Abstract
Sour taste, which is evoked by low pH, is one of the original four fundamental taste qualities, recognized as a distinct taste sensation for centuries, and universally aversive across diverse species. It is generally assumed to have evolved for detection of acids in unripe fruit and spoiled food. But despite decades of study, only recently have the receptor, the neurotransmitter, and the circuits for sour taste been identified. In this review, we describe studies leading up to the identification of the sour receptor as OTOP1, an ion channel that is selectively permeable to protons. We also describe advances in our understanding of how information is transmitted from the taste receptor cells to gustatory neurons, leading to behavioral aversion to acids.
Keywords: OTOP1, Otopetrin, serotonin, geniculate, trigeminal, PKD2L1, sensory coding, taste, sour
Introduction
Sour, the taste evoked by lemons and other acids, has for centuries been recognized to be one of the basic taste qualities detected by the tongue. Yet mechanisms of sour taste transduction and information processing have been obscure [1]. This has changed over the last 15 years, during which time spectacular progress has been made in identifying the taste bud cells that transduce sour taste, elucidating the mechanism of transduction and beginning to crack open the neural circuits that process the information en route to the brain. In this review we will describe the recent studies that have led to our current understanding of sour taste.
Sour taste is detected by taste receptor cells (TRCs), modified epithelial cells that reside in taste buds on the tongue and palate and that are innervated by gustatory nerves (chorda tympani and glossopharyngeal) with cell bodies in the petrosal and geniculate ganglia. A major advance in our understanding of sour taste came more than a decade ago, with evidence that one class of morphologically and genetically identifiable TRCs, the Type III cells, are required for the gustatory response to acids [2] (Fig1A). In these experiments, the promoter for the Pkd2l1 gene, a marker for Type III cells [3], was used to drive expression of diphtheria toxin to selectively ablate Type III cells. A marked deficit in the gustatory nerve response to acids was observed [2] confirming a role for Type III cells in sour taste. Other important experiments showed that Type III cells respond to acids with action potentials and increases in intracellular calcium [4–7].
Figure 1. Cells and receptors for sour taste.

(A) A taste bud is composed of ~50–100 cells, of which ~15% are Type III TRCs (yellow). The Type III TRCs extend an apical process to the epithelial surface through a taste pore and are innervated by nerve fibers that send information to gustatory nuclei. (B) The transduction cascade from detection of acid stimuli to release of neurotransmitter is illustrated. In response to acidic stimuli, the sour receptor, OTOP1, conducts H+ ions (protons) into the cell cytosol. This changes the membrane potential (Vm) directly, and the change in intracellular pH blocks KIR2.1 K+ channels which further depolarizes the membrane potential. With sufficient depolarization, voltage-gated Na+ channels open causing a train of action potentials that open voltage-gated calcium channels and lead to neurotransmitter release. (C) The structure of the sour receptor, OTOP1, as solved by cryo-EM. A side view shows the 12 transmembrane helices, which are divided into two structurally similar N and C domains. A top down view shows that the channel assembles as a dimer. Possible permeation pathways for protons (arrows), are found in the N domain, the C domain, and in the intrasubunit interface between N and C domains (Based on structure reported in [25]).
It is worth noting here that PKD2L1 was identified as a putative sour taste receptor by three groups simultaneously [2, 8, 9]. However, a direct role for PKD2L1 in sour transduction was not supported by subsequent studies that showed that only a slight decrease in the gustatory response to acid stimuli in mice in which PKD2L1 or its partner, PKD1L3 were knocked out, alone or in combination [10, 11]. Thus, although the function of PKD2L1 is still enigmatic, it nonetheless provides a useful marker for Type III cells and its promoter has been used as a Cre driver both to identify these cells and to selectively manipulate their gene expression, as will be described in more detail in subsequent sections.
OTOP1, a proton channel, is the sour receptor
Building on the knowledge that Type III cells are sour TRCs, a candidate sour receptor, OTOP1, was identified in 2018 [12]. Soon thereafter the essential contribution of OTOP1 to sour taste was substantiated [13, 14] (Fig 1B). This advance came after many false starts. In the absence of functional characterization of the sour receptor, several candidate genes were proposed either because they formed acid-sensitive ion channels in other systems [15–18] or were specifically enriched in sour-responsive TRCs [2, 8, 9], not because they had the expected pharmacological or functional properties.
A major advance in understanding sour taste transduction came when it was shown that responses to acids in Type III TRCs involved a proton-selective ion channel [7]. In these experiments Type III cells were identified by expression of YFP from the Pkd2l1 promoter. Lowering the extracellular pH induced an inward current specifically in Type III TRCs. The inward current was selective to protons over other cations and had functional and pharmacological properties distinct from those of other known ion channels [7, 19]. This proton current, which was sensitive to block by extracellular Zn2+, is found selectively in Type III TRCs from all three major taste fields in the tongue (circumvallate, fungiform and foliate) [19]. Importantly, activation of the current via apical uncaging of protons, in the absence of Na+, was found to be sufficient to elicit action potentials, establishing a role for the current in sensory transduction [7].
The molecular basis for the proton channel in taste cells remained a mystery for another decade. Then in 2018, in a major breakthrough, one of us reported that the proton channel in taste cells was encoded by a most unlikely gene, Otop1 [12] (Fig 2 A,B). Otop1 was previously shown to be required for vestibular function but was not known to form an ion channel or play a role in pH sensing [20–22]. To identify the gene encoding the proton channel in taste cells, Tu et al. [12] used a combination of transcriptome profiling of genetically identified taste cells, and expression cloning. Genes enriched in sour taste cells that encoded novel transmembrane proteins were tested for functional activity. Among >40 candidates tested, one, encoding the protein OTOP1, induced large inward proton currents in response to lowering the extracellular pH. The response resembled the proton currents in TRCs. As expected for a sour receptor, expression of OTOP1 in the taste bud was largely restricted to Type III TRCs [12]. Moreover, a mutation that affects trafficking of OTOP1 in mice [23], the tlt mutation, largely abolished the proton currents in TRCs [12]. Together these data suggested that OTOP1 functions as the long-sought sour receptor.
Figure 2. Identification of OTOP1 as a sour receptor.
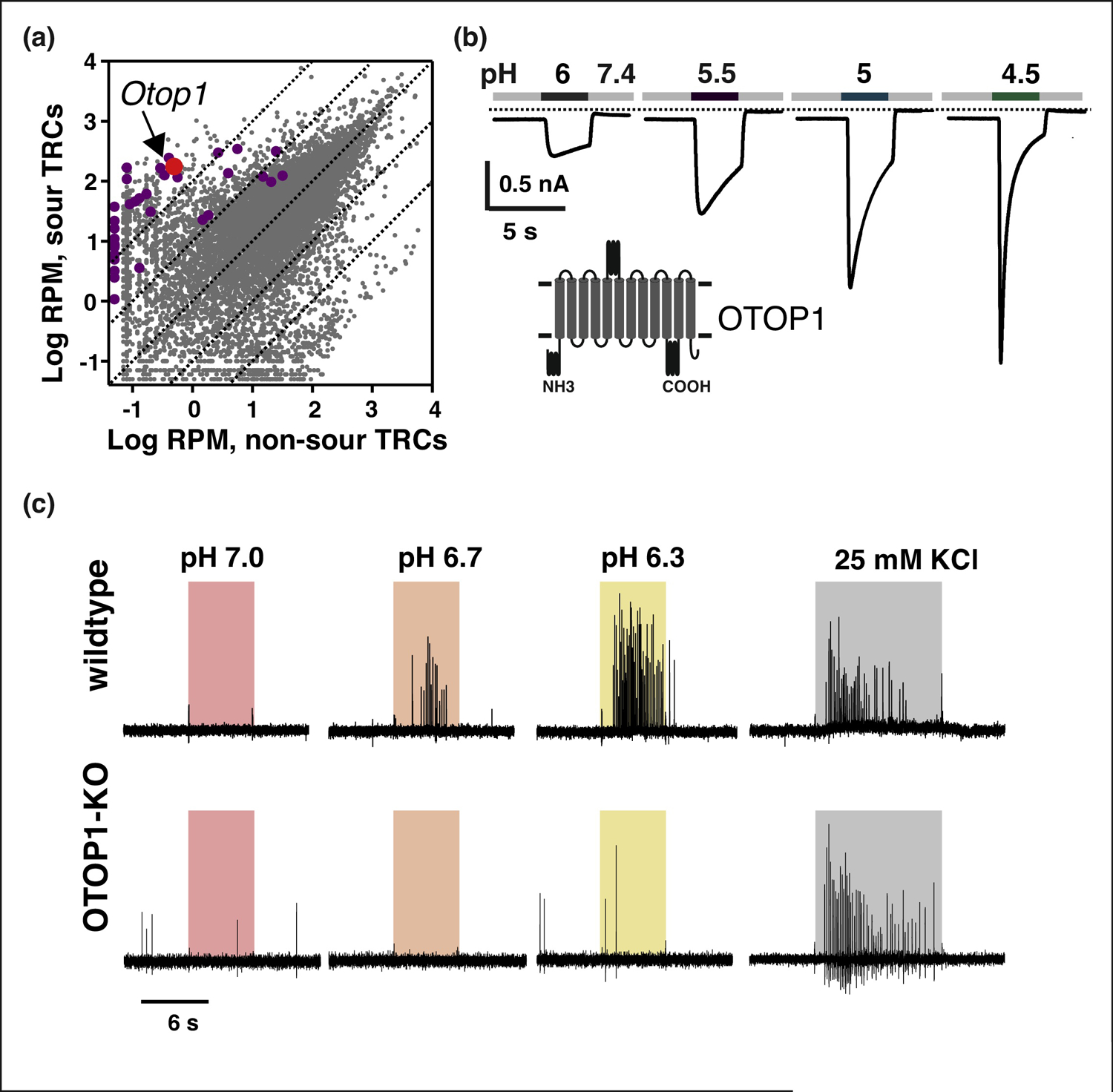
(A) A comparison of transcriptome profiling of sour-sensing taste cells (PKD2L1; Y-axis) with that of non-sour-sensing taste cells (TRPM5; X -axis) identified candidate transcripts encoding novel membrane proteins for functional testing (purple dots). Among these was Otop1 (Red dot), which is >100-fold enriched in sour-sensing taste cells (Modified from [12]). (B) Patch clamp recording of HEK-293 cells expressing OTOP1 shows that OTOP1 is an ion channel that is activated by lowering pH. In these experiments, extracellular Na+ was replaced by the large cation NMDG; inward currents are carried by H+ ions (Modified from [12]). (C) OTOP1 is required for cellular responses of Type III TRCs to acids. Here responses are measured using cell-attached patch clamp recording from genetically identified Type II TRCs from wild-type and OTOP1-KO mice. The response to acids, applied at the times indicated, are largely absent in cells from OTOP1-KO mice. These cells still respond to the positive control, high potassium (25 mM KCl). (Modified from [14]).
Affirmation that OTOP1 functions as a sour taste receptor in vivo came from two reports published simultaneously in 2019 [13, 14]. Teng et al (2019) showed that in their OTOP1 knockout mice, isolated Type III TRCs no longer responded to extracellular acidification with a proton influx, measured either with patch clamp or pH imaging. Further, Type III cells in OTOP1-KO mice did not fire action potentials to mildly acidic stimuli (Fig 2C). Collectively, this was evidence of the necessity of OTOP1 for sensory transduction. Zhang et al [13] further found that central neurons failed to respond to acid stimuli in an independent OTOP1-KO mouse strain. Finally, both groups reported that the gustatory nerve response to acids in OTOP1-KO mice were strongly attenuated, demonstrating that OTOP1 is required for sour taste signaling at the periphery in an intact animal. OTOP1-KO mice retained a small residual response to acids, which one group showed could be eliminated by exposure of the tongue to the thiol reactive compound, AITC, although the mechanism is still poorly understood [13].
Together, because OTOP1 forms a proton channel that transduces a change in extracellular pH into an electrophysiological response, and because it is required for the detection of acids by Type III TRCs, it fulfills the criteria of sufficient and necessary and can be considered a bona-fide sour taste receptor. A discussion of its contribution to behavior can be found below.
Functional properties and structure of OTOP1
The functional properties of the sour receptor OTOP1 have been best characterized in heterologous cells types, HEK-293 cells and Xenopus oocytes, which do not have endogenous proton channels. These studies [12, 14] have shown that OTOP1 mediates inward proton currents that (a) increase as the pH is lowered (Fig 2B), (b) that are not sensitive to voltage, and (c) that are blocked by Zn2+ in a dose-dependent manner. These studies have also shown that OTOP1 is highly, if not perfectly, selective for H+ ions over all other physiologically relevant ions. Thus, it could be argued that OTOP1 channels are uniquely suited to mediate sour taste transduction. Among the thousands of types of ion channels found in eukaryotes, OTOP1 and its relatives are the only proton-selective and voltage-insensitive ion channels known to date. These properties allow taste cells to respond to small changes in the concentrations of protons, and to ignore much larger changes in concentrations of other ions as different foods are sampled. Interestingly, although OTOP1 is inhibited by Zn2+, this inhibition is relieved by low pH [14,19] which may explain why Zn2+ is not a very effective blocker of sour taste.
OTOP1 is a member of a gene family conserved from C. Elegans to humans and not structurally related to any other known ion channel or transporter [24]. Many members of the family function as proton channels [12]. The structure of OTOP1 (Fig 1C) shows that it assembles as a dimeric protein containing 24 transmembrane domains that, due to structural similarity between the N and C domains of each monomer, adopt a pseudotetrameric stoichiometry [25]. Interestingly, unlike other ion channels, there is no hydrated central pore. Instead three possible permeation pathways have been identified, one in the N terminal domain of the protein, one in the C terminal domain, and one at the intrasubunit interface. Despite these advances, how the channels are gated and permeate protons remain open questions for the future.
Downstream signaling mechanisms in Type III taste receptor cells
The entry of protons through OTOP1 is expected to depolarize the cells through the positive charge that the protons carry and to acidify the cell cytosol (Fig 1B). This second affect, intracellular acidification, may also play an important role in sour taste transduction [5, 26]. The effect of intracellular pH on cell signaling is expected to be wide ranging. One molecule sensitive to pH in Type III TRCs is the K+ channel, KIR2.1, which contributes to the resting potential in these cells [27]. Intracellular acidification blocks the KIR2.1 channels, which depolarizes the cells, amplifying the response to proton influx. Whether there are other elements of transduction and cell signaling that are sensitive to intracellular pH and contribute to the sensory response remains to be determined.
From Type III cells to the brain: Sour taste circuits
In parallel to the advances in our understanding of sour transduction, the last decade has seen enormous advances in our understanding of how taste cells transmit information to the nervous system, leading to behavior. One question that has vexed the field is the nature of the neurotransmitter(s) used by taste cells to communicate with afferent nerve fibers. For Type II TRCs, the neurotransmitter is ATP [28], released by ATP permeable CALHM1 channels [29], which form a channel synapse as opposed to a typical chemical synapse with vesicles [30, 31]. In contrast, Type III TRCs, which have conventional synapses, were known for many years to contain the neurotransmitter serotonin (5-HT3; [32]) and to release 5-HT in response to acid stimulation [4]. Now several new lines of evidence show that 5HT serves as a neurotransmitter in Type III TRCs: (1) A subset of geniculate ganglion neurons that express the 5HT3 receptor, was shown to innervate the Type III TRCs using a transgenic mouse in which the 5HT3a promoter drives expression of GFP (Fig. 3A,B; [33]) (2) Pharmacological block and knockout of the 5-HT3 receptor significantly reduces chorda tympani responses to acids (Fig. 3D; [34]). However, a complication is that the knockout does not fully eliminate responses of gustatory nerves to acids and ATP/purinergic signaling is also required for communication from Type III TRCs to afferent nerve fibers [28]. Because ATP release has not been detected from Type III TRCs in response to acid stimulation [38], it cannot, as yet, be concluded that ATP functions as a neurotransmitter for sour taste.
Figure 3. Evidence for 5-HT as a neurotransmitter in Type III TRCs.

(A) Confocal z-projection of a geniculate ganglion in a 5-HT3a-GFP mouse, showing co-expression of GFP (green) and P2X3 (magenta). White box outlines area that is magnified in the inset to the left. White arrows illustrate neurons that express both markers. (B) Type III TRC labeled with an antibody against 5-HT (magenta). 5-HT3a-GFP labels a single axon that wraps around and contacts the Type III TRC at multiple locations. (C) Representative calcium imaging recording of isolated geniculate ganglion neurons in response to ATP (10 µM), 5-HT (10 μM) and KCl (50 mM). The green trace (top) is from a GFP-labeled neuron, while the black trace (bottom) is from an unlabeled neuron. All neurons responded to both ATP and KCl, but only the GFP-labeled neurons responded to 5-HT. (D) Chorda tympani nerve recordings from 5-HT3a-KO and wildtype mice in response to acid stimuli. The knockout mice showed significant reductions in the response to both citric acid and HCl compared to their wildtype littermates. (Modified from [34).
The gustatory neurons in the geniculate ganglion that innervate Type III (and other) TRCs have been further characterized in two recent studies that used single cell RNA-sequencing. The first study identified four clusters of neurons based on molecular profile -- three containing the taste specific transcription factor Phox2b and the other containing markers for mechanosensory neurons [35]. A subsequent, larger, study sampled over 800 P2X3-tdTomato expressing geniculate ganglion cells [13] and identified seven clusters of neurons, including five taste clusters and 2 somatosensory clusters. The calcium indicator gCamp6 was then targeted to the taste clusters, using the transcription factors that defined each cluster. Remarkably, sour responses were confined to the cluster marked by the transcription factor Penk, and these responses were found to be dependent on OTOP1 [13]. Utilizing viral tracing, they were able to confirm that a cluster of neurons in the nucleus of the solitary track (the prodynorphin (Pdyn)-containing neurons) that responded to sour stimuli was connected to the Penk-expressing ganglion neurons, completing the circuit from the Type III cells to the brain. Interestingly, the Penk cluster expressed high levels of 5-HT3, as expected from previous studies. Thus, we can conclude that Type III TRCs communicate with Penk+ cells in the geniculate ganglion, using 5HT as a neurotransmitter (but probably not the only) and that these cells in turn synapse onto Pdyn+ neurons in the nucleus of the solitary tract.
How do the Type III TRCs retain fidelity of signal transmission considering the constant turnover of TRCs? A clue to the specificity of connections during turnover comes from studies by Lee et al. on Type II TRCs and their target ganglion neurons [36]. They found that a class of guidance molecules, the semaphorins, are released from TRCs in a cell type-specific manner, and are detected by specific receptors in the geniculate ganglion neurons that guide the geniculate axons to their appropriate targets in taste buds. Although the specific guidance molecules that are released from Type III cells are not yet clear, some insights can be gained by mice that lack the transcription factor skn-1a, which is required for Type II cell differentiation. Skn-1a knockout mice completely lack Type II TRCs, lack responses to bitter, sweet & umami stimuli, and have a corresponding expansion of Type III TRCs [37]. Despite the large expansion of Type III TRCs, gustatory nerve responses to acids in the knockouts are not increased over those of their wildtype littermates, and the numbers of 5-HT3a expressing ganglion neurons are similarly unchanged compared to wildtype mice [38]. These data suggest that whatever signals are released by Type III TRCs to attract their ganglion cell partners, they only attract the 5-HT3a expressing ganglion cells and do not recruit the additional neurons that would have innervated Type II TRCs if they were present.
Sour taste and Behavior
How do these neural computations lead to behavior? Mice show a behavioral aversion to acids, a response that is mediated through contributions of both the taste system and the trigeminal system, which contains afferents sensitive to acids that innervate the oral and nasal cavities [39, 40]. To distinguish contributions of the two systems and test the role of the sour taste cells in behavior, two groups used optogenetics to selectively stimulate Type III TRCs in the absence of acids. Wilson et al expressed Channelrhodopsin2 (ChR2) in Type III TRCs using a PKD2L1-Cre driver and stimulated the tongue with blue light [41]. Light activation of Type III TRCs evoked a robust chorda tympani nerve response as well as a significant behavioral aversion to the light in short term 2-bottle preference tests, consistent with the notion that sour taste is innately aversive in mice. Different results were obtained by Zocchi et al [42] using a different PKD2L1-Cre driver, who showed that in the transgenic animals blue light instead evoked a powerful drive to drink [42]. This and other observations were used to argue for a role of Type III cells in the detection of water; but this conclusion remains speculative, absent a receptor mechanism.
Given evidence showing that OTOP1 functions as a sour taste receptor, it is natural to ask whether it is required for behavioral aversion to acids. Two groups showed that behavioral aversion to the taste of acids appears to be completely unchanged in mice that carry a targeted inaction of the Otop1 gene [13, 14]. This is not completely surprising as even a complete genetic ablation of taste receptor cells was shown to produce no change in behavioral aversion to acidic solutions [42]. Moreover, genetic inactivation of two ion channels implicated in the response of the trigeminal system to acids, TRPA1 and TRPV1, also did not affect the taste preference of animals towards acids [40]. Only the combination of surgical ablation of the trigeminal system with Otop1 gene inactivation produced a change in sensitivity to acids [13]. This suggests a high degree of redundancy and similar acid sensitivity of the two systems.
Finally, does stimulation of the prodynorphin-expressing (Pdyn) neurons of the nucleus of the solitary track elicit aversive behavior, as expected if they signal the presence of acid stimuli? Zhang et al. [13] targeted this cluster using pdyn-Cre to drive ChR2. Light stimulation of the Pdyn-ChR2 containing cells elicited an aversive response, similar to a sour stimulus, in thirsty mice drinking water. Further, they trained mice in an operant conditioning paradigm to distinguish sour stimuli from bitter stimuli, both aversive tastes. Upon stimulation of the Pdyn-Chr2 cells, the mice identified the light stimulus as a sour stimulus, showing that this cluster of neurons in the solitary track are directly involved in processing the sour taste signal. Further studies are needed to follow the pathway centrally to the gustatory cortex to determine if “hot spots” of sour responsive neurons are present, or if responses are more broadly distributed.
Summary and Future Directions
This is an exciting time for research on sour taste, with the identification, at long last, of the sour receptor and the development of tools to trace, study and manipulate neural circuits. Questions that remain for the future include the following:
What mechanism(s) allow the sour receptor, OTOP1, to respond to acid stimuli over the appropriate pH range so as to detect physiologically and ecologically relevant levels of acidity in foods?
What is the role of ATP in signaling from Type III TRCs to gustatory nerve fibers and is there cross talk between different taste receptor cell types?
What is the full repertoire of receptors in taste cells and trigeminal nerve fibers that underlie behavioral aversion to acids in mice?
What function(s) did sour taste play during vertebrate evolution and why do human prefer sour taste (when combined with sweeteners)?
Funding:
This work was supported by the National Institutes of Health [R01GM131235; R01DC013741; R01DC017679; R01DC012555].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
The authors declare no conflict of interests.
Annotated References
- [1].Liman ER, Zhang YV, Montell C, Peripheral coding of taste, Neuron 81(5) (2014) 984–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS, The cells and logic for mammalian sour taste detection, Nature 442(7105) (2006) 934–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sevigny J, Kinnamon JC, Finger TE, The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse, Chem Senses 33(3) (2008) 243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Huang YA, Maruyama Y, Stimac R, Roper SD, Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste, J Physiol 586(Pt 12) (2008) 2903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Richter TA, Caicedo A, Roper SD, Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells, J Physiol 547(Pt 2) (2003) 475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yoshida R, Miyauchi A, Yasuo T, Jyotaki M, Murata Y, Yasumatsu K, Shigemura N, Yanagawa Y, Obata K, Ueno H, Margolskee RF, Ninomiya Y, Discrimination of taste qualities among mouse fungiform taste bud cells, J Physiol 587(Pt 18) (2009) 4425–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chang RB, Waters H, Liman ER, A proton current drives action potentials in genetically identified sour taste cells, Proc Natl Acad Sci U S A 107(51) (2010) 22320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H, Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor, Proc Natl Acad Sci U S A 103(33) (2006) 12569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].LopezJimenez ND, Cavenagh MM, Sainz E, Cruz-Ithier MA, Battey JF, Sullivan SL, Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells, J Neurochem 98(1) (2006) 68–77. [DOI] [PubMed] [Google Scholar]
- [10].Horio N, Yoshida R, Yasumatsu K, Yanagawa Y, Ishimaru Y, Matsunami H, Ninomiya Y, Sour taste responses in mice lacking PKD channels, PLoS One 6(5) (2011) e20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nelson TM, Lopezjimenez ND, Tessarollo L, Inoue M, Bachmanov AA, Sullivan SL, Taste function in mice with a targeted mutation of the pkd1l3 gene, Chem Senses 35(7) (2010) 565–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[12].Tu YH, Cooper AJ, Teng B, Chang RB, Artiga DJ, Turner HN, Mulhall EM, Ye W, Smith AD, Liman ER, An evolutionarily conserved gene family encodes proton-selective ion channels, Science 359(6379) (2018) 1047–1050.This paper describes the identification of OTOP1 as a proton channel and candidate sour taste receptor. The approach involves functional analysis of >40 candidate genes differentially expressed as assessed by RNA-seq analysis of Type II and Type III taste cells. They show that OTOP1 is highly selective for protons over other ions (by nearly a million-fold), is immunolocalized to Type III taste cells, and is required for proton currents in these cells. Further, they show that related proteins, OTOP2 and OTOP3 (not expressed in the gustatory system), and a drosophila ortholog also function as proton channels.
- **[13].Zhang J, Jin H, Zhang W, Ding C, O’Keeffe S, Ye M, Zuker CS, Sour Sensing from the Tongue to the Brain, Cell 179(2) (2019) 392–402 e15.This paper, along with Teng et al., (2019) confirmed using a knockout strategy that OTOP1 is the sour sensor in the Type III taste cells. In addition, they profiled over 800 single geniculate ganglion neurons using RNA-seq and identified the penk-expressing ganglion neurons as the neurons that receive input from the Type III cells. Further, using viral tracing techniques they identified the central neurons in the circuit and showed, using operant behavioral strategies, that optogenetic stimulation elicited a behavioral recognition of sour stimuli that depended on a functional OTOP1 channel.
- **[14].Teng B, Wilson CE, Tu YH, Joshi NR, Kinnamon SC, Liman ER, Cellular and Neural Responses to Sour Stimuli Require the Proton Channel Otop1, Curr Biol 29(21) (2019) 3647–3656 e5.This paper, along with Zhang et al (2019) uses a knockout strain to show that OTOP1 functions as a sour taste receptor in mice in vivo. They use patch clamp recording to show that OTOP1 is the only proton channel in taste cells and that it is required for cellular responses to acids. Further, they use gustatory nerve recording to establish the requirement for OTOP1 in an intact animal. Despite the evidence that OTOP1 is the sour receptor, they, along with Zhang et al, 2019, observe no change in behavioral preference for acids in the OTOP1 KO mouse, suggesting that avoidance of acids is not simply encoded by the gustatory system.
- [15].Ugawa S, Yamamoto T, Ueda T, Ishida Y, Inagaki A, Nishigaki M, Shimada S, Amiloride-insensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ASIC2b heteromeric sour-taste receptor channel, J Neurosci 23(9) (2003) 3616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stevens DR, Seifert R, Bufe B, Muller F, Kremmer E, Gauss R, Meyerhof W, Kaupp UB, Lindemann B, Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli, Nature 413(6856) (2001) 631–5. [DOI] [PubMed] [Google Scholar]
- [17].Lin W, Burks CA, Hansen DR, Kinnamon SC, Gilbertson TA, Taste receptor cells express pH-sensitive leak K+ channels, J Neurophysiol 92(5) (2004) 2909–19. [DOI] [PubMed] [Google Scholar]
- [18].Richter TA, Dvoryanchikov GA, Chaudhari N, Roper SD, Acid-sensitive two-pore domain potassium (K2P) channels in mouse taste buds, J Neurophysiol 92(3) (2004) 1928–36. [DOI] [PubMed] [Google Scholar]
- [19].Bushman JD, Ye W, Liman ER, A proton current associated with sour taste: distribution and functional properties, FASEB J 29(7) (2015) 3014–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hurle B, Ignatova E, Massironi SM, Mashimo T, Rios X, Thalmann I, Thalmann R, Ornitz DM, Non-syndromic vestibular disorder with otoconial agenesis in tilted/mergulhador mice caused by mutations in otopetrin 1, Hum Mol Genet 12(7) (2003) 777–89. [DOI] [PubMed] [Google Scholar]
- [21].Hughes I, Blasiole B, Huss D, Warchol ME, Rath NP, Hurle B, Ignatova E, Dickman JD, Thalmann R, Levenson R, Ornitz DM, Otopetrin 1 is required for otolith formation in the zebrafish Danio rerio, Dev Biol 276(2) (2004) 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hughes I, Saito M, Schlesinger PH, Ornitz DM, Otopetrin 1 activation by purinergic nucleotides regulates intracellular calcium, Proc Natl Acad Sci U S A 104(29) (2007) 12023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim E, Hyrc KL, Speck J, Salles FT, Lundberg YW, Goldberg MP, Kachar B, Warchol ME, Ornitz DM, Missense mutations in Otopetrin 1 affect subcellular localization and inhibition of purinergic signaling in vestibular supporting cells, Mol Cell Neurosci 46(3) (2011) 655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hughes I, Binkley J, Hurle B, Green ED, Program NCS, Sidow A, Ornitz DM, Identification of the Otopetrin Domain, a conserved domain in vertebrate otopetrins and invertebrate otopetrin-like family members, BMC Evol Biol 8 (2008) 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[25].Saotome K, Teng B, Tsui CCA, Lee WH, Tu YH, Kaplan JP, Sansom MSP, Liman ER, Ward AB, Structures of the otopetrin proton channels Otop1 and Otop3, Nat Struct Mol Biol 26(6) (2019) 518–525.This paper describes the cryo-EM structure of zebrafish OTOP1 (zfOTO1), along with that of the related chicken OTOP3 (chOTOP3). The structure shows that the channel forms a dimer, with no central permeation pathway. Molecular dynamic simulations show hydration of three putative pores and single site amino acid substitution show the essential role for conserved charged residues in each putative pore.
- [26].Lyall V, Alam RI, Phan DQ, Ereso GL, Phan TH, Malik SA, Montrose MH, Chu S, Heck GL, Feldman GM, DeSimone JA, Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction, Am J Physiol Cell Physiol 281(3) (2001) C1005–13. [DOI] [PubMed] [Google Scholar]
- [27].Ye W, Chang RB, Bushman JD, Tu YH, Mulhall EM, Wilson CE, Cooper AJ, Chick WS, Hill-Eubanks DC, Nelson MT, Kinnamon SC, Liman ER, The K+ channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction, Proc Natl Acad Sci U S A 113(2) (2016) E229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC, ATP signaling is crucial for communication from taste buds to gustatory nerves, Science 310(5753) (2005) 1495–9. [DOI] [PubMed] [Google Scholar]
- [29].Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK, CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes, Nature 495(7440) (2013) 223–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[30].Romanov RA, Lasher RS, High B, Savidge LE, Lawson A, Rogachevskaja OA, Zhao H, Rogachevsky VV, Bystrova MF, Churbanov GD, Adameyko I, Harkany T, Yang R, Kidd GJ, Marambaud P, Kinnamon JC, Kolesnikov SS, Finger TE, Chemical synapses without synaptic vesicles: Purinergic neurotransmission through a CALHM1 channel-mitochondrial signaling complex, Sci Signal 11(529) (2018).This paper used a combination of scanning block face electron microscopy and electrophysiology to demonstrate that the ATP-release channel CALHM1 forms a signaling complex with atypical mitochondria in Type II cells to mediate purinergic neurotransmission. Taruno et al. [31] later termed this signaling complex a “channel synapse”.
- [31].Taruno A, Nomura K, Kusakizako T, Ma Z, Nureki O, Foskett JK, Taste transduction and channel synapses in taste buds, Pflugers Arch (2020). [DOI] [PMC free article] [PubMed]
- [32].Nada O, Hirata K, The occurrence of the cell type containing a specific monoamine in the taste bud of the rabbit’s foliate papila, Histochemistry 43(3) (1975) 237–40. [DOI] [PubMed] [Google Scholar]
- [33].Stratford JM, Larson ED, Yang R, Salcedo E, Finger TE, 5-HT3A -driven green fluorescent protein delineates gustatory fibers innervating sour-responsive taste cells: A labeled line for sour taste?, J Comp Neurol 525(10) (2017) 2358–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Larson ED, Vandenbeuch A, Voigt A, Meyerhof W, Kinnamon SC, Finger TE, The Role of 5-HT3 Receptors in Signaling from Taste Buds to Nerves, J Neurosci 35(48) (2015) 15984–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dvoryanchikov G, Hernandez D, Roebber JK, Hill DL, Roper SD, Chaudhari N, Transcriptomes and neurotransmitter profiles of classes of gustatory and somatosensory neurons in the geniculate ganglion, Nat Commun 8(1) (2017) 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lee H, Macpherson LJ, Parada CA, Zuker CS, Ryba NJP, Rewiring the taste system, Nature 548(7667) (2017) 330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y, Abe K, Skn-1a (Pou2f3) specifies taste receptor cell lineage, Nat Neurosci 14(6) (2011) 685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *[38].Larson ED, Vandenbeuch A, Anderson CB, Kinnamon SC, Function, Innervation, and Neurotransmitter Signaling in Mice Lacking Type-II Taste Cells, eNeuro 7(1) (2020).This paper used transgenic mice lacking the transcription factor skn-1a, which is required for development of Type II cells. In these mice, Type II cells are completely eliminated and Type III cells are proportionally expanded. Despite the large expansion of Type III cells, 5-HT3a expressing ganglion neurons are not concomitantly expanded and responses to sour stimuli are not different from those in wildtype mice. Further, this paper shows that while ATP release is not detected from Type III cells in these mice, ATP is still required for chorda tympani responses to sour stimuli.
- [39].Wang YY, Chang RB, Allgood SD, Silver WL, Liman ER, A TRPA1-dependent mechanism for the pungent sensation of weak acids, J Gen Physiol 137(6) (2011) 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yu T, Wilson CE, Stratford JM, Finger TE, Genetic Deletion of TrpV1 and TrpA1 Does not Alter Avoidance of or Patterns of Brainstem Activation to Citric Acid in Mice, Chem Senses (2020). [DOI] [PMC free article] [PubMed]
- *[41].Wilson CE, Vandenbeuch A, Kinnamon SC, Physiological and Behavioral Responses to Optogenetic Stimulation of PKD2L1(+) Type III Taste Cells, eNeuro 6(2) (2019).This paper utilized PKD2l1-Cre mice to drive channelrhodopsin specifically in Type III taste cells. Stimulation of the tongue with blue light evokes chorda tympani nerve responses, similar to those evoked by sour stimuli. In behavioral assays, stimulation of the lick spout with blue light evokes behavioral avoidance as would be expected for a sour stimulus.
- [42].Zocchi D, Wennemuth G, Oka Y, The cellular mechanism for water detection in the mammalian taste system, Nat Neurosci 20(7) (2017) 927–933. [DOI] [PubMed] [Google Scholar]


