Abstract
Measurements of IgG and IgA in human rectal secretions are used to evaluate the antibodies elicited by HIV vaccines or the bioaccumulation following immuno-prophylaxis at the sites of HIV exposure. To improve sampling methods and tolerability of the procedure, we optimized a balloon device (OriCol) for rectal microbiome sampling requiring 10-second inflation and compared this method to a 5-minute collection using sponges. Lubrication of the device did not interfere with IgG, IgA or hemoglobin (Hb) ELISA. Lubricated OriCols inflated to 30cc minimized Hb contamination (<4.68ng/ml) compared to collections with two sponge types (Weck-Cel 267.2ng/ml, p<0.0001; and Merocel 59.38ng/ml, p=0.003). Median human serum albumin for OriCols was 14.9μg/ml whereas Merocels and Weck-Cels were 28.57μg/ml (p=0.0005) and 106.2μg/ml (p=0.0002), respectively. Consistent with reduced systemic contamination, the median IgG measured in OriCol-collected rectal secretions (986ng) was lower than secretions from sponges (Weck-Cel 8,588ng; p<0.0001; Merocel 2,509ng; p=0.0389). The median IgA yield of samples using the OriCol method (75,253ng) was comparable to that using Merocel (71,672 ng; p=0.6942) but significantly higher than Weck-Cel sponges (16,173 ng, p=0.0336). Median recovery volumes for OriCols were 800μl whereas Merocels and Weck-Cels were 615μl (p=0.0010) and 655μl (p=0.0113), respectively. The balloon device was acceptable among 23 participants, as 85.1% experiencing their first collection ranked it as “7-acceptable-a lot” or “6-acceptable-somewhat” in a 7-point Likert scale. Therefore, lubricated OriCols inflated to 30cc allowed for a rapid, well-tolerated, blood-free collection of human rectal secretions.
INTRODUCTION
The highest probability of sexual transmission of HIV occurs through receptive anal intercourse (RAI)(1). Gaining insights into the immune responses needed to rapidly contain HIV-1 following rectal exposures is critical to lower transmission rates by vaccination. Recent studies have demonstrated retrograde migration of semen delivered to the rectal compartment after simulated intercourse(2), defining the area where infectious ejaculate interacts with rectal tissue. Similar investigations have sought to define where rectally and orally delivered microbicides/drugs localize to prevent HIV(3–6), and to identify the compartments where the interventions are most active. Similar characterizations are needed for antibodies induced by vaccines or delivered by passive prophylaxis(7), as they aim to block infection early after exposure. Establishing the rectal immunoglobulin concentrations needed to prevent HIV infection at mucosal surfaces is fundamental in the comparative evaluation of various immune strategies in current clinical testing.
Only a single layer of glandular columnar epithelium separates the rectal lumen from the lamina propria, where many HIV target cells reside(8). In the rectum, antibodies are differentially concentrated and their transport is heavily regulated by Fc receptors(9–15), with IgG dominating in blood and colonic tissue, and IgA preferentially found in secretions(9–11, 16–19). Thus, the analysis of gastrointestinal biodistribution of HIV vaccine-induced immunoglobulins and the pharmacokinetics of passively-transferred monoclonal antibodies in rectal compartments requires careful sampling, to ensure that the compartmentalization of immunoglobulins in secretions, tissue and blood is maintained. Thus, defining the precise mucosal IgG and IgA levels that can accumulate in colonic compartments following immune-based interventions, in the absence of systemic blood immunoglobulin contamination, is critical to assess their potential benefit in mediating HIV prevention.
Current rectal secretion sampling methods in humans use either Sno-Strips(20), cellulose acetate wicks(21), or sponges(22–24) which are inserted in the rectum using a syringe, pipette, or anoscope, and held against the rectal folds for up to 5 minutes to absorb secretions. Merocel sponges have crosslinked polyvinyl alcohol in an open reticulate structure to facilitate secretion recovery(22); Ultracell sponges have highly absorbent polyvinyl acetal(6) and Weck-Cel spears have more rigid, closed cellulose structure(25). The use of rectal sponges permits the collection of undiluted secretions(20, 21, 23, 24) and targets sampling of recent secretions near mucosal tissue folds(25). However, as the sponges were optimized for ocular surgery, which does not require elution or protein extraction, the different absorption materials have not been compared for recovery of immunoglobulins. Additionally, rectal sponges sample a very small surface area of the rectal compartment (~3cm2), and often do not provide sufficient material for multiple antigen-specific assays.
The OriCol balloon device is designed for rectal microbiome sampling near the mucosal surface(26). Through an anoscope, the balloon device is inflated for 10 seconds using an applicator and retracted back after collection. Inflation of 30cc to 80cc could sample rectal surfaces of 47 to 90cm2, potentially increasing the yield of secretions. Thus, we optimized procedures for collection of rectal secretions using this device. We compared rectal secretions collected via anoscopy using two different sponges versus OriCol for the measurement of rectally secreted antibody. Our overall goal was to identify rectal secretion collection procedures that 1) shortened the duration of collection for participant comfort, 2) supported the collection of mucosal immunoglobulins, and 3) decreased the likelihood of blood contamination. We demonstrate that balloon devices reduce the likelihood of Hb contamination in the secretion sample, without dramatic reductions in immunoglobulin yields.
METHODS
Study Population
For OriCol and sponge optimization studies, participants were enrolled in a Rectal Secretion Optimization Study at the Seattle Vaccine Trials Unit. To assess tolerability and feasibility for its use within a clinical trial, participants were enrolled in an experimental phase 1 HIV prevention product study at the same research clinic (HVTN 116, ClinicalTrials.gov NCT02797171). Eligibility criteria for both studies included HIV seronegative adults at low HIV risk, good general health, and no history of rectal bleeding, as well as negative screening for hemorrhoids or active rectal or perianal infection.
All participants were instructed to abstain from RAI and the use of any rectal or perianal products for 48 hours prior to the study visit.
Ethics Approvals
Informed written consent was obtained from all participants. The Fred Hutchinson Cancer Research Center Institutional Review Board characterized OriCols as a low risk device and approved all studies and procedures (IR5460 and IR8447).
OriCol Preparation
The OriCol sampling device kit (Origin Sciences) includes an anoscope, applicator with attached balloon (OriCol sampling device), connector, vial and syringe. Balloon devices were prepared with a test inflation of 30–80cc and retraction to 100cc. When lubricated, approximately 0.5ml of lubricant (PDI Lubricating Jelly II) was applied by a gloved finger around the inside rim of the device, bordering the balloon attachment. The device was then inflated and deflated twice to 30–60cc to spread the lubricant. Devices without lubrication were only assessed for inflation. For all devices, the syringe was then removed, the plunger reset to 30–80cc and the syringe reattached for sample collection.
OriCol Collection and Elution
Participants were positioned lying on their left side and a lubricated OriCol anoscope was inserted 8cm into the rectum. The balloon device was inserted into the anoscope with a syringe attached and inflated to 30–80cc and held for 10 seconds. The balloon was then withdrawn completely into the device and the device was removed from the anoscope. Several procedures were compared for processing of the OriCol secretions after sample collection:
In method 1, 1 ml of Dulbecco’s phosphate buffered Saline (PBS, Gibco) with 1× protease inhibitors type I (PI1, Calbiotech) was poured into the deflated balloon immediately following collection at the bedside. The device was inverted 10 times to mix the secretions with the media, and emptied by inversion into a 4ml container, which was shipped to the nearby lab in wet ice for centrifugation.
In method 2, the balloon device without media was shipped to the lab in wet ice immediately after collection. At the lab, 1ml of PBS with 1× PI1 was added to the balloon, and its surface was washed with the media 5 times to remove any attached secretions and stool. The media and secretions were pipetted out of the OriCol and transferred to a 2ml tube for centrifugation.
In method 3, 1 ml of PBS with 1× PI1 was poured into the balloon immediately following collection at the bedside and then shipped to the lab in wet ice for further processing. At the lab, the balloon surface was washed with the media 5 times to remove any attached secretions and stool. The media and secretions were pipetted out of the Oricol and transferred to a 2ml tube for centrifugation.
In method 4, 1 ml of RPMI 1640 with 25mM HEPES buffer and L-glutamine (Gibco) containing 500U/ml of nystatin (Gibco), 100U/ml penicillin and 100U/ml streptomycin was poured into the balloon immediately following collection at the bedside and then shipped to the lab in wet ice for further processing. At the lab, the balloon surface was washed with the media 5 times to remove any attached secretions and stool. The media and secretions were pipetted out of the OriCol and transferred to a 2ml tube for centrifugation. The supernatant received PII to a final concentration of 1X.
In all four methods, the sample was processed within 2 hours of collection. The collected sample was centrifuged at 16,000×g for 5 minutes at 4°C. The supernatant was removed from the stool/solid mucus pellet, and the volume measured with a p1000 pipette. The supernatant was aliquoted and stored at −80°C before use in assays.
Sponge Preparation
Approximately 2.6cm was cut off the narrowest end of a 5ml narrow stem disposable flexible polyethylene transfer pipette (Fisher) and the handle of a Weck-Cel surgical spear or Merocel eye sponge was inserted approximately 1.4cm into the stem of the pipette(25). A 2ml tube (Sarstedt Micro tube) was prefilled with 50μl PBS at the lab. To facilitate absorption and minimize mucosal damage with a dry sponge, each prepared spear/sponge was pre-moistened by placing the tip into the tube with PBS prior to collection.
Sponge Collection and Elution
Participants were positioned lying on their left side and a lubricated Sani-Scope Disposable Anoscope was inserted 8cm into the rectum. A moistened sponge attached to a flexible pipette was inserted into the mucosal folds for 5 minutes. Following collection, the sponge was placed in a sterile 2ml cryovial and immediately placed on dry ice for shipment to the lab.
Each frozen sponge was thawed 10–15 minutes on ice, then placed on a spinX column (Costar) without filter in a microcentrifuge tube, and the handle removed with scissors. The sponge was washed with 300μl of PBS with 1× PI1, incubated at 4°C for 5 minutes, and then centrifuged at 16,000×g for 5 minutes at 4°C. The sponge was washed a second time with 300μl of PBS with 1× PI1, incubated at 4°C for 10 minutes, and centrifuged at 16,000×g for 20 minutes at 4°C. The clarified supernatant was aliquoted and stored at −80°C before use in assays.
ELISA
For Hb concentrations, samples were diluted 1:3, 1:100 and 1:300 and assayed using the human Hb ELISA Kit (Immunology Consultants Laboratory [ICL]) according to manufacturer instructions. The standard curve was fit to a 4PL model using dilutions in the range of 200ng/ml to 1.56ng/ml. Frozen serum samples from 9 healthy volunteers also donating rectal secretions were used to determine the Hb contamination threshold in the Hb ELISA. To eliminate the possible contribution of animal Hb from ingested food, Hb was also quantitated in sera dilutions from animal species (fish, porcine, bovine, goat); the Hb ELISA was found to have minimal cross-reactivity with animal Hb. Some cross-reactivity was found when goat serum was used, because the secondary antibody is an anti-goat IgG reagent, but this was only detected at a 1:1 dilution. For statistical analysis and graphing, samples with Hb concentrations below detection were replaced by the limit of quantitation of the assay (4.68ng/ml).
For IgG and IgA yields, samples were diluted 1:10, 1:100 and 1:1000 and assayed using the human IgG or IgA ELISA Kit (ICL) according to manufacturer instructions. Comparisons to the animal sera tested above demonstrated minimal cross-reactivity with animal immunoglobulins potentially present in food. For assessing the interaction of lubricating jelly with the ELISA assays, human serum was diluted in media containing various concentrations of lubricant, including those concentrations harvested after preparation of OriCols with lubrication.
For detection of Human Serum Albumin (HSA), samples were diluted at 1:500 and 1:2000 and assayed using the Albumin Human ELISA kit (Invitrogen) according to manufacturer instructions, with a limit of quantitation of 1.228ng/ml.
All the assays were read by monitoring absorbance on a Spectramax i3X Microplate Reader (Molecular Devices). Extrapolation of duplicate samples at multiple dilutions was carried out after 4PL fitting of each standard curve using Softmax Pro 6.5.1 software.
Statistical Analysis
The measurements were analyzed with R package robustrank. For independent samples, two-sided Wilcoxon-Mann-Whitney two-sample tests were performed; for partially matched samples, two-sided rank-based two-sample tests for paired data with missing values were performed(27). Continuity correction was applied in both tests. P values <0.05 were considered significant.
RESULTS
Rectal Secretion Optimization Study Conduct
Thirty-eight participants from the Seattle area were enrolled in the Rectal Secretion Optimization Study (see Table I). Participants were 58% assigned male at birth (AMAB) and 42% female assigned at birth (AFAB). They had a median age 35, with no history of bleeding disorders, report or evidence of rectal abnormalities. Of the 38 participants, 14 had collections using both OriCols and sponges; 12 had collections only with OriCol, and 12 had collections only with sponges. No participant reported complications, including signs of rectal bleeding, following the procedures.
Table I.
Demographics of the 38 participants enrolled and evaluated in the Rectal Secretion Optimization Study.
| Characteristics | N (%) 38 (100%) |
|---|---|
| Sex Assigned at Birth | |
| Male (AMAB) | 22 (58%) |
| Female (AFAB) | 16 (42%) |
| Gender | |
| Cisgender | 37 (98%) |
| Transgender | 1 (2%) |
| Race | |
| White | 29 (76%) |
| African American/Black | 5 (13%) |
| Native American | 1 (3%) |
| Asian | 1 (3%) |
| Multiracial* | 2 (5%) |
| Ethnicity | |
| Hispanic or Latino | 3 (8%) |
| Age | |
| 18–24 | 6 (16%) |
| 25–29 | 11 (29%) |
| 30–34 | 6 (16%) |
| 35–39 | 6 (16%) |
| 40–44 | 5 (13%) |
| 45–49 | 2 (5%) |
| 50–54 | 2 (5%) |
Includes African American/Black/White
Optimizing OriCol Collections to Maximize Mucosal IgG Recovery and Minimize Blood Contamination
Manufacturer recommendations for the OriCol are to insert the lubricated anoscope in the rectal compartment, inflate the unlubricated device to 80cc for 10 seconds and then retract the balloon into the device. Fresh blood was often observed in these samples, and a small amount of fresh blood was also noted in the rectal cavity immediately following collection. We initially thought this was due to suction on rectal mucosa during balloon deflation; but carefully breaking the suction seal prior to removal of the device did not prevent blood contamination (data not shown).
To prevent bleeding during the collection procedure, we modified two other conditions: balloon inflation volume and lubrication. We tested balloon inflation at 60cc and 30cc (Figure 1A), and we added lubrication to the rim of the balloon-tube interface aiming to reduce tissue damage. OriCol collections were compared to free Hb present in serum samples, as the centrifugation of rectal secretions removes both fecal contamination and red blood cells from the secretion supernatant (Figure 1B). Rectal samples with ≥1% of serum Hb levels (median 91,373ng/ml, IQR 62,383–180,874) were deemed unacceptable for mucosal secretion IgG quantitation (marked in red), since such blood contamination would severely confound measurement of actual mucosal IgG.
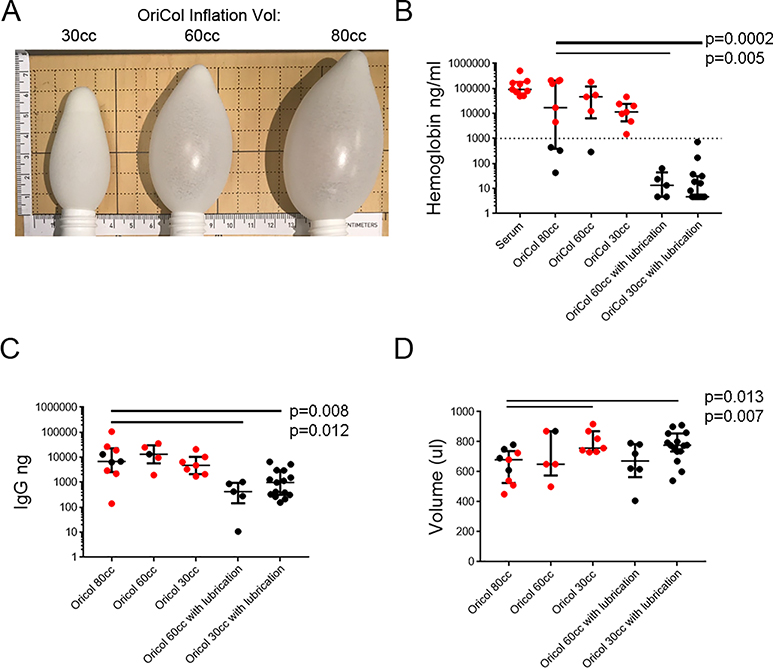
Figure 1. Hb, IgG and supernatant volume recovery from OriCol collections of rectal secretions involving 80cc, 60cc and 30cc of device inflation, with or without lubrication at the edge of the balloon.
OriCols without lubrication were inflated to either 80cc (n=9), 60cc (n=5) or 30cc (n=7). Pre-lubricated OriCols were tested with 60cc (n=5) or 30cc (n=15) inflation. Balloons inflated to 80cc with lubrication were not tested. All were processed according to method #3. The p values indicate significant comparisons when compared to manufacturer’s recommended collection (80cc without lubrication). Samples marked in red indicate Hb contamination threshold above 1% of free Hb in serum. A) Photograph of balloons inflated at 30cc, 60cc and 80cc alongside a ruler in mm. B) Hb concentration measured by ELISA. Serum samples (n=9) were used to define the Hb contamination threshold (marked by the dashed line). Hb concentrations below the detection limit were graphed at 4.68ng/ml, the limit of quantitation of the assay. C) IgG yield measured by IgG ELISA. D) Supernatant volume recovered from OriCols after centrifugation and removal of stool pellet. Lines represent medians and bars are the interquartile ranges (IQR) of each sample set.
Reducing inflation volume did not reduce Hb contamination (Figure 1B). Unlubricated OriCol collections inflated to 80cc (median 16,989ng/ml of Hb; IQR 386.5–205,130), 60cc (median Hb 91,377ng/ml; IQR 62,383–180,874), and 30cc (median Hb 46,339ng/ml; IQR 6,315–118,963) often exceeded the Hb threshold. However, OriCols tested using lubricated balloons inflated to 60cc (median Hb 13.41ng/ml; IQR 4.68–43.66) and 30cc (median Hb at limit of quantitation 4.68ng/ml; IQR 4.68–31.43) demonstrated significant reductions in Hb contamination (p=0.005 and p=0.0002, respectively).
We measured the IgG and IgA yields with and without lubrication. OriCols inflated to 30cc or 60cc with lubrication yielded slightly lower IgG than the equivalent without lubrication (Figure 1C, p=0.008 and p=0.012, respectively), but blood contamination potentially contributed to the IgG yield in samples collected without lubrication (Figure 1A). Overall, lubricated devices contained significant mucosal IgG with minimal evidence of blood contamination.
Lastly, we compared the supernatant volume of the secretions collected using the different OriCol collection methods. Samples collected with 30cc of inflation (with or without lubrication) yielded higher supernatant volumes than 60cc or 80cc inflation (Figure 1D). For example, whereas samples collected using 30cc with lubrication yielded a median recovery volume of 776μl (IQR735–855; p=0.013), manufacturer recommended collections of 80cc with no lubrication yielded a median of 680μl (IQR525–738).
Because of the reduced Hb contamination, we selected lubricated OriCols to continue our studies. We selected lubricated balloons with 30cc inflation as they required less suction upon removal from the rectum, had slightly increased IgG yield (Figure 1C) and better recovery volume (Figure 1D) when compared to 60cc with lubrication.
Effects of Lubrication on Assay Readouts
Since the optimal collection method required spreading lubricant (containing water and glycerin) on the balloon, by placing at the rim and inflating and deflating twice, we wanted to explore the possibility that lubricant contamination would affect our assays. It is not possible to collect rectal secretions without any lubrication, as most techniques involve a lubricated anoscope insertion, so we tested the effects of lubricant on IgG, IgA and Hb ELISA assay readouts.
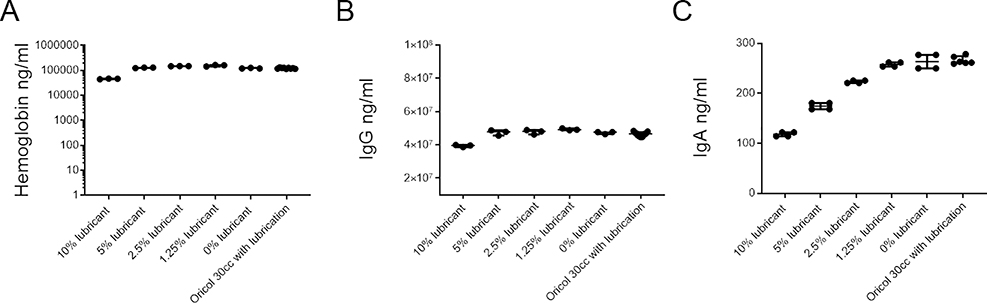
We diluted lubricant at 10%, 5%, 2.5% and 1.25% weight by volume and used these as diluents for a human serum sample with known measurements. Media collected from three pre-lubricated devices was also used to dilute serum, to examine the effects of the maximum concentration of lubricant present in the secretion collected from OriCols. Pre-lubricated balloons were washed with 1ml of media, and the lubricant-containing media was used at 1:10 dilution to test Hb (Figure 2A), IgG (Figure 2B) and IgA (Figure 2C) of the same serum sample. No rectal insertion took place in these OriCols. Although 10% lubricant had effects in all the assays, lubricant at the concentrations used in the balloons performed comparably to 0% lubricant and did not affect the quantitation of Hb, IgG or IgA.
Figure 2. Effects of lubricant in Hb, IgG and IgA detection.
The same serum sample was measured in triplicate for A) Hb concentration, B) IgG concentration, and C) IgA concentration. All were measured by ELISA. Serum was diluted in different medias, each containing different concentrations of lubricant (10%, 5%, 2.5%, 1.25% or 0% weight/volume). In addition, 1:10 of media from washing 3 lubricated OriCols (not inserted in the rectum) were also compared by ELISA. To have samples within the ELISA ranges, before addition of the lubricant-spiked medias, serum was diluted 1:1,000 for IgA, 1:10,000 for IgG, and 1:1,000 for Hb. There were no significant p values among lubricated OriCols and media containing 0% lubrication. Lines represent medians and bars are the interquartile ranges (IQR) of each sample set.
Optimizing OriCol Sample Processing for Antibody Measurements
For microbiome analysis, the manufacturer recommends immediate addition of 1–4ml media to the balloon, inverting the device to mix thoroughly, and immediate transfer of the sample with media into a single aliquot tube, which can be frozen at the clinic.
For our clinical trial applications, a short collection time was desired to improve subject tolerability and clinical operations, but the recommended downstream sample processing required modifications. First, we needed to minimize freeze-thaw cycles, which can damage antibody integrity. Second, multiple aliquots of the same sample were required for different analyses in various laboratories. Third, many of our downstream IgG assays require separation of the solid phase of fecal matter or mucus from the soluble proteins before storage. Fourth, as some of our assays aim to identify antigen-specific IgG, an initial dilution into 4ml of media was suboptimal as it would leave many rectal sample concentrations below the limit of detection.
Therefore, we assessed the effects of modifications to the manufacturer recommendations to maximize sample IgG concentrations. For optimization, all samples were collected using 1ml of media using processing methods 1) manufacturer recommended procedures at the clinic, except for a lower media volume, 2) all recovery and processing done at the lab, and 3) media added at the clinic and processing done at the lab, as described in the methods.
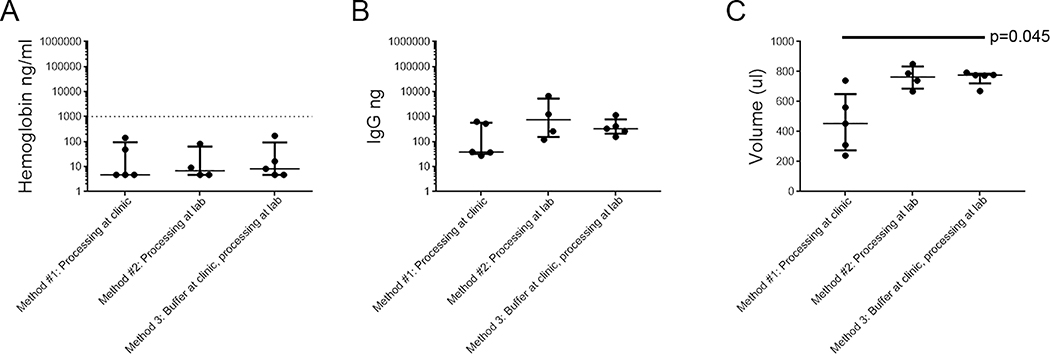
As expected from the improved collection method of 30cc inflation with lubrication, Hb contamination was minimal in all samples regardless of the sample processing method (Figure 3A; median 6.42ng/ml; IQR 4.68–39.9 ng/ml). We did not detect significant differences in IgG recovery (Figure 3B; median 296.6ng; IQR 129.1–601.9).
Figure 3. Comparison of different OriCol processing methods for Hb, IgG, and volume recovery.
Rectal secretions were collected with lubricated OriCol with 30cc inflation. The samples were processed utilizing the following methods: 1) manufacturer recommended procedures at the clinic (n=5), 2) all recovery and processing done at the lab (n=4), and 3) media added at the clinic and processing done at the lab (n=6), as described in the methods section. A) Hb concentration measured by ELISA. Serum samples (n=9) were used to define the Hb contamination threshold (1% of serum levels, marked by dashed line). For graphing purposes, Hb concentrations below the detection limit were plotted at 4.68μg/ml, the limit of quantitation of the assay. B) IgG yield measured by IgG ELISA. C) Supernatant volume recovered from balloon device after centrifugation and removal of stool pellet. The p values indicate significant comparisons when compared to manufacturer recommendations (method 1). Lines represent medians and bars are the interquartile ranges (IQR) of each sample set.
The largest difference between processing methods was in the volume of secretions recovered (Figure 3C). Decanting the devices with 1ml of PBS with PI1 into the manufacturer’s recommended tube at the clinic left a considerable volume in the folds of the device itself, with median recovery of 454μl (IQR 275–650). The methods utilizing pipette aspiration provided median recovery volumes of 764μl (IQR 686–834; p=0.097) and 776μl (IQR 722–786; p=0.045), after centrifugation of the stool/mucus pellet.
Therefore, we selected processing method #3 for the following reasons: 1) the addition of PI1-containing media at the clinic prevented protein degradation during transport to the lab (Figure 3B and data not shown); 2) pipetting at the lab improved recovery volume (Figure 3C); 3) insoluble matter could be removed without adding freeze-thaw cycles; and 4) we could generate smaller aliquots for single usage in protein assays.
Comparison of Secretions Collected Via OriCol (30cc with lubrication) and Sponges
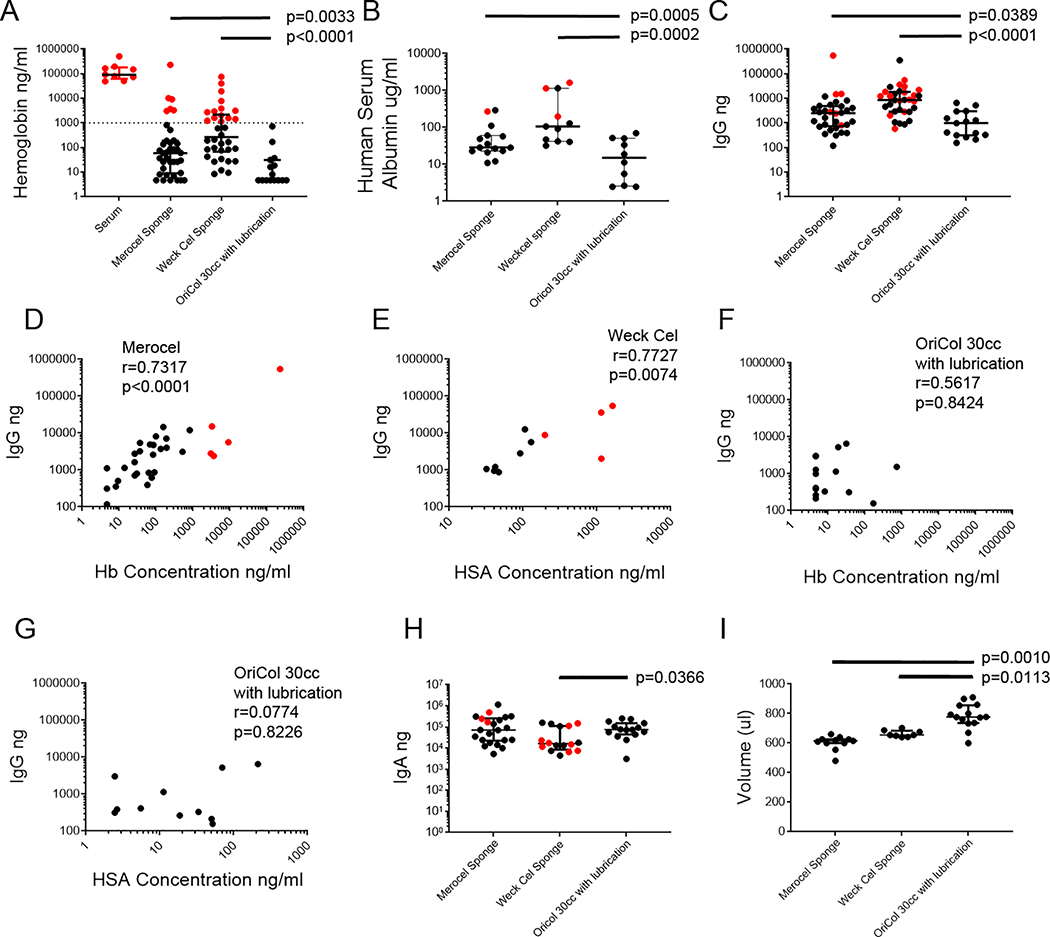
Next we compared 30cc lubricated OriCol samples collected using processing method 3 (n=17; 9 AMAB and 8 AFAB, mean age 36 years) with samples collected via two brands of sponges commonly used for the rectal collection: Merocel (n=38; 24 AMAB and 14 AFAB, mean age 37 years); and Weck-Cel (n=34; 26 AMAB, 8 AFAB, mean age 35 years). Donors in the three collection groups had comparable sex at birth ratios and age ranges (IQR 27–43), and were unlikely to have blood in their stool. Balloon device collections were all below the Hb contamination threshold and had significantly lower Hb concentrations than either type of sponge collection (Figure 4A). Median OriCol Hb concentration was below detection at 4.68ng/ml (IQR <4.68–31.43), whereas Merocel sponges had a median Hb concentration of 59.38ng/ml (IQR 9.0–191.2; p=0.003 vs. OriCol) and Weck-Cel sponges had a median Hb concentration of 267.2ng/ml (IQR 68.2– 2200; p<0.0001 vs. OriCol). We monitored HSA in a random selection of samples, to assess any contamination of the samples by plasma located in interstitial mucosal tissue (Figure 4B). Median HSA for OriCols was 14.9μg/ml (IQR 2.6–50.8) whereas Merocels and Weck-Cels were significantly higher, with a median HSA of 28.57μg/ml (IQR 22.75–60.70; p=0.0005) and 106.2 μg/ml (IQR 41.87–1,147; p=0.0002), respectively.
Figure 4. Comparison of volume, IgG, IgA and Hb recovery from rectal sponges (Weck-Cel and Merocel) and OriCol collections.
Weck-Cel (n=24) or Merocel (n=21) sponges were pre-moistened with 50μl of PBS before insertion, then placed in a mucosal fold of the rectum for 5 minutes. Sponge secretions were eluted in 600μl of PBS with PI1, after centrifugations to remove the stool pellet. OriCol collections with lubrication and 30cc inflation were processed according to method #3 (n=10). Samples marked in red indicate Hb contamination threshold above 1% of free Hb in serum. A) Hb concentration measured by ELISA. Serum samples (n=9) were used to define the Hb contamination threshold (marked by dashed line). For graphing purposes, Hb concentrations below the detection limit were plotted at 4.68μg/ml, the limit of quantitation of the assay. B) HSA concentration measured by ELISA. Concentrations below the detection limit were plotted at 1.22μg/ml, the limit of quantitation of the assay. C) IgG yield measured by IgG ELISA. D) Spearman correlation between IgG and Hb concentration in Merocel sponges. E) Spearman correlation between IgG yield and Hb concentration in Weck-Cel sponges. F) Spearman correlation between IgG yield and Hb concentration in OriCols inflated to 30cc with lubrication. G) Spearman correlation between IgG yield and HSA concentration in OriCols inflated to 30 cc with lubrication. H) IgA yield measured by ELISA. I) Supernatant volume recovered from collections after centrifugation and removal of stool pellet. The p values indicate significant comparisons in when compared to OriCols. Lines represent medians and bars are the interquartile ranges (IQR) of each sample set.
OriCol collections yielded lower IgG than sponges (Figure 4C). Balloons had a median of 986ng of IgG (IQR 316–2,965) whereas Merocel and Weck-Cel collections had medians of 2,509ng (IQR 733–4,874; p=0.0389) and 8,588ng (IQR 2,871–18,288; p>0.0001), respectively.
The IgG yield of the soft, flexible Merocel sponges correlated well with the Hb concentrations in them (Figure 4D; Spearman’s correlation r=0.7317, p<0.0001), suggesting that their IgG recovery could be due to low levels of blood contamination. Absorbent, yet rigid Weck-Cel spears were not as strongly correlated with Hb (r=0.3453, p=0.0529) but were well correlated to HSA (Figure 4E; r=0.7727, p=0.0074), suggesting that they also draw their high IgG from interstitial plasma (Figure 4B). OriCols did not show a correlation among IgG and Hb (Figure 4F; r=0.516, p=0.8424), or IgG and HSA (Figure 4G; r=0.0774, p=0.8226), suggesting the recovered IgG was mainly transudated into the colonic lumen.
Balloon sampling recovered a median of 75,253ng IgA (IQR 44,537–152,969ng; Figure 4H), which was significantly higher than Weck-Cel sponges (median IgA 16,173ng, IQR 8,546–112,225ng; p=0.0366) but indistinguishable from Merocel sponges (median IgA 71,672ng; IQR 22,488–258,184ng; p=0.694).
Lastly, we compared the recovery volumes from the sponges and balloon collections (Figure 4I). As expected from the 1ml addition of media, OriCol secretion supernatants had increased recovery volumes compared to the sponges, which receive 650μl of media during elution. Median recovery volumes for OriCols were 800μl (IQR 736–880) whereas Merocels and Weck-Cels were 615μl (IQR 600–626; p=0.0010) and 655μl (IQR 644–684; p=0.0113), respectively.
Therefore, we concluded that OriCols inflated at 30cc with lubrication provided minimal Hb contamination and increased recovery volume compared to both sponge types. The improvements minimizing Hb and maximizing recovery volume were observed even after exclusion of any sponge sample with more than 1% of free Hb in serum (data not shown). OriCols had minimal systemic IgG contamination and a significant IgA yield, making them ideal for rectal immunoglobulin studies.
Implementation of Balloon Device Collections in a Phase 1 Clinical Trial
Due to their short collection time and low blood contamination, we decided to introduce balloon devices in a phase 1 trial to test their acceptability by trial participants. The study requested donation of semen, rectal secretions using OriCols, and rectal biopsies in persons AMAB; cervicovaginal secretions, cervical biopsies, vaginal biopsies, rectal secretions using OriCols and rectal biopsies in persons AFAB. Of the 28 participants enrolled in the study, 27 were eligible for rectal sampling (see Table II). Participants who enrolled and agreed to OriCol sampling were 44% AMAB and 56% AFAB, median age 29, with no history or report of rectal abnormalities or bleeding disorders.
Table II.
Demographics of the 27 participants assessing the tolerability of rectal secretion sampling with the OriCol device in their first collection within a Phase 1 study in Seattle.
| Characteristics | All N (%) 27 (100%) |
AMAB N (%) | AFAB N (%) |
|---|---|---|---|
| Sex Assigned at Birth# | 12 (44%) | 15 (56%) | |
| Race | |||
| White | 16 (59%) | 7 | 9 |
| African American/Black | 4 (15%) | 3 | 1 |
| Native American | 0% | 0 | 0 |
| Asian | 2 (7%) | 1 | 1 |
| Multiracial and Other* | 5 (19%) | 1 | 4 |
| Ethnicity | |||
| Hispanic or Latino | 1 (4%) | 1 | 0 |
| Age | |||
| 18–24 | 4 (15%) | 1 (4%) | 3 (11%) |
| 25–29 | 10 (37.0%) | 4 (15%) | 6 (22%) |
| 30–34 | 5 (19%) | 2 (7%) | 3 (11%) |
| 35–39 | 2 (7%) | 1 (4%) | 1 (4%) |
| 40–44 | 3 (11%) | 2 (7%) | 1 (4%) |
| 45–49 | 2 (7%) | 1 (4%) | 1 (4%) |
| 50–54 | 1 (4%) | 1 (4%) | 0 |
| Experience with rectal secretion collection using a balloon | |||
| Acceptable – a lot | 17 (62.9%) | 7 (58.3%) | 10 (66.6%) |
| Acceptable – somewhat | 6 (22.2%) | 4 (33.3%) | 2 (13.3%) |
| Acceptable – a little | 1 (3.7%) | 0 (0%) | 1 (6.6%) |
| Neutral | 3 (11.1%) | 1 (8.3%) | 2 (13.3%) |
| Not acceptable – a little | 0 (0%) | 0 (0%) | 0 (0%) |
| Not acceptable – somewhat | 0 (0%) | 0 (0%) | 0 (0%) |
| Not acceptable – a lot | 0 (0%) | 0 (0%) | 0 (0%) |
All participants identified as cisgender
Includes four Asian/White and one Hispanic
Study participants donated their first mucosal samples before any study intervention was conducted. The OriCol samples were collected prior to rectal biopsy. They were interviewed two weeks later to evaluate acceptability of the mucosal sampling procedures. Participants were asked to rank using a 7-point Likert scale their “experience with rectal secretion collection using a balloon”. As seen in Table II, 62.9% ranked OriCol collections as “acceptable-a lot”; 22.2% as “somewhat acceptable”; 0% provided a “Not acceptable” score. No differences in acceptability were observed among participants based on assigned sex at birth (p=0.835). No participant had any rectal complications at the rectal secretion collection visit.
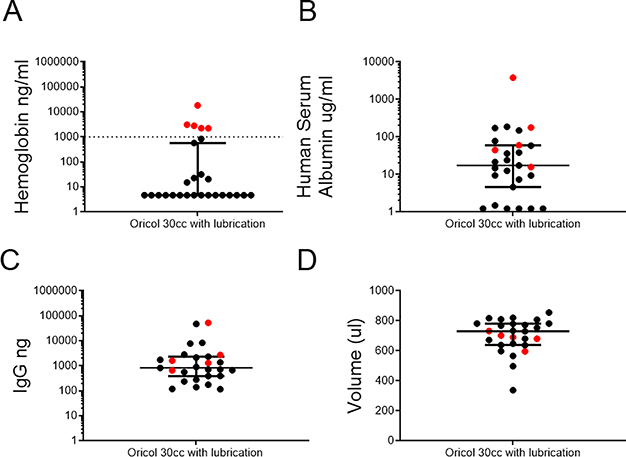
In this trial, OriCol secretions were processed according to method #4, diluted in RPMI1460 with antibiotics and antimycotics (see methods), to provide consistency with the media used to process other mucosal samples from the study. As expected, the median Hb in these samples was under the limit of detection (4.68ng/ml; IQR 4.68–474.4ng/ml; Figure 5A), highlighting the low blood contamination observed, even when collections were carried out during a complex multi-sample collection visit. HSA concentrations were also low in these samples (median 17.51μg/ml IQR 4.13–60.16 μg/ml; Figure 5B) indicating low contamination from interstitial plasma. The mean IgG was 829.9ng (IQR 389.8–2333ng; Figure 5C), similar to the levels measured in the optimization samples (Figure 4B; p=0.753). The recovery volume median was 730μl (IQR 639–782μl; Figure 5D), also comparable with the optimization samples (Figure 4C; p=0.2125). In short, during this sample-intense trial, rectal collections with lubricated balloon devices were acceptable to study participants, maintained the desired mucosal IgG yields, and avoided blood contamination.
Figure 5. Volume, IgG, and Hb recovery from OriCol collections in a phase 1 study.
OriCol collections with lubrication and 30cc inflation were processed according to method #4 (n=27). Samples marked in red indicate Hb contamination threshold above 1% of free Hb in serum. A) Hb concentration measured by ELISA. Serum samples (n=9 from Figure 4A) were used to define the Hb contamination threshold (marked by dashed line). Hb concentrations below the detection limit were plotted at 4.68μg/ml, the limit of quantitation of the assay. B) HSA concentration measured by ELISA. Concentrations below the detection limit were plotted at 1.22μg/ml, the limit of quantitation of the assay. C) IgG yield measured by IgG ELISA. D) Supernatant volume recovered from collections after centrifugation and removal of stool pellet. Lines represent medians and bars are the interquartile ranges (IQR) of each sample set.
DISCUSSION
We have optimized a method to collect rectal secretions using balloon devices that preserves the compartmentalization of rectal immunoglobulins, is well tolerated by participants, and provides multiple benefits over current practices. The use of lubricated OriCols inflated to 30cc provides benefits for the clinician related to efficiency and for the laboratory related to improved efficiency and decreased blood contamination. The device was generally well tolerated for rectal secretion collection; no adverse events were experienced and no participant complaints regarding the device were received. OriCols were easy to implement at the clinic: lubrication of the device is easy; inflation volume can be accurately controlled by syringe markings; the time that the anoscope remains inserted is under one minute (whereas the time for sponge collection is between 5 and 6 minutes). In addition, the processing of balloon secretions to remove the stool pellet can be accomplished by a short processing method (less than 30 minutes) compared to several rounds of washes and centrifugation for eluting secretions from sponges (~80 minutes). Because the ultimate goal of using lubricated OriCols is the collection of rectal secretions in large-scale studies, these operational benefits are encouraging as demonstrated in the implementation of the initial mucosal collection in a phase 1 study at a single clinical site.
In addition to these logistical benefits, we further optimized collection and processing procedures to maximize recovery of mucosal antibodies. Balloon inflation consistently samples 8cm-13cm from the anal margin, an area where seminal plasma and HIV locate after simulated RAI(2, 28). We demonstrate that lubricated OriCol sampling minimizes Hb and HSA contamination compared to pre-moistened sponges. The use of the described OriCol lubrication methodology is essential to obtaining blood-free secretions, so training clinical staff on appropriate OriCol preparation will be important as the method is implemented more widely.
There are also some drawbacks to using lubricated OriCols for collecting rectal mucosal samples. Currently, the device kit (USD $16.22) is more expensive than the sponges and anoscope (USD $1.24), but cost reductions may be possible with expanded use. The second is a slightly lower median IgG concentration in the recovered fluid, despite sampling an area 15–30 times larger than the sponges. It might be possible to concentrate the IgG, or to wash the balloon with a lower volume than 1ml without affecting optimal recovery. Third, the proposed OriCol methodology does not permit calculating the original volume of rectal secretion collected and IgG concentrations in the collected secretion volume prior to any dilution. It should be possible to subtract the weight of the pre-lubricated OriCol from the weight of the device immediately after collection, but the weight differential would be an aggregate of lubricant carryover, fecal matter contamination and soluble secretions. Therefore, estimates of concentration of rectal immunoglobulins in OriCol-collected secretions will require normalizing to another secretion protein, or calculating the initial dilution by measuring a media component not present in human secretions, to calculate the dilution factor.
In summary, we describe an innovative balloon device to accurately sample rectal secretions, that allows for analysis of mucosal proteins without blood contamination. In the two studies presented here, these balloon devices, when lubricated, supported consistent collections of mucosal IgG and IgA with minimal Hb and HSA levels while sampling a greater rectal surface area. The collection process is also faster than sponge methods and well tolerated. We expect that our standardized collection protocol will support a multitude of downstream assays including assessment of HIV-specific antibodies in vaccine or passive immunization studies. Our approach is uniquely poised to detect mucosal antibodies, which is important to evaluate HIV protection mechanisms playing a role during receptive anal sex.
KEY POINTS.
Rectal balloon collections minimize blood contamination of mucosal immunoglobulins
Rectal balloon collections require ring lubrication to minimize blood contamination
Rectal balloon collection is quick and well tolerated in humans
Acknowledgements
We would like to thank the study participants for their interest and contribution to this study. Additionally, we thank G Vargas, T Lidsky, A Treu and R Gomez for technical help processing the OriCol samples for optimization. We would like to thank the protocol team of HVTN116 for their support for the collection of OriCol samples at a phase 1 trial at the Seattle site, including LG Bekker, P Mann, Y Huang, J Hutter, J Ledgerwood, B Graham, O Ho, M Jones, M Brandon, and C Sopher.
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers UM1AI068618 (MJM), UM1AI069481 (MJM), U19AI128914 (MJM) and P30AI027757. The phase 1 study was also supported by UM1AI068614 and UM1AI068635. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, and Mermin J 2014. Estimating per-act HIV transmission risk: a systematic review. AIDS 28: 1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao YJ, Caffo BS, Fuchs EJ, Lee LA, Du Y, Li L, Bakshi RP, Macura K, Khan WA, Wahl RL, Grohskopf LA, and Hendrix CW 2012. Quantification of the spatial distribution of rectally applied surrogates for microbicide and semen in colon with SPECT and magnetic resonance imaging. Br J Clin Pharmacol 74: 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anton PA, Cranston RD, Kashuba A, Hendrix CW, Bumpus NN, Richardson-Harman N, Elliott J, Janocko L, Khanukhova E, Dennis R, Cumberland WG, Ju C, Carballo-Dieguez A, Mauck C, and McGowan I 2012. RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses 28: 1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shieh EC, Weld ED, Fuchs EJ, Hiruy H, Buckheit KW, Buckheit RW Jr., Breakey J, and Hendrix CW 2017. Lubricant Provides Poor Rectal Mucosal HIV Coverage. AIDS Res Hum Retroviruses 33: 784–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leyva FJ, Bakshi RP, Fuchs EJ, Li L, Caffo BS, Goldsmith AJ, Ventuneac A, Carballo-Dieguez A, Du Y, Leal JP, Lee LA, Torbenson MS, and Hendrix CW 2013. Isoosmolar enemas demonstrate preferential gastrointestinal distribution, safety, and acceptability compared with hyperosmolar and hypoosmolar enemas as a potential delivery vehicle for rectal microbicides. AIDS Res Hum Retroviruses 29: 1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anton PA, Saunders T, Elliott J, Khanukhova E, Dennis R, Adler A, Cortina G, Tanner K, Boscardin J, Cumberland WG, Zhou Y, Ventuneac A, Carballo-Dieguez A, Rabe L, McCormick T, Gabelnick H, Mauck C, and McGowan I 2011. First phase 1 double-blind, placebo-controlled, randomized rectal microbicide trial using UC781 gel with a novel index of ex vivo efficacy. PLoS One 6: e23243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, Wang K, Bao S, Kraemer TD, Rath T, Zeng M, Schmidt SD, Todd JP, Penzak SR, Saunders KO, Nason MC, Haase AT, Rao SS, Blumberg RS, Mascola JR, and Nabel GJ 2014. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature 514: 642–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grivel JC, Elliott J, Lisco A, Biancotto A, Condack C, Shattock RJ, McGowan I, Margolis L, and Anton P 2007. HIV-1 pathogenesis differs in rectosigmoid and tonsillar tissues infected ex vivo with CCR5- and CXCR4-tropic HIV-1. AIDS 21: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 9.Waldmann TA, and Strober W 1969. Metabolism of immunoglobulins. Prog Allergy 13: 1–110. [DOI] [PubMed] [Google Scholar]
- 10.Brandtzaeg P 1971. Human secretory immunoglobulins. V. Occurrence of secretory piece in human serum. J Immunol 106: 318–323. [PubMed] [Google Scholar]
- 11.Brandtzaeg P 1974. Mucosal and glandular distribution of immunoglobulin components. Immunohistochemistry with a cold ethanol-fixation technique. Immunology 26: 1101–1114. [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida M, Kobayashi K, Kuo TT, Bry L, Glickman JN, Claypool SM, Kaser A, Nagaishi T, Higgins DE, Mizoguchi E, Wakatsuki Y, Roopenian DC, Mizoguchi A, Lencer WI, and Blumberg RS 2006. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest 116: 2142–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida M, Masuda A, Kuo TT, Kobayashi K, Claypool SM, Takagawa T, Kutsumi H, Azuma T, Lencer WI, and Blumberg RS 2006. IgG transport across mucosal barriers by neonatal Fc receptor for IgG and mucosal immunity. Springer Semin Immunopathol 28: 397–403. [DOI] [PubMed] [Google Scholar]
- 14.Roopenian DC, and Akilesh S 2007. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 7: 715–725. [DOI] [PubMed] [Google Scholar]
- 15.Raghavan M, and Bjorkman PJ 1996. Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol 12: 181–220. [DOI] [PubMed] [Google Scholar]
- 16.Lemos MP, Karuna ST, Mize GJ, Fong Y, Montano SM, Ganoza C, Lama JR, Sanchez J, and McElrath MJ 2016. In men at risk of HIV infection, IgM, IgG1, IgG3, and IgA reach the human foreskin epidermis. Mucosal Immunol. 9: 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelzayd EA, Kraft SC, and Kirsner JB 1968. Distribution of immunoglobulins in human rectal mucosa. I. Normal control subjects. Gastroenterology 54: 334–340. [PubMed] [Google Scholar]
- 18.Brandtzaeg P, Baklien K, Fausa O, and Hoel PS 1974. Immunohistochemical characterization of local immunoglobulin formation in ulcerative colitis. Gastroenterology 66: 1123–1136. [PubMed] [Google Scholar]
- 19.Macpherson AJ, McCoy KD, Johansen FE, and Brandtzaeg P 2008. The immune geography of IgA induction and function. Mucosal Immunol 1: 11–22. [DOI] [PubMed] [Google Scholar]
- 20.Zuckerman RA, Whittington WL, Celum CL, Collis TK, Lucchetti AJ, Sanchez JL, Hughes JP, Sanchez JL, and Coombs RW 2004. Higher concentration of HIV RNA in rectal mucosa secretions than in blood and seminal plasma, among men who have sex with men, independent of antiretroviral therapy. J Infect Dis 190: 156–161. [DOI] [PubMed] [Google Scholar]
- 21.Kozlowski PA, Cu-Uvin S, Neutra MR, and Flanigan TP 1997. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun 65: 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omosa-Manyonyi G, Park H, Mutua G, Farah B, Bergin PJ, Laufer D, Lehrman J, Chinyenze K, Barin B, Fast P, Gilmour J, and Anzala O 2014. Acceptability and feasibility of repeated mucosal specimen collection in clinical trial participants in Kenya. PLoS One 9: e110228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohamed OA, Ashley R, Goldstein A, McElrath J, Dalessio J, and Corey L 1994. Detection of rectal antibodies to HIV-1 by a sensitive chemiluminescent western blot immunodetection method. J Acquir Immune Defic Syndr 7: 375–380. [PubMed] [Google Scholar]
- 24.Raux M, Finkielsztejn L, Salmon-Ceron D, Bouchez H, Excler JL, Dulioust E, Grouin JM, Sicard D, and Blondeau C 1999. Comparison of the distribution of IgG and IgA antibodies in serum and various mucosal fluids of HIV type 1-infected subjects. AIDS Res Hum Retroviruses 15: 1365–1376. [DOI] [PubMed] [Google Scholar]
- 25.Kozlowski PA, Lynch RM, Patterson RR, Cu-Uvin S, Flanigan TP, and Neutra MR 2000. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J Acquir Immune Defic Syndr 24: 297–309. [DOI] [PubMed] [Google Scholar]
- 26.Booth J, Lacy-Colson J, Norwood M, and Murray C 2014. A novel sampling device for collecting mucocellular material from the unprepared rectum. In 23rd Biennial Congress of the European Association for Cancer Research, 5–8 July 2014. European Journal of Cancer, Munich, Germany. S240. [Google Scholar]
- 27.Fong Y, Huang Y, Lemos MP, McElrath MJ. 2018. Rank-based two-sample tests for paired data with missing values. Biostatistics 19: 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louissaint NA, Nimmagadda S, Fuchs EJ, Bakshi RP, Cao YJ, Lee LA, Goldsmith J, Caffo BS, Du Y, King KE, Menendez FA, Torbenson MS, and Hendrix CW 2012. Distribution of Cell-Free and Cell-Associated HIV Surrogates in the Colon After Simulated Receptive Anal Intercourse in Men Who Have Sex With Men. J Acquir Immune Defic Syndr 59: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]