Supplemental Digital Content is available in the text.
Keywords: Preconception, Bisphenol A, Parabens, Neurodevelopment, Prenatal
Background:
Epidemiologic studies suggest that prenatal urinary bisphenol A (BPA) concentrations are associated with childhood behavior problems, but there is limited research on prenatal paraben concentrations. In rodent offspring, preconception maternal BPA exposure caused behavioral problems and paraben exposure impacted sperm quality. However, the effects of parental preconception and prenatal BPA and paraben exposure on children’s neurodevelopment are unknown.
Methods:
The Environment and Reproductive Health (EARTH) Study is a prospective cohort of couples from a fertility clinic at Massachusetts General Hospital. The Centers for Disease Control and Prevention (CDC) quantified BPA, butylparaben, ethylparaben, methylparaben, and propylparaben concentrations in multiple urine samples collected before conception and during pregnancy. From the eligible parents (N = 220), we enrolled 158 children between 2 and 9 years of age. The parents completed the Behavior-Assessment-System-for-Children-2 (BASC-2). We estimated covariate-adjusted associations of average parental preconception and prenatal ln-transformed urinary BPA and sum of paraben concentrations (∑paraben) with BASC-2 scores using linear regression with generalized estimating equations.
Results:
Median urinary BPA and Σparaben concentrations were 1.2 and 189 μg/L in mothers preconception and 1.7 and 25 μg/L in fathers preconception, respectively. Among all children, parental BPA and ∑paraben concentrations were not associated with BASC-2 behavioral symptoms index, internalizing, or externalizing problems scores. Point estimates ranged from −1.5 to 1.4 with wide 95% confidence intervals that included the null value.
Conclusion:
In this fertility clinic cohort, parental preconception and maternal prenatal BPA and paraben concentrations were not associated with problem behaviors among children. However, our small sample sizes reduced the precision of our results.
What this study adds
We investigated whether the period before conception and during pregnancy is a window of heightened susceptibility to bisphenol A and paraben exposures with respect to neurodevelopment. While animal studies suggest that preconception exposure to these compounds could impact offspring behavior, to our knowledge, this is one of the first human studies focused on childhood behavior in relation to preconception exposures.
Introduction
There is a growing body of research showing that exposure to some endocrine-disrupting chemicals (EDC) is chronic across the lifespan and that potential health effects of EDCs may depend on the timing of exposure.1,2 Particular attention has been paid to the effect of these exposures during the gestational and childhood periods. However, evidence is emerging that the period before conception may also be a window of heightened susceptibility.3,4 EDCs may alter the epigenome of gametes, affecting sperm microRNAs, DNA methylation, histones, oocytes, or ovarian follicles, which in turn could influence offspring health,4 including neurodevelopmental disorders.3,5
Although a number of EDCs have been implicated in affecting early life development, of particular concern are bisphenol A (BPA) and parabens.1,6 BPA is used to manufacture some polycarbonate plastics and epoxy resins.7,8 Parabens are used as antimicrobial preservatives in some food, pharmaceuticals, and cosmetics.9,10 Exposure to these nonpersistent chemicals can occur through dermal absorption, ingestion, and inhalation.8,9 BPA is routinely detected in urine of the US general population and exposure to parabens is also widespread, with some parabens being detected in over 99% of urine samples from the US general population.7,9
Both BPA and paraben exposure may influence the development and function of the central nervous system. In animals, both maternal and paternal preconception exposure to BPA were associated with increased anxiety behavior.11,12 Paternal preconception BPA exposure was also associated with impaired spatial memory in rats13 and maternal preconception BPA exposure increased depression behavior in male mice.14 Based on studies in both humans and animals, 2 systematic reviews concluded that prenatal BPA exposure could have an adverse effect on the neurobehavioral outcomes of children,15 including increased hyperactivity.16 In experimental studies of animals, some parabens impaired sexual17 and social18 behaviors. Using data from the US National Health and Nutrition Examination Surveys, Shiue19 reported that urinary paraben concentrations were associated with higher emotional support needs in adults. Both BPA and parabens may also affect the homeostasis of thyroid hormones essential for neurodevelopment.20–22
Because little is known about the neurodevelopmental effects of BPA and paraben exposures during the potentially sensitive preconception and prenatal periods, we evaluated the associations of maternal and paternal preconception and maternal prenatal urinary BPA and paraben concentrations with child behavior in a prospective cohort in Boston, MA.
Methods
Study participants
Starting in 2004, trained study staff recruited couples from the Massachusetts General Hospital (MGH) Fertility Center to join the Environment and Reproductive Health (EARTH) Study. This prospective preconception cohort is designed to examine environmental exposures that may affect human reproduction. To date, nearly 1,000 women and 550 men have enrolled in the EARTH Study. Specific details on the cohort and data collection have been previously published.23 Briefly, women and men were eligible to participate on their own or with their partner. Study staff followed the participants through fertility treatment, pregnancy, and children’s births. At study entry, staff administered a questionnaire to each participant to assess sociodemographic, lifestyle, and medical history. Individuals also completed questionnaires on family, reproductive, and occupational history, physical activity, personal care product use, and tobacco and drug use. Study staff also collected blood and urine samples at this time and continued to collect additional samples throughout fertility treatment and pregnancy.
In 2014, EARTH Study participants with children born after 2005 and older than 2.5 years were invited to participate in a child follow-up pilot study. Study staff gave the invitees detailed information about the study protocol. Of potential participants, approximately 69% completed questionnaires about their child’s behavior, for a total of 158 participating children. A total of 127 mothers and 93 fathers had urinary chemical concentration data available for the preconception period when they, or their partner, were undergoing fertility care but had not yet conceived. Study staff obtained written informed consent from each participating parent. The Institutional Review Boards of Partners HealthCare, Harvard T.H. Chan School of Public Health, and the Centers for Disease Control and Prevention (CDC) approved this study; Brown University deferred to the Partners and Harvard institutional review boards.
Bisphenol A and paraben exposure assessment
At study enrollment, all women and men gave a single spot urine sample in a sterile polypropylene cup. Depending on the fertility treatment cycle, women could provide up to 2 more preconception urine samples per cycle during follow-up. Male partners provided an additional urine sample if their female partner underwent oocyte retrieval or intrauterine insemination. Study staff also collected urine samples from the women during pregnancy at a median of 6, 21, and 35 weeks’ gestation.
Each urine sample was analyzed for specific gravity using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD). Samples were divided into aliquots and frozen at −80°C. Samples were then shipped on dry ice overnight to the CDC (Atlanta, GA) for quantification of BPA, butylparaben, ethylparaben, methylparaben, and propylparaben concentrations using online solid phase extraction–isotype dilution–high-performance liquid chromatography–tandem mass spectrometry.24 The limits of detection (LODs) were 0.4, 0.1, 1.0, 1.0, and 0.1 μg/L for BPA, butylparaben, ethylparaben, methylparaben, and propylparaben, respectively. Values below the LOD were assigned the LOD divided by the square root of 2.25 To adjust for urine dilution, we multiplied BPA and each paraben metabolite concentrations by [(SGc − 1)/(SGi − 1)], where SGc is the median specific gravity for all male or female participants in the study sample and SGi is the specific gravity for the ith participant’s sample. We calculated a weighted molar sum of paraben concentrations (∑paraben) by dividing each specific gravity standardized paraben concentration by its molecular weight, summing the molar concentrations together, and then weighting this sum by the molecular weight of methyl paraben (152.15 μg/μmol) to convert the molar concentration to units of μg/L.
Child behavior measurement
To assess child behavior, study staff mailed participants age-appropriate versions of the second edition of the Behavior Assessment System for Children (BASC-2) and provided written instructions for completing the questionnaire about their child.26 The BASC-2 is a valid and reliable measure of children’s problem and adaptive behaviors that assesses ≥100 individual behaviors using Likert-style responses.26 Individual items are totaled into composite scores including the behavioral symptoms index, internalizing problems, and externalizing problems composite scales. Although additional clinical subscales are assessed by the BASC-2, we focused on these 3 composite scores to assess a broad array of problem behaviors. Each BASC-2 composite score is calculated as age- and sex-standardized T-scores, with a mean and SD of 50 and 10, respectively. On these scales, higher scores indicate more problematic behaviors.
Covariates
We obtained relevant sociodemographic information about each mother or father from questionnaires administered at baseline. Specifically, staff obtained age, race, education, and smoking status. At study entry, study staff also recorded height (meters) and weight (kilograms) for each mother or father, which we used to calculate body mass index (BMI, kg/m2). The treating infertility physician used the Society of Assisted Reproductive Technology definitions to diagnose the underlying cause of infertility.27 Electronic medical records were used to collect information about infertility treatment and delivery records for child characteristics, including date of birth, child sex, and singleton or multiple birth.
Statistical analysis
We ln-transformed the specific gravity-standardized BPA and ∑paraben concentrations to reduce the influence of potential outliers. To estimate preconception exposure, we averaged the paternal and maternal preconception BPA and ∑paraben concentration obtained from study entry and each treatment cycle up to and including the index cycle of conception for the respective child. We also averaged the 3 trimester-specific urinary concentrations of BPA and ∑paraben to estimate prenatal exposures for the mothers. Most participants had at least 2 maternal preconception (N = 122, 96%), paternal preconception (N = 85, 91%), and prenatal (N = 126, 99%) urine samples. We examined the exposure distribution for BPA and ∑paraben concentrations using violin plots. We calculated parent’s sociodemographic characteristics and children’s birth characteristics by sex and age. Using Spearman correlation coefficients, we calculated the correlation between maternal preconception and prenatal and paternal preconception BPA and ∑paraben concentrations.
We estimated the associations of preconception maternal and paternal and prenatal maternal BPA and ∑paraben concentrations with behavioral symptoms index, internalizing, and externalizing problems T-scores using linear regression with generalized estimating equations (GEE) models to control for the correlation among siblings. Each beta coefficient and corresponding 95% confidence interval represents the adjusted mean T-Score difference for each ln-unit increase in BPA or ∑paraben concentration. We used the locally estimated scatterplot smoothing function in R to assess linearity assumptions.
We created a directed acyclic graph to identify factors associated with concentrations of BPA or ∑paraben with child behavior (eFigure 1; http://links.lww.com/EE/A69). In our multivariable GEE models, we applied separate covariates relevant to the mother or father. For the maternal preconception and prenatal urinary concentration model, we included maternal age, maternal race (white versus other), and maternal smoking status (never versus former or current). For the paternal preconception urinary concentration model, we included paternal age, paternal race (white versus other), and paternal smoking status (never versus former or current).
Secondary and sensitivity analysis
To evaluate potential differences by child sex, we further stratified the preconception GEE models by boys versus girls. We calculated effect modification P values using a 2-sample z-test with beta coefficients and variances from the sex-stratified models.28 To determine if results differed by age of children at time of behavioral assessment and given that we had so few children ≥6 years of age in our study sample (n = 26), we excluded these older children from our analysis. Because twins and triplets may have a different risk for neurodevelopmental outcomes compared with singletons,29 we performed a sensitivity analysis using linear regression excluding all twins and triplets. We also performed a sensitivity analysis where we excluded preterm births (<37 weeks) among singletons and multiples.
We also examined the impact of additional potential confounders on the associations of preconception maternal and paternal BPA and ∑paraben concentrations with BASC-2 T-scores: prepregnancy parental BMI (continuous), infertility cause (female related, male related, unknown), conception mode (intrauterine insemination [IUI], in vitro fertilization [IVF], natural), and maternal education status (<College, College Graduate, Graduate Degree). We adjusted the BPA models for ln-transformed maternal prenatal urinary BPA concentrations and the ∑paraben models for ln-transformed maternal prenatal urinary ∑paraben concentrations. We did a further analysis adjusting for the respective other chemical (e.g., adjusting for urinary ∑paraben concentrations in the BPA models) and adjusting for the respective partner’s chemical concentration (e.g., adjusting for the paternal preconception urinary BPA concentrations when examining the impact of maternal preconception urinary BPA concentrations with BASC-2 T-scores).
Results
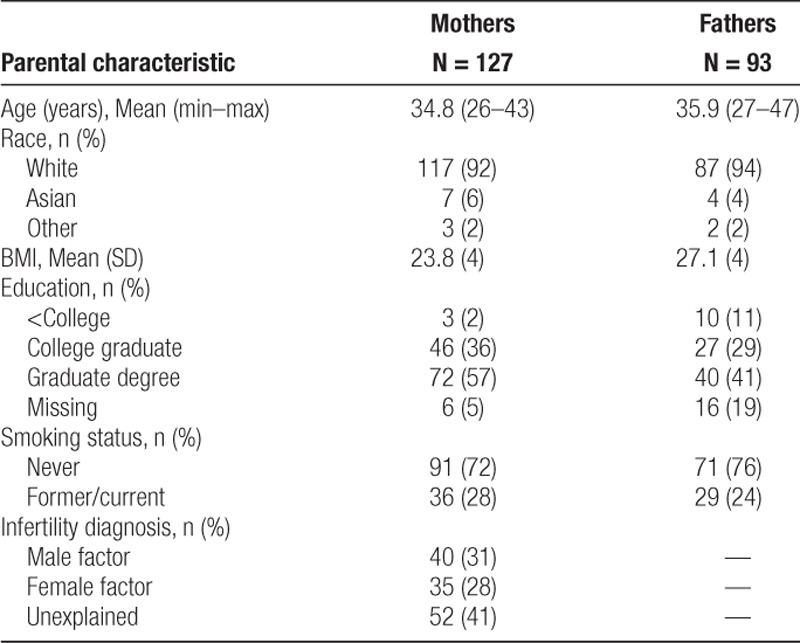
The 127 mothers and 93 fathers in our analysis had a mean age and BMI of 35 years and 24 kg/m2 and 36 years and 27 kg/m2, respectively (Table 1). Most participants were white (mothers, 92%; fathers, 94%) and never-smokers (mothers, 72%; fathers 76%). Only 2% of women and 11% of men had less than a college education. Female and male factors account for 28% and 31%, respectively, of the infertility diagnosis, although the remaining 41% was unexplained. We compared baseline characteristics of participants in the present study to the total EARTH Study cohort and found that our sample was similar in mean age and BMI; however, participants in our study were more likely to be white, mothers were more likely to have graduate degrees, and fathers were less likely to smoke (eTable 1; http://links.lww.com/EE/A69).
Table 1.
Parental characteristics of 127 mothers and 93 fathers participating in the EARTH Study
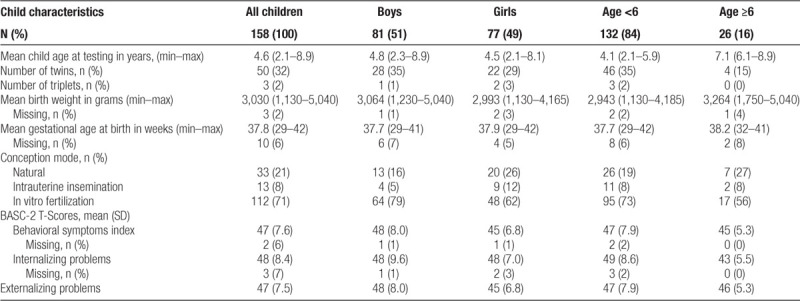
The majority of children were conceived through IVF (71%) followed by natural conception (21%) and IUI (8%). The average age among the 158 children was 4.6 years (SD = 1.53) and 49% were female (Table 2). There were 25 sets of twins and 1 set of triplets. A total of 37 children (23%) were born before 37 weeks gestation. The mean BASC-2 T-scores were 47 (SD = 7.6), 48 (SD = 8.4), and 47 (SD = 7.5) for the behavioral symptoms index, internalizing problems, and externalizing problems, respectively. Younger children and boys had slightly higher average scores.
Table 2.
Characteristics of 158 children participating in the EARTH Study
Each father provided an average of 2.7 preconception spot urine samples (25th, 75th: 1, 3) and each mother provided an average of 4.3 preconception spot urine samples (25th, 75th: 2, 5) and an average of 2.7 (25th, 75th: 2, 3) urine samples in the prenatal period. Median specific gravity-adjusted urinary concentrations of BPA and ∑paraben were 1.2 and 189 μg/L in mothers and 1.7 and 25 μg/L in fathers, respectively (Figure 1, eTable 2; http://links.lww.com/EE/A69). The percentage of detectable BPA concentrations was 58% in mothers and 81% in fathers (eTable 2; http://links.lww.com/EE/A69). Detection frequencies of the 3 parabens (butyl, methyl, and propyl) that created the ∑paraben variable ranged from 55% to 100%. We excluded ethyl paraben from the ∑paraben measure because 61% of the samples had values below the limit of detection. We also excluded paternal butylparaben from the ∑paraben measure for fathers because 78% of samples had values below the limit of detection.
Figure 1.
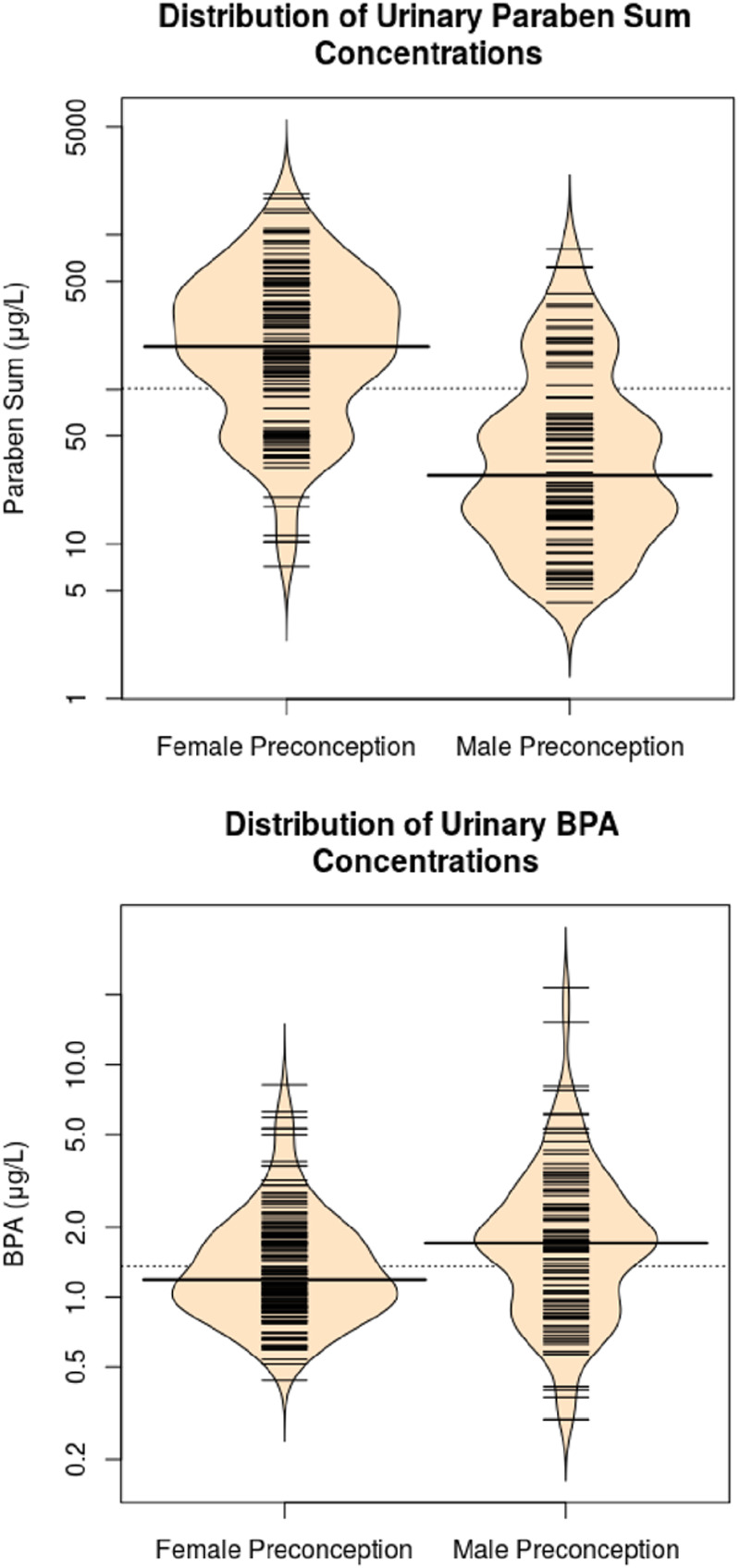
Violin plots of specific gravity-standardized maternal and paternal preconception urinary bisphenol A and ∑paraben concentrationsa,b. aViolin plots show the kernel probability density of the data. Each small line represents a participant and the longer lines are the median urinary concentration. bFor females butyl, methyl, and propyl constitute the ∑paraben. For males, methyl and propyl constitute the ∑paraben.
Maternal and paternal preconception urinary BPA and ∑paraben concentrations were not correlated (Spearman r ≤ 0.21) (eTable 3; http://links.lww.com/EE/A69). Paternal preconception urinary concentrations were also not correlated with maternal prenatal urinary concentrations (Spearman r ≤ 0.13). There was moderate correlation between maternal preconception and maternal prenatal urinary paraben concentrations (Spearman r = 0.54) and weak correlations for urinary BPA concentrations (Spearman r = 0.27).
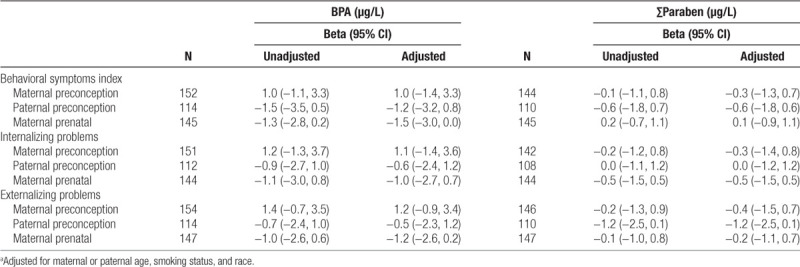
Among all children before adjustment for covariates, the associations of maternal or paternal preconception or maternal prenatal urinary BPA and ∑paraben concentrations with BASC-2 behavioral symptoms index, internalizing problems, and externalizing problems index T-Scores ranged from −1.5 to 1.4 with wide 95% confidence intervals that included the null value (Table 3). The magnitude of these associations did not change substantially after adjustment for covariates (Table 3). Most notably, we observed a weak inverse association between paternal preconception ∑paraben concentrations and externalizing problems T-scores (β = −1.2, 95% confidence interval [CI] = −2.5, 0.1). We did not find evidence for nonlinearity of these associations (nonlinearity P values ≥ 0.10).
Table 3.
Unadjusted and adjusted mean differences in the BASC-2 behavioral symptoms index, internalizing, and externalizing problems T-Scores with ln-unit increase in maternal and paternal preconception and maternal prenatal urinary bisphenol A and ∑paraben concentrationsa
Sensitivity analysis
When stratifying by child sex, we did not observe strong evidence for sex-specific associations based on the sex by urine biomarker interaction P values (eTable 4; http://links.lww.com/EE/A69). The weak inverse association between paternal preconception ∑paraben concentrations and externalizing scores was similar in female (β = −1.6, 95% CI = −3.0, −0.2) and male (β = −1.0, 95% CI = −3.0, 1.1) children (effect modification P value = 0.62). We observed higher internalizing problem scores among younger children less than 6 years of age concentrations (β = 2.4, 95% CI = −0.3, 5.1) in relation to maternal preconception BPA compared with all children in the total sample (β = 1.1, 95% CI = −1.4, 3.6) (eTable 5; http://links.lww.com/EE/A69).
Among singleton children (N = 101), each ln-unit increase in maternal preconception urinary BPA concentrations was associated with a 2.3-point increase in externalizing scores (95% CI = −0.5, 5.2) (eTable 6; http://links.lww.com/EE/A69). Moreover, among children born term, each ln-unit increase in maternal preconception urinary BPA concentrations was associated with a 2.1 increase in externalizing scores (95% CI = −0.1, 4.3) (eTable 7; http://links.lww.com/EE/A69). We performed additional analyses to further examine this association and found that among singleton children with gestation ≥37 weeks, maternal preconception urinary BPA concentrations were associated with a 3.7 increase in externalizing scores (95% CI = 1.2, 6.1; N = 83) (eTable 8; http://links.lww.com/EE/A69).
Generally, our results did not meaningfully change when we adjusted for additional confounders (eFigures 2–4; http://links.lww.com/EE/A69, eTable 9; http://links.lww.com/EE/A69). However, when we adjusted for maternal education, each ln-unit increase in maternal preconception urinary BPA concentrations was associated with a 1.8 increase in externalizing scores (95% CI = −0.2, 3.8) (N = 121).
Discussion
Among participants in this prospective cohort of couples from a fertility clinic, maternal and paternal urinary preconception and maternal prenatal concentrations of BPA or ∑paraben were not associated with BASC-2 behavioral symptoms index, internalizing problems, and externalizing problems index T-Scores. Adjusting for additional covariates, stratifying by child sex and age, and restricting to singletons or children born term did not materially change this finding. We did however find a positive association between maternal preconception urinary BPA concentrations after restricting to singleton children with a gestation length ≥37 weeks. However, due to the substantial reduction in sample size (N = 83), this association was imprecise and could be spurious given that it was one of several secondary analyses. We found some evidence of a modestly stronger positive association between maternal preconception BPA concentrations and internalizing problems in younger children. It is possible that younger children are more at risk of internalizing problems in face of maternal preconception BPA exposure; however, future studies comparing older versus younger children would be necessary to determine if age modifies any potential association.
We are unaware of other studies examining the association of preconception urinary BPA and ∑paraben concentrations with child behavior. However, substantial, albeit heterogeneous, evidence suggests that prenatal urinary BPA concentrations are associated with neurobehavioral outcomes. In a meta-analysis, Rochester et al16 concluded that early life exposure to BPA is a “presumed” hazard to human health in regards to the development of hyperactivity. In another systematic review on BPA exposure and specifically children’s behavior, Ejaredar et al15 concluded that maternal prenatal urinary BPA concentrations were associated with increased levels of hyperactivity, inattention, conduct problems, depression, and anxiety. However, we did not observe associations between prenatal urinary BPA concentrations and children’s behavior.
There is growing evidence that paternal environments before conception may influence the health of offspring via. modification of sperm DNA methylation, histones, or microRNAs.5 In animals, paternal preconception chemical exposures are associated with the neurodevelopment of their offspring. Fan et al13 found that paternal BPA exposure impaired spatial memory in rats. Paternal and maternal preconception BPA exposure were also associated with increased anxiety and depression behaviors in rats11,12 and maternal preconception BPA exposure was associated with depressive-like symptoms in male mice.14
There is some epidemiological evidence suggesting that exposure to BPA before conception may influence birth outcomes, which in turn may be related to child neurodevelopment.30,31 Using data from the EARTH Study, Mustieles et al30 found an inverse association of maternal preconception urinary BPA concentrations with birth weight and head circumference. In a cohort of 233 infants from Michigan and Texas, Smarr et al31 reported that paternal urinary BPA concentrations were associated with increased birth length. Although there is less research on the influence of parabens on neurodevelopment in animals or humans, parabens may damage semen quality by inhibiting mitochondrial function.32
Our study has several limitations. First, it is likely that there was some exposure misclassification. Although 96% of women and 91% of male participants had 2 or more urine samples, given the short-lived nature of these chemicals, more urine sample collection in the window of exposure would improve our assessment of BPA and paraben exposures during the preconception and prenatal periods.33 Second, we were limited by our small sample size, which decreased the precision of our estimates and potentially precluded us from detecting subtle associations, particularly when evaluating sex modification and associations among singletons or children born term. Third, there was a lower percent of BPA detected among female (<62%) participants compared with male participants (81%). We speculate both the overall lower frequency of detection among women and differences across sexes may be due to more health conscious behaviors among the women while attending a fertility clinic.
Our study also has many strengths. We had multiple preconception urine samples from the vast majority of participants. Furthermore, BPA and paraben concentrations among these participants were also within the ranges reported in the US National Health and Nutrition Examination Survey,34 suggesting potential generalizability. In addition, the homogeneity in the characteristics of our study population may also help control for residual confounding. Finally, these study findings may not be generalizable to families from lower socioeconomic status, because these children were born to parents seeking fertility treatments and likely came from relatively higher socioeconomic backgrounds. Nevertheless, education and socioeconomic status remain important predictors and possible modifiers of children’s neurobehavior. It is possible that the effect of parental parabens on children’s neurobehavior is only observed among children from more disadvantage backgrounds and this may have contributed to our null results.
Conclusions
In this cohort of couples from a fertility clinic, maternal and paternal preconception and maternal prenatal concentrations of BPA and parabens were not associated with child behavior problems. Because a growing body of evidence suggests that preconception and prenatal environmental exposures impact child health, additional research using well-designed studies with sufficient sample size is needed to further explore the potential effects of exposure to BPA and parabens during the preconception and prenatal period on childhood health.
Acknowledgments
The authors gratefully acknowledge all members of the Environment and Reproductive Health (EARTH) Study team, specifically the Harvard T. H. Chan School of Public Health research staff Myra G. Keller, Ramace Dadd, physicians and staff at Massachusetts General Hospital Fertility Center. We thank Ashley Truong for entering the child behavior data, and Xiaoliu Zhou, Tao Jia, Prabha Dwivedi, and the late Xiaoyun Ye for technical assistance. We would also like to thank all study participants. The EARTH Study has been conducted in collaboration with students, post-doctoral and clinical fellows, and visiting scientists and welcomes the opportunity for new and continued collaborations. All enquiries should be made to Russ Hauser, Principal Investigator of the EARTH Study, Harvard T.H. Chan School of Public Health (rhauser@hsph.harvard.edu). More information about the study and a complete list of our publications can be found at https://www.hsph.harvard.edu/earth/.
Supplementary Material
Footnotes
Published online 27 January 2020
Sponsorships or competing interests that may be relevant to content are disclosed at the end of the article.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
J.M.B. was financially compensated for serving as an expert witness for plaintiffs in litigation related to tobacco smoke exposures and served as a paid consultant to Quest Diagnostics. The other authors have no conflicts to report.
Supported by grants R01 ES027408, R01 ES024381, R01 ES009718, R01 ES020346, and P01 ES000002 from the National Institute of Environmental Health Sciences (NIEHS).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
References
- 1.Braun JM. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol. 2017; 13:161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gore AC, Chappell VA, Fenton SE, et al. EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015; 36:E1–E150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun JM, Messerlian C, Hauser R. Fathers matter: why it’s time to consider the impact of paternal environmental exposures on children’s health. Curr Epidemiol Rep. 2017; 4:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Q, Yan W, Duan E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat Rev Genet. 2016; 17:733–743. doi:10.1038/nrg.2016.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xin F, Susiarjo M, Bartolomei MS. Multigenerational and transgenerational effects of endocrine disrupting chemicals: a role for altered epigenetic regulation? Semin Cell Dev Biol. 2015; 43:66–75. doi:10.1016/j.semcdb.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M. Environmental pollutants and child health-A review of recent concerns. Int J Hyg Environ Health. 2016; 219:331–342 [DOI] [PubMed] [Google Scholar]
- 7.Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol a and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008; 116:39–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandenberg LN, Chahoud I, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Biomonitoring studies should be used by regulatory agencies to assess human exposure levels and safety of bisphenol A. Environ Health Perspect. 2010; 118:1051–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calafat AM, Ye X, Wong L-Y, Bishop AM, Needham LL. Urinary concentrations of four parabens in the U.S. population: NHANES 2005-2006. Environ Health Perspect. 2010; 118:679–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith KW, Braun JM, Williams PL, et al. Predictors and variability of urinary paraben concentrations in men and women, including before and during pregnancy. Environ Health Perspect. 2012; 120:1538–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan Y, Huang L, Jiang D, Xu X. Research on shipborne aided navigation system based on enhanced traffic environment perception. PLoS One. 2018; 13:e0206402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris EP, Allardice HA, Schenk AK, Rissman EF. Effects of maternal or paternal bisphenol a exposure on offspring behavior. Horm Behav. 2018; 101:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Y, Ding S, Ye X, et al. Does preconception paternal exposure to a physiologically relevant level of bisphenol a alter spatial memory in an adult rat? Horm Behav. 2013; 64:598–604 [DOI] [PubMed] [Google Scholar]
- 14.Xin F, Fischer E, Krapp C, et al. Mice exposed to bisphenol a exhibit depressive-like behavior with neurotransmitter and neuroactive steroid dysfunction. Horm Behav. 2018; 102:93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ejaredar M, Lee Y, Roberts DJ, Sauve R, Dewey D. Bisphenol a exposure and children’s behavior: a systematic review. J Expo Sci Environ Epidemiol. 2017; 27:175–183 [DOI] [PubMed] [Google Scholar]
- 16.Rochester JR, Bolden AL, Kwiatkowski CF. Prenatal exposure to bisphenol a and hyperactivity in children: a systematic review and meta-analysis. Environ Int. 2018; 114:343–356 [DOI] [PubMed] [Google Scholar]
- 17.Guerra MT, Sanabria M, Cagliarani SV, Leite GAA, Borges CDS, De Grava Kempinas W. Long-term effects of in utero and lactational exposure to butyl paraben in female rats. Environ Toxicol. 2017; 32:776–788 [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi M, Morohoshi K, Imai H, Morita M, Kato N, Himi T. Maternal exposure to isobutyl-paraben impairs social recognition in adult female rats. Exp Anim. 2010; 59:631–635 [DOI] [PubMed] [Google Scholar]
- 19.Shiue I. Urinary parabens and polyaromatic hydrocarbons independent of health conditions are associated with adult emotional support needs: USA NHANES, 2005-2008. Environ Sci Pollut Res Int. 2015; 22:12951–12959 [DOI] [PubMed] [Google Scholar]
- 20.Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: a repeated measures study. Environ Int. 2018; 113:341–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koeppe ES, Ferguson KK, Colacino JA, Meeker JD. Relationship between urinary triclosan and paraben concentrations and serum thyroid measures in NHANES 2007-2008. Sci Total Environ. 2013445–446:299305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romano ME, Webster GM, Vuong AM, et al. Gestational urinary bisphenol a and maternal and newborn thyroid hormone concentrations: the HOME Study. Environ Res. 2015; 138:453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messerlian C, Williams PL, Ford JB, et al. The Environment and Reproductive Health (EARTH) Study: a prospective preconception cohort. Hum Reprod Open. 2018; 2018:hoy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye X, Bishop AM, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal Chim Acta. 2008; 622:150–156 [DOI] [PubMed] [Google Scholar]
- 25.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990; 5:46–51 [Google Scholar]
- 26.Reynolds CR, Kamphaus RW. The Clinician’s Guide to the Behavior Assessment System for Children (BASC). 2002. New York, NY, US: Guilford Press [Google Scholar]
- 27.SART. 2015.
- 28.Buckley JP, Doherty BT, Keil AP, Engel SM. Statistical approaches for estimating sex-specific effects in endocrine disruptors research. Environ Health Perspect. 2017; 125:067013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen K, Basso O, Kyvik KO, et al. Fecundability of female twins. Epidemiology. 1998; 9:189–192 [PubMed] [Google Scholar]
- 30.Mustieles V, Williams PL, Fernandez MF, et al. Maternal and paternal preconception exposure to bisphenols and size at birth. Hum Reprod Oxf Engl. 2018; 33:1528–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smarr MM, Grantz KL, Sundaram R, Maisog JM, Kannan K, Louis GM. Parental urinary biomarkers of preconception exposure to bisphenol a and phthalates in relation to birth outcomes. Environ Health. 2015; 14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavares RS, Martins FC, Oliveira PJ, Ramalho-Santos J, Peixoto FP. Parabens in male infertility-is there a mitochondrial connection? Reprod Toxicol. 2009; 27:1–7 [DOI] [PubMed] [Google Scholar]
- 33.Perrier F, Giorgis-Allemand L, Slama R, Philippat C. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology. 2016; 27:378–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CDC. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables 2019. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention. https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


