin, 2019; Domingues et al., 2018; Seixas et al., 2019). Notably, actin cytoskeletal mechanisms do not function in isolation and there is often crosstalk between actin- and microtubule-based cytoskeletal activities. With regard to the OLG/OPC growth cone, however, the microtubule-rich central region has up to now received extremely little attention. The reader is referred to previously published review articles that discuss the increasingly recognized relevance of the microtubule-rich central region to motile growth cone behaviors in neurons (Cammarata et al., 2016; Kahn & Baas, 2016), as well as to those that address more general roles of the microtubule cytoskeleton in OLG lineage cells (Bauer et al., 2009; Richter-Landsberg, 2001, 2008, 2016). This review focuses on the actin cytoskeletal aspects of the OLG/OPC growth cone by providing an overview of its role in driving dynamic morphological changes at the different stages of the OLG lineage. More specifically, this review presents an introduction into the molecular basis of how actin cytoskeletal dynamics drive growth cone behaviors as they have been revealed from studies done in neurons and are emerging as critical effector mechanisms in shaping the morphologies and motile activities of OLGs and OPCs. Further, evidence is discussed in support of a model in which the regulation of cytoskeletal changes, at least at the later stages of OLG maturation, is uncoupled from the transcriptional control of gene expression traditionally associated with CNS myelination.
2 -. THE CRITICAL ROLE OF THE GROWTH CONE’S ACTIN CYTOSKELETON IN DRIVING DYNAMIC CHANGES IN MORPHOLOGY: A BRIEF INTRODUCTION ON THE NEURONAL GROWTH CONE
The unique structural feature termed the growth cone was first described by Santiago Ramón y Cajal as a ‘cone-like lump with a peripheral base’ decorated by triangular or short thorny processes and located at the distal tip of advancing axons (Ramón y Cajal, 1890a, 1890b; Tamariz & Varela-Echavarria, 2015). This structure is now known to function as a highly motile signaling center that surveys and integrates information from the extracellular environment to guide neuronal migration as well as axon outgrowth and pathfinding (Goodman, 1996; Marin et al., 2010; Tessier-Lavigne & Goodman, 1996; Vitriol & Zheng, 2012). Notably, it is becoming increasingly apparent that growth cones are also present at the distal tips of OPCs and OLGs, where they function according to principles similar to those described for neuronal growth cones. Thus, for a better understanding a brief introduction into the basic mechanisms of actin cytoskeletal dynamics with a focus on their specific roles in neuronal growth cones is presented prior to discussing their emerging functions in driving the behaviors of growth cones and associated changes in OPC motility and OLG morphology.
In general, dynamic actin cytoskeletal changes are defined by a precisely controlled interplay between the assembly of double helical actin filaments (F-actin), through polymerization of globular actin (G-actin), and their disassembly, mediated by F-actin severing and depolymerizing factors. Within this framework, well-organized F-actin growth is enabled by the highly polarized structure of F-actin, which is characterized by a ‘barbed’ or ‘plus’ end and a ‘pointed’ or ‘minus’ end (Holmes et al., 1990). Due to the kinetics for actin polymerization, G-actin monomer addition occurs predominantly at barbed ends, while disassembly takes place mainly at pointed ends (Fujiwara et al., 2018; Shekhar et al., 2016). Based on this feature, a fundamental concept of actin cytoskeletal dynamics and F-actin turnover lies in a process referred to as treadmilling (Figure 1) (Pantaloni et al., 2001; Wegner, 1976). During treadmilling, filament disassembly by net loss of ADP-bound actin monomers at the pointed end is balanced by net barbed end growth through polymerization of ATP–bound actin monomers and subsequent hydrolysis of ATP. As a result, F-actin filaments coexist with actin monomers at a ‘steady-state’ but seemingly move in one direction, leading to, for example, outward expansion of the cell membrane and forward movement of cellular protrusions (Carlier & Shekhar, 2017; Narita, 2011; Neuhaus et al., 1983).
Figure 1. Diagram illustrating actin dynamics during treadmilling and forward protrusion of cellular processes.
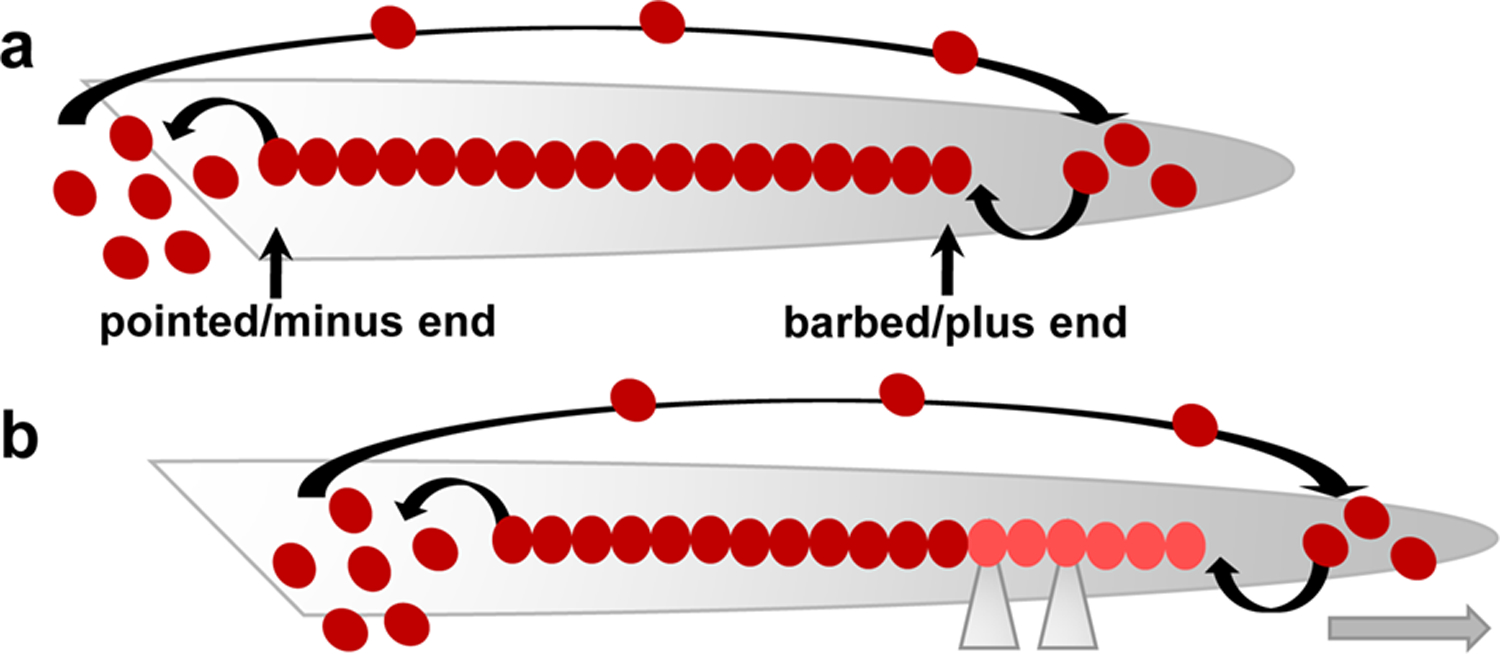
(a) F-actin filament growth occurs at the barbed/plus end and is balanced by net filament disassembly at the pointed/minus end and by recycling of G-actin subunits back to the barbed/plus end (large arrow at the top). Thus, F-actin filaments coexist with G-actin monomers at a ‘steady state’. (b) When the actin filament is anchored to a growth promoting (permissive) substrate via the action of adhesion complexes (grey triangles), actin retrograde flow (from the barbed/plus end to the pointed/minus end; not shown) is reduced, and the F-actin filament seemingly moves in one direction, leading to forward movement of cellular protrusions (grey arrow). Actin monomers are depicted as dark red filled circles; newly polymerized F-actin is shown in light red
In all cells, the assembly and disassembly of actin filaments, and their organization into higher order actin networks, is regulated by a plethora of actin-binding proteins, the activities and binding of which are controlled by various signaling pathways (Pollard & Cooper, 2009; Winder & Ayscough, 2005), and actin post-translational modifications (Varland et al., 2019). In this context, actin polymerization is energetically unfavorable until there is a nucleus of three associating monomers. Thus, de novo actin polymerization is initiated by actin nucleators such as the Arp2/3 complex, which is best known for its function in driving the formation of branched actin networks within sheet-like lamellipodial protrusions located at the leading edge of cells or the advancing growth cone (Bailly et al., 2001; Korobova & Svitkina, 2008; Mullins et al., 2018; Pollard, 2007). The nucleating function of Arp2/3 is further enhanced or activated by nucleation promoting factors such as Wiskott-Aldrich syndrome protein (WASP), Neural WASP (N-WASP), and members of the WASP-family verprolin-homologous protein (WAVE) family (Kurisu & Takenawa, 2009; Machesky & Insall, 1998; Takenawa & Suetsugu, 2007). The actin nucleating formins, on the other hand, have been primarily implicated in driving the formation of parallel-bundled actin filaments found in slender rod-like protrusions known as filopodia (Breitsprecher & Goode, 2013; Courtemanche, 2018; Pollard, 2007). Upon nucleation, F-actin filament growth is mediated by the actin polymerization machinery, which includes profilin, an actin-binding protein initially proposed to sequester G-actin monomers but now thought to promote the assembly of G-actin into F-actin (Alkam et al., 2017; Kang et al., 1999). Counterbalancing of actin polymerization is achieved by the actin disassembly machinery, the best characterized components of which are the actin-depolymerizing factor (ADF) and members of the cofilin family (Van Troys et al., 2008). These proteins enable actin turnover by binding to ADP-F-actin and promoting the dissociation of ADP-actin from the pointed ends of F-actin filaments (Kanellos & Frame, 2016). Higher order F-actin structures, which are responsible for the overall appearance of cells or particular sub-cellular structures such as dendritic spines, are generally shaped through F-actin bundling and F-actin crosslinking mechanisms, involving proteins such as α-actinin or CaMKIIβ (Lin & Redmond, 2009; Okamoto et al., 2009; Winder & Ayscough, 2005).
For growth cones of migratory neurons and outgrowing axons, it has been well-established that sensing and responding to extracellular cues is enabled by filopodia and lamellipodia, respectively, i.e. two key structural features located within the F-actin rich peripheral region (Figure 2). In a simplified model, forward movement of the growth cone is driven by Arp2/3-dependent F-actin polymerization (Korobova & Svitkina, 2008) alongside an attenuation of myosin-based F-actin retrograde flow. The latter process is enabled by selective engagement of the so-called ‘clutch’, a multimolecular point contact complex that, similar to focal adhesions, anchors F-actin with respect to an extracellular substrate (Gomez & Letourneau, 2014; Letourneau, 1981; Lowery & Van Vactor, 2009). Through engagement of the clutch, actin polymerization exceeds retrograde flow during protrusion. In contrast, during growth cone retraction, retrograde flow and/or F-actin breakdown exceed actin polymerization. An intermediate scenario occurs during growth cone turning, which is thought to be initiated by spatial alterations in growth cone calcium concentrations (Gasperini et al., 2017; Zheng, 2000) followed by asymmetrical polymerization and disruption of the growth cone’s F-actin cytoskeleton (Gallo & Letourneau, 2004; Gomez & Letourneau, 2014).
Figure 2. Schematic of the growth cone, the main driver of process outgrowth, branching and guidance.
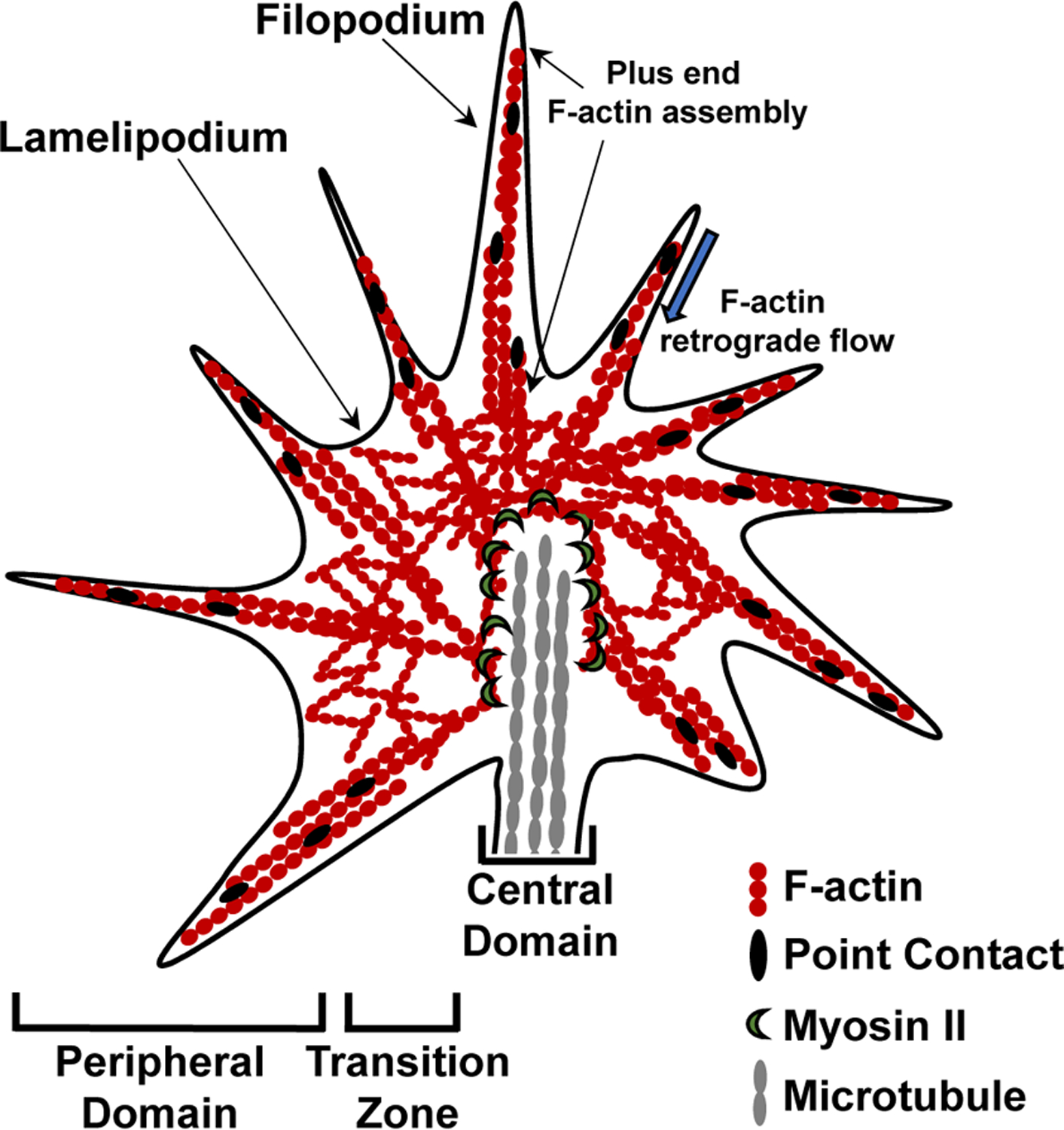
The growth cone is divided into three distinct compartments: the actin-rich peripheral region containing filopodia and lamellipodia, the central region enriched in dense microtubule arrays, and the transition zone characterized by the presence of actomyosin contractile structures. Forward movement of the growth cone, ultimately leading to process outgrowth, is initiated by actin polymerization within the peripheral domain of the growth cone, attenuation of F-actin retrograde flow, and engagement of focal contact points, also referred to as the ‘clutch’. Polymerization of microtubules is then necessary to complete process outgrowth. Process branching can be achieved through growth cone splitting, while growth cone turning in response to guidance cues involves an asymmetric regulation of F-actin cytoskeleton structure and polymerization/depolymerization.
Changes in actin cytoskeletal organization and dynamics, such as those described above, are regulated by upstream signaling cascades initiated by extracellular factors that can function as either attractive/permissive or repulsive/non-permissive cues. In this context, Rho family GTPases have emerged as key intracellular mediators governing cell morphogenesis and movement, whereby most studies have focused on the classic Rho family GTPases RhoA, Rac1, and Cdc42, which are regulated by the opposing actions of Rho-specific guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) (Lawson & Ridley, 2018; Narumiya & Thumkeo, 2018). In neuronal growth cones, activation of Rho A is most commonly associated with growth cone collapse in response to repulsive cues (Borisoff et al., 2003; Kopp et al., 2012), while Rac1 and Cdc42 are generally implicated in the regulation of lamellipodial dynamics and actin polymerization/filopodia formation, respectively (Kuhn et al., 2000). Seemingly paradoxically though, RhoA-mediated contractile forces were found in neurons to lead to both the retraction/collapse of growth cones and the extension of axons (Hall & Lalli, 2010; Jalink et al., 1994). This phenomenon may be explained by the concept that contractile forces during growth cone retraction/collapse are generated at F-actin bundles located within the growth cones’ central domain and transition zone (Zhang et al., 2003) rather than intra-axonally as described for axon extension (Gallo, 2006) or at the cell periphery as established for stress fiber formation in fibroblasts (Ridley & Hall, 1992). Interestingly, growth cone collapse induced by the repulsive cue semaphorin 3A (Sema3A, originally called collapsin-1) was found to occur by a RhoA-independent mechanism requiring the signal transducer collapsin response mediator protein 2 (CRMP2) (Goshima et al., 1995). Since CRMP2 has also been identified as a downstream target in RhoA-dependent growth cone collapse, CRMP2 may serve as a critical cytoskeleton regulatory convergent point in the process of growth cone collapse (Tan et al., 2015). The above provides a glimpse into the intricacy of growth cone regulation through the activities of actin regulatory proteins and upstream signaling pathways. Further complexity may be added by cell type-specific and/or context-dependent modulation of the growth cone’s actin-based dynamics and motile functions (Dent et al., 2011; Korobova & Svitkina, 2008; Lowery & Van Vactor, 2009; Strasser et al., 2004). Thus, it is not surprising that the functional roles of these proteins and pathways in shaping growth cone behaviors are currently not fully understood.
Taken together, it is evident that dynamic rearrangements of the actin cytoskeleton are a driving force for motile growth cone behaviors and subsequent changes in morphology. As discussed below, this fundamental concept also applies to cells of the OLG lineage, in particular bipolar and migratory progenitor cells, and differentiating OLGs that extend initially a simple and then increasingly complex process network.
3 -. THE GROWTH CONE AND ITS ACTIN CYTOSKELETON AS A DRVIER OF DYNAMIC MORPHOLOGICAL CHANGES IN OLG LINEAGE CELLS
3.1 -. Growth cone-driven OPC migration
OPCs can be characterized as migratory cells with highly motile protrusions (Hughes et al., 2013; Kirby et al., 2006) of which the leading process extends an OPC growth cone at its distal tip (Jarjour & Kennedy, 2004; Schmidt et al., 1997; Simpson & Armstrong, 1999). Next to a cytoskeletal framework in common with neuronal growth cones (Figure 2), OPC growth cones also hold components of the actin nucleating Arp2/3 complex (Bacon et al., 2007). In addtion, Arp2/3 complex actvities have been assigned an important role in establishing a lamellipodium-rich OPC growth cone and to, thereby, enable guided migratory responses (Li et al., 2015). The nucleation promoting factor N-WASP, on the other hand, has functionally been associated primarily with the extension and stabilization of filopodia rather than the formation of the OPC growth cone lamellipodium (Bacon et al., 2007). It is of note here, that the role of the Arp2/3 complex in filopodia extension still remains to some extent controversial and may apply to only certain cell types, possibly including OPCs (Korobova & Svitkina, 2008; Steffen et al., 2006; Yang & Svitkina, 2011; Young et al., 2015). Nevertheless, the above data support the idea that in OPCs the migratory guiding function of the growth cone located at the tip of the leading process is dependent on Arp2/3-mediated F-actin polymerization.
Overall, guided migratory responses can either be toward an attractive cue or away from a repulsive cue. In the context of OPC migration, PDGF-AA and FGF have been identified as attractive cues, and consistent with a critical role of actin-based motility driven by the OPC growth cone, migratory responses to these cues were found to be dependent on intracellular calcium signals, F-actin polymerization, and myosin-based contractile forces (Simpson & Armstrong, 1999). More precisely, in vitro cell culture studies imply that PDGF-AA-guided OPC migration is mediated by a signaling pathway that involves activation of Fyn tyrosine kinase, followed by sequential activation of the actin cytoskeleton regulators cyclin-dependent kinase 5 (Cdk5) and WAVE2 (Miyamoto et al., 2008). However, no OPC migration deficits were seen in conditional Cdk5 knockout mice (He et al., 2011; Luo et al., 2018), indicating that Cdk5 involvement may not represent an essential component of a molecular pathway instructing OPC migration in vivo.
Semaphorins and netrins have also been implicated in guiding the migratory trajectories of OPCs (Cohen, 2005; Jarjour et al., 2003; Ortega et al., 2012; Sugimoto et al., 2001; Tsai & Miller, 2002). In particular, in vitro assays revealed that netrin-1can trigger a rapid and persistent decrease in OPC surface area, process length, and process number (Jarjour et al., 2003). This finding is consistent with the concept that repellent cues can, in vitro, induce process retraction through a mechanism that is initiated by growth cone collapse. Consistently, activation of the Rho GTPase RhoA and its effector Rho kinase were found to be required for the OPC’s chemorepellent response to netrin-1 (Rajasekharan et al., 2010). The in vivo role of netrin-1-mediated repulsion has been associated with a dispersal of OPCs from the restricted domain of their origin in the developing spinal cord, the ventral ventricular zone (Tsai et al., 2006; Tsai et al., 2003). In agreement with such a functional role in dispersal, over-expression of netrin-1 during early phases of myelin repair was found to impair OPC recruitment and remyelination (Tepavcevic et al., 2014). Similar to netrin-1, members of the semaphorin family have been reported to function as repellent cues for OPCs by inducing growth cone collapse and altered migratory patterns (Cohen et al., 2003; Okada et al., 2007; Spassky et al., 2002). However, currently available data are variable, in particular for Sema3F for which both repulsive and attractive OPC responses have been reported (Boyd et al., 2013; Cohen et al., 2003; Piaton et al., 2011; Spassky et al., 2002; Sugimoto et al., 2001; Xiang et al., 2012). Nevertheless, an in vivo role in guiding OPC migration is indicated for Sema3A and Sema6A, which are both ligands of Plexin-A4, by the observed differences in OPC and Sema3A/6A distribution patterns when comparing the developing cortex in Plexin-A4 deficient mice with the one in wildtype mice (Okada & Tomooka, 2012). It is worth mentioning that CRMP2 has been found present in Sema3A-responsive differentiating OLGs (Ricard et al., 2001) and, it has been implicated in a RhoA-dependent pathway of process retraction in these cells (Fernandez-Gamba et al., 2012). Whether similar roles may apply for OPCs, however, still needs to be investigated.
More recently, the nervous system vasculature has been identified as a critical substrate for guiding OPC migration via a molecular interaction that is dependent on Wnt pathway activated expression of Cxcr4 (chemokine receptor 4) in OPCs and that, on the endothelial site, likely involves the presence of the Cxcr4 ligand Sdf1/Cxcl12 (stromal cell-derived factor 1/C-X-C motif chemokine 12) (Banisadr et al., 2011; Dziembowska et al., 2005; Tian et al., 2018; Tsai et al., 2016). Interestingly, tightly regulated interactions between Cxcr4 and the actin cytoskeleton have been assigned crucial functions during leucocytes migration and the reorganization of the actin cytoskeleton at the protrusive leading edge (Martinez-Munoz et al., 2018; Okabe et al., 2002). However, the precise roles of the actin cytoskeleton and the OPC growth cone in Cxcr4-mediated OPC migration still need to be determined.
From the findings described above, it becomes evident that actin cytoskeletal dynamics within the growth cone of the leading process of an OPC play a critical role in guiding OPC migration. Apart from the long-range cues discussed here, short-range cues, often represented by components of the extracellular matrix such as fibronectin (OPC migration promoting; Frost et al., 1996) or tenascin-C (OPC migration inhibiting; Garcion et al., 2001; Kiernan et al., 1996), have also been reported to regulate OPC motility (Jarjour & Kennedy, 2004). Moreover, there is evidence that expression of OPC guidance cues within demyelinated lesions may play critical roles in influencing OPC recruitment and myelin repair under pathological conditions (Boyd et al., 2013; Piaton et al., 2011; Tepavcevic et al., 2014; Williams et al., 2007). Despite the critical role of OPC guidance cues present during development and myelin repair, surprisingly little is currently known about the regulatory mechanisms directing actin cytoskeletal changes in the OPC growth cone, the main driver of OPC migratory behaviors.
3.2 -. Growth cone-driven process dynamics in differentiating and pre-myelinating OLGs
Similar to OPCs, differentiating OLGs have been found to extend OLG growth cones at the distal tips of their multiple processes (Asou et al., 1995; Fox et al., 2006; Kachar et al., 1986; Michalski et al., 2016; Rumsby et al., 2003; Song et al., 2001; Zuchero et al., 2015). Given the post-migratory and pre-myelinating characteristics of these cells, the functional importance of their growth cones lies in surveying the local environment in search of axonal segments to be myelinated and/or inappropriate targets to be avoided. As a first step toward surveillance of the surrounding area, differentiating OLGs develop a highly branched, complex process network. This process is driven by the nucleating functions of the Arp2/3 complex in combination with WASP/WAVE family members, all of which have been found present in differentiating OLGs and their growth cones (Bacon et al., 2007). The idea that actin assembly by the Arp2/3 complex drives the extension of complex process networks at pre-myelinating stages of the OLG lineage is corroborated by the observation that inhibition of Arp2/3 in vitro in cultures of differentiating OLGs impairs F-actin assembly, the establishment of lamellipodium-rich growth cones, and process outgrowth and branching (Zuchero et al., 2015). These deficits in actin-driven morphological maturation of OLGs were found to be associated with a significant reduction in myelination initiation, i.e. axon ensheathment, in vitro in a co-culture system and in vivo in conditional ArpC3 knockout mice. Similarly, loss of WAVE1 function via over-expression of dominant-negative WAVE1 or WAVE1 knockout was seen to impair growth cone formation and process outgrowth in differentiating OLGs (Kim et al., 2006). Furthermore, a critical role of Arp2/3 complex function in initial myelin ensheathment has been identified by pharmacological inhibition of N-WASP in explant cultures (Bacon et al., 2007). Interestingly though, in WAVE1 knockout mice, hypomyelination, ultimately a consequence of decreased numbers of OLG processes and myelination initiation, was observed in the corpus callosum and optic nerve but not the spinal cord (Kim et al., 2006). This finding may indicate that the regulation of actin cytoskeletal dynamics in growth cones of differentiating OLGs may follow regionally heterogeneous mechanisms due to differences in the importance of Arp2/3 in general, variances in Arp2/3 complex subunit composition or the variable nature of the functionally predominant Arp2/3 nucleation promoting factor (Pizarro-Cerda et al., 2017). It is of note that in the case of conditional ArpC3 knockout mice and pharmacological inhibition of N-WASP, available published data are currently restricted to the optic nerve (Zuchero et al., 2015).
In light of the proposed intrinsic program of myelination, the ability of OLG growth cones to recognize non-permissive targets becomes evident (Almeida, 2018). Indeed, neuronal junction adhesion molecule 2 (JAM2) has been identified as a non-permissive somatodendritic cue necessary for preventing non-axonal myelination of neurons (Redmond et al., 2016). Similarly, galectin-4 has been recently proposed to function as a repulsive cue for the initiation of myelination and to thereby define non-myelinated axonal segments (Diez-Revuelta et al., 2017). Members of the IgLON family, which represent GPI anchored adhesion molecules expressed by both OLGs and neurons, have also been identified as repulsive cues with functional importance in preventing precocious developmental myelination of specific fiber tracts (Sharma et al., 2015). Specific axon selection for myelination has additionally been reported to involve bidirectional Eph-ephrin signaling, whereby OLG process retraction in response to ephrin A1-EphA4 forward signaling was found to be mediated by a signaling cascade involving RhoA, the RhoA downstream target Rho kinase (ROCK) and the motor protein myosin II (Cohen, 2005; Harboe et al., 2018; Linneberg et al., 2015). This finding provides evidence for a critical role of RhoA in driving the actin cytoskeletal changes that ultimately lead to process retraction when differentiating OLGs respond to non-permissive cues. Interestingly, in studies on OLG growth cone turning and retraction behavior, collagen IV, a component of the capillary basement membrane, was identified as a non-permissive substrate for growth cones of differentiating OLGs (Fox et al., 2006). This observation indicates that repulsive cues may also be involved in preventing interactions with blood vessels at the later stages of the OLG lineage.
In addition to repulsion, attraction and permissive cues have also been found to play a critical role in guiding OLG processes, in particular in the context of extrinsically modulated promotion of myelination (Almeida, 2018). Such permissive cues include extracellular matrix molecules, the best characterized of which is laminin-2/merosin (Bechler et al., 2015). First, a number of in vitro studies identified laminin-2/merosin as a permissive substrate for OLG growth cones on which filopodia formation, and process outgrowth and branching are augmented (Eyermann et al., 2012; Fox et al., 2006; Lafrenaye & Fuss, 2011; Michalski et al., 2016). Collectively, these studies point toward a signaling pathway that is initiated by an interaction of laminin-2/merosin with the non-integrin extracellular matrix receptor dystroglycan, which is located within point contact complexes of OLG filopodia and process branch points. This interaction then leads to the sequential activation of Fyn tyrosine kinase, focal adhesion kinase (FAK) and the Rho GTPases Rac1 and Cdc42 (Eyermann et al., 2012; Hoshina et al., 2007; Lafrenaye & Fuss, 2011; Liang et al., 2004; Osterhout et al., 1999; Schafer et al., 2016). Consistent with an in vivo relevance of this pathway, reduced numbers of myelinated axons of particularly smaller diameter have been reported for the CNS of mice carrying a spontaneous mutation at the laminin α2 chain gene locus (Chun et al., 2003), and of knockout mice for Fyn (constitutive) (Sperber et al., 2001; Umemori et al., 1994) and FAK (conditional) (Camara et al., 2009; Forrest et al., 2009). An important role of Fyn and FAK activation in promoting OLG process outgrowth is further supported by the finding that the process outgrowth promoting effect of netrin-1 is also mediated by the activation of these two kinases (Rajasekharan et al., 2009; Rajasekharan et al., 2010). Moreover, Fyn activation was found to deactivate RhoA via p190 RhoGAP, a step thought to be critical for the morphological differentiation of OLGs (Liang et al., 2004; Rajasekharan et al., 2009; Rajasekharan et al., 2010; Wolf et al., 2001). As a step further downstream, this pathway has been implicated in mediating a downregulation of the motor protein non-muscle myosin II, which has been shown to function as an inhibitor of OLG process outgrowth and branching (Wang et al., 2012; Wang et al., 2008). Thus, activation of Fyn and FAK, associated with a deactivation of RhoA, emerges as a crucial component of the actin cytoskeletal regulatory machinery that promotes growth cone-driven process outgrowth and branching in differentiating OLGs and, thereby, the initiation of CNS myelination.
In contrast to the above described pathway involving an inactivation of RhoA, a complementary role for the Rho GTPases Rac1 and Cdc42 in regulating process outgrowth and branching in differentiating OLGs is questioned by the in vivo observation that their conditional knockout in cells of the OLG lineage does not lead to reduced numbers of myelinated fibers (Thurnherr et al., 2006). Interestingly, potential alternate molecular players have been identified. First, Ermin, which shares structural and functional characteristics with ERM proteins known to act in concert with Rho family GTPases, has been identified in differentiating OLGs and found to function as an actin-binding protein that induces process outgrowth and branching (Brockschnieder et al., 2006). Second, junction-mediating and -regulatory protein (Jmy), a multifunctional actin cytoskeleton regulator that can act as a nucleation promoting factor for the Arp2/3 complex or nucleate unbranched filaments by itself (Zuchero et al., 2009), has recently been characterized as a novel player in driving F-actin polymerization, and process outgrowth and branching in differentiating OLGs (Azevedo et al., 2018).
In addition to the extracellular matrix protein laminin-2/merosin, adhesion molecules have been proposed to function as permissive signals for the initiation of myelination; these include the neural cell adhesion molecules L1 (Laursen et al., 2009), N-cadherin (Chen et al., 2017; Schnadelbach et al., 2001), and Necl-1 (Park et al., 2008). The implicated diversity of permissive cues suggests that extrinsically modulated myelination of axons may be regulated by local molecular signatures that identify individual subpopulations of axons to be myelinated. In support of this idea, it has been shown that laminin-2/merosin is present on only a subset of axons in the CNS (Colognato et al., 2002) and that just some subtypes of neurons modulate myelination by signals that are released along their axons (Koudelka et al., 2016). In light of the increasing recognition of OLG heterogeneity (Dimou & Simons, 2017; Foerster et al., 2019; Marques et al., 2016; Ornelas et al., 2016; Spitzer et al., 2019; Trotter & Mittmann, 2019; van Bruggen et al., 2017), one might postulate that different OLG subtypes may respond in an individual fashion to the various cues provided by subpopulations of axons. It should be noted here that current knowledge related to the role of the OLG growth cone and its actin cytoskeleton during OLG differentiation and the initiation of myelination is still rather limited. This gap in knowledge is nicely illustrated by a recently published study in which myelination was characterized in mice over-expressing the extracellular domain of the cell adhesion molecule Cadm4 in OLGs (Elazar et al., 2019). This strategy is thought to interfere with cell-cell interactions involving OLG expressed Cadm4. In these mice, the formation of tight, and thus non-dynamic, OLG cell contacts was found to cause a number of phenotypic changes in myelination that reflect the inability of OLG processes to respond to positive as well as negative cues. There are, however, other myelin aberrations, such as a mixed hypo- and hypermyelination phenotype in the corpus callosum, that are, with the current knowledge available, difficult to explain.
Additional complexity comes into view when considering that extrinsically modulated myelination can also be mediated by electrical activity and vesicular release of neurotransmitters, in particular glutamate, along unmyelinated axons (Bechler et al., 2018; de Faria et al., 2018; Gibson et al., 2018). In this context, our data revealed that glutamate-mediated activation of sodium-dependent glutamate transporters, which are expressed by differentiating OLGs, initiates a signaling cascade that drives actin cytoskeletal changes leading to an increase in process outgrowth and branching (Martinez-Lozada et al., 2014; Suarez-Pozos et al., 2019; Waggener et al., 2013). In this pathway, activation of glutamate transport is thought to activate the reverse mode of sodium-calcium exchange, resulting in a transient increase in intracellular calcium concentration and subsequent inactivation of the actin binding/bundling activity of calcium/calmodulin-dependent protein kinase IIβ (CaMKIIβ). A critical in vivo role for neuronal activity-induced calcium transients within processes of differentiating OLGs and elongating myelin sheaths has been recently demonstrated in studies using the developing zebrafish as a model system (Baraban et al., 2018; Krasnow et al., 2018). Thus, analogous to the involvement of CaMKIIβ during dendritic spine remodeling in response to neuronal activity (Lin & Redmond, 2009; Okamoto et al., 2009), initiation of the glutamate transporter-CaMKIIβ pathway in OLGs may lead to a transient increase in actin cytoskeleton flexibility that enables remodeling and outgrowth of OLG processes. Details to the physical and functional interactions with other actin cytoskeleton regulators involved in this OLG process outgrowth promoting pathway have, however, not yet been investigated.
Taken together, it becomes clear that dynamic actin-cytoskeletal mechanisms initiated at the tips of outgrowing OLG processes represent key players in driving the morphological aspects of OLG differentiation, i.e. process outgrowth and branching, and the initiation of myelination. Changes in overall morphology occurring at the transition from OPC to differentiating OLG are likely regulated to a large extent by an intrinsic program. In contrast, there is increasing evidence that at later stages, especially during the initiation of myelination, both permissive and non-permissive extracellular cues regulate actin cytoskeletal dynamics at individual OLG processes, which ultimately determine their decision on whether to modulate myelination or to withdraw.
3.3 -. Leading edge-driven myelin wrapping by myelinating OLGs
Once myelination is initiated, it is completed by concentric wrapping of the myelin sheath around the axonal segment. This spiral growth is driven by the leading edge of the inner tongue, located adjacent to the axon, and it occurs simultaneously with compaction, i.e. exclusion of cytoplasm and adhesion of the different layers of myelin membrane (Snaidero et al., 2014; Snaidero & Simons, 2014). In contrast to myelin wrapping, compaction starts at the outermost layers and is thought to be mediated by myelin basic protein (MBP), one of the few proteins present within compacted myelin (Aggarwal et al., 2013; Nawaz et al., 2015; Snaidero & Simons, 2017; Zuchero et al., 2015). Somewhat unexpectedly, a peak in F-actin levels was seen in white matter at the time point when myelination starts to occur (Zuchero et al., 2015) while continuing myelin sheath wrapping was found associated with a decline in F-actin levels (Nawaz et al., 2015; Zuchero et al., 2015). Similarly, in differentiating OLG cultures, extension of membranous sheets was found associated with reduced levels of F-actin and could be induced experimentally by triggering F-actin disassembly (Nawaz et al., 2015; Wilson & Brophy, 1989; Zuchero et al., 2015). Consistent with the observed decrease in F-actin, myelin wrapping appeared unaffected when ArpC3 was deleted after initiation of myelination (Zuchero et al., 2015). Collectively, these data put forward the idea that myelin sheath wrapping is driven by F-actin disassembly. This notion is reinforced by the observation that actin assembly promoting proteins such as Arp2/3 complex components are statistically enriched in earlier stages of the OLG lineage, while actin disassembly promoting proteins, particularly gelsolin and members of the ADF/cofilin family, are prevalent in actively myelinating OLGs (Cahoy et al., 2008; Lena et al., 1994; Zhang et al., 2014). Indeed, inducing F-actin disassembly by surgically implanting latrunculin A loaded gelfoam on the dorsal spinal cord surface during the time-window of active CNS myelination caused a robust increase in myelin sheath wrapping (Zuchero et al., 2015). Conversely, myelin thickness was found slightly reduced in gelsolin knockout mice (Zuchero et al., 2015). Similar to the above, myelin sheaths in mice with constitutive and conditional deletion of ADF and cofilin1, respectively, were characterized by increased F-actin levels and reduced thickness when compared to the ones in control animals; in contrast, initiation of myelination appeared unaffected in these mice (Nawaz et al., 2015). Taken together, these data demonstrate that actual wrapping of the myelin sheath is a process that can mechanistically be distinguished from OLG process outgrowth and the initiation of myelination as it is driven by a robust increase in F-actin depolymerization that ultimately leads to the elimination of F-actin within myelin sheaths.
The importance of F-actin depolymerization in myelin sheath wrapping raises the intriguing question of which forces drive the leading edge of the inner tongue to achieve multiple layers of ensheathment. Based on measurement of tension, Nawaz et al. (2015) proposed a model in which movement of the membranous sheath occurs independently of stable adhesions with the extracellular matrix and is powered by cycles of F-actin based inflation and deflation at the leading edge of the advancing inner tongue. The idea of adhesion-independent motion is supported by the in vitro finding that OLGs are capable of concentrically wrapping their membranes around inert fibers (Bechler et al., 2015; Espinosa-Hoyos et al., 2018; Lee et al., 2012). The model proposed by Zuchero et al. (2015) is similarly based on adhesion-independent forward movement of the inner tongue but builds on the assumption of a lack of F-actin at the leading edge. In this case, movement is proposed to be driven by myosin II-independent blebbing, i.e. hydrostatic pressure generated in the cytoplasm (Paluch & Raz, 2013), whereby membrane compaction by MBP is hypothesized to sufficiently increase intracellular pressure. Notably, structure-function studies related to the maintenance of cytoplasmic channels within myelin sheaths provide evidence for a critical role of F-actin in stabilizing cytoplasm-rich areas by mechanisms that involve the myelin protein 2’/3’-cyclic nucleotide 3’phosphodiesterase (CNP) and antagonize MBP-mediated compaction (Snaidero et al., 2017). These findings highlight the complexity of actin cytoskeletal dynamics during myelination, and they uncover regional cytoskeletal heterogeneity within the myelin sheath, which may favor the presence of F-actin at the leading edge of the advancing inner tongue. Despite the still ongoing discussion about the fine details of the mechanism driving the movement of the advancing inner tongue during myelin sheath wrapping, the present data support a model in which this process is based on some form of adhesion-independent migratory movement that is associated with an overall increase in F-actin disassembly.
Most of the extracellular cues modulating myelination have thus far been associated with OLG process outgrowth and the initiation of myelination. In the case of laminin2/merosin, however, it has additionally been proposed that for late stage differentiating OLGs, initial spreading is promoted at the OLG growth cone contact site with lamini2/merosin-positive axons. The signaling pathway that has been implicated in this process involves the integrin receptor α6β1 and downstream activation of integrin-linked kinase (ILK) (Buttery & ffrench-Constant, 1999; Chun et al., 2003; Colognato et al., 2002; Michalski et al., 2016). In vivo, reduced numbers of particularly smaller diameter myelinated axons have been reported during the developmental window of active myelination for mice in which a dominant-negative form of integrin β1 is expressed in OLGs (Camara et al., 2009). OLG process outgrowth is apparently not affected in cells derived from these mice, reinforcing the idea that laminin2/merosin-integrin β1 signaling affects primarily initial membrane spreading and not, as shown for laminin2/merosin-dystroglycan signaling, process outgrowth and branching. In further support of a role of the above pathway in specifically membrane spreading, OLGs deficient in ILK show a reduced capacity to enwrap neurites in a co-coculture system (Camara et al., 2009; O’Meara et al., 2013), and there is a developmentally transient reduction in the number of myelinated axons, again with a preferential effect on smaller diameter axons, in conditional Ilk knockout mice (Camara et al., 2009; O’Meara et al., 2013). Importantly, and consistent with a critical role of F-actin disassembly during membrane spreading, aberrant F-actin accumulation was seen when ILK-deficient OLGs were differentiated on laminin2/merosin as a substrate (Michalski et al., 2016; O’Meara et al., 2013). It should be noted here, however, that ILK has additionally been shown to regulate OLG growth cones and their cytoskeleton by laminin2/merosin-independent mechanisms (Elazar et al., 2019; Michalski et al., 2016).
Overall, it is becoming increasingly apparent that myelin sheath spreading and myelin wrapping requires an increase in F-actin disassembly. Thus, there is a critical shift in the role of the OLG protrusion located actin cytoskeleton during the transition from differentiating OLGs characterized by process outgrowth to mature OLGs actively involved in myelin wrapping.
4 -. RegulatOry programs for the OLG growth cone and its actin cytoskeleton
4.1 -. Regulation of OLG morphology in coordination with the transcriptional expression of OLG differentiation genes
During CNS development, the extensive changes in cellular morphology that occur at the transition from OPC to differentiating OLG are well-coordinated with gene expression patterns that have been associated with the progression along the OLG lineage (Elbaz & Popko, 2019; Emery & Lu, 2015; Pol et al., 2017). In order to achieve synchronization, changes in morphology and gene expression may be regulated by actin cytoskeletal dynamics in both the cytoplasm and the nucleus, and they could, thereby, be directly coupled. In this context, and in general, actin cytoskeletal dynamics have been implicated in chromatin remodeling and the control of transcriptional gene expression profiles (Klages-Mundt et al., 2018; Sinha et al., 2018; Viita & Vartiainen, 2017; Virtanen & Vartiainen, 2017). In addition, contractile forces associated with the activities of non-muscle myosin II (NMII) are thought to control nuclear morphology and function (Kanellos et al., 2015; Wiggan et al., 2017). Thus, it is not surprising that NMII, shown to function as an inhibitor of process outgrowth at the transition from OPC to differentiating OLG, has also been found to affect the OLG’s transcriptional gene expression program (Rusielewicz et al., 2014; Wang et al., 2012; Wang et al., 2008). Consistent with the idea that nuclear actin cytoskeletal dynamics regulate transcriptional gene expression during these earlier stages of the OLG lineage, nuclear actin has been implicated in regulating the repressive histone marks that are necessary for heterochromatin formation and for the transition from OPC to differentiating OLG (Hernandez et al., 2016; Liu et al., 2015; Tsai & Casaccia, 2019). Interestingly, this epigenetic regulation has been found to be associated with a decrease in the levels of microtubule (i.e. stathmin) and F-actin (i.e. gelsolin) severing proteins, suggesting that the highly dynamic behavior of OPCs is supported by relatively high levels of cytoskeleton severing proteins; reducing their levels upon initiation of OPC differentiations is then thought to allow a shift toward cytoskeleton polymerization and process outgrowth (Hernandez et al., 2016; Liu et al., 2003; Liu et al., 2015).
Collectively, the above described observations suggest that, at the early stages of the OLG lineage and especially at the transition from OPC to differentiating OLG, actin cytoskeletal dynamics, OLG morphology, and transcriptional changes in OLG differentiation genes may be regulated by mechanisms that are directly coupled to each other.
4.2 -. Evidence for a regulation of OLG morphology and transcriptional myelin gene expression by mechanisms that function independently from each other
In contrast to the tightly coupled mechanisms discussed above, there is increasing evidence that, once OLGs reach pre-myelinating stages, cytoskeletal dynamics may be regulated independently from the transcriptional gene expression changes typically associated with myelination. For example, conditional knockout of ArpC3, shown to greatly affect OLG process outgrowth and the initiation of CNS myelination, was not seen associated with significant changes in MBP protein levels in this in vivo model (Zuchero et al., 2015). In cell culture studies, inactivation of the Fyn tyrosine kinase, via treatment of differentiating OLGs with the tyrosine kinase inhibitors PP1 and PP2 over a period of 4 days, has been described to lead to a robust inhibition of process outgrowth; at the same time, this treatment was not associated with apparent changes in the expression of the cell surface marker O1 and the myelin proteins myelin associated glycoprotein (MAG) and MBP (Osterhout et al., 1999). Similarly, the OLG process outgrowth regulatory role of Mayven, a kelch-related actin-binding protein shown to interact with Fyn, was found to be unrelated to a control of myelin gene expression when analyzed in primary cultures of differentiating OLGs (Jiang et al., 2005; S. K. Williams et al., 2005). Comparable observations were made when assessing myelin protein levels both in vivo and in vitro in the context of FAK-mediated process outgrowth and membrane extension in the presence of laminin-2/merosin (Buttery & ffrench-Constant, 1999; Camara et al., 2009; Lafrenaye & Fuss, 2011) and in primary cultures of differentiating OLGs upon netrin-1 stimulated process outgrowth (Rajasekharan et al., 2009). It is of note that changes in myelin gene expression seen in certain knockout mice, such as those for Fyn, are complicated to interpret due to the multifunctional character of the protein under investigation and potential functional roles in OPCs (Kramer-Albers & White, 2011; White & Kramer-Albers, 2014). A similar scenario may account for the changes seen in MBP protein levels upon knockdown of the actin nucleation factor Jmy in primary cultures of differentiating OLGs (Azevedo et al., 2018), since Jmy can also function as a transcriptional co-activator (Zuchero et al., 2009). Nevertheless, the above data strongly suggest that at the pre-myelinating stages, morphological maturation of OLGs can be regulated by the key actin cytoskeleton regulators Arp2/3, Fyn and FAK by mechanisms that apparently function independently from those regulating transcriptional myelin gene expression.
Additional evidence for a regulation of OLG morphology by mechanisms that appear to function independently from those controlling myelin gene expression comes from our own studies, in which the role of sodium-dependent glutamate transporters was investigated in differentiating OLGs (Martinez-Lozada et al., 2014). In these initial studies, cells were analyzed by immunocytochemistry six hours post-treatment. In order to confirm that mRNA levels for myelin genes remain unchanged even under conditions that allow sufficient time for potential downstream signaling events, we developed a treatment paradigm as indicated in Figure 3 and analyzed cells at 24 hours after the initial treatment. For these experiments, we used the naturally occurring amino acid D-Aspartic acid (d-Asp), since previous studies demonstrated that D-Asp stimulates OLG process outgrowth in a manner similar to glutamate (Martinez-Lozada et al., 2014). In addition, D-Asp comes with the advantages of not activating non-NMDA ionotropic or metabotropic glutamate receptors and of not being metabolized by glutamine synthetase (Martinez-Lozada et al., 2014). We also chose a multiple application paradigm since the effect of D-Asp treatment on the downstream target CaMKIIβ was found to last for no more than six hours. Using this extended paradigm, we observed an increase in process outgrowth and branching that was similar under both conditions, i.e when analyzed at eight hours post one-time D-Asp application (56 hrs) and at 24 hours post three-time D-Asp applications (72 hrs) (Figure 3). Importantly, no changes in mRNA levels were seen at 72 hrs (Figure 3). These findings provide further evidence for the existence of molecular pathways that can regulate the morphological maturation of pre-myelinating OLGs without significantly affecting transcriptional myelin gene expression.
Figure 3. Stimulation of process outgrowth via activation of sodium-dependent glutamate transport does not affect myelin gene expression.
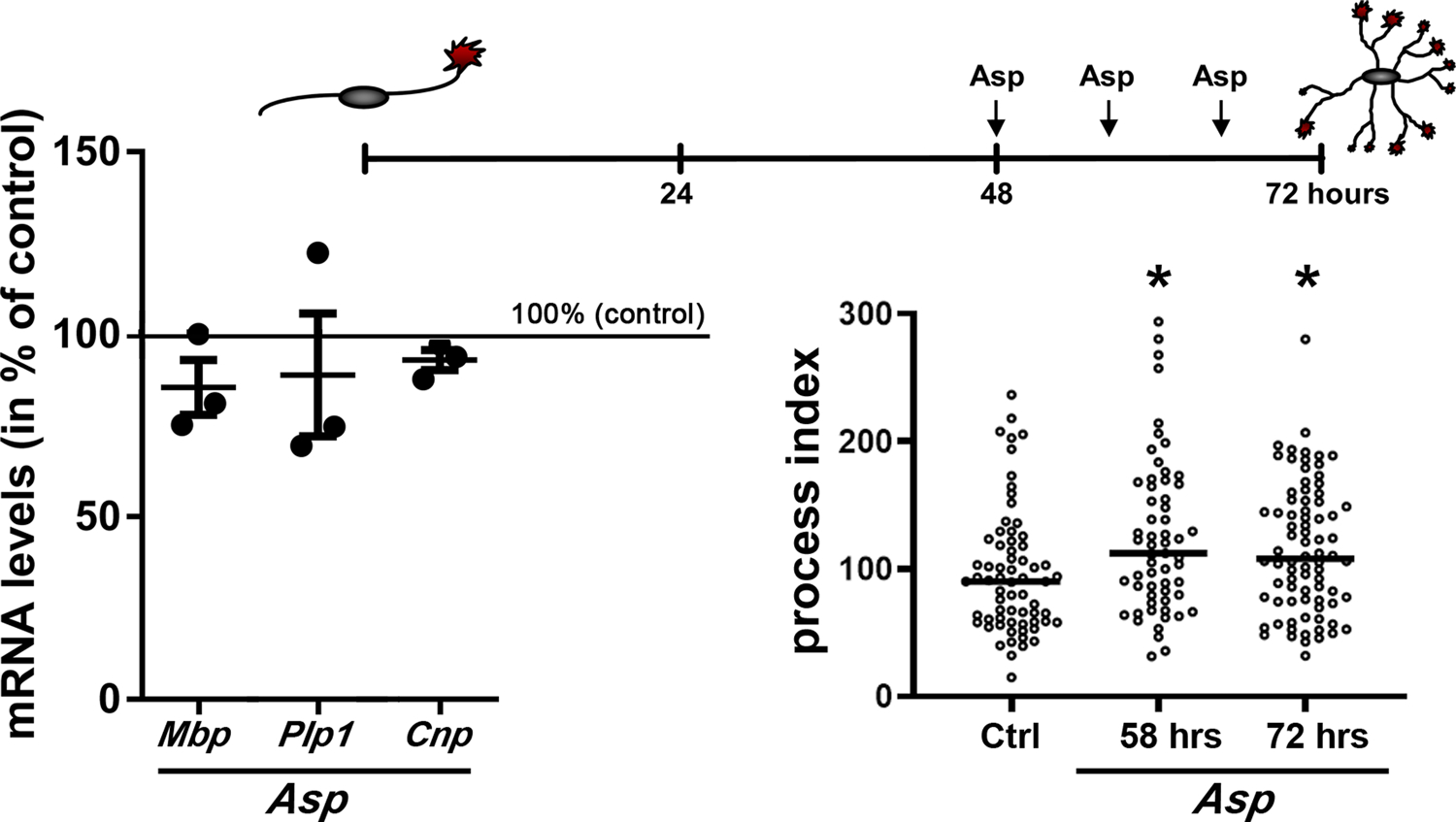
Differentiating OLGs were isolated and cultured as previously described (Martinez-Lozada et al., 2014). Cells were treated with D-Asp as indicated in the scheme shown in upper right. mRNA levels for the myelin genes myelin basic protein (Mbp), proteolipid protein (Plp1) and cyclic nucleotide phosphodiesterase (Cnp) were determined by real-time RT qPCR analysis at 72 hours (24 hours post initial D-Asp treatment) and relative expression levels were determined using the ΔΔCT method (bottom left graph) Statistical significance was assessed by ANOVA, and revealed no statistically significant changes in myelin protein mRNA levels. OLG morphology was assessed as previously described (Martinez-Lozada et al., 2014) revealing a significant increase in process index under both conditions, i.e. when analyzed at 8 hours post one-time D-Asp application (56 hrs) and at 24 hours post D-Asp application at 8-hour intervals (72 hrs) (bottom right graph; horizontal lines indicate medians). Statistical significance was assessed by ANOVA (non- parametric Kruskal Wallis and Dunn’s post hoc test). *p≥0,05 (compared to non-stimulated control (Ctrl)).
The above discussion highlights that changes in process outgrowth mediated in differentiating OLGs by mechanisms of actin cytoskeletal dynamics can occur independently from major changes in transcriptional myelin gene expression. It is interesting to note that cellular motile behaviors have been proposed to require a tight temporal link between actin cytoskeletal dynamics and transcriptional activity at gene loci encoding actin and actin regulatory proteins (Olson & Nordheim, 2010). Thus, it is tempting to speculate that in pre-myelinating OLGs, progress outgrowth-related maturation events are controlled by a morphology regulatory program that functions independently from the myelin gene expression regulatory program.
5 -. CONCLUSION
The highly dynamic actin cytoskeleton of growth cones found at the tips of OPC and OLG processes emerges as a main driver for the motile behaviors and morphological changes that are associated with OPC guidance and migration, OLG process outgrowth and branching, and the initiation of myelination (Figure 4). The actin cytoskeletal mechanisms underlying these aspects of OPC/OLG biology are characterized by varying stoichiometries of F-actin polymerizing and depolymerizing events. At the transition from myelination initiation to myelin wrapping, however, a remarkable conceptual switch occurs as morphological changes are now mediated by predominantly F-actin depolymerization and disassembly (Figure 4).
Figure 4. Summary of growth cone-driven actin cytoskeletal changes in cells of the oligodendrocyte (OLG) lineage.
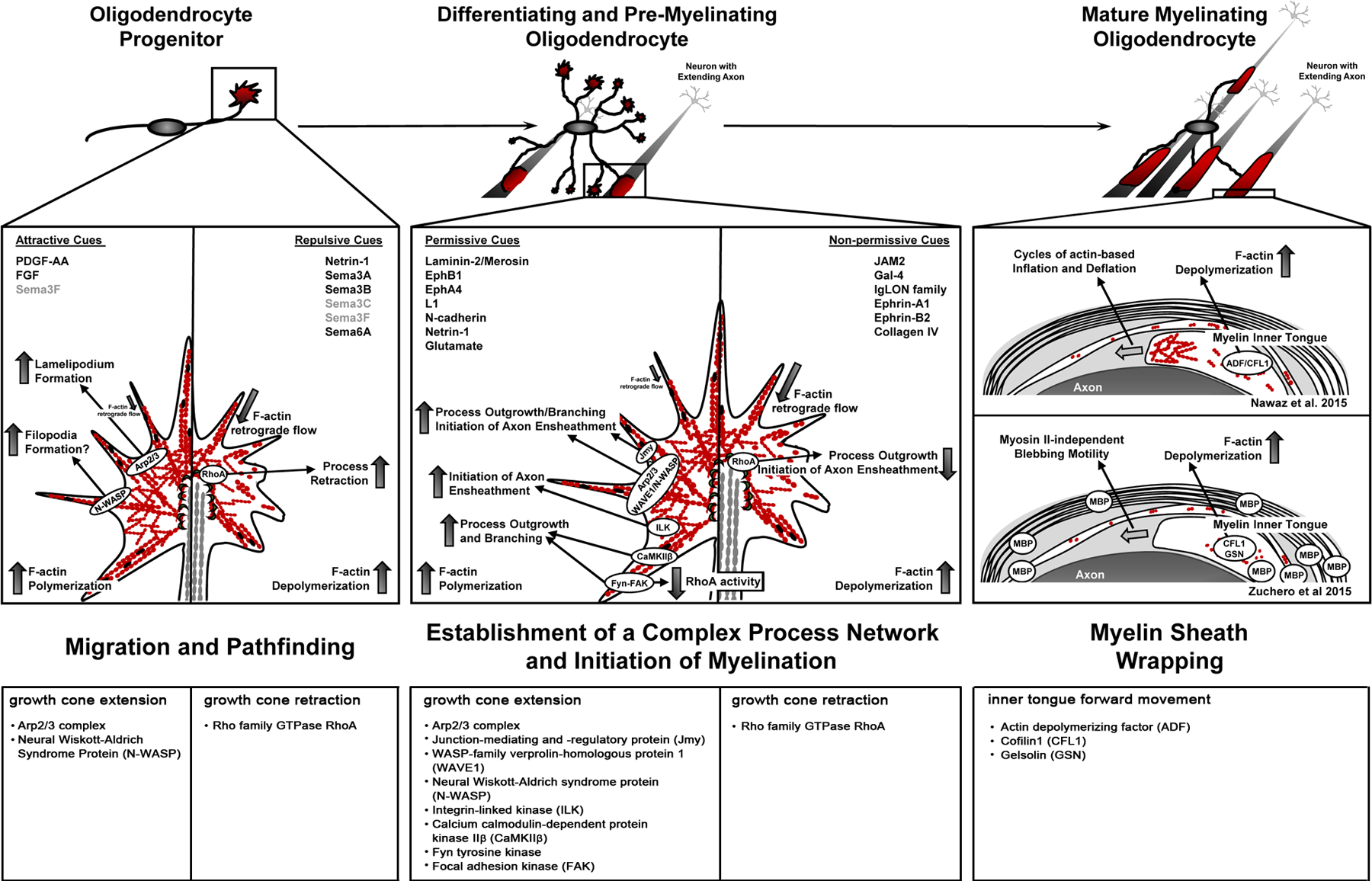
Cells of the OLG lineage undergo extensive changes in morphology when differentiating from a bipolar migratory OLG progenitor cell (OPC) first into a differentiating and pre-myelinating OLG that extends a large process network in search of axonal segments to be myelinated, and then into a mature OLG that generates and maintains a fully functional myelin sheath. At the progenitor stage (left), highly dynamic actin cytoskeletal mechanisms regulate growth cone behavior and, thereby, guide OPC migration along trajectories within the CNS in response to both attractive and repulsive extracellular cues. These motile behaviors are characterized by process extension events requiring F-actin polymerization, process retraction events driven by F-actin depolymerization, and process turning events that are mediated by an asymmetrical distribution of F-actin. Actin-binding proteins and key intracellular mediators implicated in OPC growth cone extension and retraction are depicted in the growth cone model (top) and listed in the bottom table. Differentiation into the differentiating OLG (middle) is associated with extensive process outgrowth and branching. In this process, F-actin polymerization and the actin regulatory proteins Arp2/3, Fyn, and focal adhesion kinase (FAK) play key roles in orchestrating actin cytoskeletal dynamics and motile behaviors (see growth cone model (top) and bottom table). Additional actin regulatory proteins, i.e. integrin-linked kinase (ILK), calcium calmodulin-dependent protein kinase IIβ (CaMKIIβ), and junction-mediating and -regulatory protein (Jmy), have been implicated in this process. However, the interactions, physical and/or functional, between all the potential players involved are currently not well understood. At the pre-myelinating stages, OLG processes respond to extracellular cues, which can be permissive as well as non-permissive, in an individual fashion. Growth cone responses at this stage are thought to be similar to the ones observed at the progenitor stage, and they are the determining factors regulating the initiation of myelination. The transition to the myelinating stage is associated with the loss of process extension events, as myelin wrapping is mediated largely by F-actin disassembly. F-actin severing proteins shown to be involved in this process are depicted in the model (top) and listed in the bottom table. Despite the consensus of F-actin disassembly as the major driver, there is still some controversy as to whether the force driving the myelin’s inner tongue is generated by cycles of actin-based inflation and deflation or by MBP-dependent, myosin II-independent blebbing motility.
The synchronized changes in morphology and transcriptional expression of OLG differentiation genes appear, at the early stages of the lineage, to be regulated by mechanisms that may be directly coupled to each other. At the pre-myelinating stages, on the other hand, evidence accumulates that morphological maturation may be controlled by a regulatory program that functions independently from the one targeting the transcriptional control of myelin gene expression. Interestingly, in the CNS of patients with Multiple Sclerosis (MS), the main demyelinating disease in human, OLGs have been detected that express an apparent full repertoire of myelin genes but fail to successfully initiate and/or complete myelination (Chang et al., 2002). This finding could be taken as further evidence for regulatory programs that independently control either myelin gene expression or actin-driven morphology, since under the above pathological conditions the transcriptional myelin gene expression program appears mostly unaffected while the OLG morphology regulatory program seems to be largely dysfunctional. With this interpretation in mind, further defining the mechanisms that regulate, particularly at the pre-myelinating stages, the morphological aspects of OLG maturation is likely to provide important clues on how to achieve myelin repair under pathological conditions as they are seen in MS.
Overall, the findings presented in this review highlight the still under-developed understanding of the OLG growth cone, its actin cytoskeleton and its functions in cells of the OLG lineage. Future studies will clearly be crucial to gaining a better understanding of the roles of these players during developmental myelination as well as their potential contributions to pathological conditions in which OLGs and/or the myelin sheath are affected.
MAIN POINTS.
Actin cytoskeletal dynamics initiated at the growth cone drive motility in OPCs and morphological changes in differentiating and pre-myelinating OLGs
Myelinating OLGs lose growth cone structures as myelin wrapping requires F-actin disassembly.
The OLG growth cone’s actin cytoskeleton can be regulated independently from transcriptional myelin gene expression.
Acknowledgements:
The authors would like to express their regret to any authors whose work could not be cited due to space limitations. Support for the authors was provided by grants from the National Institute of Health (B.F.), the National Multiple Sclerosis Society (B.F.), Virginia’s Commonwealth Health Research Board (B.F.) and the Upstate Foundation (DJO).
REFERENCES
- Aggarwal S, Snaidero N, Pahler G, Frey S, Sanchez P, Zweckstetter M, Janshoff A, Schneider A, Weil MT, Schaap IA, Gorlich D, & Simons M (2013). Myelin membrane assembly is driven by a phase transition of myelin basic proteins into a cohesive protein meshwork. PLoS Biol, 11(6), e1001577. 10.1371/journal.pbio.1001577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkam D, Feldman EZ, Singh A, & Kiaei M (2017). Profilin1 biology and its mutation, actin(g) in disease. Cell Mol Life Sci, 74(6), 967–981. 10.1007/s00018-016-2372-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RG (2018). The Rules of Attraction in Central Nervous System Myelination. Front Cell Neurosci, 12, 367. 10.3389/fncel.2018.00367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asou H, Hamada K, & Sakota T (1995). Visualization of a single myelination process of an oligodendrocyte in culture by video microscopy. Cell Struct Funct, 20(1), 59–70. [DOI] [PubMed] [Google Scholar]
- Azevedo MM, Domingues HS, Cordelieres FP, Sampaio P, Seixas AI, & Relvas JB (2018). Jmy regulates oligodendrocyte differentiation via modulation of actin cytoskeleton dynamics. Glia, 66(9), 1826–1844. 10.1002/glia.23342 [DOI] [PubMed] [Google Scholar]
- Bacon C, Lakics V, Machesky L, & Rumsby M (2007). N-WASP regulates extension of filopodia and processes by oligodendrocyte progenitors, oligodendrocytes, and Schwann cells-implications for axon ensheathment at myelination. Glia, 55(8), 844–858. [DOI] [PubMed] [Google Scholar]
- Bailly M, Ichetovkin I, Grant W, Zebda N, Machesky LM, Segall JE, & Condeelis J (2001). The F-actin side binding activity of the Arp2/3 complex is essential for actin nucleation and lamellipod extension. Curr Biol, 11(8), 620–625. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Frederick TJ, Freitag C, Ren D, Jung H, Miller SD, & Miller RJ (2011). The role of CXCR4 signaling in the migration of transplanted oligodendrocyte progenitors into the cerebral white matter. Neurobiol Dis, 44(1), 19–27. 10.1016/j.nbd.2011.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban M, Koudelka S, & Lyons DA (2018). Ca (2+) activity signatures of myelin sheath formation and growth in vivo. Nat Neurosci, 21(1), 19–23. 10.1038/s41593-017-0040-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer NG, Richter-Landsberg C, & Ffrench-Constant C (2009). Role of the oligodendroglial cytoskeleton in differentiation and myelination. Glia., 57(16), 1691–1705. [DOI] [PubMed] [Google Scholar]
- Bechler ME, Byrne L, & Ffrench-Constant C (2015). CNS Myelin Sheath Lengths Are an Intrinsic Property of Oligodendrocytes. Curr Biol, 25(18), 2411–2416. 10.1016/j.cub.2015.07.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechler ME, Swire M, & Ffrench-Constant C (2018). Intrinsic and adaptive myelination-A sequential mechanism for smart wiring in the brain. Dev Neurobiol, 78(2), 68–79. 10.1002/dneu.22518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisoff JF, Chan CC, Hiebert GW, Oschipok L, Robertson GS, Zamboni R, Steeves JD, & Tetzlaff W (2003). Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci, 22(3), 405–416. [DOI] [PubMed] [Google Scholar]
- Boyd A, Zhang H, & Williams A (2013). Insufficient OPC migration into demyelinated lesions is a cause of poor remyelination in MS and mouse models. Acta Neuropathol, 125(6), 841–859. 10.1007/s00401-013-1112-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D, & Goode BL (2013). Formins at a glance. J Cell Sci, 126(Pt 1), 1–7. 10.1242/jcs.107250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockschnieder D, Sabanay H, Riethmacher D, & Peles E (2006). Ermin, a myelinating oligodendrocyte-specific protein that regulates cell morphology. J Neurosci, 26(3), 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TL, & Macklin WB (2019). The Actin Cytoskeleton in Myelinating Cells. Neurochem Res. 10.1007/s11064-019-02753-0 [DOI] [PMC free article] [PubMed]
- Butt AM, Ibrahim M, & Berry M (1997). The relationship between developing oligodendrocyte units and maturing axons during myelinogenesis in the anterior medullary velum of neonatal rats. J Neurocytol, 26(5), 327–338. [DOI] [PubMed] [Google Scholar]
- Buttery PC, & ffrench-Constant C (1999). Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci, 14(3), 199–212. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, & Barres BA (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci, 28(1), 264–278. 10.1523/JNEUROSCI.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camara J, Wang Z, Nunes-Fonseca C, Friedman HC, Grove M, Sherman DL, Komiyama NH, Grant SG, Brophy PJ, Peterson A, & ffrench-Constant C (2009). Integrin-mediated axoglial interactions initiate myelination in the central nervous system. J Cell Biol, 185(4), 699–712. 10.1083/jcb.200807010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarata GM, Bearce EA, & Lowery LA (2016). Cytoskeletal social networking in the growth cone: How +TIPs mediate microtubule-actin cross-linking to drive axon outgrowth and guidance. Cytoskeleton (Hoboken), 73(9), 461–476. 10.1002/cm.21272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, & Shekhar S (2017). Global treadmilling coordinates actin turnover and controls the size of actin networks. Nat Rev Mol Cell Biol, 18(6), 389–401. 10.1038/nrm.2016.172 [DOI] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, & Trapp BD (2002). Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med, 346(3), 165–173. [DOI] [PubMed] [Google Scholar]
- Chen M, Xu Y, Huang R, Huang Y, Ge S, & Hu B (2017). N-Cadherin is Involved in Neuronal Activity-Dependent Regulation of Myelinating Capacity of Zebrafish Individual Oligodendrocytes In Vivo. Mol Neurobiol, 54(9), 6917–6930. 10.1007/s12035-016-0233-4 [DOI] [PubMed] [Google Scholar]
- Chun SJ, Rasband MN, Sidman RL, Habib AA, & Vartanian T (2003). Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J Cell Biol, 163(2), 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RI (2005). Exploring oligodendrocyte guidance: ‘to boldly go where no cell has gone before’. Cell Mol Life Sci, 62(5), 505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RI, Rottkamp DM, Maric D, Barker JL, & Hudson LD (2003). A role for semaphorins and neuropilins in oligodendrocyte guidance. J Neurochem, 85(5), 1262–1278. [DOI] [PubMed] [Google Scholar]
- Colognato H, Baron W, Avellana-Adalid V, Relvas JB, Baron-Van Evercooren A, Georges-Labouesse E, & ffrench-Constant C (2002). CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol, 4(11), 833–841. 10.1038/ncb865 [DOI] [PubMed] [Google Scholar]
- Courtemanche N (2018). Mechanisms of formin-mediated actin assembly and dynamics. Biophys Rev, 10(6), 1553–1569. 10.1007/s12551-018-0468-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Faria O Jr., Pama EAC, Evans K, Luzhynskaya A, & Karadottir RT (2018). Neuroglial interactions underpinning myelin plasticity. Dev Neurobiol, 78(2), 93–107. 10.1002/dneu.22539 [DOI] [PubMed] [Google Scholar]
- Dent EW, Gupton SL, & Gertler FB (2011). The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol, 3(3). 10.1101/cshperspect.a001800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Revuelta N, Higuero AM, Velasco S, Penas-de-la-Iglesia M, Gabius HJ, & Abad-Rodriguez J (2017). Neurons define non-myelinated axon segments by the regulation of galectin-4-containing axon membrane domains. Sci Rep, 7(1), 12246. 10.1038/s41598-017-12295-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, & Simons M (2017). Diversity of oligodendrocytes and their progenitors. Curr Opin Neurobiol, 47, 73–79. 10.1016/j.conb.2017.09.015 [DOI] [PubMed] [Google Scholar]
- Domingues HS, Cruz A, Chan JR, Relvas JB, Rubinstein B, & Pinto IM (2018). Mechanical plasticity during oligodendrocyte differentiation and myelination. Glia, 66(1), 5–14. 10.1002/glia.23206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowska M, Tham TN, Lau P, Vitry S, Lazarini F, & Dubois-Dalcq M (2005). A role for CXCR4 signaling in survival and migration of neural and oligodendrocyte precursors. Glia, 50(3), 258–269. 10.1002/glia.20170 [DOI] [PubMed] [Google Scholar]
- Elazar N, Vainshtein A, Golan N, Vijayaragavan B, Schaeren-Wiemers N, Eshed-Eisenbach Y, & Peles E (2019). Axoglial Adhesion by Cadm4 Regulates CNS Myelination. Neuron, 101(2), 224–231. 10.1016/j.neuron.2018.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz B, & Popko B (2019). Molecular Control of Oligodendrocyte Development. Trends Neurosci, 42(4), 263–277. 10.1016/j.tins.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B, & Lu QR (2015). Transcriptional and Epigenetic Regulation of Oligodendrocyte Development and Myelination in the Central Nervous System. Cold Spring Harb Perspect Biol. 10.1101/cshperspect.a020461 [DOI] [PMC free article] [PubMed]
- Espinosa-Hoyos D, Jagielska A, Homan KA, Du H, Busbee T, Anderson DG, Fang NX, Lewis JA, & Van Vliet KJ (2018). Engineered 3D-printed artificial axons. Sci Rep, 8(1), 478. 10.1038/s41598-017-18744-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyermann C, Czaplinski K, & Colognato H (2012). Dystroglycan promotes filopodial formation and process branching in differentiating oligodendroglia. J Neurochem, 120(6), 928–947. 10.1111/j.1471-4159.2011.07600.x [DOI] [PubMed] [Google Scholar]
- Fernandez-Gamba A, Leal MC, Maarouf CL, Richter-Landsberg C, Wu T, Morelli L, Roher AE, & Castano EM (2012). Collapsin response mediator protein-2 phosphorylation promotes the reversible retraction of oligodendrocyte processes in response to non-lethal oxidative stress. J Neurochem, 121(6), 985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster S, Hill MFE, & Franklin RJM (2019). Diversity in the oligodendrocyte lineage: Plasticity or heterogeneity? Glia. 10.1002/glia.23607 [DOI] [PubMed]
- Forrest AD, Beggs HE, Reichardt LF, Dupree JL, Colello RJ, & Fuss B (2009). Focal adhesion kinase (FAK): A regulator of CNS myelination. J Neurosci Res., 87(15), 3456–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster AY, Bujalka H, & Emery B (2019). Axoglial interactions in myelin plasticity: Evaluating the relationship between neuronal activity and oligodendrocyte dynamics. Glia. 10.1002/glia.23629 [DOI] [PubMed]
- Fox MA, Afshari FS, Alexander JK, Colello RJ, & Fuss B (2006). Growth conelike sensorimotor structures are characteristic features of postmigratory, premyelinating oligodendrocytes. Glia, 53(5), 563–566. [DOI] [PubMed] [Google Scholar]
- Frost E, Kiernan BW, Faissner A, & ffrench-Constant C (1996). Regulation of oligodendrocyte precursor migration by extracellular matrix: evidence for substrate-specific inhibition of migration by tenascin-C. Dev Neurosci, 18(4), 266–273. 10.1159/000111416 [DOI] [PubMed] [Google Scholar]
- Fujiwara I, Takeda S, Oda T, Honda H, Narita A, & Maeda Y (2018). Polymerization and depolymerization of actin with nucleotide states at filament ends. Biophys Rev, 10(6), 1513–1519. 10.1007/s12551-018-0483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G (2006). RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. J Cell Sci, 119(Pt 16), 3413–3423. 10.1242/jcs.03084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, & Letourneau PC (2004). Regulation of growth cone actin filaments by guidance cues. J Neurobiol, 58(1), 92–102. [DOI] [PubMed] [Google Scholar]
- Garcion E, Faissner A, & ffrench-Constant C (2001). Knockout mice reveal a contribution of the extracellular matrix molecule tenascin-C to neural precursor proliferation and migration. Development, 128(13), 2485–2496. [DOI] [PubMed] [Google Scholar]
- Gasperini RJ, Pavez M, Thompson AC, Mitchell CB, Hardy H, Young KM, Chilton JK, & Foa L (2017). How does calcium interact with the cytoskeleton to regulate growth cone motility during axon pathfinding? Mol Cell Neurosci, 84, 29–35. 10.1016/j.mcn.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Gibson EM, Geraghty AC, & Monje M (2018). Bad wrap: Myelin and myelin plasticity in health and disease. Dev Neurobiol, 78(2), 123–135. 10.1002/dneu.22541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TM, & Letourneau PC (2014). Actin dynamics in growth cone motility and navigation. J Neurochem, 129(2), 221–234. 10.1111/jnc.12506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CS (1996). Mechanisms and molecules that control growth cone guidance. Annu Rev Neurosci, 19, 341–377. 10.1146/annurev.ne.19.030196.002013 [DOI] [PubMed] [Google Scholar]
- Goshima Y, Nakamura F, Strittmatter P, & Strittmatter SM (1995). Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature, 376(6540), 509–514. 10.1038/376509a0 [DOI] [PubMed] [Google Scholar]
- Gregath A, & Lu QR (2018). Epigenetic modifications-insight into oligodendrocyte lineage progression, regeneration, and disease. FEBS Lett, 592(7), 1063–1078. 10.1002/1873-3468.12999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, & Lalli G (2010). Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol, 2(2), a001818. 10.1101/cshperspect.a001818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M, Torvund-Jensen J, Kjaer-Sorensen K, & Laursen LS (2018). Ephrin-A1-EphA4 signaling negatively regulates myelination in the central nervous system. Glia, 66(5), 934–950. 10.1002/glia.23293 [DOI] [PubMed] [Google Scholar]
- Hardy RJ, & Friedrich VL Jr. (1996). Progressive remodeling of the oligodendrocyte process arbor during myelinogenesis. Dev Neurosci, 18(4), 243–254. [DOI] [PubMed] [Google Scholar]
- He X, Takahashi S, Suzuki H, Hashikawa T, Kulkarni AB, Mikoshiba K, & Ohshima T (2011). Hypomyelination phenotype caused by impaired differentiation of oligodendrocytes in Emx1-cre mediated Cdk5 conditional knockout mice. Neurochem Res, 36(7), 1293–1303. 10.1007/s11064-010-0391-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez M, Patzig J, Mayoral SR, Costa KD, Chan JR, & Casaccia P (2016). Mechanostimulation Promotes Nuclear and Epigenetic Changes in Oligodendrocytes. J Neurosci, 36(3), 806–813. 10.1523/jneurosci.2873-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines JH, Ravanelli AM, Schwindt R, Scott EK, & Appel B (2015). Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci, 18(5), 683–689. 10.1038/nn.3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KC, Popp D, Gebhard W, & Kabsch W (1990). Atomic model of the actin filament. Nature, 347(6288), 44–49. 10.1038/347044a0 [DOI] [PubMed] [Google Scholar]
- Hoshina N, Tezuka T, Yokoyama K, Kozuka-Hata H, Oyama M, & Yamamoto T (2007). Focal adhesion kinase regulates laminin-induced oligodendroglial process outgrowth. Genes Cells, 12(11), 1245–1254. 10.1111/j.1365-2443.2007.01130.x [DOI] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, & Bergles DE (2013). Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci, 16(6), 668–676. 10.1038/nn.3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, & Moolenaar WH (1994). Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol, 126(3), 801–810. 10.1083/jcb.126.3.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjour AA, & Kennedy TE (2004). Oligodendrocyte precursors on the move: mechanisms directing migration. Neuroscientist, 10(2), 99–105. [DOI] [PubMed] [Google Scholar]
- Jarjour AA, Manitt C, Moore SW, Thompson KM, Yuh SJ, & Kennedy TE (2003). Netrin-1 is a chemorepellent for oligodendrocyte precursor cells in the embryonic spinal cord. J Neurosci, 23(9), 3735–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Avraham HK, Park SY, Kim TA, Bu X, Seng S, & Avraham S (2005). Process elongation of oligodendrocytes is promoted by the Kelch-related actin-binding protein Mayven. J Neurochem, 92(5), 1191–1203. [DOI] [PubMed] [Google Scholar]
- Kachar B, Behar T, & Dubois-Dalcq M (1986). Cell shape and motility of oligodendrocytes cultured without neurons. Cell Tissue Res, 244(1), 27–38. [DOI] [PubMed] [Google Scholar]
- Kahn OI, & Baas PW (2016). Microtubules and Growth Cones: Motors Drive the Turn. Trends Neurosci, 39(7), 433–440. 10.1016/j.tins.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellos G, & Frame MC (2016). Cellular functions of the ADF/cofilin family at a glance. J Cell Sci, 129(17), 3211–3218. 10.1242/jcs.187849 [DOI] [PubMed] [Google Scholar]
- Kanellos G, Zhou J, Patel H, Ridgway RA, Huels D, Gurniak CB, Sandilands E, Carragher NO, Sansom OJ, Witke W, Brunton VG, & Frame MC (2015). ADF and Cofilin1 Control Actin Stress Fibers, Nuclear Integrity, and Cell Survival. Cell Rep, 13(9), 1949–1964. 10.1016/j.celrep.2015.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang F, Purich DL, & Southwick FS (1999). Profilin promotes barbed-end actin filament assembly without lowering the critical concentration. J Biol Chem, 274(52), 36963–36972. [DOI] [PubMed] [Google Scholar]
- Kiernan BW, Gotz B, Faissner A, & ffrench-Constant C (1996). Tenascin-C inhibits oligodendrocyte precursor cell migration by both adhesion-dependent and adhesion-independent mechanisms. Mol Cell Neurosci, 7(4), 322–335. 10.1006/mcne.1996.0024 [DOI] [PubMed] [Google Scholar]
- Kim HJ, DiBernardo AB, Sloane JA, Rasband MN, Solomon D, Kosaras B, Kwak SP, & Vartanian TK (2006). WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination. J Neurosci, 26(21), 5849–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, & Appel B (2006). In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci, 9(12), 1506–1511. 10.1038/nn1803 [DOI] [PubMed] [Google Scholar]
- Klages-Mundt NL, Kumar A, Zhang Y, Kapoor P, & Shen X (2018). The Nature of Actin-Family Proteins in Chromatin-Modifying Complexes. Front Genet, 9, 398. 10.3389/fgene.2018.00398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp MA, Liebscher T, Niedeggen A, Laufer S, Brommer B, Jungehulsing GJ, Strittmatter SM, Dirnagl U, & Schwab JM (2012). Small-molecule-induced Rho-inhibition: NSAIDs after spinal cord injury. Cell Tissue Res, 349(1), 119–132. 10.1007/s00441-012-1334-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, & Svitkina T (2008). Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol Biol Cell, 19(4), 1561–1574. 10.1091/mbc.E07-09-0964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudelka S, Voas MG, Almeida RG, Baraban M, Soetaert J, Meyer MP, Talbot WS, & Lyons DA (2016). Individual Neuronal Subtypes Exhibit Diversity in CNS Myelination Mediated by Synaptic Vesicle Release. Curr Biol, 26(11), 1447–1455. 10.1016/j.cub.2016.03.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Albers EM, & White R (2011). From axon-glial signalling to myelination: the integrating role of oligodendroglial Fyn kinase. Cell Mol Life Sci, 68(12), 2003–2012. 10.1007/s00018-010-0616-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow AM, Ford MC, Valdivia LE, Wilson SW, & Attwell D (2018). Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo. Nat Neurosci, 21(1), 24–28. 10.1038/s41593-017-0031-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn TB, Meberg PJ, Brown MD, Bernstein BW, Minamide LS, Jensen JR, Okada K, Soda EA, & Bamburg JR (2000). Regulating actin dynamics in neuronal growth cones by ADF/cofilin and rho family GTPases. J Neurobiol, 44(2), 126–144. [PubMed] [Google Scholar]
- Kurisu S, & Takenawa T (2009). The WASP and WAVE family proteins. Genome Biol, 10(6), 226. 10.1186/gb-2009-10-6-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafrenaye AD, & Fuss B (2011). Focal adhesion kinase can play unique and opposing roles in regulating the morphology of differentiating oligodendrocytes. J Neurochem, 115(1), 269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen LS, Chan CW, & ffrench-Constant C (2009). An integrin-contactin complex regulates CNS myelination by differential Fyn phosphorylation. J Neurosci, 29(29), 9174–9185. 10.1523/jneurosci.5942-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson CD, & Ridley AJ (2018). Rho GTPase signaling complexes in cell migration and invasion. J Cell Biol, 217(2), 447–457. 10.1083/jcb.201612069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Leach MK, Redmond SA, Chong SY, Mellon SH, Tuck SJ, Feng ZQ, Corey JM, & Chan JR (2012). A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods, 9(9), 917–922. 10.1038/nmeth.2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena JY, Legrand C, Faivre-Sarrailh C, Sarlieve LL, Ferraz C, & Rabie A (1994). High gelsolin content of developing oligodendrocytes. Int J Dev Neurosci, 12(5), 375–386. [DOI] [PubMed] [Google Scholar]
- Letourneau PC (1981). Immunocytochemical evidence for colocalization in neurite growth cones of actin and myosin and their relationship to cell--substratum adhesions. Dev Biol, 85(1), 113–122. 10.1016/0012-1606(81)90240-2 [DOI] [PubMed] [Google Scholar]
- Li Y, Wang PS, Lucas G, Li R, & Yao L (2015). ARP2/3 complex is required for directional migration of neural stem cell-derived oligodendrocyte precursors in electric fields. Stem Cell Res Ther, 6, 41. 10.1186/s13287-015-0042-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Draghi NA, & Resh MD (2004). Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J Neurosci, 24(32), 7140–7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, & Redmond L (2009). Neuronal CaMKII acts as a structural kinase. Commun Integr Biol., 2(1), 40–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linneberg C, Harboe M, & Laursen LS (2015). Axo-Glia Interaction Preceding CNS Myelination Is Regulated by Bidirectional Eph-Ephrin Signaling. ASN Neuro, 7(5). 10.1177/1759091415602859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Muggironi M, Marin-Husstege M, & Casaccia-Bonnefil P (2003). Oligodendrocyte process outgrowth in vitro is modulated by epigenetic regulation of cytoskeletal severing proteins. Glia, 44(3), 264–274. [DOI] [PubMed] [Google Scholar]
- Liu J, Magri L, Zhang F, Marsh NO, Albrecht S, Huynh JL, Kaur J, Kuhlmann T, Zhang W, Slesinger PA, & Casaccia P (2015). Chromatin landscape defined by repressive histone methylation during oligodendrocyte differentiation. J Neurosci, 35(1), 352–365. 10.1523/jneurosci.2606-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Moyon S, Hernandez M, & Casaccia P (2016). Epigenetic control of oligodendrocyte development: adding new players to old keepers. Curr Opin Neurobiol, 39, 133–138. 10.1016/j.conb.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, & Van Vactor D (2009). The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol, 10(5), 332–343. 10.1038/nrm2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HO, Franklin RJ, Attwell D, & Karadottir RT (2013). Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol, 11(12), e1001743. 10.1371/journal.pbio.1001743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Zhang J, Burke K, Romito-DiGiacomo RR, Miller RH, & Yang Y (2018). Oligodendrocyte-specific loss of Cdk5 disrupts the architecture of nodes of Ranvier as well as learning and memory. Exp Neurol, 306, 92–104. 10.1016/j.expneurol.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, & Insall RH (1998). Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol, 8(25), 1347–1356. [DOI] [PubMed] [Google Scholar]
- Marin O, Valiente M, Ge X, & Tsai LH (2010). Guiding neuronal cell migrations. Cold Spring Harb Perspect Biol, 2(2), a001834. 10.1101/cshperspect.a001834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques S, Zeisel A, Codeluppi S, van Bruggen D, Mendanha Falcao A, Xiao L, Li H, Haring M, Hochgerner H, Romanov RA, Gyllborg D, Munoz Manchado A, La Manno G, Lonnerberg P, Floriddia EM, Rezayee F, Ernfors P, Arenas E, Hjerling-Leffler J, Harkany T, Richardson WD, Linnarsson S, & Castelo-Branco G (2016). Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science, 352(6291), 1326–1329. 10.1126/science.aaf6463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lozada Z, Waggener CT, Kim K, Zou S, Knapp PE, Hayashi Y, Ortega A, & Fuss B (2014). Activation of sodium-dependent glutamate transporters regulates the morphological aspects of oligodendrocyte maturation via signaling through calcium/calmodulin-dependent kinase IIbeta’s actin-binding/-stabilizing domain. Glia, 62(9), 1543–1558. 10.1002/glia.22699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Munoz L, Rodriguez-Frade JM, Barroso R, Sorzano COS, Torreno-Pina JA, Santiago CA, Manzo C, Lucas P, Garcia-Cuesta EM, Gutierrez E, Barrio L, Vargas J, Cascio G, Carrasco YR, Sanchez-Madrid F, Garcia-Parajo MF, & Mellado M (2018). Separating Actin-Dependent Chemokine Receptor Nanoclustering from Dimerization Indicates a Role for Clustering in CXCR4 Signaling and Function. Mol Cell, 70(1), 106–119. 10.1016/j.molcel.2018.02.034 [DOI] [PubMed] [Google Scholar]
- Michalski JP, Cummings SE, O’Meara RW, & Kothary R (2016). Integrin-linked kinase regulates oligodendrocyte cytoskeleton, growth cone, and adhesion dynamics. J Neurochem, 136(3), 536–549. 10.1111/jnc.13446 [DOI] [PubMed] [Google Scholar]
- Michalski JP, & Kothary R (2015). Oligodendrocytes in a Nutshell. Front Cell Neurosci, 9, 340. 10.3389/fncel.2015.00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RH (2005). Dorsally derived oligodendrocytes come of age. Neuron, 45(1), 1–3. 10.1016/j.neuron.2004.12.032 [DOI] [PubMed] [Google Scholar]
- Miller RH, Payne J, Milner L, Zhang H, & Orentas DM (1997). Spinal cord oligodendrocytes develop from a limited number of migratory highly proliferative precursors. J Neurosci Res, 50(2), 157–168. [DOI] [PubMed] [Google Scholar]
- Mitew S, Gobius I, Fenlon LR, McDougall SJ, Hawkes D, Xing YL, Bujalka H, Gundlach AL, Richards LJ, Kilpatrick TJ, Merson TD, & Emery B (2018). Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat Commun, 9(1), 306. 10.1038/s41467-017-02719-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, & Tanoue A (2008). Cdk5 phosphorylation of WAVE2 regulates oligodendrocyte precursor cell migration through nonreceptor tyrosine kinase Fyn. J Neurosci, 28(33), 8326–8337. 10.1523/jneurosci.1482-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RD, Bieling P, & Fletcher DA (2018). From solution to surface to filament: actin flux into branched networks. Biophys Rev, 10(6), 1537–1551. 10.1007/s12551-018-0469-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita A (2011). Minimum requirements for the actin-like treadmilling motor system. Bioarchitecture, 1(5), 205–208. 10.4161/bioa.18115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narumiya S, & Thumkeo D (2018). Rho signaling research: history, current status and future directions. FEBS Lett, 592(11), 1763–1776. 10.1002/1873-3468.13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse M, Ishizaki Y, Ikenaka K, Tanaka A, & Hitoshi S (2017). Origin of oligodendrocytes in mammalian forebrains: a revised perspective. J Physiol Sci, 67(1), 63–70. 10.1007/s12576-016-0479-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz S, Sanchez P, Schmitt S, Snaidero N, Mitkovski M, Velte C, Bruckner BR, Alexopoulos I, Czopka T, Jung SY, Rhee JS, Janshoff A, Witke W, Schaap IAT, Lyons DA, & Simons M (2015). Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system. Dev Cell, 34(2), 139–151. 10.1016/j.devcel.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus JM, Wanger M, Keiser T, & Wegner A (1983). Treadmilling of actin. J Muscle Res Cell Motil, 4(5), 507–527. [DOI] [PubMed] [Google Scholar]
- O’Meara RW, Michalski JP, Anderson C, Bhanot K, Rippstein P, & Kothary R (2013). Integrin-Linked Kinase Regulates Process Extension in Oligodendrocytes via Control of Actin Cytoskeletal Dynamics. J Neurosci, 33(23), 9781–9793. 10.1523/jneurosci.5582-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Fukuda S, & Broxmeyer HE (2002). Activation of Wiskott-Aldrich syndrome protein and its association with other proteins by stromal cell-derived factor-1alpha is associated with cell migration in a T-lymphocyte line. Exp Hematol, 30(7), 761–766. [DOI] [PubMed] [Google Scholar]
- Okada A, Tominaga M, Horiuchi M, & Tomooka Y (2007). Plexin-A4 is expressed in oligodendrocyte precursor cells and acts as a mediator of semaphorin signals. Biochem Biophys Res Commun, 352(1):158–163. 10.1016/j.bbrc.2006.10.176 [DOI] [PubMed] [Google Scholar]
- Okada A, & Tomooka Y (2012). Possible roles of Plexin-A4 in positioning of oligodendrocyte precursor cells in developing cerebral cortex. Neurosci Lett, 516(2), 259–264. 10.1016/j.neulet.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Okamoto K, Bosch M, & Hayashi Y (2009). The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology (Bethesda). 24, 357–366. [DOI] [PubMed] [Google Scholar]
- Olson EN, & Nordheim A (2010). Linking actin dynamics and gene transcription to drive cellular motile functions. Nat Rev Mol Cell Biol, 11(5), 353–365. 10.1038/nrm2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Hirahara Y, Gotoh H, Nomura T, Takebayashi H, Yamada H, & Ikenaka K (2018). Origin of Oligodendrocytes in the Vertebrate Optic Nerve: A Review. Neurochem Res, 43(1), 3–11. 10.1007/s11064-017-2404-8 [DOI] [PubMed] [Google Scholar]
- Ornelas IM, McLane LE, Saliu A, Evangelou AV, Khandker L, & Wood TL (2016). Heterogeneity in oligodendroglia: Is it relevant to mouse models and human disease? J Neurosci Res, 94(12), 1421–1433. 10.1002/jnr.23900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega MC, Bribian A, Peregrin S, Gil MT, Marin O, & de Castro F (2012). Neuregulin-1/ErbB4 signaling controls the migration of oligodendrocyte precursor cells during development. Exp Neurol, 235(2), 610–620. 10.1016/j.expneurol.2012.03.015 [DOI] [PubMed] [Google Scholar]
- Osterhout DJ, Wolven A, Wolf RM, Resh MD, & Chao MV (1999). Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol, 145(6), 1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluch EK, & Raz E (2013). The role and regulation of blebs in cell migration. Curr Opin Cell Biol, 25(5), 582–590. 10.1016/j.ceb.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaloni D, Le Clainche C, & Carlier MF (2001). Mechanism of actin-based motility. Science, 292(5521), 1502–1506. 10.1126/science.1059975 [DOI] [PubMed] [Google Scholar]
- Park J, Liu B, Chen T, Li H, Hu X, Gao J, Zhu Y, Zhu Q, Qiang B, Yuan J, Peng X, & Qiu M (2008). Disruption of Nectin-like 1 cell adhesion molecule leads to delayed axonal myelination in the CNS. J Neurosci, 28(48), 12815–12819. 10.1523/jneurosci.2665-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, & Bansal R (1993). The oligodendrocyte and its many cellular processes. Trends Cell Biol, 3, 191–197. [DOI] [PubMed] [Google Scholar]
- Piaton G, Aigrot MS, Williams A, Moyon S, Tepavcevic V, Moutkine I, Gras J, Matho KS, Schmitt A, Soellner H, Huber AB, Ravassard P, & Lubetzki C (2011). Class 3 semaphorins influence oligodendrocyte precursor recruitment and remyelination in adult central nervous system. Brain, 134(Pt 4), 1156–1167. 10.1093/brain/awr022 [DOI] [PubMed] [Google Scholar]
- Pizarro-Cerda J, Chorev DS, Geiger B, & Cossart P (2017). The Diverse Family of Arp2/3 Complexes. Trends Cell Biol, 27(2), 93–100. 10.1016/j.tcb.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol SU, Polanco JJ, Seidman RA, O’Bara MA, Shayya HJ, Dietz KC, & Sim FJ (2017). Network-Based Genomic Analysis of Human Oligodendrocyte Progenitor Differentiation. Stem Cell Reports, 9(2), 710–723. 10.1016/j.stemcr.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD (2007). Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct., 36, 451–477. [DOI] [PubMed] [Google Scholar]
- Pollard TD, & Cooper JA (2009). Actin, a central player in cell shape and movement. Science., 326(5957), 1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekharan S, Baker KA, Horn KE, Jarjour AA, Antel JP, & Kennedy TE (2009). Netrin 1 and Dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA. Development, 136(3), 415–426. 10.1242/dev.018234 [DOI] [PubMed] [Google Scholar]
- Rajasekharan S, Bin JM, Antel JP, & Kennedy TE (2010). A central role for RhoA during oligodendroglial maturation in the switch from netrin-1-mediated chemorepulsion to process elaboration. J Neurochem, 113(6), 1589–1597. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S (1890a). Notas anatómicas I. Sobre la aparición de las expansiones celulares en la médula embrionaria. Gac. Sanit. Barc, 12, 413–419. [Google Scholar]
- Ramón y Cajal S (1890b). A quelle époque apparaissent les expansions des cellules nerveuses de la moelle épinière du poulet. Anat Anz, 5(609–613). [Google Scholar]
- Redmond SA, Mei F, Eshed-Eisenbach Y, Osso LA, Leshkowitz D, Shen YA, Kay JN, Aurrand-Lions M, Lyons DA, Peles E, & Chan JR (2016). Somatodendritic Expression of JAM2 Inhibits Oligodendrocyte Myelination. Neuron, 91(4), 824–836. 10.1016/j.neuron.2016.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard D, Rogemond V, Charrier E, Aguera M, Bagnard D, Belin MF, Thomasset N, & Honnorat J (2001). Isolation and expression pattern of human Unc-33-like phosphoprotein 6/collapsin response mediator protein 5 (Ulip6/CRMP5): coexistence with Ulip2/CRMP2 in Sema3a- sensitive oligodendrocytes. J Neurosci, 21(18), 7203–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson WD, Kessaris N, & Pringle N (2006). Oligodendrocyte wars. Nat Rev Neurosci, 7(1), 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Landsberg C (2001). Organization and functional roles of the cytoskeleton in oligodendrocytes. Microsc Res Tech, 52(6), 628–636. [DOI] [PubMed] [Google Scholar]
- Richter-Landsberg C (2008). The cytoskeleton in oligodendrocytes. Microtubule dynamics in health and disease. J Mol Neurosci, 35(1), 55–63. 10.1007/s12031-007-9017-7 [doi] [DOI] [PubMed] [Google Scholar]
- Richter-Landsberg C (2016). Protein aggregate formation in oligodendrocytes: tau and the cytoskeleton at the intersection of neuroprotection and neurodegeneration. Biol Chem, 397(3), 185–194. 10.1515/hsz-2015-0157 [DOI] [PubMed] [Google Scholar]
- Ridley AJ, & Hall A (1992). The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell, 70(3), 389–399. [DOI] [PubMed] [Google Scholar]
- Rumsby M, Afsari F, Stark M, & Hughson E (2003). Microfilament and microtubule organization and dynamics in process extension by central glia-4 oligodendrocytes: evidence for a microtubule organizing center. Glia, 42(2), 118–129. [DOI] [PubMed] [Google Scholar]
- Rusielewicz T, Nam J, Damanakis E, John GR, Raine CS, & Melendez-Vasquez CV (2014). Accelerated repair of demyelinated CNS lesions in the absence of non-muscle myosin IIB. Glia, 62(4), 580–591. 10.1002/glia.22627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer I, Muller C, Luhmann HJ, & White R (2016). MOBP levels are regulated by Fyn kinase and affect the morphological differentiation of oligodendrocytes. J Cell Sci, 129(5), 930–942. 10.1242/jcs.172148 [DOI] [PubMed] [Google Scholar]
- Schmidt C, Ohlemeyer C, Labrakakis C, Walter T, Kettenmann H, & Schnitzer J (1997). Analysis of motile oligodendrocyte precursor cells in vitro and in brain slices. Glia, 20(4), 284–298. [PubMed] [Google Scholar]
- Schnadelbach O, Ozen I, Blaschuk OW, Meyer RL, & Fawcett JW (2001). N-cadherin is involved in axon-oligodendrocyte contact and myelination. Mol Cell Neurosci, 17(6), 1084–1093. 10.1006/mcne.2001.0961 [DOI] [PubMed] [Google Scholar]
- Seixas AI, Azevedo MM, Paes de Faria J, Fernandes D, Mendes Pinto I, & Relvas JB (2019). Evolvability of the actin cytoskeleton in oligodendrocytes during central nervous system development and aging. Cell Mol Life Sci, 76(1), 1–11. 10.1007/s00018-018-2915-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Schmitt S, Bergner CG, Tyanova S, Kannaiyan N, Manrique-Hoyos N, Kongi K, Cantuti L, Hanisch UK, Philips MA, Rossner MJ, Mann M, & Simons M (2015). Cell type- and brain region-resolved mouse brain proteome. Nat Neurosci, 18(12), 1819–1831. 10.1038/nn.4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar S, Pernier J, & Carlier MF (2016). Regulators of actin filament barbed ends at a glance. J Cell Sci, 129(6), 1085–1091. 10.1242/jcs.179994 [DOI] [PubMed] [Google Scholar]
- Simpson PB, & Armstrong RC (1999). Intracellular signals and cytoskeletal elements involved in oligodendrocyte progenitor migration. Glia, 26(1), 22–35. [PubMed] [Google Scholar]
- Sinha B, Biswas A, & Soni GV (2018). Cellular and Nuclear Forces: An Overview. Methods Mol Biol, 1805, 1–29. 10.1007/978-1-4939-8556-2_1 [DOI] [PubMed] [Google Scholar]
- Small RK, Riddle P, & Noble M (1987). Evidence for migration of oligodendrocyte--type-2 astrocyte progenitor cells into the developing rat optic nerve. Nature, 328(6126), 155–157. 10.1038/328155a0 [DOI] [PubMed] [Google Scholar]
- Snaidero N, Mobius W, Czopka T, Hekking LH, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, Nave KA, & Simons M (2014). Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell, 156(1–2), 277–290. 10.1016/j.cell.2013.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaidero N, & Simons M (2014). Myelination at a glance. J Cell Sci, 127(Pt 14), 2999–3004. 10.1242/jcs.151043 [DOI] [PubMed] [Google Scholar]
- Snaidero N, & Simons M (2017). The logistics of myelin biogenesis in the central nervous system. Glia, 65(7), 1021–1031. 10.1002/glia.23116 [DOI] [PubMed] [Google Scholar]
- Snaidero N, Velte C, Myllykoski M, Raasakka A, Ignatev A, Werner HB, Erwig MS, Mobius W, Kursula P, Nave KA, & Simons M (2017). Antagonistic Functions of MBP and CNP Establish Cytosolic Channels in CNS Myelin. Cell Rep, 18(2), 314–323. 10.1016/j.celrep.2016.12.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Goetz BD, Baas PW, & Duncan ID (2001). Cytoskeletal reorganization during the formation of oligodendrocyte processes and branches. Mol Cell Neurosci, 17(4), 624–636. [DOI] [PubMed] [Google Scholar]
- Spassky N, de Castro F, Le Bras B, Heydon K, Queraud-LeSaux F, Bloch-Gallego E, Chedotal A, Zalc B, & Thomas JL (2002). Directional guidance of oligodendroglial migration by class 3 semaphorins and netrin-1. J Neurosci, 22(14), 5992–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber BR, Boyle-Walsh EA, Engleka MJ, Gadue P, Peterson AC, Stein PL, Scherer SS, & McMorris FA (2001). A unique role for Fyn in CNS myelination. J Neurosci, 21(6), 2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer SO, Sitnikov S, Kamen Y, Evans KA, Kronenberg-Versteeg D, Dietmann S, de Faria O Jr., Agathou S, & Karadottir RT (2019). Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age. Neuron, 101(3), 459–471 10.1016/j.neuron.2018.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann C, Timmler S, Barrantes-Freer A, & Simons M (2019). Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol Rev, 99(3), 1381–1431. 10.1152/physrev.00031.2018 [DOI] [PubMed] [Google Scholar]
- Steffen A, Faix J, Resch GP, Linkner J, Wehland J, Small JV, Rottner K, & Stradal TE (2006). Filopodia formation in the absence of functional WAVE- and Arp2/3-complexes. Mol Biol Cell, 17(6), 2581–2591. 10.1091/mbc.e05-11-1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser GA, Rahim NA, VanderWaal KE, Gertler FB, & Lanier LM (2004). Arp2/3 is a negative regulator of growth cone translocation. Neuron, 43(1), 81–94. 10.1016/j.neuron.2004.05.015 [DOI] [PubMed] [Google Scholar]
- Suarez-Pozos E, Thomason EJ, & Fuss B (2019). Glutamate Transporters: Expression and Function in Oligodendrocytes. Neurochem Res. 10.1007/s11064-018-02708-x [DOI] [PMC free article] [PubMed]
- Sugimoto Y, Taniguchi M, Yagi T, Akagi Y, Nojyo Y, & Tamamaki N (2001). Guidance of glial precursor cell migration by secreted cues in the developing optic nerve. Development, 128(17), 3321–3330. [DOI] [PubMed] [Google Scholar]
- Takenawa T, & Suetsugu S (2007). The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol, 8(1), 37–48. 10.1038/nrm2069 [DOI] [PubMed] [Google Scholar]
- Tamariz E, & Varela-Echavarria A (2015). The discovery of the growth cone and its influence on the study of axon guidance. Front Neuroanat, 9, 51. 10.3389/fnana.2015.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Cha C, Ye Y, Zhang J, Li S, Wu F, Gong S, & Guo G (2015). CRMP4 and CRMP2 Interact to Coordinate Cytoskeleton Dynamics, Regulating Growth Cone Development and Axon Elongation. Neural Plast, 2015, 947423. 10.1155/2015/947423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepavcevic V, Kerninon C, Aigrot MS, Meppiel E, Mozafari S, Arnould-Laurent R, Ravassard P, Kennedy TE, Nait-Oumesmar B, & Lubetzki C (2014). Early netrin-1 expression impairs central nervous system remyelination. Ann Neurol, 76(2), 252–268. 10.1002/ana.24201 [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, & Goodman CS (1996). The molecular biology of axon guidance. Science, 274(5290), 1123–1133. 10.1126/science.274.5290.1123 [DOI] [PubMed] [Google Scholar]
- Thurnherr T, Benninger Y, Wu X, Chrostek A, Krause SM, Nave KA, Franklin RJ, Brakebusch C, Suter U, & Relvas JB (2006). Cdc42 and Rac1 signaling are both required for and act synergistically in the correct formation of myelin sheaths in the CNS. J Neurosci., 26(40), 10110–10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Yin H, Deng X, Tang B, Ren X, & Jiang T (2018). CXCL12 induces migration of oligodendrocyte precursor cells through the CXCR4activated MEK/ERK and PI3K/AKT pathways. Mol Med Rep, 18(5), 4374–4380. 10.3892/mmr.2018.9444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter J, & Mittmann T (2019). Diversity in the Oligodendrocyte Lineage: Current Evidence. Neuron, 101(3), 356–357. 10.1016/j.neuron.2019.01.032 [DOI] [PubMed] [Google Scholar]
- Tsai E, & Casaccia P (2019). Mechano-modulation of nuclear events regulating oligodendrocyte progenitor gene expression. Glia, 67(7), 1229–1239. 10.1002/glia.23595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Macklin WB, & Miller RH (2006). Netrin-1 is required for the normal development of spinal cord oligodendrocytes. J Neurosci, 26(7), 1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, & Miller RH (2002). Glial cell migration directed by axon guidance cues. Trends Neurosci, 25(4), 173–175; discussion 175–176. [DOI] [PubMed] [Google Scholar]
- Tsai HH, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien AC, Kuo CJ, Chan JR, Daneman R, & Fancy SP (2016). Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science, 351(6271), 379–384. 10.1126/science.aad3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Tessier-Lavigne M, & Miller RH (2003). Netrin 1 mediates spinal cord oligodendrocyte precursor dispersal. Development, 130(10), 2095–2105. [DOI] [PubMed] [Google Scholar]
- Umemori H, Sato S, Yagi T, Aizawa S, & Yamamoto T (1994). Initial events of myelination involve Fyn tyrosine kinase signalling. Nature, 367(6463), 572–576. 10.1038/367572a0 [DOI] [PubMed] [Google Scholar]
- van Bruggen D, Agirre E, & Castelo-Branco G (2017). Single-cell transcriptomic analysis of oligodendrocyte lineage cells. Curr Opin Neurobiol, 47, 168–175. 10.1016/j.conb.2017.10.005 [DOI] [PubMed] [Google Scholar]
- Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, & Ampe C (2008). Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol, 87(8–9), 649–667. 10.1016/j.ejcb.2008.04.001 [DOI] [PubMed] [Google Scholar]
- Varland S, Vandekerckhove J, & Drazic A (2019). Actin Post-translational Modifications: The Cinderella of Cytoskeletal Control. Trends Biochem Sci. 10.1016/j.tibs.2018.11.010 [DOI] [PubMed]
- Viita T, & Vartiainen MK (2017). From Cytoskeleton to Gene Expression: Actin in the Nucleus. Handb Exp Pharmacol, 235, 311–329. 10.1007/164_2016_27 [DOI] [PubMed] [Google Scholar]
- Virtanen JA, & Vartiainen MK (2017). Diverse functions for different forms of nuclear actin. Curr Opin Cell Biol, 46, 33–38. 10.1016/j.ceb.2016.12.004 [DOI] [PubMed] [Google Scholar]
- Vitriol EA, & Zheng JQ (2012). Growth cone travel in space and time: the cellular ensemble of cytoskeleton, adhesion, and membrane. Neuron, 73(6), 1068–1081. 10.1016/j.neuron.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggener CT, Dupree JL, Elgersma Y, & Fuss B (2013). CaMKIIbeta Regulates Oligodendrocyte Maturation and CNS Myelination. J Neurosci, 33(25), 10453–10458. 10.1523/jneurosci.5875-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Rusielewicz T, Tewari A, Leitman EM, Einheber S, & Melendez-Vasquez CV (2012). Myosin II is a negative regulator of oligodendrocyte morphological differentiation. J Neurosci Res, 90(8), 1547–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tewari A, Einheber S, Salzer JL, & Melendez-Vasquez CV (2008). Myosin II has distinct functions in PNS and CNS myelin sheath formation. J Cell Biol., 182(6), 1171–1184. Epub 2008 Sep 1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner A (1976). Head to tail polymerization of actin. J Mol Biol, 108(1), 139–150. 10.1016/s0022-2836(76)80100-3 [DOI] [PubMed] [Google Scholar]
- Wheeler NA, & Fuss B (2016). Extracellular cues influencing oligodendrocyte differentiation and (re)myelination. Exp Neurol, 283(Pt B), 512–530. 10.1016/j.expneurol.2016.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, & Kramer-Albers EM (2014). Axon-glia interaction and membrane traffic in myelin formation. Front Cell Neurosci, 7, 284. 10.3389/fncel.2013.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggan O, Schroder B, Krapf D, Bamburg JR, & DeLuca JG (2017). Cofilin Regulates Nuclear Architecture through a Myosin-II Dependent Mechanotransduction Module. Sci Rep, 7, 40953. 10.1038/srep40953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Piaton G, Aigrot MS, Belhadi A, Theaudin M, Petermann F, Thomas JL, Zalc B, & Lubetzki C (2007). Semaphorin 3A and 3F: key players in myelin repair in multiple sclerosis? Brain, 130(Pt 10), 2554–2565. 10.1093/brain/awm202 [DOI] [PubMed] [Google Scholar]
- Williams SK, Spence HJ, Rodgers RR, Ozanne BW, Fitzgerald U, & Barnett SC (2005). Role of Mayven, a kelch-related protein in oligodendrocyte process formation. J Neurosci Res, 81(5), 622–631. [DOI] [PubMed] [Google Scholar]
- Wilson R, & Brophy PJ (1989). Role for the oligodendrocyte cytoskeleton in myelination. J Neurosci Res, 22(4), 439–448. [DOI] [PubMed] [Google Scholar]
- Winder SJ, & Ayscough KR (2005). Actin-binding proteins. J Cell Sci, 118(Pt 4), 651–654. 10.1242/jcs.01670 [DOI] [PubMed] [Google Scholar]
- Wolf RM, Wilkes JJ, Chao MV, & Resh MD (2001). Tyrosine phosphorylation of p190 RhoGAP by Fyn regulates oligodendrocyte differentiation. J Neurobiol., 49(1), 62–78. [DOI] [PubMed] [Google Scholar]
- Xiang X, Zhang X, & Huang QL (2012). Plexin A3 is involved in semaphorin 3F-mediated oligodendrocyte precursor cell migration. Neurosci Lett, 530(2), 127–132. 10.1016/j.neulet.2012.09.058 [DOI] [PubMed] [Google Scholar]
- Yang C, & Svitkina T (2011). Filopodia initiation: focus on the Arp2/3 complex and formins. Cell Adh Migr, 5(5), 402–408. 10.4161/cam.5.5.16971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LE, Heimsath EG, & Higgs HN (2015). Cell type-dependent mechanisms for formin-mediated assembly of filopodia. Mol Biol Cell, 26(25), 4646–4659. 10.1091/mbc.E15-09-0626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Schaefer AW, Burnette DT, Schoonderwoert VT, & Forscher P (2003). Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron, 40(5), 931–944. 10.1016/s0896-6273(03)00754-2 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, & Wu JQ (2014). An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci, 34(36), 11929–11947. 10.1523/jneurosci.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ (2000). Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature, 403(6765), 89–93. 10.1038/47501 [DOI] [PubMed] [Google Scholar]
- Zuchero JB, Coutts AS, Quinlan ME, Thangue NB, & Mullins RD (2009). p53-cofactor JMY is a multifunctional actin nucleation factor. Nat Cell Biol, 11(4), 451–459. 10.1038/ncb1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero JB, Fu MM, Sloan SA, Ibrahim A, Olson A, Zaremba A, Dugas JC, Wienbar S, Caprariello AV, Kantor C, Leonoudakis D, Lariosa-Willingham K, Kronenberg G, Gertz K, Soderling SH, Miller RH, & Barres BA (2015). CNS myelin wrapping is driven by actin disassembly. Dev Cell, 34(2), 152–167. 10.1016/j.devcel.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]


